Abstract
Endoscopic submucosal dissection (ESD) is a standard treatment for rectal laterally spreading tumors (LSTs). This study aims to compare the efficacy and safety of the Injection Mucosa Knife (IMK) technique versus the conventional Dual Knife (DK) technique in ESD for rectal LSTs. A total of 229 patients with rectal LSTs were enrolled from four hospitals between June 2020 and June 2025 and were divided into two groups: the IMK group (n = 108) and the DK group (n = 121). The primary outcome measures included total procedure time, mucosal dissection time, and submucosal dissection rate, while the secondary outcomes encompassed intraoperative and postoperative complications as well as the R0 resection rate. Additionally, the efficacy and safety of the two groups were compared based on tumor size and endoscopic morphology. The IMK group demonstrated significantly shorter total procedure time (median 50 vs. 65 min, P < 0.001) and mucosal dissection time (median 40 vs. 56 min, P < 0.001), along with a higher submucosal dissection rate (median 0.12 vs. 0.08 cm²/min, P < 0.001). There were no significant differences in intraoperative perforation, severe hemorrhage, R0 resection, postoperative bleeding, fever, or pathology between the two groups (P > 0.05). Subgroup analyses based on tumor size (2.5 cm and ≥ 2.5 cm) and morphology (LST-G and LST-NG) consistently showed superior efficiency of the IMK technique without compromising safety. The IMK technique significantly improves the efficiency of ESD for rectal LSTs by reducing operative time and enhancing dissection rates, while maintaining comparable safety and outcomes to the conventional DK technique. IMK represents a promising advancement in ESD for rectal lesions.
Similar content being viewed by others
Introduction
Endoscopic submucosal dissection (ESD) is a minimally invasive procedure widely regarded as the standard treatment for large superficial gastrointestinal neoplasms, including rectal laterally spreading tumors (LSTs), owing to its capacity to achieve en bloc resection and curative outcomes1,2,3. Despite its high efficacy, ESD remains technically demanding, often necessitating specialized knives and extended procedural durations that may elevate the risk of complications such as perforation and bleeding4,5,6,7. This is particularly true in the rectum, where the narrow lumen, rich submucosal vascularity, and proximity to the dentate line and anal sphincter complex pose unique technical challenges and increase the risks of intraoperative bleeding, perforation, and postoperative pain. To optimize ESD performance, several novel knives have been developed with integrated functions such as submucosal injection, aiming to improve safety and reduce operative time6,8,9,10. Examples include through-the-needle injection-capable electrosurgical knives7 and waterjet-assisted devices8, which have been shown to streamline and accelerate the submucosal dissection process. Additionally, scissor-type knives like the Clutch Cutter have demonstrated potential in facilitating dissection and reducing procedural risks, particularly for less experienced endoscopists4,6,10.
Nonetheless, comparative studies specifically focused on rectal LSTs remain limited. Although some investigations have evaluated alternative techniques such as endoscopic submucosal tunnel dissection (ESTD)1 or compared various knife types within broader gastrointestinal contexts4,5,7, direct comparisons between knives with integrated injection functions and conventional devices like the Dual Knife (DK) are scarce, especially in the context of rectal ESD where procedural efficiency and safety are critically important.
Therefore, this study aims to evaluate the efficacy and safety of the Injection Mucosa Knife (IMK)—which incorporates an injection function into the dissection instrument—in comparison with the conventional DK during ESD specifically for rectal LSTs. By providing objective data on dissection efficiency and complication rates, this research addresses a significant gap in the current literature and may help inform clinical decision-making.
Materials and methods
Patients
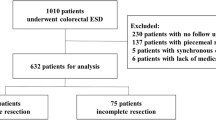
This study retrospectively analyzed data from patients who underwent endoscopic submucosal dissection (ESD) for rectal laterally spreading tumors (LSTs) at four hospitals—Suzhou BenQ Medical Center, Changshu Hospital Affiliated to Soochow University, Kunshan First People’s Hospital, and Zhangjiagang First People’s Hospital—between June 2020 and June 2025. The inclusion criteria were:1 endoscopic diagnosis of rectal LST;2 normal preoperative platelet count and coagulation parameters;3 patients who underwent ESD. The exclusion criteria were:1 presence of lymph node or distant metastasis detected on preoperative computed tomography (CT);2 poor lifting after submucosal injection;3 patients who underwent traction-assisted ESD or piecemeal endoscopic mucosal resection (EMR);4 history of previous rectal surgery;5 failure to provide signed informed consent or explicit refusal to participate in the procedure;6 incomplete data. Figure 1 illustrates the patient selection flow chart. Based on the type of electrosurgical knife used, patients were divided into an injection mucosa knife (IMK) group and a conventional dual knife (DK) group. All endoscopic procedures were performed by experienced endoscopists adhering to standardized protocols. The study protocol was approved by the Clinical Research Ethics Committee of Changshu Hospital Affiliated to Soochow University. All methods were performed in accordance with the relevant guidelines and regulations. All patients provided written informed consent prior to their inclusion in the study, ensuring adherence to ethical guidelines and patient confidentiality.
