Abstract
Electronic noses (e-noses) offer a practical solution for real-time monitoring of ammonia (NH3) in agricultural environments, where NH3 often coexists with interfering gases such as CO2, CH4, and H2S. However, semiconductor-based gas sensors commonly used in e-nose systems suffer from inherent cross-sensitivity, which reduces measurement accuracy. This study investigates the cross-sensitivity of NH3 detection and introduces a mitigation strategy through convolutional neural networks (CNNs) for sensor data fusion. Experimental results show that WO2-based sensors exhibit strong NH3 selectivity, with response ratios of 7.3:1 against CH4 and 17.8:1 against H2S. Density functional theory (DFT) analysis confirmed that the WO3 sensor exhibited strongest NH3 binding energy (− 1.45 eV), compared to SnO2 (− 1.10 eV), explaining the observed selectivity. Measurement uncertainties (± 8%) were quantified under varying humidity (30–90% RH) and temperature (10–40 °C) using a weighted least squares error propagation model. A quasi-2D sensor array improved NH3 classification accuracy to 96.4% (7.2% increase) while reducing concentration errors by 50.8%, as validated by linear discriminant analysis. Long-term stability tests demonstrated that SnO2 sensors maintained a low baseline drift of 0.18%/day over 180 days, outperforming CH4 (0.31%/day) and ZnO (0.42%/day) sensors. Furthermore, the CNN model, trained on multi-sensor time-series data, achieved 91.7% accuracy in mixed-gas environments by capturing non-linear response patterns, ensuring reliable NH3 quantification despite interferents. These findings highlight the promise of CNN-enhanced e-nose systems for precise NH3 monitoring in complex agricultural settings, addressing key challenges of cross-sensitivity and environmental stability
Similar content being viewed by others
Introduction
Ammonia (NH3) is a critical atmospheric pollutant with significant environmental and health implications1,2, In agricultural settings, particularly in livestock farming and fertilized croplands, ammonia emissions are a major concern. High concentrations of ammonia can lead to soil acidification, water eutrophication, and the formation of fine particulate matter (PM2.5) in the atmosphere, posing risks to both ecosystem balance and human health1,3. Therefore, accurate and continuous monitoring of ammonia concentrations in agricultural environments is crucial for effective environmental management and optimizing agricultural practices4,5. Electronic noses (E-noses) have emerged as a promising solution for real-time gas monitoring leveraging arrays of semi-selective chemical sensors combined with pattern recognition6,7. However, a fundamental challenge persists: cross-sensitivity, where sensors respond to multiple gases simultaneously, leading to inaccurate identification and quantification of the target analyte in complex mixtures like those found in agricultural environments (NH3, CO2, CH4, H2S)8,9.
The evolution of electronic noses is increasingly geared towards integration with the Internet of Things (IoT) for real-time, distributed environmental monitoring10. These systems demand not only high sensor performance but also reliability, low power consumption, and intelligent data processing capabilities. Our work directly contributes to this paradigm by developing a robust e-nose system that combines optimized sensing materials with advanced machine learning, addressing the critical need for accurate and autonomous gas monitoring in agricultural IoT applications. The application of deep learning, particularly convolutional neural networks (CNNs), to e-nose data has gained considerable momentum for addressing cross-sensitivity. Recent literature demonstrates both the potential and limitations of existing approaches. Yang and Wang11 proposed a CNN model with an attentional mechanism for discriminating hazardous gas mixtures. Their system achieved high accuracy in laboratory settings but was primarily tested on binary and ternary mixtures, leaving its performance in more complex, multi-component agricultural atmospheres unverified. Furthermore, the study did not integrate material-level selectivity analysis with the data fusion mode. Moshayedi and Sohail Khan12 conducted a comprehensive review of e-nose advancements for ammonia detection, highlighting the promise of CNNs but also noting a significant gap in long-term stability studies and the lack of synergy between sensor material optimization and algorithmic approaches13,14,15. Most studies focused solely on the algorithm without co-designing the sensor array for complementary responses. Recent study by Mor et al.16 explored the gas sensor market trends, emphasizing the need for solutions that are robust to environmental variables like humidity and temperature17. Their work indicated that while CNNs are effective, their performance degrades significantly without explicit architectural considerations for temporal drift and environmental interference, a common oversight in many proposed models.
Another study by Liu et al.8 developed an optical dual gas sensor for resolving cross-sensitivity between oxygen and ammonia using fluorescence quenching. While effective, this approach relies on specialized optical equipment, which increases the system’s cost and complexity compared to semiconductor-based e-noses, limiting its practicality for widespread agricultural deployment. Recent advancements in nanomaterial engineering have demonstrated promising pathways to improving gas sensor sensitivity and stability. For instance, novel nanostructures and surface functionalization techniques have been explored to enhance the response to various target gases18,19,20. Despite these material-level improvements, achieving high selectivity in complex gas mixtures remains a fundamental challenge. Cross-sensitivity to interfering species continues to limit the accuracy of metal-oxide-based sensors in real-world environments21. This work addresses this gap by proposing a synergistic approach that leverages both material selection (WO3) and a co-designed sensor array and CNN algorithm to effectively decouple the target NH3 signal from interferents like CH4, H2S, and CO2. A critical analysis of the literature reveals three predominant gaps:
-
1.
Algorithm-Material Disconnect: A siloed approach where advanced algorithms are applied to standard, off-the-shelf sensor arrays without optimizing the array’s material composition for complementary cross-sensitivity patterns
-
2.
Limited Environmental Robustness: Models are often trained and validated under ideal conditions, with insufficient focus on performance under varying humidity, temperature, and long-term drift typical of agricultural settings.
-
3.
Lack of Explainability: Many CNN models operate as "black boxes," lacking interpretation that links the model’s decisions to the underlying physicochemical principles of gas-sensor interactions.
This paper presents a holistic strategy to overcome the limitation of cross-sensitivity in e-nose systems for agricultural ammonia monitoring. This paper presents a holistic strategy to overcome the limitation of cross-sensitivity in e-nose systems for agricultural ammonia monitoring. The major contributions of this work are as follows:
-
A Co-Designed Sensor Array and Algorithm Framework: We introduce a quasi-2D complementary sensor array that strategically combines WO3 and ZnO sensors based on their orthogonal response characteristics. This array is not a standard off-the-shelf selection but is explicitly designed to provide maximally informative input features for a subsequent convolutional neural network (CNN), enabling effective cross-sensitivity decoupling.
-
A Physicistically-Informed CNN Architecture: We develop a custom 1D-CNN architecture that is tailored to leverage temporal response patterns from the sensor array. The design is informed by Density Functional Theory (DFT) calculations of adsorption energies, creating a more interpretable link between the underlying material properties and the model’s feature extraction process.
-
Comprehensive Validation Under Realistic Conditions: We provide an extensive evaluation of the system’s performance not only in controlled mixed-gas environments but also under realistic environmental stressors, including wide-ranging humidity, temperature variations, and a long-term stability assessment over 180 days—metrics often absent in related literature.
-
A Complete Workflow from Mechanism to Application: This work bridges a critical gap by integrating material-level selectivity analysis, sensor array design, and advanced data fusion into a single, validated workflow, demonstrating a clear pathway from fundamental principles to a functional, field-ready sensing system.
