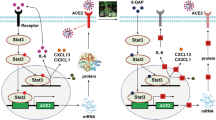
Abstract
Smoking and infection are the most common risk factors for acute exacerbation of chronic obstructive pulmonary disease (AECOPD). Irisin is a hormone-like substance that helps reduce oxidative stress and inflammation in lung diseases. This study aims to explore the potential regulatory mechanism of irisin in AECOPD induced by CSE combined with LPS. Autophagy-deficient models were established in mice and MH-S cells. Pathological changes in mouse lung tissues were assessed using HE staining, while Irisin expression was detected via immunofluorescence. Levels of IL-1β, IL-6, and TNF-α in mouse BALF and MH-S cell supernatants were measured by ELISA. Autophagic flux in MH-S cells was monitored using the Ad-mCherry-GFP-LC3B dual-fluorescence system and transmission electron microscopy. Western blotting was performed to analyze autophagy-related proteins in mice and MH-S cells, elucidating the molecular mechanism of Irisin’s protective effects. Irisin can reduce the accumulation of the autophagy marker protein P62 and promote the conversion of LC3-I to LC3-II through the AMPK-Beclin1 pathway, increase autophagosome-lysosome formation, effectively restore damaged autophagic flux, and lower the levels of pro-inflammatory factors IL-1β, IL-6, and TNF-α elevated by CSE + LPS. Irisin activates protective autophagy through the AMPK-Beclin1 pathway, restores the autophagy imbalance induced by CSE + LPS in the lungs, reduces lung inflammation levels, and provides a new therapeutic target for the treatment of AECOPD.
Similar content being viewed by others
Introduction
Chronic obstructive pulmonary disease (COPD) is a heterogeneous pulmonary condition characterized by persistent airflow limitation. Its high morbidity and mortality place a significant burden on both the physical and mental health of patients, as well as on the healthcare system1,2,3. Cigarette smoke is a major risk factor that impairs the function of the airway epithelial barrier, thereby exacerbating the invasion of viruses and bacteria, and triggering massive recruitment of immune cells and persistent inflammation in lung tissue4. These pathological processes destroy the pulmonary parenchyma, leading to emphysema formation. This cascade thereby severely compromises the airways’ innate defense and repair mechanisms and ultimately results in respiratory function impairment. The frequent exacerbation of COPD not only accelerates the disease progression of COPD but also severely impacts patients’ quality of life and significantly reduces their survival period5. Bacterial infection is generally regarded as an important factor in the exacerbation of COPD. Approximately 40–60% of AECOPD patients are hospitalized due to bacterial infection. Despite combination therapy with corticosteroids and bronchodilators, a subset of patients exhibits suboptimal response to corticosteroids or even develops corticosteroid resistance, significantly complicating clinical management. Therefore, the mechanisms underlying acute exacerbations of COPD warrant further in-depth investigation. In this study, a method of Cigarette Smoking Exposure (CSE) combined with LPS stimulation was employed to simulate AECOPD induced by bacterial infection.
Autophagy is a self-repair process that maintains cellular homeostasis by relying on the lysosomal degradation of damaged proteins, lipids, and organelles. Moderate enhancement of autophagy can mitigate cigarette smoke extract (CSE)-induced COPD/emphysema6, whereas impaired autophagy leads to the accumulation of oxidatively damaged proteins and organelles, exacerbating COPD progression. Studies have confirmed that the autophagy markers LC3II and P62 levels are higher in bronchial epithelial cells of COPD patients7. There is accumulation of autophagosomes and reduced autophagy flux due to mitochondrial dysfunction in alveolar macrophages of smokers8.
Irisin is a new hormone-like myokine that plays an important role in the activation of cellular autophagy. For example, irisin promotes autophagy and improves insulin resistance (IR) through the p38-mitogen-activated protein kinase (MAPK)-peroxisome proliferator-activated receptor-γ coactivator 1-α (PGC-1α) signaling pathway9. This finding emphasizes the potential role of irisin in regulating cellular autophagy and metabolic balance. In a PM2.5-induced acute lung injury (ALI) model, irisin has been confirmed to significantly enhance the expression of autophagy protein LC3-II and inhibit the expression of p62 protein, thereby repairing the damaged autophagy flux and reducing the inflammatory response10.
The AMPK-Beclin1 pathway represents a canonical regulatory axis of autophagy, playing a pivotal role in PM2.5-induced damage to both bronchial epithelial cells and alveolar macrophages10,11. ATG5 serves as an essential initiator protein in autophagy. ATG5 knockout inhibits autophagy and impairs the body’s self-repair capacity. The dysregulation of autophagy in COPD involves complex mechanisms with multiple contributing factors and signaling pathways. While irisin has been shown to regulate autophagy in other disease models, its potential role in modulating autophagy through the AMPK-Beclin1 signaling pathway in CSE + LPS-induced AECOPD, along with the underlying molecular mechanisms, remains to be elucidated.
This study aims to investigate whether irisin can ameliorate CSE + LPS-induced dysregulation of pulmonary autophagy and determine the involvement of the AMPK-Beclin1 signaling pathway in this process. By exploring the underlying mechanisms, we seek to evaluate irisin’s potential as a novel therapeutic target for lung diseases, thereby providing new insights for personalized treatment of AECOPD.
