Abstract
This study aimed to investigate the role of the AIM2 inflammasome pathway-mediated pyroptosis in severe acute pancreatitis-induced brain injury (SAP-IBI) utilizing a murine model. A male C57BL/6 murine model of SAP-IBI was established. Mice were randomized into five groups: sham-operated controls (SO), SAP-IBI model, adeno-associated virus negative control (SAP + AAV-NC), AIM2 silencing via AAV-delivered shRNA (SAP + AAV-AIM2 shRNA), and AAV-mediated AIM2 overexpression (SAP + AAV-AIM2 OE). Assessment at 24 h included mortality rates, modified neurological severity scores (mNSS), histopathological scoring of pancreatic and hippocampal tissues, hippocampal water content, serum biomarkers (amylase, lipase, IL-1β, IL-18, PLA2), and expression of pyroptosis-related molecules (AIM2, ASC, Caspase-1, GSDMD, IL-1β, IL-18) in the hippocampus. At 24 h post-operation, the mortality rates of the SAP-IBI group, SAP + AAV-AIM2 shRNA group and SAP + AAV-AIM2 OE group did not reach statistical significance. Compared with the SAP-IBI group, the SAP + AAV-AIM2 shRNA group exhibited significantly improved outcomes including reduced neurological deficits (mNSS: 8.63 ± 1.06 vs. 13.00 ± 1.00; P < 0.05), markedly lower histopathological scores in both hippocampal and pancreatic tissues (P < 0.05), attenuated hippocampal edema (water content: 78.11 ± 0.45 vs. 80.70 ± 0.81; P < 0.05). These improvements were accompanied by a marked reduction in serum biomarkers (P < 0.05) and downregulation of hippocampal pyroptosis-related molecules (P < 0.05). Conversely, the SAP + AAV-AIM2 OE group showed significant exacerbation in all parameters compared to the SAP-IBI group (P < 0.05). This study demonstrates that the AIM2 inflammasome promotes hippocampal pyroptosis in SAP-IBI pathogenesis. Modulating this pathway may represent a promising therapeutic strategy for enhancing neurological outcomes.
Similar content being viewed by others
Introduction
Acute pancreatitis (AP) is an inflammatory disorder initiated by the premature activation of digestive enzymes within the pancreas, leading to local tissue damage that can progress to systemic complications. Globally, the AP incidence ranges from 13 to 49 cases per 100,000 individuals annually, with reported mortality around 2.0%1,2. Notably, approximately 20% of patients develop moderately severe or severe acute pancreatitis (SAP), which is characterized by rapid clinical deterioration, systemic inflammatory response syndrome (SIRS), and multiple organ dysfunction syndrome (MODS). Despite advances in critical care, SAP mortality remains high, reaching up to 30%3,4.
A frequent and serious neurological complication of SAP is SAP-induced brain injury (SAP-IBI), also termed pancreatic encephalopathy. SAP-IBI manifests through diverse neuropsychiatric symptoms, including cognitive impairment, consciousness alterations, and affective disturbances5. The reported incidence is 11%, and the mortality rate is between 28.83% and 43.00% for SAP-IBI6. A critical pathophysiological aspect of SAP-IBI involves the disruption of the blood-brain barrier (BBB), which facilitates the entry of T lymphocytes and pro-inflammatory cytokines from the circulation into brain parenchyma, thereby exacerbating cerebral edema and neuronal injury7. Current therapeutic management of SAP-IBI remains challenging, with no established consensus on effective treatment protocols8. This underscores the urgent need to elucidate its underlying mechanisms and identify novel therapeutic targets.
Pyroptosis, a form of regulated cell death mediated by Gasdermin family proteins (particularly Gasdermin D, GSDMD) and inflammatory caspases, contributes significantly to inflammatory tissue injury. It features cell swelling, membrane perforation, and the release of pro-inflammatory cytokines9,10. In SAP, GSDMD-mediated pyroptosis in the intestinal epithelium has been linked to SIRS and barrier dysfunction11,12, while pharmacological agents like taraxasterol show potential in attenuating pyroptosis and mitigating injury13. The absent in melanoma 2 (AIM2) inflammasome, a cytosolic sensor for double-stranded DNA, plays a pivotal role in initiating pyroptosis. Upon activation, AIM2 recruits ASC and pro-Caspase-1 to form an inflammasome complex, leading to Caspase-1 activation, cleavage of GSDMD and pro-IL-1β/IL-18, and amplified inflammatory cascades14,15. In AP models, AIM2 is up-regulated in peripheral blood mononuclear cells, correlating with increased IL-1β and IL-18 levels and exacerbated pancreatic injury16. Salidroside has been suggested to ameliorate pancreatic injury possibly via AIM2 inflammasome inhibition17, though direct evidence is limited.
To date, the specific role of AIM2 inflammasome-driven pyroptosis in the progression from SAP to brain injury remains poorly defined. By systemically modulating AIM2 expression in a murine SAP-IBI model, our study aims to delineate the contribution of AIM2-mediated pyroptosis to hippocampal damage and BBB disruption. We hypothesize that AIM2 silencing will confer protection, whereas its overexpression will exacerbate injury. This mechanistic investigation may not only advance our understanding of SAP-IBI pathogenesis but also identify AIM2 as a potential target for developing much-needed therapeutic interventions.
