Abstract
This study aimed to evaluate the potential use of resveratrol on bone healing after tooth extraction in zoledronic acid-treated rats. Seventy-two Wistar rats were separated into 4 groups of 18: no drugs (control group), zoledronic acid (ZA), resveratrol (RES), zoledronic acid + resveratrol (ZA + RES). The first and second molars of each rat were extracted and nine rats from each group were sacrificed on days 14 and 28. The extraction sites and blood samples were analyzed histologically, immunohistochemically and biochemically. The epithelization, connective tissue, new bone formation in RES group was significantly higher compared to ZA and ZA + RES groups on day 28. The connective tissue in control group was significantly higher compared to ZA and ZA + RES groups on days 14 and 28. BMP-4 levels in RES group were significantly higher compared to the other groups on days 14 and 28. Serum CTX levels in ZA + RES group were significantly higher compared to control and RES groups on day 14. Serum TRACP-5b levels in ZA + RES group, were significantly higher compared to control and RES groups on day 14. TRACP-5b level of the control was significantly lower than ZA and ZA + RES groups. Although resveratrol improved new bone formation in normal rats, no significant positive effects were detected in zoledronic acid-injected rats.
Similar content being viewed by others
Introduction
Bisphosphonates define a class of drugs widely used in the treatment of benign bone diseases such as osteoporosis, osteogenesis imperfecta and Paget’s disease, and malignancies such as multiple myeloma, metastasis to the bone. They inhibit osteoclast differentiation and function, increase osteoclast apoptosis, and finally lead to decreased bone remodeling and resorption1,2,3. Their antineovascularization effect and ability to slow the growth rate of tumor cells are the other actions of this group of drugs3.
Medication- related osteonecrosis of the jaws (MRONJ) is a severe adverse effect associated with bisphosphonate use and is influenced by local factors such as tooth extraction, infection, and trauma4,5. The pathophysiological mechanisms of MRONJ have not been fully elucidated. However, there have been many hypotheses concerning the pathogenic mechanism of MRONJ, including suppression of bone remodeling, inhibition of angiogenesis, infection and inflammation, toxicity to soft tissues and oxidative stress-related theories6,7,8,9,10,11,12,13.
Although a definitive treatment strategy has not been identified for MRONJ, conservative approaches (e.g., antibiotic therapy, the local use of oral rinses), surgical interventions, and adjuvant non-surgical approaches (e.g., laser application, hyperbaric oxygen therapy, ozone therapy, application of regenerative materials such as bone morphogenetic protein (BMP) and autologous platelet concentrates, and molecular and cellular approaches such as teriparatide, mesenchymal stem cell therapy or a combination of these are some of the treatment options used6.
Recent studies have highlighted the potential of drug delivery systems as promising therapeutic strategies for the prevention and management of MRONJ. Several investigations have focused on designing and optimizing carrier-based delivery platforms capable of providing sustained or localized drug release to enhance bone regeneration and minimize systemic toxicity14,15,16,17,18. Among these, hydrogel-based composites combined with nanoparticles have gained particular attention for their dual antibacterial and osteogenic effects14. Controlled-release systems, including those incorporating BMPs, vascular endothelial growth factor (VEGF), or platelet-rich fibrin within hydrogel or chitosan matrices, have shown enhanced angiogenesis, bone remodeling, and reversal of bisphosphonate-induced bone suppression in experimental MRONJ models15,16,17,18. Collectively, these findings indicate that sustained or localized drug delivery approaches represent a promising and emerging direction for improving bone healing and reducing adverse systemic effects in MRONJ management.
In MRONJ treatment trials, antioxidant therapy with pentoxifylline and tocopherol has been investigated. Previous studies have also reported oxidative damage due to bisphosphonates in various cancer tissues and neurons and oral epithelium12,19. In a study conducted by Bagan et al., it was reported that plasma and saliva oxidative stress levels were higher in patients with bisphosphonates-related osteonecrosis of the jaws (BRONJ) than in controls12. Taniguchi et al. stated that bisphosphonates cause fibroblasts obtained from the oral cavity to generate reactive oxygen species (ROS) and that the subsequent ROS-mediated inhibition of fibroblast growth and migration definitely delays wound healing, thereby contributing to BRONJ pathogenesis13. Khandelwal et al. previously reported that treatment with zoledronic acid increased ROS in the human breast cancer cell line MCF-7, and this increase in oxidative stress was reversed by antioxidants20. Some authors have also been demonstrated that oxidative stress is associated with osteonecrosis21,22,23. It has been reported that bisphosphonates and oxidative stress may induce osteonecrosis following invasive dentoalveolar surgery and oxidative stress has been identified as an additional risk factor for the development of BRONJ24. In this context, the use of products with anti-inflammatory and antioxidant effects may be an interesting approach to prevent MRONJ.
The increasing prevalence and high morbidity of MRONJ call attention to the need for new and effective approaches with minimal adverse effects to treating and preventing these lesions25,26. The co-administration of natural products with zoledronic acid to prevent MRONJ recently been reported in a few studies. Therefore, antioxidant agents may provide a promising preventive and therapeutic option for MRONJ. As a natural polyphenolic compound, resveratrol (3,4,5-trihydroxystilbene) is found in mulberries, grapes, peanuts, and red wine and has several biological properties, such as antioxidant, anti-inflammatory, antiapoptotic, antiresorptive, phytoestrogenic, antiaging, and osteoprotective27. Many studies have reported that resveratrol can enhance bone repair, upregulation of bone remodeling, and downregulation of osteoclastogenic markers.
Other studies show that resveratrol can inhibit apoptosis, directly affecting osteonecrosis control28. In an in vitro study, resveratrol has regulated the proliferation and differentiation of zoledronic acid-treated osteoblasts and provided a protective effect on these cells. To date, only a few studies have investigated the potential protective effects of resveratrol in MRONJ models, but none have evaluated its post-extraction use in zoledronic acid–treated rats26,29,30. In this context, it is hypothesized that administration of resveratrol could interfere with MRONJ development in rats treated zoledronic acid and may represent an effective preventive therapy. Thus, this study aimed to histopathologically, immunohistochemically, and biochemically evaluate the potential effects of resveratrol administration in preventing MRONJ immediately after teeth extraction in rats treated with zoledronic acid.
Materials and methods
Ethics and animals
The study protocol was approved by the ethics committee of experimental animals at Mersin University (Approval No. 2018/03/05). All methods were performed in accordance with the relevant guidelines and regulations. This study adhered to the ARRIVE guidelines, the Animals (Scientific Procedures) Act 1986 and associated guidance documents, the European Union Directive 2010/63/EU on the protection of animals used for scientific purposes, and the Guide for the Care and Use of Laboratory Animals published by the U.S. National Institutes of Health (NIH). Seventy-two female Wistar Albino rats, weighing 200–250 g (3 to 5 months old) were obtained from the Experimental Animal Research Unit of Mersin University, and experimental procedures were performed in the same center. The selected animals were kept in plastic cages in a room set at 21 °C with a 12-hour cycle of daylight; the animals were fed ad libitum and provided water with a standard laboratory pellet diet.
