Abstract
Most previous studies on Estimated Glucose Disposal Rate (eGDR) have been conducted in general population, with few studies focusing on critically ill patients. We investigated the predictive value of eGDR in death risk in intensive care unit (ICU) patients with cardiorenal syndrome (CRS). Our study selected ICU patients from the MIMIC-IV database, and we focused on the outcomes of 30-, 90-, 180-, and 360-day mortality. We assessed the predictive value of eGDR for mortality in ICU patients with CRS by multivariate Cox regression models, restricted cubic spline (RCS), and Kaplan–Meier curves. The study included 2,398 participants with a median age of 71.0 years, and 61.2% were male. Multifactorial Cox regression models as well as RCS analysis showed that LOW eGDR was significantly associated with DECREASED 30-day mortality, 90-day mortality, 180-day mortality, and 360-day mortality, and there was a significant nonlinear relationship between eGDR and 90-day mortality, 180-day mortality, and 360-day mortality. This relationship was also found in all critically ill patients. We further stratified the eGDR and found that the significant correlation between eGDR levels and all-cause mortality was mainly present in ICU patients with CRS who were not treated with CRRT. Combined with the results of the Boruta algorithm, higher eGDR is associated with increased all-cause mortality in critically ill patients, including those with crs, and this was particularly evident in patients not treated with CRRT. This surprising result is counter to our hypothesis and may suggest that insulin resistance improves survival.
Similar content being viewed by others
Introduction
Intensive care unit (ICU) patients commonly have varying degrees of cardiac and renal dysfunction, and dysfunction in either organ, the heart or the kidneys, can cause the other to function and lead to its pathophysiologic alterations through a complex mechanism, resulting in the cardiorenal syndrome (CRS). CRS is a recognized clinical entity in which acute or chronic dysfunction of either the heart or the kidney induces dysfunction of the other organ, and its classification consists of five main subtypes1,2.A consensus on CRS in 2008 was proposed and endorsed by several fields, including nephrology, critical care medicine, cardiovascular medicine, and cardiovascular surgery; however, studies on CRS in different populations have gradually increased, but they have mainly focused on cardiovascular and nephrology, and studies on CRS in critically ill patients have not been common. In addition, hypervolemia is one of the main problems of CRS, and volume overload may be secondary to renal insufficiency or acute or chronic heart failure, leading to a significant increase in mortality in hospitalized patients, especially in the critically ill population with combined CRS3,4,5,6,7. Despite recent advances in diagnostic criteria and therapeutic strategies for CRS, the prognostic assessment of patients with CRS in the ICU still faces great challenges. As a result, CRS has a higher prevalence and poorer prognosis in critically ill patients, with a significantly higher rate of death than in patients with cardiac or renal disease alone.
Insulin resistance is one of the primary mechanisms underlying cardiovascular events8, linking the components of metabolic syndrome, including hypertension, dyslipidemia, hyperglycemia, and obesity, which are major risk factors for cardiovascular events9. However, the current gold standard for assessing insulin resistance, the hyperinsulinemic-euglycemic clamp technique, requires specialized equipment and is complex to perform, making it impractical for large-scale critical care studies or routine clinical use. Therefore, researchers have proposed using the estimated glucose disposal rate (eGDR) to estimate the severity of insulin resistance10,11. Compared to the normal glucose high insulin clamp method, eGDR calculation is based on body mass index (BMI), hypertension, and hemoglobin A1c (HbA1c)—data readily obtainable from clinical records—and demonstrates high accuracy in estimating insulin resistance12. Furthermore, eGDR has been validated across multiple studies as a prognostic marker for microvascular and macrovascular complications, including associations with coronary artery disease, heart failure, and nephropathy11,13,14,15. Studies have shown that insulin resistance is correlated with the progression of diseases such as chronic heart failure and acute kidney injury16,17.