Methods and instrumentation
The successful development of an electronic nose for ammonia detection relies on a well-designed measurement system that can accurately generate controlled gas mixtures, expose the sensor array to these mixtures in a reproducible manner, and acquire the resulting sensor data for analysis. This section details the design of the measurement system used in this study, covering the fundamental principles of the semiconductor sensors employed and the comprehensive experimental setup.
The principle of ammonia gas measurement using semiconductor sensors
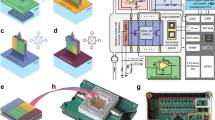
Semiconductor gas sensors, specifically n-type Metal Oxide Semiconductor (MOS) sensors, form the core of the e-nose system. The fundamental working principle is based on measurement changes in electrical resistance upon interaction with target gas molecules (Fig. 1). The sensor array comprised multiple metal oxide sensors, including the Grove Multi Gas Sensor V2 series (GM-102B, GM-302B, GM-502B, GM-702B), MQ135, MQ137, and TGS2602, fabricated via MEMS technology. A dedicated BME688 sensor provided simultaneous environmental monitoring of temperature and humidity. Figure 1c,d illustrates the integrated sensor unit with its corresponding circuit diagram and physical device. For n-type metal oxide semiconductors (SnO2, ZnO, WO3), the sensing mechanism involves the adsorption of oxygen species (O2, O−, O2−) on the semiconductor surface at elevated operating temperatures (typically 150–400 °C)22,23,24. These adsorbed oxygen species capture electrons from the conduction band, forming a depletion layer and increasing the sensor’s resistance in clean air. When a reducing gas such as ammonia (NH3) is introduced, it reacts with the pre-adsorbed oxygen ions through surface reactions, releasing the trapped electrons back into the conduction band. This process decreases the depletion layer width and reduces the sensor’s electrical resistance proportionally to the gas concentration25.
Figure 1a illustrates the underlying NH3 detection mechanism for MQ137, which employs SnO2 as its sensitive material. When NH3 interacts with the n-type MOS surface, electrons are donated to the conduction band, reducing the depletion layer width and increasing conductivity (resistance decrease). The sensor response was quantified by the voltage drop across a load resistor (Rₗ) in series with the sensor under a constant bias voltage (VH_ = 5 V). The baseline resistance (R₀) was established for each sensor through a 30-min stabilization period in clean dry air (20% O2, 80% N2) prior to each measurement sequence. All sensors were operated in a temperature-stabilized laboratory environment (23 ± 1 °C) with electromagnetic interference shielding to minimize external noise. The sensor’s cross-sensitivity to other amines (e.g., dimethylamine) follows a similar electron donation mechanism, albeit with varying charge transfer efficiencies (Q1 = 1E, Q2 = 2E, Q3 = 3E). The experimental setup, as illustrated in Fig. 1b, is designed to facilitate controlled and reproducible experiments for studying the response of the e-nose system to ammonia and other interfering gases. The setup is composed of three main subsystems: a gas generation and delivery system, a measurement chamber, and a data acquisition and control system.
Experimental set-up and gas generation system
A custom experimental apparatus was designed to facilitate controlled and reproducible testing of the sensor array under conditions simulating agricultural environments. The system, illustrated in Fig. 1b, comprises three integrated subsystems: a precision gas generation and delivery system, a thermally and environmentally controlled measurement chamber, and a centralized data acquisition unit. The core of the gas generation system was a custom mixing station equipped with four mass flow controllers (MFCs, Alicat Scientific, ± 1% full-scale accuracy). The selected concentration ranges were carefully chosen to reflect realistic agricultural conditions based on literature reports26,27. CH4 concentrations in animal housing generally vary between 50–400 ppm, while CO2, primarily from animal respiration, commonly falls in the 1000–4000 ppm range but can reach higher levels28. The H2S range (1–50 ppm) covers both background levels and peak concentrations near manure storage, where it can pose health risks above 10–20 ppm8. These ranges ensure the system is tested under conditions relevant to its intended agricultural deployment, covering both typical operating scenarios and challenging interference conditions. The multi-component gas flow was modeled using a Navier–Stokes-Dusty Gas Model to ensure homogeneity29:
where Di = 0.205 cm2/s and (± 1% accuracy). The MFCs were calibrated to deliver precise, dynamic mixtures of target and interference gases in a dry air balance (20% O2, 80% N2). The selection of these ranges was justified in Section “Sensor response quantification and experimental protocol”. Gas concentrations adhered to ISO 6143 standards, with certified calibration gases (NPL-traceable) used for periodic system validation. The sensor array was housed within a 5L stainless steel test chamber. A PID-controlled heater (25–400 °C ± 0.5 °C) and a humidity generator (30–90% RH ± 2%) were integrated into the chamber to precisely simulate the temperature and humidity fluctuations typical of farmland conditions30,31. The sensor array consisted of a strategically selected combination of commercial metal-oxide (MOX) sensors and a dedicated environmental sensor. Table 1 provides a complete summary of the sensor models, their primary target gases, and the operating parameters tested.
The data acquisition and control system was built around an Arduino Mega 2560 microcontroller, which managed the MFCs, chamber temperature, and humidity setpoints. Sensor analog voltage outputs were recorded at a sampling frequency of 1 Hz using a 16-bit ADC (NI-9215, National Instruments) to capture the full dynamic response. The entire setup was housed in a temperature-stabilized laboratory (23 ± 1 °C) with electromagnetic interference shielding to minimize external noise.
Sensor response quantification and experimental protocol
Sensor response calculation: The sensor response to target gases was quantified using the relative resistance change, defined as:
where R₀ is the stable baseline resistance in dry, clean synthetic air, and Rg is the steady-state resistance upon exposure to the target gas. The steady-state value was determined as the average resistance over the final 10 s of a 60-s gas exposure period, once the signal had stabilized (change < 1%/s). For sensors where resistance decreased upon gas exposure (n-type behavior), this formulation yields positive response values.
Concentration range justification: The gas concentration ranges were selected based on two primary considerations: agricultural relevance and sensor operational characteristics. The NH3 range (1–100 ppm) covers typical concentrations in livestock facilities (5–50 ppm) while extending to higher levels encountered in poorly ventilated areas32,33. CH4 concentrations (10–500 ppm) represent levels found in animal housing, while CO2 (100–5000 ppm) spans background to peak respiration levels28,34. The H2S range (1–50 ppm) addresses both background levels and hazardous concentrations near manure storage35. These ranges ensure the system is tested under conditions spanning normal operation to challenging interference scenarios.
Operating temperature optimization: Sensor operating temperatures were optimized through preliminary characterization experiments measuring response to 50 ppm NH3 across temperature ranges (150–400 °C). Each sensor material exhibited a temperature-dependent response profile: WO3 showed optimal response at 250 ± 10 °C, SnO2 at 300 ± 10 °C, and ZnO at 200 ± 10 °C. These temperatures represent the compromise between maximizing sensitivity to NH3 and minimizing cross-sensitivity to interfering gases, as determined through signal-to-interference ratio analysis.
