Abstract
We recently characterized the potent antiplasmodial activity of the aggregated protein dye YAT2150, whose presumed mode of action is the inhibition of protein aggregation in the malaria parasite. Using single-dose and ramping methods, assays were done to select Plasmodium falciparum parasites resistant to YAT2150 concentrations ranging from 3× to 0.25× the in vitro IC50 of the compound (in the two-digit nM range) and performed a cross-resistance assessment in P. falciparum lines harboring mutations that make them resistant to a variety of antimalarial drugs. Resistant parasites did not emerge in vitro after 60 days of incubation, which postulates YAT2150 as an ‘irresistible’ antimalarial. The lyophilized compound is stable for at least one year stored at 25 °C. Tests performed in clinical isolates indicated that YAT2150 had also strong activity against Plasmodium vivax (IC50 between 4 and 36 nM) and Leishmania infantum (1.27 and 1.11 µM), placing it as a unique compound with perspectives of becoming the first drug to be used against both malaria and leishmaniasis.
Similar content being viewed by others
Introduction
According to the last World Malaria Report1, in 2022 there had been an estimated 249 million cases of malaria worldwide causing 608,000 deaths. With the aim of reducing the global disease burden, efforts to control it in endemic areas are numerous, including the recent recommendation by the World Health Organization (WHO) of using the RTS, S/AS01 vaccine2 in children living in high Plasmodium falciparum malaria transmission areas3. Insecticide-based vector control methods as well as preventive treatment with sulfadoxine-pyrimethamine during pregnancy are other means used to avoid malaria infections4. However, prevention in high-risk areas is not enough and the reliance on antimalarial drugs for treatment is strong. The reduction in the efficacy of the gold standard malaria treatment, artemisinin combination therapies (ACTs), firstly in Asia5 and, more recently, in South America6 and Africa7,8 is dangerously threatening the progress towards a malaria-free world.
Resistance to ACTs is far from being an exception, and almost all antimalarials used in the field have given rise to the emergence and spread of drug-resistant parasites shortly after their deployment9. A striking example of how quickly P. falciparum can evolve resistance to antimalarial drugs is sulfadoxine-pyrimethamine, to which resistant parasites were detected in Thailand the same year, 1967, that the drug was introduced in the country10. In most cases, a single gene mutation is enough to trigger the resistance mechanism. For example, point mutations in the genes pfcrt or pfmdr1 confer resistance to chloroquine and other quinolines, respectively, allowing more efficient efflux from or inhibiting the entrance of the drugs into the parasite’s digestive vacuole, where the target molecules of the compounds are located11. Artemisinin resistance is produced by point mutations in the gene pfk13, and a number of mechanisms explaining it has been proposed12. Malaria incidence rate has reached in the last years a plateau phase after a reductive tendency since 2000, when ACTs started to be used as front line treatments for uncomplicated malaria13. The implementation of triple artemisinin-containing combination therapies has been proposed with the aim of avoiding ACT resistance progress, but this would only be a way to delay the problem and gain some time before multiresistant parasites appear14. Although Plasmodium vivax malaria has been often considered a benign illness, recent evidence of its significant morbidity and mortality has placed it as a health risk at the same level as falciparum malaria15, with equal concerns regarding the emergence of resistance to available drugs.
After malaria, leishmaniasis is the second parasitic disease in number of deaths16, but, despite their overlapping geographical distribution, the study of Plasmodium and Leishmania co-infections has been largely neglected17. The clinical usefulness of the few current antileishmanial treatments is severely undermined by resistance evolution in the pathogen, drug toxicity and painful administration, and by the high cost of the liposomal formulation which today represents the best therapeutic option18. This critical scenario has led the WHO to give priority to the discovery of new drugs acting on new targets and against clinical isolates, which could phenotypically diverge from the laboratory-adapted strains (e.g. in virulence or drug susceptibility), as a key strategy to achieve leishmaniasis eradication19,20.
New antimalarial compounds should have novel mechanisms of action in order to avoid cross-resistance with drugs already in use and they should target more than one Plasmodium life cycle stage, including gametocytes in order to block transmission21. Our group has recently characterized two new antiplasmodial compounds, DONE3TCl and YAT2150 (Fig. 1), whose presumed mode of action is the inhibition of protein aggregation in the parasite22. DONE3TCl is a 4-aminoquinoline compound with a half-maximal inhibitory concentration (IC50) of 80 nM against cultured P. falciparum, which inhibits protein aggregation in vitro23. On the other hand, YAT2150 features a unique scaffold, with two aminostyrylpyridinium moieties connected through an oligomethylene linker, inaugurating a chemical family where no other antimalarial drugs are known. In sexual and asexual stages of P. falciparum it shows an in vitro IC50 of 90 nM, also for chloroquine- and artemisinin-resistant lines, and at this physiologically relevant concentration it diminishes the aggregative protein load in cultured parasites22. Due to its A/T-biased genome, the P. falciparum proteome is exceptionally enriched in proteins with asparagine repeats, which have a tendency to form insoluble aggregates24,25. Moreover, 90% of P. falciparum proteins contain at least one Low Complexity Region (LCR) where asparagine is the most represented amino acid26. LCRs are stretches inside proteins that have a fluctuant tertiary structure and, as a consequence, are more prone to aggregate27. The presence of aggregative proteins in the parasite proteome has been proven in silico28 and also in live parasite cells throughout the whole life cycle, including sexual and mosquito stages29.
YAT2150 has a number of additional properties that make it highly interesting as potential new antiparasitic drug, namely: (i) it is a fast-acting antiplasmodial30, a rare property among antimalarial compounds31, and an attractive characteristic regarding the need for new drugs in front of the emergence of resistance to the fast-acting artemisinin; (ii) it interferes with the development of Plasmodium hepatic forms, a preferred target to treat the dormant stages of vivax malaria; (iii) it has shown strong activity against a Leishmania infantum laboratory strain32, (iv) its presumed mode of action (inhibition of protein aggregation in the parasite) likely targets multiple proteins, which would prevent a rapid resistance evolution by the pathogen; (v) it belongs to an unexplored chemical family where no other antimalarial or antileishmanial has been described to date, which will prevent the adaptation by the parasite of preexisting resistance mechanisms to currently used drugs; (vi) its synthesis is easy and rapid22 (only two steps), which offers good prospects for production in the low per capita income regions where malaria and leishmaniasis are endemic.