However, no previous investigation has evaluated the eGDR in critically ill patients with CRS. The present study is the first to test the hypothesis that eGDR serves as an independent predictor of all-cause mortality in this population. ICU patients are associated with hyperglycemia, which can lead to high morbidity, mortality, and incidence of infection; therefore, frequent monitoring of blood glucose levels was prominent in the ICU to avoid the dangers of persistent hyperglycemia. Some studies have found that intensive insulin therapy can lead to a significant increase in morbidity and mortality, which may be related to the increased incidence of severe hypoglycemia after intensive glycemic control18. Critically ill patients have received increasing attention due to the higher incidence of glycemic dysfunction and its impact on prognosis than general hospitalized patients19. Despite mild hypoglycemia, when blood glucose is < 3.9 mmol/L, morbidity and mortality are notably increased in ICU patients20.
The MIMIC-IV database, as an open critical care medicine research database, incorporates critically ill patients with complete laboratory examination and follow-up information, providing a high-quality research platform for exploring novel biomarkers21. In this study, we proposed to analyze the predictive ability of eGDR for all-cause mortality in CRS patients by analyzing a cohort of CRS patients in MIMIC-IV to provide a new reference index for the early identification of high-risk patients in the clinic and improve the prognosis of CRS patients. Our surprising results run counter to our hypothesis.
Methods
Data sources
In our observational study, we extracted data from the publicly accessible Medical Information Marketplace in Intensive Care (MIMIC-IV) database (https://physionet.org/content/mimiciv/). The MIMIC-IV study originated from an organization between Beth Israel Deaconess Medical Center (BIDMC) and the Massachusetts Institute of Technology (MIT). Data collected by Beth Israel Deaconess Medical Center are identified and transformed by researchers who have completed human research training and signed data use agreements. The Beth Israel Deaconess Medical Center Institutional Review Board approved the waiver of informed consent and the sharing of research resources22. In accordance with the data use guidelines, the authors of this article obtained a Cooperative Institutional Training Initiative (CITI) license and the necessary authorization to use the MIMIC-IV database.
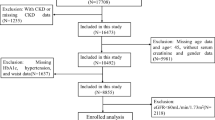
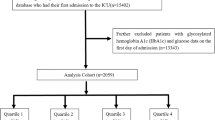
The study included 35,282 patients with CRS who were hospitalized in ICU. All study subjects were at least 18 years of age. Figure 1 shows flowchart. For patients with multiple admissions, we studied data related to their first admission. Patients with insufficient data on height, weight, hypertensive status and HbA1c levels within 48 h of admission or lack of follow-up data were also excluded from our analyses to ensure the precision and accuracy of the data. A cohort of 2398 patients was established, and these patients were categorized into four groups (Q1, Q2, Q3, and Q4) according to the quartiles of the eGDR index, and group Q1 was used as the reference group.
Data collection
We applied Structured Query Language (SQL) and PostgreSQL (version 16.2) to collect relevant data. The data in this study included patients’ demographic characteristics: age, gender, BMI; laboratory tests: red blood cells (RBC), white blood cells (WBC), platelets (PLT), HbA1c, aspartate transaminase (AST), alanine transaminase (ALT), albumin (ALB), serum creatinine (SCR), prothrombin time (PT), partial thromboplastin time (PTT), international normalized ratio ( INR), creatine kinase-MB (CKMB), troponin T (TnT); comorbidities: anemia, atrial fibrillation (AF), coronary artery disease (CAD), myocardial infarction (MI), diabetes mellitus (DM), hyperglycemia, hypertension, transient ischemic attack (TIA), peripheral vascular disease (PVD), cerebrovascular disease(CVD); continuous renal replacement therapy (CRRT), ventilation. Follow-up of the study began on the day of ICU admission and ended when the relevant endpoints (30-day, 90-day, 180-day, and 360-day mortality) were present. BMI = weight (Kg)/ height (m)2. eGDR was calculated by the following formula: eGDR = 19.02—(0.22 × BMI)—(3.26 × hypertension)—(0.61 × HbA1c) [BMI (Kg/m2), hypertension (yes = 1/no = 0), and HbA1c (%)]23.