The sample size of the study was calculated based on the mean values of the bone healing parameters reported in a previous study31. Based on a Type I error rate (α) of 0.01, a statistical power of 80%, and an effect size of 0.78, the required sample size was determined to be 8 rats per subgroup using G*Power software (version 3.1.10). Considering a possible 10% loss of animals during the experiment, 9 rats were included in each analysis period.
Study design
The 72 female Wistar Albino rats were randomly divided into four groups of 18, as follows:
-
(1)
Control group (Group 1): The animals received a placebo solution (saline solution) intraperitoneally after tooth extraction until the day of sacrifice (n = 18).
-
(2)
Resveratrol group (RES or Group 2): The upper first and second molars of the rats were extracted. The animals received a resveratrol solution after tooth extraction until the day of sacrifice (n = 18).
-
(3)
Zoledronic acid group (ZA or Group 3): The animals received zoledronic acid (0.06 mg/kg) once a week for 2 weeks prior to tooth extraction. After 3rd week of zoledronic acid treatment, the upper first and second molars of the rats were extracted (n = 18).
-
(4)
Zoledronic acid + resveratrol group (ZA + RES or Group 4): The animals received zoledronic acid (0.06 mg/kg) once a week for 2 weeks prior to tooth extraction. After 3rd week of zoledronic acid treatment, the upper first and second molars of the rats were extracted. The animals continued to receive resveratrol for an additional 14 and 28 days before being sacrificed (n = 18).
Nine rats from each group were sacrificed on day 14 and on day 28 after the teeth extraction in agreement with the AMVA guidelines of Euthanasia 2020. On the 14th and 28th day of teeth extraction, the rats were again deeply anesthetized by an intramuscular injection of ketamine (50 mg/kg) (Ketamidor® 100 mg/mL, Richter Pharma AG, Austria) and xylazine (5 mg/kg) (Rompun® 2%, Bayer Türkiye Pharmaceuticals, İstanbul, Türkiye) and blood samples (5 ml) from each animal were collected by cardiac puncture into polypropylene tubes under anesthesia. Plasma was separated by centrifugation for 10 min at 3000 rpm and 4 °C. Then, the animals were euthanized with a dose of 200 mg/kg of body weight of sodium pentobarbital (Pentothal; Abbott, Chicago, IL). After sacrifice, tissue samples from around the maxillary molars and jaws were excised and used for the histological and immunohistochemical analyses. In this study, the specific method of euthanasia applied to the rats was the injection of euthanasia agent (barbiturate), which is a chemical method. The euthanasia agent used was sodium pentobarbital and was administered intraperitoneally at a dose of 200 mg/kg (Pentothal; Abbott, Chicago, IL).
Dosage and route of administration of zoledronic acid and induction of MRONJ
Zoledronic acid (Zometa®, Novartis Pharma©, Basel, Switzerland) was administered intraperitoneally to the animals at a dose of 0.06 mg/kg once a week for 2 weeks. At the end of the third week, the upper first and second molars of all rats in the control and experimental groups were extracted.
The osteonecrosis of jaw induction protocol was developed previously by Vidal-Gutiérrez et al.32. We used the rat model of osteonecrosis described by Vidal-Gutiérrez et al.32. They reported generating osteonecrosis lesions in all cases, both macroscopically, histologically and radiologically, in their study.
Dosage, preparation and route of administration of resveratrol
Resveratrol (R5010, 500 MG; Sigma-Aldrich, St Louis, MO, USA), which has a molecular weight of 228.2 g/mol, was dissolved in ethanol (50 mg/mL) to make a stock solution. This stock solution of resveratrol was then diluted in serum physiologic to obtain working concentrations (10 mg/kg). The resveratrol treatment was administered by intraperitoneal injection to Groups 2 and 4 once daily for 14 and 28 days following the teeth extraction at a dose of 10 mg/kg/d.
Teeth extraction procedure
The rats were anesthetized using an intramuscular injection of 5 mg/kg xylazine hydrochloride and 50 mg/kg ketamine hydrochloride. Following the induction of general anesthesia, local infiltration anesthesia was performed with 4% articaine hydrochloride (Ultracain D-S Forte-Aventis, Istanbul, Turkey) containing 0.006 mg/mL epinephrine in the operation area for local hemostasis. The upper first and second molars of each animal were extracted using curved hemostatic forceps. No antibiotics were used after the extraction. For the first 3 days after the extraction, the rats were fed ground rat chow, and a standard laboratory pellet diet was restarted after postoperative day 3.
Macroscopic analysis
After euthanasia, wound healing was assessed macroscopically at 2 and 4 weeks after teeth extraction. Mucosal healing was classified into three groups as follows: score 1-closed wound (complete healing with normal mucosa covering); score 2-open wound without bone exposure; score 3-open wound with bone exposure.
Blood samples and biochemical analysis
Prior to euthanasia, the animals were anesthetized using an intramuscular injection of xylazine hydrochloride and ketamine hydrochloride and blood samples were then collected by cardiac puncture into polypropylene tubes. Collected blood samples were centrifuged at 3000 rpm for 10 min. A total of 2.4 ml of serum was aliquoted into two 1.5 ml Eppendorf tubes (1.2 ml per tube) for each animal, and stored at − 80 °C until biochemical analyses. After one month, the frozen serum aliquots were thawed at room temperature (about 24 °C) for approximately 1.5 h until completely thawed. All samples were analyzed after a single thaw; no refreezing was performed. All analyses were conducted under identical conditions and within a single run. The intra- and inter-assay coefficients of variation for these measurements (C-terminal telopeptide of type 1 collagen (CTX-1) and Tartrate-resistant acid phosphatase 5b (TRACP-5b)) were 8% and 9%, respectively.
The concentrations of serum CTX-1 (Catalogue no: E1146Ra, Rat C- telopeptide of type 1 collagen, CTX-1, ELISA Kit, Relassay, Mega Tıp, Gaziantep, Türkiye) and TRACP-5b (Catalogue no: E0384Ra, Rat tartrate-resistant acid phosphatase 5b, TRACP-5b ELISA Kit, Relassay, Mega Tıp, Gaziantep, Türkiye) were determined using commercially available kits according to the manufacturer’s instructions. The absorbance at 450 nm was determined using a microplate reader. All marker measurements were performed blinded and in duplicate.