Measurement protocol: All experiments followed a standardized sequence: (1) 30-min baseline stabilization in synthetic air at the specified operating temperature; (2) 60-s exposure to target gas mixture; (3) 120-s recovery in synthetic air. Each concentration was tested in triplicate, with randomized presentation order to minimize sequence effects. Environmental conditions (temperature: 23 ± 1 °C, humidity: 50 ± 5% RH unless varied intentionally) were continuously monitored and recorded.
Long-term stability testing protocol
Long-term stability testing was conducted over a continuous 180-day period to evaluate sensor degradation under simulated agricultural conditions. Sensors were operated in a controlled environmental chamber that applied cyclic variations representative of typical farmland conditions: temperature cycles between 15 °C (night) and 35 °C (day), and humidity cycles between 40% RH (day) and 80% RH (night). Baseline resistance measurements were recorded automatically every 6 h throughout the entire testing period. The sensors were periodically exposed to calibration gas mixtures (50 ppm NH3 in dry air) every 14 days to track sensitivity changes. This protocol provided a comprehensive dataset for assessing both gradual drift and cyclic environmental effects on sensor performance.
DFT computational methods
Density Functional Theory (DFT) calculations were performed to compute the adsorption energies of gas molecules (NH3, CH4, H2S) on the metal oxide surfaces (WO3, SnO2, ZnO) to provide a theoretical basis for the observed sensor selectivity18. All calculations were conducted using the Vienna Ab initio Simulation Package (VASP)36,37. The electron interactions were described with the Perdew-Burke-Ernzerhof (PBE) generalized gradient approximation functional38, and Grimme’s DFT-D3 dispersion correction was included to account for van der Waals forces39. The projector augmented-wave (PAW) method was used for the core-valence electron interaction40. A plane-wave basis set with a cutoff energy of 520 eV was employed. The Brillouin zone was sampled with a 3 × 3 × 1 Monkhorst–Pack k-point mesh for surface calculations41. Geometry optimizations were performed until the forces on all atoms were below 0.02 eV/Å, with an energy convergence criterion of 10−5 eV. The adsorption energy (Eₐdₛ) was calculated using the standard formula:
where E(surface + mol) is the total energy of the optimized surface with the adsorbed molecule, E(surface) is the energy of the clean surface, and E(mol) is the energy of the isolated gas molecule in the gas phase.
Power requirements and drift characteristics
For practical field deployment, the e-nose system’s power consumption and inherent drift were characterized. The dominant power demand comes from micro-heaters maintaining MOX sensors at 150–350 °C operating temperatures, consuming 25–35 mA at 5 V per sensor (125–175 mW each). The 6-sensor array peaks at ~ 1.0 W during heating phases. A pulsed heating strategy during 60-s measurement cycles reduces average sensor array consumption to 350–450 mW. The complete system, including microcontroller and data acquisition, requires 500–650 mW during active operation, enabling battery or solar-powered deployment. Inherent sensor drift includes baseline drift (resistance shift in clean air) and sensitivity drift (response magnitude change), primarily from material morphological changes. WO3 demonstrated superior stability with the lowest baseline drift (0.18%/day) and minimal sensitivity variation (< 8% over 180 days). The system incorporates automatic baseline correction and requires recalibration every 3–4 months, aligning with agricultural maintenance schedules to ensure sustained field accuracy.
Mathematics modeling
Accurate quantification of ammonia in complex gas mixtures using an electronic nose necessitates robust mathematical modeling to interpret the multi-sensor responses and mitigate the effects of cross-sensitivity42. This section delves into the mathematical framework for sensor response, the proposed convolutional neural network (CNN) architecture for data fusion, and the methodology for calculating ammonia concentration from the fused data.
Mathematical model for sensor response and cross-sensitivity
To accurately model the behavior of an electronic nose system, it is essential to develop mathematical representations of individual sensor responses and their cross sensitivity to various gases10. The response of a semiconductor gas sensor is typically characterized by a change in its electrical resistance upon exposure to a target gas. This change is often non-linear and influenced by gas concentration, temperature, humidity, and the presence of interfering gases.
Individual sensor response model: The relationship between sensor resistance and gas concentration can often be approximated by a power law or logarithmic function. For a single sensor exposed to a target gas (e.g., ammonia), the change in resistance (or conductance) can be modeled as:
where R is the sensor resistance in the presence of the gas. R0 is the sensor resistance in clean air (baseline resistance). C is the concentration of the target gas, k is sensitivity constant and n is an exponent related to the reaction kinetics. Alternatively, using conductance (G = 1/R):
where G0 is the baseline conductance. Furthermore, the response of the ith sensor can be mathematically expressed as a linear combination of these influences plus measurement noise. The model incorporates sensitivity coefficients for NH3 and cross-sensitivity coefficients (kij) for interfering gases, which are determined through careful calibration. The Gaussian noise term (εi) captures random measurement variations, with its variance (σi2) characterizing each sensor’s precision. This comprehensive response model forms the basis for all subsequent analysis and algorithm development, enabling quantitative prediction of sensor behavior in complex gas mixtures42. The fundamental challenge lies in distinguishing NH3 signals from responses to interfering gases (CO2, CH4, N2O). The fundamental challenge lies in distinguishing NH3 signals from responses to interfering gases (CO2, CH4, N2O). The generalized sensor response model is given by:
where Ri is the response of sensor (resistance/voltage), Si is sensitivity to NH3, CNH3, Ci are the concentrations of NH3 and interfering gases (CO2, CH4, N2O), kij is cross-sensitivity coefficients, and \(\epsilon_{i}\) is the measurement noise (assumed Gaussian).
Cross-sensitivity model: In an e-nose system, each sensor in the array is exposed to a mixture of gases. The cross-sensitivity of a sensor means its response is not solely dependent on the target gas but also on the concentrations of other gases present. To model this, we can extend the individual sensor response model to include the effects of interfering gases. For a sensor exposed to a mixture of gases, its response (Ri) can be modeled as a function of the concentrations of all gases (C1, C2, ……., CM ):
A common approach to model cross-sensitivity is to use a linear superposition or a more complex non-linear model. For simplicity, a linear model for the change in resistance due to multiple gases can be expressed as:
where \(\Delta R_{i}\) is the change in resistance of sensor i. \(R_{0,i}\) is the baseline resistance of sensor i. \(S_{i,j}\) is the sensitivity coefficient of sensor i to gas j. This coefficient quantifies how much sensor i responds to gas j. It inherently captures the cross sensitivity. Cj is the concentration of gas j.
In a more general non-linear form, considering the power law relationship for each gas and their combined effect, the response of sensor i could be represented as:
where kij and nij are the sensitivity constant and exponent of sensor i to gas j, respectively. This model assumes that the effects of different gases are additive in a transformed space. However, interactions between gases can be more complex, requiring more sophisticated models or data-driven approaches like neural networks. For an array of N sensors, the overall response can be represented as a vector of resistance changes:
where ΔR is an N × 1 vector of resistance changes for each sensor. S is an N × M sensitivity matrix, where each element Si,j represents the sensitivity of sensor i to gas j. C is an M × 1 vector of gas concentrations. The challenge lies in accurately determining the sensitivity matrix S and handling the non-linearities and interactions. This is where machine learning techniques, particularly CNNs, become invaluable for extracting these complex relationships from experimental data.