In our study, the main outcome was 30-day, and the secondary outcomes were 90-day, 180-day, and 360-day mortality.
Statistical analysis
Our statistical analyses were performed by SPSS (version 25.0, IBM), STATA (version 17.0) and R (version 4.1.3, Austria). Continuous variables were conducted normality tests by Shapiro–Wilk test, for normally distributed data, we used "mean ± SD" to presented by ANOVA, for non-normally distributed data, we use "median [interquartile range, IQR]" to presented by Kruskal–Wallis H test. Categorical variables were analyzed using the chi-square test or corrected chi-square test and are presented as numbers (proportions).
We performed correlations between the eGDR index and 30-day, 90-day, 180-day, and 360-day mortality by logistic regression and Cox regression models. Feature selection is very important in the model construction process, therefore, based on our clinical experience, we further screened the features that were significantly associated with the study results through Boruta analysis, and these features were incorporated into the final analytical model (Fig. 2). In model 1, we included only the eGDR index. In model 2, we modified the model to include gender, age, and BMI. In model 3, the variables corrected included age, gender, BMI, glucose, PLT, PT, INR, CVD, CRRT, TnT, and PTT. We made the above adjustments based on Boruta algorithm feature selection results and clinical experience.
Feature selection process for mortality based on Boruta’s algorithm (A, C, E, G) and the value evolution of Z-score in the screening process (B, D, F, H). In A, C, E, and G, the horizontal axis represents the variable name and the vertical axis represents the Z-values of each variable. In B, D, F and H, the horizontal axis represents the number of iterations, and the vertical axis represents the change in Z-values during the screening process. The blue boxes and lines correspond to the minimum, average, and maximum Z-scores for a shadow feature. The green boxes and lines represent the confirmed variables and the red ones represent the rejected variables in the model calculation.
To investigate the incidence of risk of death in the different stratification groups of the eGDR index, Kaplan–Meier survival analysis was performed, and the differences found were correlated using the log-rank test. In addition, the nonlinear relationship between the eGDR index and 30-day mortality, 90-day mortality, 180-day mortality, and 360-day mortality, was characterized by multivariate restricted cubic spline (RCS). Finally, subgroup analyses were performed considering gender, age, BMI, diabetes, CRRT, and CAD, and the p-value of the interaction was evaluated. Statistical significance was defined as a two-sided P value of less than 0.05.
Results
In our study, we checked 2398 CRS patients by strict inclusion and exclusion criteria. Their median age was 71.0 years old, median BMI was 28.8 kg/m2 and 1468 (61.2%) were male. Additionally, our study revealed 344 (14.3%) deaths within 30 days of follow-up, 503(21.0%) deaths within 90 days of follow-up, 603 (25.1%) deaths within 180 days of follow-up and 753 (31.4%) deaths within 360 days of follow-up.
Baseline features.
We analyzed the baseline characteristics of patients with CRS by eGDR quartiles (Table 1). The median eGDR value of the patients was 14.1. The results of the study revealed that four eGDR-quartile groups differed significantly in age, BMI, and glucose and HbA1c levels. The eGDR index was associated with a significantly lower risk of comorbid diabetes mellitus in those with a higher eGDR index. Also, patients with a relatively high eGDR have a higher mortality rate compared to those with a lower eGDR. The remaining parameters had similar values in both groups. Additionally, we analyzed the baseline characteristics of non-CRS patients among the critically ill cohort, as detailed in Supplementary Table S1.