Histological analysis and semi-quantitative histological scoring
A certified and two blinded histologists performed the histological and immunohistochemical analyses. After sacrificing the animals, samples were taken from the defect area to be examined by cutting with the help of burs. Bone tissues were harvested, weighed and decalcified in EDTA buffer (10% EDTA, pH 7.4) for 14 days. After that they were fixed in 10% neutral buffered formalin for 24 h, embedded in paraffin and sectioned at 5 μm thickness with a rotary microtome. Parasagittal sections, obtained near the center of the defect, were deparaffinized and rehydrated with decreasing concentrations of ethanol. For histopathological evaluations, hematoxylin and eosin, Masson’s trichrome and toluidine blue staining were performed. After staining completed, the slides were dehydrated with increasing concentrations of ethanol, cleared in xylene and mounted with entellan. All slides were imaged under light microscope (Olympus®BX50 OlympusGmbH, Germany) and photographed by integrated digital camera (Olympus LC30 Olympus Soft Imaging Solutions GmbH, Germany). Inflammatory cell infiltration, epithelization, connective tissue formation, new bone tissue formation and osteonecrosis were semi-quantitatively scored and examined by two blind observers. Scoring was performed using a 0 to 3 scoring system (0, no; 1, minimal; 2, moderate; 3, abundant) as previously described31. Histologic scoring criteria are presented in Table 1. We defined osteonecrosis as a loss of more than five contiguous osteocytes with confluent areas of empty lacunae and the analysis was performed in 4 zones of interest. The presence and absence of osteonecrosis were scored 1 and 0, respectively.
Immunohistochemical analysis
Immunohistochemistry was performed on tissue sections to evaluate the localization and expression of BMP-4, following the manufacturer’s protocol. Briefly, serial paraffin-embedded sections were deparaffinized in xylene and rehydrated through a graded ethanol series. Endogenous peroxidase activity was quenched by incubating the sections in 3% hydrogen peroxide solution (1:9 v/v in phosphate buffered saline, PBS) for 10 min at room temperature. The sections were then washed in phosphate-buffered saline (PBS; 137 mM NaCl, 2.7 mM KCl, 10 mM Na2HPO4, 1.8 mM KH2PO4, pH 7.4) containing 0.05% Tween-20 (PBS-T) for 10 min. Antigen retrieval was performed by microwaving the sections in 10 mM citrate buffer (pH 6.0) for 7 min at 100 °C (700 W), followed by cooling at room temperature for 20 min. After several washes with PBS-T, nonspecific binding was blocked for 10 min with PBS containing 5% normal goat serum. Sections were then incubated overnight at 4 °C with rabbit anti-mouse BMP-4 primary antibody (1:200 dilution, Catalog No. ab124715, Abcam, Cambridge, UK). The following day, sections were rinsed in PBS-T and incubated with a biotinylated secondary antibody for 20 min, followed by streptavidin–peroxidase labeling. Color development was achieved using 0.05% diaminobenzidine (DAB) solution, and the slides were counterstained with hematoxylin, rinsed in distilled water, and mounted with glycerol vinyl alcohol mounting medium (Catalog No. 00-8000, Invitrogen, USA). Positive immunolabeling was visualized as a brown staining under light microscopy. To verify the specificity of the staining, negative control sections were processed in parallel by omitting the primary antibody to assess non-specific background staining. Although a separate positive control tissue was not included in this study, the BMP-4 antibody used (rabbit anti-mouse BMP-4, Abcam, ab124715) has been previously validated by the manufacturer and in earlier studies conducted in our laboratory, showing a specific and consistent staining pattern in BMP-4–expressing tissues. For quantitative analysis, immunostained osteoblasts in alveolar socket areas were examined in five randomly selected fields per section at 400× magnification, for both experimental and control groups. The number of positive osteoblasts was determined using ITEM 5.0© software (Soft Imaging Systems®, Germany). Immunohistochemical scoring was performed using the H-score method33, which provides a semiquantitative evaluation of both the intensity and percentage of positive cells.
Statistical analysis
Statistical analyses were performed with IBM SPSS Statistics version 22.0 (IBM SPSS, Türkiye). Kolmogorov-Smirnov and Shapiro-Wilk tests were used to assess the normality of the data, and the parameters were found not to follow a normal distribution. The Kruskal–Wallis test was applied for the intergroup comparisons, and the Dunn’s post hoc test was used to identify the group that caused the difference. Significance values were adjusted using the Bonferroni correction for multiple comparisons. The Mann–Whitney U test was employed to compare the parameters between days. Fisher Freeman Halton Exact Test and Fisher’s Exact Test were used to compare qualitative data. p values < 0.05 were considered statistically significant.
Results
Macroscopic findings
The experiment was well tolerated by all rats. The clinical appearance of extraction socket healing in all experimental groups on days 14 and 28 post-extraction is shown in Fig. 1.
Macroscopic appearance of extraction sockets in all experimental groups at 14 and 28 days post-extraction. C: Control group; BF: Bisphosphonate group; RES: Resveratrol group; BF + RES: Bisphosphonate + Resveratrol group; 14D: Day 14; 28D: Day 28. At day 14, the control and RES groups showed nearly complete mucosal closure with minimal inflammatory signs, whereas in the BF and BF + RES groups, open extraction sockets and exposed bone surfaces were observed. At day 28, complete mucosal healing was evident in the control and RES groups. However, no apparent clinical healing was observed in the BF and BF + RES groups, which continued to exhibit unhealed extraction sockets and exposed necrotic bone areas.
At day 14, the control and RES groups showed nearly complete mucosal closure with minimal inflammatory signs, whereas in the ZA and ZA + RES groups, open extraction sockets and exposed bone surfaces were observed. There were statistically significant differences among the groups in macroscopic findings on day 14 (p = 0.001; p < 0.05). The proportion of specimens receiving a macroscopic observation score of 3 was significantly higher in the ZA (88.9%) and ZA + RES (88.9%) groups than in the RES (0%) and control (0%) groups (Table 2).
At day 28, complete mucosal healing was evident in the control and RES groups. However, no apparent clinical healing was observed in the ZA and ZA + RES groups, which continued to exhibit unhealed extraction sockets and exposed necrotic bone areas. The macroscopic findings at day 28 also differed significantly among the groups (p = 0.006; p < 0.05). The percentage of specimens with a macroscopic score of 3 was significantly greater in the ZA (55.6%) and ZA + RES (66.7%) groups compared to the RES (0%) and control (0%) groups. (Table 2).
Histological findings
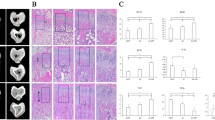
Histopathologic features of extraction socket healing in all experimental groups at 14 and 28 days post-extraction are shown in Fig. 2. Semi-quantitative comparisons of inflammation, epithelization, connective tissue formation, new bone tissue formation, and osteonecrosis among all groups are shown in Tables 3 and 4.
Representative histological images showing extraction socket healing in all experimental groups at 14 and 28 days post-extraction. (X) Hematoxylin and eosin (H&E) staining; (Y) Masson’s trichrome staining; (Z) Toluidine blue staining. Control groups (A, B); bisphosphonate-treated groups (C, D); resveratrol-treated groups (E, F); and bisphosphonate + resveratrol-treated groups (G, H) at 14 and 28 days, respectively. On day 14, control and resveratrol groups exhibited granulation tissue formation and early new bone trabeculae (NB), while bisphosphonate and bisphosphonate + resveratrol groups displayed irregular bone margins and necrotic areas characterized by empty lacunae (arrowheads). On day 28, mature bone trabeculae and well-organized connective tissue were evident in the control and resveratrol groups, whereas necrotic areas and disorganized tissue structure were observed in bisphosphonate-treated groups. Asterisks (*) indicate granulation tissue; NB: newly formed bone. Scale bar = 100 μm. 14d: Day 14; 28d: Day 28.
