Abstract
Stroke and diabetes mellitus (DM) frequently co-occur and interact through shared vascular and metabolic pathways. The comorbidity-related mortality patterns of the two diseases and the disparities across different subgroups remain insufficiently investigated on a global scale. This study aims to explore these trends using a nationally representative mortality database. We examined mortality statistics from the CDC WONDER database (1999–2023), incorporating deaths where stroke or DM was listed as an underlying or contributing condition. Age-adjusted mortality rates (AAMR) per 100,000 were computed using the 2000 U.S. Census standard. Joinpoint regression analysis assessed annual percentage change (APC) and average APC (AAPC) with 95% confidence intervals, stratified by age, sex, race/ethnicity, urbanization level, and region. Between 1999 and 2023, stroke AAMR decreased from 78.24 to 38.20 per 100,000; DM AAMR declined modestly from 38.56 to 34.48. For those with both conditions, AAMR fell from 16.47 to 9.70. This downward trend occurred across most subgroups but was slower among individuals aged ≥ 85 years, non-Hispanic Black populations, and non-metropolitan area residents. The COVID-19 pandemic caused a temporary reversal. The mortality rate associated with comorbid stroke and DM has shown an overall declining trend; however, it remains elevated among older adults and populations with limited access to healthcare services. Although based on a national database, the analytical approach employed in this study provides a generalizable framework for monitoring and addressing chronic disease mortality across diverse healthcare systems globally
Similar content being viewed by others
Introduction
Stroke and diabetes mellitus (DM) present significant public health issues worldwide, placing substantial demands on healthcare systems and impacting socio-economic conditions. Globally, stroke ranks as the second leading cause of mortality1, contributing to approximately 6.5 million fatalities each year. Research indicates that individuals with DM have a markedly increased risk of stroke2, with patients with diabetes facing nearly double the likelihood of having a stroke compared to without diabetes3. The continuous global rise in DM prevalence poses a growing threat to cerebrovascular health.
There exists a complex and bidirectional pathophysiological interaction between DM and stroke. Emerging evidence suggests that cardiovascular disease (CVD) plays a pivotal mediating role, linking the two conditions through multiple interconnected mechanisms. Chronic hyperglycemia contributes to endothelial dysfunction, oxidative stress, and a pro-inflammatory milieu, all of which accelerate atherosclerosis. This process promotes the formation and instability of atherosclerotic plaques, ultimately leading to arterial thrombosis4 and increasing the risk of ischemic stroke5. Moreover, microvascular dysfunction is common among individuals with diabetes6, further elevating their susceptibility to both ischemic and hemorrhagic cerebrovascular events. Furthermore, atrial fibrillation (AF) is a well-established and common cause of cardioembolic stroke7. Studies have shown that individuals with diabetes have a 35% higher overall risk of developing AF, although the underlying mechanisms remain unclear8. Conversely, acute stroke can induce autonomic dysfunction and activate the hypothalamic-pituitary-adrenal (HPA) axis, leading to stress-induced hyperglycemia. The severity of stroke is often positively associated with the degree of hyperglycemia observed during the acute phase9. Hyperglycemia has been shown to impair post-stroke recovery and worsen prognosis10. Therefore, the coexistence of stroke and diabetes represents a 'high-risk combination’, and the complex bidirectional interactions between the two conditions underscore the urgent need for tighter monitoring and integrated management in this vulnerable population.
In recent years, novel antidiabetic agents such as sodium-glucose cotransporter 2 inhibitors (SGLT2i) have demonstrated significant cardiovascular and renal protective effects, including reduced risk of hospitalization for heart failure and delayed progression of chronic kidney disease11,12. Emerging evidence also suggests that SGLT2i may exert potential neuroprotective effects, possibly through mechanisms such as attenuation of neuroinflammation, reduction of reactive oxygen species, and enhancement of antioxidant capacity13,14,15. Preliminary experimental and clinical studies indicate that SGLT2i may offer protective and therapeutic benefits in the context of ischemic stroke16. The rational use of these agents may hold substantial promise in alleviating the dual disease burden associated with the comorbidity of stroke and diabetes, highlighting the need for further research and clinical integration in this high-risk population.
Despite substantial progress in stroke prevention and diabetes management in recent years, studies have shown that overall stroke-related mortality has declined5. However, relatively limited attention has been given to long-term mortality among patients with coexisting stroke and diabetes, especially across different subgroups such as age, sex, race/ethnicity, urbanization level, and geographic region. Of particular concern, the COVID-19 pandemic significantly disrupted chronic disease management17, and the systemic effects of SARS-CoV-2 infection itself18 may have further exacerbated the vulnerability of individuals with coexisting stroke and diabetes, especially within socially or medically disadvantaged populations.
