Abstract
The accurate quantification of asymptomatic infections is crucial for understanding and mitigating the spread of COVID-19. However, estimating the true prevalence of asymptomatic cases remains a significant challenge. This study introduces a novel mathematical modeling approach to estimate key epidemiological parameters associated with asymptomatic infections, leveraging comprehensive COVID-19 data from the Republic of Korea. We develop a refined compartmental model that explicitly incorporates asymptomatic individuals and employs a trajectory matching method, integrating least-squares fitting with gradient matching, to align the model with confirmed cases and deaths. The model successfully reproduces the initial outbreak and subsequent epidemic waves, demonstrating strong agreement with observed data and validating the estimated parameters, which align with prior findings. We observe a progressive increase in both infectivity and the proportion of asymptomatic infections from the initial strain to the Delta and Omicron variants. Notably, across all phases, the proportion of asymptomatic cases is higher among vaccinated individuals compared to unvaccinated individuals. This study provides valuable insights into the hidden dynamics of the COVID-19 pandemic and offers a robust methodology that can be broadly applied to estimate critical epidemiological parameters for other infectious diseases, enhancing our capacity to manage future outbreaks.
Similar content being viewed by others
Introduction
The rapid global spread of COVID-19 highlighted the crucial role of asymptomatic infections in driving the pandemic. A significant portion of infected individuals exhibited no symptoms, yet played a major role in transmitting the virus1,2,3,4,5,6,7. These asymptomatic individuals, due to the lack of overt symptoms, are less likely to be identified and may continue normal social activities without diagnosis, potentially causing silent community transmission. Therefore, accurately identifying and quantifying asymptomatic cases is vital for controlling the spread of COVID-19.
Despite its importance, estimating the true prevalence of asymptomatic infections remains challenging, with reported proportions varying significantly across studies. Numerous investigations have attempted to quantify the proportion of asymptomatic cases among confirmed COVID-19 infections, reporting a wide range of values. For instance, a study conducted on the Diamond Princess cruise ship reported an asymptomatic proportion of 17.9%8, while among Japanese evacuees from Wuhan, it was estimated at 33%1,2. On the other hand, studies from Italy and a Korean call center observed much lower proportions of 1.8%1 and 4.1%9. Additionally, the Centers for Disease Control and Prevention (CDC) estimated an asymptomatic infection rate of 30%10, while other studies suggested a range of 40–45%3,4,5. Meta-analyses have further attempted to synthesize these disparate findings, reporting that 17% (95% confidential interval (CI) 14–20%)6, 15.5% (95% CI 12–18.4%)11 and 25% (95% CI 16–38%)12 of cases were asymptomatic. This inconsistency highlights the complexity of accurately assessing the asymptomatic prevalence.
One of the primary challenges in estimating asymptomatic infection rates arises from variations in testing policies, healthcare accessibility, and public awareness across different regions and time periods. Moreover, evolving viral variants with differing clinical presentations further complicates the accurate estimation of asymptomatic proportions. For instance, the Omicron variant, characterized by its high transmissibility and relatively mild symptoms, has likely led to an increase in the proportion of asymptomatic infections compared to earlier variants.
To address these challenges, this study introduces a novel mathematical modeling approach aimed at estimating key parameters associated with asymptomatic infections, specifically within the context of the Republic of Korea. Utilizing real-world data on confirmed cases and related information, we develop a refined compartmental model that explicitly incorporates the presence of asymptomatic individuals, accounting for their potential prolonged infectiousness despite remaining undetected. By employing a trajectory matching method13,14,15, we align our model with the observed epidemiological data to quantify critical characteristics of COVID-19 transmission dynamics, including the transmission rate and the proportion of asymptomatic infections. To the best of our knowledge, this study represents the first attempt to use a mathematical model in conjunction with real-world data to estimate both the proportion and detection rate of asymptomatic infections. This approach offers valuable insights into the hidden dynamics of the pandemic.
Materials and methods
To elucidate the transmission dynamics of COVID-19 in Korea, we developed a mathematical model utilizing reported epidemiological data. This model was specifically designed to estimate the disease transmission rate and the detection rates of asymptomatic infections. To comprehensively analyze the factors influencing COVID-19 transmission, we segmented the observation period into three distinct phases, each characterized by specific outbreak patterns, response policies, and the dominance of different virus variants. We investigated the role of asymptomatic individuals in transmission within the context of these phases, with the objective of evaluating the impact of asymptomatic cases on the spread of COVID-19 in a more detailed manner.
Epidemiological data
This study analyzed epidemiological data spanning January 2020–June 2022 in Korea, as illustrated in Fig. 1. The data included confirmed cases (Fig. 1A), deaths, and vaccination records, sourced from the Korea Centers for Disease Control and Prevention (KDCA)16,17. Vaccination data encompassed both the primary series (second dose) and booster shots (third dose), with the first vaccinations administered on February 26, 202118 (Fig. 1B; right y-axis). Additionally, information on the prevalence of virus variants was obtained from the CoVariants dataset, a publicly available genomic surveillance resource19 (Fig. 1B; left y-axis), focusing on the Delta and Omicron variants, which had significant impacts during the study period20,21,22. The Delta variant, first detected in April 2021, became dominant by July 2021, accounting for over 50% of cases23,24. Subsequently, the Omicron variant displaced Delta by January 2022, precipitating a rapid escalation in infection rates25.
Epidemiological data on COVID-19 in Korea (2020–2022). (A) Daily confirmed cases. (B) vaccination coverage and variant proportions. Blue shading indicates the period with non-pharmaceutical interventions (NPIs) only, while orange and green shading represent the dominance of the Delta and Omicron variants, respectively.