Materials and methods
Ethical approval
This study received ethical approval (approval code: YZYY-2023-03) from the Institutional Review Board of Yizheng Hospital, which is affiliated with the Nanjing Drum Tower Hospital Group. The Ethics Committee of Nantong University School of Medicine conducted a thorough review and formally authorized all animal experimental procedures, ensuring strict compliance with the standards set forth in the *Animal Research: Reporting of In Vivo Experiments (ARRIVE) Guidelines* and the *National Research Council (NIH) Guide for the Care and Use of Laboratory Animals* (8th Edition, 2011).
Preparation of animals
Male C57BL/6 mice, aged 8 to 10 weeks and weighing between 22 and 25 g, were procured from the Animal Research Facility at Nantong University Medical College. These animals were housed in a controlled environment that maintained a 12-hour light/dark cycle, with continuous access to standard rodent chow and water. All experimental protocols complied with institutional guidelines and were conducted under ethical certifications approved by the facility.
Mouse models of SAP-IBI
The SAP-IBI murine model was established according to the protocol described by Wu et al.18, via retrograde infusion of sodium taurocholate solution (STS). Briefly, mice were fasted for 12 h with free access to water before surgery. Anesthesia was induced by intraperitoneal injection of 2% pentobarbital sodium (0.25 mL/100 g body weight). Following laparotomy, a freshly prepared 3.5% STS (Sigma-Aldrich, MO, USA) was retrogradely infused into the pancreaticobiliary duct at a constant rate of 0.1 mL/min. Sham-operated (SO) control animals received an equal volume of sterile saline. All incisions were sutured with 3 − 0 silk. Successful induction of SAP-IBI was confirmed by the presence of neurological impairment assessed 6 h after STS injection.
Experimental procedures
Adeno-associated viral vectors (AAVs), including control (AAV-NC), AIM2- targeting short hairpin RNA (AAV-AIM2 shRNA), and AIM2 overexpression (AAV-AIM2 OE) constructs, were produced by HAMBIO under strict quality control. Fifty mice were randomly assigned to five groups: SO, SAP-IBI, SAP + AAV-NC, SAP + AAV-AIM2 shRNA, and SAP + AAV-AIM2 OE. The SAP + AAV-NC group received 3 × 10^11 viral genome (v.g.) control vectors via tail vein injection. After 21 days, the SAP-IBI model was induced. The SAP + AAV-AIM2 shRNA and SAP + AAV-AIM2 OE groups were administered the respective vectors at the same dose and schedule, following manufacturer guidelines. Following model establishment by retrograde infusion of 3.5% STS into the pancreaticobiliary duct, mice were monitored for 24 h before sample collection. Surviving mice were euthanized systematically, and tissue and blood samples were collected for further analysis.
Mortality rate
The 24-hour mortality rate was assessed for each of the five experimental groups of mice.
Blood and tissue Preparation
All surviving mice were euthanized 24 h post-intervention. Prior to euthanasia, blood was collected via left ventricular puncture under anesthesia induced by intraperitoneal injection of Zoletil/Xylazine (5:1, 0.1 mL per 10 g of body weight). Successful anesthesia was confirmed by the absence of withdrawal and corneal reflexes. Cervical dislocation was performed 3–5 min after anesthesia induction. Blood samples were collected in EDTA-containing vacutainers and centrifuged at 3000 rpm for 10 min. The resulting serum was stored at − 20 °C for subsequent biochemical analysis. Pancreatic and hippocampal tissues were harvested aseptically. Tissues for histopathology were fixed in 4% paraformaldehyde, while those for ultrastructural analysis were immersed in 2% glutaraldehyde for transmission electron microscopy (TEM). The remaining hippocampal tissues were flash-frozen in liquid nitrogen and stored at − 80 °C for subsequent analysis via Western blot (WB) and quantitative real-time polymerase chain reaction (qRT-PCR).
Evaluation of water content changes in hippocampal tissue
The hippocampal tissues were collected and immediately weighed to obtain the fresh mass. After complete dehydration, the dried samples were reweighed, and the water content was calculated using the following formula:
Histology
Pancreas histology
Pancreatic tissues were fixed in 10% neutral buffered formalin, embedded in paraffin, and sectioned at 4 μm. Sections were stained with hematoxylin and eosin (HE) and evaluated histopathologically based on Schmidt’s criteria19, which score edema, inflammatory cell infiltration, and acinar cell necrosis, as well as hemorrhage. Detailed criteria are summarized in Table 1. Ten non-overlapping fields (×200) per section were examined independently by two blinded pathologists. The final histopathological score for each sample was calculated as the mean of all assessments.
Hippocampal histology
The hippocampal tissues were stained with HE for histological evaluation and examined by TEM (Hitachi HT7700, Tokyo, Japan) after 24-hour fixation in 2% glutaraldehyde. Pathological changes were scored using Kong’s brain injury grading scale20, which assesses neuronal integrity, glial cell activation, myelin sheath preservation, and vascular morphology (each scored 0–3). Injury severity was categorized as mild (1–6), moderate (7–9), or severe (10–12). Detailed criteria are provided in Table 2.
Neurological function scores
Neurological function in surviving mice was evaluated using the modified neurological severity score (mNSS) system21. The mNSS assessed a range of sensorimotor functions, including motor coordination, balance, reflexes, and sensory responses. The scoring scale ranged from 0 (indicating normal function) to 18 (representing severe outcomes such as unconsciousness or mortality), with higher values reflecting greater neurological deficits. Detailed grading criteria are provided in Table 3.
