Abstract
Lipid metabolism disorders, which are closely linked to obesity, are significantly modulated by the enzyme 11β-hydroxysteroid dehydrogenase 1 (11β-HSD1), a key regulator of glucocorticoid (GC) activation. This study explored the therapeutic potential of H8, a curcumin analog and selective inhibitor of 11β-HSD1, in mitigating these disorders. Employing an in vitro model of GC-induced differentiation in 3T3-L1 adipocytes and an in vivo C57BL/6 mice model characterized by 11β-HSD1 overexpression, we demonstrated that treatment with H8 effectively reduced GC levels in both serum and cultured cells. Lipid accumulation, evaluated through Oil Red O and hematoxylin and eosin (HE) staining, was significantly diminished by H8 in both cellular and animal models. Mechanistic investigations revealed that H8 modulated lipid metabolism through dual mechanisms: inhibition of 11β-HSD1 and activation of the AMP-activated protein kinase (AMPK) signaling pathway. Immunohistochemical and immunofluorescence analyses corroborated these lipid-modulating effects, while RNA and protein profiling identified significant alterations in markers of lipid synthesis. Further examination of the interplay between 11β-HSD1 and AMPK led us to hypothesize that H8 ameliorates lipid metabolism disorders primarily through its inhibitory effects.
Similar content being viewed by others
Introduction
Obesity, a metabolic disorder and integral component of metabolic syndrome, represents a substantial public health challenge due to its pronounced association with an elevated risk of cardiovascular diseases and type 2 diabetes mellitus (T2DM)1. Characterized by an excessive accumulation of body fat resulting from an imbalance between energy intake and expenditure, obesity constitutes a pathological condition with significant health implications. Glucocorticoid (GC), which are essential steroid hormones, play a crucial role in the regulation of glucose and lipid metabolism. They significantly influence obesity by modulating food intake, lipid synthesis and catabolism, as well as the differentiation and maturation of adipocytes.
The hypothalamic-pituitary-adrenal (HPA) axis primarily regulates GC secretion, while 11β-hydroxysteroid dehydrogenase type 1 (11β-HSD1) modulates local GC activity2. Metabolic disorders associated with GC in tissues arise from the aberrant upregulation or downregulation of 11β-HSD1 expression. As a member of the 11β-HSDs family, 11β-HSD1 is a microsomal enzyme that predominantly facilitates the interconversion of glucocorticoids and their metabolites. It possesses dual regulatory functions, characterized by high-affinity reductase activity and low-affinity oxidase activity. The reductase activity of 11β-HSD1 catalyzes the conversion of inactive GC (cortisone in humans and dehydrocorticosterone in mice) into biologically active GC (cortisol in humans and corticosterone (CORT) in mice). Research has demonstrated that mice overexpressing 11β-HSD1 exhibit increased local GC levels3. Clinical investigations utilizing in situ hybridization have identified elevated Hsd11b1 mRNA expression in individuals with higher levels of obesity4. Moreover, research has demonstrated that 11β-HSD1 plays a role in adipocyte differentiation, with both the differentiation and maturation of adipocytes being associated with elevated levels of Hsd11b1 mRNA and increased redox enzyme activity5. These findings highlight the significant relationship between 11β-HSD1 and obesity, as well as other manifestations of metabolic syndrome. Previous studies have established that GC can inhibit AMP-activated protein kinase (AMPK) activity by promoting gluconeogenesis6. AMPK is a critical pathway that connects obesity, insulin resistance, and T2DM. Investigating the relationship between 11β-HSD1 and AMPK in the development of obesity, and exploring the inhibition of 11β-HSD1 as a potential therapeutic target, is essential for addressing lipid metabolism disorders related to obesity.
Currently, known 11β-HSD1 inhibitors can be categorized into two primary groups: natural inhibitors and chemically synthesized inhibitors. Natural inhibitors, such as glycyrrhizic acid and resveratrol, exhibit relatively fewer side effects and demonstrate anti-obesity efficacy; however, they are limited by poor bioavailability and lack of selectivity7,8. In contrast, synthetic inhibitors such as MK-0736 and MK-0916 have demonstrated therapeutic efficacy in overweight patients; however, they are associated with potential adverse effects including hypotension9. Curcumin, a low-molecular-weight phenolic compound derived from turmeric-a traditional medicinal ingredient-has exhibited significant positive effects in anticancer, anti-inflammatory, and hypoglycemic activities10,11,12. It has been established that curcumin acts as natural inhibitor of 11β-HSD113. Research has indicated that dietary administration of curcumin can prevent weight gain in rodent models14. Curcumin administration has been shown to mitigate high-fat diet-induced obesity and insulin resistance by suppressing hepatic lipogenesis and inflammatory pathways in adipocytes, while also reducing blood glucose levels by upregulating glucose transporter expression in both mice and humans15,16. Additionally, adipocytes cultured with curcumin exhibit inhibited adipocyte differentiation and lipogenesis17,18. Despite these potential health benefits, the efficacy of curcumin is limited by its low bioavailability, poor solubility, and rapid metabolism19,20. To address these limitations, we modified the chemical structure of curcumin by removing the β-diketone group, leading to the synthesis of H8: (2E,5E)-2,5-bis [2-fluoro-6-(trifluoromethyl) benzylidene] cyclopentanone. This curcumin analog possesses a reduced molecular weight and enhances structural stability. Previous experimental studies have demonstrated that H8 exhibits superior bioavailability compared to curcumin, particularly in enhancing insulin sensitivity, regulating glucose metabolism disorders, and reducing visceral fat accumulation21,22,23. However, its potential efficacy in treating obesity and the underlying mechanisms remain unclear, necessitating further investigation.