This study aimed to conduct a comprehensive analysis of mortality attributable to comorbid stroke and DM. We examined age-adjusted mortality rates from 1999 to 2023 and performed stratified analyses based on demographic and geographic variables. Our goal was to identify long-term disparities across subgroups and to provide broader public health insights that may inform chronic disease prevention and health equity strategies in diverse settings.
Methods
Study setting and population
This study is descriptive in nature and analyzes U.S. national mortality data obtained from death certificates present in CDC WONDER which is maintained by Centers for Disease Control and Prevention. This study focused on examining mortality trends from comorbid stroke and DM from 1999 to 2023. Causes of death were coded using ICD-10, with stroke defined as I60, I61, I62, I63, and I6419 and DM as E10-E1420, respectively. In this analysis, we examine deaths listed in the CDC WONDER Multiple Cause of Death Public Use data set. This dataset comprises certified death records from all registered areas and provides standardized, large-scale data suitable for population-level mortality analysis.
The CDC WONDER data provide extensive mortality data. Given the significant healthcare disruptions during the COVID-19 pandemic, trends from 2020 onward were further examined to evaluate potential impacts on mortality patterns. To qualify for inclusion, death certificates had to list either Stroke or DM as the underlying or contributing cause of death. Since this study utilized only publicly available, de-identified aggregate data from the official federal database (CDC WONDER)21, it was exempt from Institutional Review Board (IRB) approval. This research follows the guidelines set by the Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) for studies of an observational nature22.
Data abstraction
Mortality data were systematically gathered from the ‘Multiple Cause of Death Database’ maintained by the Centers for Disease Control and Prevention (CDC). This data set was organized according to important demographic and geographic factors, such as gender, race/ethnicity, level of urbanization, census region, and state of residence. Race/ethnicity was classified into categories including Hispanic, non-Hispanic White, Black, Asian/Pacific Islander, and American Indian/Alaska Native. The differentiation between urban and rural areas followed the framework developed by the National Center for Health Statistics (NCHS), which separates regions into metropolitan and non-metropolitan categories23. The census regions were classified into four broad areas: Northeast, Midwest, South, and West. The definitions used for all variables were in strict compliance with the documentation standards set by CDC WONDER, and all data were sourced exclusively from death certificate records within the public use files of the database.
Statistical analysis
Age-adjusted mortality rate (AAMR) per 100,000 population, standardized to the 2000 U.S. census, were computed to quantify the mortality burden of stroke and DM from 1999 to 202324. Temporal trends were evaluated using the Joinpoint Regression Program (Version 5.2.0.2; National Cancer Institute)25, which applied segmented log-linear modeling to derive annual percent change (APC) along with 95% confidence intervals. The Joinpoint regression model assumes a log-linear trend, independence of residuals, and homoscedasticity across time segments. To capture temporal changes while avoiding overfitting, up to three joinpoints were allowed, consistent with the guidelines of the U.S. National Cancer Institute for a 25-year study period. Multiple comparisons were adjusted using the built-in permutation testing framework, thereby controlling the overall type I error rate at the α = 0.05 level. Statistically significant trend transitions were identified through permutation testing, where APCs were classified as increasing or decreasing when the slope coefficient differed significantly from zero (two-tailed t-test, α = 0.05). As a sensitivity analysis, we repeated the joinpoint regression restricting the study period to pre-COVID years (1999–2019) to evaluate the robustness of the estimated long-term trends. Stratified analyses examined demographic and geographic subgroups, with trend parallelism assessed by comparing average annual percent changes (AAPCs) across categories26. Additionally, crude mortality rates with 95% confidence intervals were calculated to describe subgroup-specific differences.
Results
Between 1999 and 2023, the number of deaths among adults aged ≥ 25 years with both stroke and DM decreased from 29,045 in 1999 to 26,940 in 2023 (Table 1). The AAMR for stroke and DM-related deaths was 16.47 in 1999, and declined to 9.70 in 2023 per 100,000 individuals.The overall AAMR showed a consistent downward trend between 1999 and 2023, with an AAPC of -2.32 (95% CI: -3.22 to -1.42; p < 0.000001). Detailed AAPC and APC estimates are provided in Tables S1–S2.
