Abstract
Tovorafenib is a type II RAF inhibitor recently approved by the FDA for relapsed or refractory paediatric low-grade glioma in children aged 6 months and older with BRAF fusions or V600E mutations, owing to its strong central nervous system penetration and absence of paradoxical MAPK pathway activation observed with type I inhibitors. This study developed and validated a fast, robust, and environmentally friendly reversed-phase ultra-performance liquid chromatography method for Tovorafenib quantification, employing ICH Q14 development, ICH Q2(R1) validation, and assimilating Analytical Quality by Design and green analytical chemistry principles. Chromatographic analysis was performed using a Waters Acquity UPLC BEH C18 column and 0.01 N Na₂HPO₄ in water: methanol (62:38 v/v) as the mobile phase at 0.29 ml/min, with PDA detection at 292 nm. The optimized approach exhibited excellent linearity (r² = 0.9997) and reproducibility (low %RSD). Forced degradation studies demonstrated Tovorafenib stability under most conditions, except during acid hydrolysis. Assessment with green chemistry tools yielded favourable eco-scores (ComplexMoGAPI 90, AGREE 0.61, BAGI 70, RAPI 92.5, EVG Q2, and RGBfast 97.5%). This validated RP-UPLC procedure offers reliable, sensitive, and sustainable Tovorafenib analysis, supporting both efficient pharmaceutical quality control and environmental sustainability.
Similar content being viewed by others
Introduction
Tovorafenib (TRN), a type II RAF inhibitor, represents a significant advancement in the treatment of paediatric low-grade gliomas (pLGG) harbouring BRAF fusions or V600E mutations. Recently, it became the first FDA-approved drug for children aged 6 months and older with relapsed or refractory pLGG, showcasing superior central nervous system penetration and lack of paradoxical MAPK pathway activation compared to type I BRAF inhibitors1,2. The efficacy and safety of TRN have been demonstrated in phase 1 and pivotal phase 2 FIREFLY-1 trials, highlighting its clinical utility3.
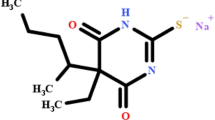
The establishment of reliable and efficient analytical procedures for TRN ((Fig. 1 illustrates the chemical structure of Tovorafenib, highlighting its key functional groups and molecular framework)) is crucial for pharmacokinetic studies, quality control, and therapeutic drug monitoring. However, traditional chromatographic approaches often rely on hazardous organic solvents, leading to environmental concerns and health risks associated with their use. Consequently, there is a growing interest in developing eco-friendly analytical techniques that adhere to the principles of Green Analytical Chemistry (GAC)4.
Analytical Quality by Design (AQbD) provides a systematic analytical approach to method development, focusing on understanding the interactions between its parameters to ensure robustness and compliance with regulatory standards5. By integrating AQbD with GAC principles, it is possible to design environmentally sustainable analytical methodology that do not compromise performance. This study reports the development of an eco-friendly reversed-phase ultra-performance liquid chromatography (RP-UPLC) framework for estimating TRN using AQbD principles. This technique not only addresses environmental concerns but also offers a reliable tool for quantifying TRN in clinical and pharmaceutical settings.
However, these strategies may involve significant solvent usage and environmental impact, highlighting the need for more sustainable analytical practices. The principles of Green Analytical Chemistry (GAC) offer a framework for reducing hazardous chemical use in analytical methods while maintaining their performance6.
This study addresses this critical gap by developing the first eco-friendly RP-UPLC procedure for estimating TRN, utilising Analytical QbD principles which provides a systematic approach to method development, ensuring robustness and compliance with regulatory standards. A 62:38 (v/v) mixture of 0.01 N Na₂HPO₄ in water and methanol was selected as a green mobile phase in the current RP-UPLC analysis because methanol is less toxic and more environmentally benign than acetonitrile or tetrahydrofuran, making it preferable for sustainable laboratory practices. The inclusion of Na₂HPO₄ buffer ensured a stable pH, enhancing the reproducibility and peak resolution for a wide range of analytes. By integrating AQbD with GAC principles, this protocol not only supports TRN’s therapeutic application of TRN but also advances the wider goal of environmentally sustainable pharmaceutical analysis.
Materials and methods
Instrumentation and software
A methodology for the analysis of tovorafenib (TRN) was developed, optimised, and validated using a water-acquity ultra-performance liquid chromatography (UPLC) system with a photodiode array (PDA) detector. An autosampler coupled with Empower-2 software (Waters Corporation, Milford, MA) and a BEH C18 column 100 A column (2.1 × 100 mm, 1.8 μm) were used to perform chromatographic separation. Design-Expert® software version 13.0.0 a statistical tool was used for the screening and optimisation of CMPs (Stat-Ease Inc., Minneapolis, USA). A PG Instruments T 60 UV-VIS spectrophotometre coupled with UV win 6 Software was used to obtain spectrophotometric measurements (PG Instruments Limited, Near Leicester, UK). Thermal degradation studies were performed using an HTLP Engineering forced-air circulating oven (Ak Industrial Estate, Maharashtra, India). The samples were dissolved using an ultrasonic bath sonicator (Analab Scientific Instruments, Vadodara, Gujarat, India), and the mobile phase pH was measured using a BVK Enterprises pH meter (Boduppal, Hyderabad, Telangana, India). Forced degradation studies were performed on a hot plate (Optima Laboratory Hot Plates, Vadodara, Gujarat, India). Pure TRN and the other chemicals were measured using a Denvar DWS-124 C balance (New Delhi, India).