Consequently, the primary objective of this study was to examine the effects of H8 on lipid metabolism in both 3T3-L1 cells and a C57BL/6 mice model. The study aims to determine whether H8, as a selective inhibitor of 11β-HSD1, exerts its effects by suppressing excessive GC levels to modulate the AMPK signaling pathway and ameliorate lipid metabolism disorders. This research endeavors to provide an experimental foundation for the development of novel therapeutics targeting obesity and other symptoms associated with metabolic syndrome.
Materials and methods
Reagent description
H8 was designed and synthesized by the Provincial Key Laboratory of Antifibrotic Biotherapy at Mudanjiang Medical University21. It is a curcumin analog with enhanced structural stability, derived from the curcumin parent structure. The chemical name of H8 is (2E,5E)-2,5-bis [2-fluoro-6-(trifluoromethyl) benzylidene] cyclopentanone, and its purity was confirmed to be 99.3% using high performance liquid chromatography.
Cell grouping and treatment
From the American Type Culture Collection (Manassas, VA, USA), 3T3-L1 mouse pre-adipocytes were purchased. The cell experiments were divided into six groups: CON group, CORT group (plus adipose differentiation medium 1), CORT + H8 group (plus adipose differentiation medium 1 + H8), dehydrocorticosterone (DHT) group (plus adipose differentiation medium 2), DHT + 11β-HSD1 group (transfected with 11β-HSD1 + adipose differentiation medium 2), and DHT + 11β-HSD1 + H8 group (transfected with 11β-HSD1 + adipose differentiation medium 2 + H8).
The pre-adipocytes were cultured in 96-well plates for 48 h at 37 °C in an environment containing 5% CO₂, using DMEM medium enriched with high glucose (Gibco) and supplemented with 10% fetal bovine serum (FBS, Gibco, New York, USA). Once the cells became confluent, the medium in the plates was switched to adipose differentiation medium 1 (Solution A: 0.5 mmol/L isobutyl-3-methylxanthine, 1.7 nmol/mL insulin, 1 µmol/L CORT, 10% FBS, high-glucose DMEM; Solution B: 1.7 nmol/mL insulin, 10% FBS, high-glucose DMEM) or adipose differentiation medium 2 (Solution A: 0.5 mmol/L isobutyl-3-methylxanthine, 1.7 nmol/mL insulin, 1 µmol/L DHT, 10% FBS, high-glucose DMEM; Solution B: 1.7 nmol/mL insulin, 10% FBS, high-glucose DMEM). The medium was switched to Solution B of the respective adipose differentiation medium after 48 h of culture. The cells were then cultured for an additional 48 h, followed by maintenance in high-glucose DMEM medium supplemented with 10% FBS. The medium was replaced every two days, and the differentiation process typically required 8–12 days. At this stage, the cells were considered fully differentiated into mature adipocytes, representing the optimal state for experimental use.
Animal modeling and grouping
Healthy male C57BL/6 mice were sourced from Beijing Vital River Laboratory Animal Technology Co., Ltd., holding the license number SCXK (Jing) 2012-0001. The ethical standards of ARRIVE Guidelines 2.0 and the Guide for the Care and Use of Laboratory Animals were followed in all animal experiments and approved by the Experimental Animal Welfare and Ethics Committee of Mudanjiang Medical University (protocol code IACUC-20200608-3). All experiments were performed in accordance with relevant institutional guidelines and regulations. The study involved 32 C57BL/6 mice, each 8 weeks of age and weighing 20–22 g. The mice were accommodated in a specific pathogen-free environment with a humidity level of 55%, a temperature of 20 °C, and a 12-hour light/dark cycle. They were given unrestricted access to food and water and acclimated for 7 days.
The 32 C57BL/6 mice were allocated randomly into four distinct groups (n = 8 per group). The control group (CON group) and the CON + H8 group received tail vein injections of blank plasmid, while the 11β-HSD1 group and the 11β-HSD1 + H8 group were injected with 11β-HSD1 lentivirus via the tail vein. Inject every other day for a total of 3 doses. Mice in the CON group and the 11β-HSD1 group were administered sodium carboxymethyl cellulose by gavage, whereas mice in the CON + H8 group and the 11β-HSD1 + H8 group received H8 (0.5 mg·kg⁻¹·d⁻¹) by gavage. Measurements of body weight and food intake were conducted every week, and changes in serum biochemical indicators were monitored. The mice were maintained for 60 days. After successful modeling, all mice were fasted for 12 h and euthanized by gradual displacement with 100% CO₂ for 5 min, and samples of serum and epididymal white adipose tissue (eWAT) were gathered and kept at -80 °C for later examination.
Biochemical analysis
According to manufacturer guidelines, the serum CORT levels in mice were assessed using a CORT ELISA Kit (Abcam, Cambridge, UK). Using an automated analyzer, biochemical indicators such as CHO, TG, LDL and HDL were measured in mice serum as per the manufacturer’s protocol (Beckman Coulter, CA, USA).
Oil red O staining
3T3-L1 cells were rinsed three times with PBS, allowed to air dry, and then fixed with a 10% formaldehyde solution for 5 min. The differentiation status of the cells was then examined through Oil Red O staining (Sigma, California, USA). The dye was dissolved in isopropanol to create a 0.5% Oil Red O solution and diluted with water at a 3:2 ratio before use, followed by filtration. The formaldehyde solution was extracted from the cell plates, and the cells were subsequently washed with 60% isopropanol and air-dried. Subsequently, 1 mL of the Oil Red O solution was used for staining, and the cells were incubated for 10 min. Excess stain was removed by washing with 60% isopropanol, followed by rinsing with distilled water. Stained cells were observed and photographed under an optical microscope (Olympus, Tokyo, Japan).