Endoscopic equipment and procedures
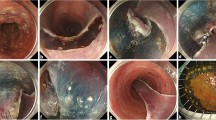
In this study, two different types of electrosurgical knives were utilized: the Kunpeng knife (Type D, Vedkang, Jiangsu, China), which enables simultaneous cutting and submucosal injection (Fig. 2), and the conventional Dual Knife (KD-650 L; Olympus, Tokyo, Japan). All procedures were performed using a single-channel endoscope (GIF-Q260J, Olympus, Tokyo, Japan or EG-550, Sonoscape, Shenzhen, China) equipped with a distal transparent cap. Electrosurgical energy was supplied by a high-frequency generator (ERBE VIO 200D, Germany). Auxiliary instruments included metallic clips, injection needles, hot biopsy forceps, and a carbon dioxide insufflation system. A comprehensive preoperative assessment was conducted for all patients, encompassing complete blood count, biochemical profiling, coagulation tests, electrocardiography, and abdominopelvic CT, to exclude any contraindications to endoscopic resection. Patients receiving anticoagulant or antiplatelet agents were instructed to withhold these medications for seven days prior to the procedure. Bowel cleansing was achieved through self-administered laxatives according to prescribed instructions. Under either intravenous sedation or general endotracheal anesthesia, patients were positioned in the left lateral decubitus position. A transparent cap was mounted on the endoscope tip, and carbon dioxide insufflation was applied throughout the procedure. Following identification of the lesion, a solution composed of 0.9% normal saline, indigo carmine, and epinephrine was injected around the peripheral margin to achieve submucosal lifting. A circumferential mucosal incision was made approximately 5 mm outside the lesion borders, after which submucosal dissection was carried out in a stepwise manner (Fig. 3).
ESD performed using the injection mucosa knife (IMK). (A) Rectal LST-G-M (granular mixed-type laterally spreading tumor) lesion; (B) Submucosal injection to lift the lesion; (C) Circumferential incision of the mucosal layer; (D) Submucosal dissection using the IMK; (E) Adequate/subtotal dissection of the submucosal layer; (F) Wound bed after complete resection; (G) Wound bed after coagulation with a hot biopsy forceps; (H) Resected specimen fixed on a foam board post-procedure.
The use of the IMK permitted integrated fluid injection during dissection, thereby maintaining submucosal elevation without instrument exchange. In contrast, procedures employing the Dual Knife required repeated switching to an injection needle to sustain adequate lifting. Active bleeding encountered during dissection was initially managed using the knife itself; refractory bleeding was controlled with hot biopsy forceps. Upon complete resection of the lesion, prophylactic coagulation of the wound bed was performed with hot biopsy forceps. The mucosal defect was subsequently closed with titanium clips, except in cases involving large defects or lesions situated adjacent to the dentate line, where clip closure was deemed unnecessary or technically unfeasible.
Postoperative management
Following resection, the specimen was pinned to a foam board for stabilization and then transferred to a sealed container with 10% formalin solution to ensure optimal fixation. After transportation to the pathology laboratory, it was processed according to conventional protocols involving paraffin embedding and sectioning for subsequent histological evaluation. Postoperatively, all patients were kept nil per os for 24 h and received supportive care including hemostatic agents and intravenous hydration to facilitate recovery. Antibiotic prophylaxis was omitted in cases where neither intraoperative perforation nor postoperative fever was observed. However, patients who exhibited signs of perforation during the procedure or developed fever afterward were administered antibiotics immediately to reduce the risk of infection. Continuous monitoring was carried out throughout the recovery phase to detect any complications such as hemorrhage, perforation, or infection, with therapeutic measures being applied as warranted. Provided no complications arose, a liquid diet was introduced 24 h after the procedure.
