Abstract
The escalating prevalence of diabetes-depression comorbidity (DDC) necessitates novel therapies targeting shared pathophysiological pathways, which needs to decipher the underlying molecular mechanisms. This study elucidates the therapeutic potential of chrysophanol, a natural anthraquinone, in streptozotocin (STZ) and chronic unpredictable mild stress (CUMS)-induced DDC rat model. Behavioral assessments, biochemical profiling, and integrated multi-omics analyses (RNA-seq and proteomics) were employed to decipher underlying mechanisms. Successful model establishment was confirmed by prolonged immobility time in the tail suspension test (p < 0.01) and reduced general health scores. Chrysophanol treatment restored serum brain-derived neurotrophic factor (BDNF) levels (p < 0.01) and ameliorated dyslipidemia (total cholesterol: p < 0.05). RNA-seq results revealed that chrysophanol regulated expression of hundreds of genes, which were enriched in synaptic vesicle cycling (downregulation of Sh3gl2, Camk5), CNS myelination, and axonal ensheathment pathways. Proteomic profile demonstrated the suppression of neurodegenerative markers and activation of axonal regeneration pathways. Notably, chrysophanol downregulated synaptic proteins associated with leukocyte chemotaxis (Pla2g7, Mdk) and glutamatergic synapses (Itpr2, Slc1a1) while upregulated axonal development, regeneration, and PPARγ signaling proteins (Apoa4, Apoa1, Apod), suggesting anti-inflammatory effects and disease-modifying potential through synaptic/axonal regulation. Integrated multi-omics identified overlapping targets linked to neuronal repair (Ankrd27) and iron metabolism (Fth1). These findings suggest chrysophanol as a multitarget agent alleviating DDC via synergistic restoration of neuroplasticity, suppression of neuroinflammation, and rebalancing of metabolic homeostasis, implying a mechanistic foundation for developing chrysophanol-based therapies of diabetes-associated neuropsychiatric disorders.
Similar content being viewed by others
Introduction
The global prevalence of diabetes mellitus (DM), a metabolic disease, is a matter of significant concern. Current trends indicate an upward trend in its incidence, with projections suggesting that by the year 2050, it is expected to affect a total of 1.31 billion individuals on a global scale1. In addition to hyperglycaemia and microvascular complications, the prevalence of depression is significantly higher in diabetics than in non-diabetics2, by a factor of about two3. This bidirectional relationship creates a vicious cycle: depression exacerbates poor glycemic control through medication non-adherence, while chronic hyperglycemia and inflammatory pathways potentiate neurochemical imbalances that worsen depressive symptomatology.
Depressive disorder represents a prevalent psychiatric condition characterized not only by affective symptoms but also by frequently comorbid cognitive dysfunction that often demonstrates treatment resistance. Notably, persistent residual cognitive impairment may endure beyond the achievement of clinical remission in affective symptoms, constituting a critical therapeutic challenge in mood disorder management4. Studies suggest that multiple converging pathogenic mechanisms in depressive disorder-associated cognitive dysfunctions, including but not limited to sustained neuroinflammatory responses, inflammatory response, dysregulated oxidative/nitrosative stress cascades, mitochondrial bioenergetic deficits, and impaired neurotrophic support5. Emerging evidence indicates that depression patients exhibit elevated levels of inflammatory biomarkers in both peripheral blood and cerebrospinal fluid, including cytokines and their soluble receptors, acute-phase proteins, chemokines, adhesion molecules, and prostaglandins. Notably, these inflammatory mediators have been mechanistically linked to core depressive symptoms such as fatigue, cognitive dysfunction, and sleep disturbances6. The central inflammatory dysfunction can induce chronic inflammation, progressive neuronal damage, and reduced brain-derived neurotrophic factor (BDNF) expression—a key neurotrophin whose depletion is directly associated with cognitive impairment7,8. The diabetes-depression comorbidity (DDC) appears driven by shared pathophysiological axes: chronic inflammation, hypothalamic-pituitary-adrenal (HPA) axis dysregulation, oxidative stress amplification, and neurotrophic factor deficiency9,10,11,12. These convergent mechanisms create a self-perpetuating cycle where hyperglycemia-induced neuroinflammation exacerbates depressive behaviors, while depression-associated cortisol dysregulation further impairs glucose metabolism. This pathological synergy underscores the urgent need for therapeutic agents targeting multiple nodes in these interacting pathways.
Chrysophanol is a 1,8-dihydroxy-3-methyl derivative of the 9,10-anthracenedionic ring isolated from the traditional Chinese medicine Rheum palmatum L. (rhubarb)13,14. Chrysophanol has a wide range of pharmacological effects and biological activities, including anti-inflammatory15,16, antioxidant17, antibacterial18, anticancer. Particularly noteworthy are its neuroprotective effects, with emerging evidence demonstrating efficacy in mitigating neurological disorders through antidepressant action19and neuronal preservation20. Chrysophanol has been shown in preclinical investigations to alleviate cognition deficits and neuronal death in streptozotocin-induced diabetic encephalopathy21. Furthermore, it demonstrates neuroprotective efficacy in motor neuron disorders including multiple sclerosis22and Parkinson’s disease through modulation of oxidative stress and inflammatory pathways20. Recent studies have shown that chrysophanol reduces oxidative stress and pyroptosis in diabetic nephropathy mice through the Keap1/Nrf2 pathway19and inhibits the production of inflammatory factors by blocking the MAPK pathway in microglia. In addition, chrysophanol targets the p38, ERK and JNK signaling pathways in cells to inhibit the expression of pro-inflammatory factors23. In hippocampal tissue, chrysophanol increases the activity of AChE and BDNF and down-regulates the levels of pro-inflammatory cytokines and oxidative stress24. It also inhibits the over-activation of astrocytes in the CA3 region of the hippocampus21. Despite these advances, a critical knowledge gap persists regarding chrysophanol’s therapeutic potential in DDC. The compound’s established effects on neuroinflammation, oxidative stress, and neurotrophic regulation - all key contributors to DDC pathogenesis - strongly suggest possible synergistic therapeutic benefits. However, systematic investigation remains lacking to delineate its specific mechanisms and efficacy in this complex comorbidity. This study aims to address this unmet need by comprehensively evaluating chrysophanol’s therapeutic effects and molecular targets in DDC models.
The aim of this study was to investigate the impact of chrysophanol on DDC by establishing an animal model of DM comorbid with depression. To this end, a multifaceted approach was adopted that encompassed biochemical assays, molecular biology, transcriptomics, and proteomics techniques. The investigation focused on elucidating its modulation of the inflammatory microenvironment in hippocampal tissue, HPA axis function, and neuroplasticity. The findings of this study serve to provide a robust experimental basis for the development of multi-targeted therapeutic strategies for the treatment of diabetes mellitus in individuals who also suffer from depression, which are based on naturally occurring products.