Overall trends in stroke and DM-related mortality
From 1999 to 2023, deaths from stroke and DM among U.S. adults changed from 137,516 and 68,199 to 103,570 and 94,875, respectively (Table 1). The AAMR for stroke decreased significantly from 78.24 per 100,000 (95% CI: 77.82–78.65) in 1999 to 38.20 (95% CI: 37.96–38.43) in 2023, with an AAPC of -2.88% (95% CI: -3.52 to -2.23; p < 0.05). In contrast, DM AAMR slightly declined from 38.56 (95% CI: 38.27–38.85) to 34.48 (95% CI: 34.25–34.70), with an AAPC of -0.48% (95% CI: -0.97 to 0.02; p = 0.06), which did not reach statistical significance (Figure S1–S2) (Table 1). The sensitivity analysis restricted to the pre-pandemic period (1999–2019) yielded results consistent with the main findings, confirming that the observed long-term decline in comorbidity-related mortality was not solely driven by pandemic-era fluctuations (Table S3) (Figure S3).
Stroke and DM-related mortality trends stratified by age groups
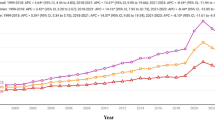
From 1999 to 2023, the AAMR increased with age. In the group aged 85 and above, it decreased from 168.13 to 103.00 (AAPC: -2.11%; p = 0.00004) (Fig. 1) (Table 1). The 75–84 age group saw a decrease from 93.08 to 48.25 (AAPC: -2.82%; p < 0.000001), while the 65–74 age group declined from 37.02 to 19.68 (AAPC: -2.62%; p < 0.000001). The AAMR in the elderly group continued to decline, with the most significant drop in the 85 + age group from 2002 to 2009 (APC: -4.75%; p < 0.000001). The rate of decline slowed from 2009 to 2018 (APC: -2.84%; p = 0.000003), followed by a slight increase from 2018 to 2021 (APC: +9.07%; p = 0.01), and then a decrease again from 2021 to 2023 (APC: -6.20%; p = 0.05). The 55–64 age group decreased from 10.85 to 8.15 (AAPC: -1.40%; p = 0.017). In contrast, the younger age groups exhibited overall non-significant changes. For those aged 45–54 years, the long-term AAPC was not significant (-0.02%; p = 0.98). Within this group, however, a significant decline occurred during 1999–2010 (APC: -2.47%; p < 0.001), followed by a plateau from 2010 to 2018 (APC: +0.15%; p = 0.85), a significant increase from 2018 to 2021 (APC: +17.7%; p = 0.01), and a significant decline from 2021 to 2023 (APC: -11.0%; p = 0.047). For the 35–44 age group, the overall trend was a non-significant increase. Joinpoint analysis indicated three phases: a significant decline from 1999 to 2018 (APC: -1.02%; p = 0.003), a non-significant rise from 2018 to 2021 (APC: +18.0%; p = 0.11), and a non-significant decline from 2021 to 2023 (APC: -8.1%; p = 0.35). The 25–34 age group remained stable throughout the study period, with a non-significant overall decline (AAPC: -0.27%; p = 0.52).
Stroke and DM-related mortality trends stratified by gender
From 1999 to 2023, the male AAMR remained consistently higher than that of females (Fig. 2) (Table 1). The male AAMR decreased from 17.66 (95% CI: 17.34–17.97) to 11.50 (95% CI: 11.31–11.70), indicating a significant overall decline (AAPC: -1.90%; 95% CI: -2.78 to -1.02; p = 0.00003). The segmented trend shows a decline from 1999 to 2010 (APC: -4.36%; p < 0.0001), a slowdown from 2010 to 2018 (APC: -1.60%; p = 0.002), a brief increase from 2018 to 2021 (APC: +11.18%; p = 0.002), and a decrease again from 2021 to 2023 (APC: -7.69%; p = 0.011). The female AAMR decreased from 15.61(95% CI: 15.37–15.84) to 8.16(95% CI༚8.02–8.30), with a slightly faster decline (AAPC༚-2.67%; 95% CI༚-3.45 to -1.90; p < 0.000001). The segmented trend for females indicates a rapid decline 2002 to 2009(APC༚-5.59%; p = 0.03), a continued decline from 2009 to 2018(APC༚-3.13%; p < 0.0001), an increase from 2018 to 2021(APC༚+10.51%; p = 0.001), and a subsequent decrease from 2021 to 2023(APC༚-8.76%; p = 0.002). Overall, the AAMR decreased from 16.47(95% CI༚16.28–16.66) to 9.7(95% CI༚9.58–9.81) ,(AAPC: -2.32%; 95% CI: -3.22 to -1.42; p < 0.000001).