Relationships of eGDR index concentration with mortality
We used three Cox regression models to examine the link between eGDR levels and the death risk (Table 2). Combined with the results of feature selection by the Boruta algorithm. For different outcomes in model 3, all models considered age, gender, BMI, glucose, PLT, PT, INR, cerebrovascular disease, PTT, CRRT, and TnT. Our analysis showed that in model 1 (unadjusted), when treated as a continuous variable, the eGDR showed a significant correlation with 30-day mortality [30-day mortality: HR (95% CI) 1.11(1.06,1.16), P < 0.001]; in model 2 (adjusted for age, sex, and BMI), when eGDR was treated as a continuous variable, the eGDR index showed a significant correlation with 180-day mortality [HR (95% CI) 1.11(1.01,1.21), P = 0.025]. Whereas, when considered as a categorical variable, the eGDR index showed a significant correlation with mortality in all three Cox regression models (P < 0.001), and all models showed a higher HR for patients with medium and high scores. The relationship between non-CRS patients in the critically ill population and mortality risk is presented in Supplementary Table S2. We found that a higher eGDR also robustly predicted increased all-cause mortality in the non-CRS population. The covariates adjusted for in the model are consistent with those in the CRS patient population. To validate that eGDR is superior to BMI alone, we conducted a Cox proportional hazards model analysis for the primary outcome variable, 30-day mortality. Detailed results are presented in Supplementary Table S3. We found that bmi alone resulted in a similar association where increased bmi was associated with decreased mortality (P = 0.001) (supplementary Table S3).
Kaplan–Meier survival analyses were performed to analyze 30-day, 90-day, 180-day, and 360-day mortality rates in different eGDR quartile groups. We observed statistically significant differences in mortality rates across quartile groups (all log-rank P < 0.001) (Fig. 3).
Non-linear relationship
Since multivariate Cox regression models showed a nonlinear relationship between the eGDR index and 90-day, 180-day, and 360-day mortality, therefore, we further analyzed this correlation by RCS analysis. Figure 4 shows the adjusted RCS curves for the nonlinear associations between eGDR and 90-day mortality (Fig. 4B, nonlinear P < 0.001), 180-day mortality (Fig. 4C, nonlinear P < 0.001), and 360-day mortality (Fig. 4D, nonlinear P < 0.001).
Stratified analyses
We stratified by gender, age, BMI, diabetes, CRRT, and ceredisease to further assess the effect of eGDR on outcome indicators (Fig. 5). We found no significant interactions for all subgroups except for the CRRT subgroup (30-day mortality: interaction P = 0.022, 90-day mortality: interaction P = 0.015, 180-day mortality: interaction P = 0.045, and 360-day mortality: interaction P = 0.049).
Discussion
In our study, a retrospective cohort analysis based on the MIMIC-IV database revealed, for the first time, that eGDR, as a surrogate indicator of insulin resistance, was significantly associated with all-cause mortality from 30 to 360 days in ICU patients with CRS, and higher eGDR is associated with increased all-cause mortality in critically ill patients with CRS. Surprisingly, our results run counter to our hypothesis. that is, low egdr (surrogate for insulin resistance) was associated with decreased mortality in all icu patients, including those with CRS. We observed that, in contrast to the earlier approach of screening variables solely based on clinical experience, we involved clinical indicators in the Multivariate Cox regression models of 30-day, 90-day, 180-day, and 360-day mortality by combining the outcomes of the Boruta algorithm with conventional risk factors. Following multivariate Cox regression models and RCS analysis, we discovered that eGDR exhibited nonlinear associations with 90-day, 180-day, and 360-day mortality and was associated with all-cause mortality in ICU patients with CRS. According to this finding, eGDR may be a potential biomarker for risk stratification in this population, and metabolic abnormalities may be a significant part of the pathophysiologic processes driving the poor prognosis of patients with CRS. And we found that a higher eGDR also robustly predicted increased all-cause mortality in the non-CRS population. We interpret these findings not as a negation of eGDR’s role in CRS, but as a refinement of its clinical implication. This indicates that eGDR is a powerful prognostic marker across a broad spectrum of critically ill patients. notably, our finding is that low eGDR is associated with decreased mortality, thereby confounding our hypothesis that insulin resistance is a common pathophysiological driver of adverse outcomes.