At day 14, all groups exhibited early-stage granulation tissue and immature small bone trabeculae within the extraction sockets. No statistically significant differences were observed among the groups in epithelialization and new bone formation on day 14 (p > 0.05). In contrast, on day 28, both parameters showed significant intergroup variation (epithelialization: p:0.002; new bone formation: p:0.007). The RES group demonstrated more epithelization and greater new bone formation than the ZA and ZA + RES groups on day 28 (p:0.021, p:0.03; p:0.029, p:0.011, p < 0.05, respectively). In the control group, socket healing was comparable to that in the RES group and markedly superior to the bisphosphonate-treated groups.
Connective tissue organization also differed significantly among the groups on both days 14 and 28 (p < 0.05). Well-developed fibrovascular connective tissue was observed in the control and RES groups, whereas disorganized connective tissue and necrotic areas were predominant in the ZA and ZA + RES groups. On days 14 and 28, connective tissue was higher in RES and control group than in the ZA and ZA + RES groups. Intragroup analyses revealed no significant time-dependent differences in epithelialization, new bone formation and connective tissue maturation (p > 0.05).
Inflammatory cell infiltration was mild to moderate in all groups and did not differ significantly between or within groups (p > 0.05).
A statistically significant difference was observed among the groups regarding the incidence of osteonecrosis on days 14 (p = 0.026) and 28 (p = 0.013). On both days, the control group showed a significantly lower incidence compared to the ZA and ZA + RES groups (p < 0.05). At day 28, the incidence in the RES group was also significantly lower than in the ZA + RES group (p < 0.05). No other intergroup or intragroup differences were statistically significant (p > 0.05) (Table 4).
Immunohistochemical findings
Immunohistochemical staining revealed cytoplasmic BMP-4 expression predominantly in osteoblasts and newly formed bone areas in all groups (Fig. 3). Mild-moderate background staining was observed in the bone matrix, which is considered typical for mineralized tissues and did not interfere with the identification of specific BMP-4 labeling.
Representative images of immunohistochemically BMP-4-stained tissue sections. Control groups (A, B); bisphosphonate-treated groups (C, D); resveratrol-treated groups (E, F) and bisphosphonate–resveratrol-treated groups (G, H) on 14 and 28 days, respectively. Scale bar: 100 μm; arrows indicate osteocytes. 14d: Day 14, 28d: Day 28.
On days 14 and 28 after extraction, BMP-4 immunoreactivity was more pronounced in the resveratrol-treated group compared with the normal and zoledronic acid–treated groups (p < 0.05). The positive osteoblasts were mainly localized along the alveolar bone surface and within the healing socket area. In contrast, the control and zoledronic acid–treated groups exhibited weak or moderate BMP-4 staining at both time points.
Quantitative evaluation using the H-score confirmed significantly higher BMP-4 expression in the resveratrol group compared with the other groups on days 14 and 28 (p < 0.05). No statistically significant differences were observed among the others groups or within intragroup comparisons over time (Table 3).
Biochemical findings
The results of biochemical analysis on day 14 after teeth extraction showed that serum CTX and TRACP-5b levels were significantly higher in the ZA + RES group compared to the Control and RES groups (p < 0.05) and CTX levels were higher in the ZA group compared to the RES group (p < 0.05). On day 28, serum CTX and TRACP-5b levels in control group significantly lower than the ZA and ZA + RES groups (p < 0.05) (Table 3).
Discussion
The increasing use of bisphosphonates in the treatment of various malignant and benign bone diseases has led to a growing incidence of MRONJ. Although the exact pathophysiology of MRONJ remains unclear, several mechanisms have been proposed, including inhibition of bone remodeling and angiogenesis, infection and inflammation, and oxidative stress6,7,8,9,10,11,12,13. Despite multiple proposed treatment protocols, an effective therapeutic approach for MRONJ has not yet been clearly established34. Several antioxidant and anti-inflammatory molecules have been reported to be effective against MRONJ and steroid-induced osteonecrosis21,26,35. Resveratrol is known for its various biological activities such as antioxidant, anti-inflammatory and angiogenesis effects27,36. The present study aimed to evaluate the potential effects of resveratrol administration in preventing MRONJ immediately after teeth extraction in rats treated with zoledronic acid.
Zoledronic acid was selected because it is the most potent nitrogen-containing bisphosphonate and has been associated with the highest risk of MRONJ development37,38. In this study, zoledronic acid was administered parenterally at 0.06 mg/kg, equivalent to the clinical dose used in oncology patients (4 mg monthly). The weekly dosing schedule was chosen to accelerate the development of MRONJ-like lesions in rats, as has been established in previous animal models. Because resveratrol exhibits low oral bioavailability in rats (~ 6%) due to extensive presystemic metabolism, the intraperitoneal route was chosen for its higher and more consistent absorption, ease of administration, and practicality compared with oral or intravenous delivery39,40,41,42,43.
Bisphosphonates have been reported to exhibit dual effects on the immune system, depending on the specific compound and the route of administration44. While first-generation bisphosphonates can deplete macrophages and decrease inflammation markers such as tumor necrosis factor-α (TNF-α), interleukin (IL)-2 and IL-6, deescalating inflammation45, nitrogen containing bisphosphonates such as zoledronic acid can either amplify or mitigate inflammation44. Continuous or intermittent administration of these agents has been shown to decrease of both γδ-T cells causing acute phase reaction and inflammatory markers44. In the present study, inflammation was observed to be higher in the ZA, RES, and ZA + RES groups than in controls, although the differences were not statistically significant. The use of resveratrol in the ZA + RES group slightly reduced local inflammation compared to the ZA group, aligning with previous findings by Vitale et al., who demonstrated that resveratrol supplementation significantly reduced local inflammatory intensity in a similar rat model26. The absence of a statistically significant reduction in inflammation despite resveratrol administration in the current study may be related to differences in dose, route, and duration of resveratrol administration, the type of the bisphosphonate used or the overall low baseline level of inflammation in the experimental model. This indicates that the anti-inflammatory effects of resveratrol may vary depending on the severity of tissue damage, the nature of the pro-inflammatory stimulus, and the compound’s overall biological characteristics.
