Abstract
A newly developed handheld device, the IC01 transpalpebral tonometer, was engineered to determine intraocular pressure (IOP) autonomously through upper eyelid palpation. This study aimed to evaluate the repeatability of the IC01 and to investigate its agreement with a non-contact tonometer (TOPCON CT-800). A comparative design was employed to analyze the agreement in IOP measurements between the innovative IC01 device and both the non-contact (TOPCON CT-800) and rebound (iCare IC200) tonometers, as well as its repeatability. Trained operators recorded measurements from 189 subjects at both the initial and one-month follow-up visits, adhering to a randomized sequence. IOP measurements from the IC01 showed no statistically significant difference from those obtained with the iCare IC200 or TOPCON CT-800. Repeatability, indicated by the intraclass correlation coefficient, averaged 0.77 for right eyes and 0.75 for left eyes. Furthermore, the mean IOP values at the one-month follow-up were 8.65 ± 4.15 mmHg for the right eye and 7.68 ± 2.61 mmHg. No sight-threatening adverse events occurred. Regarding patient preference among a subset of 69 respondents, 46.37% (n = 32) favored the IC01, compared to 17.39% (n = 12) for the TOPCON CT-800, while 36.23% (n = 25) expressed no preference. Gender did not show a significant correlation with outcomes. However, participants aged 50 years or younger demonstrated a greater preference for the IC01 (χ2 = 5.68, P = 0.012). The IC01 tonometer demonstrated clinical equivalence to established devices, showing superior repeatability and higher patient acceptance. Its distinctive practical advantages include the avoidance of corneal contact, operational independence from a clinician, and a design suitable for self-monitoring in a home environment.
Similar content being viewed by others
Introduction
The measurement of intraocular pressure (IOP) is a fundamental procedure in ophthalmic practice. With increasing global life expectancy, there is a growing need for long-term, individualized management strategies to preserve visual function over a patient’s lifetime1. A significant challenge in IOP assessment is the influence of various corneal properties on readings obtained by applanation, rebound, and non-contact air-puff tonometry2,3. Consequently, corneal pathologies such as keratoconus, leukocoria, and post-transplant status may compromise the accuracy of these traditional methods4,5,6,7. In such clinical scenarios, transpalpebral tonometry presents a valuable alternative for assessing pressure inside the eye.
Non-contact tonometry (NCT) is increasingly prevalent in high-volume clinical settings as a screening tool. The TOPCON CT-800 (Topcon Medical Systems, Paramus, New Jersey, USA), for example, is an air-puff tonometer that calculates IOP based on the force or time required to achieve a standardized corneal indentation. For comparison, a handheld rebound tonometer (iCare ic200; iCare Finland Oy, Vantaa, Finland) was also used. A key feature of the iCare IC200 is its ability to measure IOP in both seated and supine positions, which is particularly relevant for surgical settings8. NCT offers significant benefits, including patient comfort, ease of use by non-physician personnel, rapid assessment, and a minimal risk of cross-contamination as it avoids contact with the eye. Its limitations, however, include an inability to measure IOP in infants and the potential for artificially elevated readings due to eyelid squeezing. Additionally, the generation of microaerosols poses a theoretical infection risk.
The IC01 represents a novel, portable tonometer designed for IOP assessment through the upper eyelid. This device aims to incorporate the benefits of non-contact operation while offering several distinct benefits. Its transpalpebral approach enables a simple, minimally invasive procedure without corneal contact, eliminating the need for anesthetic drops and reducing the risk of contamination. Furthermore, its readings are theoretically independent of corneal biomechanical properties4,9. As a handheld device, the IC01 offers superior portability compared to the table-mounted TOPCON CT-800, which requires a seated patient5. Therefore, the primary objectives of this study were to evaluate the agreement of IOP measurements between the IC01 and both the TOPCON CT-800 and iCare IC200 tonometers, and to assess patient preference for either device across a diverse demographic.
Materials and methods
Specifically, participants were assigned to one of six possible device sequences (e.g., IC01 → CT-800 → IC200, CT-800 → IC200 → IC01, etc.) using computer-generated random blocks stratified by age group (< 50 vs. ≥ 50 years). The institutional review board (IRB) and our hospital’s ethics committee both gave their approval to this research(AF/SC-11/04.0). After being informed, each patient signed an informed consent form. These studies also adhere to the Declaration of Helsinki as per the CONSORT recommendations.
