Abstract
To investigate the effects and mechanisms of Astragalus Membranaceus Injection (AMI) on mitophagy and mitochondrial function in chronic heart failure (CHF) based on phosphoproteomic and network pharmacology analysis. Primary neonatal mouse cardiomyocytes were isolated and hypertrophy cardiomyocyte model was induced by phenylephrine (PE) stimulation. AMI’s effects on cell size, apoptosis, mitophagy, and mitochondrial function in hypertrophic cardiomyocytes were assessed. A pressure-overload CHF model was established via transverse aortic constriction (TAC) surgery in C57BL/6N mice. Echocardiography and histopathology were employed to evaluate AMI’s effects on cardiac function and structural remodeling. Transmission electron microscope (TEM) and immunofluorescence were used to detect the distribution of autophagosomes and mitochondria. Phosphorylation-antibody microarray and network pharmacology were employed to explore AMI’s cardioprotective mechanisms. The AKT/mTOR pathway’s involvement was verified through Western blotting of AKTSer473 and mTORSer2481 phosphorylation and pharmacological validation using SC79 (AKT/mTOR activator) and GSK-690693 (AKT/mTOR inhibitor) in gain/loss-of-function experiments. In vitro, AMI dose-dependently suppressed pathological hypertrophy, attenuated apoptosis, restored mitochondrial function, and enhanced mitophagic flux. In vivo, AMI treatment significantly improved left ventricular ejection fraction while attenuated cardiac hypertrophy and interstitial fibrosis in TAC-induced CHF mice. Besides, AMI treatment increased the number of mitochondria and elevated autophagy in TAC mice. Phosphoproteomic screening and network pharmacology analysis identified the PI3K/AKT/mTOR axis as the primary regulatory pathway mediating AMI’s cardioprotection. Pharmacological activation of AKT/mTOR signaling using SC79 significantly suppressed mitophagic flux, whereas AMI treatment mirrored the effects of the AKT/mTOR inhibitor GSK-690693, effectively restoring mitophagy and mitochondrial homeostasis. AMI exerts its cardioprotective effects through inhibition of the AKT/mTOR pathway, thereby ameliorating maladaptive remodeling and mitochondrial dysfunction in CHF.
Similar content being viewed by others
Introduction
Chronic Heart failure (CHF) is a significant global cause of morbidity and mortality, and its prevalence is increasing1. Concentric left ventricular hypertrophy is a specific form of cardiac remodeling induced by chronic pressure overload, as seen in systemic hypertension and aortic stenosis, and is defined by parallel sarcomeric addition leading to increased wall thickness with preserved chamber volume2. Epidemiological research demonstrate that concentric left ventricular hypertrophy is an independent predictor of incident CHF and cardiovascular mortality3. Despite decades of extensive research, CHF remains associated with a poor prognosis, demonstrating a five-year mortality rate of 40–50%4. Although various therapeutics, such as inhibitors of the renin-angiotensin system or calcium channel blockers, have been established to treat cardiac hypertrophy and prevent the progression of CHF, not all patients respond favorably to these drugs or only exhibit limited responses5. Consequently, novel therapeutic approaches are required to prevent cardiac hypertrophy and remodeling in CHF patients.
Astragalus membranaceus (Huangqi in Chinese) is a traditional Chinese medicine derived from the dried root of Astragalus membranaceus (Fisch.) Bunge, which is extensively used for treating cardiovascular diseases in China6,7. Astragalus Membranaceus Injection (AMI) is a patented Chinese herbal product that is derived from Astragalus membranaceus through the process of water extraction and alcohol precipitation. It has received approval from the China Food and Drug Administration (CFDA). Numerous studies have demonstrated its efficacy in the treatment of patients with CHF8. A systematic review and meta-analysis of 16 randomized controlled trials demonstrated that AMI combined with western medicine can improve left ventricular ejection fraction (LVEF) in CHF patients9. While the mechanism underlying Astragalus membranaceus’s active compound have been extensively characterized10,11,12,13, the pharmacological effects of AMI in CHF remain unclear. In the present study, we employed phosphoproteomic and network pharmacology analysis to investigate the effects and mechanisms of AMI on mitophagy and mitochondrial function in CHF.
Results
AMI attenuated cardiomyocyte hypertrophy and protected mitochondrial function
A cardiomyocyte hypertrophy model was established with 200 µM PE for 24 h. Triple-label fluorescent co-staining (TUNEL, WGA, and DAPI) was utilized to evaluate cellular hypertrophy and apoptosis (Fig. 1A). Treatment with 1% AMI significantly attenuated PE-induced cardiomyocyte enlargement (P < 0.05, Fig. 1B) and reduced TUNEL-positive apoptotic nuclei (P < 0.05, Fig. 1C), while 0.25% and 0.5% AMI elicited only modest reductions, demonstrating dose-dependent efficacy. Furthermore, AMI administration dose-dependently suppressed LDH release (P < 0.05, Fig. 1D) and enhanced cell viability (P < 0.05, Fig. 1E) in hypertrophic cardiomyocytes.
AMI attenuated cardiomyocyte hypertrophy and protected mitochondrial function. (A) Representative WGA (green), TUNEL (red), and DAPI (blue) immunofluorescence staining cardiomyocyte images. Scale bar: 50 μm. (B) Quantification of average cell size in cardiomyocytes (n = 4). (C) Quantification of TUNEL + cells per field in cardiomyocytes (n = 6). (D) Comparison of LDH levels released from cardiomyocytes (n = 18). (E) Comparison of cell viability in cardiomyocytes (n = 18). (F) Mitochondrial respiration measure of OCR was performed with Seahorse XF 96 Analyzer. (G) Comparison of basal mitochondrial respiration OCR in cardiomyocytes (n = 6). (H) Comparison of ATP production-linked OCR in cardiomyocytes (n = 6). (I) Comparison of ATP content in cardiomyocytes (n = 6). (J) Comparison of mitochondrial ROS production in cardiomyocytes (n = 6). *P < 0.05 and **P < 0.01.
Given the pivotal role of mitochondrial dysfunction in cardiac hypertrophy, Agilent Seahorse Mito Stress Tests were performed to evaluate AMI’s mitochondrial effects (Fig. 1F). PE-stimulated cardiomyocytes exhibited impaired mitochondrial function, characterized by depressed basal respiration (P < 0.01, Fig. 1G), attenuated ATP production (P < 0.01, Fig. 1H), reduced cellular ATP (P < 0.01, Fig. 1I) and excessive mitochondrial ROS generation (P < 0.05, Fig. 1J). Notably, 1% AMI treatment restored basal respiratory capacity (P < 0.05, Fig. 1G) and mitochondrial ATP production (P < 0.05, Fig. 1H). Furthermore, AMI treatment exhibited a dose-dependent augmentation of total cellular ATP content (P < 0.01, Fig. 1I) and suppression of mitochondrial ROS overproduction (P < 0.01, Fig. 1J) in hypertrophic cardiomyocytes.