Measurement of serological markers
Serum concentrations of amylase, lipase, IL-1β, and IL-18 were quantified using commercial enzyme-linked immunosorbent assay (ELISA) kits (Wuhan Xinqidi, China), adhering to standardized protocols. The activity of phospholipase A2 (PLA2) in serum samples was assessed according to the methodology described in Chen’s study22. These analytical techniques facilitate accurate measurement of biochemical markers that are critical for evaluating inflammatory processes and pancreatic function in SAP-IBI.
qRT-PCR Analysis
To evaluate the expression levels of messenger ribonucleic acid (mRNA) for AIM2, IL-1β, IL-18, apoptosis-associated speck-like protein containing a CARD (ASC), Caspase-1, GSDMD, and glyceraldehyde-3-phosphate dehydrogenase (GAPDH) in hippocampal tissues, qRT-PCR analysis was performed. Total RNA was extracted using Trizol reagent (Life Technologies, USA), and reverse-transcribed into cDNA. Gene-specific primers were designed with Primer3Plus (Version 3.3.0, URL: https://www.primer3plus.com/) and detailed primer sequences are provided in Table 4.
PCR amplification was performed on an Eppendorf Mastercycler (PREMIER Biosoft, USA) under the following conditions: initial denaturation at 95℃ for 20 min, followed by 40 cycles of 95℃ for 30 s, 54–55℃ for 30 s, and 72℃ for 40 s. GAPDH was used as the reference gene, and relative expression was calculated using the comparative threshold cycle method (2−ΔΔCt). This precise measurement of transcriptional activity is critical for elucidating the molecular mechanisms involved in SAP-IBI.
Western blot analysis
At 24 h post-operation, hippocampal protein expression in the five groups was assessed by Western blotting. Protein lysates were separated on 8% SDS-PAGE gels and transferred to nitrocellulose membranes. After blocking with 5% non-fat milk in TBST for 2 h, the membranes were incubated overnight at 4℃ with the following primary antibodies from Immunoway (USA) and Cohesion (UK): anti-AIM2 (1:2000), anti-ASC (1:2000), anti-GSDMD-N (1:5000), anti-cleaved-Caspase-1 (1:2000), and anti-GAPDH (1:4000). Following three washes, membranes were incubated for 2 h at room temperature with HRP-conjugated goat anti-rabbit IgG (1:4000, Pierce Biotechnology, USA). Protein bands were visualized by chemiluminescence, and band densities were quantified using ImageJ software (Version 1.48q, URL: https://imagej.net/software/imagej/). GAPDH was used for normalization to ensure measurement accuracy.
Correlation analysis
Correlation analysis was conducted on all indices with the SAP + AAV-AIM2 OE group to evaluate the correlation between the hippocampal pathological score and the other parameters. These parameters included the pancreatic pathological score, mNSS, hippocampal water content, serum biomarkers (amylase, lipase, IL-1β, IL-18, PLA2), and hippocampal expression of pyroptosis-related molecules (AIM2, ASC, Caspase-1, GSDMD, IL-1β, IL-18).
Statistical analysis
All quantitative data are presented as mean ± standard deviation (SD). Statistical analyses were performed using GraphPad Prism software (Version 10.1, URL: https://www.graphpad.com). Parametric data were analyzed by one-way ANOVA with Tukey’s post hoc test, while non-parametric data were assessed using the Kruskal-Wallis test followed by Dunn’s multiple comparisons. Group mortality rates were compared by Fisher’s exact test, and variable relationships were examined by Spearman’s rank-order correlation. A significance threshold of α = 0.05 was applied for all statistical inferences.
Results
Effect of AIM2 inflammasome Pathway-Mediated pyroptosis on mouse mortality
The survival outcomes at 24 h exhibited significant variations among the experimental groups: the SO group achieved complete survival, with a mortality rate of 0% (0 deaths out of 10 mice). The SAP-IBI group had a mortality rate of 50% (5 deaths out of 10 mice). The SAP + AAV-NC group exhibited a mortality rate of 40% (4 deaths out of 10 mice). The SAP + AAV-AIM2 shRNA group showed a reduced mortality rate of 20% (2 deaths out of 10 mice). The SAP + AAV-AIM2 OE group had a mortality rate of 50% (5 deaths out of 10 mice). There were no significant differences in mortality rates among the SAP-IBI group, SAP + AAV-NC group, SAP + AAV-AIM2 shRNA group, and SAP + AAV-AIM2 OE group (P > 0.05).
Silencing or overexpressing AIM2 inflammasome affects the progression of SAP-IBI in mice
Histopathological changes in pancreatic tissue and the regulatory effects of AIM2 inflammasome on pancreatic injury, serum levels of amylase, lipase, and PLA2 in SAP-IBI Mice
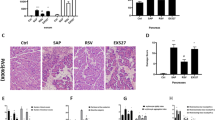
Pathological examination of pancreatic tissues revealed the following: The SO group exhibited no significant pathological abnormalities, with only occasional mild interstitial edema or scattered neutrophil infiltration (Fig. 1 A). In contrast, the SAP-IBI group at 24 h showed severe pathological injury, characterized by extensive acinar cell swelling, sublobular hemorrhage, dense neutrophil infiltration, and substantial tissue necrosis. Structural disintegration of acini, widespread lipid deposition, and parenchymal necrosis were prominent, accompanied by interstitial edema, hemorrhagic foci, inflammatory cell aggregation, and lobular architectural disruption. Distinct saponification spots were observed in both tissue samples. Compared with the SAP-IBI group, the SAP + AAV-NC group showed no significant change in pancreatic injury; whereas the SAP + AAV-AIM2 OE group demonstrated significantly aggravated pancreatic damage while the SAP + AAV-AIM2 shRNA group exhibited a marked alleviation of these pathological injuries.