Materials and reagents
Tovorafenib (TRN) in its pure form (API); (> 99%) was supplied by Spectrum Pharma Labs Pvt. Ltd., Hyderabad, India. Tovorafenib (OJEMDA, 100 mg) tablets were obtained from Day One Biopharmaceuticals, US. The mobile phase and sample solutions have been prepared with the usage of high-purity UPLC grade chemicals and solvents (including purified water, methanol, sodium hydrogen phosphate (Na2HPO4), 30% H2O2, HCl (purity of 99% or more), and NaOH (purity of 98% or more) provided by Rankem in Gurugram (Haryana), India. Millipore water was provided by Millipore Technologies (Mumbai, India).
Chromatographic conditions
The separation of TRN, its impurities, and associated peaks was performed using a Waters Acquity UPLC BEH C18 column (100 mm × 2.1 mm, 1.8 μm particle size). An isocratic mobile phase comprising 0.01 N sodium phosphate (Na₂HPO₄) in water and methanol at a 62:38 (v/v) ratio was employed, with a flow rate set to 0.29 mL/min, resulting in a total run time of 3 min for TRN analysis. The column temperature was maintained at 29.5 °C, and all sample solutions were pre-filtered using 0.2 μm PVDF membranes. Detection was performed at 292 nm, with each analysis involving the injection of 3.0 µL of the sample solution.
Preparation of diluent: Based on the solubility profile of TRN, a diluent composed of methanol and water in a 60:40 (v/v) ratio was selected as the most suitable solvent.
Preparation of 0.01 N Na2HPO4 solution
Weighed 0.89 g of anhydrous Na2HPO4. The solvent mixture was subsequently dissolved in a small amount of UPLC-grade water and measured in a 1-liter volumetric flask. The samples were diluted to a final volume of 1 L with sufficient water. The mixture was blended, sonicated for 15 min, autoclaved, and allowed to stand at room temperature.
Preparation of standard stock solutions
An accurately weighed 10 mg portion of TRN was transferred to a 25 mL volumetric flask. To prepare a standardised stock solution with a final concentration of 400 µg/mL, 20 mL of the designated diluent was added, and the mixture was sonicated for 10 min to ensure complete dissolution of TRN. The solution was then brought to volume using the same diluent and mixed thoroughly. Subsequently, 1 mL of this stock solution was transferred to a separate 10 mL volumetric flask and diluted to the mark with the diluent, resulting in a final concentration of 40 µg/mL. This was designated as the standard working solution7.
Preparation of sample stock solutions
Twenty OJEMDA (tovorafenib) 100 mg tablets were weighed and thoroughly homogenised. From this mixture, a precisely measured 50 mg portion of TRN was transferred into a 50 mL volumetric flask, followed by the addition of approximately 40 mL methanol. The suspension was sonicated for 25 min to ensure complete dissolution of the analyte. The volume was then increased to 50 mL with the same solvent, yielding a TRN concentration of 1000 µg/mL. The resulting solution was filtered using UPLC-compatible filters to remove any particles. Subsequently, 0.4 mL of this stock solution was transferred to a 10 mL volumetric flask and diluted to the mark with the diluent, achieving a working sample concentration of 40 µg/mL.
Stress degradation
TRN was subjected to an extensive degradation series under thermal, oxidative (hydrogen peroxide), hydrolytic (both acidic and alkaline), neutral (with a diluent), and photolytic conditions. These analyses were performed according to the ICH guidelines Q1A (R2) and Q1B requirements to determine the stability profile8,9. All experiments were carried out at a final TRN concentration of 40 g/mL, and 10 µL aliquots were used to inject the UPLC system. To degrade, one millilitre of TRN was added to one millilitre of 2 N HCl, refluxed at 60 °C for 30 min, neutralised with 2 N NaOH, and diluted to the required concentration. Base degradation was also performed using a similar protocol with 2 N NaOH and neutralisation using 2 N HCl, as described above. To oxidatively degrade the TRN, 1 mL of TRN stock was added to 1 mL of 20% H₂O₂ and maintained at 60 °C for 30 min. The photolytic study used a 400 µg/mL solution of TRN with UV light at 200 W.h/m² for seven days. Neutral degradation was evaluated by refluxing the drug with water at 60 °C for 6 h. In the case of thermal degradation, the standard TRN solution was heated at 105 °C for 6 h to ensure that all recommended ICH stress conditions for pharmaceutical products were satisfied10,11,12.