AMI regulated mitophagy and mitochondrial morphology in hypertrophic cardiomyocytes
Mitophagy constitutes an essential quality-control mechanism for mitochondrial homeostasis. A triple-fluorescence mitophagy detection kit was employed for visualization of mitophagyosome (red), lysosomes (green), and mitochondria (orange) in cardiomyocytes (Fig. 2A). Quantitative morphometric analysis of mitochondrial networks was performed using the “Mitochondria Analyzer” plugin in ImageJ, based on MitoTracker-stained images (Fig. 2B). PE-induced hypertrophic cardiomyocytes exhibited impaired mitochondrial architecture, manifesting as diminished mitochondrial quantity (P < 0.01, Fig. 2C), reduced cross-sectional area (P < 0.01, Fig. 2D), and abbreviated branching length (P < 0.01, Fig. 2E) versus controls. Notably, 1% AMI treatment rescued these morphological aberrations, restoring mitochondrial density (P < 0.01, Fig. 2C), mitochondrial area (P < 0.05, Fig. 2D), and branch complexity (P < 0.01, Fig. 2E).
AMI regulated mitophagy and mitochondrial morphology in hypertrophic cardiomyocytes. (A) Representative Lyso Dye (green), Mtphagy Dye (red), and MitoTracker Dye (orange) fluorescence images in cardiomyocytes. Scale bar: 50 μm. (B) Representative mitochondrial morphology images processed by ‘Mitochondria Analyzer’ plugin in ImageJ. Scale bar: 5 μm. (C) Quantification of the mitochondrial number per cell (n = 6). (D) Quantification of the mitochondrial area per cell (n = 6). (E) Quantification of the mean branch length per mitochondrion (n = 6). (F) Quantification of LC3-II/LC3-I ratio in cardiomyocytes (n = 3). (G) Quantification of p62 expression in cardiomyocytes (n = 3). (H) Quantification of Bnip3 expression in cardiomyocytes (n = 3). (I) Representative western blots of p62, Bnip3, LC3, and β-actin in cardiomyocytes. Original blots are presented in Supplementary Fig. 2. *P < 0.05 and **P < 0.01.
To investigate mitophagic flux, we quantified LC3-II/LC3-I ratio, Bnip3, and p62 expression in hypertrophic cardiomyocytes using western blots (Fig. 2F and I). PE-stressed cardiomyocytes exhibited suppressed mitophagic activity, evidenced by depressed LC3-II/LC3-I ratio (P > 0.05, Fig. 2F) and p62 accumulation (P < 0.01, Fig. 2G) alongside Bnip3 downregulation (P < 0.05, Fig. 2H). Therapeutic intervention with 1% AMI significantly augmented LC3-II/LC3-I ratio (P < 0.05, Fig. 2F), reduced p62 protein levels (P < 0.05, Fig. 2G), and upregulated Bnip3 expression (P < 0.01, Fig. 2H), indicating enhanced mitophagic flux.
AMI treatment improved cardiac function and ameliorated cardiac remodeling in CHF mice
To investigate the role of AMI in CHF, we established a pressure overload-induced CHF mouse model via transverse aortic constriction (TAC) surgery. AMI at 2.5 mL/kg/day (LAMI) and 5 mL/kg/day (HAMI) was administered continuously for 4 weeks, alongside captopril (20 mg/kg/day) as the positive control. Echocardiographic analysis (Fig. 3A) demonstrated that the TAC mice exhibited significantly impaired systolic function compared to the Sham mice, with a reduction in EF (P < 0.01, Fig. 3B) and FS (P < 0.01, Fig. 3C). Concurrently, TAC-operated mice developed remarked ventricular wall thickening, showing increases in LVAW (P < 0.01, Fig. 3D) and LVPW (P < 0.01, Fig. 3E). While LAMI treatment induced a nonsignificant EF improvement (P > 0.05), both HAMI and ACEI mice achieved substantial functional recovery, with EF increasing (P < 0.05, Fig. 3B) and LVAW decreasing (P < 0.01, Fig. 3D). Diastolic dysfunction, evidenced by an elevation in the E/Ea ratio in TAC mice, was ameliorated by LAMI, HAMI, and ACEI interventions (P < 0.01, Fig. 3F).
AMI treatment improved cardiac function and ameliorated cardiac remodeling in CHF mice. (A) Representative left ventricular M-mode, PW Dopplar and Tissue Dopplar ultrasound images. (B) EF comparison between groups (n = 6). (C) FS comparison between groups (n = 6). (D) LVAW comparison between groups (n = 6). (E) LVPW comparison between groups (n = 6). (F) E/Ea ratio comparison between groups (n = 6). (G) HW/BW ratio comparison between groups (n = 6). (H) Representative heart images from mice in groups. *P < 0.05 and **P < 0.01.
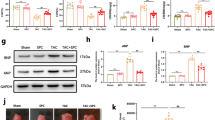
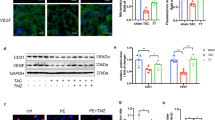
After 4 weeks of treatment, cardiac weight and size were assessed. Post-TAC hearts exhibited significant increases in both weight and size compared with Sham hearts, whereas AMI- and ACEI- treated hearts demonstrated significant reductions in weight (P < 0.05, Fig. 3G) and size (Fig. 3H). Furthermore, Masson’s trichrome staining revealed that both HAMI and ACEI interventions substantially attenuated myocardial fibrosis and reduced cardiomyocyte cross-sectional areas relative to the TAC group (Fig. 4A and B). TEM analysis revealed that TAC hearts demonstrated severe mitochondrial degeneration characterized by outer membrane disruption, matrix swelling, and cristae disorganization. Both HAMI and ACEI groups showed significant mitochondrial restoration compared to TAC models (Fig. 4C). Besides, compared with the model group, we observed more autophagosomes in the HAMI group (Fig. 4C and D). Next, we used LC3B and TOMM20 immunofluorescence to detect the distribution of autophagosomes and mitochondria (Fig. 4E). The results showed that the fluorescence intensities of LC3B (Fig. 4F) and TOMM20 (Fig. 4G) were significantly higher in the HAMI group than in the model group, and they were co-localized, indicating that the HAMI group had an increased number of mitochondria and elevated mitophagy compared to the TAC group.