Consistent with histological observations, the SAP-IBI group demonstrated significantly higher pancreatic histopathological scores and elevated serum levels of amylase, lipase, and PLA2 than the SO group at 24 h (P < 0.05), confirming successful establishment of the SAP model. Furthermore, the SAP + AAV-AIM2 OE group exhibited aggravated pathological and biochemical indices (P < 0.05). In contrast, the SAP + AAV-AIM2 shRNA group showed marked reductions in these indices (P < 0.05). Collectively, these results highlight the critical regulatory role of the AIM2 inflammasome in modulating SAP progression (Fig. 1B and E).
Pancreatic histopathology and serum injury markers in SAP-IBI mice. A: HE-stained pancreatic sections at 24-hour intervals (×200), SO group (n = 10), SAP-IBI group (n = 5), SAP + AAV-NC group (n = 6), SAP + AAV-AIM2 shRNA group (n = 8), SAP + AAV-AIM2 OE group (n = 5). B: Quantitative analysis of pancreatic histopathological scores. C: Quantitative analysis of serum amylase. D: Quantitative analysis of serum lipase. E: Quantitative analysis of serum PLA2. SO group: Sham-operation control group; SAP-IBI group: Severe acute pancreatitis-induced brain injury model group; SAP + AAV-NC group: Mice administered 3 × 10^11 v.g. of control AAV via tail vein and subjected to SAP-IBI modeling after a 21-day interval; SAP + AAV-AIM2 shRNA group: Mice administered 3 × 10^11 v.g. of AIM2-targeting shRNA AAV via tail vein and subjected to SAP-IBI modeling after a 21-day interval; SAP + AAV-AIM2 OE group: Mice administered 3 × 10^11 v.g. of AIM2-overexpressing AAV via tail vein and subjected to SAP-IBI modeling after a 21-day interval. Statistical annotations: * P < 0.05 compared with the SO group; # P < 0.05 compared with the SAP-IBI group.
Comprehensive assessment of hippocampal pathology and water Content, as well as the role of the AIM2 inflammasome in regulating these parameters in murine models of SAP-IBI
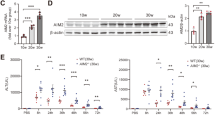
Pathological examination of hippocampal tissues revealed the following: The SO group exhibited dispersed neuronal enlargement accompanied by minor leukocytic aggregation, with no evidence of inflammatory cell infiltration (Fig. 2 A and B). In contrast, the SAP-IBI group at 24 h showed multiple pathological features, including neuronal hypertrophy with ballooning morphology, vascular congestion, multifocal capillary dilation, hemorrhagic foci, perivascular edema, inflammatory cell infiltration, and leukocyte-endothelial adhesion within microvessels. Compared with the SAP-IBI group, the SAP + AAV-AIM2 OE group demonstrated significantly aggravated neuropathological injury, whereas the SAP + AAV-AIM2 shRNA group exhibited marked alleviation of these pathological alterations.
Ultrastructural analysis via TEM corroborated these findings. The SO group displayed normal cerebral cytoarchitecture, characterized by preserved neuronal morphology and intact mitochondrial structures (Fig. 2 C). In contrast, the SAP-IBI group at 24 h exhibited multiple cytopathological abnormalities, including nuclear chromatin margination, nucleolar disappearance, cytoplasmic edema with reduced cellular density, mitochondrial dilatation, vacuolization patterns, fragmentation of the nuclear membrane, expansion of the extracellular matrix, and partial degradation of the myelin sheath. Furthermore, the SAP + AAV-AIM2 OE group showed exacerbated ultrastructural damage compared with the SAP-IBI group, while the SAP + AAV-AIM2 shRNA group showed attenuated pathological manifestations relative to the 24-hour SAP-IBI group.
At the 24-hour time point, hippocampal pathological scores were significantly elevated in the SAP-IBI group compared with the SO group (P < 0.01), confirming the successful modeling of SAP-IBI. Furthermore, pathological injury was markedly exacerbated in the SAP + AAV-AIM2 OE group relative to the SAP-IBI group (P < 0.05), whereas the SAP + AAV-AIM2 shRNA group showed substantial alleviation of these injuries (P < 0.05), indicating the regulatory role of the AIM2 inflammasome in SAP-IBI progression (Fig. 2D). Consistent with these histopathological changes, hippocampal edema assessed by tissue water content was significantly more severe in the SAP-IBI group than in the SO group (P < 0.05). Moreover, the SAP + AAV-AIM2 OE group further increased hippocampal water content compared to the SAP-IBI group (P < 0.05), while the SAP + AAV-AIM2 shRNA group effectively attenuated edema formation (P < 0.05), further supporting the involvement of the AIM2 inflammasome in the pathogenesis of SAP-IBI (Fig. 2E).
Silencing or overexpressing AIM2 inflammasome impacts neurological function of SAP-IBI in mice
At the 24-hour time point, the mNSS was significantly higher in the SAP-IBI group than in the SO group (P < 0.05). Furthermore, the score was markedly increased in the SAP + AAV-AIM2 OE group compared with the SAP-IBI group (P < 0.05), whereas the SAP + AAV-AIM2 shRNA group showed a substantial decrease (P < 0.05), underscoring the critical regulatory role of the AIM2 inflammasome in neurological impairment during SAP-IBI (Fig. 2 F).