Data collection
The study analyzed a range of patient− related variables, encompassing demographic characteristics (sex, age), comorbidities (hypertension, diabetes mellitus, coronary artery disease), and prior use of anticoagulant or antiplatelet agents. Tumor features—including location, dimensions (size and area), endoscopic morphology, and distance from the dentate line—were systematically documented. Intraprocedural metrics such as total resection time and mucosal dissection duration were also collected. Key outcomes assessed were R0 resection status, intraoperative events (perforation and significant bleeding), postoperative complications (bleeding, perforation, and fever), and postoperative hospital stay. Histopathological diagnoses based on the Vienna Classification of gastrointestinal tumors were classified as low-grade intraepithelial neoplasia (LGIN), high-grade intraepithelial neoplasia (HGIN), intramucosal carcinoma, or submucosal carcinoma11.
Definitions
Tumor location, distance from the dentate line, and endoscopic morphology were assessed using preoperative colonoscopy. LST-G-H-type lesions display a homogeneous granular surface pattern characterized by uniformly sized nodules, regular contours, and minimal variation in elevation. LST-G-M lesions are distinguished by predominant granular elevations with heterogeneous nodule size and height, sometimes accompanied by focal depressions or ulcerated areas. LST-NG-F lesions manifest as broadly elevated yet flat masses with smooth surfaces lacking discernible granularity, typically merging imperceptibly with adjacent normal mucosa. LST-NG-PD lesions are mainly flat with central shallow depressions or erosions, bordered by mildly raised edges and an absence of granular architecture11. Tumor size was determined from post-resection specimens, and the tumor area was derived mathematically based on measured dimensions. As all resection beds in this study assumed a near-circular configuration, the area was calculated applying the formula for a circle’s area. Total procedure time was defined as the interval from the initial submucosal injection to the complete excision of the lesion, excluding time allocated to post-resection wound management. Mucosal dissection time denoted the period from the circumferential mucosal incision to full lesion removal. The submucosal dissection rate was expressed as the resected lesion area divided by the mucosal dissection time. An R0 resection was confirmed when the lesion was excised completely with histologically negative horizontal and vertical margins12. Intraoperative perforation was recognized as an iatrogenic full-thickness rectal wall defect establishing communication between the rectal lumen and the pelvic space13. Major intraoperative bleeding was defined as hemorrhage necessitating repeated endoscopic hemostatic attempts, with an associated postoperative hemoglobin reduction exceeding 2 g/dL, or requiring surgical assistance14,15. Postoperative bleeding was strictly identified by clinically overt hematochezia accompanied by a hemoglobin decline of ≥ 2 g/dL, alterations in vital signs, and endoscopic confirmation of a bleeding source related to the resection site during emergent examination16. Postoperative fever was defined as any body temperature elevation above 37.3℃ following the procedure. Postoperative perforation was diagnosed in cases where no perforation was identified intraprocedurally or immediately post-ESD, but subsequent clinical signs of peritoneal irritation were observed, or imaging studies (e.g., abdominal radiography or CT revealed subdiaphragmatic free air)17.
Follow-up
All patients were required to attend regular follow-up appointments in the gastroenterology outpatient clinic on a monthly basis after discharge. Colonoscopic examinations were scheduled at 6 and 12 months postoperatively, followed by annual CT scans of the chest, abdomen, and pelvis. The primary objectives of follow-up were to monitor wound healing, detect potential disease recurrence at an early stage, and comprehensively evaluate the possibility of lymph node or distant metastasis.
Sample size calculation
A priori sample size calculation was performed using G*Power software (version 3.1.9.7). Based on a pilot study involving 30 patients (15 per group), the mean total procedure time was 55 min in the DK group and 45 min in the IMK group, with a pooled standard deviation of 15 min. To detect a minimum clinically important difference of 10 min in total procedure time with a two-tailed α of 0.05 and a power (1–β) of 90%, a minimum of 105 patients per group was required. The final analysis included 108 patients in the IMK group and 121 in the DK group, which met the estimated sample size requirement.
