Abstract
Early detection of hypertension remains challenging due to its asymptomatic nature and limited screening tools. While surrogate indices of insulin resistance (IR) have emerged as potential screening tools for various metabolic disorders, their predictive value for hypertension remains unclear, particularly in different demographic groups. In this cross-sectional study, we analyzed data from 1,840 adults (aged 35–70 years) participating in the PERSIAN Dena cohort study (PDCS). Eight IR indices were evaluated: triglyceride-to-high-density lipoprotein cholesterol ratio (TG/HDL-C), visceral adiposity index (VAI), lipid accumulation product (LAP), triglyceride-glucose index (TyG), TyG-body mass index (TyG-BMI), TyG-waist circumference (TyG-WC), metabolic score for insulin resistance (METS-IR), and atherogenic index of plasma (AIP). Associations were assessed using adjusted binary logistic regression and receiver operating characteristic (ROC) curve analyses, controlling for age, sex, and other potential confounders (P < 0.05). Among the study participants, 171 (9.3%) had hypertension. After adjusting for confounders, six surrogate indices were significantly associated with hypertension in males: TyG-index (odds ratio [OR]: 2.58, P < 0.001), TyG-BMI (OR: 3.53, P < 0.001), TyG-WC (OR: 2.76, P < 0.001), VAI (OR: 1.8, P = 0.008), LAP (OR: 2.7, P < 0.001), and METS-IR (OR: 2.5, P < 0.001). Notably, TyG-BMI demonstrated the strongest association and highest predictive power (AUC: 0.712). In females, only TyG-BMI remained an independent factor associated with hypertension (OR: 2.0, P = 0.029, AUC: 0.623). Overall, TyG-BMI exhibited the highest effectiveness across all participants (AUC: 0.66, Youden index: 0.26). This study demonstrates that surrogate IR indices, particularly TyG-BMI, may serve as valuable screening tools for hypertension risk, especially in males. The sex-specific differences in these associations suggest the need for tailored screening approaches. Further research is needed to validate their clinical applications and establish population-specific thresholds.
Similar content being viewed by others
Introduction
Hypertension, characterized by persistently elevated arterial pressure, is a widespread chronic condition that leads to serious health consequences, including stroke, myocardial infarction, heart failure, and kidney failure, contributing significantly to mortality and disability worldwide1. The current consensus defines hypertension as sustained systolic blood pressure (SBP) and diastolic blood pressure (DBP) readings of 140/90 mmHg or higher. Treatment is necessary to achieve readings of 130/80 mmHg or lower2. Hypertension affects more than one in every eight adults worldwide and increases to 60% in those aged 60 years and above, regardless of socioeconomic status or income level3.
Insulin resistance, in which the body’s cells become less responsive to insulin, leads to impaired glucose uptake and utilization, acting as a major risk factor for type 2 diabetes and cardiovascular disease. The body responds by producing excess insulin to maintain blood glucose homeostasis, which can trigger chronic metabolic conditions, including hyperinsulinemia, now a significant public health concern globally4,5. Understanding the complex interaction between insulin resistance and other metabolic dysregulations is essential for comprehending the development of chronic non-communicable diseases.
Although direct methods like the hyperinsulinemic-euglycemic clamp are considered the gold standard for measuring insulin sensitivity, they are not practical for everyday clinical use or large-scale epidemiological studies. Therefore, alternative models using fasting glucose and insulin levels, such as the Homeostatic Model Assessment (HOMA) and the Quantitative Insulin Sensitivity Check Index (QUICKI), as well as the oral glucose tolerance test (OGTT), are commonly used to provide indirect estimates of insulin resistance. However, these indirect indices show varying reference values depending on age, sex, and ethnicity, limiting their clinical applicability6.