Stroke and DM-related mortality trends stratified by race
The AAMR for non-Hispanic Black individuals (NH Black) was highest in 1999 at 33.42 (95% CI: 32.47–34.36), decreasing to 18.04 in 2023 (95% CI: 17.52–18.56), with an average annual percentage change (AAPC) of -2.80% (p = 0.0003) (Fig. 3) (Table 1). From 1999 to 2018, there was a decline (annual percentage change, APC: -4.45%; p < 0.0001), followed by an increase from 2018 to 2021 (APC: +14.39%; p = 0.021), and a subsequent decrease from 2021 to 2023 (APC: -10.37%; p = 0.045). For Hispanic individuals, the AAMR decreased from 20.94 to 11.23 (AAPC: -2.84%; p = 0.00003). The rate declined from 1999 to 2017 (APC: -4.39%; p < 0.0001), increased from 2017 to 2021 (APC: +7.90%; p = 0.017), and showed a slight decrease from 2021 to 2023 (APC: -8.90%; p = 0.107). The AAMR for non-Hispanic other populations (NH Other) decreased from 19.81 to 9.03 (AAPC: -3.28%; p = 0.000006). Between 1999 and 2016, it showed a decline (APC: -4.82%; p < 0.0001), followed by an increase from 2016 to 2021 (APC: +5.61%; p = 0.025), and a subsequent decrease from 2021 to 2023 (APC: -11.04%; p = 0.083). The AAMR for non-Hispanic whites (NH White) was the lowest, recorded at 14.33 in 1999 and 8.35 in 2023 (AAPC: -2.20%; p < 0.000001). There was a significant decline from 2002 to 2009 (APC: -5.38%; p < 0.0001), an increase from 2018 to 2021 (APC: +10.22%; p = 0.0003), followed by another decrease from 2021 to 2023 (APC: -6.91%; p = 0.002).
Stroke and DM-related mortality trends stratified by geographic regions
Stroke and DM-related mortality trends stratified by urbanization
From 1999 to 2023, the AAMR in non-metropolitan areas has consistently been higher than that in metropolitan areas (Fig. 4) (Table 1). The AAMR in non-metropolitan areas decreased from 18.59 (95% CI: 18.12–19.05) to 13.82 (95% CI: 13.47–14.18), indicating a significant overall decline (AAPC: -1.47%; p = 0.000036), with the 2023 AAMR estimated based on 2020 data. The segmented trend shows that the most significant decline occurred between 2002 and 2011 (APC: -4.35%; p < 0.0001), while there was a brief increase in AAMR from 2018 to 2020 (APC: +12.48%; p = 0.0008). The AAMR in metropolitan areas decreased from 15.93 to 10.32 (AAPC: -2.39%; p < 0.000001). There was a significant decline from 1999 to 2010 (APC: -4.73%; p < 0.0001), with a brief rebound also observed from 2018 to 2020 (APC: +12.07%; p = 0.0018).
Stroke and DM-related mortality trends stratified by census region
The AAMR shows a declining trend across all regions, with the southern region generally having the highest rates (16.96 in 1999, 95% CI: 16.63–17.28; 11.59 in 2023, 95% CI: 11.39–11.80; AAPC: -1.65%, p = 0.0036) (Fig. 5) (Table 1). Segment analysis indicates that the most rapid decline occurred between 2002 and 2011 (APC: -4.82%, p < 0.001), followed by a brief increase from 2018 to 2021 (APC: +12.15%, p = 0.005), and a subsequent decrease from 2021 to 2023 (APC: -6.66%, p = 0.045). The northeastern region has the lowest AAMR, decreasing from 13.61 in 1999 to 6.93 in 2023 (AAPC: -2.50%, p = 0.000002), with a rapid decline observed between 1999 and 2009 (APC: -5.44%, p < 0.001). The midwestern (AAPC: -2.84%, p < 0.0001) and western regions (AAPC: -2.55%, p = 0.0002) also experienced significant declines, although both regions saw a rebound from 2018 to 2021 (APC: +7.30%, p = 0.01 and APC: +10.72%, p = 0.036, respectively).
Discussion
This study presents a comprehensive assessment of the 25-year trends in mortality associated with stroke and DM, with particular attention to variations across demographic and geographic subgroups. Findings revealed that AAMR for stroke and DM have both experienced a decline, albeit at markedly different rates. In particular, the AAMR for stroke saw a nearly 50% reduction during this timeframe, with an AAPC of -2.88%. In contrast, although the AAMR for DM showed a 4.1% decline, this trend was not statistically significant and should therefore be interpreted with caution. In individuals suffering from both stroke and DM, a notable reduction in AAMR was observed (AAPC − 2.32%), a result that remained robust in sensitivity analyses restricted to the pre-COVID period (1999–2019); nonetheless, this drop was less significant compared to that noted in the broader stroke population. These findings suggest that, despite substantial progress in the management of both conditions, the persistent mortality risk associated with comorbid stroke and DM may remain a significant concern. Nevertheless, it is important to highlight that during the timeframe of 2018 to 2021, many subgroup mortality curves showed a temporary increase, coinciding with the onset of the COVID-19 pandemic. This suggests that the pandemic may have interfered with the management of chronic conditions and care for strokes. After 2021, the decline in mortality rates continued.