Period-based analysis of transmission dynamics
To enhance the accuracy of estimating the COVID-19 transmission rates and the proportion of asymptomatic cases, we incorporated key epidemiological and policy factors associated with the disease. We then partitioned the observation interval into three major periods, each spanning six months, to evaluate transmission patterns, variant characteristics, and the impacts of vaccination and non-pharmaceutical interventions (NPIs) across distinct phases of the pandemic. The six-month window was chosen to mitigate distortion from short-term fluctuations, including rapid and repeated changes in public health policies, thereby providing a clearer representation of the sustained effects of public health measures and variant dynamics. Within each six-month period, we additionally performed month-by-month calibration to capture meaningful short-term changes in transmission dynamics, such as shifts in behavior, testing practices, or intervention intensity. The characteristics of each observation period are as follows:
-
Period I (Only NPIs: September 2020 ~ February 2021) This initial phase preceded the availability of COVID-19 vaccines. Consequently, public health responses relied exclusively on Non-Pharmaceutical Interventions – such as mandated social distancing, limitations on facility operations, and mask requirements – to curtail the transmission of the disease.
-
Period II (Delta Variant Predominance: July 2021–December 2021) This period was marked by the dominance of the Delta variant, which posed significant challenges due to its rapid transmission despite ongoing vaccination deployment. As shown in Fig. 1A, prior to this period, South Korea successfully maintained low-level transmission through robust public health interventions, but the emergence of the Delta variant caused a rapid, several-fold increase in infections.
-
Period III (Omicron Variant Predominance: January 2022 ~ June 2022) The Omicron variant emerged as the predominant strain during this period. While widespread vaccination and booster doses contributed to a decrease in severe illness, the high transmissibility and relatively lower fatality rate of Omicron underscored the importance of managing asymptomatic cases.
Mathematical models for COVID-19
To investigate the transmission dynamics of COVID-19, we developed two compartmental models based on the Susceptible \(-\) Exposed \(-\) Infectious \(-\) Confirmed \(-\) Recovered \(/\) Dead (SEICRD) framework, an extension of the well-established SEIR model. These models are specifically designed to account for asymptomatic transmission, capturing the distinct dynamics of COVID-19 while considering the varying impact of interventions across different observation periods.
Model 1: COVID-19 transmission model without vaccination
Model 1 is a COVID-19 transmission model that does not account for vaccination effects. Based on the SEICRD framework, it focuses on the natural transmission dynamics of the virus, incorporating both asymptomatic individuals (\(A\)) and detected asymptomatic cases (\({C}_{A}\)). Here, \({C}_{A}\) represents asymptomatic individuals identified through surveillance measures, such as preemptive testing or voluntary screening, and is considered to be in a confirmed and isolated state.
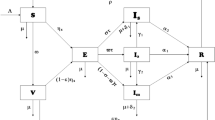
The model includes the following compartments: Susceptible (\(S\)), Exposed (or Latent) (\(E\)), Asymptomatic Infectious (\(A\)), Symptomatic Infectious (\(I\)), Confirmed Asymptomatic (\({C}_{A}\)), Confirmed Symptomatic (\({C}_{I}\)), Recovered (\(R\)), and Deceased (\(D\)). The schematic diagram for the model is illustrated in Fig. 2.
The model equations are governed by a system of ordinary differential equations (ODEs) that describe the transitions between the compartments.
where \(\Lambda =\frac{\beta \left(\theta A+I\right)}{N}\) represents the force of infection, which quantifies the per capita rate at which susceptible individuals acquire infection through contact with infectious individuals (\(A, I\)). Here, \(N=S+E+A+I+R\) denotes the population segment contributing to community transmission, excluding individuals who have been isolated following confirmation. The parameters of the system (1) were calibrated using empirical data from Korea alongside values reported in relevant prior studies. A detailed description of the parameter values is provided in Table 1.
This model assumes that the recovery rate of asymptomatic individuals (\({\gamma }_{A}\)) is equivalent to that of symptomatic individuals (\({\gamma }_{H}\)), based on the quarantine policy implemented during the early COVID-19 response in Korea. At that time, both confirmed cases and close contacts were required to undergo a 14-day quarantine period, which remained in place until it was shortened to 10 days on November 1, 2021. Due to the high level of uncertainty about the virus’s spread during the early phase of the pandemic, maintaining strict adherence to the 14-day quarantine was regarded as essential to reduce transmission risk.
To quantitatively assess the transmission dynamics, we calculated the effective reproduction number \({R}_{t}\). Defined as the average number of secondary cases generated by an infectious individual within a population comprising both susceptible and non-susceptible individuals, \({R}_{t}\) provides a crucial measure of epidemic spread. For the system (1), \({R}_{t}\) was derived using the next-generation matrix method and is given by:
Where \(S\left(0\right)={S}_{0}\). A detailed derivation of Rt is written in S1 Text.
Model 2: COVID-19 transmission model with vaccination
Model 2 builds upon Model 1 by incorporating the effects of vaccination, focusing on transmission dynamics after vaccines became available and widely administered. This model specifically accounts for changes in transmission patterns introduced by vaccination, including the protective effects of both the primary series and booster doses, as well as the waning of vaccine-induced immunity over time.
The model includes the following compartments: Susceptible (\(S\)), Vaccinated Susceptible after Primary series (\({V}_{P}\)), Vaccinated Susceptible after Booster dose (\({V}_{B}\)), Exposed (\(E\)), Vaccinated Exposed (\({E}_{V}\)), Asymptomatic Infectious (\(A\)), Vaccinated Asymptomatic Infectious (\({A}_{V}\)), Symptomatic Infectious (\(I\)), Vaccinated Symptomatic Infectious (\({I}_{V}\)), Confirmed Asymptomatic (\({C}_{A}\)), Confirmed Symptomatic (\({C}_{I}\)), Recovered (\(R\)), and Deceased (\(D\)). The schematic diagram for the model is illustrated in Fig. 3.