Statistical analysis
Categorical data were summarized as frequencies and percentages, and group differences were assessed using the chi-square test. Continuous variables with non-normal distributions were expressed as median and interquartile range (IQR), and the Mann–Whitney U test was applied for comparative analyses between groups. A p-value below 0.05 was considered statistically significant. All statistical analyses were performed with SPSS version 26.0 (IBM Corp., Chicago, IL, USA).
Results
Baseline characteristics
This study included a total of 229 patients, with 108 in the IMK group and 121 in the DK group. The proportion of male patients was higher than that of female patients (55.9% vs. 44.1%). Patients over 60 years of age accounted for 61.6% of the cohort, 34.9% had underlying diseases, and only 10.0% had a history of anticoagulant or antiplatelet medication use. Nearly half (48.5%) of the patients exhibited an endoscopic morphology of LST-G-M. The median tumor size was 2.3 cm, and the median tumor area was 4.2 cm². No statistically significant differences were observed in baseline characteristics between the two groups (P > 0.05) (Table 1).
Intraoperative and postoperative clinicopathological characteristics
The IMK group exhibited a significantly reduced median procedure time relative to the DK group (50.0 min vs. 65.0 min, P < 0.001). Consistently, the median mucosal dissection time was also lower in the IMK group (40.0 min vs. 56.0 min, P < 0.001). Furthermore, a higher median submucosal dissection rate was observed in the IMK group compared to the DK group (0.12 cm²/min vs. 0.08 cm²/min, P < 0.001). With respect to adverse events, intraoperative perforation was documented in one patient, while intraoperative and postoperative bleeding occurred in nine and two patients, respectively. Postoperative fever was noted in nine cases; however, no instances of postoperative perforation were recorded. Pathological assessment indicated LGIN in 49.3% of the resected specimens. These parameters did not differ significantly between the two groups (all P > 0.05). The R0 resection rates were comparable between the IMK and DK groups (93.5% vs. 92.6%, P > 0.05), as detailed in Table 2.
Subgroup analyses
To further evaluate the differential efficacy between the IMK and DK techniques across various lesion characteristics, subgroup analyses were conducted based on tumor size and endoscopic morphology. Across all subgroups, the IMK technique consistently demonstrated superior performance compared to the DK approach. In both tumor size categories (2.5 cm and ≥ 2.5 cm), the IMK group showed significantly shorter median procedure times (50.0 vs. 61.0 min and 51.0 vs. 70.0 min, respectively; both P < 0.001), reduced mucosal dissection times (40.0 vs. 52.0 min and 41.0 vs. 60.0 min; both P < 0.001), and higher submucosal dissection rates (0.09 vs. 0.06 cm²/min and 0.21 vs. 0.12 cm²/min; both P < 0.001) (Tables 3 and 4). Similarly, for both endoscopic morphology subtypes (LST-G and LST-NG), the IMK technique maintained significantly better outcomes in procedure time (50.0 vs. 65.0 min and 50.0 vs. 67.0 min; both P < 0.001), mucosal dissection time (40.0 vs. 55.0 min and 40.0 vs. 57.0 min; both P < 0.001), and submucosal dissection rate (0.11 vs. 0.07 cm²/min and 0.14 vs. 0.09 cm²/min; both P < 0.001) (Tables 5 and 6).
Discussion
This study demonstrates that the Injection Mucosa Knife (IMK) technique significantly enhances the efficiency of ESD for rectal LSTs compared to the conventional DK approach, without compromising safety or pathological outcomes. Specifically, the IMK group exhibited markedly shorter total procedure and mucosal dissection times, along with a higher submucosal dissection rate, across all subgroups stratified by tumor size and morphology. These findings suggest that the integrated injection function of the IMK reduces the need for instrument exchange, thereby streamlining the dissection process and improving operational fluency. Importantly, both techniques showed comparable rates of R0 resection, intraoperative and postoperative complications, and histopathological outcomes, reinforcing the clinical viability of the IMK as an effective and efficient tool for the management of rectal LSTs.
Rectal LSTs are a type of superficial colorectal neoplasia that predominantly extend laterally along the mucosal surface rather than invading deeply into the layers of the bowel wall, typically measuring more than 10 mm in diameter18. Based on endoscopic morphological features, LSTs are classified into granular and non-granular types. The granular type can be further subdivided into homogeneous granular and mixed nodular subtypes, while the non-granular category includes flat elevated and pseudo-depressed subtypes19,20. Consistent with previous studies18,21,22, we also observed in this research that LST-G-M was the most prevalent subtype. Although most LSTs are benign or exhibit low-grade malignancy, certain pathological types carry a higher potential for malignant progression. Therefore, complete resection via endoscopic techniques is essential for both curative treatment and accurate pathological assessment23,24,25.