Materials and methods
Animals and model
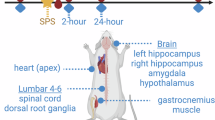
Previous study has demonstrated that thyroid hormones and associated diseases showed obvious difference between female and male patients25, so we used one gender rats to eliminate the impact of gender. Male Sprague-Dawley rats (6–8 weeks old, SPF grade) were purchased from the Beijing Huafukang Bio-technology Co., Ltd, and the animals were acclimatized for one week under standard laboratory conditions (22 ± 1 °C, 12-h light/dark cycle, ad libitum access to food and water). We have presented the working flow of animal experiment and following multi-omics experiments (Fig. 1A). Rats were then randomly assigned to a normal control group and a diabetes mellitus (DM) model group. The DM model group received high-fat diet (Feed formula: 20% lard, 1% cholesterol, 0.2% bile salt, 20% sugar, 58.8% breeding feed, Medicience Jiangsu) feeding for 4 weeks followed by a single intraperitoneal injection of 30 mg·kg⁻¹ streptozotocin (STZ, CasNo: 18883-66-4, ≥ 75% α-anomer basis, ≥ 98% (HPLC)). After the administration of STZ, the rats were observed for a period of three days. Those rats with fasting blood glucose levels of ≥ 16.7mmol·L⁻¹, as determined on two consecutive days, were designated as diabetic and thus included in the study.
Subsequently, DM rats underwent chronic unpredictable mild stress (CUMS) for 5 weeks to induce depression-like phenotypes. The CUMS paradigm consisted of daily exposure to one of nine randomized stressors: water deprivation (12 h); Damp bedding (24 h); Light/dark cycle reversal (24 h); Cage tilting (24 h); stroboscopic illumination (24 h); Tail pinch (1 min); Swimming in cold water (4 °C, 5 min); Food deprivation (12 h); restraint (24 h). Stressors were administered in a pseudorandom sequence to ensure no consecutive repetition7,26. All CUMS animals were individually housed throughout the stress protocol to enhance social isolation effects. The normal control group remained group-housed under standard conditions without stress exposure.
Following successful establishment of the diabetic depression model, rats were randomly stratified into three experimental groups after a statistical justification for the chosen group sizes (n = 6/group):
Diabetic depression model (DDC) control.
DDC + chrysophanol low-dose (CHR-L, 0.1 mg·kg⁻¹·d⁻¹).
DDC + chrysophanol high-dose (CHR-H, 10 mg·kg⁻¹·d⁻¹).
All treatments were administered via oral gavage once daily for 14 consecutive days. Chrysophanol (CasNo: 481-74-3, ≥ 97% purity, Jskchem manufacturer). Dose selection was based on preliminary pharmacokinetic studies and literature reports of effective antidepressant ranges21,24,27,28,29,30.
All experimental procedures involving animals were approved by the Animal Experimental Ethic Committee of the Second Hospital of Lanzhou University (Approval No. D2021-208). All methods were carried out in accordance with relevant guidelines and regulations of the Lanzhou City, and are reported in accordance with the ARRIVE guidelines (https://arriveguidelines.org). Rats were humanely euthanized at the end of the experiment using overdose of sodium pentobarbital, in accordance with the ethical protocols approved by the committee.
Validation of depression in animals
General condition score
3 model groups and 3 blank groups were randomly selected and scored for general condition: Healthy condition was marked as 3 points, erect or dirty hair was marked as 1 point, and intermediate condition was recorded as 2 points. Each score was recorded by single-blind scoring method.
Tail suspension test
The other behavioral despair test is the tail suspension test (TST). Each rat was suspended upside down by the tail at 30 cm above the ground and recorded with a camera for six minutes31. Two observers who were blinded to the kind of treatment measured the duration of immobility time.
Sucrose preference test
The sucrose preference test (SPT) was performed at the end of the intervention. First day of training, two bottles of 1% sugared water; second day, one bottle of sugared water and one bottle of plain water, changing position every two hours; 24 h fasting and water fasting from the end of the shift on the second night; single cage feeding with one bottle of water and one bottle of sugared water on the third shift day; end of experiment before the end of the shift on the fourth day. The sucrose preference rate was calculated by the percentage between sucrose consumption and surcrose + water consumption.
Water maze test
It is imperative that each rat is tested four times. Ideally, a minimum interval of 15 min should be observed between each test. Initially, each rat should be tested once, and then returned to the initial group to repeat the process. The rats and mice were positioned in the pool with their heads facing upwards. The size of water maze was 160 cm in diameter and 40 cm in depth. The temperature of water was 20 ± 1 ℃. The green sign on the side of the pool served as visual cues for spatial orientation. In each iteration of the experiment, they were gradually immersed in the pool in accordance with the directions E, S, W, and EN, respectively. The observation of the rats and mice ascending the platform was recorded on video. If the platform was reached within 60 s, the time taken to ascend was immediately recorded, and the recording was terminated. If the subjects did not ascend to the platform within 60 s, they were guided to do so with the assistance of an object. Thereafter, they were permitted a period of five seconds to acclimatize to the platform before being returned to their cages. The initial five-day period was dedicated to training for four trials per day, with each of the four directions being trained on an individual basis. The initial training day involved a sequence of movements directed from S → E → westsouth (WS) → eastnorth (EN). The subsequent day’s training sequence proceeded from WS → S → EN → E. The third day’s training involved a sequence from EN → WS → E → S. The fourth day’s training involved a sequence from E → EN → S → WS. The fifth day’s training involved a sequence from S → E → WS → EN. The sixth day focused exclusively on the SE direction, and the final day marked the conclusion of the formal experiments using probe test without the platform. After saving the experiment to the relevant file, the data and video from the preceding day’s experiment are exported and analyzed by SuperMaze.
Enzyme-linked immunosorbent assay
In line with the manufacturer’s guidelines, rat-specific enzyme-linked immunosorbent assay (ELISA) kits (Catalog No: E-EL-R1235, Elabscience, WuHan, China) were used to assess BDNF (Brain Derived Neurotrophic Factor) in rat serum.
Fasting blood sugar levels (FBG)
A portable glucometer (Roche Accu-Chek) was used to assess blood glucose levels 15 h after the last treatment. A drop of blood was taken from each rat using the tail vein puncture technique and placed on the glucometer strip loaded in the device for blood glucose measurement.
Blood sampling and analysis
At the experimental endpoint, rats were anaesthetized with pentobarbital overdose and blood samples were collected. After achieving total anesthesia, the abdomen was dissected to allow access to the vena cava, and blood sampling. Afterward, the blood sample was centrifuged at 3,500 rpm for 10 min, and the serum was separated and used for subsequent analyses. Serum total cholesterol (TC), low-density lipoprotein (LDL-C) concentrations were assessed through enzymatic colorimetric methods using commercial kits (Catalog No: 701132 and 70104, Biobase, Shandong, China) on an automated biochemical analyzer (Biobase, BK-600, China). Complex calibrator (Catalog No: 91072, Biobase, Shandong, China).
RNA-seq and data analysis
Total RNA was treated with RQ1 DNase (Promega) to remove DNA. The quality and quantity of the purified RNA were determined by measuring the absorbance at 260 nm/280nm (A260/A280) using smartspec plus (BioRad). RNA integrity was further verified by 1.5% agarose gel electrophoresis.
For each sample, 1 µg of total RNA was used for RNA-seq library preparation. mRNAs were captured by VAHTS mRNA capture Beads (Vazyme, N401). The purified RNA was used for directional RNA-seq library preparation by KAPA Stranded mRNA-Seq Kit for Illumina® Platforms (KK8544). Polyadenylated mRNAs were purified and fragmented. Fragmented mRNAs were converted into double strand cDNA. Following end repair and A tailing, the DNAs were ligated to Diluted Roche Adaptor (KK8726). After purification of ligation product and size fractioning to 300–500 bps, the ligated products were amplified, purified, quantified, stored at -80℃ before sequencing. The strand marked with dUTP (the 2nd cDNA strand) was not amplified, allowing strand-specific sequencing. For sequencing, the libraries were prepared following the manufacturer’s instructions and applied to illumina Novaseq 6000 system for 150 nt paired-end sequencing.