Results and discussion
Preliminary investigation
The preliminary method development focused on optimising the UPLC procedure for analysing TRN in both tablet formulations and as an active pharmaceutical ingredient (API). The detection wavelength was set at 292.0 nm, chosen for its capacity to maximise sensitivity while reducing baseline noise. The Waters Acquity UPLC BEH C18 (100 × 2.1 mm, 1.8 μm) column, utilised with Empower-2 software, exhibited the best overall chromatographic performance following a systematic comparison of various columns. Methodology optimisation revealed that a mobile phase comprising methanol and 0.01 N Na₂HPO₄ in water (38:62, v/v) and a flow rate of 0.29 mL/min were optimal, resulting in significant improvements in key chromatographic metrics such as retention time, tailing factor, and theoretical plate count. These results emphasise the importance of precise control over the mobile phase composition and flow rate to ensure optimal separation, sensitivity, and reproducibility in UPLC analysis. Through careful and systematic optimisation, the procedure consistently delivered high sensitivity, effective component separation, and robust and repeatable results, underscoring its reliability for the quantitative estimation of TRN in tablet dosage forms and its suitability for routine pharmaceutical applications.
Analytical target profile (ATP)-critical method parameters (CMPs)-and-critical quality attributes (CQAs)
The Quality Target Product Profile (QTPP) lays out the essential goals for a drug, such as its purity, effectiveness, safety, and delivery, serving as a blueprint for development. The ATP defines what the analytical procedure should achieve within the AQbD guidelines. This study focused on creating a quick and accurate procedure for TRN measurement in drug bulk and oral forms. Identifying CQAs and key CMPs, particularly flow rate, column temperature, and organic solvent proportion, was fundamental. Initial screening narrowed the CMPs, while CQAs such as retention time (Rt), theoretical plates, and tailing factor were used to assess method performance13.
Analytical quality risk assessment
Risk assessment is used to identify key method parameters (CMPs) that significantly impact both the quality attributes (CQAs) and analytical objectives (ATP) of a technique. In QbD-driven RP-UPLC development, it is vital to thoroughly evaluate the factors that could compromise these outcomes. The Ishikawa fishbone diagram visually maps how CMPs, namely mobile phase composition, column temperature, and flow rate, can affect the analytical performance, highlighting the main risks and their subcategories. Each of these risks was carefully assessed during procedure screening and optimisation, and a detailed cause-and-effect diagram is provided in Supplementary Figure S114.
AQbD-method development
During AQbD-based method development, initial experiments were conducted with different solvents, columns, and operating conditions to identify the most effective parameters for TRN analysis. Three main factors—column temperature (27–33 °C), flow rate (0.27–0.33 mL/min), and organic solvent content in the mobile phase (35–45%)—were systematically tested at various levels, with the organic percentage identified as a critical risk that required careful adjustment. The process used statistical designs to fine-tune these variables, and an optimal combination was selected: a mobile phase of 0.01 N Na₂HPO₄ in water and methanol (62:38), a flow rate of 0.29 mL/min, a column temperature of 27.5 °C, and UV detection at 292 nm. The sample diluent was methanol: water (60:40), with a 3 µL injection volume. These conditions provided a selective and efficient analysis of both stable and degraded samples. Table 1 presents the AQBD-guided optimised chromatographic conditions and their key responses for the RP-UPLC technique developed for TRN.
Optimizing methods using experimental design
Response Surface Methodology (RSM) is an effective statistical approach for optimising methods by evaluating the individual and combined effects of CMPs on CQAs. In this study, a Central Composite Design (CCD), which is part of RSM, was used to systematically test 20 sets of conditions, enabling the analysis of variables such as flow rate, organic solvent percentage, and column temperature at different levels. This approach captured linear, quadratic, and interaction effects, with data fitted to second-order polynomial equations to model how these factors influenced outcomes such as retention time, theoretical plates, and tailing factor. Figure 2 shows 3D response surface plots with matching 2D contour plots illustrating how the independent variables affect the dependent variables. The dependent variables analysed were retention time (Rt) (Response 1; min; Fig. 2a), number of theoretical plates (NTP) (Response 2; number; Fig. 2b), and tailing factor (Response 3; number; Fig. 2c), and Table 2 presents the experimental factors and responses used in the Central Composite Design-based optimization of RP-UPLC for TRN.
Replicate centre-point runs helped gauge the experimental error, whereas star points allowed the assessment of response curvature. Statistical analysis (ANOVA) validated the significance of each parameter, and graphical tools, such as contour and 3D response plots, illustrated these relationships. The outcomes of the ANOVA analyses for retention time (RT), number of theoretical plates (NTP), and tailing factor (TF), evaluated using a central composite design for each response variable, are summarised in Table 3 (a, b, and c). Numerical optimisation based on acceptance criteria predicted the best chromatographic conditions, ensuring robust and efficient TRN quantification in the samples.