Recent years have witnessed the emergence of novel and accessible tools for predicting insulin resistance. The Triglyceride-to-High-Density Lipoprotein Cholesterol (TG/HDL-C) ratio, derived from simple blood lipid parameters, effectively predicts insulin resistance and cardiovascular disease risk7. Additionally, the Triglyceride-Glucose (TyG) index, calculated from fasting triglyceride and glucose levels, along with TyG-BMI, TyG-WC, and the Metabolic Score for Insulin Resistance (METS-IR), has emerged as a reliable surrogate marker when compared to HOMA-IR, capable of accurately evaluating insulin resistance in individuals with or without diabetes8. Furthermore, the Visceral Adiposity Index (VAI) and Lipid Accumulation Product (LAP) serve as reliable indicators of visceral adiposity9,10,11. The TyG index and TG/HDL-C ratio are particularly notable for being simple, cost-effective, reproducible, and dependable tools for assessing insulin resistance12.
Given the steady rise of hypertension in Iran and the lack of comprehensive studies at the provincial level in Kohgiluyeh and Boyer-Ahmad, this study sought to investigate the relationship between alternative indices of insulin resistance and blood pressure status among adults participating in the PERSIAN Dena cohort study (PDCS).
Materials and methods
Study design and participants
This cross-sectional study was conducted among the adult population using baseline phase data from the PDCS13,14. The Prospective Epidemiological Research Studies in Iran (PERSIAN) Cohort (http://persiancohort.com) is a large longitudinal study focused on non-communicable diseases, encompassing various geographical, ethnic, and climatic groups across 18 provinces of Iran. The PDCS includes nearly all individuals aged 35 to 70 years residing in both urban and rural areas of Dena County, which is located near Yasuj city in the Kohgiluyeh and Boyer-Ahmad Province in southwestern Iran14. Baseline phase data were collected from February 2016 to March 2018.
The inclusion criteria required that residents have lived in the study area for at least one year, possess adequate physical and mental capacity to participate in the evaluation program, and provide written consent. Participants were excluded if they were lactating or pregnant, following specific diets, or suffering from conditions such as cancer, liver diseases, stroke, or severe kidney failure requiring specialized medications15. More detailed information regarding the study design, participants, and data collection methods has been published previously14. Additionally, participants who reported daily energy intake below 800 Kcal or above 4800 Kcal were excluded from the study.
Ethics approval and consent to participate
This study was conducted by the principles of the Declaration of Helsinki. The study protocol was approved by the Research Ethics Committee of Yasuj University of Medical Sciences (Ethics code: IR.YUMS.REC.1403.030). All participants provided written informed consent and were informed of their right to withdraw from the study at any time.
Dietary assessment
Dietary intake was assessed using a validated 113-item Food Frequency Questionnaire (FFQ), supplemented with a 127-item FFQ specifically designed for indigenous foods16,17. Participants reported their food consumption frequency (daily, weekly, monthly, seasonal, or annual) and portion sizes over the past year. Foods were categorized into nine groups: breads and cereals, legumes, meats, vegetables, fruits, dairy products, sugars, oils, nuts and seeds, and miscellaneous items. All portion sizes were converted to grams per day, and nutrient intake was calculated using NUTRITIONIST IV software (N-Squared Computing, Salem, OR, USA), modified for Iranian populations.
Assessment of physical activity level
Physical activity levels (PAL) were evaluated using the International Physical Activity Questionnaire (IPAQ). The questionnaire assessed various forms of physical activity, including work-related and leisure activities. Total physical activity was calculated in MET hours per week by multiplying the duration of each activity by its corresponding metabolic equivalent (MET) factor18.
Blood pressure measurement
Blood pressure was measured using a mercury sphygmomanometer (Richter, MTM Munich, Germany) on both arms at 15-minute intervals. Measurements were taken while participants were seated comfortably with their arms supported at heart level. Participants were instructed to avoid tea, coffee, smoking, and strenuous activities before measurement. The average of these readings was recorded as the final blood pressure value.
Blood pressure classifications were as follows:
-
Normal: SBP < 120 mmHg and DBP < 80 mmHg.
-
Prehypertension: SBP 120–139 mmHg and DBP 80–89 mmHg.
-
Hypertension: SBP ≥ 140 mmHg and/or DBP ≥ 90 mmHg, or use of antihypertensive medications19.
Anthropometric measurements
Weight and height were measured using standardized equipment (Seca 765 1021949, Germany). Weight was recorded with participants wearing minimal clothing and no shoes. Body Mass Index (BMI) was calculated as weight in kilograms divided by height in meters squared. WC was measured at the midpoint between the greater trochanter and iliac crest using a flexible measuring tape14.