Generation of mutant resistant P. falciparum in vitro cultures has been used to (i) follow the development of resistance to current drugs (e.g. chloroquine33,34,35), (ii) discover the molecular targets of a compound during its characterization process36, and (iii) gauge the propensity of parasites to develop resistance to potential new antimalarials37. Since parasite resistance evolution is one of the main challenges that future antimalarial drugs need to face, we have explored if resistance to YAT2150 evolves easily in vitro. To determine if YAT2150 could also be active against other Plasmodium species, we have evaluated its activity against blood stages of Plasmodium vivax, the most geographically widespread human malaria species. Finally, we have assessed as well the activity of YAT2150 against two Leishmania clinical isolates with different susceptibilities to antileishmanial drugs in clinical use to explore if the antiparasitic effect of YAT2150 is not only restricted to Plasmodium species.
Results
In vitro selection of YAT2150- and DONE3TCl-resistant parasites using a dose of 3×IC50
The attempt to generate resistant parasites was performed using a concentration equal to 3×IC50 of YAT2150, DONE3TCl and KAE609, a control drug that targets the P-type cation transporter PfATP438 and that had already been tested in this kind of experiment with an established minimum inoculum for resistance39. To increase the possibilities of obtaining resistant parasites, a Dd2-Pol∂ P. falciparum strain was used. This line has point mutations in the DNA polymerase delta subunit, resulting in a mutation rate during DNA replication approximately 5- to 8-fold higher than wild type Dd239. During this assay, P. falciparum treated with 3×IC50 of KAE609 re-emerged in all three replicates after 15–17 days of parasite clearance (Table 1), at both tested inocula of 1 × 108 and 1 × 109. These parasites were resistant to KAE609 since the drug’s IC50 against them was at least 70 times higher than the IC50 of this compound against the parental Dd2-Pol∂ P. falciparum (Supplementary Fig. 1A).
In DONE3TCl-treated cultures, 4 replicates re-emerged, which did not show a clear shift in their IC50 values compared to the Dd2-Pol∂ parental line (Supplementary Fig. 1B). Despite the small increase in the IC50 values of the bulk cultures from the 4 resistant replicates, cloning plates with the two of them showing the biggest shifts, 109 (1) and 109 (2), were prepared. The objective was to check if any particular clone was more resistant to DONE3TCl than the mixed bulk culture. However, none of the tested clones showed an IC50 value significantly different from the Dd2-Pol∂ control (Table 2). After 60 days of parasite clearance, no resistant parasites to YAT2150 came back upon treatment of 108 or 109 parasites with 3 times the IC50 (Table 1).
In vitro selection of YAT2150- and DONE3TCl-resistant parasites using reduced compound concentrations
As resistant parasite appearance was not observed in cultures treated with 3×IC50 concentrations of DONE3TCl and YAT2150, a less aggressive resistance generation strategy was tested. In this case, 1 × 108 parasites were treated with 1×IC50, 0.5×IC50 or 0.25×IC50 of YAT2150 or 1×IC50 of DONE3TCl (Table 1), and the compound concentration was stepwise increased up to the respective IC50s to avoid a total parasite clearance in the cultures. In the event that a strong arrest in parasite growth was observed, compounds would be removed from the medium to allow parasites’ recovery.
Despite the tight control to which cultures were subjected, it was not possible to increase YAT2150 concentrations up to values higher than its IC50 as parasite clearance was observed after treatment with the IC50 for two consecutive days, with or without previous treatment at lower concentrations. In the case of DONE3TCl, total parasite clearance was also observed when cultures were treated with its IC50. Thus, no resistant parasites to either compound appeared using this strategy.
Cross-resistance assessment of YAT2150 and DONE3TCl in mutant P. falciparum strains
Although de novo in vitro resistance to YAT2150 and DONE3TCl did not appear in P. falciparum cultures, an alternative scenario to be considered in the wild is cross-resistance, i.e. that resistance mechanisms already developed by the parasite against certain drugs could serve for new ones40,41. If the only mode of action of YAT2150 and DONE3TCl was the inhibition of protein aggregation, which has not been described for other antimalarials, cross-resistance might be difficult to arise. However, other parasite processes targeted by currently used drugs and next-generation compounds in development might be affected by the new compounds and in this case cross-resistances could be expected. To explore this possibility, a pool of 47 barcoded parasite lines with known mutations (Supplementary Table 1) that confer resistance to a variety of antimalarial drugs was exposed to different concentrations of YAT2150 or DONE3TCl with the objective of identifying whether specific drug-resistant parasites would be enriched. This approach was based on the ability to detect changes in parasite abundance in a pool by barcode sequencing42. After 14 days of compound exposure, parasites were harvested and sequenced.
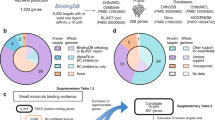
All tested concentrations of YAT2150 (0.25×IC50, 0.5×IC50, 1×IC50 and 3×IC50) drastically inhibited the growth of the culture after a couple of days of treatment (Fig. 2A). In the case of DONE3TCl, the lowest concentration tested (0.5×IC50) allowed parasite growth comparable to that of the untreated culture, while the other two concentrations (1×IC50 and 3×IC50) totally arrested parasite growth after the second day of treatment. Sequencing of barcodes associated with YAT2150-treated cultures showed that there was no enriched population on day 14 (Fig. 2B, C), consistent with the no-growth profiles. The same outcome was found in the cultures treated with the two doses of DONE3TCl that inhibited parasite growth. The parasite population of the culture treated with 0.5×IC50 of DONE3TCl, which grew at a regular rate after 14 days, was compared to the untreated culture at day 14. In this case, the strains with a Dd2 background showed no differences between treated and untreated cultures, whereas the 3D7 background strains were less enriched in the treated culture than in the untreated one. However, no particular population inside the 3D7 background lines was dominant.
Cross-resistance assessment of YAT2150 and DONE3TCl using a mutant P. falciparum pool. (A) Cumulative parasitemia of the pool culture of mutant parasites left untreated or treated with different concentrations (0.25×IC50, 0.5×IC50, 1×IC50 and 3×IC50) of YAT2150 or DONE3TCl. (B) Relative proportion of each parasite line in the pool culture (represented with different colors). See Supplementary Table 1 for the description of the parasite lines present in the pool. DONE3TCl and YAT2150-treated samples represent parasite population at day 14 of the experiment, and the untreated controls are shown for day 0 and 14. (C) Relative change in proportion of DONE3TCl- or YAT2150-treated pool compared to the appropriate untreated control based on growth (day 14 for DONE3TCl at 0.5×IC50, day 0 for all other treatments). Each triangle (3D7 background) or circle (Dd2 background) represents one parasite line. The change in proportion of each line in treated vs. untreated cultures is represented by the log fold change (y axis). A log fold change (LFC) < ‒2.5 (red color) or > 2.5 (green color) is considered to be significant (dashed lines frame the significance boundary). The x-axis shows barcode read counts of each line (mean of untreated and treated samples).