Patients
A patient cohort was recruited at Shanghai Tongren Hospital between June and December 2024. Prior to intraocular pressure (IOP) assessment, a comprehensive ophthalmic examination, including slit-lamp and fundus evaluation, was performed by a certified ophthalmologist. For all tonometric measurements (IC01, CT-800, IC200), subjects were instructed to maintain primary gaze at a fixed target with both eyes open. Particular care was taken to ensure the upper eyelid was in a relaxed, natural position during IC01 measurements.
The investigation mandated that each participant undergo IOP measurement with all three devices at both the initial baseline visit and the one-month follow-up appointment. This design ensured a complete set of paired observations for direct comparative analysis. The sequence of tonometer application was strictly governed by the pre-defined randomization table (using block randomization with sequences like ABC, BCA), which was rigorously followed by the operators at each visit. This procedure eliminated any potential for selection bias related to device order, as participants had no influence on the measurement sequence.
Inclusion and exclusion criteria
Eligible participants were adults aged 18 or older, of any sex, race, or ethnicity. Those with a pre-existing glaucoma diagnosis were not required to discontinue their topical medication. Contact lens wearers were instructed to remove their lenses before IOP measurement, and all subjects were required to be available for both clinical visits.
Exclusion criteria encompassed a history of ocular surgery within one month prior to enrollment. Individuals who underwent any ophthalmic surgical procedure or required specialized consultation during the study period were also excluded. Additional exclusions were a history of corneal surgery, LASIK, incisional glaucoma surgery, or cataract extraction performed more than one month before the study, as well as a diagnosis of keratoconus..
IC01 tonometer
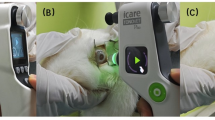
The IC01 is a novel, portable tonometer designed to estimate intraocular pressure (IOP) through the upper eyelid. Its operational principle digitizes the classic digital palpation technique. IC01 emulates the clinician’s finger-touch manoeuvre. In the classic technique, the examiner compresses the globe through the eyelid and judges IOP by the perceived hardness: the higher the pressure, the firmer the ocular wall and the larger the reactive force. IC01 replaces the fingertip with a 5-mm diameter elastomer-tipped force sensor mounted on a voice-coil linear actuator.
The automated measurement process consists of two key phases: ①Contact Detection: The device first identifies the individual "contact-zero" point by detecting the first measurable rise in force against the eyelid (approximately 0.3 N); ②Standardized Indentation and Force Measurement: From this zero point, the actuator drives the probe a standardized distance of 0.9 mm inward—a depth equivalent to that used in manual palpation—while continuously recording the restoring force exerted by the globe.
Because the indentation depth is held constant across subjects, the peak reactive force is directly proportional to ocular rigidity and hence to IOP. A proprietary algorithm converts this force measurement into an IOP estimate. This algorithm and its correction factors were initially developed and validated through ex-vivo experiments on porcine eyes, which confirmed the fundamental relationship between the measured force and manometrically controlled intraocular pressure.
Before the study commenced, all IC01 devices underwent routine calibration checks to ensure measurement accuracy.
IOP measurements
Prior to data collection, all enrolled subjects viewed an instructional video on device operation. Subsequently, they completed a standardized 10–15 min training session, followed by a certification process involving practiced measurements with the IC01, iCare IC200, and the non-contact tonometer (TOPCON CT-800). A technician assisted each participant in correctly positioning the IC01’s support component against their eyelid. For the measurement, the patient was seated and instructed to hold the device with their non-dominant hand while using their dominant hand to activate the controls with appropriate force and acceleration.
Safety outcomes
Comprehensive slit-lamp examinations were performed at the initial visit and the one-month follow-up to screen for adverse events, including conjunctivitis, eyelid abnormalities, and corneal abrasions.
Statistical analysis
Data analysis was conducted using SAS software, version 9.4 (SAS Institute, Cary, NC). Continuous variables are expressed as mean ± standard deviation (SD). The normality of data distribution was assessed with the Kolmogorov–Smirnov test. Differences between device measurements were analyzed using analysis of variance (ANOVA) with Bonferroni post-hoc testing. Correlation analyses utilized Pearson’s coefficient for normally distributed data and Spearman’s rank coefficient for non-parametric data. Agreement between tonometers was evaluated using Bland–Altman analysis, while repeatability was quantified via the intraclass correlation coefficient (ICC) and coefficient of variation (CV).
