Abstract
In-hospital mortality among patients with acute myocardial infarction (AMI)-related cardiogenic shock (CS) remains approximately 30–45%. While costly mechanical circulatory support (MCS) devices were expected to improve prognosis, previous studies have demonstrated limited benefits. This retrospective study aimed to evaluate contemporary mortality, discharge rates to home, and associated medical costs in patients with AMI-related CS, using the Japanese nationwide administrative dataset, JROAD-DPC, between 2012 and 2023. Of 75,619 patients with AMI-related CS, in-hospital mortality occurred in 33,869 (45%). Among 41,750 survivors, 9850 (24%) were not discharged home. Costs for all patients averaged 15,500 USD, with 26,300 USD for survivors treated with MCS, 13,600 USD for survivors without, 19,700 USD for non-survivors treated with MCS, and 1200 USD for non-survivors without. Among survivors treated with MCS, costs were 24,100 USD for those discharged home and 34,700 USD for those not discharged home. After its approval in 2018, the use of microaxial flow pump increased and in-hospital mortality decreased from 46.2% in 2018 to 43.3% in 2022 (p < 0.001). However, during the study period, the proportion of patients not discharged home increased from 10.5 to 14.0% (p < 0.001) and medical costs rose from 13,900 USD to 15,600 USD. (p < 0.001). Over the decade, in-hospital mortality of AMI-related CS decreased slightly, while healthcare costs increased, particularly for patients treated with MCS who either died or were not discharged home. These findings warranted future studies to optimize MCS use and construct effective treatment strategies to improve prognosis and minimize potentially avoidable spending.
Similar content being viewed by others
Introduction
In-hospital mortality among patients with acute myocardial infarction (AMI) has declined to around 5% in recent years, owing to improvements in management and advances in device therapy. However, among those who develop heart failure and cardiogenic shock (CS), which affects approximately 10% of AMI cases, mortality remains as high as 30–45%1,2. In recent years, the advent of mechanical circulatory support (MCS) devices has shown some promise for patients with CS. While a recent randomized controlled trial (RCT) suggested potential benefits of a microaxial flow pump on survival prognosis3, a meta-analysis demonstrated no reduction in six-month mortality among ST-elevation CS patients overall, except in those without risk of hypoxic brain injury4. Moreover, other RCTs have failed to demonstrate clear survival advantages and have reported serious complications, such as bleeding5,6.
As MCS devices gain wider use, concerns regarding optimal resource utilization have intensified in patients with CS. Healthcare expenses are known to escalate substantially as patients approach the end of life, particularly in the final month, posing complex ethical and economic challenges7. There is thus an urgent need to examine contemporary mortality, discharge rates to home, and associated medical costs in patients with AMI-related CS, using a nationwide administrative dataset.
Accordingly, we aimed to evaluate contemporary mortality, discharge rates to home, and associated medical costs in patients with AMI-related cardiogenic shock, using a nationwide administrative dataset. We also sought to clarify how MCS use correlates with healthcare expenditures over time, including variations by discharge status.
Methods
Data source and patient population
Patient-level data for the present study was extracted from the Japanese Registry Of All cardiac and vascular Diseases-Diagnostic Procedure Combination (JROAD-DPC)8. JROAD is a nationwide database established by the Japanese Circulation Society to monitor and assess the clinical activities and outcomes of cardiovascular care across Japan (a total of 1119 institutions provide the data annually). The registry gathers extensive institution-level information through annual surveys, and links this with detailed patient-level data extracted from the Diagnosis Procedure Combination (DPC) system. The DPC system is a comprehensive healthcare insurance claims database that provides detailed information on patient demographics, clinical characteristics, and healthcare utilization. This system collects extensive data on primary diagnoses, disease-specific information, comorbidities, complications post-admission, medications, procedures, and devices. In addition, it records essential administrative data such as the length of hospitalization, medical costs, and discharge status. Notably, the documentation of medical agents, procedures, and devices is based on healthcare receipt data, ensuring a robust and systematic capture of clinical and financial information. Data entry was completed by consultant cardiologists before discharge. The reliability of the DPC system is supported by its validated diagnostic and procedural records. Studies have demonstrated that primary diagnoses recorded in the DPC database exhibit a sensitivity of 78.9% and specificity of 93.2% for primary diagnoses, as well as both sensitivity and specificity exceeding 90% for procedures9.