The governing equation of the vaccination model is written as follows:
where \(\Lambda =\frac{\beta \left(\theta \left({A}_{S}+{A}_{V}\right)+I\right)}{N}\), extending the force of infection in Model 1 to include transmission driven by infections in vaccinated individuals (\({A}_{V}\)), and \(N=S+{V}_{P}+{V}_{B}+{E}_{S}+{E}_{V}+{A}_{S}+{A}_{V}+I+R\), encompassing both vaccinated and unvaccinated individuals across susceptible, exposed, and infectious states. The description of the parameter values is given in Table 2.
In this model, the vaccination rates for the primary series (\({\phi }_{P}\)) and booster dose (\({\phi }_{B}\)) were treated as variable parameters, as they directly depended on daily updates from actual vaccination data. Additionally, the model considers the waning effectiveness of COVID-19 vaccines as a factor reflecting the gradual reduction in vaccine-induced immunity over time. The vaccine effectiveness loss rate was set to 1/90 (0.0111) based on previous studies indicating significant waning of immunity approximately 90 days after the second dose34,35,36, which aligns with the guidelines issued by the Korean government37, recommending a booster dose shot three months after the primary series.
The effective reproduction number for Model 2, incorporating vaccination, is given by:
Where \(S\left(0\right)={S}_{0}\), \({V}_{P}\left(0\right)={V}_{P0}\), and \({V}_{B}\left(0\right)={V}_{B0}\). A detailed derivation is written in S1 Text.
Trajectory — based parameter calibration
In this section, we introduce the trajectory-based parameter calibration approach to quantify key parameters related to the transmission dynamics of COVID-19 during the observation period, including the transmission rate (\(\beta\)), the detection rate of asymptomatic infections (\({q}_{A}\)), and the probability of asymptomatic cases (\(\rho\)).
To effectively calibrate the COVID-19 transmission models, we applied data smoothing using a seven-day moving average on the confirmed case data to reduce variability and obtain a stable trend, minimizing short-term fluctuations that could distort parameter estimates. Additionally, for vaccination data, including both the primary series and booster dose records, we derived the daily increments (\({dV}_{P}\) and \({dV}_{B}\)) and directly incorporated them into the model by updating the vaccinated compartments (\({V}_{P}\) and \({V}_{B}\)) daily. This approach allowed the model to dynamically reflect real-time changes in vaccination coverage.
We estimated initial compartment values and monthly transmission rates (\({\beta }_{1}\) through \({\beta }_{6}\)) for each period, along with the asymptomatic case rate (\(\rho\)). The parameters were calibrated for two types; Model 1 was applied to Period I, excluding vaccination effects, while Model 2 was used for Period II and III, each with distinct parameter sets. For Model 1, a total of 16 parameters were estimated, including the monthly transmission rates for six months, the asymptomatic rate, and the detection rate of asymptomatic cases. Additionally, we estimated the initial compartment values for the states \(E,P,A,I,{C}_{A},{C}_{I},R,\) and \(D\) at \(t={t}_{1}\). The Model 2, which accounts for the impact of vaccinated individuals, required more parameters and compartments, resulting in 21 unknown parameters. In particular, we estimated an additional parameter \({\rho }_{V}\), representing the probability of asymptomatic cases among vaccinated individuals. Overall, we calibrated a total of 58 parameters independently for the three periods (Period I, II and III). However, some parameters, such as \({q}_{A}\) (the detection rate of asymptomatic cases), posed challenges in certain periods. In Period I, significant variability in testing and detection rates occurred due to frequent changes in public policies, reducing the reliability of asymptomatic data. Similarly, in Period III, the emergence of the Omicron variant disrupted comprehensive testing, leading to potential data incompleteness or underreporting. To address this issue, we calibrated \({q}_{A}\) based on data from Period II (Delta variant predominance) and applied it uniformly across other periods.
For model simulations, we employed the fourth-order Runge Kutta (RK4) method with a step size of 0.1. The explicit fourth-order Runge–Kutta (RK4) method was used instead of a built-in adaptive solver because the proposed models are not stiff and thus do not require adaptive step-size control. Moreover, the empirical data were available at uniform daily intervals, for which a fixed-step integration method enables a more direct and consistent comparison between simulated and observed trajectories. All computations were carried out in MATLAB R2023b. Parameter calibration was achieved by minimizing the sum of squared errors between model predictions and observed data using nonlinear least squares. The main error term for trajectory matching \({\upepsilon }_{T}\) was defined as:
Where \({H}_{k}\) is the confirmed number of hospitalized on day \(k\), and \(dC\left({t}_{k}\right)=C\left({t}_{k}\right)-C({t}_{k-1})\). Here \({c}_{1}\) is a normalizing constant equal to \(1/\sum_{k=1}^{N}{H}_{k}^{2}\). Additionally, we incorporated an extra penalty term \({\upepsilon }_{A}\) to account for large-scale asymptomatic case observations that at least 25% of confirmed cases were reported as asymptomatic12. The penalty term was formulated as:
Where \(r=0.25\) represents the lower bound of asymptomatic case estimates, and \({c}_{2}\) is a normalizing constant defined as \(1/r\sum_{k=1}^{N}{H}_{k}^{2}\). The total error function for parameter fitting was expressed as:
The penalty rate \(\lambda\) was set to \({10}^{-2}\), the smallest value ensuring a unique optimization result across multiple trials with varying initial guesses.
To assess the robustness of parameter estimation, we conducted multiple fitting procedures starting from various initial parameter values. In all cases, the optimization process consistently converged to the same parameter set, suggesting that the model is numerically identifiable and the estimated parameters are stable with respect to initial conditions. Although a formal structural identifiability analysis was not performed in this study, these consistent convergence results support the practical identifiability of the proposed models within the scope of the available data.
Results
We employed the trajectory matching approach to estimate key epidemiological parameters, including the transmission rate, the probabilities of asymptomatic cases, and the detection rate of asymptomatic cases across the three defined periods. These parameters were estimated on a monthly basis to capture temporal variations in transmission dynamics. Reflecting the national vaccination timeline, which began in February 2021, Period I (preceding widespread vaccination) was simulated using the COVID-19 transmission model without vaccination (Model 1), while Period II and III (during widespread vaccination) were analyzed with a model incorporating vaccination effects (Model 2).