Assessment of YAT2150 efficacy against P. vivax
In order to assess the effect of YAT2150 on P. vivax, we performed ex vivo growth inhibition assays on four field isolates collected in Mondulkiri province, Cambodia. Isolate 1, Isolate 2 and Isolate 4 presented mostly young to mid-stage ring forms at the start of drug treatment with parasite densities of 15,751, 4661 and 11,563 parasites/µL, respectively. The samples were incubated with YAT2150 in six concentrations for 42, 39 and 38 h, respectively. The third blood sample (Isolate 3) contained mostly mature trophozoite forms at the start of drug treatment with a parasite density of 3073 parasites/µL and was incubated with the compound for 16 h. YAT2150 was strongly active against all three isolates that were treated at the ring stage and incubated with the compound for at least 38 h with IC50 values of 36 nM, 9 nM and 4 nM, respectively (Fig. 3). The compound was also active against the trophozoite stage sample with shorter incubation giving a slightly higher IC50 value of 56 nM. The half-maximal cytotoxic concentration (CC50) of YAT2150 for Caco-2 cells was 18.2 ± 2.6 µM, similar to the half-maximal in vivo toxicity concentration determined in the Caenorhabditis elegans model (16.2 ± 1.4 µM)30, which indicates a selectivity index (SI, CC50/IC50) for P. falciparum and P. vivax safely above the 100-fold threshold suggested as a generic lead selection criteria in drug discovery for infectious diseases43.
Assessment of YAT2150 efficacy against L. infantum clinical isolates
YAT2150 was strongly active against two clinical L. infantum isolates tested, LLM-1936 and LLM-1689. Their phenotypic characterization showed that both isolates were more sensitive to the antileishmanial drug miltefosine compared to the reference L. infantum JPC strain. Nevertheless, LLM-1936 was less susceptible to two of the available drugs for leishmaniasis treatment, pentamidine and paromomycin (Supplementary Fig. 2). Interestingly, YAT2150 showed a similar activity in both L. infantum clinical strains, with the IC50 estimated at 1.27 ± 0.30 µM for LLM-1936 and 1.11 ± 0.69 µM for LLM-1689 (Fig. 4). Considering the CC50 and in vivo toxicity values reported above, the SI for YAT2150 in L. infantum is around 15.
Assessment of YAT2150 shelf stability
An adequate stability at room temperature is always a desirable attribute for any drug, as it enables a simpler, cheaper, and safer conservation, storage, distribution, and administration than that of drugs requiring conditions out of the normal, e.g., refrigeration, while ensuring the expected efficacy and safety. We have monitored by 1H NMR spectroscopy the stability of solid samples of YAT2150 at 25 °C either exposed to light or protected from it, during 11 months. At the end of this period, the purity of the YAT2150 sample exposed to light had decreased by 8.5% (Supplementary Fig. 3), whereas the sample that was kept in the darkness remained essentially unchanged, observing only 0.5% reduction in purity compared with the initial sample (Supplementary Fig. 4). These results were confirmed by a parallel HPLC analysis, which showed a purity reduction of 8.4% after 10 months in the sample exposed to light (Supplementary Fig. 5) and only 1.9% reduced purity in the sample protected from light (Supplementary Fig. 6); both aged YAT2150 samples maintained their antiplasmodial activity in P. falciparum cultures, with IC50s of 93.0 and 84.0 nM, respectively (Supplementary Fig. 7). Thus, YAT2150 has a very favorable shelf stability if kept protected from light, a condition which can be readily attained and is generally recommended for most drugs.
Discussion
The rampant evolution of resistance to currently used antimalarial compounds, especially artemisinin derivatives, is seriously endangering the future of malaria treatments, and calls for the urgent discovery of new drugs44. These novel antimalarials need to be able to escape the parasite resistance mechanisms, i.e., they should be active against known resistant parasites as well as in vitro proofed towards potential resistance emergence21. Regarding the first characteristic, we have recently shown that YAT2150 is effective towards P. falciparum strains resistant to chloroquine and artemisinin22. This promising property has been here thoroughly studied and consistently confirmed by the lack of parasite growth when a broadly-resistant parasite pool was treated with DONE3TCl or YAT2150, the latter even at low concentrations. This pool contained 47 different parasite lines, including some carrying mutations in three of the most widespread resistance markers: pfmdr1, pfdhfr and pfcrt45. Intriguingly, DONE3TCl at concentrations lower than its IC50 seemed to be more effective against parasites with the 3D7 background than against parasites with Dd2 background. One of the main phenotypical differences between these two strains is their sensitivity to chloroquine, which is moderately reduced in Dd2 compared to 3D746. The fact that DONE3TCl, like chloroquine, is an aminoquinoline, could account for the observed increased sensitivity to it of 3D7 parasites. Protein homeostasis in Plasmodium has been explored as a potential antimalarial target, and proteasome inhibitors47 or compounds affecting the regular function of chaperones48 have shown good antimalarial properties. However, in some cases, in vitro resistance has appeared49. Interestingly, one of the mutations conferring resistance to proteasome inhibitors in Plasmodium (Dd2-PROTB5-A20V) was included in the pool of parasites tested against YAT2150 and DONE3TCl and was not enriched by either compound. This is in accordance with their protein aggregation inhibitory effect, as an efficient proteasome does not confer an advantage towards a less aggregated proteome.
Regarding the necessity of new antimalarial drugs to be tested for in vitro de novo resistance, neither YAT2150 nor DONE3TCl resistant parasites emerged. This lack of resistance evolution could be due to the presumed mode of action of these compounds (inhibition of protein aggregation in the parasite) and their likely multiple targets, i.e., the numerous aggregative proteins present in Plasmodium cells. A single gene mutation is not expected to confer resistance to compounds with such characteristics50, except in the case of a change in a transporter that allows a more efficient drug efflux. However, the entry of YAT2150 into cells is presumably based on its crossing of biological membranes without the need for a specific carrier, as suggested by its activity as intracellular protein aggregate stain in live cells22,29 and by its experimentally demonstrated interaction with lipid bilayers32. The re-emergence of DONE3TCl-treated parasites during in vitro resistance selection experiments suggests that this compound is not as lethal as YAT2150, and despite no resistant parasites appearing, this characteristic makes DONE3TCl less attractive for future development than YAT2150.