AMI improves myocardial mitochondrial structure and promotes mitophagy. (A) Representative Masson’s trichrome staining images. Scale bar: 50 μm. (B) Quantitative analysis of myocardial fibrosis levels (n = 3). (C) Representative TEM images. Scale bar: 1 μm, arrows indicate autophagosomes. (D) Quantitative analysis of autophagosome numbers (n = 6). (E) Immunofluorescence staining of myocardial LC3B (green), TOMM20 (red), and DAPI (blue). Scale bar: 20 μm. (F) Quantitative analysis of LC3B fluorescence intensity (n = 3). (G) Quantitative analysis of TOMM20 fluorescence intensity (n = 3). *P < 0.05 and **P < 0.01.
AMI inhibited the phosphorylation of AKT/mTOR signaling in hypertrophic cardiomyocytes
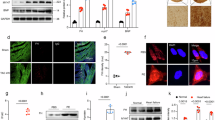
To elucidate AMI’s cardioprotective mechanisms against pathological hypertrophy, we conducted phosphoproteomic profiling of hypertrophic cardiomyocytes using a phosphorylation antibody microarray. A total of 304 phosphorylated sites across 16 signaling pathways were screened. Filtered with fold change ≥ 1.3 for upregulation or ≤ 0.7 for downregulation, AMI treatment regulated 25 phosphosites – enhancing 14 and suppressing 11 compared to untreated hypertrophic controls (Fig. 5A).
AMI inhibited the phosphorylation of AKT/mTOR signaling in hypertrophic cardiomyocytes and CHF hearts. (A) AMI-induced downregulated (fold change ≤ 0.7) and upregulated (fold change ≥ 1.3) phosphorylation sites in cardiomyocytes using a phosphorylation-profiling antibody microarray. (B) KEGG pathway analysis of the AMI-induced differential phosphorylation sites in hypertrophic cardiomyocytes. (C) PPI network analysis of the AMI-induced differential phosphorylation sites in hypertrophic cardiomyocytes. (D) Quantification of p-AKT(Ser473) in cardiomyocytes (n = 3). (E) Quantification of p-mTOR(Ser2481) in cardiomyoyctes (n = 3). (F) Representative western blots of p-mTOR(Ser2481), mTOR, p-AKT(Ser473), AKT, and β-actin in cardiomyoyctes. Original blots are presented in Supplementary Fig. 3. (G) Quantification of p-AKT(Ser473) in post-TAC hearts (n = 3). (H) Quantification of p-mTOR(Ser2481) in post-TAC hearts (n = 3). (I) Representative western blots of p-mTOR(Ser2481), mTOR, p-AKT(Ser473), AKT, and β-actin in post-TAC hearts. Original blots are presented in Supplementary Fig. 4. *P < 0.05 and **P < 0.01.
KEGG pathway enrichment analysis revealed AMI’s significant impact on four critical pathways: ROS homeostasis (P = 7.99 × 10− 14), MAPK signaling (P = 1.06 × 10− 13), PI3K-AKT signaling (P = 2.95 × 10− 11), and autophagy regulation (P = 1.62 × 10− 11) (Fig. 5B). Protein-protein interaction (PPI) network topology analysis identified AKT as the central hub node, with the PI3K/AKT/mTOR axis emerging as the primary regulatory pathway (Fig. 5C).
Western blot validation confirmed AMI’s dose-dependent attenuation of AKTSer473 (Fig. 5D) and mTORSer2481 (Fig. 5E) phosphorylation in vitro (Fig. 5F). Consistently, HAMI mice exhibited significantly suppressed cardiac phosphorylation of AKT Ser473 (Fig. 5G) and mTORSer2481 (Fig. 5H) compared with the control mice (Fig. 5I).
AKT/mTOR activation suppressed mitophagy and AMI enhanced mitophagy via AKT/mTOR inhibition
To investigate AMI’s mechanism in modulating mitophagy through AKT/mTOR signaling, pharmacological interventions were employed using SC79 (AKT/mTOR activator) and GSK-690693 (AKT/mTOR inhibitor). Optimal concentrations of 4 µM SC79 (Fig. 6A) and 5 µg/mL GSK-690693 (Fig. 6B) were established through CCK-8 viability assays, with subsequent western blot confirmation of pathway modulation efficacy (Fig. 6C). SC79 treatment induced slightly increase in cellular hypertrophy (Fig. 6D and E), reduction in mitophagy (Fig. 6F) and decrease in mitochondrial density (Fig. 6G and H), branch length (Fig. 6I) and ATP production (Fig. 6J) in PE + SC79 cells compared with PE cells. Co-treatment with AMI reversed SC79-mediated effects by normalizing cell size, restoring mitophagy, increasing mitochondrial density, branch length, and ATP production (Fig. 6D and J). Both GSK-690693 and AMI + GSK-690693 treatments demonstrated comparable attenuation of cellular hypertrophy, synergistic mitophagy activation and ATP production increase. These results demonstrate a causal relationship between AKT/mTOR activation and mitophagy suppress, while AMI restores mitochondrial homeostasis through AKT/mTOR pathway inhibition. Our results confirm AKT/mTOR signaling as the primary mechanistic pathway through which AMI exerts its pro-mitophagy effects.
AKT/mTOR activation suppressed mitophagy and AMI enhanced mitophagy via AKT/mTOR inhibition. (A) Detection of cell viability in cells treated with different concentrations of SC79 (n = 6). (B) Detection of cell viability in cells treated with different concentrations of GSK-690693 (n = 6). (C) Representative western blots of p-AKT, p-mTOR and β-actin in cardiomyocytes following treatment. Original blots are presented in Supplementary Fig. 5. (D) Quantification of average cell size in each group (n = 5). (E) Representative immunofluorescence staining of WGA (green) and DAPI (blue) in each group. Scale bar: 50 μm. (F) Representative immunofluorescence staining of Lyso Dye (green) and Mtphagy dye (red) in each group. Scale bar: 50 μm. (G) Representative mitochondrial morphology. Scale bar: 5 μm. (H) Quantification of the mitochondrial number per cell in each group (n = 6). (I) Quantification of the mean branch length per mitochondrion in each group (n = 6). (J) Quantification of ATP content in each group (n = 6). *P < 0.05 and **P < 0.01.