Transmission dynamics by period
To evaluate how well the model captures the spread dynamics, we compared the predicted and observed trends in newly confirmed and cumulative cases. As shown in Fig. 4, the predictions closely matched the reported case numbers across all three periods, indicating that the estimated transmission rates effectively captured the key dynamics of COVID-19 spread. Model performance was further evaluated using root mean squared error (RMSE), representing the average prediction error (lower values indicate smaller discrepancies), and the coefficient of determination (\({R}^{2}\)), indicating the proportion of observed variation explained by model (value closer to 1 indicate greater consistency with observations). Across Periods I-III, RMSE values were 103.05, 633.85, and 39,496.33, respectively, and \({R}^{2}\) values were 0.8799, 0.8578, and 0.8988. Although RMSE was higher in Period III due to larger case counts during the Omicron wave, the consistently high \({R}^{2}\) values (> 0.85) indicate that the model successfully reproduced both the magnitude and temporal patterns of reported cases in each period.
In Period I (Figs. 4A, D, 5A) the model successfully captured the early transmission trends, demonstrating a mean transmission rate of 0.3110 with a range between 0.1482 and 0.5206. The average effective reproduction number (Rt) during this period was estimated at 1.0854, though it exhibited considerable fluctuation from 0.5171 to 1.8178. These variations reflect the inherent instability in transmission dynamics before the introduction of vaccines, where NPIs were the primary means of control. The reliance on NPIs alone contributed to variability in the transmission rate, particularly during periods of increased social interactions or changes in public health measures27,52.
During Period II (Figs. 4B, E, 5B), characterized by the predominance of the Delta variant, the mean transmission rate increased to 0.4271, with an average Rt of 1.1115. This period showed the least variability in transmission rates, likely due to the combined effects of vaccination rollout and stable public health policies.
In Period III (Figs. 4C, F, 5C), dominated by the Omicron variant, the peak transmission rate rose to 0.6883. Despite the high transmissibility of Omicron, leading to a rapid increase in confirmed cases, cumulative vaccination coverage and a reduction in the susceptible population contributed to a gradual decline in the effective reproduction number, averaging 1.0361. This declining trend highlights the combined effect of vaccination-induced immunity and behavioral adaptations, such as increased self-isolation during symptomatic phases, despite the variant’s heightened infectiousness.
Figure 5 illustrates the temporal variations in the transmission rate and the corresponding Rt for each period. The estimated monthly transmission rates and Rt values are summarized in Table 3, presenting the progression from the early unstable transmission in Period I to the relatively stabilized dynamics in Period II. In Period III, a phase of rapid transmission followed by a decline is observed, reflecting the cumulative effects of vaccination and public health measures.
Probability and detection rate of asymptomatic cases
Our model also estimated the proportion of asymptomatic infections and detection rate across different periods and vaccination statuses. As summarized in Table 4, the estimated probability of asymptomatic cases varied between vaccinated and unvaccinated populations throughout the study periods.
In Period I, the probability of asymptomatic infections was estimated at 31.55%. This proportion increased during Period II, reaching 37.57% among unvaccinated individuals and 41.79% among vaccinated individuals. The upward trend continued into Period III, where the proportion rose to 44.71% among unvaccinated individuals and 49.70% among vaccinated individuals. This temporal increase likely reflects the combined impact of increased viral infectivity, changes in clinical presentation associated with evolving dominant variants, and the transition from comprehensive surveillance to more self-diagnosis and isolation53,54,55. Notably, in all periods, the probability of asymptomatic cases was consistently higher among vaccinated individuals compared to unvaccinated ones. This pattern suggests that vaccination, while effective in preventing severe disease outcomes, may also lead to a greater proportion of mild or asymptomatic infections.
The estimated detection rate for asymptomatic cases throughout the observation period was relatively low, at approximately 0.093. This finding underscores the persistent challenge of identifying asymptomatic carriers, even with widespread testing strategies. The difficulty in detecting asymptomatic cases may contribute to ongoing community transmission, emphasizing the need for continuous surveillance and more sensitive detection methodologies.
Sensitivity analysis of asymptomatic infection estimates
Given the inherent uncertainties in epidemiological modeling, arising from complex model structures and multiple free parameters, a systematic approach is required to interpret the influence of individual variables on overall transmission dynamics48,50.
Accordingly, we performed sensitivity analysis focusing on key parameters related to asymptomatic infection estimates: probability of asymptomatic infection in the unvaccinated individuals (\({\rho }_{S}\)), in the vaccinated individuals (\({\rho }_{V}\)) and detection rate of asymptomatic cases (\({q}_{A}\)), play a central role in defining the influence of asymptomatic transmission and size. Given their interdependence, we limited our analysis to the probability of asymptomatic infection in the unvaccinated group (\({\rho }_{S}\)) and detection rate (\({q}_{A}\)) to ensure clarity and consistency in evaluating the impact of asymptomatic transmission on model outcomes.
To comprehensively assess parameter sensitivities, both the elasticity analysis and elementary effect of the Morris method were applied in this study, as shown in Fig. 6.
Sensitivity of cumulative infections to asymptomatic-related estimates across periods: Panels (A, D) correspond to Period I, (B, E) to Period II, and (C, F) to Period III. The results of the elasticity analysis are presented in (A–C), where the dashed line indicates a zero-sensitivity boundary. The elementary effects from the Morris method are displayed in (D–F), with the dashed line showing the baseline value for relative infectiousness (\(\theta =0.75\)).