Materials and methods
Materials and reagents
Cell Signaling Technology (Danvers, MA, USA) provided AMPK(1:1000, 5831T), p-AMPK(1:1000, 2535T), ATG5(1:1000, 12994T); LC3(1:1000, PM036), P62(1:1000, PM045) and Beclin1(1:1000, PD017) were obtained from MBL(Beijing China); FNDC5(1:200, bs-8486R) was obtained from Bioss (Beijing China); Lipofectamine 2000 was purchased from Thermo Fisher Scientific (MA, USA); ATG5 siRNA/control siRNA was purchased from (Genepharma, Suzhou, China); Compound C were obtained from AmBeed (Arlington Hts, IL USA); The reagents required for cell culture, including RPMI 1640, Fetal Bovine Serum (FBS), penicillin–streptomycin (100×), Phosphate Buffered Saline (PBS) were purchased from Gibco or Solarbio; TNF-α Elisa Kit, IL-6 Elisa Kit, and IL-1β Elisa Kit were purchased from Multi Sciences (China); Ad-mCherry-GFP-LC3B were obtained from Beyotime (C3011, Nantong, China); Irisin was purchased from Cayman Chemical Company. LPS were obtained from Sigma–Aldrich (L2630, MO, USA). Diamond filter cigarettes (flue-cured tobacco, 11 mg coke, 1 mg nicotine, 13 mg carbon monoxide, Hebei China Tobacco Industry Company).
Preparation of CSE
Cigarette smoke was extracted according to previous research methods12. 2 cigarettes were placed on the duct and attached to a conical flask, the smoke was collected in 10 ml of serum-free RPMI1640 through a 50 ml syringe, and the smoke was repeatedly aspirated to make a suspension by completely dissolving the smoke, the pH was adjusted to 7.4 with 1 mol/L NaOH, and it was filtered for sterilization through a 0.22 pm filter, and the absorbance of the CSE was measured using an enzyme marker at 320 nm (OD 0.76 ± 0.05), which was defined as 100% CSE. CSE is easily decomposed in the presence of light and should be freshly prepared before each use.
Cell culture
Mouse alveolar macrophages (MH-S cells) used in this experiment were purchased from ATCC (USA) and preserved in RPMI 1640 medium containing 10% fetal bovine serum (FBS, Gibco, USA). Afterwards, cells were incubated at 37 °C in 5% CO2 and 95% air and grown to the desired density for subsequent experiments.
Cell viability assay
The MH-S cells in the logarithmic growth phase were seeded in a 96-well plate at a density of 1 × 104 cells per well. After the cells adhered, different concentrations of CSE and a fixed concentration of LPS (10 µg/ml) were added. The plate was incubated in the incubator for an appropriate amount of time (12–24 h). After aspirating the culture supernatant, 90 µL of RPMI 1640 complete medium was added to each well, followed by 10 µL of CCK reagent. The plate was incubated for 2 h in the incubator. The absorbance at 450 nm of each well was measured using a microplate reader. Cell viability was calculated using the formula.
ATG5 SiRNA transfection
ATG5 siRNA and a control nonspecific siRNA was used as negative control (GenePharma, China). The expression of ATG5 in MH-S cells was silenced by using Lipofectmine 2000 transfection reagent (Thermo Fisher Scientific, MA, USA). The cells were cultured with a mixture of ATG5 siRNA/control siRNA and transfection reagent in serum-free RPMI 1640 medium for 6 h. The medium was then replaced with RPMI 1640 containing 10% serum for continued culture. After 48 h, the cells were treated with LPS (10 µg/ml) in combination with either 2% CSE or Irisin (20 nM)10 for 12 h. Finally, Western Blot analysis was performed to assess the knockdown efficiency.
Transmission electron microscopy (TEM) and Ad-mCherry-GFP-LC3B transfection
MHS cells were seeded in 6-well plates. After treatment with different stimulants, the cells were washed with 1× PBS (pH 7.2) and collected by centrifugation at 1000 rpm for 5 min. The cell pellets were fixed overnight with 2.5% glutaraldehyde at 4℃. The cells were dehydrated stepwise with ethanol, embedded, sectioned and stained. Finally, the cells were imaged using a Hitachi 7500 transmission electron microscope at an acceleration voltage of 80 kV. Cells were transfected with Ad-mCherry-GFP-LC3B adenovirus (Beyotime, Beijing, China) at an MOI of 20, and then incubated in the incubator. After 24 h, the corresponding stimuli were added according to the different groups, and the cells were cultured for an additional 12 h before being observed under a fluorescence microscope.
Animal experiments
Wild-type (Wild-type WT), ATG5 knockout male C57BL/6J mice13 (7–8 weeks old, 20 ± 2 g) were gifted by the Key Laboratory of Immunity Mechanism and Major Disease Intervention of Hebei Province, and the mice were housed in a sterile environment at 24 °C and given normal water and feed. All experimental protocols were approved by the Animal Care and Use Committee of the Second Hospital of Hebei Medical University, China (2023-AE245), and were performed in accordance with the National Institutes of Health Guide for the Care and Use of Laboratory Animals, and reported in accordance with ARRIVE guidelines. According to published articles14,15,16, the experimental model was established using a combined induction approach with CSE and LPS. The protocol involved intratracheal instillation of LPS (1 mg/kg 20ul, on day 1 and day 14; 3 mg/kg 60ul, on day 28), along with exposure to cigarette smoke for 30 days. Mice were exposed to smoking in SM-120 Single-channel Intelligent Smoking Machine (Huironghe, Beijing, China) 2 times/day (10 sticks, 60 min/time) for 30 days.
Thirty male C57BL/6J mice were randomly divided into 6 groups (5 mice in each group): (1) WT-saline group (Con group); (2) WT-Irisin group (Irisin group); (3) WT-CSE + LPS group (CSE + LPS group); (4) WT-CSE + LPS + Irisin group (CSE + LPS + Irisin group); (5) ATG5-KO + CSE + LPS group; (6) ATG5-KO + CSE + LPS + Irisin group.
3, 4, 5, and 6 groups CSE + LPS treatment: mice were exposed to smoking in cigarette smoke fumigation box 2 times/d (10 cigarettes, 60 min/time) for 30 days. Tracheal intubation with intra-airway drip of LPS solution (1 mg/kg 20ul, on day 1 and day 14; 3 mg/kg 60ul, on day 28). Groups 2, 4, and 6 were injected with Irisin: Irisin (0.5 µg/g body weight)17 was administered via intraperitoneal injection 0.5 h before the airway instillation procedure. Groups 1, 2, which did not require CSE + LPS, exposed the mice to fresh air. Groups 1, 3, and 5 were injected intraperitoneally with the same volume of saline as irisin, with the number of injection times remaining the same as that for irisin injection.