Evaluation of hippocampal injury and neurological function in SAP-IBI mice. A: HE- stained hippocampal sections at 24-hour intervals (×40), SO group (n = 10), SAP-IBI group (n = 5), SAP + AAV-NC group (n = 6), SAP + AAV-AIM2 shRNA group (n = 8), SAP + AAV-AIM2 OE group (n = 5). B: HE-stained hippocampal sections at 24-hour intervals (×200). C: Representative TEM images depicting hippocampal ultrastructure (scale bar, 5.0 μm). D: Quantitative analysis of pathological score. E: Quantitative analysis of hippocampal water content. F: Quantitative analysis of modified neurological severity scores (mNSS). Statistical annotations: * P < 0.05 compared with the SO group; # P < 0.05 compared with the SAP-IBI group.
AIM2 inflammasome pathway affects the progression of SAP-IBI in mice
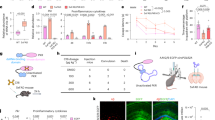
At the 24-hour time point, serum levels of IL-1β and IL-18, alongside hippocampal expression of AIM2, IL-1β mRNA and IL-18 mRNA, were significantly elevated in the SAP-IBI group compared to the SO group (P < 0.05). These increases were further enhanced in the SAP + AAV-AIM2 OE group (P < 0.05) but were markedly suppressed in the SAP + AAV-AIM2 shRNA group (P < 0.05), underscoring the pivotal role of the AIM2 inflammasome pathway in SAP-IBI pathogenesis (Fig. 3).
Effects of AIM2 modulation on systemic inflammation. A: Serum levels of IL-1β, SO group (n = 10), SAP-IBI group (n = 5), SAP + AAV-NC group (n = 6), SAP + AAV-AIM2 shRNA group (n = 8), SAP + AAV-AIM2 OE group (n = 5). B: Serum levels of IL-18. C: Relative mRNA levels of IL-1β. D: Relative mRNA levels of IL-18. Statistical annotations: * P < 0.05 compared with the SO group; # P < 0.05 compared with the SAP-IBI group.
Correlation analysis
At the 24-hour time point, the hippocampal pathological score showed significant positive correlations (all r > 0.7, P < 0.05) with the following parameters in the SAP + AAV-AIM2 OE group: serum levels of amylase, IL-1β, IL-18, PLA2; mRNA and protein expression levels of AIM2, ASC, IL-1β, IL-18, GSDMD, GSDMD-N, Caspase-1, cleaved Caspase-1; the mNSS and hippocampal water content (Fig. 4).
Notably, despite strong correlation coefficients (r > 0.7), the correlations between the hippocampal pathological score and the pancreatic pathological score or serum levels of lipase did not reach statistical significance (P > 0.05).
AIM2 inflammasome pathway-mediated pyroptosis plays a critical role in the onset and progression of brain injury induced by SAP in mice
At 24 h post-modeling, hippocampal tissues from the SAP-IBI group exhibited significant upregulation of key pyroptosis-related molecules compared to the SO group (P < 0.05), including the levels of AIM2 mRNA and protein, ASC mRNA and protein, GSDMD mRNA, Caspase-1 mRNA, and the cleaved forms of Caspase-1 and GSDMD (GSDMD-N) protein. These elevations were further enhanced in the SAP + AAV-AIM2 OE group (P < 0.05) but were significantly suppressed in the SAP + AAV-AIM2 shRNA group (P < 0.05). Together, these data demonstrate that the AIM2 inflammasome pathway drives hippocampal pyroptosis in SAP-IBI mice (Fig. 5).
Activation of the AIM2 inflammasome pathway and pyroptosis signaling in the hippocampus of SAP-IBI mice. A: Quantitative mRNA expression levels of AIM2, SO group (n = 10), SAP-IBI group (n = 5), SAP + AAV-NC group (n = 6), SAP + AAV-AIM2 shRNA group (n = 8), SAP + AAV-AIM2 OE group (n = 5). B: Quantitative mRNA expression levels of ASC. C: Quantitative mRNA expression levels of GSDMD. D: Quantitative mRNA expression levels of Caspase-1. E: Representative blots illustrating the levels of AIM2, ASC, GSDMD-N, cleaved-Caspase-1, and GAPDH for each group. F: Quantitative protein expression levels of AIM2. G: Quantitative protein expression levels of ASC. H: Quantitative protein expression levels of cleaved Caspase-1. I: Quantitative protein expression levels of GSDMD-N. Statistical annotations: * P < 0.05 compared with the SO group; # P < 0.05 compared with the SAP-IBI group.
Discussion
The retrograde infusion of STS into the biliopancreatic duct represents a reliable and widely adopted protocol for inducing SAP in laboratory rodents. Its consistent reproducibility and proven efficacy have established this methodology as an essential approach for exploring the mechanistic pathways underlying SAP-IBI23,24,25,26. In line with this, our study first confirmed the successful establishment of the SAP-IBI model. Compared to the SO group, SAP-IBI mice exhibited significantly elevated serum markers of pancreatic injury (amylase, lipase, PLA2), severe pancreatic histopathological damage (acinar cell necrosis, inflammatory infiltration), and concurrent hippocampal injury (neuronal loss, edema) alongside impaired neurological function (increased mNSS). These findings align with the core pathological features of the clinical SAP-IBI, laying a solid foundation for subsequent mechanistic exploration.
AAV-based gene delivery systems have emerged as a fundamental tool for gene function investigation, offering distinct advantages including high safety profiles, precise tissue targeting capabilities, and sustained transgene expression. Particularly valuable for neurological research, certain AAV serotypes demonstrate efficient blood-brain barrier penetration, enabling effective gene modulation within the central nervous system. Critical to their performance are optimized viral production protocols and strategic selection of serotype-promoter combinations for tissue-specific expression. In this study, all AAV vectors (control, AIM2-shRNA, and AIM2-OE) were produced by HAMBIO under stringent quality control standards27,28, ensuring experimental validity and reproducibility.