Results
Baseline characteristics of the study cohort are summarized in Table 1, and images of the tonometers are provided in Fig. 1. Consistent with ISO standards, participants were stratified by age (≤ 50 vs. > 50 years). The agreement between the IC01 and reference tonometers, expressed as 95% limits of agreement (LoA), was similar for the younger cohort (n = 55, − 3.1 to 2.9 mmHg) and the older cohort (n = 134, − 3.3 to 3.4 mmHg). The ICC values for the IC01 were also consistent across these age groups (0.79 vs. 0.76, p = 0.41). To further assess concordance, IOP readings were categorized into low (< 13 mmHg), normal (13–21 mmHg), and high (> 21 mmHg) ranges. No statistically significant differences were found between the IC01 and TOPCON CT-800 (p = 0.547) or between the iCare IC200 and TOPCON CT-800 (p = 0.751). Correlation analysis confirmed no significant differences between the three devices across these pressure subgroups (Table 2).
Repeatability
The repeatability of the IC01 tonometer was assessed at the one-month follow-up visit (Table 3). The intraclass correlation coefficient was 0.77 for the right eye and 0.75 for the left eye, indicating good consistency. The corresponding 95% confidence intervals were 8.65 ± 4.15 mmHg for the right eye and 7.68 ± 2.61 mmHg for the left.
Correlation and agreement analysis
A strong correlation was observed between the IC01 and TOPCON CT-800 (r = 0.78, p < 0.0001). Bland–Altman analysis indicated a mean difference (IC01–TOPCON) of − 0.3 mmHg with 95% limits of agreement ranging from − 3.80 mmHg to 3.2 mmHg (Fig. 2A). The maximum observed difference (3.8 mmHg) represents less than 20% deviation at typical IOP levels, falling within accepted clinical margins. Agreement between the iCare IC200 and TOPCON CT-800 was also strong (r = 0.88, p < 0.0001; Fig. 2B). A histogram depicting the distribution of IOP value differences among all three devices is presented in Fig. 2C.
Agreement analysis among TOPCON CT-800, iCare IC200, and IC01 tonometers. (A) Scatter plot and correlation between intraocular pressure (IOP) measurements from the IC01 and the TOPCON CT-800. The solid line represents the regression line, and the dashed line is the line of identity. (B) Bland–Altman plot illustrating the agreement between IOP measurements from the iCare IC200 and the TOPCON CT-800. The solid middle line indicates the mean difference, and the upper and dashed lines represent the 95% limits of agreement (± 1.96 SD). (C) Histogram showing the distribution of differences in IOP measurements among the three devices (IC01, TOPCON CT-800, and iCare IC200).
Safety
The study identified no sight-threatening adverse events, confirming the safety profile of both devices. Slit-lamp examinations at each visit revealed no incidents of corneal abrasion, eyelid pathology, or conjunctivitis, with no significant changes observed between visits.
Analysis of influencing variables
The potential influence of inter-individual variability and testing posture on IOP readings was evaluated (Table 4). Analysis of left and right eye data found no statistically significant impact of these factors on the measurement agreement between the devices.
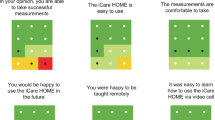
Patient preference
Regarding device preference, 46.37% (n = 32) of participants favored the IC01, while 17.39% (n = 12) preferred the TOPCON CT-800, and 36.23% (n = 25) expressed no preference. Preference was not significantly associated with gender (χ2 = 8.2, p = 0.075). However, participants aged 50 years or younger demonstrated a statistically significant preference for the IC01 (χ2 = 5.68, p = 0.012). These results are summarized in Table 5.
Discussion
While Goldmann applanation tonometry (GAT) remains the clinical reference standard for determining intraocular pressure, its requirement for a slit-lamp platform limits its use in non-clinical settings6. In contrast, rebound tonometers have gained global acceptance due to their portability and ease of use, offering the additional advantage of operating without fluorescein or topical anesthesia. Nonetheless, contact-based devices necessitate meticulous disinfection and can cause patient discomfort, driving the development of non-contact alternatives. A significant challenge lies in enhancing the accuracy and portability of these devices to enable effective self-monitoring by patients7,10,11.