ESD has become the preferred treatment for rectal LSTs due to its ability to achieve en bloc resection and accurate pathological staging1. However, the relatively narrow lumen of the rectum and the rich vascularity of its submucosa make rectal ESD more technically challenging compared to gastric ESD, with higher risks of intraoperative bleeding and perforation. In this study, we observed an intraoperative bleeding rate of 3.9%, which is consistent with previously reported rates ranging from 0.0% to 11.9%26,27. In contrast, the intraoperative perforation rate in our study was only 0.4%, with no perforations occurring in the IMK group. This result is notably lower than the previously reported range of 1.4%–20.4%28,29. We attribute this significantly reduced perforation rate primarily to the integrated injection and cutting function of the IMK, which allows continuous maintenance of submucosal lifting without the need for instrument exchange. This feature reduces the risk of inadvertent injury to the muscularis propria or deep layers caused by repeated device insertion or withdrawal or by poor visual field stability. Furthermore, the exclusion of cases with poor lifting after submucosal injection in this study also contributed, to some extent, to lowering the incidence of technical complications.
Previous studies have demonstrated various endoscopic techniques for treating rectal LSTs, including endoscopic mucosal resection (EMR)30,31, ESD1, and hybrid approaches combining EMR with endoscopic full-thickness resection (EFTR)32. ESD has emerged as a standard method for en bloc resection, particularly for large LSTs (≥ 10 cm)33,34, though it carries higher technical difficulty35. Comparative studies show ESD achieves superior R0 resection rates compared to piecemeal EMR (pEMR)31,36, while endoscopic submucosal tunnel dissection (ESTD) demonstrates comparable efficacy to ESD for rectal LSTs1. Novel techniques like hybrid ESD37 and underwater EMR (UEMR)38 are being explored to improve outcomes. Band ligation-assisted EMR has been used for smaller LSTs (mean 40 mm) with 70% complete eradication rates39. For complex cases involving hemorrhoids or appendiceal orifice involvement, ESD remains feasible40. However, during rectal ESD procedures, the rapid diffusion of submucosally injected fluid often necessitates repeated injections to maintain adequate lifting for safe and effective submucosal dissection41. This requirement not only prolongs the total procedure time but also increases the risk of intraoperative complications. In the present study, we observed that the use of the IMK significantly reduced the total operation time and was associated with a lower incidence of intraoperative complications.
Prior research has established a significant association between procedural duration and specific lesion characteristics. Features such as non-granular pseudodepressed LSTs (which demonstrate a submucosal invasion rate of 19.4%), granular nodular mixed LSTs (15.9%), and invasive pit patterns have been identified as independent predictors of both technical complexity and extended resection times exceeding 120 min35,42. Tumor size represents another consistent risk factor; meta-regression analysis indicates an inverse correlation between lesion diameter and the efficiency of traction-assisted ESD (estimate: − 1.02, 95% CI − 1.58 to − 0.46)43. Additionally, Wu et al.44 reported that involvement of the dentate line (OR = 3.881, P = 0.026) and lesions measuring ≥ 50 mm (OR = 5.047, P = 0.009) independently predict prolonged ESD duration, with circumferential involvement of ≥ 2/3 further extending operative time (P = 0.001). In alignment with these findings, Miyaguchi et al.45 observed that non-granular morphology, especially the LST-NG-PD subtype, correlates strongly with increased technical difficulty and longer procedure times (OR = 2.618, P = 0.001). This study confirms that the selection of electrosurgical devices also substantially affects operating time, and specifically demonstrates that the use of the IMK markedly reduces procedure duration.
Several limitations should be considered when interpreting the results of this study. First, this was a retrospective analysis with inherent selection bias, and the non-randomized assignment of knives may have influenced the outcomes despite comparable baseline characteristics. Second, all procedures were performed by experienced endoscopists at high-volume centers, which may limit the generalizability of the findings to less experienced operators or low-resource settings. Third, long-term outcomes including local recurrence and survival were not evaluated; thus, the durability of the IMK technique requires further validation through prospective studies with extended follow-up.