During AP, the pyroptosis of acinar cells triggers the release of various inflammatory mediators that stimulate immune responses and promote leukocyte recruitment. This cascade exacerbates pancreatic inflammation, initiates SIRS, and ultimately progresses to SAP29. The underlying molecular mechanisms involve dual activation pathways: Caspase-1 mediates the classical pathway, while Caspase-4/5/11 orchestrate the non-classical pathway, both converging on GSDMD activation through proteolytic processing to execute pancreatic cell death programs30. Experimental suppression of pyroptosis in murine models has demonstrated a reduction in SAP severity, underscoring its pathogenic significance31. Our previous investigation32 identified salidroside’s therapeutic potential in alleviating SAP-related pancreatic damage, inflammatory responses, and pyroptotic mechanisms through coordinated regulation of Akt/NF-κB signaling and Caspase-3/GSDME activation pathways. Subsequent research by Wu et al.33 revealed that GSDMD-dependent pyroptosis contributes to pulmonary damage associated with SAP; pharmacological inhibition using disulfiram exhibited dual protective effects on both pancreatic and respiratory systems. Complementary findings from Shao et al.34 highlighted Caspase-11-mediated pyroptotic pathways in renal impairment induced by SAP, where targeted inhibition of these pathways ameliorated kidney dysfunction. Collectively, these studies establish pyroptosis as a pivotal mechanism driving not only the development of SAP but also its systemic complications such as MODS.
Recent studies have underscored pyroptosis as a critical pathological process in ischemic stroke (IS), leading to cellular demise through membrane disruption and the release of inflammatory cytokines. This particular form of cell death is intricately linked to neuroinflammatory processes, exacerbating inflammatory cascades during cerebral ischemia. Observations following IS indicate elevated levels of inflammasome components, including GSDMD and Caspase molecules, alongside pro-inflammatory mediators that collectively drive pyroptotic pathways and exacerbate neurological damage. Therapeutic interventions aimed at inhibiting pyroptosis demonstrate promising potential for the management of IS35. Research conducted by Liu and colleagues36 demonstrated that the application of electroacupuncture significantly mitigated neurological impairments resulting from delayed thrombolytic therapy in rodent models. This intervention not only reduced cerebral lesions but also enhanced neural preservation. The underlying neuroprotective mechanism is attributed to the suppression of GSDMD-related protein expressions, specifically IL-1β and IL-18, as well as the modulation of pyroptotic signaling through GSDMD pathways, which collectively contribute to the stabilization of the blood-brain barrier. These findings highlight the critical interplay between pyroptotic mechanisms, neuroinflammatory responses, and the progression of cerebral tissue damage.
The experimental findings revealed significantly elevated levels of serum amylase, lipase, PLA2, pancreatic histopathological scores, hippocampal neuropathological scores, and mNSS measurements in the SAP-IBI group compared to the SO group. This confirms the successful establishment of the SAP-IBI animal model. Notably, a substantial upregulation of pyroptosis-related biomarkers was observed; specifically, there was an increase in mRNA expression levels of ASC, GSDMD, and Caspase-1 alongside heightened protein levels of ASC, GSDMD-N terminal fragments, and activated Caspase-1 in SAP-IBI specimens relative to the SO group. These molecular changes strongly indicate that pyroptosis plays a pivotal role in both the initiation and progression of SAP-IBI.
The HIN200 domain located at the C-terminus of AIM2 demonstrates a binding capacity for microbial double-stranded DNA derived from both viral and bacterial sources. Through its interaction with ASC, AIM2 initiates the formation of the inflammasome complex by recruiting pro-Caspase-1. This macromolecular assembly triggers the proteolytic activation of Caspase-1, which subsequently mediates the maturation of pro-inflammatory cytokines IL-1β and IL-18, thereby amplifying inflammatory signaling through leukocyte recruitment. Concurrently, Caspase-1 cleaves GSDMD to generate its pore-forming N-terminal fragment that executes pyroptosis. Canonical activation of the AIM2 inflammasome typically induces substantial release of inflammatory mediators and precipitates irreversible cell death in damaged tissues. Emerging evidence suggests that pharmacological regulation of this pathway can modulate inflammatory progression and pyroptotic processes37,38. Experimental studies conducted by Li et al.39 revealed that AIM2 enhances neuronal pyroptosis via the AIM2/Caspase-1/GSDMD axis, exacerbating cerebral damage following hypoxic-ischemic insults. Zhao et al.40 reported that Nicorandil, an ATP-sensitive potassium channel activator, exhibits neuroprotective effects in the ischemic penumbra through dual mechanisms involving suppression of NF-κB signaling and inhibition of AIM2-mediated pyroptosis. Our prior investigation41 identified administration of forsythoside A to mitigate SAP-IBI manifestations in murine models, potentially through modulation of the AIM2 pathway and suppression of pyroptosis. The current experimental data demonstrate that silencing AIM2 disrupts inflammasome signaling, reduces pyroptosis, alleviates SAP-IBI symptoms, and enhances neurological recovery in mice. Conversely, overexpression of AIM2 activates the inflammasome cascade, intensifies pyroptosis, aggravates SAP-IBI progression, and impairs neural function.