On day 30, lung tissue and bronchoalveolar lavage fluid (BALF) samples were collected for follow-up experiments (Fig. 1) .
Hematoxylin and Eosin (H&E) and immunofluorescence staining
Prepare a 1% pentobarbital sodium solution (35 mg/kg), and administer it intraperitoneally for deep anesthesia in mice. Then, mice were euthanized by cervical dislocation, and the lungs were harvested. The specimens were fixed in 4% paraformaldehyde solution, dehydrated, and embedded in paraffin for sectioning. H&E staining was performed, and pathological changes in lung tissues were evaluated using light microscopy. Lung injury score was measured according to the methods reported previously18. The method for measuring the Mean Linear Intercept (MLI) involved randomly selecting five different fields of view on each H&E-stained section, drawing 10 equidistant horizontal lines and 11 vertical lines per field, and counting the number of alveolar septa intersected by these lines (NS). The total length of the intersecting lines (L) is measured, and the MLI is obtained by dividing this length by the number of alveolar septa intersected. The formula MLI = L / NS is used to calculate the mean linear intercept. The expression of irisin in lung tissues and the expression of P62 and LC3B in MH-S cells from different treatment groups were detected by immunofluorescence staining. The images were then observed using confocal microscopy.
ELISA
BALF collection: The right lung of mice was lavaged three times with 0.5 ml of sterile saline to ensure that more than 50% of the fluid was obtained. The collected fluid is centrifuged at 252 g for 10 min at 4℃ The upper layer of clear fluid was taken as BALF.
Cell supernatant collection: Centrifuge at 300 g for 10 min to remove the precipitate and remove the upper layer of clear liquid as cell supernatant.
Then, the levels of IL-1β, IL-6 and TNF-α in each group of specimens were measured by mouse-specific ELISA kit according to the instructions.
Western blot analysis
After lung tissues or cells are lysed by RIPA buffer, total proteins in frozen lung tissues and cell lysates are extracted. Then, the protein concentration is detected by NanoDrop 2000 C spectrophotometer. Next, the total proteins are loaded into 10%, 12%, or 15% SDS-PAGE gels. After electrophoretic separation of proteins, they are transferred to a 0.45 μm PVDF membrane. Subsequently, after blocking in 5% BSA for 1 h, the membrane is incubated overnight at 4 °C with ATG5 (1:1000, CST, USA), p-AMPK (1:1000, CST, USA), AMPK (1:1000, CST, USA), LC3 (1:1000, MBL China), P62 (1:1000, MBL, China), and Beclin1 (1:1000, MBL, China). After washing three times with TBST, the membrane is incubated with the secondary antibody at room temperature for 1 h. After washing, screening and visualization are performed using an ECL chemiluminescence imager. Protein levels are normalized to GADPH (1:1000, Proteintech, USA) or GADPH(1:10000,Abways, China). Finally, the expression of proteins is quantified using Image J software.
Statistical analysis
Data are expressed as means ± standard deviations (SD). Data are derived from at least three independent trials. Statistical analysis was performed using Graphpad Prism 10.3. Bartlett’s test was performed to test the homogeneity of variance. Statistical analyses for multiple groups were performed using one-way analysis of variance (ANOVA) and Tukey’s post hoc multiple comparisons test. The comparison between the two groups was conducted using t-test. P value of < 0.05 was considered statistically significant.
Results
CSE and LPS cause alveolar cavity expansion and increased secretion of inflammatory factors. Irisin can alleviate the lung inflammation induced by CSE + LPS
Histopathological analysis of lung tissues from different treatment groups revealed the following findings: In control mice, the alveolar structure remained intact with continuous septal walls, and no significant inflammatory cell infiltration was observed. In contrast, exposure to CSE + LPS induced marked pulmonary damage, including thinning and disruption of the alveolar walls, breakdown of alveolar septa, expansion of alveolar spaces, and partial alveolar rupture and fusion, which ultimately leads to bullae formation. A significant reduction in alveolar number. Increased inflammatory cell infiltration. These pathological changes collectively indicated the development of emphysema. Exogenous supplementation of irisin can alleviate pulmonary inflammatory injury. (Fig. 2A,C,D).
To evaluate irisin expression in lung tissue following CSE + LPS exposure, we performed immunofluorescence analysis on lung tissues from control and CSE + LPS-treated mice. The results showed that combined CSE and LPS stimulation significantly increased irisin levels in lung tissue compared to the control group. The results indicate that CSE + LPS stimulation upregulates irisin expression in mouse lung tissue (Fig. 2B,E).
We observed that in control and ATG5-KO mice following CSE + LPS stimulation, the levels of inflammatory cytokines IL-1β, IL-6, and TNF-α in bronchoalveolar lavage fluid (BALF) were all significantly elevated (P < 0.05). Irisin treatment reduced these CSE + LPS-induced increases in IL-1β, IL-6, and TNF-α levels in control and ATG5-KO mice BALF (P < 0.05). These results demonstrate that: CSE + LPS stimulation significantly upregulates inflammatory cytokine levels (IL-1β, IL-6, and TNF-α) in mouse BALF, indicating pulmonary inflammatory response induction. Irisin effectively reduces these elevated inflammatory levels in mice, demonstrating significant anti-inflammatory activity (Fig. 2F–H).