By combining administrative claims data with targeted institutional surveys, the JROAD-DPC serves as a powerful platform for epidemiological research, quality improvement, and health policy formulation in cardiovascular medicine. Researchers have used JROAD to evaluate trends in treatment practices, outcomes, and resource utilization, thereby informing clinical guidelines and contributing to a more efficient allocation of medical resources in Japanese healthcare system.
Inclusion criteria
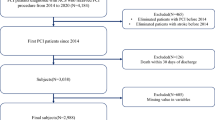
Patients who developed AMI-related CS between April 2012 and March 2023 were included regardless of any revascularizations such as percutaneous coronary intervention (PCI) and coronary artery graft bypass (CABG). AMI-related CS was determined using Killip classification within DPC records. AMI was defined by the International Classification of Diseases, Tenth Revision (ICD-10) codes of I21, I22, I23, or I24 (n = 1,017,687). AMI classified as Killip 4 was classified as AMI-related CS.
Exclusion criteria
Of 1,017,687 registered patients with AMI, 942,054 patients (92.6%) who were not classified as Killip class 4, 213 under 18 years of age, or 6 without information on medical costs were excluded.
Cost data acquisition and health insurance system in Japan
The DPC system for hospitalization consists of bundled and additional payment. Hospitals acquire a fixed daily payment based on the patients’ diagnosis, severity, and procedures. The daily payment is determined by the length of hospitalization and typically decreases after a specified period. In contrast, high-cost services (e.g. surgeries, expensive medical devices, and advanced diagnostic tests) are reimbursed separately based on the actual costs incurred, making accurate input into the DPC system essential for proper reimbursement. Japan’s comprehensive universal health insurance system requires all residents, regardless of age, income, or employment status, to be insured, thereby ensuring access to healthcare services. This system is comprised of two main types: employee health insurance and national health insurance. Employee health insurance, provided through employers, covers both employees and their dependents, with premiums shared equally between the employer and employee. National health insurance, managed by local municipal governments, is designed for self-employed, unemployed, or retired individuals. Typically, patients are responsible for 10–30% of their medical costs depending on age and income, although children and older adults often pay a lower percentage (0–20%). Additionally, under the high-cost medical expense benefit system, if a patient’s monthly out-of-pocket expenses exceed approximately 530 USD to 1700 USD, depending on each patient’s income, the excess amount is reduced to about 1% of the total medical expenses. The system covers a wide range of services, including doctor visits, hospitalizations, procedures, and medications. In contrast, welfare recipients are not covered by this system, as their medical costs are fully financed through a separate program. Moreover, patients have the freedom to choose their healthcare provider without needing a referral, irrespective of their welfare status. For reporting purposes, costs originally presented in Japanese yen were converted to U.S. dollars (USD) at an exchange rate of 150 yen per 1 USD, which is the most recent rate as of 2025.
Endpoints
The primary endpoint was in-hospital all-cause mortality. The secondary endpoints were medical costs and discharge destinations. Medical costs were described in Yen and USD. These clinical endpoints were verified by consultant cardiologists. Moreover, the medical costs reported in the JROAD-DPC accurately reflect the actual payments made to hospitals.
Definitions
The severity of dementia was classified according to the Japanese Ministry of Health, Labour and Welfare as following: rank 1, individuals who have some form of dementia but are almost fully independent in daily life, both at home and socially; rank 2, individuals who have any symptoms and behaviors that hinder daily life or undergo any communication difficulties to some extent, but can maintain independence under supervision; rank 3, individuals who have any symptoms and behaviors that hinder daily life or undergo any communication difficulties occasionally, requiring intermittent caregiving support; rank 4, individuals who have any symptoms and behaviors that hinder daily life or undergo any communication difficulties frequently, requiring constant caregiving support; rank M, individuals who have serious psychiatric symptoms, problematic behaviors, or severe physical illness, requiring specialized medical care. Rank 1 was classified as mild dementia, rank 2 and 3 as moderate, rank 4 and M as severe.
Ethics statement
This study protocol followed the ethical guidelines established in the 1975 Declaration of Helsinki and the Ethical Guidelines for Epidemiological Research issued by the Japanese government. The Ethics Committee of Showa University granted approval for the study on May 21, 2024 (approval number: 2024-050-A). The study met the criteria for a waiver of written informed consent, which was approved by the ethics committee.