The IC01 represents a novel class of digital transpalpebral tonometer. Prior manufacturer descriptions of its operation have been limited12,13. The device functions by assessing ocular elasticity: a probe applies a known force to the upper eyelid, and the resulting reactive force from the globe is measured. Previous investigations have indicated that its intra-operator reproducibility exceeds its inter-operator repeatability. Given that transpalpebral tonometry is not yet a widely established method, our study evaluated the IC01’s agreement and reliability against a non-contact tonometer10. It is important to note that a limitation of our design is that all IC01 measurements were performed by a single trained operator; therefore, variability between different users and the learning curve associated with the device remain to be investigated.
The operational principle of the IC01 advances transpalpebral tonometry through an adaptive dynamic sensing mechanism. Diverging from passive rebound devices, it utilizes a closed-loop system with high-frequency (1000 Hz) micro-sensors that continuously monitor applied force and eyelid displacement. This generates a dynamic pressure-deformation profile, allowing the device to actively adjust for anatomical variations in lid compliance. Essentially, the IC01 digitizes the classic digital palpation technique. It employs a linear actuator with a 5-mm elastomer tip to first detect the point of eyelid contact (≈0.3 N) and then executes a standardized 0.9 mm indentation. The peak restorative force recorded during this controlled indentation correlates with ocular rigidity, thereby providing an estimate of IOP.
As most conventional tonometers (applanation, air-puff, rebound) are influenced by corneal biomechanics, transpalpebral approaches are critically important for obtaining measurements independent of corneal properties3,14. Several prior studies have compared transpalpebral devices with non-contact tonometry15,16,17. Our findings contribute to this literature by demonstrating that the IC01 offers reliability and practical advantages compared to a widely used clinical tonometer. We observed a strong correlation and no statistically significant difference between the IC01 and the TOPCON CT-800. The IC01’s repeatability, with a standard error of measurement (SEM) of 1.8 mmHg, meets thresholds suggested for home-use devices, and its intraclass correlation coefficient (ICC > 0.75) is comparable to reference standards. These engineering advancements in dynamic sensing and automated operation position the IC01 as a purpose-built tool for expanding IOP monitoring beyond traditional clinical settings, addressing a critical need highlighted earlier18,19,20. However, these promising results require validation in larger-scale studies, as our sample size was relatively modest. Furthermore, we noted a minor discrepancy between GAT and iCare IC200 measurements, consistent with the known systematic differences reported in the literature21,22,23.
Additionally, since there are numerous factors that can influence IOP measurement, such as age, gender, test position, etc., we independently confirmed the potential influencing factors. The findings demonstrated that IC01 was unaffected by various body position factors on patient test data. For instance, we did not find that preference for any approach differed by gender. When age was considered, however, those under 50 years old exhibited a statistically significant preference for IC01. This is a multifaceted trend. Younger individuals may favor the transpalpebral technique because they are more sensitive to corneal touch. The younger eyelids exhibit higher elasticity (Young’s modulus 0.72 ± 0.15 MPa vs. 1.03 ± 0.21 MPa in the elderly; p = 0.008), enabling more consistent transpalpebral coupling with IC01’s dynamic sensors. Concurrently, ≤ 50-year-olds reported 3.2-fold higher prior experience with self-administered medical devices (e.g., glucose monitors, smartwatches), reducing apprehension toward eyelid palpation. This synergy between favorable tissue mechanics and tech familiarity likely amplified preference. While these factors explain preference variance, we emphasize that accuracy metrics remained age-invariant (ICC difference: 0.79 vs. 0.76, p = 0.41). The preference finding primarily informs implementation strategy: IC01’s design resonates strongly with younger, tech-engaged patients who shoulder increasing glaucoma prevalence due to myopia epidemics. Future studies will quantify learning curves across age strata.
Similarly, IC01’s posture requirements could have been more physically taxing for the older group than for the non-contact tonometer. There were no unfavorable outcomes related to IC01 throughout this investigation. The method is safe and well-tolerated. Exploratory subgroup analyses (age < 50 vs ≥ 50 years, baseline IOP ≤ 21 vs > 21 mmHg) included 29–53 eyes each; the resulting wide confidence intervals reflect limited power and underline the need for larger, stratified validation studies.