Relapsing and chronic low-density infections significantly contribute to the transmission of P. vivax15, making its control and elimination more difficult than for P. falciparum51. In 2022, clinical vivax malaria cases raised to an estimated 6.9 million1, representing a major cause of morbidity in endemic regions, especially considering the existence of a significant number of asymptomatic infections52,53,54. P. vivax can cause severe and fatal malaria, characterized by malnutrition and anemia, derived from its chronicity and propensity to cause relapses15,53, together with organ dysfunction and hypotension in acute infections55,56. Although in recent years our understanding of the life cycle and pathogenesis of P. vivax has significantly grown, management of this major pathogen urgently requires new efficient drugs to be added to the limited therapeutic arsenal currently available51. The strong activity of YAT2150 against P. vivax clinical isolates, with an IC50 down to single-digit nM concentrations, offers good prospects for this compound, or some of its derivatives, to be part of future treatments for vivax malaria.
The activity of YAT2150 in clinical isolates of L. infantum (IC50 of 1.27 µM for LLM-1936 and of 1.11 µM for LLM-1689) is significantly better than that of the currently used antileishmanial drugs miltefosine, paromomycin, and pentamidine57,58,59, being only inferior to amphotericin B60,61, whose liposomal formulation, however, has a high cost taking into account most areas where leishmaniasis is endemic. Finally, it should be noted that, despite what has been described by Hefnawy et al., where 55% of the compounds active against the L. infantum reference strain lost their activity against at least one of the two clinical isolates used, YAT2150 not only is active against strains currently in circulation, which represent the actual clinical situation of leishmaniasis, but also does not lose potency against a strain with reduced susceptibility to two of the currently used antileishmanial drugs20. The relatively low selectivity index of YAT2150 can be raised to yet safer levels through its encapsulation in targeted drug delivery nanocarriers. As an example of this, immunoliposomal formulations of YAT2150 targeted with specific antibodies to P. falciparum and L. infantum resulted in increased antiparasitic in vitro activities and reduced cytotoxicities which led to respective SIs of > 98030 and > 10032.
Malaria and leishmaniasis are the most deadly parasitic diseases, and the two malaria parasite species with the widest distribution, P. falciparum and P. vivax, are together responsible for 95% of global malaria deaths1. It is extremely unusual, and highly desirable, for a single compound like YAT2150 to be highly potent against clinical isolates of P. vivax and L. infantum, and irresistible in P. falciparum assays. The significantly different IC50 values of YAT2150 for Plasmodium and Leishmania complicate its use as a single-drug treatment for malaria and leishmaniasis co-infections. However, future eventual chemical derivatives with less toxicity might allow for the administration of safe, high doses capable of eliminating both pathogens in a co-infection scenario. Because drug development is expensive, entering into it with a drug that will be a good medicine for leishmaniasis and malaria would contribute to reduce the associated costs and make the process sufficiently cost-efficient to have realistic perspectives of obtaining a cure that can fit into the economic landscape of the endemic areas of these two diseases. This, in the end, will significantly contribute to their eradication.
Methods
In vitro culture of asexual forms of P. falciparum
P. falciparum parasites were grown in human O+ red blood cells (RBCs) provided by anonymous healthy donors from the UK National Health Services Blood and Transplant (NHSBT). NHSBT obtained the informed consent from donors, and the use of RBCs was performed with approval from the NHS Cambridgeshire Research Ethics Committee and the Wellcome Sanger Institute Human Materials and Data Management Committee. Prior to use, in order to discard blood material different from RBCs, these were washed twice (10 min, 400× g) with Roswell Park Memorial Institute 1640 medium (RPMI) containing L-glutamine and sodium bicarbonate (complete RPMI, RPMIc; Sigma-Aldrich, St. Louis, MO, US), and supplemented with 0.5% AlbuMAX II (Thermo Fisher Scientific, Waltham, MA, US) and 25 µg/L gentamycin (Sigma-Aldrich). After washing them, RBCs were kept at 4 °C diluted in a 1:1 proportion with RPMIc. Cultures at 3% hematocrit in RPMIc were established and maintained continuously infected with P. falciparum at parasitemias no higher than 8%. Medium was changed every other day and cultures were kept with a gas mix of 1% O2, 3% CO2 and 96% N2 at 37 °C.
In vitro selection of P. falciparum parasites resistant to YAT2150, DONE3TCl and KAE609
In vitro selection of YAT2150-, DONE3TCl- (both synthesized as previously described22) and KAE609-resistant parasites was performed on the Dd2-derived P. falciparum line Dd2-Pol∂. Triplicate cultures containing 1 × 108 or 1 × 109 parasites were initially exposed to a concentration of 3×IC50 of each drug. Culture medium supplemented with the compounds was changed every day until total parasite clearance was observed in Giemsa-stained blood smears. At this point, drug exposure was stopped and cultures were maintained in regular medium, which was renewed every two days and supplemented with fresh RBCs once a week. Cultures were grown in this way until parasite reemergence or for a maximum of 60 days. When parasites were seen again in blood smears, cultures were expanded and the IC50 values of the bulk cultures for the corresponding compounds were calculated in order to determine if resistant parasites had arisen.
As a parallel strategy, 1 × 108 parasites were exposed to progressively increasing YAT2150 or DONE3TCl concentrations starting at 0.25×IC50 or the IC50 respectively. Parasite growth was monitored by observation of Giemsa-stained blood smears every day or every other day and compound concentrations were stepwise-adjusted with the objective of not allowing total parasite clearance.
SYBR™ Green I growth determination assay
P. falciparum cultures enriched in ring stages were diluted to 1% parasitemia and 1% hematocrit and plated in duplicates in 96-well plates. To determine the IC50 of the tested compounds, they were included in the cultures (100 µL culture/well) at different concentrations, and incubated in standard culturing conditions. As growth controls were used untreated infected cultures and a 1% suspension of uninfected RBCs. After 72 h of incubation, 100 µL of lysis buffer (20 mM tris-HCl, 5 mM EDTA, 0.1% w/v saponin, and 1% v/v Triton X-100) containing SYBR™ Green I (1:10000, Invitrogen, Thermo Fisher Scientific) were added to each well and plates were incubated in the dark for 30 min. Afterwards, the fluorescence signal of each well was measured in a Synergy HTX Multi-Mode Microplate Reader (BioTek Instruments, Winooski, VT, US) by exciting the samples at 485 nm and collecting the emission at 535 nm. Growth inhibition data was transformed through sigmoidal fitting using GraphPad Prism 9 (GraphPad Software Inc., La Jolla, CA, US) and used to determine the IC50 values.