Statistical analysis
Continuous variables that follow a normal distribution are described as mean ± standard deviation. Otherwise, variables are presented as medians with interquartile range values. Categorical variables are shown as absolute numbers and percentages. Continuous variables were evaluated using unpaired Student’s t-tests or Mann–Whitney U tests, as appropriate. The chi-squared test was applied for categorical variables. The Jonckheere-Terpstra test was used to evaluate the significance of trends in the mean age, while the Cochran-Armitage test was applied to analyze trends in in-hospital mortality, the proportion of patients unable to be discharged home, and the use of each type of MCS. Medical costs were compared according to outcomes at discharge and any MCS use. In the first scenario, all patients were classified into 4 groups based on a 2 × 2 combination of survival status at discharge and any MCS use. In the second scenario, patients discharged alive were divided into 4 groups based on a 2 × 2 combination of discharge destinations and any MCS use. Differences in each classification were evaluated using the Kruskal–Wallis test. Post-hoc multiple comparisons were conducted using Bonferroni corrections.
Propensity score (PS) matching was applied to assess in-hospital mortality and medical costs between patients who received MCS and those who did not, accounting for potential confounding factors. The PS was estimated using a logistic regression model including the patient-level, hospital-level, and prefecture-level variables: age, sex, body mass index (BMI), hypertension, dyslipidemia, diabetes mellitus, homecare before admission, dementia, chronic kidney disease, malignancy, previous heart failure, previous ischemic stroke, cardiopulmonary arrest, chronic obstructive pulmonary disease, anemia, PCI or CABG, gastroenterological bleeding, acute ischemic stroke, head injury on admission, bacteremia, pneumonia, urinary tract infection, dopamine, dobutamine, noradrenaline, epinephrine, PDE3 inhibitors, vasopressin, blood transfusion products (red blood cells, albumin, fresh-frozen plasma, and platelet), hospital bed capacity, and physician distribution index, defined as the standardized number of physicians in each prefecture divided by the prefectural population, adjusted for the prefecture-specific standardized consultation rate ratio10. Continuous variables (age, BMI, hospital bed capacity, and physician distribution index) were classified into three groups divided by tertiles. Many-to-one nearest-neighbor matching was performed within a caliper width of 0.25 multiplied by the SD of the PS. Covariate balance was assessed using standardized mean differences, variance ratios, and PS distribution. A standardized mean difference < 0.10 and a variance ratio between 0.50 and 2.00 were regarded as acceptable balance.
Because the JROAD-DPC does not include detailed hemodynamic data, we conducted a sensitivity analysis using the administration of vasoactive drugs, including dopamine, dobutamine, noradrenaline, phosphodiesterase-3 inhibitors, and vasopressin.
Statistical significance was set at a p-value of < 0.05. Only for post-hoc multiple comparison, a p-value of < 0.008 (0.05 divided by 4C2) was regarded as significant. Statistical analyses were performed using Stata software, version 17 (Stata Corp; College Station, TX, USA).
Results
Baseline characteristics
The JROAD-DPC dataset used in this study included 1,017,687 AMI patients from 2012 to 2023, averaging approximately 92,517 patients per year. Across Japanese hospitals, the median bed capacity was 487 (345–643). Of these patients, 75,619 (7%) were complicated with CS (mean age, 73 ± 13 years; male, 69%). Among patients with CS, 37,233 (49%) were treated with any MCS (intra-aortic balloon pump [IABP], 33,178 [44%]; venoarterial extracorporeal membrane oxygenation [VA-ECMO], 10,159 [13%]; microaxial flow pump, 1950 [3%]). Patient characteristics are summarized in Table 1. The prevalence of hypertension, dyslipidemia, diabetes mellitus, and chronic kidney disease was 38%, 32%, 25%, and 8%, respectively. At the time of hospital presentation, cardiopulmonary arrest and ventricular tachycardia/fibrillation were observed in 13% (n = 9493) and 10% (n = 7473) of patients, respectively. Regarding functional status, 3% (n = 2084) were homecare residents prior to admission, and 15% (n = 11,552) had dementia, categorized as mild (7%, n = 5369), moderate (6%, n = 4784), or severe (2%, n = 1399). Notably, 12% (n = 4392) of MCS patients had dementia (mild, 6% [n = 2330]; moderate, 4% [n = 1602]; severe, 1% [n = 460]).