Calibrating the model-to-response data
The results from 20 experimental runs were analysed using Design-Expert software, which determined that a quadratic model provided the best fit based on high R² values and minimal error. The relationships between key responses, such as retention time, theoretical plates, and tailing factor, and experimental variables are displayed using two-dimensional and three-dimensional plots. A composite desirability function identified the optimal settings, with the best outcome (value of 1) recommending a 0.29 mL/min flow rate. The method’s accuracy was confirmed by six repeated injections of 40 µg/mL TRN, with observed results falling within 5% of the model predictions, demonstrating the reliability of the approach. Additional plots show the distribution of the experimental results and highlight the optimised zones where all the method criteria were met. Supplementary Figure S2 presents 2D-normal plots illustrating the distribution of experimental responses for (a) retention time (RT), (b) number of theoretical plates (NTP), and (c) tailing factor (TF) of TRN, along with interaction effect plots generated from the screening D-optimal split-plot design. Panels (d), (e), and (f) display residuals versus run order for RT, NTP, and TF, respectively, enabling the visualisation of model fit and potential outliers across all screened chromatographic conditions. Supplementary Figure S3 displays desirability and overlay plots that illustrate the optimised chromatographic conditions for predicting retention time, theoretical plates, and peak area, thereby identifying the design space that meets all specified analytical objectives.
Fitting of responses to the model
The optimal model was identified by evaluating each response from 20 experimental runs against various mathematical models using Design-Expert software. Selection was based on a high R², low SD, CV, and PRESS, as well as a close agreement between the adjusted and predicted R² values. For all responses, retention time (Rt), theoretical plates, and tailing factor, the quadratic model provided the best balance of fit and predictive accuracy. This approach avoids the overfitting seen with cubic models and aligns with established chromatographic methodology optimisation practices, where quadratic models reliably capture nonlinear interactions while maintaining model simplicity and robustness. The results are presented in Supplementary Table S1.
The 3D response surface plots, along with the corresponding 2D contour plots, illustrate the influence of independent variables on the dependent variables. (a) Three-dimensional response surface plots (1–2–3) and two-dimensional contour diagrams (4–5–6) were generated to illustrate how flow rate (A), mobile phase composition (B), and temperature (C), designated as critical method parameters (CMPs), affect retention time (Response 1) as a critical analytical attribute (CAA). (b) Effects of flow rate (A), mobile phase composition (B), and temperature (C) on theoretical plates (Response 2) were visualised using 3D response surfaces (1–2–3) and 2D contour plots (4–5–6), highlighting the influence of these CMPs on this specific CAA. (c) 3D response surface graphs (1–2–3) alongside 2D contour diagrams (4–5–6) depict how the key method parameters—flow rate (A), mobile phase composition (B), and temperature (C)—impact the tailing factor (Response 3) considered as the CAA. (d) A 2D plot of overall desirability (1) was constructed under the optimised conditions to predict the retention time, theoretical plates, and peak area.
Analytical method validation (AMV)
Optimised settings, including eluents, column type, flow rate, temperature, and sample preparation steps, were selected from the experimental process and used to validate the procedure following the ICH Q2 (R1) guidelines. This involved assessing the method’s precision, accuracy, specificity, linearity, robustness, and other key parameters to ensure that the analytical procedure met international regulatory standards for drug testing.
System suitability
The prospective analytical procedure was ensured to be suitable with the use of the standard solution which was injected ‘six times. The tailing factor, theoretical plates, and retention time were calculated. In the overall validation study, the tailing factor, NTP, and retention time were 1.2, greater than 2000, and 1.489 (mean), respectively. The system suitability parameters for TRN data indicate that the system is appropriate for the current research, as shown in Table 4.
LOD and LOQ
The sensitivity of the analytical approach was evaluated by determining the limit of detection (LOD) and limit of quantitation (LOQ) for the analyte peaks. These values were calculated using the standard deviation (σ) and slope (S) of the calibration curve according to the following formulas: LOD = 3.3 × σ/S and LOQ = 10 × σ/S. The detection limit (DL) and quantification limit (QL) were obtained using the slope approach based on linearity, resulting in DL values at or below 0.14 µg/mL and QL values of 0.44 µg/mL.
Linearity
The effect of concentration was assessed by preparing TRN solutions ranging from 10 to 60 µg/mL and analysing them. The relationship between concentration and peak area was summarised using linear regression, which was used to establish a mathematical correlation between the analyte amount and detector response. A calibration curve depicting area versus concentration within this range was generated, yielding a straight-line equation (y = 9036.4x + 1503.3) and a correlation coefficient of 0.9997, indicating excellent linearity of the assay. The standard error of the estimate (STEYX) of 3625.5 further supports the strong fit of the regression model to the information. Figure 3 displays the calibration curve of Tovorafenib, illustrating the linear relationship between concentration and response. Table 5 complements this by summarizing the linearity parameters, confirming the method’s reliability across the tested range.
Specificity
The specificity of this analysis technique was assessed by injecting a placebo, diluent, and standard into the UPLC system. The chromatograms revealed no interference between TRN and the placebo peaks. Figure 4 displays chromatograms from the interference study, including blank, placebo, and Tovorafenib (TRN) sample, analysed using the finalized RP-UPLC method with optimized chromatographic conditions.