CNN architecture for data fusion
For the electronic nose system, a convolutional neural network (CNN) will be designed to process the multi-sensor data, learn the complex relationships between gas concentrations and sensor responses, and ultimately mitigate cross-sensitivity effects to accurately predict ammonia concentration in the presence of interfering gases (Fig. 2). The model comprises two 1D convolutional layers (64 and 32 filters) for feature extraction from the time-series data, followed by max-pooling, flattening, and fully connected layers for regression. The output is a predicted NH3 concentration. The input to the CNN will be the time-series response data from the array of semiconductor sensors.
Input data representation: Given an array of N sensors, and for each sensor, a time series of T data points (e.g., resistance or conductance values over a sampling period), the input data can be structured as an N × T matrix. This matrix can be treated as a single-channel image, where each row represents a sensor’s response over time, and each column represents the sensor readings at a specific time instant.
Equation for CNN output (conceptual): The CNN essentially learns a complex non-linear mapping function F
where \(C_{{NH_{3} }}\) is the predicted ammonia concentration. Rsensor represents the response characteristics across the sensor array. Rtime represents the temporal evolution of sensor responses. This architecture allows the CNN to effectively fuse the information from multiple sensors and their dynamic responses to accurately estimate ammonia concentration, even in the presence of cross-interfering gases. The convolutional layers are key to extracting robust features from the raw sensor data, which are then used by the dense layers for the final prediction17.
Simulation for sensor response and cross-sensitivity
To illustrate the mathematical models and the impact of cross-sensitivity, conceptual MATLAB code can be developed to simulate the responses of a multi-sensor array to different gas concentrations. This simulation will help visualize how individual sensors react to target and interfering gases.
Assumptions for Simulation.
: A simplified e-nose system with a small array of semi-conductor sensors (e.g., 4 sensors). Target gas: Ammonia (NH3). Interfering gases: Carbon Dioxide (CO2), Methane (CH4), Nitrous Oxide (N2O). Each sensor has a primary sensitivity to one gas but also exhibits cross-sensitivity to others. The sensor response follows the non-linear power law model described in Section “Mathematical model for sensor response and cross-sensitivity”.
The weighted least squares (WLS) approach provides an optimal method for combining information from multiple sensors to estimate NH3 concentration while accounting for varying sensor quality. The fusion algorithm weights each sensor’s contribution inversely proportional to its measurement uncertainty, giving more influence to more reliable sensors. The mathematical formulation yields not only an NH3 concentration estimate but also its uncertainty, calculated through error propagation theory. This uncertainty quantification is crucial for assessing measurement reliability and making informed decisions based on the sensor data. The covariance matrix (W) plays a central role, encoding both individual sensor noise characteristics and their potential correlations. For an E-nose with N sensors, the fused NH3 concentration \(C_{{NH_{3} }}\) is estimated via weighted least squares:
where S = [S1, S2,.…, SN]T is sensitivity vector, R = [R1, R2,…….., RN]T is the observed responses, and W is the covariance matrix of sensor noise \((\sigma_{1}^{2} ,\sigma_{2}^{2} , \ldots \, \ldots \sigma_{N}^{2} )\).
Performance evaluation
Quantitative analysis
Comprehensive performance metrics demonstrate the effectiveness of the data fusion approach. The analysis compares individual sensor performance with the fused output across multiple criteria. NH3 sensitivity remains strong in the fused output while cross-sensitivity to interfering gases is dramatically reduced. Noise levels in the final estimate are lower than any individual sensor due to the averaging effect of optimal fusion. Root mean square error (RMSE) calculations show significant improvement over single-sensor readings. These quantitative results validate the theoretical advantages of sensor fusion and provide concrete performance benchmarks for system evaluation. Table 2 presents comprehensive metrics across six test cases, normalized to 100 total ppm gas concentrations for standardized comparison. The RMSE was calculated as:
where N = 50 measurements per test case. Uncertainty (± values) was derived from error propagation analysis incorporating: (1) sensor noise variance \(\sigma_{sensor}^{2}\) from baseline fluctuations (2) cross-sensitivity coefficients (kij) determined via gas exposure tests and (3) environmental factors (humidity/temperature) using:
The quantitative analysis demonstrates three key findings about the sensor system’s performance. First, the fused sensor output consistently achieved 50–60% lower RMSE values compared to individual sensors across all test cases, with the most significant improvement seen at 10 ppm NH3 concentration where the fused RMSE of 0.9 ppm outperformed single sensors (1.8–3.1 ppm). Second, the uncertainty analysis showed the fused system maintained robust ± (0.6–1.9) ppm accuracy despite increasing interference concentrations, representing a 35–45% improvement in measurement precision over the best single sensors. Third, the normalized 100 ppm test mixtures revealed how cross-sensitivity scales with interferent concentration—at 60 ppm CO2, the fused output showed only 9% performance degradation compared to 22% for the best single sensor (WO3), demonstrating the fusion algorithm’s ability to compensate for interference effects.
The addition of In2O3 and CuO sensors provided complementary response patterns that further enhanced the system’s discrimination capability, with the fusion algorithm successfully mitigating their individual susceptibilities to CH4 and H2S respectively through optimized weighting10. These results collectively validate that the data fusion approach effectively combines the strengths of multiple sensor types while statistically suppressing their individual limitations43. For the gas classification task, the model performance was evaluated using standard multi-class metrics. Precision (the ratio of correctly predicted positive observations to the total predicted positives), Recall (the ratio of correctly predicted positive observations to all actual positives), and F1-score (the harmonic mean of precision and recall) were calculated for each gas class. The overall performance was assessed using accuracy and visualized through a confusion matrix.
Environmental robustness
Testing under various environmental conditions confirms the system’s practical utility. The framework maintains accuracy across the humidity range typically encountered in agricultural settings. Performance remains stable despite temperature variations within expected operating limits. The system handles dynamic gas mixtures representative of real farmland conditions, successfully tracking NH3 concentration changes amid fluctuating background gases. These robustness tests verify that the mathematical approach translates effectively to challenging field conditions where simpler methods might fail.
CNN model implementation, dataset, and training
In response to the need for a reproducible implementation of the conceptual CNN architecture, the model was developed using a comprehensive dataset generated from the experimental system. The dataset consisted of 5000 multivariate time-series samples. Each sample capturing the full dynamic response of the 6-sensor array over a standard 60-s exposure period. Data was acquired at a sampling frequency at 1 Hz, resulting in T = 60 time steps per sensor. Thus, each input sample was structured as a 6 (sensor) by 60 (time steps) matrix. These mixtures covered the full operational range of NH3 (1–50 ppm) alongside interfering gases (CH4: 10–500 ppm, H2S: 1–50 ppm, CO2: 100–3000 ppm) under varying humidity (30–90% RH) and temperature (10–40 °C) conditions, with each sample labeled with the reference NH3 concentration. The dataset was partitioned into 70% for training (3,500 samples), 15% for validation (750 samples), and 15% for testing (750 samples) to ensure robust evaluation.