Table 2 provides details on medications and interventions during hospitalization, including catecholamine use, blood transfusions, and invasive procedures such as PCI and CABG, all of which were more frequently used in individuals receiving MCS. It is noteworthy that each blood transfusion product was administrated 5 to 20 times more frequently in patients treated with MCS compared to those without.
Outcomes
Table 3 summarizes clinical events during hospitalization and discharge destinations. Table 4 details noncardiac adverse events during hospitalization. All-cause mortality occurred in 17,534 patients within 24 h of arrival (23%), 31,697 within 30 days (42%), and 33,869 beyond 30 days (45%). Among 41,750 patients discharged alive, 9,850 (24%) were not discharged home, including 1256 (3%) to homecare and 8,594 (21%) to chronic care facilities. Figure 1 demonstrates PS distribution before and after matching. After PS matching, all standardized mean differences were < 0.10, and most variance ratios ranged from 0.80 to 1.05, indicating good covariate balance between groups (Supplemental Table 1). After PS matching, the risk difference in in-hospital mortality after PS matching was 0.87% (95% CI, − 2.88 to 1.13%), indicating no significant difference between patients treated with and without MCS.
Medical costs
The median medical cost was 2.32 million yen (15,500 USD) (Table 3). Costs for patients discharged alive averaged 2.82 million yen (18,800 USD), with 3.94 million yen (26,300 USD) for those treated with MCS and 2.04 million yen (13,600 USD) for those without (Fig. 2A). Among patients who died, costs averaged 1.36 million yen (9100 USD), including 2.96 million yen (19,700 USD) for those treated with MCS and 0.18 million yen (1200 USD) for those without (Fig. 2A). There was significant difference of medical costs among the four groups (p < 0.001). Daily medical costs for patients who died despite MCS use were 0.62 million yen (4100 USD), whereas costs for the others were around 0.15 million yen (1000 USD) (Supplemental Table 2). Post-hoc comparisons between each pair of the four groups showed significant differences in medical costs across all combinations (p < 0.001 for each comparison). Medical costs were higher for patients who died and underwent MCS (2.96 million yen [19,700 USD]), compared to those discharged alive without MCS (2.04 million yen [13,600]) (p < 0.001).
Medical costs in patients with and without MCS. (A) The medical costs for patients with MCS were higher than those without regardless of survival (p < 0.001, respectively). (B) The medical costs were higher for patients not discharged home compared to those discharged home regardless of MCS use (p < 0.001, respectively). It is noteworthy that the highest medical costs were observed in patients not discharged home following MCS use. MCS, mechanical circulatory support.
Among survivors, medical costs differed between patients who discharged to home (2.63 million yen [17,500 USD]) and did not (3.99 million yen [26,600 USD]). Figure 2B demonstrates a comparison of medical costs in those classified by discharge destinations and MCS use, which indicated significant differences (p < 0.001). Post-hoc analysis demonstrated significant differences across all combinations (p < 0.001 for each comparison). Regardless of MCS use, the medical costs were higher in patients not discharged home than in those who were (p < 0.001, respectively), while daily medical costs for each group ranged from 0.10 to 0.16 million yen (670 USD to 1070 USD) (Supplemental Table 2).
These results persisted after excluding ≤ 1-day admissions (Supplemental Table 3). Finally, after PS matching, patients treated with MCS required an additional 0.89 million yen (5900 USD) in medical costs compared with those without MCS.
Sensitivity analysis using vasoactive agents
Among 61,845 patients treated with any vasoactive agents, in-hospital death occurred in 15,871 patients who received MCS (46%) and 14,677 patients who did not (54%) (p < 0.001). Medical costs were 3.72 million yen (24,800 USD) for patients with MCS and 1.41 million yen (9400 USD) for those without MCS (p < 0.001).