Degradation
The samples were exposed to different stress phenomena to establish the stability-indicating property of the analysis framework. The acid, base, oxidative, and thermal degradation states were 2.0 M HCl/1mL/30 min at 60 °C, 2.0 M NaOH/1 mL/30 min at 60 °C, 20% H2O2/1 mL/30 min at 60 °C, and 105 °C, 6 h, respectively. Photolytic degradation was carried out using UV irradiation at 200 W h/d m2 for 7 days, and neutral degradation was evaluated by refluxing in water at 60 °C for 6 h. Degradation conditions were conducted as per the recommendations of the ICH Q1B. Degradation was studied on the formulation, and the degraded sample was injected. The analysis of the injected samples was factored, and all the samples were within the degradation limits. Forced degradation experiments on TRN indicated that the drug had low degradation (below 2%) under photolytic and neutral conditions. It was gradually degraded by 7.76% under acid hydrolysis conditions, and the highest degradation points were attained at 1.150 and 2.175 min. In contrast, with basic hydrolysis, the drug exhibited a degradation rate of 4.63%, which corresponds to a degradation peak of 1.038 min. During oxidative and thermal degradation, TRN reflected a 4.46 and 2.72 degradation, respectively. Notably, the analysis did not provide any interference with the degradation peaks. The % degradation and peak purity outcomes are presented in Table 6, and the relative chromatograms are shown in Fig. 5.
Chromatograms from the interference study: (A) blank, (B) placebo, and (C) tavorafenib (TRN) sample obtained using the finalised method. Chromatographic parameters: mobile phase consisted of 0.01 N Na₂HPO₄ in water and methanol mixed in a 62:38 (v/v) ratio; column used was Waters Acquity UPLC-BEH C18 (100 × 2.1 mm, 1.8 μm); flow rate set at 0.29 mL/min; total run time of 3 min; column temperature maintained at 29.5 °C; injection volume was 3 µL; and wavelength detection performed at 292.0 nm.
Method precision
Repeatability (Intra-Day)
Repeatability was evaluated by determining the relative standard deviation (%RSD) across six replicate measurements of a 40 µg/mL sample, all performed within a single day under consistent laboratory conditions. The %RSD obtained was 0.3%, indicating excellent precision and demonstrating the high reliability of this analytical technique.
Intermediate accuracy (Inter-Day)
Inter-day precision was determined by calculating the relative standard deviation (%RSD) across six independent measurements of the drug at a concentration of 40 µg/mL. This assessment was conducted over multiple days and involved the use of different instruments, columns, and sample preparations while remaining within a single laboratory. The resulting %RSD for inter-day precision was 0.2%, reflecting a high degree of reproducibility and consistency of the process. The detailed precision results are listed in Table 7.
Accuracy
The precision of the current procedure was determined using the standard addition method. TRN was added to the test solutions at concentrations of 50%, 100%, and 150% of the test concentration (20, 40, and 60 µg/mL). The average recovery rate was 99.92% with a %RSD of less than 2.0, indicating acceptable accuracy. Table 8 records the accuracy results. The accuracy results with statistics are presented in detail in Supplementary Table S6.
Robustness
The robust method conditions of the current approach were determined based on the MODR of the CCD. The developed procedure is robust in terms of MODR, and the results were independent of the differences in the conditions of the protocols. The derived technique was demonstrated to be resilient. The fundamental strength of the proposed strategy was confirmed at different flow rates, mobile phase compositions, and column temperatures, and the method was not influenced by the alteration of the flow rate between 0.19 and 0.39 mL/min, column temperature between 22 and 32 °C, and mobile phase composition between 55B: 45 A and 65B: 35 A which demonstrates the robustness of the developed methodology. The parameters with respect to system suitability did not change significantly, where all parameters met the necessary standards, and the % RSD fell within the acceptable range. Table 9 demonstrates the robustness of the approach used.
Solution-stability
The stability of the Tovorafenib (TRN) standard and sample solutions was evaluated at both room temperature and refrigerated conditions. The results indicated that both the standard and sample solutions remained stable for up to 48 h when stored in a refrigerator (0–4 °C). However, at room temperature, the solutions were unstable over the same period. Therefore, it is recommended that sample solutions be stored under refrigerated conditions to maintain their stability. Supplementary Figure S4 presents the chromatograms showing the stability profile of the TRN sample solution at (a) 0 h and (b) 48 h (Refrigerator (0–4 °C).
Assay
The tablet product designated as OJEMDA (tovorafenib), labelled with the claim of TRN at a dosage of 100 mg, underwent an assay utilizing the aforementioned formulation. The analysis produced an average assay result for TRN, which was determined to be 99.81%. These assay results have been systematically compiled and are displayed in Table 10.
Methods’ sustainability assessment
Green analytical practices aim to minimise or eliminate the use of organic solvents, energy consumption, sample processing steps, and waste generation to create more sustainable laboratory workflows. Although it is not feasible to eliminate solvents from liquid chromatography, sustainability can be enhanced by employing safer, biodegradable solvents, reducing waste, streamlining procedures, and adopting miniaturised sample preparation methods. Assessing the environmental impact of an analytical technique is complex and necessitates a thorough evaluation of multiple factors.