The overall decline in the AAMR among patients with stroke and DM may be attributed to the combined effect of multiple factors. The optimization of the acute stroke treatment process27, the application of advanced imaging technologies, and the widespread use of emergency treatments such as thrombolysis and mechanical thrombectomy28 have played a crucial role in reducing the mortality rate associated with stroke. Concurrently, improvements in hypertension management29 and the extensive use of biguanide hypoglycemic drugs30 and statins31 have contributed to a reduction in both the incidence and recurrence rates of stroke. This aligns with previous studies that indicate that strengthening chronic disease management can improve the survival rates of stroke patients. However, the brief rebound in AAMR from 2018 to 2021 warrants attention, as this phenomenon coincided with the COVID-19 pandemic, suggesting a secondary impact of the epidemic32.
The direct biological impacts of COVID-19, along with the indirect interruption of healthcare services, together account for the shift in mortality rates observed during this time. Research has indicated that the novel coronavirus can elevate the likelihood of stroke by triggering the coagulation cascade, provoking inflammatory responses, and worsening endothelial dysfunction18. Throughout the pandemic, the frequency of emergency visits due to strokes and hyperglycemic events saw a decline of 20% and 10%33, respectively. In addition, delays in managing chronic conditions17, rising psychological stressors34, and diminished social networks35 could collectively worsen the disease burden for individuals suffering from strokes and DM. As we progress, it is crucial to consistently track the health trends of this demographic and adopt strategies that strengthen the management of chronic diseases to better prepare for any possible future public health emergencies. This highlights the vulnerability of individuals with comorbid conditions during public health crises.
The examination categorized by age group indicated that among older patients, the mortality rate of stroke in conjunction with DM was markedly higher compared to younger individuals and tended to rise with advancing age36. Individuals aged 85 and above exhibited the highest AAMR. Previous research has suggested that once individuals reach the age of 55, the likelihood of experiencing a stroke approximately doubles approximately every ten years36. Older adults frequently face the risk of cerebrovascular problems, which include microvascular damage and endothelial dysfunction37. Additionally, the presence of DM in this demographic intensifies the risk of other health complications38. Fortunately, throughout the research period, although the baseline AAMR figures for each older age cohort were relatively elevated, they displayed a declining pattern. It is important to recognize that mortality rates among adults aged 85 and older increased markedly during the pandemic, likely reflecting the combined effects of multiple factors. Beyond the widespread disruption of healthcare services, older adults are generally more frail, have more comorbidities, and are more prone to rapid clinical deterioration39. Moreover, physical limitations, social isolation policies, and financial constraints may have hindered timely access to medical care in this population40,41. These vulnerabilities, compounded by their heightened susceptibility to severe COVID-19 infection42, substantially intensified the disease burden and mortality risk among the oldest-old during the pandemic.
Significant sex-related differences were observed, with males consistently exhibiting higher AAMR than females. This disparity may be attributed to underlying physiological mechanisms. It is thought that estrogen provides a protective effect on both cardiovascular and cerebrovascular health43; however, this advantage declines after menopause, resulting in an elevated stroke risk for older women44. In comparison, men typically encounter more difficulties in managing DM, displaying poorer control of blood sugar and more pronounced fluctuations in blood glucose levels45. Additionally, behavioral factors may notably influence the gender disparities observed. Generally, men show lower adherence to health-promoting practices than women and tend to postpone seeking medical care or neglect managing their conditions46. Strategies for health education and management may need to be specifically designed for each gender, such as improving compliance management for male patients and considering hormone replacement therapy for women after menopause.
Ethnic and racial disparities were pronounced, with higher AAMR observed in historically disadvantaged groups. Non-White populations—especially individuals of African ancestry—faced disproportionately high comorbidity-related mortality. The reasons behind this inequality are intricate and multifaceted, incorporating biological vulnerabilities alongside socioeconomic influences, as well as systemic inequities and cultural variances47. Although these disparities were observed in one national context, they reflect broader global patterns of health inequality and highlight the potential need for more inclusive and equitable interventions.
Similarly, geographic disparities contributed to differences in mortality rates, with AAMR consistently higher in rural areas compared to urban areas. When compared to urban contexts, factors contributing to stroke risk, including hypertension, obesity, and smoking, are more frequently observed in rural areas. These disparities are associated with socioeconomic conditions, health-related behaviors, the distribution of healthcare resources, and the presence of “medical deserts.” Despite significant advancements in telehealth and community health initiatives in recent years, the divide between urban and rural settings remains stark. The variation in AAMRs across geographic regions indicates the necessity of formulating region-specific policies that take into account local healthcare capacity and community characteristics.