After obtaining the raw RNA-seq data, the fragments were evaluated using FastQC software. Clean reads were compared to the rat genome using HISAT2 software32 with a tolerance of one mismatch. DESeq233 was fitted to a negative binomial distribution model, and a Wald test or a likelihood ratio test was performed for differential expression analysis of genes. The results were analyzed using fold change (FC) and P-value to determine whether a gene was differentially expressed. The criteria for significant differential expression were: log₂FC ≥ 0.5 and p-value < 0.05.
Proteome and data analysis
Samples were taken from the − 80 °C refrigerator, and an appropriate amount of tissue samples were thoroughly ground into powder in a mortar pre-cooled with liquid nitrogen, the powder was collected in a new centrifuge tube, and the pre-mixed lysis solution (8 M urea, 1% protease inhibitor) was slowly added to the sample tube, and the sample was lysed by ultrasonic lysis in the ultrasonic crusher. Centrifugation (4 °C, 15000 g, 10 min) was performed to remove the cell residue, and the supernatant was transferred to a new centrifuge tube, and the supernatant protein concentration was determined using the BCA method.
The analysis utilizes the nanoflow rate Vanquish Neo system (Thermo Fisher Scientific) for chromatographic separation, and samples post high-efficiency liquid chromatography separation are subjected to Data Independent Acquisition (DIA) mass spectrometry using the Astral high-resolution mass spectrometer (Thermo Scientific). Protein Functional Enrichment GO terms, KEGG pathway and protein domain enrichment analysis of DEPs was performed using Fisher’s exact test (with the identified proteins as background). To identify proteins with significant expression differences between groups, the threshold for differential expression was set as an absolute fold change (FC) > 1.3 and P-value < 0.05.
Reverse transcription and quantitative polymerase chain reaction (RT-qPCR)
After reverse transcription, the cDNA was used for qPCR, which was performed on the ABI QuantStudio 5, followed by denaturing at 95˚C for 10 min, 40 cycles of denaturing at 95˚C for 15 s and annealing and extension at 60˚C for 1 min. Each sample had three technical replicates. The concentration of each transcript was then normalized to GAPDH (glyceraldehyde-3-phosphate dehydrogenase) and mRNA level using 2− ΔΔCT method to analysis34. Comparisons were performed with the two-way ANOVA or the paired Student’s t-test by using GraphPad Prism software (Version number 8.0, San Diego, CA). The primer sequences were shown in Table S1.
Statistical analysis
For data presentation, the results were shown as mean ± standard deviation. Student’s unpaired t-test and one-way ANOVA followed by Dunnett’s test were used to analyze data. A P-value < 0.05 was considered as statistically significant. All statistical analyses were done with the GraphPad Prism 10.1.2 software.
Results
Chrysophanol affects the progression of DDC in rats
To explore how chrysophanol affects the behaviors of DDC rats, we first established the DDC rat model and treated them with low- and high-dose chrysophanol, then we tested their behaviors and assessed expression pattern of genes and proteins by multi-omics methods (Fig. 1A). To validate the establishment of the DDC rat model, systematic serum biochemical testing and behavioral assessments were performed. The model group exhibited significantly increased FBG compared to age-matched controls (Fig. 1B, F = 22.31, P = 0.0003). Concurrently, lipid profile assessments demonstrated marked elevations in both total cholesterol (TC) and low-density lipoprotein cholesterol (LDL-C) levels in the model group relative to controls (p < 0.01) (Fig. 1C-D, F = 8.91, P = 0.0008 for C, and F = 9.95, P = 0.0004 for D), consistent with characteristic dyslipidemia observed in diabetic pathophysiology. The changes in depression-related behaviors in rats were monitored using the general condition score, tail suspension test (TST), sucrose preference test (SPT), and water maze test (Fig. 1E-J, F = 7.47, P = 0.0017 for G; F = 10.44, P = 0.0004 for H; F = 4.21, P = 0.0239 for I; F = 17.11, P < 0.0001 for J). Compared to the control group, the general condition score and sucrose preference rate in the DDC rats were significantly reduced, but immobility rate significantly increased. The water maze test showed that chrysophanol could reverse the effect of modeling on the average swimming speed (p < 0.01), number of platform crossings (p < 0.05) and first crossing of the platform latency (p < 0.001) of rats. Serum biochemical analysis revealed distinct metabolic alterations among experimental groups. Notably, BDNF levels were significantly elevated in the high-dose chrysophanol group compared with the model group (p < 0.01) (Fig. 1K, F = 8.10, P = 0.0007). These data demonstrate two key findings: (1) the successful establishment of a depressive-like phenotype under diabetic conditions; and (2) chrysophanol administration significantly ameliorates depression-associated behaviors, potentially exerting antidepressant effects via targeted modulation of neurotrophic factor signaling pathways.
Construction of rat model of DDC. (A) Working flow and pipeline of this study. (B) FBG levels after intervention. (C) Serum concentration of TC. (D) Serum concentration of LDL-C. (E) General condition score. (F) The immobility rate in tail suspension test. (G) Percentage of sucrose preference of all groups. (H) The test’s overall average swimming speed. (I) Number of platform crossings. (J) First crossing of the platform latency. (K) Serum concentration of BDNF. (B–D & G–K: *p-value < 0.05, **p-value < 0.01, ***p-value < 0.001, ****p-value < 0.0001, one-way ANOVA followed by Dunnett’s test.); (E & F: n = 3 for each group, *p-value < 0.05, Student’s t-test.). Each dot point represented individual animal data point.
Chrysophanol affects DDC by upregulating neurogenesis-related genes
To further explore the effect of chrysophanol on diabetes-depression comorbidity, we performed RNA-seq to identify the potential mechanisms of chrysophanol using the tissues from the hippocampal region obtained from model and chrysophanol high-dose treatment groups, as well as their control samples. To analyze the expression profile of the samples between the different groups, we performed PCA analysis (Fig. 2A) using the list of FPKM values of all expressed genes as input, which showed obvious clustering for the three groups. The differentially expressed genes (DEGs) between these two groups were conducted by applying the DESeq2 software. Based on the result, there were a total of 395 DEGs in the ChryM group compared with the model group, of which 187 DEGs were up-regulated and 208 DEGs were down-regulated. To visualize the differential expression patterns, heatmap and volcano plots were generated where each row represents one gene (Fig. 2B-C). Gene Ontology (GO) and Kyoto Encyclopedia of Genes and Genomes (KEGG) pathway enrichment analysis was performed on the DEGs to further investigate the mechanisms by which chrysophanol acts Down-regulated GO functional pathways revealed that the synaptic vesicle recycling (SVC) (Sh3gl2, Camk5), neuroepithelial cell differentiation, astrocyte differentiation was significantly enriched in the comparison between ChryM group versus model group (Fig. 2D), suggesting chrysophanol’s suppression of hyperactive neurotransmitter release and glial dysregulation in DDC. Concurrently the central nervous system myelination (Plp1/Mal/Nkx6-2/Mobp/Mag), axon ensheathment in central nervous system and regulation of neurogenesis (Ankrd27/Ist1/Rgs14/Mbp/Atf5/Mag) was Up-regulated (Fig. 2E-F). The synaptic vesicle cycle, a fundamental biological mechanism governing neurotransmitter release and synaptic plasticity, emerged as a key regulatory node in chrysophanol’s anti-DDC action. Our transcriptomic profiling revealed targeted suppression of synaptic vesicle recycling components, particularly Sh3gl2 (synaptic endocytosis regulator) and Scamp5 (secretory vesicle trafficking modulator), suggesting chrysophanol’s capacity to modulate neurotransmission in DDC pathophysiology. Concurrently, chrysophanol enhanced CNS axon ensheathment pathways, a dual-layered glial process involving both myelinating oligodendrocytes and non-myelinating astrocytes. This bidirectional regulation, suppressing pathogenic synaptic overactivity while promoting axonal insulation, positions chrysophanol as a unique neuro-glial interface modulator. In summary, we speculate that chrysophanol may influence depression development through a number of different molecular mechanisms in DDC.