The sustainability of the current approach was assessed using the following tools:
-
1.
Methods’ practicality (Blueness) assessment: Blue Applicability Grade Index (BAGI) tool.
-
2.
Analytical performance assessment (redness): Red analytical performance index (RAPI).
-
3.
Analytical methodology greenness assessment: Analytical GREEnness (AGREE) metric & ComplexMoGAPI.
-
4.
Review analytical procedure efficiency, validation, and environmental impact: The EVG (Efficient, Valid, Green) tool.
-
5.
Assessments of analytical approach’s “greenness” and “whiteness”: Red-Green-Blue-fast (RGBfast).
Evaluation of the sustainability assessment of the proposed RP-UPLC method: (a) BAGI asteroid pictogram; (b) RAPI pictogram; (c) AGREE pictogram score; (d) ComplexMoGAPI pictogram; (e) EVG Radar chart; (f) RGBfast model pictogram; (g) Graph of the proposed technique showing saturation levels of red, green, and blue colours, along with the overall whiteness as a holistic evaluation.
The RP-UPLC procedure assessed using multiple modern analytical sustainability and performance tools showed strong overall applicability, excellent analytical performance, and significant but improvable environmental sustainability.
BAGI
This framework achieved a score of 70 (Fig. 6a; BAGI pictogram), highlighting its suitability for routine applications owing to the accessibility of reagents, minimal sample volume, high throughput, and widespread instrumental availability. However, modest scores were noted for multi-analyte capability and sample preparation complexity, indicating operational aspects that could be refined. The BAGI pictogram and scores provide rapid benchmarking of strengths and limitations, making it easy to compare this protocol with other alternatives. Notably, although BAGI quickly identifies practical aspects, it does not thoroughly account for reagent safety and waste impact15,16.
RAPI
The current approach receives very high RAPI scores—typically 10/10 for core parameters—illustrating its outstanding repeatability, precision, recovery, and robustness with a wide working range. Minor deductions (for example, 7.5/10 for trueness, ruggedness, and selectivity) still indicate strong reliability but allow room for minor optimisation. The RAPI score of 92.5 (Fig. 6b; RAPI pictogram) reflects the exceptional analytical quality and reproducibility17.
ComplexMoGAPI
This tool demonstrates the method’s operational advantages (low sample/solvent use, concise steps, reduced energy demand), but flags environmental drawbacks in hazardous reagent use (notably methanol), lack of waste treatment, and partial compliance with Green Chemistry principles. An overall score of 70 (Fig. 6d; ComplexMoGAPI pictogram) indicates respectable sustainability, but the yellow pictogram icons highlight notable limitations compared to ideal eco-friendly protocols18,19.
AGREE
The score of 0.61 (Fig. 6c; AGREE pictogram) demonstrates good—but not outstanding—green chemistry compliance. The method’s greenness benefits from sample minimisation, automation, derivatisation avoidance, and energy efficiency, but is penalised for hazardous solvent use and gaps in comprehensive operator safety and in situ analysis opportunities. Pictograms assist in quickly identifying opportunities for greener practices, such as switching to less hazardous reagents20,21.
EVG: The visual radar chart and scores (Fig. 6e; EVG radar chart) indicate balanced performance: efficiency (1.8/3), validation (2.2/3), and greenness (2/3), with an overall “very good” Q2 rating. These results show that the current strategy successfully harmonises analytical rigor, sustainability, and effectiveness22,23. The evaluation of the EVG radar chart is readily available as a supplementary Excel file.
RGBfast: Using six graded criteria, the RP-UPLC methodology obtained moderate (50.0/100) scores in the red, green, and blue domains, translating to an overall whiteness of 50.0 (Fig. 6f: RGBfast model pictogram; & 6 g: graph of the proposed technique showing saturation levels of red, green, and blue colours, along with the overall whiteness as a holistic evaluation). This profile suggests that the procedure is among the most “green and white” of those assessed, aligning with the White Analytical Chemistry paradigm by balancing robustness, eco-friendliness, and practical usability. However, its blue score (sample throughput) is limited by a 24-hour cycle, which may constrain high-volume real-world applications24,25. The evaluation of the RGBfast tool is readily available as a supplementary excel.
Collectively, these tool-based assessments underscore the analytical robustness and broad practicality of the RP-UPLC protocol, which demonstrates meaningful but not absolute environmental sustainability. The areas most in need of improvement include the use of safer (greener) reagents, waste management, and multi-analyte analysis capacity. Supplementary Tables S2 to S5 (S2-RAPI, S3-BAGI; S4-ComplexMOGAPI; S5-AGREE).
Figure 7 provides a concise graphical summary of the eco-conscious AQbD-based RP-UPLC method developed for Tovorafenib. It highlights key chromatographic parameters, robustness, and stability-indicating features, supporting precise and environmentally responsible analysis in bulk and dosage forms.