Studies have found that insulin resistance is linked with diabetes, disorders of lipid metabolism, and elevated blood pressure24,25. Low eGDR, one of the main indicators of insulin resistance response, is correlated with a low glucose disposal rate measured by a normoglycemic-hyperinsulinemic clamp11. Most of the previous studies have been conducted in the general population or patients with chronic diseases26,27,28. At this point, low eGDR (high IR) is a marker of cardiovascular risk and is strongly associated with obesity and metabolic syndrome. At the same time, given the invasiveness and cost of traditional methods for assessing insulin resistance, eGDR is based on patient height, weight, glycated hemoglobin levels, and the presence of hypertension, it is more suitable for large-scale clinical application. One study found that lower levels of eGDR were associated with an increased risk of CVD events in a nondiabetic population29. Furthermore, in nondiabetic patients, there was a sex-specific association between eGDR and all-cause and CVD mortality. In women, eGDR was negatively associated with all-cause and CVD mortality, whereas in men, eGDR was L-shapedly associated with all-cause and CVD mortality30. Lower eGDR is a predictor of the progression of renal function in patients with T2DM31. Insulin resistance has been found to be associated with the presence of autonomic neuropathy and subclinical atherosclerosis32. In conclusion, the value of LOW eGDR in the assessment of HIGH insulin resistance has been widely recognized and in other disease states low eGDR is associated with adverse outcomes. Remarkably, we find the exact opposite, that is, low eGDR is associated with improved outcomes, even in a disease state such as CRS.
On the other hand, studies on eGDR in ICU population, insulin resistance is associated with diabetes, lipid metabolism disorders, and elevated blood pressure24,25. When ICU patients are in a state of stress, a large number of neuroendocrine hormones and inflammatory mediators are released, which can lead to metabolic disorders and stress hyperglycemia, and stress hyperglycemia can amplify the systemic inflammatory response and trigger immune suppression, which delays the recovery of brain function and tissue repair, and leads to an increase in the morbidity and mortality rate. It has been found that in ICU patients under stress, with or without diabetes mellitus, abnormalities of glucose metabolism are prevalent, and the incidence of acute hyperglycemia in ICU patients is as high as 43–50%33, and hypoglycemia is 12.59–26.5%34, and even if only one mild-to-moderate hypoglycemia occurs, it will lead to an increased risk of death and a significantly prolonged stay in the ICU. The risk of hypoglycemia in the ICU is increased, and the length of stay in the ICU is significantly prolonged, even if only one mild to moderate hypoglycemia occurs35. ICU patients are at increased risk of severe hypoglycemia due to a lack of specific signs and undetectable early warning signs36. Hypoglycemia has a deleterious effect on critically ill patients in many ways and may even lead directly to death, with the cardiovascular system being the most susceptible37. The blood flow to the kidneys is also affected when the blood glucose concentration in the body is low. Deterioration of renal function frequently accompanies heart disease, with an incidence of up to 40% in patients hospitalized for HF38. In addition, the incidence of hypoglycemia is higher in patients who develop organ failure than in those who do not39. In contrast, the present study was conducted on patients in the intensive care unit with concomitant cardiorenal syndrome, and such patients were in a state of acute multi-organ failure with significant metabolic disturbances40. High eGDR may reflect a compensatory response after hypercatabolism or stress hyperglycemia rather than a simple improvement in insulin sensitivity. At this point, excessively high glucose handling rates may suggest energy depletion or inflammatory storms in the organism, which are instead associated with a poor prognosis.
We investigated the predictive value of eGDR and all-cause mortality in ICU patients with CRS by multivariate Cox regression models. Compared with previous multivariate regression analyses based on clinical experience, we assessed the effect of eGDR on all-cause mortality more accurately by combining the most relevant features of the dependent variable with those screened by the Boruta algorithm. Our analysis found that low eGDR was significantly associated decreased with all-cause mortality in ICU patients, including those with CRS and was nonlinearly associated with decreased 90-day mortality, 180-day mortality, and 360-day mortality. Further stratified analysis showed a significant interaction with whether CRRT treatment was applied or not. Specifically, the correlation between low eGDR levels and decreased all-cause mortality was mainly seen in patients not treated with CRRT. Notably, the predictive efficacy of low eGDR for decreased mortality risk remained stable over time (30 to 360 days), suggesting that it can be used for early warning of improved outcomes in the acute phase. However, low eGDR in the icu setting appears to be different from low eGDR in the non-critically ill setting where low eGDR may be used for identifying high-risk populations in need of long-term metabolic interventions.