CSE + LPS increase P62 and promote LC3I to LC3II conversion. Irisin can reduce the increased P62 caused by CSE + LPS stimulation and further promote the conversion of LC3I to LC3II in vivo and in vitro
We performed quantitative detection of autophagy-related proteins P62 and LC3 in lung tissues using Western blotting. The results revealed that P62 expression was significantly elevated in the CSE + LPS group compared to the control group, while the conversion of LC3I to LC3II was also promoted (P < 0.05). Irisin treatment reduced the CSE + LPS-induced elevation of P62 and further enhanced the conversion of LC3I to LC3II (P < 0.05). In contrast, ATG5-KO mice stimulated with CSE + LPS and treated with irisin showed no significant changes in P62 or LC3II levels. Compared to the CSE + LPS-stimulated group, ATG5 knockout failed to induce the expected accumulation of P62 under CSE + LPS stimulation, accompanied by reduced conversion of LC3I to LC3II (P < 0.05). When compared with the CSE + LPS + Irisin-treated group, the ATG5-KO + CSE + LPS + Irisin group exhibited higher P62 levels and diminished LC3I-to-LC3II conversion (P < 0.05). These findings suggest that inhibiting autophagy can prevent CSE + LPS-induced autophagic dysregulation in mouse lung tissue, and that Irisin’s lung-protective effects depend on the autophagy pathway—when key autophagy genes are knocked out, this protective effect is lost (Fig. 3).
Effects of Different Intervention Conditions on Pulmonary Histopathology, Irisin Expression, and Inflammatory Response in Mice. (A, C, D) H&E staining of pulmonary tissue (100× magnification, scale bar = 200 μm) (n = 5). Morphometric measurements of Mean Linear Intercept (MLI) (µm) (n = 5). Lung injury scores were assessed from histological analyses of mouse lung tissues (n = 5). (B, E) Immunofuorescence of FNDC5 (irisin) in lung tissues. (400× magnification, scale bar = 50 μm) (n = 5). (F–H) The levels of IL-1β, IL-6, and TNF-α in bronchoalveolar lavage fluid (BALF) from mice (n = 5). Data are presented as mean ± SD. Signifcant differences were presented as *P < 0.05, **P < 0.01, ***P < 0.001,****P < 0.0001. ns indicates no statistical significance.
Irisin regulated the expression of autophagy related proteins in lung tissues of mice. (A) Assessment of P62 and LC3II protein expression in lung tissue by Western blotting. (B) Intensity of protein bands in different treatments were quantified using Image J software and normalized to GADPH. Data are presented as mean ± SD. Signifcant differences were presented as *P < 0.05, **P < 0.01, ***P < 0.001, ****P < 0.0001.
We fixed the LPS concentration at 10 µg/ml and co-stimulated MH-S cells with varying concentrations of CSE. Cell viability assays (Fig. 4A) demonstrated that when treated with 2% CSE + 10 µg/ml LPS for 12 h, MH-S cells showed significant viability reduction. Therefore, we selected 12-hour stimulation with 2% CSE + 10 µg/ml LPS as the final treatment condition.
Irisin regulated autophagy related protein expression in MH-S cells. (A) Cell viability of MH-S cells at different time point after exposed to CSE + LPS (10ug/ml) with different concentration.(n = 3) (B) P62 and LC3 protein expression by Western blotting (normalized to GAPDH). MH-S cells were treated with CSE2%+LPS(10ug/ml), irisin(20nM) for 12 h. (C) Intensity of protein bands in different treatments were quantified using Image J software. Data are presented as mean ± SD. Signifcant differences were presented as *P < 0.05, **P < 0.01, ***P < 0.001, ****P < 0.0001.
Consistent with in vivo findings, we observed that CSE + LPS stimulation induced P62 accumulation and increased LC3II levels in MH-S cells (P < 0.05). Irisin treatment effectively reduced P62 accumulation and further elevated LC3II levels (P < 0.05). Following ATG5 siRNA treatment, MH-S cells showed no significant changes in P62 or LC3II levels upon either CSE + LPS stimulation or subsequent Irisin administration. ATG5 siRNA-mediated autophagy inhibition reversed both the CSE + LPS-induced P62 accumulation and LC3II increase (P < 0.05). Notably, ATG5 siRNA abolished Irisin’s effects on P62 degradation and LC3II conversion (P < 0.05). These results demonstrate that autophagy inhibition eliminates Irisin’s protective effects, indicating that Irisin’s cytoprotective action against CSE + LPS-induced damage in MH-S cells is autophagy-dependent (Fig. 4).
Irisin has the effect of promoting the fusion of autophagosomes and lysosomes and repairing the autophagic flux obstruction in MH-S cells induced by CSE + LPS
We infected MH-S cells with Ad-mCherry-GFP-LC3B double-labeled fusion adenovirus to monitor autophagic flux (Fig. 5A,G). Upon autophagy activation, mCherry-GFP-LC3B accumulates on the autophagosome membrane, appearing as yellow fluorescent spots. When autophagosomes fuse with lysosomes, the GFP green fluorescence is partially quenched, resulting in red fluorescent spots. In the CSE + LPS group, the increased intensity of yellow fluorescence and fluorescent spots indicates autophagy activation with substantial autophagosome accumulation. However, the inability of autophagosomes to fuse with lysosomes suggests impaired autophagic flux. Following irisin treatment, both red fluorescence intensity and fluorescent spots increased compared to the CSE + LPS group, accompanied by GFP green fluorescence quenching. This demonstrates that irisin promotes autophagosome-lysosome fusion and restores autophagic flux, effectively repairing the CSE + LPS-induced autophagic flux obstruction in MH-S cells.
Furthermore, we conducted TEM analysis to evaluate the protective effects of Irisin on CSE + LPS-induced MH-S cells at the ultrastructural level (Fig. 5BH). TEM observations revealed increase in autophagosome quantity accompanied by a reduction in lysosome number in the CSE + LPS group. While CSE + LPS treatment activated autophagy, the impaired autophagic-lysosomal function prevented successful fusion between autophagosomes and lysosomes, consequently inhibiting the degradation of damaged organelles and resulting in autophagic flux imbalance. In contrast, the CSE + LPS + Irisin group demonstrated a marked increase in autolysosome formation and a corresponding decrease in autophagosome accumulation compared to the CSE + LPS group. These morphological findings indicate that Irisin facilitates the fusion process between autophagosomes and lysosomes, thereby restoring the impaired autophagic flux in CSE + LPS-induced MH-S cells.