Resveratrol has been shown to exhibit a dual osteoprotective effect by both promoting bone formation and inhibiting bone resorption27,46. In the present study, the resveratrol group exhibited significantly higher new bone formation compared to the ZA and ZA + RES groups at day 28 (p = 0.029 and p = 0.011, respectively). However, the addition of resveratrol to zoledronic acid treatment did not result in a significant increase in alveolar bone formation compared to zoledronic acid alone. This partial effect may be due to the strong and prolonged suppressive activity of zoledronic acid, which could not be completely counteracted by resveratrol at the applied dose. Similar findings were reported by Movahedian Attar et al.30, who observed no significant improvement in bone formation in alendronate/dexamethasone-treated rats receiving resveratrol. These results suggest that the osteogenic effects of resveratrol may be dose-dependent and may require longer healing periods to manifest fully. In contrast, an in vitro study by Borsani et al.29 demonsrated that resveratrol enhanced the proliferation and differentiation of zoledronic acid-treated osteoblasts and exerted a protective effect on these cells by positively modulating sirtuin 1, BMP-2 and osteoprotegerin (OPG). Although these findings support the osteogenic potential of resveratrol, the inconsistency with our in vivo results is likely related to differences in experimental conditions, particularly the resveratrol dose, route of administration, and the potent, long-lasting suppressive effect of zoledronic acid.
Although the etiopathogenesis of osteonecrosis has not been fully elucidated, there is evidence suggesting that it is associated with the initiation of endothelial damage, intravascular thrombosis, and insufficient vascularization47. In a steroid-induced osteonecrosis model, resveratrol was shown to reduce osteonecrosis by enhancing blood flow and preventing thrombosis through upregulation of VEGF expression, downregulation of thrombomodulin production, and inhibition of transcription factors48. Movahedian Attar et al.30 reported that resveratrol significantly reduced the incidence of jaw osteonecrosis in rats treated with alendronate and dexamethasone, although it did not enhance new bone formation during the short healing period. These findings suggest that resveratrol may primarily prevent early necrotic changes rather than accelerate bone formation during the early healing phase. This delayed osteogenic response may be related to the prolonged suppressive effect of alendronate or the need for a longer healing period for resveratrol’s action to become evident30. A study by Vitale et al.26 reported that the group receiving a combination of zoledronic acid and resveratrol exhibited a significantly improved healing pattern in the extraction sockets. Nonetheless, the same study emphasized that resveratrol alone was not sufficient to fully prevent the development of MRONJ26. In the present study, no statistically significant difference was found in terms of osteonecrosis between the ZA and ZA + RES groups on days 14 and 28, which is consistent with the findings of Vitale et al.21.
In this study, epithelialization was lower in the ZA group than the other groups on day 14, although the difference was not significant. On day 28, epithelialization in the RES group was significantly higher compared to the ZA-treated groups. Resveratrol use in the ZA + RES group improved epithelialization compared to ZA alone at both time points, though not significantly. Vitale et al.26 similarly reported that resveratrol combined with zoledronic acid significantly improved epithelial structure compared to zoledronate alone in ovariectomized rats. Resveratrol has been shown to enhance wound healing by promoting VEGF-mediated angiogenesis and suppressing pro-inflammatory cytokines such as TNF-α, IL-1β, and IL-649. It also supports keratinocyte migration and proliferation through its antioxidant and anti-inflammatory properties50,51,52.
BMPs promote bone formation by stimulating of osteoblastic proliferation and differentiation. Specifically, BMP-4 induces osteocalcin synthesis and VEGF release through p38 mitogen-activated protein (MAP) kinase pathway in osteoblast-like MC3T3-E1 cells53,54. In vivo studies have shown that during bone repair, BMP-2 and BMP-7 levels increase in the regenerated bone tissue in both healthy and diabetic rats55,56. Previous in vitro research demonstrated that resveratrol significantly upregulated the mRNA expression of key osteogenic marker, including alkaline phosphatase (ALP), BMP-4, and osterix during osteogenic differentiation in qRT-PCR analyses57. Additionally, resveratrol affected the proliferation and differentiation of zoledronic acid-treated osteoblasts and presented a protective effect by positively modulating sirtuin 1, BMP-2, and osteoprotegrin29. In the present study, consistent with previous literature55,56,57, the number of BMP-4-positive cells were significantly higher in the RES group compared to other groups on days 14 and 28.
Although immunohistochemical analyses revealed no statistically significant difference in the number of BMP-4 positive cells among the control, ZA and ZA + RES groups, both the control and ZA + RES groups exhibited higher BMP-4 expression compared to the ZA group, consistent with the findings of Borsani et al.29. Similarly, Huang et al.58 demonstrated that increasing concentrations of zoledronic acid reduced BMP-2 expression at both gene and protein levels in cultured osteoblasts.
Serum CTX-1 values have been used as a biomarker of bone resorption. During bone resorption, type I collagen is degraded, releasing CTX, so when bone turnover is suppressed by a bisphosphonate, then the CTX level is expected to decrease. The first clinical use of CTX measurement for predict the BRONJ was reported by Marx et al.59. However, the 2014 position paper of the American Association of Oral and Maxillofacial Surgeons (AAOMS) stated that the use of systemic bone turnover markers, such as CTX, to assess the risk of developing MRONJ has not been validated60. Moreover, there is wide individual variation in CTX to identical doses of bisphosphonates61. In the study by Ağaçayak et al.62, although all rats received the same dose of zoledronic acid (0.3 mg/kg), serum CTX levels varied among the traumatic extraction, atraumatic extraction, and non-extraction groups. Interestingly, the highest CTX levels were observed in the traumatic extraction group, suggesting that local surgical trauma and extraction type may influence bone turnover activity and CTX levels, even under identical bisphosphonate exposure62. Resveratrol has also shown inconsistent effects on CTX levels in previous studies. Hairi et al.27 and Zhang et al.46 reported that resveratrol significantly increased bone formation markers (such as osteocalcin and ALP) and bone mineral density, while reducing bone resorption markers including TRACP and CTX. Conversely, Lee et al.63 observed significantly elevated CTX-1 levels in ageing rats (6–9 months) following resveratrol treatment, suggesting that resveratrol may exert differential effects depending on the bone loss model, potentially even negatively influencing bone metabolism in certain conditions. In the present study, the serum CTX-1 levels were significantly higher in the ZA + RES group than in the control (p = 0.015) and RES (p = 0.001) groups, and higher in the ZA group than in the RES group (p = 0.001) on day 14. Additionally, serum CTX levels in the ZA (p = 0.001) and ZA + RES (p = 0.043) groups were significantly lower than those in the control group on day 28. This transient elevation of CTX-1 is noteworthy, as bisphosphonate therapy would typically be expected to reduce CTX levels over time. Such variability may reflect an acute post-extraction response, a delay in the suppressive effect of zoledronic acid due to treatment duration and cumulative dose of anti-resorptive agent, a potential negative effect of resveratrol. Technical factors such as sampling protocol, timing of blood collection (e.g., fasting status), and storage conditions (e.g., temperature) may also have contributed to these fluctuations63,64,65.