The strengths of this study include its long observational period, the use of large-scale population-based data, and the incorporation of subgroup analyses. Nonetheless, several limitations of this study should be acknowledged. First, mortality data were derived from death certificates, which are subject to misclassification and underreporting, particularly for diabetes, potentially leading to an underestimation of the true burden. Second, the ecological design precludes causal inference at the individual level; hence, the findings should be interpreted as population-level trends rather than individual-level associations. Third, the absence of individual-level information—such as body mass index, smoking status, socioeconomic indicators, and treatment details—limited our ability to adjust for potential confounders. Fourth, although we described mortality fluctuations during the COVID-19 pandemic, the study design cannot directly attribute these changes to pandemic-related mechanisms, and such interpretations should be regarded as speculative. Finally, as the analysis was restricted to U.S. mortality data, caution is warranted when generalizing the findings to other populations.
This study demonstrates that although mortality and AAMR associated with comorbid stroke and DM have shown a declining trend over time, they remain markedly elevated in specific populations. The analysis provides a reproducible framework that can be applied across diverse healthcare systems to monitor comorbidity-related mortality. Future research should focus on international comparisons, the identification of predictive biomarkers for stroke-DM comorbidity to facilitate personalized treatment, and the evaluation of the effectiveness of multimodal interventions tailored to different population groups to mitigate the overall disease burden.
Conclusion
In conclusion, although stroke- and DM-related mortality has declined over time, substantial disparities persist. Targeted strategies are needed to address the burden of comorbidity and to ensure more equitable health outcomes across diverse populations.
The authors declare no competing interests.
Nomenclature
This study utilized the Joinpoint Regression Program (RRID:SCR_003999) and R statistical computing environment (RRID:SCR_001905) with packages including tidyverse (RRID:SCR_019186), ggplot2 (RRID:SCR_014601), ggpubr (RRID:SCR_021243), and openxlsx (RRID:SCR_021322). These resources were employed for data analysis and visualization.
Data availability
Data used for this study is publicly available at https://wonder.cdc.gov. For further access to the data or inquiries regarding this study, please contact the corresponding author, Dr. Jing Teng (60170099@sdutcm.edu.cn).
References
Pu, L. et al. Projected global trends in ischemic stroke Incidence, deaths and disability-adjusted life years from 2020 to 2030. Stroke 54 (5), 1330–1339. https://doi.org/10.1161/strokeaha.122.040073 (2023).
Mosenzon, O. et al. Diabetes and stroke: What are the connections? J. Stroke. 25 (1), 26–38. https://doi.org/10.5853/jos.2022.02306 (2023).
Luitse, M. J. et al. Diabetes, hyperglycaemia, and acute ischaemic stroke. Lancet Neurol. 11 (3), 261 – 71. (2012). https://doi.org/10.1016/s1474-4422(12)70005-4
Yaghi, S. et al. Intracranial atherosclerotic disease. Stroke 50 (5), 1286–1293. https://doi.org/10.1161/strokeaha.118.024147 (2019).
Kim, J. S. et al. Risk factors and stroke mechanisms in atherosclerotic stroke: Intracranial compared with extracranial and anterior compared with posterior circulation disease. Stroke 43 (12), 3313–3318. https://doi.org/10.1161/strokeaha.112.658500 (2012).
van Sloten, T. T. et al. Cerebral microvascular complications of type 2 diabetes: Stroke, cognitive dysfunction, and depression. Lancet Diabetes Endocrinol. 8 (4), 325–336. https://doi.org/10.1016/s2213-8587(19)30405-x (2020).
Katsanos, A. H. et al. Stroke prevention in atrial fibrillation: Looking forward. Circulation 142 (24), 2371–2388. https://doi.org/10.1161/circulationaha.120.049768 (2020).
Seyed Ahmadi, S. et al. Risk of atrial fibrillation in persons with type 2 diabetes and the excess risk in relation to glycaemic control and renal function: A Swedish cohort study. Cardiovasc. Diabetol. 19 (1), 9. https://doi.org/10.1186/s12933-019-0983-1 (2020).
Maida, C. D. et al. Diabetes and ischemic stroke: An old and new relationship an overview of the close interaction between these diseases. Int. J. Mol. Sci.. https://doi.org/10.3390/ijms23042397 (2022).
Bladin, C. F. et al. Management of poststroke hyperglycemia: Results of the TEXAIS randomized clinical trial. Stroke 54 (12), 2962–2971. https://doi.org/10.1161/strokeaha.123.044568 (2023).