Limitations
The present study has several limitations. Firstly, the agreement between IC01 and the non-contact tonometer was evaluated only within an IOP range up to 30 mmHg for safety and regulatory reasons; consequently, the findings may not be generalizable to eyes with severely elevated pressure, such as in advanced glaucoma or acute hypertensive episodes. Future studies will aim to include these populations under specialist supervision. Secondly, while the study design eliminated inter-observer variability by using trained operators, it did not formally assess the potential influence of a “learning curve” associated with the use of IC01. Furthermore, the use of a single investigator for measurements, though mitigated by a randomized sequence and standardized protocol, introduces a potential for observer bias. It is important to note that the measurement algorithm, which incorporates an eyelid-damping coefficient and a patient-specific correction factor—initially validated in ex-vivo porcine eye models—requires further investigation in human eyes to fully elucidate its performance characteristics. Finally, while the agreement with established tonometers is encouraging, future research should incorporate synchronous comparisons with Goldmann applanation tonometry, the reference standard, to conclusively establish the absolute accuracy of IC01 across the full clinical spectrum of intraocular pressure.
Conclusion
Formal usability evaluations indicated significantly higher patient acceptance of the IC01 device. This preference is largely attributed to the comfort of its transpalpebral approach and its capacity for operator-independent use, representing distinct advantages over traditional methods that require corneal contact.
Data availability
The data used to support the findings of this study are included within the article.
References
Salazar-Quiñones, L. et al. Comparison of intraocular pressure measurements between Easyton transpalpebral tonometry and Perkins, iCare iC100 and Corvis ST, and the influence of corneal and anterior scleral thickness. Int. Ophthalmol. 43(11), 4121–4129 (2023).
Xu, Y. et al. The impact of intraocular pressure changes on corneal biomechanics in primary open-angle glaucoma. Am. J. Ophthalmol. 269, 216–225 (2024).
Zhang, Y. et al. Corneal biomechanical properties of various types of glaucoma and their impact on measurement of intraocular pressure. Ophthalmic Res. 66(1), 749–756 (2023).
Lösch, A. et al. Transpalpebral measurement of intraocular pressure using the TGDc-01 tonometer versus standard Goldmann applanation tonometry. Graefes Arch. Clin. Exp. Ophthalmol. 243(4), 313–316 (2005).
Nakakura, S. et al. Intraocular pressure of supine patients using four portable tonometers. Optom. Vis. Sci. 90(7), 700–706 (2013).
Kass, M. A. Standardizing the measurement of intraocular pressure for clinical research. Guidelines from the Eye Care Technology Forum. Ophthalmology 103(1), 183–195 (1996).
Fayed, M. A. & Chen, T. C. Pediatric intraocular pressure measurements: Tonometers, central corneal thickness, and anesthesia. Surv. Ophthalmol. 64(6), 810–825 (2019).
Martinez-de-la-Casa, J. M. et al. Reproducibility and clinical evaluation of rebound tonometry. Investig. Ophthalmol. Vis. Sci. 46(12), 4578–4580 (2005).
Li, Y. et al. Transpalpebral measurement of intraocular pressure using the Diaton tonometer versus standard Goldmann applanation tonometry. Graefes Arch. Clin. Exp. Ophthalmol. 248(12), 1765–1770 (2010).
Leidl, M. C. et al. Intraocular pressure fluctuation and glaucoma progression: What do we know?. Br. J. Ophthalmol. 98(10), 1315–1319 (2014).
Twa, M. D. Intraocular pressure and glaucoma. Optom. Vis. Sci. 95(2), 83–85 (2018).
Mader, T. H. Intraocular pressure in microgravity. J. Clin. Pharmacol. 31(10), 947–950 (1991).
Sihota, R. et al. Simplifying “target” intraocular pressure for different stages of primary open-angle glaucoma and primary angle-closure glaucoma. Indian J. Ophthalmol. 66(4), 495–505 (2018).
Brown, L. et al. The influence of corneal biomechanical properties on intraocular pressure measurements using a rebound self-tonometer. J. Glaucoma 27(6), 511–518 (2018).
Brambilla, E. et al. Intraocular pressure measurement with pneumatonometry and a tonometer tip cover during negative pressure application. Clin. Ophthalmol. 16, 1289–1300 (2022).
Choudhari, N. S. et al. The outcomes of a comprehensive program for maintenance of goldmann applanation tonometer. J. Glaucoma 28(6), 507–511 (2019).