Cloning of DONE3TCl-resistant P. falciparum candidates by limiting dilution
Bulk cultures possibly resistant to DONE3TCl were diluted to 0.8 parasites/200 µL and each culture was distributed in a 96-well plate. Parasites were maintained for 17 days in standard culturing conditions, and medium containing 0.4% fresh RBCs was renewed once a week. After 17 days, parasite viability was assessed using the SYBR™ Green I assay previously described, but using in this case 50 µL of both culture (taken from each well and transferred to a new plate) and lysis buffer containing SYBR™ Green I. Cultures in wells emitting the highest fluorescence values were discarded, as they could contain a mixed population with more than one initial clone; in a similar way, cultures in wells emitting the lowest fluorescence were also discarded, as possibly no parasites were initially seeded in them. From the medium-range fluorescence emission samples, at least six clones per plate were selected and grown in standard culturing conditions for three days, when the IC50 of some of them was calculated.
Cross-resistance studies
To perform cross-resistance studies with known drug-resistance mutations, we used a set of parasite lines (Supplementary Table 1) representing a broad cross-section of mutations in current and next-generation drug targets. The 47 parasite lines, from both the Dd2 or 3D7 strain backgrounds, were derived largely from resistance evolutions by the MalDA consortium62,63,64. The parasite lines were barcoded by CRISPR editing by inserting a short 101 bp cassette into the non-essential pfpare locus65. These lines were mixed in a pool culture, with relative abundance detected by barcode sequencing as described42. The pool was plated in duplicates in 24-well plates at 1% parasitemia. YAT2150 was tested at four different concentrations: 0.25×IC50 (22.5 nM), 0.5×IC50 (45 nM), 1×IC50 (90 nM) and 3×IC50 (270 nM), and DONE3TCl was tested at three different concentrations: 0.5×IC50 (40 nM), 1×IC50 (80 nM) and 3×IC50 (240 nM); an untreated control was also included in the assay. Parasites were grown for 14 days during which medium containing the query compounds was changed every other day and parasite growth was regularly monitored by flow cytometry. For flow cytometry, 2 µL of culture was mixed in 96-well plates with 198 µL of phosphate buffered saline (PBS) containing 1× SYBR™ Green I and 200 nM MitoTracker™ Deep Red FM (Thermo Fisher Scientific). After 30 min incubation, samples were read in a CytoFLEX 5 cytometer (Beckman Coulter, Brea, CA, US) using excitation/emission values of 644/665 nm for MitoTracker™ Deep Red FM and 488/530 nm for SYBR™ Green I. If parasitemia was higher than 8%, cultures were diluted to avoid overgrowth. At day 14 (and day 0 in the case of the untreated control), cultures were transferred to 1.5-mL tubes and lysed with 0.05% saponin. Pellets were collected by centrifugation (400×g, 5 min) and washed with PBS twice in order to remove hemoglobin. After the last washing step, pellets were resuspended in 30 µL of PBS and frozen at ‒20 °C until sequencing.
Next generation sequencing
For barcoding amplification, two sequential PCRs were performed. For the first one, 5 µL of each frozen pellet were mixed with CloneAmp HIFI PCR premix (1×, Takara Bio Inc., Kusatsu, Japan) and 10 µM of each Illumina adapter-containing primers: p1356 (TCGGCATTCCTGCTGAACCGCTCTTCCGATCTGTAATTCGTGCGCGTCAG) and p1357 (ACACTCTTTCCCTACACGACGCTCTTCCGATCTCCTTCAATTTCGATGGGTAC) in a total volume of 25 µL/reaction. Five µL of each PCR product were run in a gel to check that no contamination occurred and that amplification worked. Positive PCR products were purified with Ampure beads (Beckman Coulter) following the manufacturer’s instructions and quantified with PicoGreen® (Invitrogen, P7589). Briefly, 1 µL of each purified sample was mixed with 100 µL of PicoGreen® reagent in black 96-well plates and incubated at room temperature for 2 min before reading fluorescence in a FLUOstar Omega plate reader (BMG LABTECH GmbH, Ortenberg, Germany) by exciting the samples at 480 nm and collecting the emission at 520 nm. DNA concentration was calculated after generating a standard curve using known concentrations of DNA standards. For the second PCR, 20 ng of DNA of the first PCR were mixed with CloneAmp HIFI PCR premix (1×, Takara Bio Inc.) and with 10 µM of the appropriate paired-end index primers (Nextera; Illumina, San Diego, CA, US). PCR products were purified and quantified as explained above and diluted to a final concentration of 4 nM. Samples were loaded onto an Illumina MiSeq sequencer, using a MiSeq Reagent Kit v2 (300 cycles). They were loaded at a low cluster density (< 400 k), and 50% of PhiX was spiked in, as described elsewhere for low complexity libraries66. Raw reads obtained after sequencing were separated according to their unique index tags and barcodes. Reads without an intact barcode or with the barcode flanked by the incorrect genomic context were eliminated.
P. vivax IC50 determination from clinical isolates
The ex vivo P. vivax field isolate assay was conducted at Institut Pasteur du Cambodge (Phnom Penh, Cambodia). Venous blood (7 to 8 mL) was collected by venipuncture into heparinized tubes from four symptomatic P. vivax-infected patients (P. vivax monoinfection was confirmed by species-specific PCR). After washing and depletion of host leucocytes by centrifugation and filtration (NWF single use filter) the infected red blood cell pellet was distributed into wells of a 96-well plate each containing either medium with YAT2150 in six different concentrations (10-fold dilution series from 10,000 nM to 0.1 nM), or control medium (Iscove’s Modified Dulbecco’s Medium with 5% AB+ human serum, 0.2 mM hypoxanthine, 0.18% D-glucose and 0.4% AlbuMAX) with dimethyl sulfoxide (DMSO). The cultures were incubated at 3% hematocrit under standard conditions (37 °C, 5% O2, 5% CO2) until the majority of parasites reached the mature schizont stage in the drug-free control (for 42, 39, 38 and 16 h, respectively, for the different isolates). At the end of incubation thick blood films were prepared and stained with 5% Giemsa (Merck, Germany). The number of schizonts (> 4 nuclei visible) per 200 asexual parasites (free merozoites and gametocytes excluded) was determined and normalized to the drug-free controls. IC50 values were determined using GraphPad Prism 9.