Hematoxylin and Eosin (HE) staining
Paraffin-embedded sections were prepared for mouse adipose tissue. The adipose tissue was preserved in 4% paraformaldehyde for a duration of 48 h, followed by gradient dehydration using increasing concentrations of ethanol (70% to absolute ethanol). After wax immersion, the tissue was embedded using an embedding machine. Pathological changes in adipose tissue were observed using hematoxylin and eosin (HE) staining (Solarbio, Beijing, China). Mouse adipose tissue embedded in paraffin was sliced into 4 μm thick sections with a microtome (Leica, Wetzlar, Germany). The sections were fixed, dehydrated, stained with HE, and mounted with neutral resin. After air-drying, microscopic observation and photography were conducted on the sections.
RNA extraction and real-time PCR
Following instructions provided by the manufacturer, total RNA was extracted from cells and mouse adipose tissue using an RNA extraction kit (Omega, USA). The concentration of RNA was determined with a Nanodrop 2000 (Thermo Fisher, Massachusetts, USA). Using a reverse transcription kit (Roche, Basel, Switzerland), the RNA was converted into cDNA according to the manufacturer’s instructions. For real-time quantitative PCR, 2 µL of diluted cDNA was mixed with 9 µL of SYBR Green Mix (ORIGENE, Maryland, USA), 0.8 µL each of forward and reverse primers for Hsd11b1, Srebp1c, Fas, Acc1, Cpt1, Ucp1, and Hsl, and 7.4 µL of the internal reference Ribosomal Protein S16 (Rps16) and ddH₂O. A real-time PCR system (Bio Rad, California, USA) was utilized to perform the reaction under the following conditions: pre-denaturation at 95 °C for 5 min, denaturation at 95 °C for 30 s, annealing at 53–60 °C for 30 s and 40 amplification cycles. The Ct values for both the target genes and the internal reference were measured, and the relative mRNA expression levels were derived using the standard curve. Primer sequences can be found in Table 1.
Western blotting
Mouse adipose tissue or cells were used to extract proteins, and their concentration was assessed using a Nanodrop 2000. A total volume of 16 µL of protein samples was loaded in sequence, separated by SDS-PAGE gel electrophoresis, and then transferred onto a polyvinylidene fluoride (PVDF) membrane (Roche, Basel, Switzerland). The membrane made of PVDF was treated with a 10% BSA solution at room temperature for 1 h, followed by incubation with primary antibodies at a dilution of 1:1000 [11β-HSD1, CPT1, UCP1, AMPK, p-AMPK (Abcam, Cambridge, UK), and β-actin (Cell Signaling, Boston, USA)] at 4 °C overnight. After the washing step, secondary antibodies diluted at 1:10000 were used to incubate the PVDF membrane at room temperature for 60 min. Following thorough washing, the membrane was developed using a DAB chromogenic solution (TIANGEN, Beijing, China) and imaged using a highly sensitive multifunctional imaging analyzer (General Electric, Boston, USA). Band intensity analysis was conducted using Image J software (version 1.8.0), and protein expression levels were calculated.
Immunofluorescence staining
Immunofluorescence staining was performed on paraffin-embedded sections of mouse fat tissue. Xylene was used to deparaffinize the sections, which were then rehydrated using a series of graded alcohols. The adipose tissue was immersed in 3% H₂O₂ solution for 10 min, rinsed with distilled water for 2 min, and then subjected to antigen retrieval by boiling in a citrate buffer until just boiling, followed by cooling to room temperature to expose antigenic sites. The sections underwent a 1-hour blocking process with 3% skim milk at 37 °C. Subsequently, overnight incubation of the sections was done using a 1:55 dilution of the F4/80 antibody (Abcam, Cambridge, UK), this was followed by a one-hour incubation with a fluorescent secondary antibody diluted at 1:1000. The sections were then stained with 1 µg/mL of DAPI solution (Sigma, California, USA) for 10 min, mounted with neutral resin, and imaged using an optical microscope. Green fluorescence protein intensity was analyzed.
Immunohistochemistry
For immunohistochemical staining of FAS and HSL in mouse adipose tissue, paraffin sections were dried in an oven at 65 °C, deparaffinized and then rehydrated with a graded series of alcohols. An H₂O₂ solution and citrate buffer were used for antigen retrieval. The sections were treated with 3% skim milk and left to incubate overnight at 4 °C with FAS and HSL antibodies diluted at 1:100, followed by incubation with a 1:1000 dilution of secondary antibody for 1 h. After mounting, the sections were allowed to dry in a ventilated area, imaged, and analyzed for protein intensity.
Statistical analysis
GraphPad Prism 8.2.1 software (GraphPad Software Inc., San Diego, CA, USA) was used for statistical analysis and data processing. All data are expressed as mean ± standard deviation (SD). One-way analysis of variance (ANOVA) was used to analyze comparisons among more than two groups, with a P-value under 0.05 indicating statistical significance.
Results
H8 alleviates obesity in 11β-HSD1-overexpressing mice
We successfully developed a model of 11β-HSD1 overexpression and administered H8 treatment (Fig. 1A). Through monitoring food intake and body weight changes, it was observed that the 11β-HSD1 group consistently exhibited greater body weight compared to the 11β-HSD1 + H8 group following model induction. There were no statistically significant differences in food intake observed among the groups (Fig. 1B-C). Biochemical analysis demonstrated H8 intervention significantly mitigated weight gain in mice relative to the Con and 11β-HSD1 groups (Fig. 1D). In mice, overexpression of 11β-HSD1disrupted lipid metabolism, resulting in significantly elevated serum levels of CORT, cholesterol (CHO), triglycerides (TG), low-density lipoprotein (LDL), and high-density lipoprotein (HDL) compared to the control group. The H8 intervention significantly reduced serum concentrations of CORT, CHO, TG, LDL, and HDL in mice (Fig. 1E-I).