In summary, this study demonstrates that the IMK technique significantly enhances the efficiency of ESD for rectal LSTs by reducing total procedure time and mucosal dissection time, while increasing the submucosal dissection rate, compared to the conventional DK technique. Importantly, these improvements in operational efficiency were achieved without compromising safety, as evidenced by comparable rates of R0 resection, intraoperative and postoperative complications, and histopathological outcomes between the two groups. The integrated injection function of the IMK minimizes instrument exchange and maintains submucosal elevation, thereby streamlining the dissection process. These findings support the adoption of the IMK as a valuable tool in the endoscopic management of rectal LSTs, particularly for lesions of varying sizes and morphological subtypes.
Data availability
Data availability can be requested from the corresponding author with a reasonable justification.
References
Zou, J. et al. Efficacy and safety of endoscopic submucosal tunnel dissection for rectal laterally spreading tumors. Surg. Endosc. 35(8), 4356–4362. https://doi.org/10.1007/s00464-020-07927-4 (2021).
Kouladouros, K., Warkentin, V. & Kähler, G. Transanal endoscopic microsurgical submucosal dissection: Are there advantages over conventional ESD? Minim. Invasive Ther. Allied Technol. 31(5), 720–727. https://doi.org/10.1080/13645706.2021.1967999 (2022).
Kuwai, T. et al. Efficacy and safety comparison of scissor-type knives with needle-type knives for colorectal endoscopic submucosal dissection: a post-hoc propensity score-matched analysis (with videos). Gastrointest. Endosc. 96(1), 108–117 (2022).
Visrodia, K. et al. Scissor-type knife improves the safety of endoscopic submucosal dissection (ESD) among endoscopists without experience in ESD: A randomized ex vivo study. Endosc Int. Open. 9(8), E1207–E1213. https://doi.org/10.1055/a-1487-5469 (2021).
Shen, J. et al. Efficacy of two kinds of scissor-type knives for colorectal endoscopic submucosal dissection: A retrospective comparative study. Dig. Dis. Sci. 69(11), 4214–4223. https://doi.org/10.1007/s10620-024-08525-3 (2024).
Ichijima, R. et al. Ex vivo porcine model study on the treatment outcomes of scissor-type knife versus needle-type knife in endoscopic submucosal dissection performed by trainees. BMC Surg. 20(1), 287. https://doi.org/10.1186/s12893-020-00955-w (2020).
Aihara, H. et al. A multicenter, retrospective study of a through-the-needle injection-capable electrosurgical knife for endoscopic submucosal dissection. Gastrointest. Endosc. 100 (6), 1034–1042 (2024).
Cecinato, P. et al. Endoscopic submucosal dissection in colorectal neoplasia performed with a waterjet system-assisted knife: higher en-bloc resection rate than conventional technique. Clin. Endosc. 55 (6), 775–783. https://doi.org/10.5946/ce.2022.099 (2022).
Ryu, D. G. et al. Efficacy and safety of one-step knife compared to conventional insulated-tip knife for endoscopic submucosal dissection: a preliminary study with prospective data collection and retrospective review. Surg. Endosc. 37 (1), 329–336. https://doi.org/10.1007/s00464-022-09515-0 (2023).
Veras Ayres da Silva PH et al. Scissor-assisted vs. conventional endoscopic submucosal dissection for colorectal lesions: systematic review and meta-analysis. Dig. Endosc. 36 (11), 1213–1224. https://doi.org/10.1111/den.14829 (2024).
Dixon, M. F. Gastrointestinal epithelial neoplasia: Vienna revisited. Gut 51(1), 130–131. https://doi.org/10.1136/gut.51.1.130 (2002).
Pérez-Cuadrado-Robles, E. et al. Risk factors for conversion to snare resection during colorectal endoscopic submucosal dissection in an expert Western center. Endoscopy 51(2), 152–160. https://doi.org/10.1055/a-0650-4562 (2019).
Nie, X. et al. Curative effect analysis of endoscopic submucosal dissection in giant laterally spreading rectal tumors. J. Clin. Gastroenterol. 58(2), 169–175. https://doi.org/10.1097/MCG.0000000000001844 (2024).
Oda, I. et al. Complications of gastric endoscopic submucosal dissection. Dig. Endosc. 25(Suppl 1), 71 –78. https://doi.org/10.1111/j.1443-1661.2012.01376.x (2013).