In the comparison between model group vs. control group, a total of 590 DEGs were found, of which 258 were up-regulated and 332 were down-regulated (Figure S1A-B). In contrast to control group, GO enrichment analysis in the model group revealed axonogenesis, telencephalon development, glial cell differentiation, oligodendrocyte differentiation was significantly enriched (Figure S1C-D). KEGG pathway classification showed that glutamatergic synapse was significantly enriched in the ChryM group compared to the model group (Figure S1E). Glutamatergic synapses, which use glutamate (Glu) as their main neurotransmitter, are an important neurotransmitter transmission mechanism. The ionotropic glutamate receptor in glutamatergic synapses plays an important role in nerve signaling and brain function. The results of the present study suggest that chrysophanol may play an important role in the progression of DDC in rats by modulating neural signaling. This includes, but is not limited to, synaptic vesicle cycling, neuroepithelial, astrocyte glial and oligodendrocyte cell differentiation, central nervous system myelination, axon ensheathment in central nervous system, axonogenesis and glutamatergic synapses.
Chrysophanol affects DDC by modulating synaptic and inflammation-related proteins
To explore mechanisms underlying the chrysophanol on diabetes-depression comorbidity, MS-based high-throughput proteomics was performed for large-scale protein characterization. To assess the reproducibility across samples, we calculated the Pearson’s correlation coefficient (PCC) analysis and heatmap presentation effectively illustrated the degree of correlation between any two samples (Fig. 3A). We also performed principal component analysis (PCA), and found that the three groups were clearly separated at the first and second components (Figure S2A). A threshold of absolute FC > 1.3 and p-value < 0.05 was applied to identify proteins with significant expression differences between groups. Comparative proteomic analysis revealed distinct expression profiles among experimental groups. Compared with the model group, the ChryM-treated group exhibited differential expression of 470 proteins, including 252 upregulated and 218 downregulated proteins (Fig. 3B). In contrast, the model group showed substantial proteomic dysregulation relative to the control group, with 437 proteins upregulated and 352 downregulated (Figure S2B).
These quantitative alterations suggest that ChryM intervention may partially reverse disease-associated protein expression patterns observed in the diabetic depression model. Functional classification analysis of differential proteins revealed distinct expression patterns between the model group and the ChryM group across multiple biological systems (Fig. 3C). Specifically, in the nervous system, 31 proteins were significantly downregulated whereas only 4 proteins exhibited upregulation following chrysophanol intervention. The immune system demonstrated a similar trend with predominant downregulation (17 proteins) over upregulation (9 proteins). Within the lipid metabolism group, the treatment induced downregulation of 8 proteins and upregulation of 6 proteins, indicating a moderate regulatory effect. Notably, under Human Diseases classification, proteins associated with neurodegenerative diseases showed the most pronounced suppression, with 24 downregulated versus 14 upregulated species. Intriguingly, substance dependence-related proteins presented discordant regulatory patterns between subgroups − 13 downregulated with 3 upregulated in the primary analysis, contrasting with 2 downregulated and 4 upregulated proteins observed in immune disease-related pathways. These differential expressions suggest system-specific pharmacological effects of chrysophanol intervention. Comparative proteomic analysis demonstrated significant alterations across multiple biological systems between the model and control groups (Figure S2C). In neurological parameters, we identified 16 downregulated and 21 upregulated proteins associated with neural signaling pathways. The endocrine system exhibited notable changes, with 41 proteins showing decreased expression versus 29 displaying increased abundance. Immune-related proteins presented a distinct pattern, demonstrating 34 downregulations compared to 16 upregulations. Metabolic pathway analysis revealed system-specific modulations: Lipid metabolism: 16 downregulated vs. 12 upregulated proteins; Amino acid metabolism: 13 downregulated vs. 30 upregulated proteins. Notably, disease-associated protein clusters showed marked differential expression: Neurodegenerative pathways: 17 downregulated vs. 34 upregulated proteins; Endocrine-metabolic disorders: 5 downregulated vs. 9 upregulated proteins; Immune-related pathologies: 4 downregulated vs. 2 upregulated proteins.
The experimental results demonstrated that chrysophanol treatment significantly downregulated the expression of neural-associated proteins, immune-related proteins, and neurodegenerative disease-associated proteins in diabetic depression model rats. This regulatory pattern suggests that chrysophanol may exert therapeutic effects through multiple biological pathways: (1) The reduction of neural system-related proteins potentially modulates neuronal excitability and pathological processes, thereby attenuating neurological damage; (2) The inhibition of inflammatory mediators in immune-related pathways indicates its anti-inflammatory properties and possible immunomodulatory potential; (3) The downregulation of neurodegenerative markers, including β-amyloid and α-synuclein proteins, implies a neuroprotective mechanism that may decelerate disease progression. Notably, chrysophanol’s regulatory effects on lipid metabolism-associated histoproteins further suggest its involvement in maintaining metabolic homeostasis. Collectively, these findings propose that chrysophanol ameliorates diabetic depression through a multi-target mechanism involving coordinated suppression of neuroinflammatory pathways, mitigation of neurodegenerative processes, and regulation of metabolic dysfunction.
To delineate the functional enrichment patterns of differentially expressed proteins, we conducted Gene Ontology (GO) and pathway analyses, visualized through bubble charts. The top 20 significantly enriched functional categories (P < 0.05) were systematically identified, revealing distinct biological pathway modulations across experimental groups. Comparative GO analysis of biological processes showed pronounced upregulation of immune-related pathways in the ChryM group versus the model group, including immune response, complement activation, and classical pathway regulation. Concurrently, neural repair mechanisms were markedly enhanced, as evidenced by enriched terms such as neuronal projection regeneration, axonal development, axonal regeneration, peripheral nervous system axon regeneration, and response to axonal injury. Conversely, pro-inflammatory processes were significantly suppressed, particularly leukocyte chemotaxis and leukocyte migration during inflammatory responses (Fig. 4). In the model group, biological processes associated with metabolic homeostasis exhibited notable downregulation. Specifically, lipid biosynthetic processes and NLRP3 inflammasome complex assembly were significantly attenuated compared to controls (Figure S3). In conclusion, the above results suggest that chrysophanol achieves its antidepressant function by promoting axonal development and regeneration and inhibiting inflammatory responses.