H8 modulates biochemical profiles in mice models. (A) Flowchart of C57BL/6 mice model establishment experiment. (B-C) Monitoring of food intake and body weight during model induction. (D-I) Serum biochemical profiling of mice. The data represent the mean ± SD (n = 8). *P < 0.05, **P < 0.01, ***P < 0.001 vs. Con group, ### P < 0.001 vs. 11β-HSD1 group.
H8 reduces the size of adipocytes in adipose tissue
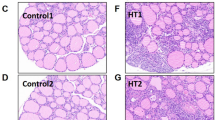
HE staining of adipose tissue sections from each experimental group was examined (Fig. 2A-B). The 11β-HSD1 group demonstrated a significant increase in adipocyte size compared to the Con group. However, after treatment with H8, there was a significant improvement in adipocyte morphology.
H8 modulates pathological remodeling of adipose tissue. (A-B) HE staining images and quantitative analysis of adipocytes (black arrow) in adipose tissue in mice (original magnification, × 100 and × 200). The data represent the mean ± SD (n = 8). ***P < 0.001 vs. Con group, ### P < 0.001 vs. 11β-HSD1 group.
H8 suppresses 11β-HSD1 to ameliorate adipose tissue lipid metabolic disorders in mice
As shown in Fig. 3A-E, in the 11β-HSD1 group the protein expression levels of 11β-HSD1 were significantly elevated relative to the Con group. Conversely, the 11β-HSD1 + H8 group demonstrated a notable decrease in 11β-HSD1 protein expression compared to the 11β-HSD1 group (Fig. 3B). Furthermore, the protein expression levels of UCP1 and the p-AMPK/AMPK ratio were significantly reduced in the 11β-HSD1 group compared to the Con group. In contrast, the 11β-HSD1 + H8 group exhibited a substantial increase in the protein expression levels of UCP1, the p-AMPK/AMPK ratio, and CPT1 relative to the 11β-HSD1 group.
Regarding mRNA expression, the levels of Hsd11b1, Srebp1c, Fas, and Acc1 were significantly higher in the 11β-HSD1 group compared to the Con group. However, the 11β-HSD1 + H8 group showed a significant reduction in the mRNA expression of these genes compared to the 11β-HSD1 group (Fig. 3F-I). Additionally, the mRNA expression levels of Cpt1, Ucp1, and Hsl were significantly lower in the 11β-HSD1 group compared to the Con group. The 11β-HSD1 + H8 group, however, exhibited a marked increase in the expression levels of these genes relative to the 11β-HSD1 group (Fig. 3J-L).
H8 alleviates lipid metabolic disorders in murine adipose tissue via suppression of 11β-HSD1. (A) The representative western blotting images. (B-E) Protein expression level. (F-L) Relative mRNA expression level. The data represent the mean ± SD (n = 8). *P < 0.05, **P < 0.01, ***P < 0.001 vs. Con group, #P < 0.05, ##P < 0.01, ###P < 0.001 vs. 11β-HSD1 group.
H8 ameliorates adipocyte hypertrophy and inflammation in mouse adipose tissue
Figure 4 (A-D) presents the immunohistochemical staining employed to detect FAS and HSL protein expression in murine adipose tissue. The 11β-HSD1 group exhibited a significant elevation in FAS protein expression in adipose tissue when compared to both the Con and Con + H8 groups. Conversely, treatment with H8 resulted in a substantial reduction in FAS protein expression. Furthermore, post-H8 administration, there was a notable upregulation of HSL protein in the Con + H8 and 11β-HSD1 + H8 groups compared to the untreated controls. Figure 4 (E-F) displays the results of immunofluorescence staining for F4/80 in mouse adipose tissue. The 11β-HSD1 group demonstrated a pronounced increase in F4/80 protein expression relative to both the Con and Con + H8 groups. In contrast, H8 treatment significantly reduced F4/80 protein expression in adipose tissue.
H8 reduces adipocyte hypertrophy and ameliorates inflammatory responses in murine adipose tissue. Immunohistochemical staining images and quantitative analysis of (A-B) FAS, (C-D) HSL (original magnification, × 100 and × 200). (E-F) Immunofluorescence staining images and quantitative analysis (scale bar, 50 μm). The data represent the mean ± SD (n = 8). **P < 0.01, ***P < 0.001 vs. Con group, ###P < 0.001 vs. 11β-HSD1 group.
H8 suppresses 11β-HSD1 to ameliorate lipid metabolic disorders in 3T3-L1 adipocytes
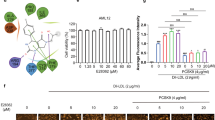
As shown in Fig. 5A-D, the influence of H8 on lipogenesis in 3T3-L1 cells was assessed by quantifying the expression levels of the proteins 11β-HSD1, SREBP1c, and ACC1 across different experimental groups. The results revealed a significant upregulation of 11β-HSD1 and ACC1 protein expression in the DHT + 11β-HSD1 group compared to the DHT group. Conversely, the DHT + 11β-HSD1 + H8 group exhibited a substantial reduction in the expression of 11β-HSD1, SREBP1c, and ACC1 proteins relative to the DHT + 11β-HSD1 group. Furthermore, the CORT + H8 group showed significantly decreased expression levels of 11β-HSD1 and ACC1 proteins compared to the CORT group.