Conclusion
The RP-UPLC method, developed using AQbD and green chemistry principles, provides a rapid (3 min, Rt) and reliable assay for TRN suitable for routine quality-control. Systematic risk assessment and experimental design identified and optimised critical method parameters, ensuring robustness and reproducibility. Optimised sample preparation-maintained solution stability, and forced-degradation studies demonstrated the method is stability-indicating, clearly separating TRN from degradation products, especially under acidic conditions. Full validation confirmed high precision, sensitivity, linearity, specificity, solution stability, and robustness for assay of TRN in drug substance and tablet formulations. Multiple greenness assessment tools verified the method’s environmental sustainability and alignment with contemporary green analytical standards.
Data availability
The datasets/information used for this study is available on reasonable request to the corresponding authors.
Abbreviations
- TRN:
-
Tovorafenib
- AQbD:
-
Analytical quality-by-design
- pLGG:
-
Paediatric low-grade gliomas
- GAC:
-
Green analytical chemistry
- LOD:
-
Limit of detection
- LOQ:
-
Limit of quantitation
- ATP:
-
analytical target profile
- CMPs:
-
Critical method parameters
- CQAs:
-
Critical quality attributes
- AMV:
-
Analytical method validation
- RSD:
-
Relative standard deviation
- RSM:
-
Response surface methodology
- PRESS:
-
Predicted residual sums of squares
- NTP:
-
Number of theoretical plates
- AGREE:
-
Analytical greenness
- BAGI:
-
Blue applicability grade index
- EVG:
-
Efficient valid green
- ComplexMoGAPI:
-
Complex modified GAPI
- RGBfast:
-
Red-green-blue fast model
- RAPI:
-
Red analytical performance index
- MODR:
-
Method operable design region
- CCD:
-
Central composite design
- STEYX:
-
Standard error of the estimate
References
Zhang, T., Xu, B., Tang, F., He, Z. & Zhou, J. Type II RAF inhibitor Tovorafenib for the treatment of pediatric Low-Grade glioma. Expert Rev. Clin. Pharmacol. 17 (11), 999–1008. https://doi.org/10.1080/17512433.2024.2418405 (2024).
Khuong-Quang, D. A. et al. CTNI-37. LOW-GRADE GLIOMA ARM OF THE PHASE 2 FIREFLY-1 (PNOC026) STUDY. Neuro-Oncology 25 (Supplement_5), v82–v83. (2023). https://doi.org/10.1093/neuonc/noad179.0319
Kilburn, L. B. et al. The type II RAF inhibitor Tovorafenib in Relapsed/Refractory pediatric Low-Grade glioma: the phase 2 FIREFLY-1 trial. Nat. Med. 30 (1), 207–217. https://doi.org/10.1038/s41591-023-02668-y (2024)., E. E. K.
Gałuszka, A., Migaszewski, Z. & Namieśnik, J. The 12 principles of green analytical chemistry and the SIGNIFICANCE mnemonic of green analytical practices. TRAC Trends Anal. Chem. 50, 78–84. https://doi.org/10.1016/j.trac.2013.04.010 (2013).
Bairagi, A., Kothrukar, R., Chikhale, H., Kosanam, S. & Borse, L. AQbD-Novel strategy for analytical methods. Futur J. Pharm. Sci. 10 (1), 138. https://doi.org/10.1186/s43094-024-00706-1 (2024).
Ražić, S., Arsenijević, J., Đogo Mračević, S., Mušović, J. & Trtić-Petrović, T. Greener chemistry in analytical sciences: from green solvents to applications in complex matrices. Curr. Challenges Future Perspectives: Crit. Rev. Analyst. 148 (14), 3130–3152. https://doi.org/10.1039/D3AN00498H (2023).
Venkatesan, K., Sundarababu, J. & Anandan, S. S. The recent developments of green and sustainable chemistry in multidimensional way: current trends and challenges. Green Chem. Lett. Rev. 17 (1), 2312848. https://doi.org/10.1080/17518253.2024.2312848 (2024).
Muchakayala, S. K. et al. AQbD based green UPLC method to determine mycophenolate mofetil impurities and identification of degradation products by QToF LCMS. Sci. Rep. 12 (1), 19138. https://doi.org/10.1038/s41598-022-22998-0 (2022).
El-Hanboushy, S., Marzouk, M., Ayish, H. S. & Sustainable Multi-Task, N. A. HPLC–UV method for simultaneous analysis of top neuromodulating agents in diverse pharmaceutical formulations. Sci. Rep. 15 (1), 22724. https://doi.org/10.1038/s41598-025-07502-8 (2025).
ICH Q1A (R2. ) Stability Testing of New Drug Substances and Drug Products - Scientific Guideline, (2003). https://www.ema.europa.eu/en/ich-q1a-r2-stability-testing-new-drug-substances-drug-products-scientific-guideline
Bakshi, M. & Singh, S. Development of validated Stability-Indicating assay Methods–Critical review. J. Pharm. Biomed. Anal. 28 (6), 1011–1040. https://doi.org/10.1016/s0731-7085(02)00047-x (2002).
Center for Drug Evaluation and Research. Center for Biologics Evaluation and Research. Q1B Photostability Testing of New Drug Substances and Products, (1996).