Biochemical measurements
Venous blood samples were collected after overnight fasting and centrifuged at 3000 RPM for 8 min at 4 °C. Serum samples were stored at −80 °C until analysis. Parameters measured included fasting blood glucose (FBG), total cholesterol, triglycerides, HDL-C, LDL-C, blood urea nitrogen (BUN), creatinine, alanine aminotransferase (ALT), alkaline phosphatase (ALP), gamma-glutamyl transferase (GGT), and aspartate aminotransferase (AST). All measurements were performed using Pars Azmoon kits (Pars Azmoon Co, Iran) on a biochemistry autoanalyzer (BT1500, 47173738, Italy) and hematology cell counter (Sysmex, XP B0463, Japan)14. Estimated glomerular filtration rate (eGFR) was calculated using the CKD-Epidemiology Collaboration (CKD-EPI) equation: eGFR = GFR = 175 × standardized Scr − 1.154 × age − 0.203 × 1.212 (if black) × 0.742 (if female), where Scr represents serum creatinine. To align with the CKD-EPI equation requirements, serum creatinine values were adjusted downward by 5% before calculating eGFR20,21.
Insulin resistance indices
Eight surrogate indices of insulin resistance were calculated using the following formulas10,22,23,24:
-
1.
TG/HDL-C ratio = Serum triglycerides (mg/dL)/HDL-C (mg/dL).
-
2.
Visceral Adiposity Index (VAI):
-
Male: (WC [cm]/(39.68 + (1.88 × BMI)) × (TG [mM/L]/1.03) × (1.31/HDL-C [mM/L])
-
Female: (WC [cm]/36.58 + (1.89 × BMI)) × (TG [mM/L]/0.81) × (1.52/HDL-C [mM/L])
-
-
3.
Lipid Accumulation Product (LAP):
-
Male: (WC [cm] − 65) × TG [mM/L].
-
Female: (WC [cm] − 58) × TG [mM/L].
-
-
4.
TyG-BMI = TyG index × BMI.
-
5.
TyG-WC = TyG index × WC.
-
6.
METS-IR = (Ln [2 × FBG (mg/dL) + TG (mg/dL)] × BMI)/(Ln [HDL-C (mg/dL)])
-
7.
AIP = log (TG [mmol/L]/HDL-C[mmol/L])
Socioeconomic status assessment
The Wealth Status Index (WSI) was derived from nine household characteristics: ownership of a washing machine, computer, vacuum cleaner, freezer, motorcycle, car, and home, along with internet access and the number of people per room25. The calculation methodology involved a comprehensive correlation analysis between these variables, followed by coefficient assignments based on correlation strengths. The final index score was computed by multiplying these coefficients by their corresponding variable values. Based on these calculations, participants were categorized into three socioeconomic groups: poor, average, and wealthy.
Statistical analyses
Statistical analyses were performed using SPSS software version 26 (SPSS Inc., Chicago, IL, USA). The Kolmogorov-Smirnov test was used to assess data normality. Values deviating beyond 3 standard deviations from the mean were considered outliers. Continuous variables were expressed as mean ± standard deviation (SD), while categorical variables were presented as counts and percentages. For variables with normal distribution, differences between blood pressure levels were evaluated using the Independent Two Samples T-Test, while the Mann-Whitney U test was applied for non-normally distributed variables. Binary logistic regression was used to calculate odds ratios (ORs) with 95% confidence intervals (95% CI) for hypertension in both unadjusted and adjusted models. The adjusted models accounted for age, sex, FBG (only for AIP and TG/HDL), ALP, ALK, AST, and GGT.
The predictive ability of insulin resistance surrogate indicators was assessed using receiver operating characteristic (ROC) curve analysis. Optimal cut-off points were determined by maximizing the Youden index (sensitivity + specificity − 1)26. Statistical significance was set at P < 0.05.
Results
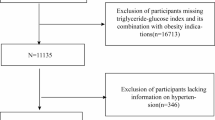
The participant selection process is illustrated in Fig. 1. Of the initial 3,203 participants, 1,363 were excluded based on predetermined criteria, resulting in 1,840 eligible participants for the final analysis. Of these, 171 (9.3%) had prehypertension/hypertension, and 1,669 (90.7%) had normal blood pressure.