The effect of H8 on lipolysis in 3T3-L1 cells was evaluated by measuring the expression levels of HSL and the p-AMPK/AMPK proteins (Fig. 5E-F). The CORT + H8 group demonstrated a significant increase in HSL protein expression compared to the CORT group. In contrast, the DHT + 11β-HSD1 group showed a pronounced decrease in p-AMPK/AMPK protein expression relative to the DHT group.
H8 alleviates lipid metabolic disorders in 3T3-L1 adipocytes via suppression of 11β-HSD1. (A) The representative western blotting images. (B-F) Protein expression level. The data represent the mean ± SD (n = 4). *P < 0.05, **P < 0.01, ***P < 0.001 vs. CON group and DHT group, #P < 0.05, ###P < 0.001 vs. CORT group and DHT + HSD group.
H8 reduces lipid accumulation in 3T3-L1 adipocytes
Oil Red O staining was employed to assess lipid accumulation in 3T3-L1 adipocytes, as shown in Fig. 6A-B. Intracellular lipid accumulation was significantly elevated in the CORT group compared to the CON group, and in the DHT + 11β-HSD1 group compared to the DHT group. Conversely, adipocytes treated with H8 exhibited a pronounced reduction in lipid synthesis, suggesting that H8 is effective in inhibiting lipid accumulation in adipocytes.
Discussion
Obesity is a consequence of metabolic disorders characterized by the excessive accumulation of fat within the body. Consequently, research into the underlying mechanisms of obesity predominantly focuses on elucidating the processes that facilitate adipogenesis, fat accumulation, and differentiation. Under physiological conditions, glucocorticoid (GC) play a dual role in lipid metabolism: they not only promote lipogenesis and induce the differentiation of preadipocytes into mature adipocytes but also stimulate lipolysis, thereby maintaining a dynamic equilibrium within the body. GC are crucial for the differentiation of human adipose stromal cells and 3T3-L1 preadipocytes24,25, primarily through the activation of key transcription factors involved in adipocyte differentiation. However, chronic elevation of GC levels can result in increased mobilization of energy reserves and enhanced fat storage at the adipocyte level, leading to lipid synthesis and accumulation. CORT and dehydrocorticosterone represent the biologically active and inactive forms of GC respectively, within their interconversion being regulated by 11β-HSD1 reductase activity. We successfully developed an in vitro differentiation model in which 3T3-L1 preadipocytes were induced to differentiate into mature adipocytes using these respective forms of GC. This model was subsequently utilized to examine the effects of H8 on adipogenesis and lipid metabolism in differentiated adipocytes.
The enzyme 11β-HSD1 plays a crucial role in controlling glucocorticoid production at the pre-receptor stage in target tissues26. Its primary function is to convert inactive GC into active cortisol, thereby enabling their physiological effects. To further investigate the effects of H8 on lipid metabolism in vivo, we constructed an 11β-HSD1 gene overexpression model by packaging and administering 11β-HSD1 viral particle into C57BL/6 mice via tail vein injection. Previous studies have demonstrated that 11β-HSD1 is expressed in the anterior pituitary, paraventricular nucleus, and hypothalamus within the central nervous system, all of which are involved in the regulation of the HPA axis27,28. Research on adipose tissue, adipocytes, and myoblasts suggests that 11β-HSD1 facilitates local GC production and is upregulated by cortisol, indicating a potential positive feedback loop between 11β-HSD1 and GC29. This hypothesis is corroborated by our findings, which demonstrate elevated corticosterone (CORT) levels in the serum of mice in the 11β-HSD1 group compared to the Con group. Notably, treatment with H8 significantly reduced CORT levels, indicating that while 11β-HSD1 overexpression elevates systemic GC levels, this effect can be counteracted by H8. These findings were further validated by in vitro experiments. Overexpression of 11β-HSD1 in adipose tissue has been linked to obesity4, and similarly, we have previously improved 11β-HSD1 overexpression in diet-induced obese mice22. The current pharmacological intervention with H8 not only mitigated weight gain but also improved dyslipidemia, as evidenced by significant reductions in serum TC, TG and LDL concentrations. These results suggest the potential of H8 to ameliorate lipid metabolism abnormalities associated with 11β-HSD1 overexpression. However, in this study we observed a significant reduction in HDL levels following H8 treatment, which contrasts with previously reported findings30. This discrepancy may be attributed to variations in experimental models. We intend to further investigate this phenomenon in future studies. Adipose tissue growth results from an increase in both adipocyte size and number, driven by lipid droplet accumulation and preadipocyte differentiation, respectively31. Oil Red O staining and HE staining revealed significant lipid droplet accumulation in the 11β-HSD1 + DHT group of cells, and well as degenerative changes in the liver adipose tissue of the 11β-HSD1 group of mice, indicative of obesity characterized by lipid metabolism disorders. These abnormalities were ameliorated following H8 intervention, suggesting that H8 may enhance lipid metabolism.
AMPK is a key molecule in regulating bioenergy metabolism and chronic diseases associated with metabolic syndrome32. As a serine/threonine protein kinase, the phosphorylation of the threonine 172 site on its α-subunit is crucial for modulating AMPK activity33,34. Research indicates that the AMPK signaling pathway plays a significant role in glucose metabolism, fatty acid metabolism, and appetite regulation35.