The study has some limitations: firstly, as retrospective observational and single-center research, a causal relationship between eGDR and mortality could not be confirmed, In Supplementary Table S2, we conducted a Cox regression analysis for the non-CRS population, treating eGDR as both a continuous and categorical variable. The results are intriguing. We found that a higher eGDR also robustly predicted increased all-cause mortality in the non-CRS population. Thus, the eGDR–mortality association is not unique to CRS and may simply reflect a general ICU phenomenon. And our single-arm design precludes comparison with non-CRS critically ill patients; consequently, we cannot determine whether the eGDR–mortality association is unique to CRS or simply reflects a general ICU phenomenon. Future case–control or cohort studies comparing CRS with non-CRS ICU populations are required to establish disease specificity. Secondly, BMI from admission may overestimate adiposity in fluid-overloaded ICU patients, and single HbA1c on ICU admission may not reflect pre-admission glycaemic control. Third, residual unmeasured confounding cannot be excluded, and we could not completely exclude the effect of residual confounders. Fourth, our study did not consider whether patients with CRS and hypertension received hypertension-related treatment management, nor did it examine whether these patients underwent glucose tolerance tests. Therefore, more detailed research is needed in the future to support these findings. Fifth, the absence of repeated HbA1c and standardized Blood pressure recordings precluded us from validating whether HbA1c or hypertension adds prognostic value beyond BMI; this question awaits a prospective cohort with protocolized early laboratory and hemodynamic measurements. Furthermore, in our study, there was insufficient evidence to demonstrate that eGDR is superior to BMI alone, necessitating more precise research to establish this. It may be that our surprising results are simply due to the ‘obesity paradox’. In addition, this study only analyzed short-term follow-up data from 30 to 360 days for CRS patients, which may result in an inaccurate assessment of the long-term prognosis for such patients. Therefore, future studies should combine dynamic metabolic monitoring, inflammatory markers, and other methods to further elucidate the underlying mechanisms and consider extending the follow-up period to provide stronger support for optimizing clinical treatment protocols.
Conclusion
This study observed that higher eGDR is associated with increased all-cause mortality in critically ill patients including those with CRS, especially in those who were not treated with CRRT. This contradicts our initial hypothesis which was that lower eGDR (indicating more severe insulin resistance) should be associated with poor prognosis.
Data availability
The datasets can be accessed via https://mimic.mit.edu.
References
Goh, C. Y. & Ronco, C. Cardio-renal syndromes [J]. J. Ren. Care 36(Suppl 1), 9–17 (2010).
Ronco, C. et al. Cardiorenal syndrome [J]. J. Am. Coll. Cardiol. 52(19), 1527–1539 (2008).
Ahmed, M. S., Wong, C. F. & Pai, P. Cardiorenal syndrome - a new classification and current evidence on its management [J]. Clin. Nephrol. 74(4), 245–257 (2010).
Drazner, M. H. et al. Prognostic importance of elevated jugular venous pressure and a third heart sound in patients with heart failure [J]. N. Engl. J. Med. 345(8), 574–581 (2001).
Jain, P. et al. Current medical treatment for the exacerbation of chronic heart failure resulting in hospitalization [J]. Am. Heart J. 145(2 Suppl), S3-17 (2003).
Androne, A. S. et al. Hemodilution is common in patients with advanced heart failure [J]. Circulation 107(2), 226–229 (2003).
Godin, M., Bouchard, J. & Mehta, R. L. Fluid balance in patients with acute kidney injury: emerging concepts [J]. Nephron Clin. Pract. 123(3–4), 238–245 (2013).
Laakso, M. & Kuusisto, J. Insulin resistance and hyperglycaemia in cardiovascular disease development [J]. Nat. Rev. Endocrinol. 10(5), 293–302 (2014).