L. infantum IC50 determination from clinical isolates
L. infantum MHOM/ES/2011/LLM-1936 and MHOM/ES/2008/LLM-1689 strains, isolated from two Spanish leishmaniasis patients and belonging to the Instituto de Salud Carlos III collection, were defrosted and initially cultured on Novy-MacNeal-Nicolle medium for 2 to 3 weeks. After this primary culture, the adaptation of these strains to the laboratory culture Schneider medium consisted of at least three additional passages. In total, no more than 12 passages were generally performed before in vitro susceptibility assay. Culture of the promastigotes was performed at 27 ºC in Schneider supplemented with 10 µg/mL of hemin, 20% (v/v) heat-inactivated fetal bovine serum, and an antibiotic cocktail containing 100 U/mL penicillin and 100 µg/mL streptomycin. For IC50 value calculations, promastigotes were collected in the exponential growth phase and seeded into black, clear-bottom 384-well plates, at a density of 50,000 cells per well. The susceptibility of both strains to antileishmanial clinical drugs compared to the reference L. infantum JPC strain (MCAN/ES/98/LLM-722) was also assessed. For this purpose, a nine-point, three-fold dilution curve of YAT2150 was generated with a highest concentration of 10 µM, and 2-fold dilutions of the clinical drugs were dispensed in duplicate into the microplates containing the parasites. After an incubation period of 72 h, promastigote viability was monitored with the Alamar Blue assay (Invitrogen) according to the manufacturer’s recommendations. The antileishmanial effect of the compounds was calculated as the percentage inhibition in relation to a negative control containing 0.11% DMSO, using GraphPad Prism 9.
Shelf stability determination
A sample of 120 mg of YAT2150 was split into two vials, containing 60 mg each. One vial was stored at 25 °C without taking any measure to protect it from light and the other vial was stored in the same conditions but protecting it from light by covering it with aluminum foil. Samples of 6 mg from each vial were taken at different time points (0.5, 1.5, 2.5, and 11 months) and dissolved in 0.7 mL of CDCl3, to register the corresponding 1H NMR spectra (400 MHz, Mercury-400 spectrometer, registered at the NMR Unit of the Centres Científics i Tecnològics de la Universitat de Barcelona (CCiTUB)). The purity of the samples was measured by comparing the integration of the signal of 3(5)-H of the 4-diethylaminostyryl group of YAT2150 at δ = 6.66 ppm (d, J = 8.8 Hz) with that of an additional signal that appeared at δ = 6.61 ppm (d, J = 8.8 Hz). Additionally, the purity of the solid samples of YAT2150 kept for 10 months at 25 °C either in the dark or exposed to light was assessed by RP-HPLC (Agilent 1260 Infinity II, coupled to a photodiode array): column Poroshell 120 EC-C18 (50 × 4.6 mm, 2.7 μm); 40 °C; as mobile phase mixtures of A (0.05% formic acid in water) and B (0.05% formic acid in acetonitrile) (gradient A/B 95:5, 3 min; B, 3 min; gradient A/B 95/5, 3 min); flow rate 0.6 mL/min; and detection wavelength λ = 465 nm. Growth inhibition assays in P. falciparum cultures were performed as previously described22.
Ethical issues
All methods were carried out in accordance with the relevant ethical guidelines and regulations. The human blood used for P. falciparum cultures was commercially obtained from the Banc de Sang i Teixits (www.bancsang.net). Blood was not specifically collected for this research; the purchased units had been discarded for transfusion, usually because of an excess of blood relative to anticoagulant solution. Prior to their use, blood units underwent the analytical checks specified in the current legislation. Before being delivered to us, unit data were anonymized and irreversibly dissociated, and any identification tag or label had been removed in order to guarantee the non-identification of the donor. No personal data were or will be supplied, in accordance with the current Spanish Ley Orgánica de Protección de Datos and Ley de Investigación Biomédica and with the protocol reviewed and approved by the Ethics Committee on Drug Research from the Hospital Clínic de Barcelona (www.clinicbarcelona.org/ceim; Reg. HCB/2021/1258, February 17, 2022). The blood samples will not be used for studies other than those made explicit in this research.
Statistical analysis
Statistical differences between IC50 values were analysed by two-tailed Student’s t test using GraphPad Prism 9 software. For the barcode data statistical analysis, the DESeq2 R package67 was used. The determination of differentially represented barcodes was carried out by comparing YAT2150 and DONE3TCl samples to either the day 0 untreated control for samples that did not grow during the assay or the day 14 untreated control if they grew. A negative binomial generalized linear model was fit for significance testing with a Wald test in which a log2 fold change > 2.5 and p value ≤ 0.001 were considered significantly different.
Data availability
Data is provided within the manuscript or supplementary information files.
References
World Health Organization. World Malaria Report 2023 (Internet Communication, 2023). https://www.who.int/publications/i/item/9789240086173
Laurens, M. B. RTS,S/AS01 vaccine (MosquirixTM): an overview. Hum. Vaccin. Immunother. 16, 480–489 (2020).
World Health Organization. WHO Recommends Groundbreaking Malaria Vaccine for Children at Risk (Internet Communication, 2022).
Tizifa, T. A. et al. Prevention efforts for malaria. Curr. Trop. Med. Rep. 5, 41–50 (2018).
Ménard, D. & Fidock, D. A. Accelerated evolution and spread of multidrug-resistant Plasmodium falciparum takes down the latest first-line antimalarial drug in southeast Asia. Lancet Infect. Dis. 19, 916–917 (2019).
Mathieu, L. C. et al. Local emergence in Amazonia of Plasmodium falciparum k13 C580Y mutants associated with in vitro artemisinin resistance. eLife 9, e51015 (2020).
Stokes, B. H., Ward, K. E. & Fidock, D. A. Evidence of artemisinin-resistant malaria in Africa. N. Engl. J. Med. 386, 1385–1386 (2022).
Rosenthal, P. J. et al. The emergence of artemisinin partial resistance in Africa: how do we respond? Lancet Infect. Dis. 24, e591–e600 (2024).
Ippolito, M. M., Moser, K. A., Kabuya, J. B., Cunningham, C. & Juliano, J. J. Antimalarial drug resistance and implications for the WHO Global Technical Strategy. Curr. Epidemiol. Rep. 8, 46–62 (2021).
Wongsrichanalai, C., Pickard, A. L., Wernsdorfer, W. H. & Meshnick, S. R. Epidemiology of drug-resistant malaria. Lancet Infect. Dis. 2, 209–218 (2002).