TRACP-5b is a bone metabolic biomarker specifically secreted by osteoclasts and is widely used as an indicator of bone resorption and cancer metastasis. Serum TRACP-5b levels are elevated in patients with bone diseases and decreased following antiresorptive treatments such as with bisphosphonates. However, recent studies have shown that secreted TRACP-5b activity may reflect the number of osteoclasts rather than their activity66. Its serum levels are not affected by feeding, exhibit low biological and analytical variability, and are not affected by renal or hepatic failure. The only concern when measuring TRACP-5b is that the enzyme is relatively unstable when serum samples are stored at − 20 °C for > 1 month, and for longer periods, serum samples should be stored at − 70 °C where the enzyme is stable67. In the present study, increased serum TRACP-5b levels were observed in the zoledronate-treated groups, suggesting that osteoclast numbers might be elevated in these groups. This finding could represent a compensatory response where osteoclast precursors are present but unable to adhere and function effectively due to the inhibitory effects of zoledronate on osteoclast maturation and activity68,69,70. Alternatively, technical factors such as sampling protocol and storage conditions (e.g., storage period and temperature) might also contribute to this observation.
Our study has several limitations, including small sample size, the testing of only a single dose in a limited number of animals and the lack of additional analysis such as evaluation of oxidative stress markers. Another limitation is the lack of radiographic assessment and measurement of additional serum indicators. We choose to limit the group number and, consequently, the number of sacrificed rats for ethical and economic reasons. Although rodents are widely used in MRONJ studies, the dosage of zoledronic acid and the anatomy and metabolism of rodents are different from those of humans. Another limitation of the study is that controlled or sustained-release drug delivery systems were not employed, which might have provided more consistent and localized therapeutic effects in the MRONJ model. Future experimental and prospective clinical studies with larger sample sizes, various doses and application methods of resveratrol, and extended follow-up periods are therefore recommended. Another limitation concerns the immunohistochemical analysis, where mild to moderate background staining was observed in some sections. This aspect should be carefully controlled in future studies to improve staining clarity and reproducibility.
Conclusion
Although resveratrol improved new bone formation in normal rats, no significant positive effects were detected in zoledronic acid-injected rats. However, its effectiveness on MRONJ may vary depending on factors such as the route of administration, dosage, and duration of treatment. Furthermore, the combined use of agents with potential synergistic effects with resveratrol may represent a more effective therapeutic strategy for the management of MRONJ. To confirm its effects on MRONJ, further studies are required with different study designs.
Data availability
All data generated or analysed during this study are included in this published article.
References
Baron, R., Ferrari, S. & Russell, R. G. Denosumab and bisphosphonates: different mechanisms of action and effects. Bone 48, 677–692. https://doi.org/10.1016/j.bone.2010.11.020 (2011).
Russell, R. G., Watts, N. B., Ebetino, F. H. & Rogers, M. J. Mechanisms of action of bisphosphonates: similarities and differences and their potential influence on clinical efficacy. Osteoporos. Int. 19, 733–759. https://doi.org/10.1007/s00198-007-0540-8 (2008).
Fleisch, H. & Bisphosphonates Mechanisms of action. Endocr. Rev. 19, 80–100. https://doi.org/10.1210/edrv.19.1.0327 (1998).
Campisi, G. et al. Medication-related osteonecrosis of jaws (MRONJ) prevention and diagnosis: Italian consensus update 2020. Int. J. Environ. Res. Public. Health. 17, 5998. https://doi.org/10.3390/ijerph17165998 (2020).
Hoefert, S., Schmitz, I., Tannapfel, A. & Eufinger, H. Importance of microcracks in etiology of bisphosphonate-related osteonecrosis of the jaw: A possible pathogenetic model of symptomatic and non-symptomatic osteonecrosis of the jaw based on scanning electron microscopy findings. Clin. Oral Investig.. 14, 271–284. https://doi.org/10.1007/s00784-009-0281-3 (2010).
On, S. W., Cho, S. W., Byun, S. H. & Yang, B. E. Various therapeutic methods for the treatment of medication-related osteonecrosis of the jaw (MRONJ) and their limitations: A narrative review on new molecular and cellular therapeutic approaches. Antioxidants 10, 680. https://doi.org/10.3390/antiox10050680 (2021).
Allen, M. R. & Burr, D. B. The pathogenesis of bisphosphonate-related osteonecrosis of the jaw: so many hypotheses, so few data. J. Oral Maxillofac. Surg. 67, 61–70. https://doi.org/10.1016/j.joms.2008.10.010 (2009).
Chang, J., Hakam, A. E. & McCauley, L. K. Current Understanding of the pathophysiology of osteonecrosis of the jaw. Curr. Osteoporos. Rep. 16, 584–595. https://doi.org/10.1007/s11914-018-0473-5 (2018).
Lopez-Jornet, P. et al. Perioperative antibiotic regimen in rats treated with pamidronate plus dexamethasone and subjected to dental extraction: A study of the changes in the jaws. J. Oral Maxillofac. Surg. 69, 2488–2493. https://doi.org/10.1016/j.joms.2011.02.116 (2011).
Landesberg, R. et al. Potential pathophysiological mechanisms in osteonecrosis of the jaw. Ann. N. Y. Acad. Sci. 1218, 62–79. https://doi.org/10.1111/j.1749-6632.2010.05836.x (2011).
Allam, E., Allen, M., Chu, T. M., Ghoneima, A. & Windsor, L. J. In vivo effects of Zoledronic acid on oral mucosal epithelial cells. Oral Dis. 17, 291–297 (2011).
Bagan, J. et al. Oxidative stress in bisphosphonate-related osteonecrosis of the jaws. J. Oral Pathol. Med. 43, 371–377 (2014).
Taniguchi, N. et al. Bisphosphonate-induced reactive oxygen species inhibit proliferation and migration of oral fibroblasts: A pathogenesis of bisphosphonate-related osteonecrosis of the jaw. J. Periodontol.. 91, 947–955 (2020).
Sungkhaphan, P. et al. Dual-Functional drug delivery system for Bisphosphonate-Related osteonecrosis prevention and its bioinspired releasing model and in vitro assessment. ACS Omega. 8, 26561–26576. https://doi.org/10.1021/acsomega.3c03440 (2023).
Brierly, G. I. et al. Investigation of sustained BMP delivery in the prevention of medication-related osteonecrosis of the jaw (MRONJ) in a rat model. Macromol. Biosci. 19, e1900226. https://doi.org/10.1002/mabi.201900226 (2019).
Sharma, D. et al. Local delivery of hydrogel-encapsulated vascular endothelial growth factor for the prevention of medication-related osteonecrosis of the jaw. Sci. Rep. 11, 23371. https://doi.org/10.1038/s41598-021-02637-w (2021).
Erten Taysi, A. et al. The efficacy of sustained-release Chitosan microspheres containing Recombinant human parathyroid hormone on MRONJ. Braz. Oral. Res. 33, e086. https://doi.org/10.1590/1807-3107bor-2019.vol33.0086 (2019).