AMPK modulates its activity through acetyl-CoA carboxylase (ACC), thereby influencing the synthesis of triglycerides and fatty acids. Acetyl-CoA carboxylase 1 (ACC1) serves as the primary rate-limiting enzyme in the synthesis of malonyl-CoA, a vital precursor for fatty acid production and a potent inhibitor of carnitine palmitoyltransferase 1 (CPT1), thereby preventing its mitochondrial entry for β-oxidation36. Consequently, the activation of AMPK can inhibit fatty acid synthesis and enhance the oxidation of free fatty acids by decreasing malonyl-CoA levels, thus mitigating its inhibitory effect on CPT1 activity. Furthermore, studies have demonstrated that elevated AMPK expression can modulate downstream protein expression, resulting in reduced levels of sterol regulatory element-binding protein (SREBP), which subsequently decreases fatty acid synthase (FAS) expression and suppresses lipogenesis37. Uncoupling protein 1 (UCP1), predominantly expressed in brown adipose tissue, primarily functions to counteract obesity by decomposing white adipose tissue and is often regarded as a marker protein for brown adipose tissue38. Notably, UCP1 expression has also been documented in eWAT in a limited number of reports, which may be associated with the induction of a brown adipose gene program in eWAT under specific conditions39,40. Consistent with these observations, our study detected upregulated UCP1 expression in eWAT following H8 treatment. We are interested in further validating and investigating this phenomenon in subsequent experiments. Additionally, hormone-sensitive lipase (HSL) plays a crucial role in catalyzing the hydrolysis of triglycerides into free fatty acids41.
The enzyme11β-HSD1 is associated with obesity due to its influence on enzymes that govern lipid synthesis and degradation within adipose tissue. Key enzymes involved in the de novo fatty acid synthesis pathway in adipose tissue include ACC1, FAS, and CPT1. UCP1 and HSL are essential for the regulation of lipolysis. Furthermore, SREBPs are involved not only in lipid synthesis but also in promoting adipocyte differentiation at the transcriptional level. Studies have shown that mice deficient in SREBP1c exhibit reduced adipose tissue deposition, underscoring the importance of SREBP1c as a critical regulator of lipogenesis in adipose tissue42. Based on the relationship between these enzymes, it is indicated that AMPK and 11β-HSD1 are correlated.
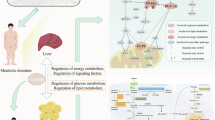
Studies have indicated that curcumin functions as an AMPK agonist, which enhances AMPK phosphorylation and regulates lipid metabolism43. In contrast, we have synthesized H8, a structurally more stable analog of curcumin. Based on our preliminary investigations into the pharmacological activity, pharmacokinetics, and toxicological profile of H8, we have confirmed its significant therapeutic efficacy21,22. Therefore, to further develop H8 and elucidate the relationship between its mechanism of action in ameliorating lipid metabolism disorders and the AMPK, we quantified indicators related to lipid synthesis and metabolism by extracting RNA and proteins from both cellular and adipose tissue samples. The experimental findings revealed that H8 markedly inhibited the mRNA and protein expression levels of enzymes involved in lipid synthesis, while concurrently enhancing the expression levels of enzymes associated with lipolysis. Nonetheless, the relationship between these findings and 11β-HSD1 necessitates further investigation. Consequently, we conducted more comprehensive studies and observed that the CORT group (differentiated with active GC) exhibited higher levels of p-AMPK compared to the CON group. In contrast, the reductase activity of 11β-HSD1 was found to suppress AMPK phosphorylation in cells differentiated with inactive GC (DHT + 11β-HSD1 group versus DHT group). Consistently, mice overexpressing 11β-HSD1 also displayed reduced p-AMPK levels, whereas H8 treatment significantly increased p-AMPK expression across all conditions. These findings collectively indicate an inverse correlation between 11β-HSD1 expression and the activation of the AMPK pathway. Based on our experimental findings, we have verified that H8 potentially ameliorates lipid metabolism disorders through the inhibition of 11β-HSD1 activity, which subsequently activates the AMPK signaling pathway (Fig. 7). Specifically, H8 modulates glucocorticoid levels via 11β-HSD1 inhibition, thereby initiating the activation of the AMPK pathway. This mechanism results in the upregulation of HSL and UCP1 expression while concurrently suppressing SREBP and ACC1 activity, leading to reduced FAS and elevated CPT1 levels, ultimately enhancing lipid metabolic function. Previous studies have indicated that the expression of 11β-HSD1 is also linked to adipogenic factors, such as adipose triglyceride lipase (ATGL), peroxisome proliferator-activated receptor gamma (PPARγ) and diacylglycerol O-acyltransferase 1/2 (DGAT1/2)44,45. In subsequent research, we aim to further investigate the potential regulatory effects of compound H8 on these targets to comprehensively elucidate its pharmacological mechanisms.
To substantiate the notable enhancement of H8 on lipid metabolism disorders, we performed immunohistochemical analyses focusing on two critical enzymes: FAS (a lipogenic enzyme) and HSL (a lipolysis-related enzyme), which produced affirmative outcomes. Furthermore, macrophages are predominantly responsible for inflammation in adipose tissue, which consequently results in chronic low-grade inflammation in obesity46. Therefore, we also investigated F4/80, a specific marker protein for adipose tissue macrophages47. Our immunofluorescence staining results for F4/80 indicated that H8 mitigated adipocyte hypertrophy and inflammation within adipose tissue. Nonetheless, the specific anti-inflammatory mechanisms warrant further exploration.
In conclusion, our study confirms that H8 modulates GC activity by inhibiting 11β-HSD1 and activates the AMPK signaling pathway, thereby playing a crucial role in ameliorating lipid metabolism disorders. These findings provide a theoretical foundation for the development and application of H8 as a therapeutic agent for obesity.
Data availability
All relevant data is included within the manuscript.
References
Heymsfield, S. B., Wadden, T. A. & Mechanisms Pathophysiology, and management of obesity. N Engl. J. Med. 376, 254–266 (2017).