Liu, L. et al. Development and validation of a preoperative difficulty scoring system for endoscopic resection of gastric gastrointestinal stromal tumor: a multi-center study. Surg. Endosc. 37(8), 6255–6266. https://doi.org/10.1007/s00464-023-10106-w (2023).
Takahashi, S. et al. Characteristics of factors contributing to follow-up for suspected delayed bleeding after colorectal endoscopic submucosal dissection. Gastrointest. Endosc. 100(4), 718–727. https://doi.org/10.1016/j.gie.2024.03.021 (2024).
Shichijo, S. et al. Safety and feasibility of intensive endoscopic interventions for delayed perforation after colorectal endoscopic submucosal dissection (with video). Gastrointest. Endosc. https://doi.org/10.1016/j.gie.2025.03.1328 (2025).
Cong, Z. J. et al. A long-term follow-up study on the prognosis of endoscopic submucosal dissection for colorectal laterally spreading tumors. Gastrointest. Endosc. 83(4), 800–807 (2016).
Kudo, T. et al. Diagnostic performance of endocytoscopy for evaluating the invasion depth of different morphological types of colorectal tumors. Dig. Endosc. 27(7), 754–761. https://doi.org/10.1111/den.12469 (2015).
Vleugels, J. L. A., Hazewinkel, Y. & Dekker, E. Morphological classifications of gastrointestinal lesions. Best Pract. Res. Clin. Gastroenterol. 31(4), 359–367. https://doi.org/10.1016/j.bpg.2017.05.005 (2017).
Chiba, H. et al. The feasibility of endoscopic submucosal dissection for colorectal lesions larger than 10 cm. Surg. Endosc. 36(7), 5348–5355. https://doi.org/10.1007/s00464-021-08916-x (2022).
Nishiyama, H. et al. Endoscopic submucosal dissection for laterally spreading tumours of the colorectum in 200 consecutive cases. Surg. Endosc. 24(11), 2881–2887. https://doi.org/10.1007/s00464-010-1071-5 (2010).
Tamura, S. et al. Evaluation of endoscopic mucosal resection for laterally spreading rectal tumors.Endoscopy 36(4), 306– 312. https://doi.org/10.1055/s-2004-814204 (2004).
Tanaka, S. et al. Japanese society of Gastroenterology. Evidence-based clinical practice guidelines for management of colorectal polyps. J. Gastroenterol. 50(3), 252–260. https://doi.org/10.1007/s00535-014-1021-4 (2015).
Osera, S. et al. Endoscopic treatment outcomes of laterally spreading tumors with a skirt (with video). Gastrointest. Endosc. 86(3), 533–541. https://doi.org/10.1016/j.gie.2017.01.037 (2017).
Tanaka, S. et al. JGES guidelines for colorectal endoscopic submucosal dissection/endoscopic mucosal resection. Dig. Endosc. 27(4), 417–434. https://doi.org/10.1111/den.12456 (2015).
Yamamoto, K. et al. Colorectal endoscopic submucosal dissection: Recent technical advances for safe and successful procedures. World J. Gastrointest. Endosc. 7(14), 1114–1128. https://doi.org/10.4253/wjge.v7.i14.1114 (2015).
Tanaka, S. et al. Current status and future perspectives of endoscopic submucosal dissection for colorectal tumors. Dig. Endosc. 24(Suppl 1), 73–79. https://doi.org/10.1111/j.1443-1661.2012.01252.x (2012).
Kim, E. R. & Chang, D. K. Management of complications of colorectal submucosal dissection. Clin. Endosc. 52(2), 114–119. https://doi.org/10.5946/ce.2019.063 (2019).
Russo, P. et al. Management of colorectal laterally spreading tumors: A systematic review and meta-analysis. Endosc Int. Open. 7(2), E239–E259. https://doi.org/10.1055/a-0732-487 (2019).
Zhao, H. J. et al. Endoscopic mucosal resection versus endoscopic submucosal dissection for colorectal laterally spreading tumors: A meta-analysis. Rev. Esp. Enferm. Dig. 112(12), 941–947. https://doi.org/10.17235/reed.2020.6681/2019 (2020).
Chua, J. S. et al. Dutch eFTR working Group. Hybrid endoscopic mucosal resection and full-thickness resection for large colonic polyps harboring a small focus of invasive cancer: a case series. Endosc Int. Open. 9(11), E1686–E1691. https://doi.org/10.1055/a-1529-1447 (2021).