First, to quantitatively evaluate the relative impact of our target parameters on cumulative infection counts (\(CI\)), we performed elasticity analysis by applying a 10% perturbation to each target parameter (\(p\)) and calculating the normalized forward sensitivity index51:
As shown in Fig. 6A–C, the detection rate (\({q}_{A}\)) consistently exhibited a negative relationship with \(CI\) across all periods: higher detection rate led to fewer infections, as more asymptomatic cases were identified and isolated, thereby reducing onward transmission. In contrast, the probability of asymptomatic infection in unvaccinated individuals (\({\rho }_{S}\)) showed a period-specific pattern. While higher \({\rho }_{S}\) was associated with increased \(CI\) (positive elasticity) in Periods I and II, this relationship reversed in Period III, suggesting possible interactions or nonlinear effects with other infection-related variables.
To further investigate these nonlinear effects, particularly those related to the cumulative burden of asymptomatic infections, we examined the relative infectiousness of asymptomatic individuals (\(\theta\)) using the Morris method49. For the target parameter, the elementary effect (\({S}_{p})\) was calculated using a central finite difference approximation:
where \(f\) is the cumulative incidence, \({x}_{p}\) is the target parameter, and \(\epsilon\) is a small perturbation set to \({10}^{-4}\). Figure 6D–F illustrates the sensitivity of \(CI\) to variations in \({q}_{A}\) and \({\rho }_{S}\) across the full plausible range of \(\theta\). The results demonstrate that increasing the detection rate consistently decreased \(CI\), regardless of the relative infectiousness of asymptomatic individuals. However, the influence of the probability of asymptomatic infection depends on \(\theta\), with distinct threshold values observed in each period (0.4–0.5 in Period I, 0.6–0.7 in Period II, and 0.9–1.0 in Period III). This indicates that the epidemiological impact of asymptomatic infections depends on both their prevalence and their relative ability to transmit the virus, with relationships that are often nonlinear. Notably, in Period III, the threshold for \(\theta\) was higher than in earlier periods, which suggests that the influence of asymptomatic transmission was relatively reduced during this period. This diminished role appears to result from the shorter infectious period implemented in this period (5 days for unvaccinated individuals and 3 days for vaccinated individuals), which likely limited the overall contribution of asymptomatic transmission to epidemic dynamics.
Discussion
In this study, we developed a compartmental mathematical model to investigate the transmission dynamics of COVID-19 in the Republic of Korea, with a particular focus on quantifying unknown parameters related to asymptomatic infection. By applying a trajectory matching method incorporating gradient information to incidence data, we estimated the probability of asymptomatic infections, the detection rate, and the transmission rate on a monthly basis to capture the impact of temporal factors, including governmental interventions and seasonal variations. Our modeling framework successfully reproduced the observed epidemic trajectory, including the initial outbreak and subsequent large-scale waves driven by the Delta and Omicron variants, thereby lending credibility to the estimated parameters.
Two key findings emerged from this analysis, both of which hold significant public health implications. First, we observed a clear temporal increase in the estimated probability of asymptomatic infections from the pre-vaccination period (Period I) through the Delta (Period II) and Omicron (Period III) waves. This trend likely reflects a complex interplay of factors, including the evolving clinical characteristics and potentially higher intrinsic infectivity of successive variants25,38,39, shifting population immunity profiles, and changes in testing behaviors, such as the increased reliance on self-diagnosis kits during later phases40,41. These results emphasize the importance of robust detection systems in mitigating silent transmission, as asymptomatic cases are less likely to be identified and isolated promptly.
Second, the probability of asymptomatic cases was consistently estimated to be higher among vaccinated individuals compared to their unvaccinated counterparts across all observed periods. This observation aligns with previous studies suggesting that while vaccination effectively reduces the risk of severe disease42,43,44,45,46, it may also alter the clinical manifestation, leading to a greater proportion of mild or asymptomatic infections. This finding suggests a potential unintended consequence of mass vaccination, where individuals with mild or no symptoms may remain active in the community, thereby contributing to ongoing transmission. While beneficial at the individual level, this phenomenon could potentially facilitate continued silent transmission, highlighting the need for ongoing surveillance and mitigation strategies that account for asymptomatic spread, even within highly vaccinated populations.
Our analysis also captured the impact of specific variants and public health contexts on transmission dynamics. The gradual increase in mean transmission rates from Period I through Period III corresponds to be the known higher transmissibility of the Delta and Omicron variants compared to earlier strains25,38,47. The relative stability observed during the Delta-dominant period (Period II) could be attributed to consistent public health policies in Korea, which likely provide a robust dataset for parameter estimation. On the other hand, the Omicron wave (Period III), characterized by the highest transmission rates, also exhibited a subsequent decline in the effective reproduction number (Rt), attributable to the rapid accumulation of population immunity through vaccination and infection. Behavioral adaptations, such as increased self-isolation despite challenges in testing capacity, may have also played a role. Korea’s comprehensive public health infrastructure, encouraging testing and supporting isolation, undoubtedly influenced these observed dynamics.
Our sensitivity analysis revealed that the epidemiological impact of asymptomatic infections depends on a complex nonlinear interplay between their prevalence, detection rate, and relative infectiousness. While enhancing the detection of asymptomatic cases consistently reduced viral transmission, the impact of a higher prevalence of these cases was conditional on a period-specific infectiousness threshold, which rose in the later period of Omicron variant predominance as shorter infectious durations limited the overall opportunity for silent spread.
One of the strengths of this study lies in the utilization of detailed national-level data from Korea, coupled with a monthly parameter estimation approach that captures the evolving nature of the pandemic. The explicit modeling of asymptomatic states and the stratification by vaccination status allowed for insights into their respective roles of these groups in sustaining transmission. Furthermore, the strong concordance between model predictions and empirical data for both confirmed cases and deaths validates the overall approach and the plausibility of our parameter estimates, which also align broadly with values reported in other studies.