Abrahams, L. et al. Biomarkers of hypothalamic-pituitary-adrenal axis activity in mice lacking 11β-HSD1 and H6PDH. J. Endocrinol. 214, 367–372 (2012).
Masuzaki, H. et al. A Transgenic model of visceral obesity and the metabolic syndrome. Science 294, 2166–2170 (2001).
Paulmyer-Lacroix, O., Boullu, S., Oliver, C., Alessi, M. C. & Grino, M. Expression of the mRNA coding for 11beta-hydroxysteroid dehydrogenase type 1 in adipose tissue from obese patients: an in situ hybridization study. J. Clin. Endocrinol. Metab. 87, 2701–2705 (2002).
Liu, Y. et al. Suppression of 11beta-hydroxysteroid dehydrogenase type 1 with RNA interference substantially attenuates 3T3-L1 adipogenesis. Physiol. Genomics. 32, 343–351 (2008).
Gupta, A. P. et al. Pancreastatin inhibitor activates AMPK pathway via GRP78 and ameliorates dexamethasone induced fatty liver disease in C57BL/6 mice. Biomed. Pharmacother. 116, 108959 (2019).
Classen-Houben, D. et al. Selective Inhibition of 11beta-hydroxysteroid dehydrogenase 1 by 18alpha-glycyrrhetinic acid but not 18beta-glycyrrhetinic acid. J. Steroid Biochem. Mol. Biol. 113, 248–252 (2009).
Tagawa, N., Kubota, S., Kato, I. & Kobayashi, Y. Resveratrol inhibits 11β-hydroxysteroid dehydrogenase type 1 activity in rat adipose microsomes. J. Endocrinol. 218, 311–320 (2013).
Su, X. et al. Adamantyl carboxamides and acetamides as potent human 11β-hydroxysteroid dehydrogenase type 1 inhibitors. Bioorg. Med. Chem. 20, 6394–6402 (2012).
Alam, S., Lee, J. & Sahebkar, A. Curcumin in cancer prevention: insights from clinical trials and strategies to enhance bioavailability. Curr. Pharm. Des. 30, 1838–1851 (2024).
Hsu, K. Y., Majeed, A., Ho, C. T. & Pan, M. H. Bisdemethoxycurcumin and Curcumin alleviate inflammatory bowel disease by maintaining intestinal epithelial integrity and regulating gut microbiota in mice. J. Agric. Food Chem. 73, 3494–3506 (2025).
Servida, S. et al. Curcumin and Gut Microbiota: A Narrative Overview with Focus on Glycemic Control. Int J. Mol. Sci 25(14), 7710 (2024).
Hu, G. X. et al. Curcumin as a potent and selective inhibitor of 11β-hydroxysteroid dehydrogenase 1: improving lipid profiles in high-fat-diet-treated rats. PLoS One. 8, e49976 (2013).
Shehzad, A., Ha, T., Subhan, F. & Lee, Y. S. New mechanisms and the anti-inflammatory role of Curcumin in obesity and obesity-related metabolic diseases. Eur. J. Nutr. 50, 151–161 (2011).
Shao, W. et al. Curcumin prevents high fat diet induced insulin resistance and obesity via attenuating lipogenesis in liver and inflammatory pathway in adipocytes. PLoS One. 7, e28784 (2012).
Jazayeri-Tehrani, S. A. et al. Nano-curcumin improves glucose indices, lipids, inflammation, and Nesfatin in overweight and obese patients with non-alcoholic fatty liver disease (NAFLD): a double-blind randomized placebo-controlled clinical trial. Nutr. Metab. (Lond). 16, 8 (2019).
Tian, L. et al. Curcumin represses mouse 3T3-L1 cell adipogenic differentiation via inhibiting miR-17-5p and stimulating the Wnt signalling pathway effector Tcf7l2. Cell. Death Dis. 8, e2559 (2017).
Wu, L. Y. et al. Curcumin attenuates adipogenesis by inducing preadipocyte apoptosis and inhibiting adipocyte differentiation. Nutrients 11(10), 2307 (2019).
Yang, K. Y., Lin, L. C., Tseng, T. Y., Wang, S. C. & Tsai, T. H. Oral bioavailability of Curcumin in rat and the herbal analysis from curcuma longa by LC-MS/MS. J. Chromatogr. B Analyt Technol. Biomed. Life Sci. 853, 183–189 (2007).
Shabbir, U. et al. Curcumin, Quercetin, Catechins and Metabolic Diseases: The Role of Gut Microbiota. Nutrients 13(1), 206 (2021).
Yuan, X. et al. Synthesis of novel Curcumin analogues for Inhibition of 11β-hydroxysteroid dehydrogenase type 1 with anti-diabetic properties. Eur. J. Med. Chem. 77, 223–230 (2014).
Yuan, X. et al. The 11β-hydroxysteroid dehydrogenase type 1 inhibitor protects against the insulin resistance and hepatic steatosis in db/db mice. Eur. J. Pharmacol. 788, 140–151 (2016).
Liu, H. et al. 11β-Hydroxysteroid dehydrogenase type 1 inhibitor development by lentiviral screening based on computational modeling. Pharmacology 102, 169–179 (2018).
Pantoja, C., Huff, J. T. & Yamamoto, K. R. Glucocorticoid signaling defines a novel commitment state during adipogenesis in vitro. Mol. Biol. Cell. 19, 4032–4041 (2008).
Hauner, H. et al. Promoting effect of glucocorticoids on the differentiation of human adipocyte precursor cells cultured in a chemically defined medium. J. Clin. Invest. 84, 1663–1670 (1989).
Zhong, C. et al. Targeting osteoblastic 11β-HSD1 to combat high-fat diet-induced bone loss and obesity. Nat. Commun. 15, 8588 (2024).