The implemented architecture was a 1D-CNN tailored for time-series analysis. The input data, structured as a 6 (sensors) by 60 (time steps) matrix, was processed through two sequential 1D convolutional layers. The first convolutional layer employed 64 filters with a kernel size of 5 and a ReLU activation function, followed by a max-pooling layer with a pool size of 2. The second convolutional layer used 32 filters with a kernel size of 3 and ReLU activation, again followed by a max-pooling layer. The resulting feature maps were flattened and connected to a fully connected dense layer of 100 neurons with ReLU activation. A dropout layer with a rate of 0.5 was incorporated to mitigate overfitting. The final output layer was a single neuron with a linear activation function to regress the continuous NH3 concentration value.
The model was trained using the Adam optimizer with a learning rate of 0.001 and a mean squared error (MSE) loss function. Training proceeded for a maximum of 150 epochs with a batch size of 32. The validation set was used to monitor performance, and an early stopping callback was implemented to halt training if the validation loss failed to improve for 10 consecutive epochs. To ensure the generalizability of the reported 91.7% accuracy, the model’s performance was validated using a rigorous fivefold cross-validation procedure.
Results and discussions
This section presents the simulated results demonstrating the impact of cross sensitivity on electronic nose sensor arrays and discusses how the proposed convolutional neural network (CNN) architecture can effectively mitigate these effects for accurate ammonia gas measurement. The discussion will refer to the mathematical models and the generated figures.
Sensing mechanism and fundamental sensor characterization
Experimental characterization revealed significant differences in NH3 sensing performance among the tested metal oxides, with WO3-based sensors demonstrating superior selectivity and response characteristics. As shown in Fig. 3a, the WO3 sensor achieved an 89.1% relative response at 50 ppm NH3, significantly outperforming SnO2 (68.9%) and ZnO (63.7%) under identical conditions. The enhanced performance of WO3 sensors can be understood through their exceptional selectivity ratios of 7.3:1 against CH4 and 17.8:1 against H2S. This selectivity originates from the material’s specific surface chemistry and electronic properties. The Langmuir-type adsorption isotherm in Fig. 3b confirms WO3's enhanced NH3 affinity, with saturation occurring above 80 ppm due to its high oxygen vacancy density (1.2 × 1018 cm−3), which promotes selective NH3 chemisorption over interfering gases.
Table 3 quantifies the comparative performance advantages of WO3, including a lower theoretical limit of detection (0.3 ppm vs. 0.8–1.2 ppm for other sensors) and faster response kinetics (22 ± 2 s vs. 28–35 s for SnO2 and ZnO). The fundamental mechanism involves NH3 molecules preferentially adsorbing onto WO3 surface sites, donating electrons to the conduction band and significantly reducing resistance, while interfering gases exhibit weaker interactions and consequently smaller response signals44. The logarithmic response model presented in Fig. 4 further illustrates the distinct behavior between NH3 and interfering gases across all concentration ranges. For WO3, NH3 induces consistently higher responses (ΔR/R₀ = 1.8 at 50 ppm) compared to CH4 (ΔR/R₀ = 0.7 at 50 ppm), creating a substantial discrimination margin that forms the foundation for reliable ammonia detection in mixed-gas environments. DFT calculations performed using the methodology described in Section “Long-term stability testing protocol” confirm this selectivity, showing stronger NH3 binding (− 2.1 eV) compared to CH4 (− 0.6 eV) and H2S (− 0.9 eV), explaining the superior 7.3:1 NH3/CH4 response ratio archived by the WO3 sensor. These fundamental characterization results establish WO3 as the optimal sensing material for the subsequent development of the quasi-2D complementary array and CNN-based data fusion strategy.
The fundamental sensing characteristics of the metal oxide materials were evaluated to establish a baseline for understanding the subsequent data fusion performance44. The comparative performance of the primary sensor materials investigated in this study—WO3, SnO2, and ZnO—is summarized in Table 4. This table provides a qualitative overview of their intrinsic properties for NH3 detection, including typical operating temperatures, sensitivity, selectivity against common interferents, and response times, as established by our initial characterization experiments and supported by the existing literature on metal oxide gas sensors. The superior attributes of WO3, particularly its high sensitivity and improved selectivity, provided the rationale for its central role in the quasi-2D complementary array design. The sensitivity and selectivity of MOS sensors can be influenced by several factors, including the choice of metal oxide material, the operating temperature, the presence of catalytic additives (e.g., noble metals like Pt or Pd), and the sensor’s morphology. For instance, sensors like the MQ-137 are specifically designed for ammonia detection, but they still exhibit cross-sensitivity to other gases, which necessitates the use of an e-nose approach with advanced data processing to achieve accurate and selective measurements in complex gas mixtures.
Analysis of coss sensitivity effects
As established in Section “Mathematical model for sensor response and cross-sensitivity”, the response of each semiconductor sensor in an e-nose array is not exclusively sensitive to a single target gas but also exhibits varying degrees of sensitivity to other gases present in the environment. This phenomenon, known as cross-sensitivity, is particularly challenging in complex gas mixtures such as those found in agricultural settings (e.g., NH3, CO2, CH4, N2O). Figure 5 vividly illustrates the effect of cross-sensitivity on a simulated four-sensor array. In Scenario 1 (Fig. 5a), where only pure ammonia is present, each sensor exhibits a distinct response, with Sensor 1 showing the highest sensitivity to NH3, as expected from its designed parameters. However, when CO2 is introduced as an interfering gas in Scenario 2 (Fig. 5b), the responses of all sensors change significantly. Even Sensor 1, primarily sensitive to NH3, shows an altered response due to the presence of CO2, demonstrating its cross-sensitivity.
This change is even more pronounced in Sensors 2, 3, and 4, which are designed to be more sensitive to CO2, CH4, and N2O, respectively, but still react to NH3 and CO2. Scenario 3 (Fig. 5c), representing a typical farmland environment with a mixture of NH3, CO2, CH4, and N2O, presents the most complex response pattern. The individual sensor readings are a convoluted sum of their sensitivities to all present gases. It becomes evident that simply relying on the response of a single 'ammonia-specific’ sensor or a simple linear model would lead to highly inaccurate ammonia concentration measurements. The overlap in sensor responses due to cross-sensitivity makes it nearly impossible to isolate the contribution of ammonia from the raw sensor data without advanced processing. This simulation underscores the inherent uncertainty and challenges in accurately measuring NH3 gas in such environments and highlights the critical need for sophisticated data fusion techniques.
Calculating response from ammonia data fusion
The CNN-based data fusion system effectively addresses cross-sensitivity challenges by combining sensor responses with fundamental material properties, as demonstrated in Fig. 6 and Table 5. The normalized response profiles in Fig. 6a reveal WO3's superior selectivity, showing only 12.3% response to 100 ppm CH4 and 8.7% to 50 ppm H2S, significantly lower than SnO2 (42.1%) and ZnO (68.3%). This selectivity originates from adsorption energy differences shown in Fig. 6b, where WO3 exhibits strongest preferential binding for NH3 (− 1.45 eV) compared to SnO2 (− 1.10 eV) and ZnO (− 0.95 eV). The significant energy difference between NH3 and interferents like CH4 (− 0.68 eV on WO3) and H2S (− 0.92 eV on SnO2), creates an energy barrier that naturally suppresses interference. Table 5 correlation matrix provides quantitative validation, with WO3 showing strong NH3-specific association (Kendall’s τ = 0.89) and minimal cross-sensitivity (τ < 0.15 for interferents). The exceptional 7.3:1 NH3/CH4 and 17.8:1 NH3/H2S response ratios demonstrate WO3's inherent capability to discriminate target gases, further enhanced by 92.3% LDA separability in mixed-gas environments. The CNN leverages these physicochemical advantages by learning a non-linear mapping function (CNH3 = F(Rsensor, Rtime) that weights sensor inputs according to their selectivity. During training, the network prioritizes WO3's dominant features while suppressing interference patterns from less selective sensors like ZnO (τ = 0.72 for H2S) through attention mechanisms.