However, the study is subject to limitations. The parameter estimation method utilized the fitting criterion that integrates the least-squares trajectory matching with the gradient matching at the initial date where the parameter λ determines the relative importance of the gradient matching. There are some composite difficulties arising in the gradient matching part. The gradients of the state variables except the confirmed are not available. We assumed that they were effectively zeros at the initial fitting date, based on the observation that the initial fitting date falls on the latter part of the six-month-long lull. However, this assumption is rather weak and requires further empirical evidences. The parameter λ should be set reasonably small, otherwise it makes the model solution diverge from the actual trajectory with large forward prediction errors, due to overrated importance of the initial gradient matching and also due to the validity of the zero-gradient assumption. In contrast, it turned out that the fitting with λ = 0 (the fitting that only uses the trajectory data) leads to multiple possible parameter sets with similar size of errors. In the result presented in this paper, we chose a smallest possible value of λ, \({10}^{-9}\), which consistently produces a unique parameter estimation in the fitting. Future studies could extend the present framework by addressing the limitation that a relatively large number of parameters must be inferred from a small set of observable outputs (e.g., confirmed cases and deaths). Such complexity may limit the structural identifiability of the models and thus the precision of parameter estimation. To overcome this issue, future research should aim to incorporate additional epidemiological data sources—such as serological surveys, viral load information, or contact-tracing statistics—or to constrain certain parameters based on independently measured values from experimental or clinical studies. Simplified model formulations that preserve essential transmission dynamics while reducing parameter dimensionality may also improve interpretability and real-time applicability. These developments would strengthen the capacity of mathematical models like ours to inform public health responses and enhance preparedness for future infectious disease outbreaks.
Despite these limitations, this study provides valuable quantitative insights into the complex, time-varying epidemiology of COVID-19, particularly highlighting the significant and dynamic role of asymptomatic infections. The demonstrated interplay between viral evolution (variant characteristics), public health interventions (NPIs and testing strategies), and population immunity (vaccination and prior infection) underscores the need for adaptive public health policies.
In conclusion, our findings emphasize the critical importance of understanding and accounting for asymptomatic transmission in pandemic control efforts. The modeling framework and estimation methodology presented here offer a potentially valuable tool for estimating key epidemiological parameters, including those related to asymptomatic spread, which could be broadly applicable to the study and surveillance of other infectious diseases where silent transmission is a major factor. Continued monitoring and modeling efforts remain essential to navigate the ongoing challenges posed by COVID-19 and future respiratory virus threats.
Data availability
The summary of the data is included in Fig. 1. The COVID-19 outbreak data accessed from publicly available sources at https://dportal.kdca.go.kr/pot/cv/trend/dmstc/selectMntrgSttus.do. The vaccination information in Korea from 2021 to 2023 and information on the prevalence of virus variants were available at https://data.kma.go.kr/cmmn/main.do, and https://covariants.org/, respectively.
References
Caturano, V. et al. Estimating asymptomatic SARS-CoV-2 infections in a geographic area of low disease incidence. BMC Infect Dis. 21, 1–4. https://doi.org/10.1186/s12879-021-06054-2 (2021).
Nishiura, H. et al. The rate of underascertainment of novel coronavirus (2019-nCoV) infection: Estimation using Japanese passengers data on evacuation flights. J Clin Med. 9(2), 419. https://doi.org/10.3390/jcm9020419 (2020).
He, X. et al. Temporal dynamics in viral shedding and transmissibility of COVID-19. Nat Med. 26(5), 672–675. https://doi.org/10.1038/s41591-020-0869-5 (2020).
Ma, S. et al. Epidemiological parameters of COVID-19: Case series study. J Med Internet Res. 22(10), e19994. https://doi.org/10.2196/19994 (2020).
Oran, D. P. & Topol, E. J. Prevalence of asymptomatic SARS-CoV-2 infection: A narrative review. Ann Intern Med. 173(5), 362–367. https://doi.org/10.7326/M20-3012 (2020).
Byambasuren, O. et al. Estimating the extent of asymptomatic COVID-19 and its potential for community transmission: Systematic review and meta-analysis. J Assoc Med Microbiol Infect Dis Can. 5(4), 223–234. https://doi.org/10.3138/jammi-2020-0030 (2020).
Snider, B., Patel, B. & McBean, E. Asymptomatic cases, the hidden challenge in predicting COVID-19 caseload increases. Infect Dis Rep. 13(2), 340–347. https://doi.org/10.3390/idr13020033 (2021).
Mizumoto, K., Kagaya, K., Zarebski, A. & Chowell, G. Estimating the asymptomatic proportion of coronavirus disease 2019 (COVID-19) cases on board the Diamond Princess cruise ship, Yokohama, Japan, 2020. Euro Surveill. 25(10), 2000180. https://doi.org/10.2807/1560-7917.ES.2020.25.10.2000180 (2020).
Park, S. Y. et al. Coronavirus disease outbreak in call Center, South Korea emerging infectious diseases. Centers Disease Control Prev 26(8), 1666–16670. https://doi.org/10.3201/eid2608.201274 (2020).
Centers for Disease Control and Prevention. COVID-19 pandemic planning scenarios. Archived 6/28/2024. https://archive.cdc.gov/#/details?q=https://www.cdc.gov/coronavirus/2019-ncov/hcp/planning-scenarios.html&start=0&rows=10&url=https://www.cdc.gov/coronavirus/2019-ncov/hcp/planning-scenarios.html (Accessed 23 June 2025).
Huang, Z. et al. The rate of asymptomatic COVID-19 infection: A systematic review and meta-analysis including 12,713 infections from 136 studies. Res Sq. https://doi.org/10.21203/rs.3.rs-126538/v1 (2020).
Alene, M. et al. Magnitude of asymptomatic COVID-19 cases throughout the course of infection: A systematic review and meta-analysis. PLoS ONE 16(3), e0249090. https://doi.org/10.1371/journal.pone.0249090 (2021).
Ramsay, J. & Hooker, G. Dynamic data analysis: Modeling data with differential equations (Springer, Berlin, 2019).
Seber, G. A. F. & Wild, C. J. Nonlinear regression (Wiley, Hoboken, 2003).
Banks, H. T. & Tran, H. T. Mathematical and experimental modeling of physical and biological processes (CRC Press, Boca Raton, 2009).