We then detected the expression levels of P62 and LC3 by immunofluorescence, and found that compared with the control group, the expression of P62 (green fluorescence) was significantly increased in the CSE + LPS group, and LC3 (red fluorescence) was slightly increased. Irisin decreased the fluorescence intensity of P62 stimulated by CSE + LPS and further increased LC3. This is consistent with our previous results of WB quantitative detection of autophagy protein expression (Fig. 5C–F).
We quantified the levels of inflammatory cytokines IL-1β, IL-6, and TNF-α in the supernatants of MH-S cells from different treatment groups using ELISA. The results showed that CSE + LPS stimulation significantly increased the levels of inflammatory factors IL-1β, IL-6, and TNF-α in the cell supernatant, indicating that CSE + LPS induced an inflammatory response in MH-S cells. Irisin reduced the elevated levels of inflammatory factors IL-1β, IL-6, and TNF-α induced by CSE + LPS, suggesting its anti-inflammatory effect. Compared to control MH-S cells, ATG5 siRNA-treated MH-S cells exhibited higher inflammatory levels after CSE + LPS stimulation, indicating that CSE + LPS-induced inflammatory responses were more severe under autophagy-deficient conditions. Irisin effectively reduced CSE + LPS-induced secretion of inflammatory factors in MH-S cells, demonstrating its significant anti-inflammatory effect (Fig. 5I–K).
In summary, inhibiting autophagy can reverse the CSE + LPS-induced imbalance of pulmonary autophagy, while the protective effect of irisin is attenuated by autophagy inhibition. Therefore, we also verified in our in vitro experiments that CSE + LPS stimulation resulted in impaired autophagy, blocked autophagic flux. Irisin treatment was able to restore the impaired autophagic flux, thereby exerting its protective function.
Irisin promotes autophagosome-lysosome fusion and restores impaired autophagic flux in MH-S cells induced by CSE + LPS. (A, G) Confocal fluorescence images of MH-S cells expressing mCherry-GFP-LC3B in different treatment groups (100× magnification, scale bar = 50 μm). The number of yellow and red fluorescent spots in MH-S cells transfected with Ad-mCherry-GFP-LC3B. (n = 3) (B, H) Transmission electron microscope images of MH-S cells in different treatment groups (scale bar = 50 μm). The green arrow represents autophagosomes, and the red arrow represents autolysosomes. (n = 3) (C–F) The number of P62 and LC3 fluorescent puncta per MH-S cell in different treatment groups (400× magnification, scale bar = 50 μm) (n = 3). (I–K) Inflammatory cytokines level in MH-S cell culture supernatant. (n = 3) Data are presented as mean ± SD. Signifcant differences were presented as *P < 0.05, **P < 0.01, ***P < 0.001, ****P < 0.0001.
Irisin activates protective autophagy through the AMPK-Beclin1 pathway
Beclin1 is a crucial component of the autophagy pathway and is involved in the formation of autophagosomes, and AMPK is able to activate the Beclin1 pathway to activate autophagy. To further explore the effects of CSE + LPS stimulation on AMPK and Beclin1 expression and their roles in autophagy regulation, we performed Western Blot analysis to assess how AMPK activation influences the expression levels of Beclin1 and other autophagy-associated proteins (Fig. 6).
Irisin repairs the blocked autophagic flux in CSE + LPS-induced MH-S cells through the AMPK-Beclin1 pathway. (A) Western blotting was used to detect the expression of AMPK, p-AMPK, Beclin1, P62, and LC3II proteins. (n = 3) MH-S cells were treated with CSE2%+LPS(10ug/ml), irisin(20nM), and CC(10µM) for 12 h. (B-F) Intensity of protein bands in different treatments were quantified using Image J software and normalized to GADPH. Data are presented as mean ± SD. Signifcant differences were presented as *P < 0.05, **P < 0.01, ***P < 0.001, ****P < 0.0001.
Our results demonstrated that co-treatment with CSE + LPS and Irisin significantly enhanced AMPK activation and Beclin1 expression compared to CSE + LPS treatment alone. This activation promotes the conversion of LC3I to LC3II and the degradation of P62, indicating that the AMPK-Beclin1 signaling pathway positively regulates CSE + LPS-induced autophagy. Under CSE + LPS stimulation, Irisin further activates the AMPK-Beclin1 signaling pathway, thereby restoring the impaired autophagic flux. The AMPK inhibitor Compound C (10 µM)19 suppressed Irisin-induced activation of the AMPK-Beclin1 pathway, accompanied by reduced LC3I-to-LC3II conversion and increased P62 accumulation. These results further confirm that Irisin restores CSE + LPS-induced autophagic imbalance through the AMPK-Beclin1 signaling pathway.
Discussion
COPD is a heterogeneous condition characterized by chronic respiratory symptoms caused by abnormalities in the airways and/or alveoli, leading to persistent airflow limitation. Currently, nearly 400 million people worldwide are affected by COPD, posing a significant threat to public health. It is projected to become the third leading cause of death globally by 203020,21. Environmental factors and individual biological factors are the primary causes of COPD. Particularly, the airway inflammatory response and the lung’s heightened reaction to harmful gases or particles in tobacco smoke are significantly increased. Exposure to cigarette smoke is the most critical risk factor for developing COPD22. Long-term exposure to cigarette smoke and airborne particulate matter, along with the recruitment of various immune cells such as neutrophils and macrophages and the release of inflammatory mediators, can lead to lung parenchymal damage and emphysema23.