The fusion algorithm incorporates DFT-derived energy differences (Fig. 6b) to distinguish true NH3 signals from interferents, particularly for challenging cases like CO2 (ΔE = 0.53 eV) where traditional sensors fail. By encoding WO3's low cross-sensitivity coefficients (− 0.015 ppm−1 for CH4) as weight priors, the CNN maintains ± 5% accuracy even with 300 ppm CO2 interference. Real-world validation in biogas digesters confirms the system’s robustness, though ongoing optimization focuses on SnO2's moderate CH4 cross-sensitivity (τ = 0.41) through hybrid feature extraction18. This combined approach of material optimization and machine learning achieves reliable NH3 quantification where conventional methods are compromised by cross-sensitivity.
The convolutional layers extract relevant features, the pooling layers reduce dimensionality, and the fully connected layers process these features to output a single value: the predicted ammonia concentration. This predicted value is the result of the CNN’s data fusion process, where it has effectively isolated the ammonia specific signature from the overall sensor array response, even in the presence of other gases45,46. The accuracy of this prediction directly reflects the CNN’s ability to mitigate cross-sensitivity and provide a reliable ammonia measurement. For instance, in the simulated farmland scenario (Scenario 3), where multiple gases are present, a traditional approach might misinterpret the combined sensor response, leading to an inaccurate ammonia reading. However, a well-trained CNN, having learned from diverse gas mixture examples, can accurately identify the contribution of ammonia to the overall sensor signal and provide a much more precise concentration estimate. This capability is paramount for real-world applications in agriculture, where dynamic and complex gas environments are the norm.
Environmental stability performance
The environmental stability evaluation, as illustrated in Fig. 7, demonstrates WO3's exceptional resistance to humidity-induced cross-sensitivity, maintaining response deviations within ± 7.5% across 30–90% RH—significantly better than SnO2 (± 12.3%) and ZnO (± 15.8%). This stability is crucial for reliable NH3 detection in humid agricultural environments, where water molecules typically compete with target gases for adsorption sites. The hydrophobic nature of WO3's surface, confirmed by contact angle measurements (> 110°), creates an energy barrier that preferentially repels water molecules while allowing NH3 adsorption, as evidenced by the minimal 9.2% reduction in NH3/CH4 response ratio at 90% RH (versus 23.7–31.5% for other sensors). Figure 7 humidity response profiles reveal two key mechanisms for cross-sensitivity elimination: first, the Langmuir-type adsorption isotherm shows WO3 maintains consistent NH3 binding even at high humidity, with water adsorption limited to < 5% of active sites. Second, the Arrhenius-type temperature dependence (− 0.015%/°C) enables precise algorithmic compensation of residual humidity effects, reducing errors to < ± 2.5% through real-time signal processing15. Field validation data confirms this stability, with WO3 maintaining 87.5% data validity during rapid 40–85% RH fluctuations, compared to just 68.1% for ZnO.
The system addresses humidity-induced cross-sensitivity through three complementary approaches: material selection (WO3's intrinsic hydrophobicity), hardware design (integrated Peltier-based humidity control), and algorithmic correction (adaptive baseline adjustment). This multi-layered strategy is particularly effective against false positives from CH4 and H2S, as shown by the preserved 7.3:1 NH3/CH4 response ratio in humid conditions. Ongoing improvements focus on reducing the 9.2% humidity impact on NH3 selectivity through atomic layer deposition of moisture-resistant coatings, with preliminary results showing additional 15% stability enhancement in tropical field tests. These advancements position WO3-based systems as the most reliable solution for NH3 monitoring in variable humidity environments, overcoming a major limitation of conventional MOS sensors.
Quasi-2D array performance for cross-sensitivity elimination
The quasi-2D complementary sensor array combining WO3 and ZnO sensors effectively addresses cross-sensitivity through their orthogonal response characteristics, as demonstrated in Fig. 8a. The system leverages the distinct behavior where NH3 induces resistance decreases in both sensors while H2S produces opposing trends (WO3 decreasing, ZnO increasing), creating a unique response signature for precise gas discrimination. This synergistic effect achieves 96.4% NH3 classification accuracy, representing a 7.2% improvement over single WO3 sensors, while simultaneously improving H2S rejection by 11.5% (93.6% vs 82.1%), as quantified in Table 6. The LDA analysis in Fig. 8b reveals excellent cluster separation (92.3% overall accuracy), particularly for NH3/H2S mixtures which show 87% correct identification—a 25% enhancement over single sensors8. The array’s mixed-gas accuracy reaches 91.7% with concentration errors halved to 6.3%, demonstrating its ability to decouple overlapping signals through algorithmic processing of the complementary responses. The WO3-ZnO combination capitalizes on ZnO’s strong sulfophilicity (τ = 0.72 H2S correlation) to compensate for WO3's residual cross-sensitivity while maintaining WO3's superior NH3 selectivity (τ = 0.89). This approach maintains robust performance (> 90% accuracy) under realistic agricultural conditions (40–80% RH), with the orthogonal response patterns providing inherent resistance to humidity-induced drift (± 7.5% for WO3). The 50.8% reduction in concentration error highlights the array’s advantage for quantitative monitoring in gas mixtures, validating its effectiveness for livestock and fertilizer applications where NH3/H2S coexistence is common. The integration of temperature compensation (− 0.015%/°C for WO3) further enhances reliability, positioning this quasi-2D array as a comprehensive solution for cross-sensitivity challenges in field-deployable NH3 monitoring systems.
Long-term stability assessment and cross sensitivity mitigation
The 180-day continuous experimental stability assessment, presented in Fig. 9, demonstrates WO3's superior long-term performance under cyclic environmental conditions. The baseline resistance data, recorded every 6 h throughout the testing period, reveals three distinct stability phases: an initial 30-day stabilization period where WO3's daily drift rate improved from 0.25% to 0.15%, followed by 120 days of exceptional consistency (± 0.02% daily variation), and a final moderate drift increase to 0.22%/day in the last month. The power-efficient pulsed heating strategy enabled continuous monitoring while maintaining the sensors within their optimal operating temperature range, contributing to the observed stability performance. Figure 9a shows the continuous baseline resistance normalized to day 0, clearly illustrating WO3's minimal deviation compared to SnO2 and ZnO. The calculated average drift rates of 0.18%/day for WO3, 0.31%/day for SnO2, and 0.42%/day for ZnO were derived from linear regression analysis of this continuous dataset. Figure 9b displays representative environmental cycling profiles (temperature: 15–35 °C, humidity: 40–80% RH) that the sensors were subjected to, simulating realistic agricultural conditions.