Time-trend analysis
Figure 3 illustrates trends in age, MCS utilization, in-hospital mortality, and discharge outcomes from 2012 to 2022. The average age of patients increased from 72.5 years in 2012 to 73.8 years in 2022 (p < 0.001). Venoarterial extracorporeal membrane oxygenation (VA-ECMO) utilization rose from 11.2% in 2012 to 15.9% in 2019, followed by a decline to 12.9% in 2022 (p < 0.001). IABP usage peaked at 46.9% in 2017, then declined to 38.9% in 2022 (p < 0.001), while microaxial flow pump use increased from 1.2% in 2018 to 9.1% in 2022. In-hospital mortality decreased significantly (p < 0.001) during the study period, but the proportion of patients unable to be discharged home rose from 10.5% in 2012 to 14.0% in 2022 (p < 0.001). Supplemental Fig. 1 shows the time trend of all-cause mortality within 24 h, which remained around 8–10% in most years, with minor fluctuations outside this range. As for the medical cost, the median medical cost increased from 2.09 million yen (13,900 USD) in 2012 to 2.34 million yen (15,600 USD) in 2022 (p < 0.001).
Time trend of MCS, clinical outcomes, and the medical cost. The utilization rates of VA-ECMO increased from 11.2% in 2012 to 15.9% in 2019 and then gradually decreased from 13.3% in 2020 to 12.9% in 2022 (p < 0.001). While the induction rate of IABP increased from 43.1% in 2012 to 46.9% in 2017 and decreased from 43.3% in 2018 to 38.9% in 2022 (p < 0.001), that of microaxial flow pump increased since 2018 (1.2% in 2018 and 9.1% in 2022, respectively). Older patients and the medical costs increased significantly (p < 0.001). Although in-hospital mortality decreased (p < 0.001), the rates of those unable to be discharged home increased (p < 0.001). IABP, intra-aortic balloon pumping; MCS, mechanical circulatory support; VA-ECMO, venoarterial extracorporeal membrane oxygenation.
Discussion
The present study demonstrated that (1) approximately half of patients with AMI-related CS were discharged alive, and among them, about a quarter were not discharged home; (2) while in-hospital mortality decreased over the decade, the number of patients could not be discharged home and medical costs increased; (3) the medical costs were higher for non-survivors treated with MCS than for survivors who were not treated with MCS. Further, among patients undergoing MCS, the costs were higher for those who were not discharged home compared to those who were.
Our findings suggest that resource use and costs for AMI complicated by CS may be suboptimal. Although ethically and clinically challenging, optimizing care could improve survival and contain expenses, especially given evidence that higher hospital-bed capacity, more board-certified cardiologists, and greater AMI volume are linked with lower in-hospital mortality11. Japan has also been criticized for having hospitals that perform relatively few annual PCI procedures (median 281 [IQR, 182–414]) despite a high PCI-to-CABG ratio12. Consolidating underutilized centers is therefore an urgent priority, although practical issues such as regional accessibility may complicate its implementation.
Our time-trend analysis provided several important observations. Although in-hospital mortality declined significantly over the past decade, the proportion of patients unable to return home increased, suggesting that some who survive may experience impaired ADL. While a rise in microaxial flow pump use seems temporally associated with lower in-hospital mortality, this does not necessarily imply causation. Although the DanGer Shock trial showed positive results, microaxial flow pump use for AMI-related CS is not universally recommended, as indicated by the meta-analysis4. Even considering that the current observational study could not establish a causal relationship, PS matching showed that MCS use was not associated with in-hospital mortality. The reduction in mortality between 2020 and 2022 likely reflects multiple factors and merits further investigation.
Many previous studies regarding patients with AMI evaluated survival rates or rehospitalization albeit few studies investigated the rate of home discharge, which reflect functional status of the patients. In Japan, even older patients in their late 80s and 90s with limited ADL may have access to intensive and advanced treatment options13. The finding that more than 10% of patients receiving MCS in the present cohort had dementia is noteworthy and warrants careful consideration. Our nationwide cohort study also demonstrated that medical costs for patients who died despite receiving MCS were higher than those for survivors treated without these devices, consistent with previous reports from the UK 7. While MCS can be a vital therapeutic option, its high cost is accompanied by increased risks of complications such as bleeding, infection, longer hospital stays, and disuse syndrome, all of which require substantial medical resources. Importantly, it remains unclear whether MCS consistently improves prognosis. Among MCS modalities, VA-ECMO and microaxial flow pumps have been associated with higher complication risks3,6 Clinicians should carefully weigh the potential benefits and risks of MCS devices, considering patient-specific factors and device characteristics, to ensure their use is both judicious and outcome-driven14.