Discussion
CHF represents the culmination of various cardiovascular conditions, with cardiac hypertrophy, apoptosis, and autophagy intricately involved in its progression14. The imbalance between inadequate energy supply and increased ATP demand contributes to adverse remodeling and the advancement of cardiac hypertrophy15. Normal cardiac energy metabolism heavily relies on the role of mitochondria and its oxidative phosphorylation process16. Dysfunction in mitochondrial oxidative phosphorylation has been observed in both HF models and patients17. Research reports that long-term intake of barley (1–3)-β-D-glucan can improve survival rates after myocardial ischemia/reperfusion, reduce infarct size, and is associated with mitophagy18. Astragalus Membranaceus is widely used in China for its cardiac protective property19. While numerous studies have documented the cardioprotective effects of Astragalus membranaceus in heart failure—improving myocardial contractility, exerting antioxidant, anti-inflammatory and antifibrotic actions8; modulating TLR4/NF-κB and other inflammatory signaling pathways20; enhancing coronary perfusion, reducing infarct size, inhibiting calcium overload and boosting antioxidant enzyme activity via Astragaloside IV21; and correcting metabolic disturbances through PPARα signaling22—no prior work has systematically examined its dual regulation of energy metabolism and mitophagy in cardiomyocytes or elucidated the underlying mechanisms. The novelty of our study lies in (1) systematically assessing the effects of Astragalus extract on mitophagy alongside quantitative measurement of mitochondrial respiratory function; (2) establishing in vitro heart failure models combined with PE-induced mitochondrial injury to validate dual reparative effects on oxidative stress–autophagy imbalance; and (3) elucidating how Astragalus extract coordinates energy metabolism and mitochondrial homeostasis through the AKT/mTOR axis. By integrating cellular energetics and mitochondrial biology, our work provides novel insights into the systemic pharmacological mechanisms by which Astragalus extract ameliorates heart failure, offering significant theoretical advancement and potential clinical implications.
Mitophagy is a type of autophagy that regulates mitochondrial quality and quantity by removing and degrading damaged mitochondria, thereby, perceiving mitochondrial and cellular homeostasis23. During mitophagy, specific ubiquitinated outer mitochondrial membrane proteins, such as translocase of the outer mitochondrial membrane 20 (TOMM20), bind to mitophagy receptors, leading to proteasomal degradation of these mitochondrial proteins independently of microtubule-associated protein I light chain 3 beta (LC3B)24. SQSTEM1/p62 (p62), an outer mitochondrial membrane receptor, binds to LC3 to mediate mitophagy. Abnormal p62 levels have been shown to disrupt the balance of mitophagy and impair mitochondrial quality control25. Under stressful conditions, mitophagy is induced as an adaptive or defense mechanism to preserve mitochondrial function and promote cell survival. Defective mitophagy has been implicated in the pathogenesis of cardiovascular disease26. Studies have demonstrated that active ingredients in Astragali Radix can induce mitophagy in injured cells. Xia et al. reported that Astragaloside IV promoted mitophagy, reducing damaged mitochondrial accumulation, mitochondrial ROS generation, and astrocyte senescence. The inhibition of autophagy reversed the suppressive effects of Astragaloside IV on astrocyte senescence27. Yao et al. revealed that Astragalus polysaccharide induced mitophagy via the AMPT/mTOR pathway to alleviate hepatocyte senescence in vitro and in vivo28. Su et al. also reported that Astragaloside II ameliorated podocyte injury and mitochondrial dysfunction in diabetic rats, increasing expression of mitophagy-related proteins29. In this study, network pharmacological analysis enlightened the targets of AMI may involve in the process of apoptosis, autophagy, and ROS production. We found that AMI activated mitophagy, improved mitochondrial morphology, increased the expression of BNIP3 and LC3-II/LC3-I ratio, decreased the expression of p62 in PE-treated cardiomyocytes. This study showed that AMI intervention may activate mitophagy by increasing the number of mitophagosomes, promoting the conversion of mitophagosomes to mitolysosome, then remove the damaged mitochondrion, restore mitochondrial function and homeostasis.
The AKT/mTOR signaling pathway plays a crucial role in cardiac remodeling and mitophagy30,31. Excessive activation of the PI3K/AKT cascade has been observed in cardiac-selective transgenic over-expression of AKT in mice and other hypertrophy models, contributing to the development of cardiac hypertrophy. Suppression of PI3K/AKT signaling effectively counteracts cardiac hypertrophy under conditions of chronic pressure overload32. Additionally, the mTOR kinase acts as a key regulator of protein synthesis, autophagy, and cell survival33. Inhibition of mTOR signaling, either through pharmacological or genetic approaches, promotes cardiomyocyte autophagy34,35. However, clinical trials of AKT/mTOR signaling inhibitors have been hindered by their poor pharmacokinetic properties, liver toxicity, and negative side effects36. Previous studies have indeed explored aspects of Astragalus extracts’ effects on cardiac function and related molecular pathways. Zhang et. found that Astragaloside IV can activate autophagy in hypertrophic cardiomyocytes and reduce inflammatory responses37. Astragalus polysaccharides can restore mitochondrial dysfunction and affect mitophagy processes in exercise-induced chronic fatigue models38. Recent studies have shown AMI can modulate the AMPK/mTOR pathway in inflammatory context39. However, our study provides several distinct contributions that advance the understanding of AMI’s mechanisms: (1) While previous studies have examined autophagy or AKT/mTOR signaling separately, our study is the first to systematically demonstrate the direct mechanistic link between AMI-induced AKT/mTOR modulation and subsequent improvements in mitochondrial autophagy specifically in heart failure cardiomyocytes. (2) We employed high-throughput phosphorylated protein chip analysis to comprehensively map the signaling networks activated by AMI, providing unbiased identification of key pathways rather than hypothesis-driven approaches used in previous studies. (3) Our study uniquely connects mitophagy regulation with quantitative energy metabolism parameters, demonstrating how AKT/mTOR-mediated mitophagy improvements translate to functional metabolic recovery in failing cardiomyocytes. In the present study, we employed high-throughput phosphorylated protein chip analysis to comprehensively map the signaling networks activated by AMI, providing unbiased identification of key pathways rather than hypothesis-driven approaches used in previous studies. Our study uniquely connects mitophagy regulation with quantitative energy metabolism parameters, demonstrating how AKT/mTOR-mediated mitophagy improvements translate to functional metabolic recovery in failing cardiomyocytes. In validation, we found that AMI intervention activated mitophagy and attenuated cardiac hypertrophy by inhibiting AKT/mTOR signaling pathway, which could be abolished by SC79.