Comparative KEGG pathway analysis revealed distinct metabolic and neurological pathway modulations in ChryM group compared to the model group. The significant downregulation of Alzheimer’s disease-associated pathways and synaptic dysfunction markers (cholinergic/glutamatergic/dopaminergic synapses) in ChryM-treated rats suggests chrysophanol may mitigate diabetes-associated cognitive decline by targeting amyloidogenic and synaptic vesicle cycling processes. Enhanced axonal regeneration coupled with suppression of synaptic transmission hyperactivation implies a dual-action mechanism—repairing neural damage while preventing excitotoxicity. The ChryM group upregulated the Peroxisomal proliferating-activated receptor (PPAR) signaling pathway compared to the diabetic model group, indicating the potent PPARγ agonism of chrysophanol. This nuclear receptor activation mechanism effectively reversed diabetes-induced lipid dysregulation by modulating downstream targets including FABP4 (fatty acid binding protein 4) and CD36 (fatty acid translocase), thereby restoring hepatic insulin sensitivity. The coordinated upregulation of cholesterol metabolism and complement cascades further suggests that chrysophanol orchestrates lipid-inflammatory crosstalk through PPAR-mediated transcriptional reprogramming. Coordinated activation of cholesterol metabolism and complement cascades suggests a regulatory loop where lipid modulation influences inflammatory responses, critical in diabetic depression pathophysiology (Fig. 5). The model group’s upregulated glycolysis/TCA cycle and fatty acid biosynthesis reflect compensatory hypermetabolism, a hallmark of insulin resistance and mitochondrial dysfunction in diabetic encephalopathy. Downregulated lysosomal function, PPAR signalling pathway and complement cascades in the model group imply defective protein clearance and chronic low-grade inflammation, potentially exacerbating depressive phenotypes (Figure S4). In summary, these results indicate that chrysophanol can modulate the synapse pathways and inflammatory processes, which is associated with diabetic depressive disorder and in line with previous findings35.
Based on the above analysis of the functional enrichment results, key pathways involved in the regulation of chrysophanol were screened and heatmap analysis was performed to reveal its regulatory effects on key proteins and their underlying mechanisms (Fig. 6). It is worth noting that chrysophanol is involved in the regulation of inflammatory pathways through the dynamic regulation of Lgals3 (galectin-3), Adam17 (ADAM metallopeptidase domain 17), Itgam (integrin subunit alpha M), Fadd (FAS-associated death domain protein), and other proteins, and enhances neuroplasticity through the regulation of synaptic proteins such as Mdk (midkine), Grin3a (glutamate ionotropic receptor NMDA type subunit 3 A), Slc1a1 (solute carrier family 1 member 1), etc. It also exerts a potential neuroprotective effect through the regulation of Mdk, Grin3a, Slc1a1 and other synaptic proteins. The above results suggest that chrysophanol may ameliorate diabetic depression-like behavior in rats through its neuroprotective and anti-inflammatory functions.
Overlap results between RNA-seq and proteome results
Integrative analysis of DEGs and DEPs revealed that Ankrd27, Fth1, Smad3, and Cryab were upregulated in both the transcriptome and proteome, whereas Gpr37l1, Sncg, Pla2g7, and B2m were downregulated in both multi-omics datasets (Fig. 7). This cross-omics validation identifies Ankrd27 (neuronal vesicle trafficking), Fth1 (iron homeostasis), Smad3 (TGF-β signaling), and Cryab (stress chaperone) as core effectors mediating chrysophanol’s dual regulation of synaptic resilience and metabolic reprogramming in DDC pathogenesis. Finally, we selected five genes, including Ankrd27, Fth1, Cryab, Sncg, and Gpr37l1, and validated their changed expression levels using RT-qPCR experiment. We found that Ankrd27, Fth1, and Cryab were significantly increased in ChyM group and showed consistent expression pattern with the RNA-seq result (Fig. 7D), while Sncg and Gpr37l1 were downregulated in ChyM group (Fig. 7E).
Overlap Gene. (A) Venn plot of the overlapped genes between DEGs and DEPs. (B) Heatmap presented the expression pattern of overlapped genes in RNA-seq. (C) Heatmap presented the expression pattern of overlapped proteins in Proteome results (n = 3 for each group). (D) Bar plot presented the RNA-seq and RT-qPCR results of up-regulated genes. (E) The same as (D) but for the down-regulated genes. For RT-qPCR, each of the three biological replicates had three technical replicates. **P-value < 0.01, ****P-value < 0.0001, two-way ANOVA.
Discussion
The escalating global burden of DM and major depressive disorder (MDD) comorbidity represents a critical public health challenge. To elucidate the underlying molecular mechanisms linking these pathologies, this preclinical investigation systematically evaluated the therapeutic potential of chrysophanol, a bioactive anthraquinone derivative from rhubarb, on depression-like phenotypes in a STZ and CUMS induced DDC rat model. The present study elucidates the multi-target mechanisms underlying the therapeutic effects of chrysophanol in a DDC model through integrated transcriptomic and proteomic analyses. Our findings reveal that chrysophanol modulates critical biological pathways associated with neuroprotection, synaptic plasticity, immune regulation, and metabolic homeostasis, providing a molecular foundation for its efficacy in mitigating DDC progression.
Our experimental results demonstrated the successful establishment of a depression model through general condition scoring, TST, SPT and water maze test, which aligns with the findings reported by Kilic et al.7. SPT experiment shows lower sucrose preference in model group36. Furthermore, accumulating evidence implicates dysregulation of the brain-derived neurotrophic factor (BDNF) system in the pathophysiology of depression4,37. The present study contributes novel insights to this field, as our serum BDNF analysis revealed that chrysophanol may exert antidepressant effects through modulation of BDNF levels. Consistent with the diabetes modeling methodology employed in our study, Zhao et al. observed depression-like behavioral alterations in type 2 diabetes mellitus mice induced by high-fat diet combined with intraperitoneal STZ injection38. In agreement with the findings of Hoseini et al.39, our lipid profile analysis demonstrated significant elevations in total cholesterol (TC) and low-density lipoprotein cholesterol (LDL-C) levels in diabetic rats. The dual verification through behavioral and metabolic parameters strengthens the construct validity of our comorbid depression-diabetes model.
RNA-seq data revealed that chrysophanol significantly modulates genes involved in synaptic vesicle cycling, central nervous system (CNS) myelination, and axon ensheathment. The downregulation of synaptic vesicle cycling pathways (Fig. 2C), in concert with proteomic validation (Fig. 5), suggests a potential normalization of neurotransmitter release dynamics, which may counteract synaptic dysfunction induced by STZ and CUMS. The synaptic vesicle transport related genes (sh3gl2, scamp5, sncg and amph) are down-regulated, resulting in the accumulation of intracellular glutamate40. Concurrently, the upregulation of myelination-related pathways (Fig. 2D) implies enhanced axonal integrity and neuronal communication, aligning with prior studies highlighting myelination deficits in diabetic encephalopathy and depression41. Proteomic profiling further corroborated these findings, demonstrating chrysophanol-induced suppression of neurodegenerative markers (e.g., β-amyloid and α-synuclein) and potentiation of axonal regeneration pathways (Fig. 4). This dual action—repairing neuronal damage and mitigating excitotoxicity—may synergistically ameliorate cognitive and affective symptoms in diabetic depression comorbidity (DDC). Meanwhile, further experiments should be conducted to validate the anti-amyloid and anti-synuclein effects of chrysophanol by performing immunofluorescence in hippocampus, which will be considered in following study.