Alberti, K. G. et al. Harmonizing the metabolic syndrome: a joint interim statement of the International Diabetes Federation Task Force on Epidemiology and Prevention; National Heart, Lung, and Blood Institute; American Heart Association; World Heart Federation; International Atherosclerosis Society; and International Association for the Study of Obesity [J]. Circulation 120(16), 1640–1645 (2009).
Williams, K. V. et al. Can clinical factors estimate insulin resistance in type 1 diabetes? [J]. Diabetes 49(4), 626–632 (2000).
Penno, G. et al. Insulin resistance, diabetic kidney disease, and all-cause mortality in individuals with type 2 diabetes: a prospective cohort study [J]. BMC Med 19(1), 66 (2021).
Nyström T, Holzmann M J, Eliasson B, et al. Estimated glucose disposal rate predicts mortality in adults with type 1 diabetes [J]. Diabetes, obesity & metabolism, 2018, 20(3): 556–63.
Chen H Y, Kuo S, Su P F, et al. Health Care Costs Associated With Macrovascular, Microvascular, and Metabolic Complications of Type 2 Diabetes Across Time: Estimates From a Population-Based Cohort of More Than 0.8 Million Individuals With Up to 15 Years of Follow-up [J]. Diabetes care, 2020, 43(8): 1732–40.
Orchard, T. J. et al. Nephropathy in type 1 diabetes: a manifestation of insulin resistance and multiple genetic susceptibilities? Further evidence from the Pittsburgh Epidemiology of Diabetes Complication Study [J]. Kidney Int. 62(3), 963–970 (2002).
Mao, Y. & Zhong, W. Changes of insulin resistance status and development of complications in type 1 diabetes mellitus: Analysis of DCCT/EDIC study [J]. Diabetes Res Clin Pract 184, 109211 (2022).
Dong B, Chen Y, Yang X, et al. Estimated glucose disposal rate outperforms other insulin resistance surrogates in predicting incident cardiovascular diseases in cardiovascular-kidney-metabolic syndrome stages 0–3 and the development of a machine learning prediction model: a nationwide prospective cohort study [J]. Cardiovascular Diabetology, 2025, 24(1).
Tao S, Yu L, Li J, et al. Insulin resistance quantified by estimated glucose disposal rate predicts cardiovascular disease incidence: a nationwide prospective cohort study [J]. Cardiovascular Diabetology, 2025, 24(1).
Finfer, S. et al. Intensive versus conventional glucose control in critically ill patients [J]. N. Engl. J. Med. 360(13), 1283–1297 (2009).
Aramendi I, Burghi G, Manzanares W. Dysglycemia in the critically ill patient: current evidence and future perspectives [J]. Revista Brasileira de Terapia Intensiva, 2017, 29(3).
Krinsley J S, Schultz M J, Spronk P E, et al. Mild hypoglycemia is independently associated with increased mortality in the critically ill [J]. Critical Care, 2011, 15(4).
Lou, J. et al. A retrospective study utilized MIMIC-IV database to explore the potential association between triglyceride-glucose index and mortality in critically ill patients with sepsis [J]. Sci. Rep. 14(1), 24081 (2024).
Johnson, A. E. W. et al. MIMIC-IV, a freely accessible electronic health record dataset [J]. Sci Data 10(1), 1 (2023).
Nyström T, Holzmann M J, Eliasson B, et al. Estimated glucose disposal rate and long-term survival in type 2 diabetes after coronary artery bypass grafting [J]. Heart and vessels, 2017, 32(3): 269–78.
Jin, A. et al. Mediation of Systemic Inflammation on Insulin Resistance and Prognosis of Nondiabetic Patients With Ischemic Stroke [J]. Stroke 54(3), 759–769 (2023).
Da Silva A A, Do Carmo J M, Li X, et al. Role of Hyperinsulinemia and Insulin Resistance in Hypertension: Metabolic Syndrome Revisited [J]. The Canadian journal of cardiology, 2020, 36(5): 671–82.