Irisin is a novel myokine secreted by skeletal muscle24, which maintains energy metabolism and metabolic homeostasis by promoting the browning of white adipose tissue25,26,27,28. It not only reduces oxidative stress and improves endothelial cell function29, but also resists apoptosis and inhibits the production of inflammatory factors30,31,32. Therefore, irisin may serve as a potential biomarker and therapeutic target for lung diseases. Skeletal muscle dysfunction in COPD is associated with severe airflow obstruction, emphysema, increased dyspnea scores (mMRC), reduced quality of life and exercise capacity, frequent exacerbations, and increased mortality33. Studies have shown that impaired skeletal muscle function in a COPD mouse model exposed to long-term cigarette smoke leads to increased expression of myostatin (Mstn), thereby suppressing Fndc5/Irisin expression34. Reduced irisin levels are closely linked to cigarette smoke exposure and the development of emphysema35,36,37,38. However, our study found that combined stimulation with CSE and LPS significantly increased irisin levels in lung tissue compared to the control group. Under stress conditions, irisin is secreted by skeletal muscle into the bloodstream and reaches alveolar cells through circulation, alleviating lung injury39. This may explain the differing irisin levels between the stable and acute exacerbation phases of COPD.
The deposition of cigarette smoke particles in the airways and lungs significantly promotes chronic airway inflammation and mucus hypersecretion, leading to repeated injury and repair of airway walls and lung tissue, ultimately resulting in structural remodeling and scar formation in the airways and lungs40,41. These pathological changes constitute the primary pathological basis of COPD. In this study, we confirmed pathological alterations in lung tissue during AECOPD, including thinning of alveolar walls, expansion of alveolar spaces, partial alveolar rupture with formation of emphysematous bullae, and a significant reduction in alveolar number - all of which exacerbate COPD progression.Furthermore, Irisin treatment effectively reduced the release of inflammatory factors in BALF, demonstrating its ability to attenuate pulmonary inflammatory responses during AECOPD progression.
Irisin prevents cardiac hypertrophy and heart failure induced by sustained pressure overload by activating protective autophagy and restoring impaired autophagic flux19. This aligns with our findings, as both transmission electron microscopy and Ad-mCherry-LC3B-GFP revealed lysosomal dysfunction, autophagosome accumulation, and blocked autophagic flux under CSE + LPS stimulation. Irisin further activated LC3II and removed accumulated P62, increased the number of autolysosomes, and facilitated the fusion of autophagic vesicles with lysosomes, thereby restoring impaired autophagic flux induced by CSE + LPS both in vitro and in vivo. Furthermore, the protective effects of irisin were abolished in both in vitro and in vivo autophagy deficiency models, reaffirming that the protective role of irisin in CSE + LPS-induced AECOPD is associated with autophagy.
AMPK, a key regulator of cellular energy metabolism, can be activated under stress conditions and modulates autophagy through Beclin1 phosphorylation42. CSE increases intracellular reactive oxygen species levels, thereby activating AMPK43. Irisin plays an important role in anti-inflammatory processes by activating AMPK44. In this study, we observed that CSE combined with LPS stimulation activated the AMPK-Beclin1 signaling pathway, leading to p62 accumulation and LC3I-to-LC3II conversion. Irisin further enhanced this pathway, increasing LC3II levels while promoting p62 degradation and restoring impaired autophagic flux. ATG5, as an essential autophagy initiation protein, regulates autophagosome formation. Autophagy inhibition reversed the CSE + LPS-induced blockade of autophagic flux. Notably, the protective effects of Irisin were attenuated or abolished upon autophagy suppression, indicating that Irisin exerts its benefits by activating protective autophagy, restoring defective autophagic flux, maintaining autophagic homeostasis, and mitigating inflammatory responses. These findings demonstrate that Irisin protects against CSE + LPS-induced lung injury by activating the AMPK-Beclin1 pathway to restore autophagic balance, highlighting its therapeutic potential in autophagy-related pulmonary disorders (Fig. 7).
Prospects
The goal of AE treatment is to minimize exacerbations and prevent their recurrence. Early identification and active intervention treatment can significantly reduce the readmission rate and mortality among COPD patients, thereby improving their quality of life. Research indicates that inhalation of LAMA can stimulate the secretion of irisin in COPD patients45. COPD patients are predominantly middle-aged and elderly individuals, often accompanied by muscle atrophy and declined skeletal muscle function46, which subsequently leads to insufficient irisin secretion. Therefore, enhancing patients’ exercise capacity, administering LAMA in adequate doses and durations, and supplementing with exogenous irisin can improve COPD treatment outcomes and reduce adverse events during the disease course. However, current research on irisin is primarily focused on mouse and cellular levels, with limited clinical translational studies in COPD patients. Therefore, the mechanisms of irisin in COPD and its potential as a therapeutic target warrant further investigation.
Conclusions
Irisin activates protective autophagy through the AMPK-Beclin1 pathway, restores the autophagy imbalance induced by CSE + LPS in the lungs, reduces lung inflammation levels, and provides a new therapeutic target for the treatment of AECOPD.
Data availability
All data generated or analyzed during this study are available from the corresponding author upon reasonable request.
References
Barnes, P. J. Targeting cellular senescence as a new approach to chronic obstructive pulmonary disease therapy. Curr. Opin. Pharmacol. 56, 68–73. https://doi.org/10.1016/j.coph.2021.03.003 (2021).
Barnes, P. J. Oxidative stress in chronic obstructive pulmonary disease. Antioxidants 11, 965. https://doi.org/10.3390/antiox11050965 (2022).
López-Campos, J. L., Tan, W. & Soriano, J. B. Global burden of COPD. Respirology 21, 14–23. https://doi.org/10.1111/resp.12660 (2016).
Tatsuta, M. et al. Effects of cigarette smoke on barrier function and tight junction proteins in the bronchial epithelium: protective role of Cathelicidin LL-37. Respir Res. 20, 251. https://doi.org/10.1186/s12931-019-1222-8 (2019).
Anzueto, A. Impact of exacerbations on COPD. Eur. Respir Rev. 19, 113–118. https://doi.org/10.1183/09059180.00002610 (2010).
Bodas, M. et al. Autophagy augmentation alleviates cigarette smoke-induced CFTR-dysfunction, ceramide-accumulation and COPD-emphysema pathogenesis. Free Radic Biol. Med. 131, 81–97. https://doi.org/10.1016/j.freeradbiomed.2018.11.023 (2019).