Table 1 presents the baseline characteristics of the study population. The prehypertensive/hypertensive group was significantly older and had higher BMI, WC, and waist-to-hip ratio (WHR) than the normotensive group (P < 0.01). Among biochemical parameters, FBG, liver enzymes (AST, ALT, GGT, ALP), triglycerides, and total cholesterol were significantly higher in the hypertensive group (P < 0.05), while HDL and LDL cholesterol remained comparable between groups.
Table 2 shows the demographic characteristics of participants. Hypertension prevalence was higher in males compared to females (11.1% vs. 7.2%, P = 0.004), while residential status, education level, and smoking status showed no significant differences between groups.
Table 3 demonstrates the association between insulin resistance indices and hypertension risk. In the adjusted models, the TyG index, TyG-BMI, TyG-WC, VAI, LAP, and METS-IR showed significant associations with hypertension in males, with TyG-BMI demonstrating the strongest association (OR: 3.53, P < 0.001).
Table 4; Fig. 2 present the receiver operating characteristic analysis results. TyG-BMI emerged as the strongest predictor of hypertension in both the overall population and males, with AUC values of 0.660 and 0.712, respectively. While TyG-BMI maintained the highest predictive value in females (AUC: 0.623), several indices, including TyG, VAI, and TG/HDL-C, showed no significant predictive ability in females. Sex-specific ROC curves for all eight insulin resistance indices are presented in Supplementary Figs. 1 and 2, demonstrating the differential predictive abilities between males and females, with consistently higher AUC values observed in males across all indices.
Receiver operating characteristic (ROC) analysis of insulin resistance surrogate indices for hypertension prediction. AIP atherogenic index of plasma, LAP lipid accumulation product, MET-IRR metabolic score for insulin resistance, TyGindex, triglyceride glucose, TyG-BMI TyG index with body mass index, TyG-WC TyG index with waist circumference, TG/HDL-C the ratio of triglycerides to high-density lipoprotein cholesterol ratio, VAI visceral obesity index.
Discussion
In this cross-sectional study, we investigated the relationship between various surrogate indices of insulin resistance and hypertension in adults using data from the Dena Cohort Study, part of the broader PERSIAN Cohort Study. Our findings revealed that the hypertensive group had significantly higher anthropometric indices (BMI, WC, WHR), blood pressure measurements (systolic and diastolic), metabolic parameters (fasting blood glucose, cholesterol, triglycerides), and all eight surrogate indices of insulin resistance. Age was also significantly higher in this group, while physical activity, BUN, HDL-C, and LDL-C did not significantly differ between groups.
The risk of hypertension was significantly elevated across all eight insulin resistance indices in both crude and adjusted models, particularly in males. Among these indices, TyG-BMI and MET-IR demonstrated particularly robust associations, highlighting the potential importance of insulin resistance management in cardiovascular health, specifically among males. Our findings are consistent with previous studies. A cross-sectional study in Romania reported that high levels of TG/HDL-C, VAI, LAP, TyG, TyG-BMI, and TyG-WC were associated with an increased risk of hypertension27. Similarly, a study of Thai police officers found that higher values of all eight insulin resistance indices correlated with elevated hypertension risk, with TyG-BMI showing the highest accuracy and VAI the lowest in predicting hypertension risk22. These findings align with our results, despite the differences in study populations. It is important to note that the observed AUC values (0.56–0.71) indicate moderate predictive ability, consistent with the multifactorial nature of hypertension. These indices should be interpreted as practical screening tools for population-level risk stratification rather than definitive diagnostic markers, and are best used in combination with other clinical parameters for comprehensive cardiovascular risk assessment.
Additional support comes from the Ravansar non-communicable diseases study (RaNCD) in Iran, which demonstrated TyG-BMI’s significant discriminatory effect on hypertension28, and the Rich Healthcare Group study in China, which identified TyG-BMI as the strongest predictor of hypertension prevalence29. While two prospective studies have shown VAI to be a strong predictor of incident hypertension30,31, they did not adjust for confounding factors, unlike our analysis. In contrast, a cross-sectional study of American adults found no association between LAP and systolic blood pressure, though LAP significantly affected other cardiovascular risk factors32. However, a seven-year cohort study of individuals with a family history of type 2 diabetes found that elevated VAI and hypertriglyceridemic-waist increased hypertension risk, even after adjusting for confounders33.