Fu C, Li Y, Gao X, et al. Association between estimated glucose disposal rate with the all-cause and cause-specific mortality among the population with cardiometabolic syndrome [J]. Diabetology & metabolic syndrome, 2025, 17(1).
Xing, D. et al. Correlation between estimated glucose disposal rate, insulin resistance, and cardiovascular mortality among individuals with metabolic syndrome: a population-based analysis, evidence from NHANES 1999–2018 [J]. Diabetol. Metab. Syndr. 17(1), 11 (2025).
Chen, X., Li, A. & Ma, Q. Association of estimated glucose disposal rate with metabolic syndrome prevalence and mortality risks: a population-based study [J]. Cardiovasc Diabetol 24(1), 38 (2025).
Kong X, Wang W. Estimated glucose disposal rate and risk of cardiovascular disease and mortality in U.S. adults with prediabetes: a nationwide cross-sectional and prospective cohort study [J]. Acta Diabetologica, 2024, 61(11): 1413–21.
Wang H, Zhou Z, Liu X, et al. Gender differences in the association between insulin resistance assessed by estimated glucose disposal rate and the risk of all-cause and cardiovascular deaths in adults without diabetes [J]. Diabetes Research and Clinical Practice, 2025, 219.
Peng J, Li A, Yin L, et al. Estimated Glucose Disposal Rate Predicts Renal Progression in Type 2 Diabetes Mellitus: A Retrospective Cohort Study [J]. Journal of the Endocrine Society, 2023, 7(7).
Karamanakos, G. et al. The association of insulin resistance measured through the estimated glucose disposal rate with predictors of micro-and macrovascular complications in patients with type 1 diabetes [J]. Prim. Care Diabetes 16(6), 837–843 (2022).
Brealey, D. & Singer, M. Hyperglycemia in critical illness: a review [J]. J. Diabetes Sci. Technol. 3(6), 1250–1260 (2009).
Krinsley, J. S. et al. Mild hypoglycemia is independently associated with increased mortality in the critically ill [J]. Critical care (London, England) 15(4), R173 (2011).
Wei, M. et al. Low fasting plasma glucose level as a predictor of cardiovascular disease and all-cause mortality [J]. Circulation 101(17), 2047–2052 (2000).
Ichai C, Preiser J-C. International recommendations for glucose control in adult non diabetic critically ill patients [J]. Critical Care, 2010, 14(5).
Adler, G. K. et al. Antecedent hypoglycemia impairs autonomic cardiovascular function: implications for rigorous glycemic control [J]. Diabetes 58(2), 360–366 (2009).
Damman, K. et al. Both in- and out-hospital worsening of renal function predict outcome in patients with heart failure: results from the Coordinating Study Evaluating Outcome of Advising and Counseling in Heart Failure (COACH) [J]. Eur. J. Heart Fail. 11(9), 847–854 (2009).
van Hooijdonk, R. T. et al. Sweet Spot: Glucose Control in the Intensive Care Unit [J]. Seminars in respiratory and critical care medicine 37(1), 57–67 (2016).
Preiser J-C, van Zanten A R H, Berger M M, et al. Metabolic and nutritional support of critically ill patients: consensus and controversies [J]. Critical Care, 2015, 19(1).
Acknowledgements
Not applicable.
Funding
No fundings.
Author information
Authors and Affiliations
Contributions
Data collection, organization, and statistical analysis were conducted by YZ, and XS. The project was led by YZ, while YC, YY, and HS played key roles in developing the methodology, drafting the manuscript’s initial version, and reviewing it. Each author has assessed and approved the final manuscript version.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Zhou, Y., Su, X., Chen, Y. et al. Low estimated glucose disposal rate (eGDR) predicts decreased all-cause mortality in critically ill patients with cardiorenal syndrome (CRS): analysis of the MIMIC-IV database. Sci Rep 16, 1692 (2026). https://doi.org/10.1038/s41598-025-31240-6
Received:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s41598-025-31240-6