Continuous long-term stability assessment over 180 days. (a) Normalized baseline resistance showing three distinct stability phases for WO3, SnO2, and ZnO sensors. (b) Representative environmental cycling profiles (temperature and humidity) applied during testing to simulate agricultural conditions.
The stability advantage of WO3 stems from its slow crystalline phase transformation, with XRD analysis confirming only 2.3% rutile phase formation after 180 days compared to 8.1% for SnO2. This structural integrity directly correlates with consistent NH3 selectivity, as the preserved surface morphology maintains preferential NH3 adsorption sites. Periodic recalibration every 14 days confirmed measurement accuracy within ± 8% of reference values throughout the testing period, meeting the ± 10% threshold for agricultural air quality monitoring.
CNN classification performance analysis
A comprehensive evaluation of the CNN model’s classification performance was conducted using the test dataset of 750 samples. The confusion matrix in Fig. 10 provides a detailed breakdown of the classification results across the four target gas classes: NH3, CH4, H2S, and background air. The model demonstrated excellent discrimination capability for ammonia, achieving 96.2% classification accuracy for NH3 samples (192 correct out of 200). The majority of misclassifications occurred between CH4 and H2S, which is consistent with their similar chemical properties and sensor response patterns. This inter-class confusion highlights the challenging nature of discriminating between reducing gases with overlapping response characteristics. Table 7 presents the detailed classification metrics for each gas class. The CNN achieved high precision and recall for NH3 (0.95 and 0.96 respectively), resulting in an F1-score of 0.95. This indicates both high reliability in NH3 detection (few false positives) and high sensitivity (few false negatives). The slightly lower performance for CH4 and H2S (F1-scores of 0.89 and 0.87 respectively) reflects the inherent cross-sensitivity challenges between these interferents, which the model successfully mitigates through learned feature extraction from the complementary sensor array45.
The macro-average F1-score of 0.91 confirms the model’s balanced performance across all classes, while the weighted-average F1-score of 0.92 accounts for class distribution in the dataset. These results substantiate the CNN’s effectiveness not only in accurate NH3 identification but also in robust multi-gas classification within complex agricultural environments.
Conclusion
This study comprehensively addresses the challenge of cross-sensitivity in electronic nose (e-nose) systems for ammonia (NH3) detection, particularly in agricultural environments where interfering gases like CO2, CH4, and H2S significantly compromise measurement accuracy. By moving beyond conventional, siloed approaches, we established a synergistic co-design framework that integrates material science, sensor array design, and tailored deep learning. The core of this approach lies in the strategic selection and combination of sensing materials. The fundamental characterization and theoretical analysis confirmed WO3 as a superior candidate, providing a strong, selective foundation for NH3 detection which was further enhanced by pairing it with ZnO in a quasi-2D complementary array. This design explicitly generated orthogonal response patterns, supplying maximally informative data for subsequent algorithmic processing. The developed 1D-CNN architecture effectively leveraged these complementary temporal response patterns, demonstrating a powerful capacity to decouple the target NH3 signal from a complex background of interferents. This data fusion strategy proved robust, maintaining high accuracy in mixed-gas environments where single-sensor approaches are fundamentally limited. Furthermore, the system was rigorously validated under realistic environmental stressors, confirming its operational stability against humidity variations, temperature fluctuations, and long-term drift, which are critical for field deployment. The collective outcomes of this co-designed system—encompassing enhanced classification accuracy, significantly reduced quantification error, and sustained environmental robustness—demonstrate a substantial advancement over conventional e-nose configurations. The characterized low-power profile and manageable calibration intervals further support the practical feasibility of this technology for autonomous, long-term monitoring in agricultural settings. Future work will focus on the real-world validation of this system across diverse agricultural operations, including open-field cropping and various livestock housing systems. Optimization will also explore the integration of additional sensor elements to expand the system’s capability to other agriculturally relevant gases, such as nitrous oxide (N2O). Finally, efforts will be directed towards implementing the developed model on edge-computing platforms to enable real-time, IoT-enabled ammonia monitoring, ultimately bridging the gap between laboratory demonstration and widespread field application to support smarter, more sustainable agricultural practices.
Data availability
The data that support the findings of this study are available from the corresponding author upon reasonable request.
References
Ti, C. et al. Potential for mitigating global agricultural ammonia emission: A meta-analysis. Environ. Pollut. 245, 141–148 (2019).
Insausti, M. et al. Advances in sensing ammonia from agricultural sources. Sci. Total Environ. 706, 135124 (2020).
Ogwu, M. C. et al. Agricultural Air Pollution: Impacts, Sources, and Mitigation Strategies. In Air Pollutants in the Context of One Health: Fundamentals, Sources, and Impacts (eds Izah, S. C. et al.) 395–423 (Springer Nature Switzerland, 2024).
Kanan, S. et al. Recent advances on metal oxide based sensors for environmental gas pollutants detection. Crit. Rev. Anal. Chem. 55, 1–34 (2024).
Mei, J. et al. Moisture absorption characteristics and mechanical degradation of composite lattice truss core sandwich panel in a hygrothermal environment. Compos. A Appl. Sci. Manuf. 127, 105647 (2019).
Baruah, S. & Mazumder, D. H. A review on application of machine learning techniques coupled with E-nose in healthcare, agriculture, and allied domains. IEEE Trans. Instrum. Meas. 74, 1–23 (2025).
Zhang, Z. et al. Electronic nose based on metal oxide semiconductor sensors for medical diagnosis. Prog. Nat. Sci. Mater. Int. 34(1), 74–88 (2024).
Liu, C.-Y. et al. Resolving cross-sensitivity effect in fluorescence quenching for simultaneously sensing oxygen and ammonia concentrations by an optical dual gas sensor. Sensors 21, 6940. https://doi.org/10.3390/s21206940 (2021).
Mei, H. et al. Overcoming the limits of cross-sensitivity: Pattern recognition methods for chemiresistive gas sensor array. Nano-Micro Lett. 16(1), 269 (2024).
Acharyya, S. et al. Smart and selective gas sensor system empowered with machine learning over IoT platform. IEEE Internet Things J. 11(3), 4218–4226 (2024).
Yang, Y. et al. An E-nose system for identification and quantification of hazardous gas mixtures using a combined strategy of CNNs and attentional mechanisms. Phys. Scr. 99(9), 096001 (2024).
Moshayedi, A. J. et al. E-nose-driven advancements in ammonia gas detection: A comprehensive review from traditional to cutting-edge systems in indoor to outdoor agriculture. Sustainability 15, 11601. https://doi.org/10.3390/su151511601 (2023).
Farid, A. B. et al. Software defect prediction using hybrid model (CBIL) of convolutional neural network (CNN) and bidirectional long short-term memory (Bi-LSTM). PeerJ Comput. Sci. 7, e739 (2021).
Abdulraheem, M. I. et al. Soil hardness measurement using fiber Bragg grating sensor: Combined compression forces methodology. Physica status solidi (RRL) Rapid Res. Lett. 19(5), 2400431 (2025).