The pathophysiology of hypertension is multifactorial, encompassing increased sodium retention, volume overload, dysregulation of the renin-angiotensin-aldosterone system (RAAS), and heightened sympathetic nervous system (SNS) activity34. Insulin resistance plays a central mechanistic role in hypertension development through several interconnected pathways. First, hyperinsulinemia resulting from insulin resistance directly stimulates renal sodium reabsorption in the proximal tubules, leading to volume expansion and increased cardiac output. Second, insulin resistance impairs endothelial nitric oxide production, reducing vasodilation capacity and increasing peripheral vascular resistance. Third, compensatory hyperinsulinemia enhances SNS activity, promoting vasoconstriction and increasing heart rate. Fourth, insulin resistance promotes oxidative stress and inflammatory cytokine release from adipose tissue, contributing to vascular remodeling and arterial stiffness35. These alterations lead to increased peripheral resistance and elevated afterload, ultimately contributing to hypertension development.
The superior predictive performance of TyG-BMI in our study can be explained by its unique ability to capture both metabolic dysregulation (through the TyG component reflecting glucose-lipid metabolism) and overall adiposity (through BMI). This composite index effectively integrates information about insulin resistance severity with body composition, making it a more comprehensive marker than indices based solely on lipid parameters or anthropometric measurements. The strong association observed particularly in males may reflect sex-specific differences in fat distribution patterns, with males typically exhibiting more visceral adiposity, which is more metabolically active and closely linked to insulin resistance than subcutaneous fat36.
A key mechanism linking obesity, particularly abdominal obesity, to hypertension is its relationship with insulin resistance and hyperinsulinemia, which represents a major cause of hypertension in overweight and obese individuals37. Obesity additionally promotes pro-inflammatory and oxidative processes, further exacerbating insulin resistance. Expanded adipose tissue significantly impacts renal function, disrupting blood pressure regulation. The primary mechanism involves inappropriate activation of RAAS and SNS through adipose tissue’s autocrine and paracrine actions, resulting in renal microvascular remodeling, increased vascular stiffness, and enhanced sodium retention. The observed associations between composite indices like TyG-BMI and TyG-WC and blood pressure can be attributed to obesity’s impact on insulin sensitivity38,39,40. While increased adipose tissue may compensate for hyperinsulinemia, this adaptation paradoxically increases blood pressure risk. These complex interactions underscore why anthropometric-metabolic composite indices demonstrate superior predictive ability compared to single-component markers. Despite methodological variations across studies, a consistent association exists between insulin resistance indices and hypertension. However, results should be interpreted with caution, as potential biases and heterogeneous outcomes can exist. The prediction of multifactorial diseases like cardiovascular conditions requires consideration of complex interactions between genetic and environmental factors, which traditional analyses may not fully capture41.
Our study has several limitations. First, its cross-sectional nature precludes the establishment of causal relationships, necessitating long-term prospective cohort studies. Second, the lack of insulin level measurements in PDCS participants prevented comparisons with common insulin resistance indices such as HOMA and QUICKI. Third, all surrogate indices were measured at a single time point, which may not fully capture individual variability in insulin resistance status. Fourth, generalizing our results to the entire country requires integration with findings from other regional cohort studies. However, our study strengths include a large sample size, recruitment from a relatively homogeneous community with low migration rates, which facilitates better control of confounding factors. Additionally, the exclusion of patients with cancers, liver and kidney diseases allowed us to focus on the direct relationship between study variables and blood pressure.
Conclusion
This study demonstrates significant associations between insulin resistance indices and hypertension risk, particularly in males. These findings suggest that improving insulin sensitivity may be crucial for preventing or delaying hypertension onset. Future research should focus on longitudinal studies examining the temporal relationship between insulin resistance and hypertension, and investigating underlying mechanisms, including inflammation, oxidative stress, and vascular dysfunction. Additionally, the potential use of insulin resistance indices as early biomarkers for hypertension risk warrants further investigation. Healthcare systems should consider incorporating these indices into screening programs, particularly for high-risk populations, to support early intervention strategies.