Tan, W. S. D., Shen, H. M. & Wong, W. S. F. Dysregulated autophagy in COPD: A pathogenic process to be Deciphered. Pharmacol. Res. 144, 1–7. https://doi.org/10.1016/j.phrs.2019.04.005 (2019).
Monick, M. M. et al. Identification of an autophagy defect in smokers’ alveolar macrophages. J. Immunol. 185, 5425–5435. https://doi.org/10.4049/jimmunol.1001603 (2010).
Ye, X. et al. Irisin reverses insulin resistance in C2C12 cells via the p38-MAPK-PGC-1α pathway. Peptides 119, 170120. https://doi.org/10.1016/j.peptides.2019.170120 (2019).
Ma, J. et al. Irisin ameliorates PM2.5-induced acute lung injury by regulation of autophagy through AMPK/mTOR pathway. J. Inflamm. Res. 16, 1045–1057. https://doi.org/10.2147/JIR.S401893 (2023).
Cao, J. et al. Interleukin-37 relieves PM2.5-triggered lung injury by inhibiting autophagy through the AKT/mTOR signaling pathway in vivo and in vitro. Ecotoxicol. Environ. Saf. 269, 115816. https://doi.org/10.1016/j.ecoenv.2023.115816 (2024).
Qi, C. et al. Cigarette smoke extract combined with lipopolysaccharide reduces OCTN1/2 expression in human alveolar epithelial cells in vitro and rat lung in vivo under inflammatory conditions. Int. Immunopharmacol. 87, 106812. https://doi.org/10.1016/j.intimp.2020.106812 (2020).
Wang, J. et al. Integrin α5β1, as a receptor of fibronectin, binds the FbaA protein of group A Streptococcus to initiate autophagy during infection. mBio 11, e00771–e00720. https://doi.org/10.1128/mBio.00771-20 (2020).
Guo, B. et al. Targeting Immunoproteasome in polarized macrophages ameliorates experimental emphysema via activating NRF1/2-P62 axis and suppressing IRF4 transcription. Adv. Sci. 11, e2405318. https://doi.org/10.1002/advs.202405318 (2024).
Kobayashi, S. et al. A single dose of lipopolysaccharide into mice with emphysema mimics human chronic obstructive pulmonary disease exacerbation as assessed by micro-computed tomography. Am. J. Respir Cell. Mol. Biol. 49, 971–977. https://doi.org/10.1165/rcmb.2013-0074OC (2013).
Cheng, Q. et al. Memantine ameliorates pulmonary inflammation in a mice model of COPD induced by cigarette smoke combined with LPS. Biomed. Pharmacother. 109, 2005–2013. https://doi.org/10.1016/j.biopha.2018.11.086 (2019).
Han, Z. et al. Irisin attenuates acute lung injury by suppressing the pyroptosis of alveolar macrophages. Int. J. Mol. Med. 51, 32. https://doi.org/10.3892/ijmm.2023.5235 (2023).
Jiang, Z., He, R., Zhong, Y., Liu, B. & He, Z. Fumarate hydratase restrains MtDNA attenuates LPS-induced acute lung injury through cGAS-STING pathways. J. Inflamm. Res. 18, 5399–5413. https://doi.org/10.2147/JIR.S518589 (2025).
Li, R. L. et al. Irisin alleviates pressure overload-induced cardiac hypertrophy by inducing protective autophagy via mTOR-independent activation of the AMPK-ULK1 pathway. J. Mol. Cell. Cardiol. 121, 242–255. https://doi.org/10.1016/j.yjmcc.2018.07.249 (2018).
Venkatesan, P. & GOLD COPD report: 2024 update. Lancet Respir Med. 12, 15–16. https://doi.org/10.1016/S2213-2600(23)00418-9 (2024).
Confalonieri, M., Braga, L., Salton, F., Ruaro, B. & Confalonieri, P. Chronic obstructive pulmonary disease definition: Is it time to incorporate the concept of failure of lung regeneration? Am. J. Respir Crit. Care Med. 207, 366–367. https://doi.org/10.1164/rccm.202211-2063LE (2023).
Chung, K. F. & Adcock, I. M. Multifaceted mechanisms in COPD: Inflammation, immunity, and tissue repair and destruction. Eur. Respir J. 31, 1334–1356. https://doi.org/10.1183/09031936.00018908 (2008).
Hogg, J. C. Pathophysiology of airflow limitation in chronic obstructive pulmonary disease. Lancet 364, 709–721. https://doi.org/10.1016/S0140-6736(04)16900-6 (2004).
Boström, P. et al. A PGC1-α-dependent myokine that drives brown-fat-like development of white fat and thermogenesis. Nature 481, 463–468. https://doi.org/10.1038/nature10777 (2012).
Wang, S. & Pan, J. Irisin ameliorates depressive-like behaviors in rats by regulating energy metabolism. Biochem. Biophys. Res. Commun. 474, 22–28. https://doi.org/10.1016/j.bbrc.2016.04.047 (2016).
Xiong, Y. et al. Fndc5 loss-of-function attenuates exercise-induced Browning of white adipose tissue in mice. FASEB J. 33, 5876–5886. https://doi.org/10.1096/fj.201801754RR (2019).
Chen, Y. et al. Irisin induces white adipose tissue Browning in mice as assessed by magnetic resonance imaging. Exp. Biol. Med. 246, 1597–1606. https://doi.org/10.1177/15353702211006038 (2021).
Aladag, T., Mogulkoc, R. & Baltaci, A. K. Irisin and energy metabolism and the role of Irisin on metabolic syndrome. Mini Rev. Med. Chem. 23, 1942–1958. https://doi.org/10.2174/1389557523666230502153209 (2023).
Bi, J. et al. Exercise hormone Irisin mitigates endothelial barrier dysfunction and microvascular leakage-related diseases. JCI Insight. 5, e136277. https://doi.org/10.1172/jci.insight.136277 (2020).