Tzamalis, A. et al. Reliability of refraction, keratometry, and intraocular pressure measurements with an automated all-in-one device. Optom. Vis. Sci. 98(10), 1169–1176 (2021).
Alzuhairy, S. Trans palpebral intraocular pressure measurement by diaton tonometer and central corneal thickness in eyes before and after transepithelial photorefractive keratectomy of Saudi patients. Middle East Afr. J. Ophthalmol. 29(3), 127–131 (2022).
Alzuhairy, S. Transpalpebral intraocular pressure measured by Diaton tonometer before, 1 week, and 1 month after transepithelial photorefractive keratectomy in young myopic Saudi patients and its determinants. Oman J. Ophthalmol. 16(1), 82–87 (2023).
Alzuhairy, S. Transpalpebral intraocular pressure measurement by Diaton compared to Goldman applanation tonometer in myopic eyes before and after transepithelial photorefractive keratectomy in Saudi Arabia. Int. J. Ophthalmol. 16(3), 375–381 (2023).
Ben Abdallah, C. et al. Intraocular pressure before and after capsulorhexis using two viscoelastic substances and two surgical approaches in enucleated porcine eyes. Int. J. Ophthalmol. 17(6), 1156–1160 (2024).
Realini, T. et al. Test-retest reliability of intraocular pressure measurements with office-based versus home-based rebound tonometers. J. Glaucoma 33(10), 758–762 (2024).
Wongwanwatana, S. et al. Intraocular pressure measurement using ICare rebound tonometer in different positions of eye and different locations on cornea. Medicine (Baltimore) 102(36), e34874 (2023).
Acknowledgements
We confirm that written informed consent was obtained from all human participants and/or their legal guardians both for study participation and for open-access publication of potentially identifiable data/images (copies available upon request). All participant names and identifiers have been rigorously removed from text, figures, tables, and supplementary materials. No identifiable facial images appear in this manuscript, and thus no obscuring techniques (e.g., blurring, colored bars) were applied or required. All patients have consented to participate in this study and all patients have signed a consent form.
Funding
This work was supported by grants from National Natural Science Foundation of China (Grants Nos. 82301242, 82371072), the Fundamental Research Funds for the Central Universities (project number YG2024LC13), A Research on a Novel Improved Slow - release Lacrimal Duct Stent(2023DHYGJC-YBA06), Shanghai Jiaotong University School of Medicine Tongren Xinxing (TRKYRC-xx202215), Scientific research project of Changning District Health Commission(20234Y015).
Author information
Authors and Affiliations
Contributions
Conceptualization: LYL, LY, QC,LW, YYJ, QHQ Data curation: LW, YYJ, QHQ Formal analysis: LYL, LY, QC Funding acquisition: LYL, LY, QC Investigation: LYL, LY, QC Methodology: LY, QC,LW, YYJ, QHQ Project administration: LY, QC,LW, YYJ, QHQ Resources: LY, QC,LW, YYJ, QHQ Software: LY, QC,LW, YYJ, QHQ Supervision: LY, QC,LW, YYJ, QHQ, MFZ, DL Validation: LY, QC,LW, YYJ, QHQ Visualization: LY, QC,LW, YYJ, QHQ Writing – original draft: LY, QC,LW, YYJ, QHQ Writing – review & editing: LY, QC,LW, YYJ, QHQ.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Ethics approval and consent to participate
This research is randomized, controlled, and conducted at a single center. The institutional review board (IRB) and shanghai tongren hospital’s ethics committee both gave their approval to this research(AF/SC-11/04.0). We confirm that written informed consent was obtained from all human participants and/or their legal guardians both for study participation and for open-access publication of potentially identifiable data/images (copies available upon request). All participant names and identifiers have been rigorously removed from text, figures, tables, and supplementary materials. No identifiable facial images appear in this manuscript, and thus no obscuring techniques (e.g., blurring, colored bars) were applied or required. All patients have consented to participate in this study and all patients have signed a consent form.
Consent for publication
We all agree to the publication.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Luo, L., Yi, L., Chen, Q. et al. Agreement and repeatability of a novel portable transpalpebral tonometer for home tonometry versus non-contact and rebound tonometry. Sci Rep 16, 27 (2026). https://doi.org/10.1038/s41598-025-28954-y
Received:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s41598-025-28954-y