Lu, J. et al. Endoscopic submucosal dissection for rectal-sigmoid laterally spreading tumors ≥ 10 cm: An analysis of 10 cases. Transl Cancer Res. 10(2), 867–875. https://doi.org/10.21037/tcr-20-2659 (2021).
Tang, X. W. et al. Endoscopic submucosal dissection for laterally spreading tumors in the rectum ≥ 40 mm. Tech. Coloproctol. 20(7), 437–443. https://doi.org/10.1007/s10151-016-1459-x (2016).
Zhu, M. et al. Endoscopic submucosal dissection for colorectal laterally spreading tumors: Clinical outcomes and predictors of technical difficulty. J. Dig. Dis. 23(4), 228–236. https://doi.org/10.1111/1751-2980.13091 (2022).
Sekiguchi, M. et al. Cost-effectiveness analysis of endoscopic resection for colorectal laterally spreading tumors: endoscopic submucosal dissection versus piecemeal endoscopic mucosal resection. Dig. Endosc. 34(3), 553–568. https://doi.org/10.1111/den.14058 (2022).
Li, H. et al. Hybrid versus conventional endoscopic submucosal dissection for laterally spreading tumors (LSTs): A retrospective multicenter study. JGH Open. 8(12), e70066. https://doi.org/10.1002/jgh3.70066 (2024).
Le, Q. D., Le, N. Q. & Quach, D. T. Underwater versus conventional endoscopic mucosal resection for colorectal laterally spreading tumors: A post hoc analysis of efficacy. JGH Open. 8(12), e70075. https://doi.org/10.1002/jgh3.70075 (2024).
Romutis, S. et al. Safety and efficacy of band ligation and auto-amputation as adjunct to EMR of colonic large laterally spreading tumors, and polyps not amenable to routine polypectomy. Ther. Adv. Gastrointest. Endosc. 14, 26317745211001750 (2021).
Peng, D. et al. Safety and efficacy of ESD for laterally spreading tumors with hemorrhoids close to the dentate line. Minim. Invasive Ther. Allied Technol. 33(4), 215–223 (2024).
Hihara, D. et al. Factors associated with increased duration of endoscopic submucosal dissection for rectal tumors: A 22-year retrospective analysis. Gastrointest. Endosc. 98(3), 420–427e1 (2023).
Soliman, H. et al. Invasive pit pattern, macronodule and depression are predictive factors of submucosal invasion in colorectal laterally spreading tumours from a Western population. United Eur. Gastroenterol. J. 6(10), 1569–1577 (2018).
Cheng, S. W. et al. How does lesion size affect the pooled effect of traction-assisted endoscopic submucosal dissection on procedure time? A meta-regression. World J. Surg. Oncol. 17(1), 157. https://doi.org/10.1186/s12957-019-1699-0 (2019).
Wu, Y. et al. Endoscopic submucosal dissection for superficial ultra-low rectal tumors: Outcomes and predictive factors for procedure difficulty. Am. J. Cancer Res. 14(12), 5784–5797 (2024).
Miyaguchi, K. et al. A retrospective cohort study of factors influencing long procedure times in colorectal endoscopic submucosal dissection. Scand. J. Gastroenterol. 56(10), 1255–1263 (2021).
Funding
This study was supported by the Changshu Science and Technology Program (CY202339), the Suzhou Youth Science and Technology Project for the Advancement of Science, Education, and Health (KJXW2023067), and the Suzhou 23rd Batch of Science and Technology Development Plan (Clinical Trial Institution Capability Enhancement) Project (SLT 2023006).
Author information
Authors and Affiliations
Contributions
F.Z. contributed to data collection and writing. B.H., Y.F., and Y.Y. contributed to data collection. L.L. was responsible for statistical analysis. L.L. and Y.D. contributed to revising this dissertation. L.L. and Y.D. managed this project. All authors listed have made a substantial, direct, and intellectual contribution to the work and approved it for publication.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Zang, F., He, B., Feng, Y. et al. Comparative efficacy and safety of injection mucosa knife versus conventional techniques in endoscopic submucosal dissection for rectal laterally spreading tumors. Sci Rep 15, 43497 (2025). https://doi.org/10.1038/s41598-025-30684-0
Received:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s41598-025-30684-0