Tao, L. et al. The preventive effect of photocrosslinked Hep/GelMA hydrogel loaded with PRF on MRONJ. BMC Oral. Health. 24, 1010. https://doi.org/10.1186/s12903-024-04792-8 (2024).
Koçer, G. et al. Basic fibroblast growth factor attenuates bisphosphonate-induced oxidative injury but decreases zinc and copper levels in oral epithelium of rat. Biol. Trace Elem. Res. 153, 251–256 (2013).
Khandelwal, V. K. et al. Oxidative stress plays an important role in Zoledronic acid-induced autophagy. Physiol. Res. 63Suppl (4), S601–S612 (2014).
Kuribayashi, M. et al. Vitamin E prevents steroid-induced osteonecrosis in rabbits. Acta Orthop. 81, 154–160 (2010).
Ichiseki, T. et al. Oxidative stress and vascular permeability in steroid-induced osteonecrosis model. J. Orthop. Sci. 9, 509–515 (2004).
Ichiseki, T. et al. DNA oxidation injury in bone early after steroid administration is involved in the pathogenesis of steroid-induced osteonecrosis. Rheumatol. (Oxford). 44, 456–460 (2005).
Tamaoka, J. et al. Osteonecrosis of the jaws caused by bisphosphonate treatment and oxidative stress in mice. Exp. Ther. Med. 17, 1440–1448 (2019).
Dos Santos Ferreira, L. et al. Is teriparatide therapy effective for medication-related osteonecrosis of the jaw? A systematic review and meta-analysis. Osteoporos. Int. 32, 2449–2459 (2021).
Vitale, M. et al. Resveratrol for preventing medication-related osteonecrosis of the jaws in rats. Oral Dis. 30, 1462–1474. https://doi.org/10.1111/odi.14544 (2024).
Ahmad Hairi, H., Jayusman, P. A. & Shuid, A. N. Revisiting Resveratrol as an osteoprotective agent: molecular evidence from in vivo and in vitro studies. Biomedicines 11, 1453 (2023).
Kumar, B., Iqbal, M. A., Singh, R. K. & Bamezai, R. N. Resveratrol inhibits TIGAR to promote ROS induced apoptosis and autophagy. Biochimie 118, 26–35. https://doi.org/10.1016/j.biochi.2015.07.016 (2015).
Borsani, E. et al. Beneficial effects of concentrated growth factors and resveratrol on human osteoblasts in vitro treated with bisphosphonates. Biomed. Res. Int. 4597321 (2018). (2018).
Movahedian Attar, B., Razavi, S. M., Daneshmand, M. & Davoudi, A. Protective effects of Resveratrol against osteonecrosis at the extraction site in bisphosphonate-treated rats. Int. J. Oral Maxillofac. Surg. 49, 1518–1522 (2020).
Ozcan-Kucuk, A., Alan, H., Gul, M. & Yolcu, U. Evaluating the effect of Resveratrol on the healing of extraction sockets in cyclosporine A-treated rats. J. Oral Maxillofac. Surg. 76, 1404–1413. https://doi.org/10.1016/j.joms.2018.02.030 (2018).
Vidal-Gutiérrez, X., Gómez-Clavel, J. F. & Gaitán-Cepeda, L. A. Dental extraction following zoledronate induces osteonecrosis in rat’s jaw. Med. Oral Patol. Oral Cir. Bucal. 22, e177–e184. https://doi.org/10.4317/medoral.21609 (2017).
McCarty, K. S. Jr. et al. Estrogen receptor analyses. Correlation of biochemical and immunohistochemical methods using monoclonal antireceptor antibodies. Arch. Pathol. Lab. Med. 109, 716–721 (1985).
Beth-Tasdogan, N. H. et al. Interventions for managing medication-related osteonecrosis of the jaw. Cochrane Database Syst. Rev. CD013993 (2022).
Isleyen, M. et al. The preventive effect of preoperative and postoperative selenium on the medication-related osteonecrosis of the jaw: an animal study in rats. J. Oral Maxillofac. Surg. 82, 828–839. https://doi.org/10.1016/j.joms.2024.03.026 (2024).
Tseng, S. H. et al. Resveratrol suppresses the angiogenesis and tumor growth of gliomas in rats. Clin. Cancer Res. 10, 2190–2202 (2004).
Zandi, M. et al. Introducing a protocol to create bisphosphonate-related osteonecrosis of the jaw in rat animal model. J. Craniomaxillofac. Surg. 44, 271–278 (2016).
Dodson, T. B. Intravenous bisphosphonate therapy and bisphosphonate-related osteonecrosis of the jaws. J. Oral Maxillofac. Surg. 67, 44e1–4452 (2009).
Akinwumi, B. C., Bordun, K. A. M. & Anderson, H. D. Biological activities of stilbenoids. Int. J. Mol. Sci. 19, 792. https://doi.org/10.3390/ijms19030792 (2018).
Chen, W. et al. Oxyresveratrol: A bioavailable dietary polyphenol. J. Funct. Foods. 22, 122–131. https://doi.org/10.1016/j.jff.2016.01.034 (2016).
Shoyaib, A. A., Archie, S. R. & Karamyan, V. T. Intraperitoneal route of drug administration: should it be used in experimental animal studies? Pharm. Res. 37, 12. https://doi.org/10.1007/s11095-019-2764-4 (2019).
Zhong, L. X. et al. Efficacy and safety of intraperitoneally administered Resveratrol against rat orthotopic ovarian cancers. Cancer Manag. Res. 11, 6113–6124. https://doi.org/10.2147/CMAR.S206301 (2019).
Lukas, G., Brindle, S. D. & Greengard, P. The route of absorption of intraperitoneally administered compounds. J. Pharmacol. Exp. Ther. 178, 562–566 (1971).
Panagiotakou, A. et al. Extra-skeletal effects of bisphosphonates. Metabolism 110, 154264. https://doi.org/10.1016/j.metabol.2020.154264 (2020).
Sousa, S. et al. Liposome encapsulated zoledronate favours M1-like behaviour in murine macrophages cultured with soluble factors from breast cancer cells. BMC Cancer. 15, 1–11. https://doi.org/10.1186/s12885-015-1950-9 (2015).
Zhang, Y., Deng, L., Fan, J. & Zhang, Y. Effects of Resveratrol on bone metabolism and bone turnover related indexes in ovariectomized osteoporosis rats. Cell. Mol. Biol. 66, 92–97. https://doi.org/10.14715/cmb/2020.66.5.17 (2020).
Zhang, G. et al. A novel semisynthesized small molecule Icaritin reduces incidence of steroid-associated osteonecrosis with Inhibition of both thrombosis and lipid-deposition in a dose-dependent manner. Bone 44, 345–356 (2009).
Zhai, L., Weng, X. S., Wu, Z. H. & Guo, S. G. Effect of Resveratrol on preventing steroid-induced osteonecrosis in a rabbit model. Chin. Med. J. (Engl). 129, 824–830 (2016).