Data availability
On reasonable request, the corresponding author will provide the datasets used and analyzed during the current work.
Abbreviations
- AUC:
-
Area under the curve
- AIP:
-
Atherogenic index of plasma
- ALT:
-
Alanine aminotransferase
- ALP:
-
Alkaline phosphatase
- AST:
-
Aspartate aminotransferase
- BUN:
-
Blood urea nitrogen
- BMI:
-
Body mass index
- CI:
-
Confidence interval
- DBP:
-
Diastolic blood pressure
- FBG:
-
Fasting blood glucose
- FFQ:
-
Food frequency questionnaire
- GGT:
-
Gamma-glutamyl transferase
- HDL-C:
-
High-density lipoprotein cholesterol
- HOMA:
-
Homeostatic model assessment
- IPAQ:
-
International physical activity questionnaire
- IR:
-
Insulin resistance
- LAP:
-
Lipid accumulation product
- LDL-C:
-
Low-density lipoprotein cholesterol
- MET:
-
Metabolic equivalent
- METS-IR:
-
Metabolic score for insulin resistance
- OR:
-
Odds ratio
- OGTT:
-
Oral glucose tolerance test
- PDCS:
-
Persian dena cohort study
- PAL:
-
Physical activity level
- PERSIAN:
-
Prospective epidemiological research studies in Iran
- QUICKI:
-
Quantitative insulin sensitivity check index
- RaNCD:
-
Ravansar non-communicable diseases study
- ROC:
-
Receiver operating characteristic
- RAAS:
-
Renin-angiotensin-aldosterone system
- SD:
-
Standard deviation
- SNS:
-
Sympathetic nervous system
- SBP:
-
Systolic blood pressure
- TC:
-
Total cholesterol
- TG:
-
Triglyceride
- TyG:
-
Triglyceride-glucose index
- TyG-BMI:
-
TyG-body mass index
- TyG-WC:
-
TyG-waist circumference
- TG/HDL-C:
-
Triglyceride-to-high-density lipoprotein cholesterol ratio
- VAI:
-
Visceral adiposity index
- WC:
-
Waist circumference
- WHR:
-
Waist-to-hip ratio
- WSI:
-
Wealth status index
References
Whelton, P. K. et al. 2017 ACC/AHA/AAPA/ABC/ACPM/AGS/APhA/ASH/ASPC/NMA/PCNA guideline for the prevention, detection, evaluation, and management of high blood pressure in adults: a report of the american college of cardiology/american heart association task force on clinical practice guidelines. J. Am. Coll. Cardiol. 71, e127–e248 (2018).
Chianelli, M. et al. Obesity in prediabetic patients: management of metabolic complications and strategies for prevention of overt diabetes. Endocr. Metabolic Immune Disorders-Drug Targets. 25, 8–36 (2025).
Danaei, G. et al. The preventable causes of death in the united states: comparative risk assessment of dietary, lifestyle, and metabolic risk factors. PLoS Med. 6, e1000058 (2009).
Dhondge, R. H., Agrawal, S., Patil, R., Kadu, A. & Kothari, M. A comprehensive review of metabolic syndrome and its role in cardiovascular disease and type 2 diabetes mellitus: Mechanisms, risk factors, and management. Cureus https://doi.org/10.7759/cureus.67428 (2024).
Petrie, J. R., Guzik, T. J. & Touyz, R. M. Diabetes, hypertension, and cardiovascular disease: clinical insights and vascular mechanisms. Can. J. Cardiol. 34, 575–584 (2018).
Muniyappa, R., Lee, S., Chen, H. & Quon, M. J. Current approaches for assessing insulin sensitivity and resistance in vivo: advantages, limitations, and appropriate usage. Am. J. Physiology-Endocrinology Metabolism. 294, E15–E26 (2008).
Mercado-Gonzales, S. I., Carpio-Rodríguez, A. N., Carrillo-Larco, R. M. & Bernabé-Ortiz, A. Sleep duration and risk of obesity by sex: nine-year follow-up of the young lives study in Peru. Child. Obes. 15, 237–243 (2019).