Wicht, K. J., Mok, S. & Fidock, D. A. Molecular mechanisms of drug resistance in Plasmodium falciparum malaria. Annu. Rev. Microbiol. 74, 431–454 (2020).
Noreen, N. et al. New insights into the spread of resistance to artemisinin and its analogues. J. Glob. Antimicrob. Resist. 27, 142–149 (2021).
World Health Organization. World Malaria Report 2021 (Internet Communication, 2021). https://www.who.int/teams/global-malaria-programme/reports/world-malaria-report-2021
White, N. J. Triple artemisinin-containing combination anti-malarial treatments should be implemented now to delay the emergence of resistance. Malar. J. 18, 338 (2019).
Price, R. N., Commons, R. J., Battle, K. E., Thriemer, K. & Mendis, K. Plasmodium vivax in the era of the shrinking P. falciparum map. Trends Parasitol. 36, 560–570 (2020).
Leishmania: an urgent need for new treatments. EBioMedicine 87, 104440 (2023).
Ornellas-Garcia, U., Cuervo, P. & Ribeiro-Gomes, F. L. Malaria and leishmaniasis: updates on co-infection. Front. Immunol. 14, 1122411 (2023).
Ponte-Sucre, A. et al. Drug resistance and treatment failure in leishmaniasis: a 21st century challenge. PLoS Negl. Trop. Dis. 11, e0006052 (2017).
Sundar, S. & Singh, B. Emerging therapeutic targets for treatment of leishmaniasis. Expert Opin. Ther. Targets 22, 467–486 (2018).
Hefnawy, A. et al. Importance of secondary screening with clinical isolates for anti-leishmania drug discovery. Sci. Rep. 8, 11765 (2018).
Burrows, J. N. et al. New developments in anti-malarial target candidate and product profiles. Malar. J. 16, 26 (2017).
Bouzón-Arnáiz, I. et al. The protein aggregation inhibitor YAT2150 has potent antimalarial activity in Plasmodium falciparum in vitro cultures. BMC Biol. 20, 197 (2022).
Espargaró, A., Pont, C., Gamez, P., Muñoz-Torrero, D. & Sabate, R. Amyloid pan-inhibitors: one family of compounds to cope with all conformational diseases. ACS Chem. Neurosci. 10, 1311–1317 (2019).
Halfmann, R. et al. Opposing effects of glutamine and asparagine govern prion formation by intrinsically disordered proteins. Mol. Cell 43, 72–84 (2011).
Perutz, M. F., Finch, J. T., Berriman, J. & Lesk, A. Amyloid fibers are water-filled nanotubes. Proc. Natl. Acad. Sci. U. S. A. 99, 5591–5595 (2002).
DePristo, M. A., Zilversmit, M. M. & Hartl, D. L. On the abundance, amino acid composition, and evolutionary dynamics of low-complexity regions in proteins. Gene 378, 19–30 (2006).
Romero, P. et al. Sequence complexity of disordered protein. Proteins 42, 38–48 (2001).
Pallarès, I. et al. Discovering putative prion-like proteins in Plasmodium falciparum: a computational and experimental analysis. Front. Microbiol. 9, 1737 (2018).
Biosca, A. et al. Detection of protein aggregation in live Plasmodium parasites. Antimicrob. Agents Chemother. 64, e02135–e02119 (2020).
Camarero-Hoyos, C. et al. Leveraging the aggregated protein dye YAT2150 for malaria chemotherapy. Pharmaceutics 16, 1290 (2024).
Kabeche, S., Meister, T. & Yeh, E. A fast-acting inhibitor of blood-stage P. falciparum with mechanism distinct from artemisinin and chloroquine. bioRxiv (2024).
Román-Álamo, L. et al. Effect of the aggregated protein dye YAT2150 on Leishmania parasite viability. Antimicrob. Agents Chemother. 68, e0112723 (2024).
Nguyen-Dinh, P. & Trager, W. Chloroquine resistance produced in vitro in an African strain of human malaria. Science 200, 1397–1398 (1978).
Barnes, D. A., Foote, S. J., Galatis, D., Kemp, D. J. & Cowman, A. F. Selection for high-level chloroquine resistance results in deamplification of the pfmdr1 gene and increased sensitivity to mefloquine in Plasmodium falciparum. EMBO J. 11, 3067–3075 (1992).
Sidhu, A. B. et al. In vitro efficacy, resistance selection, and structural modeling studies implicate the malarial parasite apicoplast as the target of azithromycin. J. Biol. Chem. 282, 2494–2504 (2007).
Baragaña, B. et al. A novel multiple-stage antimalarial agent that inhibits protein synthesis. Nature 522, 315–320 (2015).
Stokes, B. H. et al. Covalent Plasmodium falciparum-selective proteasome inhibitors exhibit a low propensity for generating resistance in vitro and synergize with multiple antimalarial agents. PLoS Pathog. 15, e1007722 (2019).
Rottmann, M. et al. Spiroindolones, a potent compound class for the treatment of malaria. Science 329, 1175–1180 (2010).
Kümpornsin, K. et al. Generation of a mutator parasite to drive resistome discovery in Plasmodium falciparum. Nat. Commun. 14, 3059 (2023).
Siriwardana, A., Iyengar, K. & Roepe, P. D. Endoperoxide drug cross-resistance patterns for Plasmodium falciparum exhibiting an artemisinin delayed-clearance phenotype. Antimicrob. Agents Chemother. 60, 6952–6956 (2016).
Cowman, A. F., Galatis, D. & Thompson, J. K. Selection for mefloquine resistance in Plasmodium falciparum is linked to amplification of the pfmdr1 gene and cross-resistance to halofantrine and quinine. Proc. Natl. Acad. Sci. U. S. A. 91, 1143–1147 (1994).
Carrasquilla, M. et al. Barcoding genetically distinct Plasmodium falciparum strains for comparative assessment of fitness and antimalarial drug resistance. mBio 13, e0093722 (2022).
Katsuno, K. et al. Hit and lead criteria in drug discovery for infectious diseases of the developing world. Nat. Rev. Drug Discov. 14, 751–758 (2015).
Schäfer, T. M., Pessanha de Carvalho, L., Inoue, J., Kreidenweiss, A. & Held, J. The problem of antimalarial resistance and its implications for drug discovery. Expert Opin. Drug Discov. 19, 209–224 (2024).
Ahouidi, A. et al. An open dataset of Plasmodium falciparum genome variation in 7,000 worldwide samples. Wellcome Open. Res. 6, 42 (2021).