Zelniker, T. A. et al. SGLT2 inhibitors for primary and secondary prevention of cardiovascular and renal outcomes in type 2 diabetes: A systematic review and meta-analysis of cardiovascular outcome trials. Lancet 393 (10166), 31–39. https://doi.org/10.1016/s0140-6736(18)32590-x (2019).
Rossing, P. et al. KDIGO 2022 Clinical Practice Guideline for Diabetes Management in Chronic Kidney Disease. Kidney Int., 102(5s), S1. . https://doi.org/10.1016/j.kint.2022.06.008(2022).
Hong, B. et al. Sodium-glucose cotransporter-2 inhibitors, dulaglutide, and risk for dementia: A population-based cohort study. Ann. Intern. Med. 177 (10), 1319–1329. https://doi.org/10.7326/m23-3220 (2024).
Sim, A. Y. et al. Neuroprotective roles of SGLT2 and DPP4 inhibitors: Modulating ketone metabolism and suppressing NLRP3 inflammasome in T2D induced alzheimer’s disease. Exp. Neurol. 390, 115271. https://doi.org/10.1016/j.expneurol.2025.115271 (2025).
Nakhal, M. M. et al. Repurposing SGLT2 inhibitors for neurological disorders: A focus on the autism spectrum disorder. Molecules. https://doi.org/10.3390/molecules27217174 (2022).
Al Hamed, F. A. & Elewa, H. Potential therapeutic effects of sodium Glucose-linked cotransporter 2 inhibitors in stroke. Clin. Ther. 42 (11), e242–e249. https://doi.org/10.1016/j.clinthera.2020.09.008 (2020).
Ogungbe, O. et al. Disruption to diabetes and hypertension care during the COVID-19 pandemic in Latin America and the Caribbean and mitigation approaches: A scoping review. BMC Health Serv. Res. 25 (1), 660. https://doi.org/10.1186/s12913-025-12760-3 (2025).
Tan, C. W. et al. Critically ill COVID-19 infected patients exhibit increased clot waveform analysis parameters consistent with hypercoagulability. Am. J. Hematol. 95 (7), E156–e158. https://doi.org/10.1002/ajh.25822 (2020).
Ahmad, O. et al. Temporal trends in mortality related to stroke and atrial fibrillation in the united states: A 21-year retrospective analysis of CDC-WONDER database. Clin. Cardiol. 47 (12), e70058.https://doi.org/10.1002/clc.70058 (2024).
Sohail, M. U. et al. Trends and disparities in diabetes mellitus and atrial fibrillation related mortality in the united states: 1999–2020. Diabetes Res. Clin. Pract. 223, 112112. https://doi.org/10.1016/j.diabres.2025.112112 (2025).
Mar multiple cause of death data on CDC WONDER. Available from: https://wonder.cdc.gov/mcd.html (2025).
von Elm, E. et al. The strengthening the reporting of observational studies in epidemiology (STROBE) statement: Guidelines for reporting observational studies. J. Clin. Epidemiol. 61 (4), 344–349. https://doi.org/10.1016/j.jclinepi.2007.11.008 (2008).
Ingram, D. D. & Franco, S. J. NCHS urban-rural classification scheme for counties. Vital Health Stat. 2 (154), 1–65 (2012).
Anderson, R. N. & Rosenberg, H. M. Age standardization of death rates: Implementation of the year 2000 standard. Natl. Vital Stat. Rep. 47 (3), 1–16 (1998). 20.DOI:.
Joinpoint Regression Program. Available from: https://surveillance.cancer.gov/joinpoint(2024)
Kim, H. J. et al. Comparability of segmented line regression models. Biometrics 60 (4), 1005–1014. https://doi.org/10.1111/j.0006-341X.2004.00256.x (2004).
Ye, Z. et al. Acute stroke care coordination in the united states: variation in state laws for emergency medical services and hospitals. J. Stroke Cerebrovasc. Dis. 34 (2), 108174. https://doi.org/10.1016/j.jstrokecerebrovasdis.2024.108174 (2025).
Aghaebrahim, A. et al. Use of new aspiration thrombectomy delivery catheter: A multicenter experience. J. Neurointerv Surg. https://doi.org/10.1136/jnis-2025-023389 (2025).
Yu, D. et al. Comparative efficacy of different antihypertensive drug classes for stroke prevention: A network meta-analysis of randomized controlled trials. PLoS One. 20 (2), e0313309. https://doi.org/10.1371/journal.pone.0313309 (2025).
Sharma, S. et al. Repurposing Metformin to treat age-related neurodegenerative disorders and ischemic stroke. Life Sci. 274, 119343. https://doi.org/10.1016/j.lfs.2021.119343 (2021).
Mustanoja, S. et al. Association of prestroke statin use and lipid levels with outcome of intracerebral hemorrhage. Stroke 44 (8), 2330–2332. https://doi.org/10.1161/strokeaha.113.001829 (2013).