Miura, Y. et al. Prevalence and clinical implication of metabolic syndrome in chronic heart Failure–Report from MetS-CHF Study–. Circ. J. 74, 2612–2621 (2010).
Fu, W. et al. Association of adiposity with diabetes: a National research among Chinese adults. Diab./Metab. Res. Rev. 37, e3380 (2021).
Wan, H. et al. Associations between abdominal obesity indices and diabetic complications: Chinese visceral adiposity index and neck circumference. Cardiovasc. Diabetol. 19, 1–12 (2020).
Han, M. et al. Chinese visceral adiposity index: a reliable indicator of visceral fat function associated with risk of type 2 diabetes. Diab./Metab. Res. Rev. 37, e3370 (2021).
Paulmichl, K. et al. Modification and validation of the Triglyceride-to–HDL cholesterol ratio as a surrogate of insulin sensitivity in white juveniles and adults without diabetes mellitus: the single point insulin sensitivity estimator (SPISE). Clin. Chem. 62, 1211–1219 (2016).
Poustchi, H. et al. Prospective epidemiological research studies in Iran (the PERSIAN cohort Study): rationale, objectives, and design. Am. J. Epidemiol. 187, 647–655 (2018).
Harooni, J. et al. Cohort profile: the PERSIAN dena cohort study (PDCS) of non-communicable diseases in Southwest Iran. BMJ open. 14, e079697 (2024).
Hamedi-Shahraki, S., Jowshan, M. R., Zolghadrpour, M. A., Amirkhizi, F. & Asghari, S. Dietary phytochemical index is favorably associated with oxidative stress status and cardiovascular risk factors in adults with obesity. Sci. Rep. 13, 7035 (2023).
Eghtesad, S. et al. Validity and reproducibility of the PERSIAN cohort food frequency questionnaire: assessment of major dietary patterns. Nutr. J. 23, 35 (2024).
Eghtesad, S. et al. Validity and reproducibility of a food frequency questionnaire assessing food group intake in the PERSIAN cohort study. Front. Nutr. 10, 1059870 (2023).
Moghaddam, M. B. et al. The Iranian version of international physical activity questionnaire (IPAQ) in iran: content and construct validity, factor structure, internal consistency and stability. World Appl. Sci. J. 18, 1073–1080 (2012).
Shakeri Shamsi, F. & Taheri Soodejani, M. Platelet Indices and Hypertension: Results from Shahedieh Cohort Study, Yazd, Iran. Int. J. Hypertens. 2024, 3705771 (2024).
Akbari, M. et al. Effect of sleep characteristics on rapid estimated GFR decline in adults with normal kidney function. Kidney Int. Rep. https://doi.org/10.1016/j.ekir.2025.03.042 (2025).
Levey, A. S. et al. Expressing the modification of diet in renal disease study equation for estimating glomerular filtration rate with standardized serum creatinine values. Clin. Chem. 53, 766–772 (2007).
Rattanatham, R. et al. Assessment of eight insulin resistance surrogate indexes for predicting metabolic syndrome and hypertension in Thai law enforcement officers. PeerJ 11, e15463 (2023).
Su, W. et al. Metabolic score for insulin Resistance, a novel score to evaluate insulin sensitivity, is associated with the urinary albumin-to‐creatinine ratio in Chinese adults: A cross‐sectional REACTION study. J. Diabetes Invest. 13, 1222–1234 (2022).
Shi, Y. & Wen, M. Sex-specific differences in the effect of the atherogenic index of plasma on prediabetes and diabetes in the NHANES 2011–2018 population. Cardiovasc. Diabetol. 22, 19 (2023).
Sadeghianpour, Z., Cheraghian, B., Farshchi, H. R. & Asadi-Lari, M. Non-alcoholic fatty liver disease and socioeconomic determinants in an Iranian cohort study. BMC Gastroenterol. 23, 350 (2023).
Menber, Y., Gashaw, S., Belachew, T. & Fentahun, N. Validation of the minimum dietary diversity for women as a predictor of micronutrient adequacy among lactating women in Ethiopia. Front. Nutr. 11, 1459041 (2024).