Korea Disease Control and Prevention Agency. Cumulative confirmed COVID-19 cases (complete enumeration monitoring). https://dportal.kdca.go.kr/pot/cv/trend/dmstc/selectMntrgSttus.do
Statistics Korea; Public Data Portal. KDCA: COVID-19 vaccination statistics. https://www.data.go.kr/data/15131145/fileData.do?recommendDataYn=Y
Korea Disease Control and Prevention Agency. COVID-19 vaccination begins for the return to normal life. 2021 Feb 25. https://kdca.go.kr/board/board.es?mid=a20501010000&bid=0015&list_no=712528&cg_code=&act=view&nPage=1&newsField=202102 (accessed 23 June 2025).
Hodcroft EB. CoVariants: SARS-CoV-2 mutations and variants of interest. 2021 Mar. https://covariants.org/ (accessed 23 June 2025).
Seoul National University Hospital. White paper of Seoul National University Hospital. 2022. http://www.snuh.org/m/board/B003/view.do?bbs_no=5963& (accessed 23 June 2025).
Ahn, S. et al. Outbreak report of COVID-19 during designation of class 1 infectious disease in the Republic of Korea (January 20, 2020–April 24, 2022). Public Health Wkly Rep. 15(30), 2126–2136 (2022).
Ha, J.H., Lee, J.Y., Choi, S.Y., Park, S.K. COVID-19 waves and their characteristics in the Seoul Metropolitan Area (Jan 20, 2020–Aug 31, 2022). 2023.
Kim, I. H. et al. Status and characteristics of the COVID-19 variant virus outbreak in the Republic of Korea. Public Health Wkly Rep. 2021, 3388–3396 (2021).
Ryu, B. H. et al. Clinical features of adult COVID-19 patients without risk factors before and after the nationwide SARS-CoV-2 B.1.617.2 (Delta)-variant outbreak in Korea: Experience from Gyeongsangnam-do. J Korean Med Sci. 36(49), e341. https://doi.org/10.3346/jkms.2021.36.e341 (2021).
Kim, D. et al. Estimation of serial interval and reproduction number to quantify the transmissibility of SARS-CoV-2 omicron variant in South Korea. Viruses 14(3), 533. https://doi.org/10.3390/v14030533 (2022).
Buckner, J. H., Chowell, G. & Springborn, M. R. Dynamic prioritization of COVID-19 vaccines when social distancing is limited for essential workers. Proc Natl Acad Sci U S A. 118(16), e2025786118. https://doi.org/10.1073/pnas.2025786118 (2021).
Choi, Y., Kim, J. S., Choi, H., Lee, H. & Lee, C. H. Assessment of social distancing for controlling COVID-19 in Korea: An age-structured modeling approach. Int J Environ Res Public Health. 17(20), 7474. https://doi.org/10.3390/ijerph17207474 (2020).
Lee, H. R., Kim, R. K., Kim, J., Lee, J. J., Lee, H. Y., Park, H. et al. Analysis of the time from exposure to the diagnosis of Omicron infection to establish the ideal duration of quarantine for traced contacts. Public Health Weekly Report (COVID-19 Special Report), 5(7), 408–413, 2022. https://neweng-phwr.inforang.com/journal/view.html?uid=222 (accessed 15 November 2025)
Kim, J. E., Choi, H., Choi, Y. & Lee, C. H. The economic impact of COVID-19 interventions: A mathematical modeling approach. Front Public Health. 10, 993745. https://doi.org/10.3389/fpubh.2022.993745 (2022).
Lee, B. et al. An analysis of the waning effect of COVID-19 vaccinations. Genom Inform. 21(4), e50. https://doi.org/10.5808/gi.23070 (2023).
Langlete, P. et al. Estimated vaccine effectiveness against SARS-CoV-2 Delta and Omicron infections among health care workers and the general adult population in Norway, August 2021–January 2022. Vaccine. 41(26), 3923–3929. https://doi.org/10.1016/j.vaccine.2023.05.019 (2023).
Tchuem, C. R. T. et al. Vaccine effectiveness and duration of protection of COVID-19 mRNA vaccines against Delta and Omicron BA.1 symptomatic and severe COVID-19 outcomes in adults aged 50 years and over in France. Vaccine. 41(13), 2280–2288. https://doi.org/10.1016/j.vaccine.2023.01.066 (2023).
Menni, C. et al. Symptom prevalence, duration, and risk of hospital admission in individuals infected with SARS-CoV-2 during periods of omicron and delta variant dominance: A prospective observational study from the ZOE COVID Study. Lancet 399(10335), 1618–1624. https://doi.org/10.1016/S0140-6736(22)00327-0 (2022).
Robinson, L. et al. Time-varying effects of COVID-19 vaccination on symptomatic and asymptomatic infections in a prospective university cohort in the USA. BMJ Open 15(2), e084408. https://doi.org/10.1136/bmjopen-2023-084408 (2025).
Haq, M. A. et al. Antibody longevity and waning following COVID-19 vaccination in a 1-year longitudinal cohort in Bangladesh. Sci Rep. 14(1), 11467. https://doi.org/10.1038/s41598-024-58667-5 (2024).
Hernandez-Suarez, C. & Murillo-Zamora, E. Waning immunity to SARS-CoV-2 following vaccination or infection. Front Med 9, 972083. https://doi.org/10.3389/fmed.2022.972083 (2022).
Korea Disease Control and Prevention Agency. Booster dose is essential: Lifesaver for the elderly (Press briefing, Dec 16, 2021). 2021. https://www.kdca.go.kr/board/board.es?mid=a20501010000&bid=0015&list_no=717953&cg_code=&act=view&nPage=1&newsField=202112 (Accessed 23 June 2025).
Markov, P. V. et al. The evolution of SARS-CoV-2. Nat Rev Microbiol. 21(6), 361–379. https://doi.org/10.1038/s41579-023-00878-2 (2023).