Our direct analysis of cost data revealed a significant rise in medical expenses, despite little change in in-hospital mortality rates. Higher medical costs were observed in patients treated with MCS, as supported by propensity score matching and sensitivity analyses. This increase may partly reflect suboptimal resource utilization. For instance, the use of VA-ECMO may face further downgrades in future guidelines, as both the ECLS-SHOCK trial(4) and the ECMO-CS trial15 caution against its routine use in CS patients. Ongoing clinical trials are expected to provide clearer recommendations for VA-ECMO indications16. Until more definitive guidance is available, careful consideration of its use is crucial to minimize device-related complications and sustain the current insurance framework. Additionally, analyzing the utilization of medical resources other than MCS may provide important insights for optimizing intensive care. Notably, blood transfusions were administered significantly more often in patients treated with MCS17. However, the appropriate threshold for blood product administration in AMI-related CS remains uncertain18,19. Given the current practices surrounding MCS and blood transfusion in Japan, there may be opportunities to reduce adverse clinical events and associated medical costs in managing AMI-related CS.
Our analysis of AMI-related CS highlighted two important issues to address, high mortality and home discharge failure, both of which are associated with increased healthcare costs. Future studies should focus on evaluating outcomes among MCS users who either do not survive or who survive but are not discharged to their homes. Such investigations should aim to identify clinically meaningful endpoints and develop strategies that optimize patient care while ensuring responsible resource use. For patients who do not survive, it is imperative to refine treatment protocols and implement robust risk stratification to improve survival outcomes and minimize unnecessary spending. However, our findings suggest that merely increasing the use of MCS is unlikely to yield substantially better prognoses. Similarly, survivors who are not discharged home often represent a particularly severe clinical subgroup, characterized by prolonged hospitalization and complications such as disuse syndrome. Ethically, it is essential to pursue research that not only improves survival rates but also enhances patients’ quality of life. In this context, evaluating whether the intensity and quality of supervised cardiac rehabilitation can influence discharge outcomes is crucial. Such studies should be designed to respect patient autonomy, ensure equitable care, and balance the benefits of advanced therapies with their costs, thereby upholding both scientific integrity and ethical standards in clinical practice.
Our study has several limitations. While data on the use of medical resources, including MCS, medical costs, and discharge destinations are likely accurate because the information was obtained from health insurance claim records, concomitant comorbidities such as bleeding which did not require endoscopic hemostasis might be overlooked, leading to potential underestimation of the incidences. The JROAD-DPC database does not include detailed angiographic findings including culprit lesions, single- or multivessel disease, and coronary artery dominance. These findings are necessary for understanding the severity of ACS and for guiding more appropriate patient selection for MCS. In the current study, cardiogenic shock was defined as AMI patients classified as Killip IV, which may not fully correspond to SCAI (Society for Cardiovascular Angiography & Interventions) shock stages C–E, because this cohort lacks detailed hemodynamic data. To address this, we conducted a sensitivity analysis using the use of vasoactive agents as a proxy, which supports the robustness of our results. Further, the absence of blood count data makes it difficult to evaluate the appropriateness of blood transfusion. Finally, the health insurance systems and the costs of medical resources differ across countries. The results from JROAD-DPC are informative for other countries, but should not be directly applied to them.
Conclusions
The nationwide cohort study revealed that approximately half of patients with AMI-related CS were discharged alive, and among them, about a quarter were not discharged home. Although in-hospital mortality decreased over the past decade, the number of patients not discharged home and the medical costs increased. Considering prognosis, ADL, and medical costs, the next study should identify the risk factors for mortality and failure to achieve home discharge despite the use of MCS to improve the management of AMI-related CS.
Data availability
The data supporting the findings of this study are available upon request with permission from the JROAD IT Database Committee.
References
Miyachi, H. et al. 10-year temporal trends of in-hospital mortality and emergency percutaneous coronary intervention for acute myocardial infarction. JACC Asia. 2 (6), 677–688 (2022).
Pramudyo, M., Bijaksana, T. L., Yahya, A. F. & Putra, I. C. S. Novel scoring system based on clinical examination for prediction of in-hospital mortality in acute coronary syndrome patients: A retrospective cohort study. Open. Heart. 9 (2), e002095 (2022).
Møller, J. E. et al. Microaxial flow pump or standard care in infarct-related cardiogenic shock. N Engl. J. Med. 390 (15), 1382–1393 (2024).