The present study has several limitations. First, this study was conducted in a TAC-induced mouse heart failure model and a PE-induced hypertrophic cardiomyocyte model, which only partially reflects the pathophysiological process of human heart failure, limiting the translational relevance of our findings. Second, to minimize variability from hormonal cycling, our in vivo experiments were conducted exclusively in male mice. However, sex differences critically influence heart failure pathogenesis, signaling pathway activation, and mitochondrial regulation. Previous studies have shown that female mice in the TAC model better preserve myocardial contractility and exhibit reduced cardiomyocyte apoptosis and fibrosis compared to males40, suggesting that our results may differ in female models. Finally, although we observed that PE treatment induces p62 accumulation and impaired degradation—hallmarks of disrupted mitophagic flux—we did not employ autophagic flux inhibitors to directly validate this impairment, which constitutes a limitation of the present study.
Future research should employ more translationally relevant models, include both sexes, and directly validate mitophagic flux impairment using specific autophagy inhibitors and advanced imaging techniques. Extending the work beyond TAC-induced mouse hearts and PE-treated cardiomyocytes to large-animal models or human induced pluripotent stem cell–derived cardiomyocytes will enhance clinical relevance. Incorporating female mice will clarify how hormonal and genetic differences influence the effect of AMI on AKT/mTOR signaling, mitochondrial regulation, and cardiac remodeling under stress. Finally, using pharmacological inhibitors such as bafilomycin A1, chloroquine, or genetic reporters like tandem fluorescent-tagged LC3 will allow direct measurement of autophagic and mitophagic flux, solidifying the mechanistic link between AMI treatment and mitochondrial quality control.
Methods
Drugs preparation and quality control
The AMI (batch number: 210703A2) used in this study was purchased from China Shineway Pharmaceutical Group Limited. According to the product manual, AMI is used to benefit qi and to replenish vital essence, to nourish heart and to remove obstruction of channels, to strengthen spleen and to eliminate dampness. It is clinically used for treatment of viral cardiac muscle inflammation, insufficiency of cardiac function and hepatitis caused by weak spleen. Usage and dosage instructions specify intramuscular injection at 2–4 ml each time, 1–2 times daily; intravenous infusion at 10–20 ml once daily, or according to physician guidance. These dosages have been appropriately converted for mouse use in our experiments (5 mL/kg/day).
To identify the active ingredients and conduct quality assessment, the main chemical components of AMI were analyzed using ultraperformance liquid chromatography mass spectrometry (UPLC-MS). Within a 35-minuate timeframe, a total of 32 marker ingredients were separated, including Sucrose, Nicotinic acid, Adenine, Cis-P-Coumarate, Guanosine, Adeninenucleoside, Ferulaic Acid, Astragaloside VI, Astragaloside V, Soyasaponin 1, Astragaloside VII. The positive and negative ion chromatograms of AMI were illustrated in Supplementary Fig. 1, and the identified ingredients were presented in Supplementary Tables 1 and Supplementary Table 2, respectively.
Ethics approval and consent to participate
The experimental protocols were approved by the Institutional Animal Care and Use Committee (IACUC) of Beijing Hospital of Traditional Chinese Medicine (BJTCM-M-2023-07-01) and followed the ARRIVE guidelines (https://arriveguidelines.org). In addition, we confirm that all experiments were performed in accordance with relevant guidelines and regulations.
Establishment of pressure-overload CHF mouse model
C57BL/6N mice (Vital River Laboratory Animal Technology Company Limited, China) were used for experimental procedures. The mice were housed in cages at 22 ± 2 °C, with humidity of 40 ± 5% and under a 12 h light/dark cycle with standard diet and water ad libitum. Male Mice (8–10 weeks old) with body weight from 22 to 24 g were subjected to pressure overload by transverse aortic constriction (TAC). In brief, mice were anaesthetized with ketamine (90 mg/kg) and xylazine (10 mg/kg) intraperitoneally. The chest was opened, and a horizontal skin incision was made at the level of the two to three intercostal spaces. The start of the descending aorta was identified right after the subclavian branch. A 7 − 0 silk suture was placed between the innominate and left common carotid arteries and tied around a 27-gauge blunt needle, which was subsequently removed. At the end of the procedure, the chest and skin were closed. The mice were kept on a heating pad until responsive to stimuli. Sham-operated animals underwent the identical procedure, except that the aortic constriction was not placed. The integrity of the TAC operation was confirmed by measuring the systolic pressure gradient shortly after the operation.
A total of 30 male C57BL/6 N mice were allocated equally into five groups (n = 6 per group): Sham-operated mice (Sham), TAC-operated mice (TAC), TAC-operated mice with low-dose AMI treatment (LAMI), TAC-operated mice with high-dose AMI treatment (HAMI), and TAC-operated mice with ACEI treatment (ACEI). After 3 days of surgery, mice in Sham and TAC group were administrated normal saline with an equal volume via intraperitoneal injection. AMI (2.5 mL/kg/day for LAMI, 5 mL/kg/day for HAMI) and Captopril (Sigma, MO, USA; 20 mg/kg/day for ACEI) dissolved in normal saline was administered intraperitoneally. 6 mice in each group were treated for four weeks (once a day). Administration doses of AMI and Captopril in mice were determined according to the equivalent patient dose and previously research. The heart weight (HW) and body weight (BW) of the mice were measured before harvesting the heart.
Echocardiography
Trans-thoracic 2-D, M-mode, Doppler echocardiography measurements were acquired with a high-frequency linear probe (30 MHz) on the Vevo 3100 ultrasound system (Visual Sonics, Canada). Mice were anesthetized with 3% isoflurane for induction and maintained on 1.0–1.5% isoflurane in 100% oxygen, delivered via a nose cone. Body temperature was maintained at 36.5–37.0 °C using a feedback-controlled heating platform. All M-mode tracings were recorded through the anterior and posterior left ventricle (LV) walls at the level of the papillary muscle. LV end-diastolic dimension (LVEDD), LV end-systolic dimension (LVESD), LV anterior wall thickness (LVAW), and LV posterior wall thickness (LVPW) were measured and used to calculate ejection fraction (EF) and fractional shortening (FS). Early diastolic mitral valve flow velocity peak (E) and early diastolic mitral annular velocity peak (Ea) were measured using Pulsed-Wave (PW) Doppler and Tissue Doppler from a four-chamber view and the ratio of E/Ea was calculated. Measurements were performed in accordance with the guidelines presented by the American Society of Echocardiography41. At least three measurements were carried out and the values were averaged for each parameter. The echocardiography parameters were shown in Supplementary Table 3.
Histopathology
The mice were euthanized by cervical dislocation before the heart removed from the chest. Heart specimens were fixed in 10% neutral buffered formalin and then embedded into paraffin and sectioned at a thickness of 5 μm. Masson’s trichrome staining kit (Sigma-Aldrich, USA) was used to examine the changes in cardiac pathology. Each section was assessed and the images were taken by using an inverted microscope (Zeiss, Germany). Fibrotic area was measured using ImageJ by calculating the collagen-positive region as a percentage of total myocardial area in three randomly selected fields per section.