Sharma, R. et al. Excess cerebrovascular mortality in the United States during the COVID-19 pandemic. Stroke 52 (2), 563–572. https://doi.org/10.1161/strokeaha.120.031975 (2021).
Lange, S. J. et al. Potential indirect effects of the COVID-19 pandemic on use of emergency departments for acute life-threatening conditions—United States, January-May 2020. MMWR Morb. Mortal. Wkly. Rep. 69 (25), 795–800. https://doi.org/10.15585/mmwr.mm6925e2 (2020).
Mantica, G. et al. Non-COVID-19 visits to emergency departments during the pandemic: The impact of fear. Public. Health. 183, 40–41. https://doi.org/10.1016/j.puhe.2020.04.046 (2020).
Dhand, A. et al. Social networks and risk of delayed hospital arrival after acute stroke. Nat. Commun. 10 (1), 1206. https://doi.org/10.1038/s41467-019-09073-5 (2019).
Bushnell, C. et al. Guidelines for the prevention of stroke in women: A statement for healthcare professionals from the American heart association/American stroke association. Stroke 45 (5), 1545–1588. https://doi.org/10.1161/01.str.0000442009.06663.48 (2014).
Yousufuddin, M. & Young, N. Aging and ischemic stroke. Aging. 11 (9), 2542–2544. https://doi.org/10.18632/aging.101931 (2019).
Khan, M. M. et al. Prevalence and predictors of stroke among individuals with prediabetes and diabetes in Florida. BMC Public Health. 22 (1), 243. https://doi.org/10.1186/s12889-022-12666-3 (2022).
Liu, K. et al. Clinical features of COVID-19 in elderly patients: A comparison with young and middle-aged patients. J. Infect. 80 (6), e14–e18. https://doi.org/10.1016/j.jinf.2020.03.005 (2020).
Kyriazis, M. et al. COVID-19 isolation and risk of death in Cyprus elderly people. Front. Med.. 8, 717692. https://doi.org/10.3389/fmed.2021.717692 (2021).
Ding, S. et al. The satisfaction of elderly people with elderly caring social organizations and its relationship with social support and anxiety during the COVID-19 pandemic: A cross-sectional study. BMC Public. Health. 23 (1), 1206. https://doi.org/10.1186/s12889-023-15951-x (2023).
Du, Y. et al. Clinical features of 85 fatal cases of COVID-19 from Wuhan. A retrospective observational study. Am. J. Respir Crit. Care Med. 201 (11), 1372–1379. https://doi.org/10.1164/rccm.202003-0543OC (2020).
Li, J. et al. Estrogen enhances neurogenesis and behavioral recovery after stroke. J. Cereb. Blood Flow. Metab. 31 (2), 413–425. https://doi.org/10.1038/jcbfm.2010.181 (2011).
Kaufhold, C. J. et al. Sex differences in neurological disorders: Insights from ischemic stroke, parkinson’s disease, and multiple sclerosis. Brain Behav. Immun. 129, 335–347. https://doi.org/10.1016/j.bbi.2025.06.026 (2025).
Yeo, J. L. et al. Sex differences and determinants of coronary microvascular function in asymptomatic adults with type 2 diabetes. J. Cardiovasc. Magn. Reson. 27 (1), 101132. https://doi.org/10.1016/j.jocmr.2024.101132 (2025).
Lyons, J. et al. Pandemic telehealth utilization among ambulatory care diabetes patients managed at an urban teaching hospital in Washington, DC. Telemed J. E Health. https://doi.org/10.1089/tmj.2024.0468 (2025).
Renedo, D. et al. Impact of social determinants of health on outcomes of nontraumatic subarachnoid hemorrhage. J. Am. Heart Assoc. 14 (8), e037199. https://doi.org/10.1161/jaha.124.037199 (2025).
Acknowledgements
The authors thank the Centers for Disease Control and Prevention for access to the CDC WONDER database.
Funding
This work was supported by the study on the Association Between Cardiovascular Diseases and Amyotrophic Lateral Sclerosis Based on Case-Control and Mendelian Randomization Methods (YJSTZCX2025109).
Author information
Authors and Affiliations
Contributions
HM: Conceptualization, Data analysis, Writing - original draft. ML: Software, Writing - original draft. JT: Supervision, Writing - review & editing. All authors reviewed and approved the final manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Ma, H., Liu, M. & Teng, J. Temporal trends and disparities in stroke and diabetes mellitus comorbidity-related mortality from 1999 to 2023. Sci Rep 15, 43967 (2025). https://doi.org/10.1038/s41598-025-27754-8
Received:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s41598-025-27754-8