Llinás, M., Bozdech, Z., Wong, E. D., Adai, A. T. & DeRisi, J. L. Comparative whole genome transcriptome analysis of three Plasmodium falciparum strains. Nucleic Acids Res. 34, 1166–1173 (2006).
Zhan, W. et al. Development of a highly selective Plasmodium falciparum proteasome inhibitor with anti-malaria activity in humanized mice. Angew Chem. Int. Ed. Engl. 60, 9279–9283 (2021).
Everson, N. et al. Identification of Plasmodium falciparum heat shock 90 inhibitors via molecular docking. Bioorg. Med. Chem. Lett. 35, 127818 (2021).
Xie, S. C. et al. Design of proteasome inhibitors with oral efficacy in vivo against Plasmodium falciparum and selectivity over the human proteasome. Proc. Natl. Acad. Sci. U. S. A. 118, e2107213118 (2021).
Cowell, A. N. & Winzeler, E. A. The genomic architecture of antimalarial drug resistance. Brief. Funct. Genomics 18, 314–328 (2019).
Anstey, N. M. et al. The biology and pathogenesis of vivax malaria. Trends Parasitol. 40, 573–590 (2024).
Nguyen, T. N. et al. The persistence and oscillations of submicroscopic Plasmodium falciparum and Plasmodium vivax infections over time in Vietnam: an open cohort study. Lancet Infect. Dis. 18, 565–572 (2018).
Poespoprodjo, J. R., Douglas, N. M., Ansong, D., Kho, S. & Anstey, N. M. Malaria. Lancet 402, 2328–2345 (2023).
Battle, K. E. & Baird, J. K. The global burden of Plasmodium vivax malaria is obscure and insidious. PLoS Med. 18, e1003799 (2021).
Phyo, A. P., Dahal, P., Mayxay, M. & Ashley, E. A. Clinical impact of vivax malaria: a collection review. PLoS Med. 19, e1003890 (2022).
Barber, B. E. et al. Parasite biomass-related inflammation, endothelial activation, microvascular dysfunction and disease severity in vivax malaria. PLoS Pathog. 11, e1004558 (2015).
Román-Álamo, L. et al. In vitro evaluation of aerosol therapy with pentamidine-loaded liposomes coated with chondroitin sulfate or heparin for the treatment of leishmaniasis. Pharmaceutics 15, 1163 (2023).
Martínez-Orellana, P., Baxarias, M., Good, L. & Solano-Gallego, L. The effects of polyhexamethylene biguanide (PHMB) and TLR agonists alone or as polyplex nanoparticles against Leishmania infantum promastigotes and amastigotes. Vet. Sci. 7, 179 (2020).
Hendrickx, S. et al. Transmission potential of paromomycin-resistant Leishmania infantum and Leishmania donovani. J. Antimicrob. Chemother. 75, 951–957 (2020).
Ganguly, S., Bandyopadhyay, S., Sarkar, A. & Chatterjee, M. Development of a semi-automated colorimetric assay for screening anti-leishmanial agents. J. Microbiol. Methods 66, 79–86 (2006).
Amato, V. S. et al. Successful treatment of cutaneous leishmaniasis with lipid formulations of amphotericin B in two immunocompromised patients. Acta Trop. 92, 127–132 (2004).
Corey, V. C. et al. A broad analysis of resistance development in the malaria parasite. Nat. Commun. 7, 11901 (2016).
Cowell, A. N. et al. Mapping the malaria parasite druggable genome by using in vitro evolution and chemogenomics. Science 359, 191–199 (2018).
Forte, B. et al. Prioritization of molecular targets for antimalarial drug discovery. ACS Infect. Dis. 7, 2764–2776 (2021).
Istvan, E. S. et al. Esterase mutation is a mechanism of resistance to antimalarial compounds. Nat. Commun. 8, 14240 (2017).
Gomes, A. R. et al. A genome-scale vector resource enables high-throughput reverse genetic screening in a malaria parasite. Cell Host Microbe 17, 404–413 (2015).
Love, M. I., Huber, W. & Anders, S. Moderated estimation of fold change and dispersion for RNA-seq data with DESeq2. Genome Biol. 15, 550 (2014).
Acknowledgements
This work was supported by grants (i) PID2021-128325OB-I00 and PDC2022-133085-I00 (XF-B), PID2020-118127RB-I00 (DM-T) and FJC2021-047571-I (AM-A), funded by Ministerio de Ciencia, Innovación y Universidades/Agencia Estatal de Investigación (MCIU/AEI/ https://doi.org/10.13039/501100011033), which included FEDER and European Union NextGenerationEU/PRTR funds; and (ii) Generalitat de Catalunya, Spain (http://agaur.gencat.cat/), grant numbers 2021-SGR-00635 (XF-B) and 2021-SGR-00357 (DM-T). MCSL gratefully acknowledges funding from the Bill and Melinda Gates Foundation (OPP1054480). JP is supported by the NIH (grants R01AI175134 and R01AI173171). ISGlobal and IBEC are members of the CERCA Programme, Generalitat de Catalunya. We acknowledge support from MCIU/AEI through the “Centro de Excelencia Severo Ochoa 2019–2023” Program (CEX2018-000806-S). This research is part of ISGlobal’s Program on the Molecular Mechanisms of Malaria which is partially supported by the Fundación Ramón Areces. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Author information
Authors and Affiliations
Contributions
IB-A performed resistance studies, with input from MR and RC and supervision from MCSL. LBF-D, ME and AO performed P. vivax assays with supervision from JP. BD-A and JM performed L. infantum assays, with input from LR-A. EMA and AM-A performed chemical synthesis with supervision from DM-T. ANF performed P. falciparum growth inhibition assays with supervision from XF-B. XF-B conceived the project with key input from IB-A. XF-B and IB-A wrote the manuscript with input from all authors.
Corresponding author
Ethics declarations
Competing interests
A patent application (priority number: EP21382949.2; application number: PCT/EP2022/079438; application date: 21/10/2022) has been filed to protect some of the results presented in the paper, which includes as inventors IB-A, EMA, DM-T and XF-B. All other authors declare they have no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Bouzón-Arnáiz, I., Rawat, M., Coyle, R. et al. YAT2150 is irresistible in Plasmodium falciparum and active against Plasmodium vivax and Leishmania clinical isolates. Sci Rep 15, 2941 (2025). https://doi.org/10.1038/s41598-025-85346-y
Received:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s41598-025-85346-y