Culture and treatment of neonatal mouse cardiomyocytes
Neonatal mice (1–3 days old) were sacrificed by decapitation. Cardiomyocytes from the neonatal hearts were isolated as previously described42. The harvested hearts were finely chopped and kept on ice in Dulbecco’s phosphate-buffered saline solution (PBS, Solarbio, China) before enzyme digestion with 1% type II collagenase (Worthington, OH, USA). Cells released from the initial digestion were discarded, with cells from subsequent digestion transferred into DMEM/F12 medium (Gibco, CA, USA) supplemented with 10% fetal bovine serum (FBS, Gibco) until cardiomyocyte isolation was achieved (around 4 repetitions). The resulting cell mixture was centrifuged at 500 g for 5 min, resuspended in DMEM/F12 medium, and preplated for 3 h to remove non-cardiomyocytes as non-muscle cells tended to adhere more quickly to the substrate. The purified cardiomyocytes were then aliquoted onto dishes coated with laminin and incubated at 37 °C with 5% CO2 for 48 h before conducting further experiments.
To establish a hypertrophic cardiomyocyte model, the isolated cells were exposed to 200 µM phenylephrine (PE, Selleck Chemicals, China) for 24 h42. AMI was administered at concentrations of 0.25% (LAMI), 0.5% (MAMI), and 1% (HAMI), with the treatments being diluted in serum-free DMEM/F12. The cardiomyocytes in the control group were incubated solely in serum-free DMEM/F12.
Cell viability and injury assays
Cell viability and injury were assessed using the Cell Proliferation/Cytotoxicity Assay Kit (Dojindo Molecular Technologies, Japan). Cardiomyocytes were cultured in 96-well plates at a density of 3000 cells per well in 200 µL of culture medium and incubated overnight. After incubation with PE, AMI, SC79, or GSK-690693 for 24 h, 20 µL of the CCK-8 agent was added to each well 2 h prior to the experiment’s termination. The absorbance at 450 nm was measured using a microplate reader (Bioteck, VT, USA). The relative cell viability was determined by calculating the ratio of optical density (OD) values to that of the control cells. The optimal SC79 and GSK-690693 concentrations were selected for subsequent experiments. For the LDH assay, 100 µL of the supernatant was transferred into a new 96-well plate, and 100 µL of the working solution was added to each well. The plate was incubated at room temperature for 30 min and the absorbance at 490 nm was measured using a plate reader.
Apoptosis assay
Cardiomyocytes were cultured on gelatin-coated coverslips and subjected to staining using TUNEL (Beyotime Biotechnology, China), wheat germ agglutinin (WGA, Invitrogen, MA, USA), and DAPI (Sigma-Aldrich, Germany), following the manufacturer’s instructions. The size and apoptosis of the cells were recorded and analyzed using a confocal microscopy (Zeiss, Germany) and ImageJ software (NIH, MD, USA).
Measurement of mitochondrial respiration
Bioenergetic determinations were conducted using a Seahorse XF96 Analyzer (Agilent Technologies, CA, USA) in accordance with the manufacturer’s instructions. Cardiomyocytes were cultured in 96-well plates, rinsed, and the culture medium was replaced with Seahorse medium. Subsequently, the cells were incubated in a non-CO2 incubator at 37 °C for at least 30 min. The assay cartridge was hydrated with XF calibrant solution one day prior to experiment and stored at 37 °C overnight. The oxygen consumption rate (OCR) was measured both at rest and after the sequential addition of oligomycin (1.5 µM), FCCP (1 µM), and finally rotenone (0.5 µM) combined with antimycin A (0.5 µM). OCR values were normalized to the number of cells in each well.
ATP production and mitochondrial reactive oxygen species (ROS) assays
ATP content was measured using a luminescent ATP detection assay kit (ab113849, Abcam) according to the manufacturer’s protocol. Mitochondrial ROS were detected using mitochondrial superoxide assay kit (ab219943, Abcam) according to the manufacturer’s protocols.
Assessment of mitochondrial morphology and mitophagy
Cardiomyocytes were stained with MitoTracker Dye (Invitrogen, MA, USA) in accordance with the manufacturer’s instructions for mitochondrial labeling. Initially, the cells were seeded in eight-well chambers slides. After treatment, the cells were washed twice, and the dye was applied at the recommended dilution provided by the manufacturer. Subsequently, a mitophagy detection kit (Dojindo Molecular Technologies) was used to evaluate mitophagy in cardiomyocytes. Following the manufacturer’s protocol, the Lyso Dye and the Mtphagy Dye were added to the medium and incubated for 30 min prior to observation using confocal fluorescence microscopy (Zeiss). Measures of mitochondrial number, area, and mean branch length were determined using the ‘Mitochondria Analyzer’ plugin in ImageJ/Fiji Software43,44.
Myocardial tissue (~ 1 mm³) was post-fixed in 1% osmium tetroxide for 1 h, then dehydrated through a graded ethanol series (30%–100%) and cleared in propylene oxide. Tissue blocks were embedded in epoxy resin and polymerized at 60 °C for 24 h; ultra‐thin sections (~ 70 nm) were cut with an ultramicrotome and mounted on copper grids. Sections were stained with 2% aqueous uranyl acetate followed by lead citrate, then examined under a transmission electron microscope (TEM), capturing representative fields for mitochondrial and autophagosome assessment. Autophagosomes were counted manually in three randomly chosen fields per sample in TEM images.
LC3B and TOMM20 immunofluorescence staining was performed on heart samples fixed with 4% paraformaldehyde. After permeabilization with 0.1% Triton X-100 and blocking with 5% BSA, samples were incubated overnight at 4 °C with primary antibodies against LC3B and TOMM20. Following washes, samples were incubated with appropriate fluorescent secondary antibodies for 1 h at room temperature in the dark. Nuclei were counterstained with DAPI, and the samples were mounted with antifade reagent before imaging using a fluorescence microscope.
Phosphorylation-profiling antibody microarray
To validate the phosphorylation targets of AMI, a phosphorylation-profiling antibody microarray (CSP100 PLUS, Full Moon Microsystems, MI, USA) was performed according to the provided protocols. A total of 304 phosphorylated sites across 16 signaling pathways were screened. The fluorescence signal for each antibody was determined by measuring the intensity of the corresponding antibody spot. The extent of protein phosphorylation was quantified using a ratio computation method, which compared the phosphor value to the unphosphor value. Differentially phosphorylated sites between the cells in the PE and PE + AMI groups were identified by applying a fold change threshold of ≥ 1.3 or ≤ 0.7. KEGG pathway enrichment analysis was used to identify the functional pathways associated with phosphorylation sites affected by AMI45,46,47.