Alqahtani, A., Alqahtani, T., Al Fatease, A. & Tolba, E. H. A quality by design HPLC method for cephalosporin analysis in pharmaceuticals and water samples with environmental impact assessment. Sci. Rep. 15 (1), 33. https://doi.org/10.1038/s41598-024-84647-y (2025).
Fares, M. Y., Hegazy, M. A., El-Sayed, G. M., Abdelrahman, M. M. & Abdelwahab, N. S. Quality by design approach for green HPLC method development for simultaneous analysis of two thalassemia drugs in biological fluid with Pharmacokinetic study. RSC Adv. 12 (22), 13896–13916. https://doi.org/10.1039/D2RA00966H (2022).
Manousi, N., Wojnowski, W., Płotka-Wasylka, J. & Samanidou, V. Blue applicability grade index (BAGI) and software: A new tool for the evaluation of method practicality. Green. Chem. 25 (19), 7598–7604. https://doi.org/10.1039/D3GC02347H (2023).
Mahdavi, R., Talebpour, Z. & Noori, M. Application of new green evaluation tools to assess the environmental impact of analytical procedures based on solid phase Microextraction techniques. Green. Anal. Chem. 10, 100144. https://doi.org/10.1016/j.greeac.2024.100144 (2024).
Nowak, P. M., Wojnowski, W., Manousi, N., Samanidou, V. & Płotka-Wasylka, J. Red analytical performance index (RAPI) and software: the missing tool for assessing methods in terms of analytical performance. Green. Chem. 27 (19), 5546–5553. https://doi.org/10.1039/D4GC05298F (2025).
Mansour, F. R., Omer, K. M., Płotka-Wasylka, J. A. & Total Scoring System and software for complex modified GAPI (ComplexMoGAPI) application in the assessment of method greenness. Green. Anal. Chem. 10, 100126. https://doi.org/10.1016/j.greeac.2024.100126 (2024).
Płotka-Wasylka, J. & Wojnowski, W. Complementary green analytical procedure index (ComplexGAPI) and software. Green. Chem. 23 (21), 8657–8665. https://doi.org/10.1039/D1GC02318G (2021).
Pena-Pereira, F., Wojnowski, W. & Tobiszewski, M. AGREE—Analytical greenness metric approach and software. Anal. Chem. 92 (14), 10076–10082. https://doi.org/10.1021/acs.analchem.0c01887 (2020).
Manukonda, V., Dandamudi, S. P., Kusuma, P. K., Thumma, G. & Gangarapu, K. Development and validation of a UPLC-MS/MS method for the simultaneous Estimation of Ertugliflozin and sitagliptin in bulk and tablet dosage forms: assessment of greenness and blueness. Green. Anal. Chem. 12, 100193. https://doi.org/10.1016/j.greeac.2024.100193 (2025).
Saleh, S. S., Lotfy, H. M. & Elbalkiny, H. T. An integrated framework to develop an efficient valid green (EVG) HPLC method for the assessment of antimicrobial pollutants with potential threats to human health in aquatic systems. Environ. Sci. : Processes Impacts. 25 (12), 2125–2138. https://doi.org/10.1039/D3EM00339F (2023).
Bukke, S. P. N. et al. RP-HPLC Method Development, Validation, and Greenness Profile Evaluation: Concurrent Quantification of Telmisartan and Atorvastatin Calcium in Combined Pharmaceutical Formulation. AChrom (2025). https://doi.org/10.1556/1326.2025.01374
Nowak, P. M., Arduini, F. & RGBfast – A User-Friendly version of the Red-Green-Blue model for assessing greenness and whiteness of analytical methods. Green. Anal. Chem. 10, 100120. https://doi.org/10.1016/j.greeac.2024.100120 (2024).
Nowak, P. M. How to correctly evaluate Greenness, whiteness and other colours? Introducing general rules of a good evaluation practice. Green. Chem. 27 (23), 6699–6710. https://doi.org/10.1039/D5GC00615E (2025).
Acknowledgements
The authors extend their appreciation to the Deanship of Research and Graduate studies at King Khalid University for supporting this work through the Large Research Project under the grant number RGP2/639/46. The authors also extend their appreciation to the JBR Educational Society, Kampala International University, DPSR University.
Funding
The author(s) received no specific funding for this work.
Author information
Authors and Affiliations
Contributions
VMG and KPK initiated the conception. SPNB, SSR, MSR and CT developed the design. VMG, SSR, and SMR conducted the experiments. KPK, SPNB and CT analyzed the results. VMG, KPK, SPNB, and BNR prepared the first draft of the manuscript. VMG, SPNB, KPK, SSR, MSR, and CT reviewed and edited the manuscript. The authors read and approved the final manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Consent for publication
All the authors have read and agreed to the final copy of the finding as contained in the manuscript.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Goud, V.M., Bukke, S.P.N., Kusuma, P.K. et al. Eco-conscious AQbD-guided stability-indicating RP-UPLC method for analysing Tovorafenib in bulk and dosage forms. Sci Rep 16, 42 (2026). https://doi.org/10.1038/s41598-025-28683-2
Received:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s41598-025-28683-2