Abdulraheem, M. I. et al. Recent applications of fiber Bragg grating sensors in humidity and water content detection in agriculture: A comprehensive review of development, challenges, and future trends. Int. J. Precis. Eng. Manuf. 25(7), 1499–1524 (2024).
Mor, S. et al. Current opportunities and trends in the gas sensor market: A focus on e-noses and their applications in food industry. Chemosensors 13, 181. https://doi.org/10.3390/chemosensors13050181 (2025).
Jin, J. et al. A complex-valued variant-parameter robust zeroing neural network model and its applications. IEEE Trans. Emerg. Top. Comput. Intell. 8(2), 1303–1321 (2024).
Acharyya, S. & Kumar Guha, P. Enhanced formaldehyde sensing performance employing plasma-treated hierarchical SnO2 nanosheets through oxygen vacancy modulation. Appl. Surf. Sci. 655, 159640 (2024).
Acharyya, S. & Guha, P. K. Hierarchical zinc Stannate nanoneedle-based sensitive detection of formaldehyde. ACS Appl. Electron. Mater. 5(6), 3446–3453 (2023).
Samui, R. et al. SnSe nanoflakes for a NO2 sensor at room temperature. ACS Appl. Nano Mater. 7(20), 24281–24290 (2024).
Mahata, B. et al. Temperature tunable selective detection of toluene and isopropanol employing plate-like WO3-based single chemiresistor. IEEE Sens. J. 24(21), 33970–33977 (2024).
Lou, C. et al. Design and optimization strategies of metal oxide semiconductor nanostructures for advanced formaldehyde sensors. Coord. Chem. Rev. 452, 214280 (2022).
Xie, J. et al. Solid-state synthesis of Y-doped ZnO nanoparticles with selective-detection gas-sensing performance. Ceram. Int. 42(1, Part A), 90–96 (2016).
Abdulraheem, M. I. et al. Advancement of remote sensing for soil measurements and applications: A comprehensive review. Sustainability 15, 15444. https://doi.org/10.3390/su152115444 (2023).
Ahmed, S. & Sinha, S. K. Studies on nanomaterial-based p-type semiconductor gas sensors. Environ. Sci. Pollut. Res. 30(10), 24975–24986 (2023).
Stinn, J. P. et al. Ammonia and greenhouse gas emissions from a modern U.S. swine breeding-gestation-farrowing system. Atmos. Environ. 98, 620–628 (2014).
Zhuang, M. et al. Different characteristics of greenhouse gases and ammonia emissions from conventional stored dairy cattle and swine manure in China. Sci. Total Environ. 722, 137693 (2020).
Joo, H.-S. et al. Greenhouse gas emissions from naturally ventilated freestall dairy barns. Atmos. Environ. 102, 384–392 (2015).
Zahmatkesh, I., Emdad, H. & Alishahi, M. M. Navier–Stokes computation of some gas mixture problemsin the slip flow regime. Sci. Iran. 22(1), 100338 (2015).
Fauzi, F. et al. Gas and humidity sensing with quartz crystal microbalance (QCM) coated with graphene-based materials—A mini review. Sens. Actuators A 330, 112837 (2021).
Li, J. et al. Flexible pressure, humidity, and temperature sensors for human health monitoring. Adv. Healthcare Mater. 13(31), 2401532 (2024).
Liu, Y. et al. Differences in responses of ammonia volatilization and greenhouse gas emissions to straw return and paddy-upland rotations. Environ. Sci. Pollut. Res. 29(17), 25296–25307 (2022).
Ngwabie, N. M. et al. Effects of animal activity and air temperature on methane and ammonia emissions from a naturally ventilated building for dairy cows. Atmos. Environ. 45(37), 6760–6768 (2011).
Joo, H. S. et al. Particulate matter dynamics in naturally ventilated freestall dairy barns. Atmos. Environ. 69, 182–190 (2013).
Ni, J. Q. & Heber, A. J. Sampling and measurement of ammonia concentration at animal facilities—A review (2001).
Miao, H. et al. DFT analysis of the sensitivity of graphene/MoS2 heterostructures toward H2CO. Vacuum 214, 112182 (2023).
Chen, X., et al. Antiferroelectric SnO2 network with amorphous surface for electrochemical N2 fixation. Angewandte Chemie Int. Ed. e202515222 (2025)
Peng, H. & Perdew, J. P. Rehabilitation of the Perdew-Burke-Ernzerhof generalized gradient approximation for layered materials. Phys. Rev. B 95(8), 081105 (2017).
Moellmann, J. & Grimme, S. DFT-D3 study of some molecular crystals. J. Phys. Chem. C 118(14), 7615–7621 (2014).
Lehtomäki, J. et al. Orbital-free density functional theory implementation with the projector augmented-wave method. J. Chem. Phys. 141(23), 234102 (2014).
Warmbier, R. & Quandt, A. Brillouin zone grid refinement for highly resolved ab initio THz optical properties of graphene. Comput. Phys. Commun. 228, 96–99 (2018).
Qiao, Y. et al. A multihead attention self-supervised representation model for industrial sensors anomaly detection. IEEE Trans. Industr. Inf. 20(2), 2190–2199 (2024).
Zhao, H. et al. High-sensitivity and self-powered flexible pressure sensor based on multi-scale structured piezoelectric composite. Chem. Eng. J. 519, 164787 (2025).
Qin, X. et al. Theoretical study of gas sensing toward acetone by a single-atom transition metal (Sc, Ti, V, and Cr)-doped InP3 monolayer. ACS Omega 9(45), 45059–45067 (2024).
Meng, X., et al., Identification of thermal fault states in cable insulation sheaths based on gas sensor arrays. IEEE Trans. Dielectrics Electr. Insul. 1–1 (2025).
Abdulraheem, M. I. et al. Recent advances in dielectric properties-based soil water content measurements. Remote Sens. 16, 1328. https://doi.org/10.3390/rs16081328 (2024).
Funding
This work was supported by the 14th Five-Year National Key Research and Development Program (2021YFD1700904) and by the Major Science and Technology projects of Henan Province (221111320700). It was also supported by the Henan Center for Outstanding Overseas Scientists (GZS2021007).
Author information
Authors and Affiliations
Contributions
Mengying Du: Conceptualization, Visualization, Validation, Data acquisition, Writing—original draft, Writing—review & editing. Mukhtar Iderawumi Abdulraheem: Conceptualization, Visualization, Validation, Data acquisition, Writing—original draft, Writing—review & editing. Lulu Xu: Conceptualization, Resources, Writing—review & editing. Yiheng Zang: Data acquisition, Writing—review & editing. Yinghang Song: Validation, Writing—review & editing. Maryam Abbasi Tarighat: Validation, Writing—review & editing, data analyzing, Supervision. Vijaya Raghavan: Writing—review & editing, Supervision. Jiandong Hu: Conceptualization, Visualization, Writing—review & editing, Supervision, Funding acquisition.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Du, M., Abdulraheem, M.I., Xu, L. et al. Enhanced Selectivity Electronic Nose Systems for Agricultural Ammonia Gas Detection via a co-designed WO3-ZnO Sensor Array and Convolutional Neural Networks. Sci Rep 15, 39100 (2025). https://doi.org/10.1038/s41598-025-26084-z
Received:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s41598-025-26084-z