Western blotting
The hearts and cardiomyocytes were lysed in radio immunoprecipitation assay (RIPA) lysis buffer (Beyotime, China) containing protease inhibitors (Beyotime) for 30 min under rotating agitation to extract total protein. Protein concentration was measured using a BCA protein assay kit (ThermoFisher, MA, USA). Each sample was loaded with 20 µg of total protein and separated by 10% sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) at 120 V for 1.5 h. The proteins were then transferred onto polyvinylidene difluoride (PVDF) membranes. After blocking with 5% nonfat milk, the PVDF membranes were incubated with primary antibodies against p-AKT (Ser473) (1:1000, #9271, Cell Signal Technology, MA, USA), AKT (1:2000, #9272, Cell Signal Technology), p-mTOR (Ser2481) (1:1000, #2974, Cell Signal Technology), mTOR (1:1000, #2972, Cell Signal Technology), LC3 (1:1000, #12741, Cell Signal Technology), p62 (1:1000, #23214, Cell Signal Technology), and β-actin (1:1000, TA-09, ZSGB-BIO, China). Blots were washed with TBS (containing 0.1% Tween-20) before incubation with horseradish peroxidase (HRP)-conjugated goat anti-mouse (1:10000, #115-035-003, Jackson) or goat anti-rabbit (1:10000, #111-035-003, Jackson) secondary antibodies. After thorough washing, the blots were visualized using an ECL kit (Millipore, MA, USA), and densitometric analysis was performed with image acquisition and analysis software (Tanon, China).
Statistical analysis
All data are presented as mean ± standard error of the mean (SEM). The Shapiro-Wilk test was used to evaluate the normality assumption for the datasets before the statistical analysis. If data met the normality criterion, one-way analysis of variance (ANOVA) followed by Bonferroni or Fisher’s LSD post-hoc tests was applied to compare differences among multiple groups. When data did not satisfy the normality assumption, we employed the Kruskal-Wallis nonparametric test accordingly. Data analysis and visualization were conducted using GraphPad Prism 8.0 (GraphPad Software Inc., CA, USA). A significance level of P < 0.05 was considered statistically significant.
Data availability
The datasets used and analyzed during the current study are available from the corresponding author on reasonable request.
References
Heidenreich, P. A. et al. AHA/ACC/HFSA guideline for the management of heart failure: a report of the American college of cardiology/American heart association joint committee on clinical practice guidelines. J. Am. Coll. Cardiol. 79, e263 (2022).
Platt, M. J., Huber, J. S., Romanova, N., Brunt, K. R. & Simpson, J. A. Pathophysiological mapping of experimental heart failure: left and right ventricular remodeling in transverse aortic constriction is Temporally, kinetically and structurally distinct. Front. Physiol. 9, 472 (2018).
Pitoulis, F. G. & Terracciano, C. M. Heart plasticity in response to Pressure- and Volume-Overload: A review of findings in compensated and decompensated phenotypes. Front. Physiol. 11, 92 (2020).
Ravassa, S. et al. Prediction of left ventricular reverse remodeling and outcomes by Circulating Collagen-Derived peptides. JACC Heart Fail. 11, 58–72 (2023).
Euler, G. et al. Matrix metalloproteinases repress hypertrophic growth in cardiac myocytes. Cardiovasc. Drugs Ther. 35, 353–365 (2021).
Kanaan, H. et al. Screening for antibacterial and antibiofilm activities in astragalus angulosus. J. Intercult Ethnopharmacol. 6, 50–57 (2017).
Liu, K. et al. Astragalus injection ameliorates lipopolysaccharide-induced cognitive decline via relieving acute neuroinflammation and BBB damage and upregulating the BDNF-CREB pathway in mice. Pharm. Biol. 60, 825–839 (2022).
Han, X. et al. Effect of astragalus Membranaceus on left ventricular remodeling in hfref: a systematic review and meta-analysis. Front. Pharmacol. 15, 1345797 (2024).
Wang, K. et al. Huangqi injection in the treatment of chronic heart failure: A systematic review and meta-analysis. Med. (Baltim). 96, e8167 (2017).
Han, R. et al. Astragalus polysaccharide ameliorates H2O2-induced human umbilical vein endothelial cell injury. Mol. Med. Rep. 15, 4027–4034 (2017).
Liu, Z. H., Liu, H. B. & Wang, J. Astragaloside IV protects against the pathological cardiac hypertrophy in mice. Biomed. Pharmacother. 97, 1468–1478 (2018).
Zang, Y. et al. An updated role of Astragaloside IV in heart failure. Biomed. Pharmacother. 126, 110012 (2020).
Xu, J. et al. Integration of network Pharmacology and molecular Docking technology reveals the mechanism of the herbal pairing of Codonopsis pilosula (Franch.) Nannf and astragalus Membranaceus (Fisch.) Bge on chronic heart failure. Ann. Palliat. Med. 10, 7942–7959 (2021).
Xu, Y. et al. Sonodynamic therapy reduces cardiomyocyte apoptosis through autophagy activated by reactive oxygen species in myocardial infarction. Free Radic Biol. Med. 195, 36–46 (2023).
Witjas-Paalberends, E. R. et al. Gene-specific increase in the energetic cost of contraction in hypertrophic cardiomyopathy caused by Thick filament mutations. Cardiovasc. Res. 103, 248–257 (2014).
Kolwicz, S. C. Jr, Purohit, S. & Tian, R. Cardiac metabolism and its interactions with contraction, growth, and survival of cardiomyocytes. Circ. Res. 113, 603–616 (2013).
Ranjbarvaziri, S. et al. Altered cardiac energetics and mitochondrial dysfunction in hypertrophic cardiomyopathy. Circulation 144, 1714–1731 (2021).
Casieri, V. et al. Long-term intake of pasta containing barley (1–3)Beta-D-Glucan increases Neovascularization-mediated cardioprotection through endothelial upregulation of vascular endothelial growth factor and parkin. Sci. Rep. 7, 13424 (2017).
Li, C. et al. Study of the mechanism of astragali radix in treating type 2 diabetes mellitus and its renal protection based on enzyme Activity, network Pharmacology, and experimental verification. Molecules 28, 8030 (2023).