Bala, C. et al. The association between six surrogate insulin resistance indexes and hypertension: a population-based study. Metab. Syndr. Relat. Disord. 17, 328–333 (2019).
Nikbakht, H. R. et al. Triglyceride glucose-body mass index and hypertension risk in Iranian adults: a population-based study. BMC Endocr. Disorders. 23, 156 (2023).
Cheng, W., Kong, F. & Chen, S. Comparison of the predictive value of four insulin resistance surrogates for the prevalence of hypertension: a population-based study. Diabetol. Metab. Syndr. 14, 137 (2022).
Zhang, Z. et al. Visceral adiposity index (VAI), a powerful predictor of incident hypertension in prehypertensives. Intern. Emerg. Med. 13, 509–516 (2018).
Fiorentino, T. V. Visceral adiposity index (VAI), a powerful predictor of incident hypertension in prehypertensives. Intern. Emerg. Med. 13, 471–473 (2018).
Kahn, H. S. The lipid accumulation product performs better than the body mass index for recognizing cardiovascular risk: a population-based comparison. BMC Cardiovasc. Disord. 5, 1–10 (2005).
Janghorbani, M., Aminorroaya, A. & Amini, M. Comparison of different obesity indices for predicting incident hypertension. High. Blood Press. Cardiovasc. Prev. 24, 157–166 (2017).
Goff Jr, D. C. et al. 2013 ACC/AHA guideline on the assessment of cardiovascular risk: a report of the american college of cardiology/american heart association task force on practice guidelines. Circulation 129, S49–S73 (2013).
Sakr, H. F., Sirasanagandla, S. R., Das, S., Bima, A. I. & Elsamanoudy A. Z. Insulin resistance and hypertension: mechanisms involved and modifying factors for effective glucose control. Biomedicines 11, 2271 (2023).
Valencak, T. G., Osterrieder, A. & Schulz, T. J. Sex matters: the effects of biological sex on adipose tissue biology and energy metabolism. Redox Biol. 12, 806–813 (2017).
Masuo, K., Rakugi, H., Ogihara, T., Esler, M. D. & Lambert, G. W. Cardiovascular and renal complications of type 2 diabetes in obesity: role of sympathetic nerve activity and insulin resistance. Curr. Diabetes. Rev. 6, 58–67 (2010).
Wang, K. et al. Association of triglyceride-glucose index and its interaction with obesity on hypertension risk in chinese: a population-based study. J. Hum. Hypertens. 35, 232–239 (2021).
Rao, A., Pandya, V. & Whaley-Connell, A. Obesity and insulin resistance in resistant hypertension: implications for the kidney. Adv. Chronic Kidney Dis. 22, 211–217 (2015).
Morales-Villegas, E. Dyslipidemia, hypertension and diabetes metaflammation: a unique mechanism for 3 risk factors. Curr. Hypertens. Reviews. 9, 278–296 (2013).
Mirjalili, S. R. et al. Which surrogate insulin resistance indices best predict coronary artery disease? A machine learning approach. Cardiovasc. Diabetol. 23, 1–13 (2024).
Acknowledgements
We thank all participants of this study and the PDCS staff for their support. This work was supported by Yasuj University of Medical Sciences, Vice-Chancellor for Research and Technology.
Funding
This study was financially supported by Yasuj University of Medical Sciences, Vice-Chancellor for Research and Technology (Grant Number: 4030044).
Author information
Authors and Affiliations
Contributions
Conception and Design: B.EK; Data Collection and Management: B.EK, SAH; Analysis and Interpretation of Data: B.EK, MRJ; Drafting the Manuscript: SAH, B.EK; Revising Manuscript for Intellectual Content: MRJ, B.EK; Project Administration and Supervision: B.EK; Funding Acquisition: B.EK. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Hosseini, A., Ebrahimzadehkour, B. & Jowshan, MR. Association of surrogate indices of insulin resistance with hypertension: a cross-sectional analysis from PERSIAN Dena cohort study (PDCS). Sci Rep 15, 44723 (2025). https://doi.org/10.1038/s41598-025-28527-z
Received:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s41598-025-28527-z