Andre, M. et al. From alpha to omicron: How different variants of concern of the SARS-Coronavirus-2 impacted the world. Biology (Basel). 12(9), 1267. https://doi.org/10.3390/biology12091267 (2023).
Ritchey, M. D. COVID-19 self-test data: Challenges and opportunities—United States, October 31, 2021–June 11, 2022. MMWR Morb Mortal Wkly Rep. 71, 1004–1009. https://doi.org/10.15585/mmwr.mm7131a3 (2022).
Moonan, P. K. et al. Home-based testing and COVID-19 isolation recommendations United States. Emerg Infect Dis. 29(9), 1921–1929. https://doi.org/10.3201/eid2909.230685 (2023).
Watson, O. J. et al. Global impact of the first year of COVID-19 vaccination: A mathematical modelling study. Lancet Infect Dis. 22(9), 1293–1302. https://doi.org/10.1016/S1473-3099(22)00320-6 (2022).
Tenforde, M. W. Effectiveness of mRNA vaccination in preventing COVID-19–associated invasive mechanical ventilation and death—United States, March 2021–January 2022. MMWR Morb Mortal Wkly Rep. 71, 459–465. https://doi.org/10.15585/mmwr.mm7110e1 (2022).
Fatima, S. et al. COVID-19 infection among vaccinated and unvaccinated: Does it make any difference?. PLoS ONE 17(7), e0270485. https://doi.org/10.1371/journal.pone.0270485 (2022).
Feng, S. et al. Correlates of protection against symptomatic and asymptomatic SARS-CoV-2 infection. Nat Med. 27(11), 2032–2040. https://doi.org/10.1038/s41591-021-01540-1 (2021).
Shang, W. et al. Percentage of asymptomatic infections among SARS-CoV-2 omicron variant-positive individuals: A systematic review and meta-analysis. Vaccines (Basel). 10(7), 1049. https://doi.org/10.3390/vaccines10071049 (2022).
Korea Disease Control and Prevention Agency. COVID-19 response white paper. 2023. https://www.kdca.go.kr/board/board.es?mid=a20504000000&bid=0014&list_no=727575&act=view (accessed 23 June 2025).
Zi, Z. Sensitivity analysis approaches applied to systems biology models. IET Syst. Biol. 5(6), 336–346 (2011).
Qian, G. & Mahdi, A. Sensitivity analysis methods in the biomedical sciences. Math. Biosci. 323, 108306 (2020).
Marino, S., Hogue, I. B., Ray, C. J. & Kirschner, D. E. A methodology for performing global uncertainty and sensitivity analysis in systems biology. J. Theor. Biol. 254(1), 178–196 (2008).
Rodrigues, H. S., Monteiro, M. T. T., & Torres, D. F. Sensitivity analysis in a dengue epidemiological model. In Conference papers in science (Vol. 2013, No. 1, p. 721406). Hindawi Publishing Corporation. (2013)
Cho, G., Park, J. R., Choi, Y., Ahn, H. & Lee, H. Detection of COVID-19 epidemic outbreak using machine learning. Front. Public Health 11, 1252357 (2023).
Jarju, S. et al. High SARS-CoV-2 incidence and asymptomatic fraction during Delta and Omicron BA. 1 waves in The Gambia. Nat Commun 15(1), 3814 (2024).
Yu, W. et al. Proportion of asymptomatic infection and nonsevere disease caused by SARS-CoV-2 Omicron variant: A systematic review and analysis. J. Med. Virol. 94(12), 5790–5801 (2022).
Mitchell, E. C. et al. Home testing for SARS-CoV-2 and impact on surveillance in New York State. Ann Epidemiol 91, 74–81 (2024).
Funding
P. Kim and C.H. Lee were supported by the BK21 Program (Next Generation Education Program for Mathematical Sciences, 4299990414089), funded by the Ministry of Education (MOE, Korea) and National Research Foundation of Korea (NRF). Y. Choi was supported by a National Research Foundation (NRF) of Korea grant funded by the Korean Government (MSIT) (No. NRF-2022R1A5A1033624, RS-2024–00407300). P. Kim was supported by National Research Foundation of Korea (NRF) grant funded by the Korea government (2017R1D1A1B04032921). C.H. Lee was supported by National Research Foundation of Korea (NRF) grant funded by the Korea government (MSIT) (2022R1F1A1064487, RS-2025-21072981).
Author information
Authors and Affiliations
Contributions
Yongin Choi: Formal analysis, Data curation, Methodology, Visualization, Writing – original draft, Writing – review and editing. Pilwon Kim: Project administration, Methodology, Formal analysis, Writing – original draft, Writing – review and editing. Chang Hyeong Lee: Conceptualization, Supervision, Formal analysis, Writing – original draft, Writing – review and editing.
Author Summary
This study developed a novel mathematical model using COVID-19 data of South Korea to estimate key parameters of asymptomatic infections. The findings reveal a significant progressive increase in both the infectivity and the proportion of asymptomatic cases from the initial strain to the Delta and Omicron variants. Notably, vaccinated individuals consistently showed a higher probability of asymptomatic infection compared to their unvaccinated counterparts across all pandemic phases. The significance of this research lies in providing valuable insights into the hidden dynamics of COVID-19 transmission, highlighting the crucial role of asymptomatic infections. The model offers a robust methodology for quantifying these dynamics, which can be adapted for other infectious diseases. The implications underscore the necessity for robust detection systems to control silent transmission and for ongoing surveillance strategies that account for asymptomatic spread, even within vaccinated populations, to effectively manage future pandemics.
Corresponding authors
Ethics declarations
Competing interests
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Ethics approval
This study did not require any ethical approval as it is computational and theoretical work, based on mathematical modeling and analysis of publicly available and fully anonymized data.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Choi, Y., Kim, P. & Lee, C.H. Estimation of undetected asymptomatic infections of COVID-19: a mathematical modeling approach. Sci Rep 15, 45719 (2025). https://doi.org/10.1038/s41598-025-28374-y
Received:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s41598-025-28374-y