Integrated transcriptomic and proteomic analyses unveiled the anti-inflammatory properties of chrysophanol. The downregulation of leukocyte chemotaxis and migration pathways in the proteomic profile (Fig. 4), coupled with KEGG enrichment of glutamatergic synapse regulation (Figure S1E), indicates suppression of neuroinflammation, a hallmark of diabetic depression38. This observation aligns with previous study that identified leukocyte chemotaxis as a critical mediator in mild cognitive impairment (MCI) and Alzheimer’s disease (AD) pathogenesis42. Notably, the upregulation of peroxisome proliferator-activated receptor (PPAR) signaling (Fig. 5) and lipid metabolism-associated proteins (FABP4 and CD36) suggests chrysophanol restores metabolic homeostasis via PPARγ agonism. In support of this mechanism, it is also demonstrated that naringenin and naringenin-loaded hybridized nanoparticles (NAR-HNPs) alleviate diabetes mellitus (DM)-induced depression by activating the PPAR-γ/NLRP3 axis, thereby modulating neurotransmission and exerting antioxidative/anti-inflammatory effects43. Neurotransmission is involved in the regulation of mood, appetite, energy expenditure, and behavior, and is highly associated with T2D-induced depression44,45. Consistently, Zhao et al. reported chrysophanol-mediated upregulation of PPARG expression suppresses neuroinflammation and apoptosis46.
Proteomic profiling further revealed chrysophanol-induced activation of axonal development/regeneration pathways and enhanced responses to axonal injury (Fig. 4), implicating axonal plasticity modulation in its antidepressant action. These findings resonate with Silva et al., who established axonal remodeling as a key mediator in chronic stress-induced depression47. Intriguingly, significant downregulation of Alzheimer’s disease-associated pathways highlights chrysophanol’s potential to mitigate CNS pathologies. The coordinated downregulation of multiple synaptic pathways—including cholinergic, serotonergic, dopaminergic, and glutamatergic synapses—suggests dynamic synaptic remodeling may underpin chrysophanol’s neuroprotective efficacy against neurological disorders48.
Integrative analysis identified overlapping molecules (e.g., Ankrd27, Fth1) showing concordant regulation at both mRNA and protein levels (Fig. 6). Ankrd27, implicated in vesicular trafficking and involved in the regulation of neurite growth49. Fth1, a ferroptosis regulator, associated with neurodegeneration with brain iron accumulation50, also related vesicle-mediated transport, may serve as key nodes in chrysophanol’s therapeutic network. The downregulation of neurodegenerative51,52 and immune-related53,54 proteins (e.g., B2m, Pla2g7) further underscores its multi-target action. These findings align with emerging paradigms advocating polypharmacology for complex comorbidities like DDC55,56.
While this study provides mechanistic insights, there are several limitations, including the lack of functional validation (e.g., knock-out models), the lack of protein or immunofluorescence validation, and restricted sample size for generalization. Meanwhile, one standard positive control drug, such as fluoxetine, was not properly included in the omics study. As the omics datasets provided potential pathways and molecular targets of chrysophanol with small sample size, future work and long-term efficacy assessment should be conducted to explore dose-dependent effects, in vivo behavioral correlations, and direct interactions between chrysophanol and identified targets (e.g. PPARγ) with more samples in each group. Additionally, investigating sex-specific responses and long-term safety profiles will be critical for clinical translation. Finally, several studies have also showed the drawbacks of chrysophanol, such as limited pharmacokinetic data, possible toxicity, and bioavailability issues57,58, it is necessary to make a more objective evaluation on the efficacy of chrysophanol and further investigate a proper therapeutic method for chrysophanol in future.
Conclusion
The present study delineates a comprehensive molecular framework underlying chrysophanol’s potential therapeutic efficacy in DDC, revealing its multimodal action through two synergistic axes: (1) Neuroplasticity Restoration. Transcriptomic profiling identified chrysophanol-mediated suppression of synaptic vesicle cycling components (Sh3gl2 and Camk5) and enhancement of CNS myelination (Plp1 and Mal), indicating normalization of glutamate neurotransmission hyperactivation and oligodendrocyte-mediated axonal repair; Proteomic validation demonstrated upregulation of axonal regeneration markers (ANKRD27 and CRYAB) and downregulation of neurodegenerative proteins, synergistically reversing diabetes-induced synaptic dysfunction. (2) Anti-Inflammatory Reprogramming. Dual-omics convergence revealed suppression of leukocyte chemotaxis (Pla2g7, Mdk) and NLRP3 inflammasome activation, coupled with PPARγ pathway potentiation (FABP4, CD36). This dual modulation attenuates neuroinflammation while restoring lipid metabolic homeostasis. Spatial regulation of glutamatergic synapse components (Grin3a, Slc1a1) further highlights chrysophanol’s capacity to mitigate excitotoxicity-driven neuronal damage.
In summary, chrysophanol ameliorates DDC probably through coordinated modulation of neuroplasticity, inflammation, and metabolism. Its ability to target overlapping pathways at both transcriptional and translational levels implies its potential as a multi-modal therapeutic agent. These findings pave the way for further preclinical optimization and clinical evaluation of chrysophanol in diabetes-associated neuropsychiatric disorders.
Data availability
The data discussed in this publication have been deposited in NCBI’s Gene Expression Omnibus and can be viewed through GEO Series accession number GSE296320 for RNA-seq.
References
Ong, K. L. et al. Global, regional, and National burden of diabetes from 1990 to 2021, with projections of prevalence to 2050: a systematic analysis for the global burden of disease study 2021. Lancet 402, 203–234. https://doi.org/10.1016/S0140-6736(23)01301-6 (2023).
Ho, M. T. H. et al. Risk of mortality and complications in people with depressive disorder and diabetes mellitus: A 20-year population-based propensity score-matched cohort study. Eur. Neuropsychopharmacol. 92, 10–18. https://doi.org/10.1016/j.euroneuro.2024.11.011 (2025).
Wong, J. & Mehta, G. Efficacy of depression management in an integrated Psychiatric-Diabetes education clinic for comorbid depression and diabetes mellitus types 1 and 2. Can. J. Diabetes. 44, 455–460. https://doi.org/10.1016/j.jcjd.2020.03.013 (2020).
Diniz, B. S. et al. Reduced cerebrospinal fluid levels of brain-derived neurotrophic factor is associated with cognitive impairment in late-life major depression. J. Gerontol. B Psychol. Sci. Soc. Sci. 69, 845–851. https://doi.org/10.1093/geronb/gbu096 (2014).
Bakunina, N., Pariante, C. M. & Zunszain, P. A. Immune mechanisms linked to depression via oxidative stress and neuroprogression. Immunology 144, 365–373. https://doi.org/10.1111/imm.12443 (2015).
Miller, A. H., Maletic, V. & Raison, C. L. Inflammation and its discontents: the role of cytokines in the pathophysiology of major depression. Biol. Psychiatry. 65, 732–741. https://doi.org/10.1016/j.biopsych.2008.11.029 (2009).