However, several limitations of this study warrant consideration. The systemic administration of AAV vectors, while effective for global gene modulation, complicates the definitive segregation of direct CNS effects from secondary benefits of attenuated systemic inflammation. Furthermore, the reduction in 24-hour mortality observed in the AIM2 silencing group, though notable, did not reach statistical significance, potentially due to the model’s acute severity and sample size constraints, indicating that the impact on survival requires further elucidation. The absence of AIM2-knockout models or specific pharmacological inhibitors also precludes the exploration of post-onset therapeutic intervention. Finally, while the STS model is a valuable tool, its translational relevance to the more heterogeneous and complex human SAP-IBI condition necessitates future validation through clinical correlation studies.
Conclusion
This investigation examines the role of pyroptosis mediated by the AIM2 inflammasome pathway in the onset and progression of SAP-IBI. Our findings indicate that pyroptotic processes regulated through this molecular pathway represent an important mechanism underlying the pathological features of SAP-IBI. Targeting AIM2 inflammasome signaling and its associated pyroptosis mechanisms may provide a promising therapeutic strategy for alleviating manifestations of SAP-IBI. However, further research is necessary to fully elucidate the complex biological interactions within this pathway, particularly concerning its broader implications for neuroinflammatory disorders and therapeutic development.
Data availability
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
References
Sohail, Z., Shaikh, H., Iqbal, N. & Parkash, O. Acute pancreatitis: A narrative review. J. Pak Med. Assoc. 74, 953–958 (2024).
Trikudanathan, G., Yazici, C., Phillips, E., Forsmark, C. E. & A., & Diagnosis and management of acute pancreatitis. Gastroenterology 167, 673–688 (2024).
Beij, A., Verdonk, R. C., van Santvoort, H. C., de-Madaria, E. & Voermans, R. P. Acute pancreatitis: an update of Evidence-Based management and recent trends in treatment strategies. United Eur. Gastroenterol. J. 13, 97–106 (2025).
Hamesch, K., Hollenbach, M., Guilabert, L., Lahmer, T. & Koch, A. Practical management of severe acute pancreatitis. Eur. J. Intern. Med. 133, 1–13 (2025).
Wu, Y. et al. Progress in diagnosis and treatment of severe acute pancreatitis complicated with pancreatic encephalopathy. Chin. J. Crit. Care Med. (Chinese). 43, 156–160 (2023).
Meng, Y. et al. Incidence and outcomes of pancreatic encephalopathy in patients with acute pancreatitis: a systematic review and meta-analysis. Intern. Emerg. Med. 18, 1203–1212 (2023).
Albacete Ródenas, P., Ortiz Sánchez, M. L., Gajownik, U., Alberca de Las Parras, F. & Pons Miñano, J. A. Pancreatic encephalopathy, a little known complication of acute pancreatitis. Rev. Esp. Enferm Dig. 114, 122–123 (2022).
Beburishvili, A. G., Burchuladze, N. S., Mikhin, V. S., Kitaeva, A. V. & Mikhin, I. V. Prediction and diagnosis of pancreatogenic encephalopathy in patients with destructive pancreatitis. Khirurgiia 7, 58–63 (2022).
Liu, Y. et al. Pyroptosis in health and disease: mechanisms, regulation and clinical perspective. Signal. Transduct. Target. Ther. 9, 245 (2024).
Lin, T., Peng, M., Zhu, Q. & Pan, X. S1PR2 participates in intestinal injury in severe acute pancreatitis by regulating macrophage pyroptosis. Front. Immunol. 15, 1405622 (2024).
Li, Y. & Guo, B. GSDMD-mediated pyroptosis: molecular mechanisms, diseases and therapeutic targets. Mol. Biomed. 6, 11 (2025).
Lin, T. et al. Mechanism of gasdermin D on intestinal injury in severe acute pancreatitis by mediating pyroptosis. Chin. Crit. Care Med. (Chinese). 33, 89–94 (2021).
Cao, P., Chen, S., Wang, H. & Chen, Y. Taraxasterol mediated autophagy Inhibition in pancreatic encephalopathy involves its regulation on L1 cell adhesion molecule. Cytotechnology 77, 72 (2025).
Zhang, Y. et al. Neutrophil extracellular traps facilitate liver inflammation/ fibrosis progression by entering macrophages and triggering AIM2 inflammasome-dependent pyroptosis. Cell. Commun. Signal. 22, 556 (2024).
Xu, H., Xiao, H. & Tang, Q. Lipopolysaccharide-induced intestinal inflammation on AIM2-mediated pyroptosis in the brain of rats with cerebral small vessel disease. Exp. Neurol. 375, 114746 (2024).
Yang, F., Zhu, X., Zhang, J. & Yang, Y. Expression of absent in melanoma 2 in acute pancreatitis of mice model. Chin. J. Clin. Pharmacol. Ther. (Chinese). 23, 51–54 (2018).
Wang, X. et al. The study on the mechanism of Salidroside in the adjuvant treatment of patients with moderately severe acute pancreatitis. Tianjin Med. J. (Chinese). 51, 762–765 (2023).
Wu, Y. et al. Exploration on the mechanism of Dachengqi Decoction in treating severe acute pancreatitis complicated with acute kidney injury in mice based on Nrf2/HO-1 pathway. Chin. J. Inf. TCM (Chinese). 31, 55–60 (2024).
Schmidt, J. et al. A better model of acute pancreatitis for evaluating therapy. Ann. Surg. 215, 44–56 (1992).
Kong, L., Han, T., Yu, Y., Tang, Y. & Zhang, S. Effects of pancreatic lipase, cytokine, hypertonicity and infection on brain injury of rats: a morphological study. Chin. J. Pancreatol (Chinese). 4, 98–101 (2004).