Zhang, F., Wu, Y., Cheng, C., Cheng, Y. & Gao, R. Mechanistic Insights into Astragalus Membranaceus for Oral Submucosal Fibrosis: A Network Pharmacology and Experimental Approach. Curr. Pharm. Des. https://doi.org/10.2174/0113816128374420250707120128 (2025).
Zhao, J. et al. Therapeutic effects of Astragaloside IV on myocardial injuries: multi-target identification and network analysis. PLoS One. 7, e44938 (2012).
Chen, W. et al. The critical role of astragalus polysaccharides for the improvement of PPARα [ correction of PPRAα]-mediated lipotoxicity in diabetic cardiomyopathy. PLoS One. 7, e45541 (2012).
Ajoolabady, A., Chiong, M., Lavandero, S., Klionsky, D. J. & Ren, J. Mitophagy in cardiovascular diseases: molecular mechanisms, pathogenesis, and treatment. Trends Mol. Med. 28, 836–849 (2022).
Stolz, A., Ernst, A. & Dikic, I. Cargo recognition and trafficking in selective autophagy. Nat. Cell. Biol. 16, 495–501 (2014).
Liu, H. et al. From autophagy to mitophagy: the roles of P62 in neurodegenerative diseases. J. Bioenerg Biomembr. 49, 413–422 (2017).
Killackey, S. A., Philpott, D. J. & Girardin, S. E. Mitophagy pathways in health and disease. J. Cell. Biol. 219, e202004029 (2020).
Xia, M. L., Xie, X. H., Ding, J. H., Du, R. H. & Hu, G. Astragaloside IV inhibits astrocyte senescence: implication in parkinson’s disease. J. Neuroinflammation. 17, 105 (2020).
Yao, T. et al. Astragalus polysaccharide alleviated hepatocyte senescence via autophagy pathway. Kaohsiung J. Med. Sci. 38, 457–468 (2022).
Su, J. et al. Astragaloside II ameliorated podocyte injury and mitochondrial dysfunction in Streptozotocin-Induced diabetic rats. Front. Pharmacol. 12, 638422 (2021).
Wang, X. et al. ELA-11 protects the heart against oxidative stress injury induced apoptosis through ERK/MAPK and PI3K/AKT signaling pathways. Front. Pharmacol. 13, 873614 (2022).
Wei, X. et al. Hypoglycemia-Exacerbated mitochondrial connexin 43 accumulation aggravates cardiac dysfunction in diabetic cardiomyopathy. Front. Cardiovasc. Med. 9, 800185 (2022).
Yu, X. et al. A network pharmacology-based study on the mechanism of Astragaloside IV alleviating renal fibrosis through the AKT1/GSK-3β pathway. J. Ethnopharmacol. 297, 115535 (2022).
Feng, Y. et al. Interplay of energy metabolism and autophagy. Autophagy 20, 4–14 (2024).
Wang, H. et al. Neuregulin-4 attenuates diabetic cardiomyopathy by regulating autophagy via the AMPK/mTOR signalling pathway. Cardiovasc. Diabetol. 21, 205 (2022).
Zhang, S. et al. Doxorubicin downregulates autophagy to promote apoptosis-induced dilated cardiomyopathy via regulating the AMPK/mTOR pathway. Biomed. Pharmacother. 162, 114691 (2023).
Schreiber, K. H. et al. A novel Rapamycin analog is highly selective for mTORC1 in vivo. Nat. Commun. 10, 3194 (2019).
Yang, J., Tian, S., Zhao, J. & Zhang, W. Exploring the mechanism of TCM formulae in the treatment of different types of coronary heart disease by network Pharmacology and machining learning. Pharmacol. Res. 159, 105034 (2020).
Huang, Y. F. et al. Effects of Astragalus Polysaccharides on Dysfunction of Mitochondrial Dynamics Induced by Oxidative Stress. Oxid. Med. Cell Longev. 2016, 9573291 (2016).
Zhang, X. et al. Astragalus membranaceus Injection Suppresses Production of Interleukin-6 by Activating Autophagy through the AMPK-mTOR Pathway in Lipopolysaccharide-Stimulated Macrophages. Oxid. Med. Cell Longev. 2020, 1364147 (2020).
Justice, M. J. Sex matters in preclinical research. Dis. Model. Mech. 17(3), dmm050759 (2024).
Gao, S., Ho, D., Vatner, D. E. & Vatner, S. F. Echocardiography in mice. Curr. Protoc. Mouse Biol. 1, 71–83 (2011).
Lin, H. B. et al. Innate immune Nod1/RIP2 signaling is essential for cardiac hypertrophy but requires mitochondrial antiviral signaling protein for signal transductions and energy balance. Circulation 142, 2240–2258 (2020).
Chaudhry, A., Shi, R. & Luciani, D. S. A pipeline for multidimensional confocal analysis of mitochondrial morphology, function, and dynamics in pancreatic β-cells. Am. J. Physiol. Endocrinol. Metab. 318, E87–87E101 (2020).
Hemel, I., Engelen, B., Luber, N. & Gerards, M. A hitchhiker’s guide to mitochondrial quantification. Mitochondrion 59, 216–224 (2021).
Kanehisa, M., Furumichi, M., Sato, Y., Matsuura, Y. & Ishiguro-Watanabe, M. KEGG: biological systems database as a model of the real world. Nucleic Acids Res. 53, D672–D677 (2025).
Kanehisa, M. & Goto, S. KEGG: Kyoto encyclopedia of genes and genomes. Nucleic Acids Res. 28, 27–30 (2000).
Kanehisa, M., Sato, Y., Kawashima, M., Furumichi, M. & Tanabe, M. KEGG as a reference resource for gene and protein annotation. Nucleic Acids Res. 44, D457–462 (2016).
Acknowledgements
This study was supported by grants from the National Natural Science Foundation of China (82174106, 82205098).
Author information
Authors and Affiliations
Contributions
Sinai Li made contributions to the acquisition, analysis or interpretation of data for this work and drafted the work. Juju Shang and Wenlong Xing made substantial contributions to the conception or design of the work. Mingxue Zhou, Shenglei Qiu, Weihong Liu, Lei Zhang and contributions to immunofluorescence staining. Haoyue Shi, Huanning Niu, Songtao Kuang, Tong Wu, Mengting Liu, Yanjie Lian, and Heyi Zhang made contributions to the data analysis in western-blotting. Hongxu Liu revised the work for important intellectual content.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Li, S., Shang, J., Xing, W. et al. Astragalus membranaceus injection activates mitophagy and protects mitochondrial function in chronic heart failure via inhibiting AKT/mTOR pathway. Sci Rep 15, 42770 (2025). https://doi.org/10.1038/s41598-025-27065-y
Received:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s41598-025-27065-y