Thiele, H. et al. Temporary mechanical circulatory support in infarct-related cardiogenic shock: An individual patient data meta-analysis of randomised trials with 6-month follow-up. Lancet 404 (10457), 1019–1028 (2024).
Thiele, H. et al. Intraaortic balloon support for myocardial infarction with cardiogenic shock. N Engl. J. Med. 367 (14), 1287–1296 (2012).
Thiele, H. et al. Extracorporeal life support in infarct-related cardiogenic shock. N Engl. J. Med. 389 (14), 1286–1297 (2023).
Luta, X. et al. Healthcare trajectories and costs in the last year of life: A retrospective primary care and hospital analysis. BMJ Support Palliat. Care 14:e807 (2020).
Yasuda, S. et al. The current status of cardiovascular medicine in Japan—analysis of a large number of health records from a nationwide claim-based database, JROAD-DPC. Circ. J. 80 (11), 2327–2335 (2016).
Yamana, H. et al. Validity of diagnoses, procedures, and laboratory data in Japanese administrative data. J. Epidemiol. 27 (10), 476–482 (2017).
Ministry of Health Labour and welfare. [19-Sep-25] https://www.mhlw.go.jp/content/10800000/000665196.pdf
Matoba, T. et al. Institutional characteristics and prognosis of acute myocardial infarction with cardiogenic shock in Japan—Analysis from the JROAD/JROAD-DPC database. Circ. J. 85 (10), 1797–1805 (2021).
Yamamoto, K. et al. Percutaneous coronary intervention versus coronary artery bypass graftinge among patients with unprotected left main coronary artery disease in the new-generation drug-eluting stents era (From the CREDO-Kyoto PCI/CABG registry Cohort-3). Am. J. Cardiol. 145, 47–57 (2021).
Shimura, T. et al. Impact of the clinical frailty scale on outcomes after transcatheter aortic valve replacement. Circulation 135 (21), 2013–2024 (2017).
Salter, B. S. et al. Temporary mechanical circulatory support devices: Practical considerations for all stakeholders. Nat. Rev. Cardiol. 20 (4), 263–277 (2023).
Ostadal, P. et al. Extracorporeal membrane oxygenation in the therapy of cardiogenic shock: Results of the ECMO-CS randomized clinical trial. Circulation 147 (6), 454–464 (2023).
Lüsebrink, E. et al. Scrutinizing the role of venoarterial extracorporeal membrane oxygenation: Has clinical practice outpaced the evidence? Circulation 149 (13), 1033–1052 (2024).
Jentzer, J. C. et al. Red blood cell transfusion threshold and mortality in cardiac intensive care unit patients. Am. Heart J. 235, 24–35 (2021).
Frenette, A. J. et al. Albumin administration is associated with acute kidney injury in cardiac surgery: A propensity score analysis. Crit. Care. 18 (6), 602 (2014).
Li, Z. et al. Impact of albumin infusion on prognosis of intensive care unit patients with congestive heart failure-hypoalbuminemia overlap: A retrospective cohort study. J. Thorac. Dis. 14 (6), 2235–2246 (2022).
Acknowledgements
We appreciate all contributors to JROAD-DPC.
Funding
This study was supported by a research grant from Showa University (TS) and by a Grant-in-Aid for Scientific Research (C) from the Japan Society for the Promotion of Science (JSPS) (25K14387, SH).
Author information
Authors and Affiliations
Contributions
Conceptualization: SH and SK; Methodology: SH, SK, YS, and KK; Software: SH and YS; Validation: SK, YS, and KK; Formal analysis: SH, YS, and KK; Investigation: SH, SY, and KK; Resources: YS and KK; Data Curation: SH and YS; Writing—original draft: SH and SK; Review and editing: SY, KK, YY, HM, and TS; Visualization: SH, YS, and KK; Supervision: SK; Project administration: SH, YS, and KK; Funding acquisition: ST.
Corresponding author
Ethics declarations
Competing interests
TS received honoraria for speakers from Abiomed, Abbott Japan, Terumo, Medtronic Japan, and Boston Scientific Japan. All other authors report no conflicts of interest.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Higuchi, S., Kohsaka, S., Sumita, Y. et al. Evolving trends in cardiogenic shock management in acute myocardial infarction: mortality, discharge outcomes, and economic implications. Sci Rep 16, 789 (2026). https://doi.org/10.1038/s41598-025-30300-1
Received:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s41598-025-30300-1