Shao, L. et al. Irisin-mediated protective effect on LPS-induced acute lung injury via suppressing inflammation and apoptosis of alveolar epithelial cells. Biochem. Biophys. Res. Commun. 487, 194–200. https://doi.org/10.1016/j.bbrc.2017.04.020 (2017).
Ma, L. Y. et al. Irisin attenuates lipopolysaccharide-induced acute lung injury by downregulating inflammatory cytokine expression through miR-199a-mediated Rad23b overexpression. Exp. Cell. Res. 404, 112593. https://doi.org/10.1016/j.yexcr.2021.112593 (2021).
Jiao, R. et al. Irisin attenuates fine particulate matter induced acute lung injury by regulating Nod2/NF-κB signaling pathway. Immunobiology 228, 152358. https://doi.org/10.1016/j.imbio.2023.152358 (2023).
Bak, S. H. et al. Computed tomography-derived area and density of pectoralis muscle associated disease severity and longitudinal changes in chronic obstructive pulmonary disease: a case control study. Respir Res. 20, 226. https://doi.org/10.1186/s12931-019-1196-6 (2019).
Zhang, L. et al. Dysregulated myokines and signaling pathways in skeletal muscle dysfunction in a cigarette smoke-induced model of chronic obstructive pulmonary disease. Front. Physiol. 13, 929926. https://doi.org/10.3389/fphys.2022.929926 (2022).
Ijiri, N. et al. Irisin, a newly discovered myokine, is a novel biomarker associated with physical activity in patients with chronic obstructive pulmonary disease. Respirology 20, 612–617. https://doi.org/10.1111/resp.12513 (2015).
Sugiyama, Y. et al. Decreased levels of irisin, a skeletal muscle cell-derived myokine, are related to emphysema associated with chronic obstructive pulmonary disease. Int. J. Chron. Obstruct Pulmon Dis. 12, 765–772. https://doi.org/10.2147/COPD.S126604 (2017).
Kureya, Y. et al. Down-regulation of soluble α-Klotho is associated with reduction in serum Irisin levels in chronic obstructive pulmonary disease. Lung 194, 345–351. https://doi.org/10.1007/s00408-016-9864-5 (2016).
Cuttitta, G. et al. Relationship among body composition, adipocytokines, and Irisin on exercise capacity and quality of life in COPD: a pilot study. Biomolecules 13, 48. https://doi.org/10.3390/biom13010048 (2022).
Chen, K. et al. Irisin protects mitochondria function during pulmonary ischemia/reperfusion injury. Sci. Transl Med. 9, eaao6298. https://doi.org/10.1126/scitranslmed.aao6298 (2017).
Celli, B. et al. Definition and nomenclature of chronic obstructive pulmonary disease: time for its revision. Am. J. Respir Crit. Care Med. 206, 1317–1325. https://doi.org/10.1164/rccm.202204-0671PP (2022).
Cerveri, I. et al. Assessment of emphysema in COPD: a functional and radiologic study. Chest 125, 1714–1718. https://doi.org/10.1378/chest.125.5.1714 (2004).
Herzig, S. & Shaw, R. J. AMPK: guardian of metabolism and mitochondrial homeostasis. Nat. Rev. Mol. Cell. Biol. 19, 121–135. https://doi.org/10.1038/nrm.2017.95 (2018).
Ko, H. K. et al. Regulation of cigarette smoke induction of IL-8 in macrophages by AMP-activated protein kinase signaling. J. Cell. Physiol. 230, 1781–1793. https://doi.org/10.1002/jcp.24903 (2015).
Li, X. et al. Irisin alleviates pulmonary epithelial barrier dysfunction in sepsis-induced acute lung injury via activation of AMPK/SIRT1 pathways. Biomed. Pharmacother. 118, 109363. https://doi.org/10.1016/j.biopha.2019.109363 (2019).
Mandal, J. et al. Treatment with long acting muscarinic antagonists stimulates serum levels of Irisin in patients with COPD. Pulm Pharmacol. Ther. 48, 111–116. https://doi.org/10.1016/j.pupt.2017.11.001 (2018).
Wang, X., Hu, W. & Zhang, J. Advances in pathophysiology and assessment methods of chronic obstructive pulmonary disease with frailty. Chin. Med. J. Pulm Crit. Care Med. 3, 22–28. https://doi.org/10.1016/j.pccm.2024.03.002 (2025).
Acknowledgements
We thank Key Laboratory of Immune Mechanisms and Major Disease Intervention, Hebei Province, China for the generous gift of the Wild-type and ATG5 knockout male C57BL/6J mice in this study. This study was supported by Hebei Natural Science Foundation Joint Research Program for Basic Science Cooperation in the Beijing-Tianjin-Hebei Region (Grant No.H2023206909/J230030/S23ZX16013) and Key Project of Precision Medicine Joint Fund of Hebei Province (C2021206011).
Funding
This study was supported by Hebei Natural Science Foundation Joint Research Program for Basic Science Cooperation in the Beijing-Tianjin-Hebei Region (Grant No.H2023206909/J230030/S23ZX16013) and.Key Project of Precision Medicine Joint Fund of Hebei Province (C2021206011).
Author information
Authors and Affiliations
Contributions
Z.C. collected and analyzed data, performed experiments and wrote the manuscript; S.W. , Z.B. ,T.S. performed experiments analyzed and data; C.M., Y.C. and P.J.provided resources and edited the manuscript. A.M. reviewed and edited the article, supervised the methods, obtained funds and conceptualized.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Ethical approval
This study was approved by the Research Ethics Committee of the Second Hospital of Hebei Medical University (permit number 2023-AE245).
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Chen, Z., Wu, S., Bai, Z. et al. Irisin activates the AMPK-Beclin1 signaling pathway to regulate pulmonary autophagy induced by CSE + LPS. Sci Rep 15, 44320 (2025). https://doi.org/10.1038/s41598-025-31824-2
Received:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s41598-025-31824-2