Kilic, A. et al. Alamandine enhanced Spatial memory in rats by reducing neuroinflammation and altering BDNF levels in the hippocampus and prefrontal cortex. Sci. Rep. 15, 12205. https://doi.org/10.1038/s41598-025-95683-7 (2025).
Fernandes, B. S., Berk, M., Turck, C. W., Steiner, J. & Gonçalves, C. A. Decreased peripheral brain-derived neurotrophic factor levels are a biomarker of disease activity in major psychiatric disorders: a comparative meta-analysis. Mol. Psychiatry. 19, 750–751. https://doi.org/10.1038/mp.2013.172 (2014).
Sharma, A., Bhalla, S. & Mehan, S. PI3K/AKT/mTOR signalling inhibitor Chrysophanol ameliorates neurobehavioural and neurochemical defects in propionic acid-induced experimental model of autism in adult rats. Metab. Brain Dis. 37, 1909–1929. https://doi.org/10.1007/s11011-022-01026-0 (2022).
Hamed, S. A. Brain injury with diabetes mellitus: evidence, mechanisms and treatment implications. Expert Rev. Clin. Pharmacol. 10, 409–428. https://doi.org/10.1080/17512433.2017.1293521 (2017).
Lu, Y. et al. Depression with comorbid diabetes: what evidence exists for treatments using traditional Chinese medicine and natural products? Front. Pharmacol. 11, 596362. https://doi.org/10.3389/fphar.2020.596362 (2020).
Li, S. et al. Neurological and metabolic related pathophysiologies and treatment of comorbid diabetes with depression. CNS Neurosci. Ther. 30, e14497. https://doi.org/10.1111/cns.14497 (2024).
Singh, D., Rawat, M. S. M., Semalty, A. & Semalty, M. Chrysophanol–phospholipid complex. J. Therm. Anal. Calorim. 111, 2069–2077. https://doi.org/10.1007/s10973-012-2448-6 (2013).
Gu, M. et al. Chrysophanol, a main anthraquinone from rheum palmatum L. (rhubarb), protects against renal fibrosis by suppressing NKD2/NF-κB pathway. Phytomedicine 105, 154381. https://doi.org/10.1016/j.phymed.2022.154381 (2022).
Su, S. et al. The Pharmacological properties of chrysophanol, the recent advances. Biomed. Pharmacother. 125, 110002. https://doi.org/10.1016/j.biopha.2020.110002 (2020).
Jeong, H. J., Kim, H. Y. & Kim, H. M. Molecular mechanisms of anti-inflammatory effect of chrysophanol, an active component of AST2017-01 on atopic dermatitis in vitro models. Int. Immunopharmacol. 54, 238–244. https://doi.org/10.1016/j.intimp.2017.11.019 (2018).
Lian, Y., Xia, X., Zhao, H. & Zhu, Y. The potential of Chrysophanol in protecting against high fat-induced cardiac injury through Nrf2-regulated anti-inflammation, anti-oxidant and anti-fibrosis in Nrf2 knockout mice. Biomed. Pharmacother. 93, 1175–1189. https://doi.org/10.1016/j.biopha.2017.05.148 (2017).
Rokaya, M. B., Münzbergová, Z., Timsina, B. & Bhattarai, K. R. Rheum australe D. Don: a review of its botany, ethnobotany, phytochemistry and Pharmacology. J. Ethnopharmacol. 141, 761–774. https://doi.org/10.1016/j.jep.2012.03.048 (2012).
Yuan, X., Tang, W., Lin, C., He, H. & Li, L. Chrysophanol ameliorates oxidative stress and pyroptosis in mice with diabetic nephropathy through the Kelch-like ECH-associated protein 1/nuclear factor erythroid 2-related factor 2 signaling pathway. Acta Biochim. Pol. 70, 891–897. https://doi.org/10.18388/abp.2020_6778 (2023).
Chae, U. et al. Chrysophanol suppressed Glutamate-Induced hippocampal neuronal cell death via regulation of Dynamin-Related protein 1-Dependent mitochondrial fission. Pharmacology 100, 153–160. https://doi.org/10.1159/000477814 (2017).
Chu, X. et al. Chrysophanol relieves cognition deficits and neuronal loss through Inhibition of inflammation in diabetic mice. Neurochem Res. 43, 972–983. https://doi.org/10.1007/s11064-018-2503-1 (2018).
Lee, J. M., Kyeong, S., Kim, E. & Cheon, K. A. Abnormalities of Inter- and Intra-Hemispheric functional connectivity in autism spectrum disorders: A study using the autism brain imaging data exchange database. Front. Neurosci. 10, 191. https://doi.org/10.3389/fnins.2016.00191 (2016).
Lin, F. et al. Chrysophanol affords neuroprotection against microglial activation and free radical-mediated oxidative damage in BV2 murine microglia. Int. J. Clin. Exp. Med. 8, 3447–3455 (2015).
Khan, J. Z. et al. Chrysophanol attenuates cognitive impairment, neuroinflammation, and oxidative stress by TLR4/NFκB-Nrf2/HO-1 and BDNF/VEGF signaling in stress-intensified PTZ induced epilepsy in mice. Front. Pharmacol. 15, 1446304. https://doi.org/10.3389/fphar.2024.1446304 (2024).
Bauer, M., Glenn, T., Pilhatsch, M., Pfennig, A. & Whybrow, P. C. Gender differences in thyroid system function: relevance to bipolar disorder and its treatment. Bipolar Disord. 16, 58–71. https://doi.org/10.1111/bdi.12150 (2014).
Guo, J. et al. Enhancing mPFC to BLA information transmission through chemical genetics to improve exploratory behavior in chronic stress rats. Brain Res. Bull. 225, 111335. https://doi.org/10.1016/j.brainresbull.2025.111335 (2025).
Zhao, Y. et al. Neuroprotective effects of Chrysophanol against inflammation in middle cerebral artery occlusion mice. Neurosci. Lett. 630, 16–22. https://doi.org/10.1016/j.neulet.2016.07.036 (2016).
Zhang, K. et al. P2X7 as a new target for chrysophanol to treat lipopolysaccharide-induced depression in mice. Neurosci. Lett. 613, 60–65. https://doi.org/10.1016/j.neulet.2015.12.043 (2016).
Zhang, J. et al. Chrysophanol attenuates lead exposure-induced injury to hippocampal neurons in neonatal mice. Neural Regeneration Research 9 (2014).
Zhang, J., Yan, C. L., Zhang, D. S., Zhang, L. & Wang, S. Effects of Chrysophanol on learning and memory impairment induced by lead in mice and the study of its mechanisms. Chin. Pharmacol. Bull. 27, 1614–1618. https://doi.org/10.3969/j.issn.1001-1978.2011.11.033 (2011).
Fan, Q. et al. Chaihu-Shugan-San inhibits neuroinflammation in the treatment of post-stroke depression through the JAK/STAT3-GSK3β/PTEN/Akt pathway. Biomed. Pharmacother. 160, 114385. https://doi.org/10.1016/j.biopha.2023.114385 (2023).
Kim, D., Paggi, J. M., Park, C., Bennett, C. & Salzberg, S. L. Graph-based genome alignment and genotyping with HISAT2 and HISAT-genotype. Nat. Biotechnol. 37, 907–915. https://doi.org/10.1038/s41587-019-0201-4 (2019).