Pignet, A. L. et al. Resveratrol-induced signal transduction in wound healing. Int. J. Mol. Sci. 22, 12614. https://doi.org/10.3390/ijms222312614 (2021).
Liu, Y. et al. Resveratrol promotes skin wound healing by regulating the miR-212/CASP8 axis. Lab. Investig.. 101, 1363–1370. https://doi.org/10.1038/s41374-021-00621-6 (2021).
Shin, J. W. et al. Resveratrol inhibits particulate matter-induced inflammatory responses in human keratinocytes. Int. J. Mol. Sci. 21, 3446. https://doi.org/10.3390/ijms21103446 (2020).
Khanna, S. et al. Dermal wound healing properties of redox-active grape seed proanthocyanidins. Free Radic. Biol. Med. 33, 1089–1096. https://doi.org/10.1016/S0891-5849(02)00999-1 (2002).
Kozawa, O., Hatakeyama, D. & Uematsu, T. Divergent regulation by p44/p42 MAP kinase and p38 MAP kinase of bone morphogenetic protein 4 stimulated osteocalcin synthesis in osteoblasts. J. Cell. Biochem. 84, 583–589. https://doi.org/10.1002/jcb.10189 (2002).
Tokuda, H. et al. p38 MAP kinase regulates BMP-4-stimulated VEGF synthesis via p70 S6 kinase in osteoblasts. Am. J. Physiol. Endocrinol. Metab. 284, E1202–E1209. https://doi.org/10.1152/ajpendo.00515.2002 (2003).
Casarin, R. C. et al. Resveratrol improves bone repair by modulation of bone morphogenetic proteins and osteopontin gene expression in rats. Int. J. Oral Maxillofac. Surg. 43, 900–906. https://doi.org/10.1016/j.ijom.2014.03.005 (2014).
Pino, D. S. et al. Effect of resveratrol on critical-sized calvarial defects of diabetic rats: Histometric and gene expression analysis. J. Oral Maxillofac. Surg. 75 https://doi.org/10.1016/j.joms.2017.06.185 (2017). 2561.e1–2561.e10.
Li, J., Xin, Z. & Cai, M. The role of Resveratrol in bone marrow-derived mesenchymal stem cells from patients with osteoporosis. J. Cell. Biochem. 120, 16634–16642. https://doi.org/10.1002/jcb.29071 (2019).
Huang, X. et al. Dose-dependent inhibitory effects of Zoledronic acid on osteoblast viability and function in vitro. Mol. Med. Rep. 13, 613–622. https://doi.org/10.3892/mmr.2015.4560 (2016).
Marx, R. E., Cillo, J. E. Jr. & Ulloa, J. J. Oral bisphosphonate induced osteonecrosis: risk factors, prediction of risk using serum CTX testing, prevention, and treatment. J. Oral Maxillofac. Surg. 65, 2397–2410. https://doi.org/10.1016/j.joms.2007.08.003 (2007).
Ruggiero, S. L. et al. American association of oral and maxillofacial surgeons position paper on medication-related osteonecrosis of the jaw – 2014 update. J. Oral Maxillofac. Surg. 72, 1938–1956. https://doi.org/10.1016/j.joms.2014.04.031 (2014).
Khosla, S. Oral bisphosphonate-induced osteonecrosis: risk factors, prediction of risk using serum CTX testing, prevention, and treatment. J. Oral Maxillofac. Surg. 66, 1320–1321. https://doi.org/10.1016/j.joms.2008.03.013 (2008).
Agaçayak, K. S., Yuksel, H., Atilgan, S., Koparal, M., Uçan, M. C., Özgöz, M. E. H.M. E. T., Acikan, I. Experimental investigation of relationship between trauma and bisphosphonate-related osteonecrosis.Niger J. Clin. Pract.. 17(5), 559–564 (2014). doi:10.4103/1119-3077.141417.
Lee, A. M. et al. Effects of Resveratrol supplementation on bone growth in young rats and microarchitecture and remodeling in ageing rats. Nutrients 6, 5871–5887. https://doi.org/10.3390/nu6125871 (2014).
Chubb, S. A. Measurement of C-terminal telopeptide of type I collagen (CTX) in serum. Clin. Biochem. 45, 928 – 35 . https://doi.org/10.1016/j.clinbiochem.2012.03.035 (2012).
Ruggiero, S. L. et al. American association of oral and maxillofacial surgeons’ position paper on medication-related osteonecrosis of the jaws—2022 update. J. Oral Maxillofac. Surg. 80, 920–943. https://doi.org/10.1016/j.joms.2022.02.008 (2022).
Rissanen, J. P., Suominen, M. I., Peng, Z. & Halleen, J. M. Secreted tartrate-resistant acid phosphatase 5b is a marker of osteoclast number in human osteoclast cultures and the rat ovariectomy model. Calcif Tissue Int. 82, 108–115. https://doi.org/10.1007/s00223-007-9091-4 (2008).
Nenonen, A. et al. Serum TRACP 5b is a useful marker for monitoring alendronate treatment: comparison with other markers of bone turnover. J. Bone Min. Res. 20, 1804–1812. https://doi.org/10.1359/JBMR.050403 (2005).
Gross, C. et al. Osteoclast profile of medication-related osteonecrosis of the jaw secondary to bisphosphonate therapy: a comparison with osteoradionecrosis and osteomyelitis. J. Transl. Med. 15 (1), 128. https://doi.org/10.1186/s12967-017-1230-8 (2017).
Kuroshima, S., Al-Omari, F. A., Sasaki, M. & Sawase, T. Medication-related osteonecrosis of the jaw: A literature review and update. Genesis 60, e23500. https://doi.org/10.1002/dvg.23500 (2022).
Kasagawa, M. et al. Analysis of risedronate analog on extraction socket healing in mice. Vivo 39, 648–655. https://doi.org/10.21873/invivo.13870 (2025).
Acknowledgements
This study was supported by the Unit of Scientific Research Projects Fund of Mersin University, Mersin, Turkey (Grant number: 2018-1-AP4-2907). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Author information
Authors and Affiliations
Contributions
Ayse OZCAN KUCUK : Conceptualization, Data Curation, Investigation, Methodology, Project Administration, Supervision, Validation, Writing-Original Draft, Writing-Review & Editing. Burak AK : Investigation, Methodology. Sakir Necat YILMAZ : Methodology, Investigation, Validation, Visualization, Writing-Review & Editing. Deniz KIBAR : Investigation, Validation, Visualization. Ayşe Betul GUK : Data Curation, Visualization, Writing-Original Draft. All authors interpreted the findings, reviewed the final version of the manuscript, and approved it for publication.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Ozcan Kucuk, A., Ak, B., Yilmaz, S.N. et al. Evaluation of the effect of resveratrol on bone healing after tooth extraction in rats treated with zoledronic acid. Sci Rep 16, 3864 (2026). https://doi.org/10.1038/s41598-025-34009-z
Received:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s41598-025-34009-z