Zhou, H. et al. Tat-NTS peptide protects neurons against cerebral ischemia-reperfusion injury via ANXA1 sumoylation in microglia. Theranostics 13, 5561–5583 (2023).
Chen, S. & Wu, Z. A simple and rapid method for the determination of phospholipase A2 in body fluids and tissues. Acad. J. Second Mil Med. Univ. (Chinese). 10, 254–256 (1989).
Wen, E. et al. Activation of TLR4 induces severe acute pancreatitis-associated spleen injury via ROS-disrupted mitophagy pathway. Mol. Immunol. 142, 63–75 (2022).
Yan, D. et al. Effect of Chaihuang Qingyi Huoxue granules on renin-angiotensin system in rats with severe acute pancreatitis. Tradit Chin. Drug Res. Clin. Pharmacol. (Chinese). 35, 639–645 (2024).
Yu, F. et al. A metabonomic study of intragastric local hypothermia intervention in rats with pancreatitis. Chin. J. Emerg. Med. (Chinese). 32, 1206–1214 (2023).
Li, H. et al. Effect of electroacupuncture at Zusanli (ST36) on inflammatory response and sIgA expression in small intestine of rats with severe acute pancreatitis induced by sodium taurocholate. J. Emerg. Traditional Chin. Med. (Chinese). 31, 213–217 (2022).
Zhu, H. et al. Moderate UV exposure enhances learning and memory by promoting a novel glutamate biosynthetic pathway in the brain. Cell 173, 1716–1727 (2018).
Xiao, Y. Z. et al. Reducing hypothalamic stem cell senescence protects against Aging-Associated physiological decline. Cell. Metab. 31, 534–548 (2020).
Li, H., Wu, D., Zhang, H. & Li, P. New insights into regulatory cell death and acute pancreatitis. Heliyon 9, e18036 (2023).
Al Mamun, A. et al. Pyroptosis in acute pancreatitis and its therapeutic regulation. Apoptosis 27, 465–481 (2022).
Zhao, M. et al. Octreotide attenuates experimental severe acute pancreatitis through inhibiting pyroptosis and modulating intestinal homeostasis. Eur. J. Pharmacol. 994, 177314 (2025).
Wang, X. et al. Salidroside ameliorates severe acute pancreatitis-induced cell injury and pyroptosis by inactivating Akt/NF-κB and caspase-3/GSDME pathways. Heliyon 9, e13225 (2023).
Wu, J. et al. Treatment of severe acute pancreatitis and related lung injury by targeting gasdermin D-Mediated pyroptosis. Front. Cell. Dev. Biol. 9, 780142 (2021).
Shao, Y. et al. Inhibition of Caspase-11-Mediated pyroptosis alleviates acute kidney injury associated with severe acute pancreatitis in rats. J. Invest. Surg. 36, 1–7 (2023).
Li, L. et al. Targeting pyroptosis to treat ischemic stroke: from molecular pathways to treatment strategy. Int. Immunopharmacol. 133, 112168 (2024).
Liu, H. et al. Electroacupuncture extends the time window of thrombolytic therapy in rats by reducing disruptions of blood-brain barrier and inhibiting GSDMD-mediated pyroptosis. Brain Res. 1845, 149296 (2024).
Fu, R. et al. AIM2 inflammasome: A potential therapeutic target in ischemic stroke. Clin. Immunol. 259, 109881 (2024).
Li, Y. K., Chen, J. G. & Wang, F. The emerging roles of absent in melanoma 2 (AIM2) inflammasome in central nervous system disorders. Neurochem Int. 149, 105122 (2021).
Li, Q. AIM2 exacerbates hypoxic-ischemic brain damage in neonatal rats via promoting neuronal pyroptosis. Brain Res. Bull. 224, 111305 (2025).
Zhao, C., Fu, X., Yang, Z., Zhang, Q. & Zhao, Y. ATP-sensitive potassium channel opener, Nicorandil, inhibits NF-κB/AIM2/GSDMD pathway activation to protect against neuroinflammation in ischemic stroke. Neurochem Int. 179, 105810 (2024).
Wang, X. et al. Protective effects of Forsythoside A against severe acute pancreatitis- induced brain injury in mice. Biomed. Pharmacother. 178, 117301 (2024).
Acknowledgements
We sincerely acknowledge the technical support provided by the Experimental Animal Center of Nantong University, the Jiangsu Provincial Key Laboratory of Inflammation and Molecular Drug Targets, the Nantong University Key Laboratory of Neural Regeneration, and the Pathology Department of the Affiliated Hospital of Nantong University.
Funding
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Author information
Authors and Affiliations
Contributions
Ying Fei: Validation, Resources, Investigation, Formal analysis. Ping Wang: Validation, Resources, Investigation, Formal analysis. Yun Meng: Visualization, Methodology, Investigation, Data curation. Xiaoyan Wang: Visualization, Methodology, Investigation, Data curation. Yanjie Li: Visualization, Validation, Software, Methodology, Investigation, Data curation, Conceptualization. Bengzhong Wei: Writing – original draft, Methodology, Investigation, Data curation, Conceptualization. Xiaohong Wang: Writing – review & editing, Writing – original draft, Supervision, Resources, Project administration, Methodology, Investigation, Formal analysis, Conceptualization.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Wang, X., li, Y., Wang, X. et al. The role of AIM2 inflammasome pathway-mediated pyroptosis on brain injury induced by severe acute pancreatitis in mice. Sci Rep 15, 45384 (2025). https://doi.org/10.1038/s41598-025-29833-2
Received:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s41598-025-29833-2