Lakshmi, V., Sakai, R. R., McEwen, B. S. & Monder, C. Regional distribution of 11 beta-hydroxysteroid dehydrogenase in rat brain. Endocrinology 128, 1741–1748 (1991).
Moisan, M. P., Seckl, J. R. & Edwards, C. R. 11 beta-hydroxysteroid dehydrogenase bioactivity and messenger RNA expression in rat forebrain: localization in hypothalamus, hippocampus, and cortex. Endocrinology 127, 1450–1455 (1990).
Bujalska, I. J., Kumar, S., Hewison, M. & Stewart, P. M. Differentiation of adipose stromal cells: the roles of glucocorticoids and 11beta-hydroxysteroid dehydrogenase. Endocrinology 140, 3188–3196 (1999).
Hardy, R. S. et al. 11βHSD1 Inhibition with AZD4017 improves lipid profiles and lean muscle mass in idiopathic intracranial hypertension. J. Clin. Endocrinol. Metab. 106, 174–187 (2021).
Wang, L. & Di, L. J. Wnt/β-Catenin mediates AICAR effect to increase GATA3 expression and inhibit adipogenesis. J. Biol. Chem. 290, 19458–19468 (2015).
Dihingia, A., Bordoloi, J., Dutta, P., Kalita, J. & Manna, P. Hexane-Isopropanolic extract of Tungrymbai, a North-East Indian fermented soybean food prevents hepatic steatosis via regulating AMPK-mediated SREBP/FAS/ACC/HMGCR and PPARα/CPT1A/UCP2 pathways. Sci. Rep. 8, 10021 (2018).
Yan, Y., Zhou, X. E., Xu, H. E. & Melcher, K. Structure and Physiological Regulation of AMPK. Int J. Mol. Sci 19(11), 3534 (2018).
Pang, Y. et al. High Fat Activates O-GlcNAcylation and Affects AMPK/ACC Pathway to Regulate Lipid Metabolism. Nutrients 13(6), 1740 (2021).
Lin, Z., Zhang, B. & Liu, X. Advances of researches on AMPK-ACC signaling pathway in metabolic diseases. Chin. J. Diabetes. 21, 474–477 (2013).
Ke, R., Xu, Q., Li, C., Luo, L. & Huang, D. Mechanisms of AMPK in the maintenance of ATP balance during energy metabolism. Cell. Biol. Int. 42, 384–392 (2018).
Moseti, D., Regassa, A. & Kim, W. K. Molecular Regulation of Adipogenesis and Potential Anti-Adipogenic Bioactive Molecules. Int J. Mol. Sci 17(1), 124 (2016).
Parson, J. C. et al. Development and expansion of intramuscular adipose tissue is not dependent on UCP-1-lineage cells in mice. J. Orthop. Res. 41, 2599–2609 (2023).
Ma, B. et al. Rutin promotes white adipose tissue Browning and brown adipose tissue activation partially through the calmodulin-dependent protein kinase kinase β/AMP-activated protein kinase pathway. Endocr. J. 69, 385–397 (2022).
Ohno, H., Shinoda, K., Spiegelman, B. M. & Kajimura, S. PPARγ agonists induce a white-to-brown fat conversion through stabilization of PRDM16 protein. Cell. Metab. 15, 395–404 (2012).
Kraemer, F. B. & Shen, W. J. Hormone-sensitive lipase: control of intracellular tri-(di-)acylglycerol and cholesteryl ester hydrolysis. J. Lipid Res. 43, 1585–1594 (2002).
Ouyang, S. et al. Glycerol kinase drives hepatic de Novo lipogenesis and triglyceride synthesis in nonalcoholic fatty liver by activating SREBP-1c Transcription, upregulating DGAT1/2 Expression, and promoting glycerol metabolism. Adv. Sci. (Weinh). 11, e2401311 (2024).
Liu, Z. et al. Curcumin activates AMPK pathway and regulates lipid metabolism in rats following prolonged clozapine exposure. Front. Neurosci. 11, 558 (2017).
Shih, C. C., Ciou, J. L., Lin, C. H., Wu, J. B. & Ho, H. Y. Cell suspension culture of eriobotrya Japonica regulates the diabetic and hyperlipidemic signs of high-fat-fed mice. Molecules 18, 2726–2753 (2013).
Legeza, B., Balázs, Z. & Odermatt, A. Fructose promotes the differentiation of 3T3-L1 adipocytes and accelerates lipid metabolism. FEBS Lett. 588, 490–496 (2014).
Bettaieb, A. et al. Anti-inflammatory actions of (-)-epicatechin in the adipose tissue of obese mice. Int. J. Biochem. Cell. Biol. 81, 383–392 (2016).
Masi, L. N. et al. Combination of a high-fat diet with sweetened condensed milk exacerbates inflammation and insulin resistance induced by each separately in mice. Sci. Rep. 7, 3937 (2017).
Funding
This study was supported by the Scientific Research Project of the Health Commission of Heilongjiang Province of China (Project No. 20220202020954).
Author information
Authors and Affiliations
Contributions
Conceptualization, Y.H. and H.B.; methodology, Y.H., L.L., X.Y. and H.B.; validation, Y.H.,L.L., X.Y. and H.B.; data curation, L.L.; writing—original draft, Y.H.; writing—review and editing, H.B.; project administration, H.B. All authors reviewed the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Han, Y., Li, L., Yuan, X. et al. 11β-HSD1 inhibitor alleviates lipid metabolism disorder by activating the AMPK signaling pathway. Sci Rep 15, 41968 (2025). https://doi.org/10.1038/s41598-025-25941-1
Received:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s41598-025-25941-1