Love, M. I., Huber, W. & Anders, S. Moderated Estimation of fold change and dispersion for RNA-seq data with DESeq2. Genome Biol. 15, 550. https://doi.org/10.1186/s13059-014-0550-8 (2014).
Livak, K. J. & Schmittgen, T. D. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods 25, 402–408, (2001). https://doi.org/10.1006/meth.2001.1262
Herder, C. & Hermanns, N. Subclinical inflammation and depressive symptoms in patients with type 1 and type 2 diabetes. Semin Immunopathol. 41, 477–489. https://doi.org/10.1007/s00281-019-00730-x (2019).
Zhan, T. et al. Implication of LncRNA MSTRG.81401 in hippocampal pyroptosis induced by P2X7 receptor in type 2 diabetic rats with neuropathic pain combined with depression. Int. J. Mol. Sci. 25 https://doi.org/10.3390/ijms25021186 (2024).
Xi, Y. Q. et al. Association of inflammation cytokines with cognitive function in first-episode major depressive disorder. Front. Psychiatry. 15, 1473418. https://doi.org/10.3389/fpsyt.2024.1473418 (2024).
Zhao, W. et al. AdipoRon attenuates depression-like behavior in T2DM mice via inhibiting inflammation and regulating autophagy. Brain Res. Bull. 224, 111308. https://doi.org/10.1016/j.brainresbull.2025.111308 (2025).
Hoseini, Z., Behpour, N. & Hoseini, R. Aerobic training and vitamin D supplementation effects on diabetes-related parameters in a rat model of type 2 diabetes. BMC Sports Sci. Med. Rehabil. 17, 79. https://doi.org/10.1186/s13102-025-01125-2 (2025).
Li, N., Gao, Y., Zhang, Y. & Deng, Y. An integrated multi-level analysis reveals learning-memory deficits and synaptic dysfunction in the rat model exposure to austere environment. J. Proteom. 279, 104887. https://doi.org/10.1016/j.jprot.2023.104887 (2023).
Bu, J. et al. Paroxetine ameliorates corticosterone-induced Myelin damage by promoting the proliferation and differentiation of oligodendrocyte precursor cells. Neuroscience 573, 344–354. https://doi.org/10.1016/j.neuroscience.2025.03.065 (2025).
Rehman, H. et al. Plasma protein risk scores for mild cognitive impairment and alzheimer’s disease in the Framingham heart study. Alzheimers Dement. 21, e70066. https://doi.org/10.1002/alz.70066 (2025).
El-Marasy, S. A. et al. Anti-depressant effect of Naringenin-loaded hybridized nanoparticles in diabetic rats via PPARγ/NLRP3 pathway. Sci. Rep. 14, 13559. https://doi.org/10.1038/s41598-024-62676-x (2024).
Khawagi, W. Y. et al. Depression and type 2 diabetes: A causal relationship and mechanistic pathway. Diabetes Obes. Metab. 26, 3031–3044. https://doi.org/10.1111/dom.15630 (2024).
Luo, C. et al. Breaking the diabetes-depression cycle: exploring shared mechanisms, neuroinflammation, and emerging interventions for metabolic-mood comorbidities. World J. Diabetes. 16 https://doi.org/10.4239/wjd.v16.i7.107406 (2025).
Zhao, H., Wang, Y. & Zhu, X. Chrysophanol exerts a protective effect against sepsis-induced acute myocardial injury through modulating the microRNA-27b-3p/Peroxisomal proliferating-activated receptor gamma axis. Bioengineered 13, 12673–12690. https://doi.org/10.1080/21655979.2022.2063560 (2022).
Silva, J. P. et al. Comprehensive analysis of circrna expression and circrna-miRNA-mRNA networks in the ventral hippocampus of the rat: impact of chronic stress and biological sex. ACS Chem. Neurosci. https://doi.org/10.1021/acschemneuro.4c00681 (2025).
Tang, Q. et al. The effects and mechanisms of Chai Shao Jie Yu granules on chronic unpredictable mild stress (CUMS)-induced depressive rats based on network Pharmacology. J. Ethnopharmacol. 340, 119268. https://doi.org/10.1016/j.jep.2024.119268 (2025).
Burgo, A. et al. A molecular network for the transport of the TI-VAMP/VAMP7 vesicles from cell center to periphery. Dev. Cell. 23, 166–180. https://doi.org/10.1016/j.devcel.2012.04.019 (2012).
Shieh, J. T. et al. Heterozygous nonsense variants in the ferritin heavy-chain gene FTH1 cause a neuroferritinopathy. HGG Adv. 4, 100236. https://doi.org/10.1016/j.xhgg.2023.100236 (2023).
Babovic, B. et al. Association of uremic toxins and systemic inflammation with depression and anxiety among Hemodialysis patients in Montenegro. Int. J. Psychiatry Med. 912174241298837 https://doi.org/10.1177/00912174241298837 (2024).
Liu, J. et al. Comprehensive variant analysis of phospholipase A2 superfamily genes in large Chinese parkinson’ s disease cohorts. Mech. Ageing Dev. 219, 111940. https://doi.org/10.1016/j.mad.2024.111940 (2024).
Song, Y. et al. Identification of potential biomarkers and immune cell signatures in COVID-19 myocarditis through bioinformatic analysis. Cardiol. Res. Pract. 2025, 2349610. https://doi.org/10.1155/crp/2349610 (2025).
Peng, D. et al. PLA2G7 promotes immune evasion of bladder cancer through the JAK-STAT-PDL1 axis. Cell. Death Dis. 16, 234. https://doi.org/10.1038/s41419-025-07593-1 (2025).
Hu, W. et al. The association between C-reactive protein-albumin-lymphocyte index and depression in adults with type 2 diabetes mellitus: A cross-sectional study from NHANES. Psychoneuroendocrinology 176, 107442. https://doi.org/10.1016/j.psyneuen.2025.107442 (2025).
Hu, G. & Cao, H. Nuclear factor erythroid 2-related factor improves depression and cognitive dysfunction in rats with ischemic stroke by mediating Wolfram syndrome 1. Brain Res. 1856, 149572. https://doi.org/10.1016/j.brainres.2025.149572 (2025).
Xie, L. et al. Chrysophanol: a review of its pharmacology, toxicity and pharmacokinetics. J. Pharm. Pharmacol. 71, 1475–1487. https://doi.org/10.1111/jphp.13143 (2019).
Prateeksha et al. Chrysophanol: A natural anthraquinone with multifaceted biotherapeutic potential. Biomolecules 9 https://doi.org/10.3390/biom9020068 (2019).
Acknowledgements
We gratefully acknowledge the assistance and instruction from colleagues of Wuhan Nissi Biotech.
Funding
This work was supported by grants from the Natural Science Foundation of Gansu Province. (grant No. 21JR11RA125).
Author information
Authors and Affiliations
Contributions
Xue-Jian Hu and Yan Yang: Conception and design of the research, Data collection, Data analysis, Manuscript writing; Dan Ge and Xiao-Lan Ma: Conception and design of the research, Data collection, Data analysis, Manuscript writing. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Hu, Xj., Ge, D., Ma, XL. et al. Multi-omics exploration demonstrated the antidepressant effects of chrysophanol on diabetes-depression comorbidity rats via inflammatory, and neurodegenerative axes. Sci Rep 15, 42678 (2025). https://doi.org/10.1038/s41598-025-26745-z
Received:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s41598-025-26745-z