Abstract
The heat shock protein 90 (Hsp90) family of molecular chaperones mediates the folding and activation of ~ 400 client proteins, many of which contribute to oncogenesis. As a result, Hsp90 pan-inhibitors, which inhibit all four Hsp90 isoforms, have been investigated in the clinic for the treatment of cancer. Unfortunately, detrimental side effects were observed and hindered the clinical development of pan-Hsp90 inhibitors. The two most common on-target toxicities, cardio-toxicity and ocular-toxicity, have been attributed to inhibition of the Hsp90α isoform. As an alternative strategy, Hsp90β-selective inhibitors have been developed, which have shown promising anti-cancer activity in vitro and in vivo in combination with immune-checkpoint blockade therapy. This study aims to assess the potential risks of cardio-toxicity and ocular-toxicity exhibited by Hsp90β-selective inhibitors in vitro. In summary, the Hsp90β-selective NDNB1182 was found to avoid the cardio- and ocular-toxicity typical of Hsp90 pan-inhibitors (e.g. 17-AAG), providing a promising path toward the generation of isoform-selective Hsp90 inhibitors.
Similar content being viewed by others
Introduction
The 90 kDa heat shock protein (Hsp90) family are highly evolutionarily conserved and ubiquitously expressed molecular chaperones that become highly induced to regulate protein homeostasis in response to cellular stresses1,2. Hsp90 family members consist of cytosolic Hsp90α (inducible) and Hsp90β (constitutive), endoplasmic reticulum localized glucose-regulated protein 94 (Grp94), and mitochondrial tumor necrosis factor receptor-associated protein-1 (Trap1). Many of the > 400 client proteins that depend upon Hsp90 for folding, disaggregation, and trafficking have been associated with all ten hallmarks of cancer, which has made Hsp90 a highly sought after drug target for cancer3,4. In fact, there have been 22 small molecule ATP-competitive Hsp90 N-terminal domain (NTD) pan-inhibitors evaluated in clinical trials for the treatment of cancer that simultaneously target several oncogenic pathways5. However, the inhibition of all four isoforms of Hsp90 has presented challenges in terms of dosing and toxicity, resulting in less than favorable efficacy and safety profiles. Consequently, most of these investigational drugs have failed or are likely to fail in the clinical setting.
Inhibition of Hsp90’s NTD induces phosphorylation of heat shock factor 1 (HSF1), activation of the heat shock response (HSR), and subsequent increased expression of heat shock proteins such as Hsp90α and Hsp70. Increased levels of Hsp90α decreases the overall activity of Hsp90 inhibitors6. As a result, Hsp90 NTD inhibitors often require escalating doses which can result in dose-limiting toxicities. The two most common on-target concerns of Hsp90 pan-inhibition are ocular- and cardio-toxicity. Hsp90 pan-inhibitors such as Geldanamycin (17-AAG) have been observed to be toxic to human retinal pigment epithelial cells in vitro7. Ocular toxicities were also noted in vivo in beagle dogs in response to two structurally diverse Hsp90 inhibitors, CH5164840 and CH54493028. Notably, Kanamaru et al. observed visual disorders including electroretinography loss of pupillary light reflex and histopathological changes in the photoreceptor cell layer and the outer nuclear layer of the retina8. A mechanistic study of AUY922-induced retinal-toxicity was performed by Shen et al. and found that the Transient Receptor Potential cation channel subfamily M member 1 (TRPM1) is a client protein of Hsp90, providing rationale for Hsp90 inhibitor-induced apoptosis, ROS production, and growth inhibition in retinal cells9. Similar retinal damage was observed by Zhou et al. in a rat model, however, the damage was correlated with Hsp90 inhibitor retinal concentration and a lack of drug elimination from the retina10. Therefore, it appears that the pharmacokinetic profile of an Hsp90 inhibitor may significantly impact whether ocular toxicities occur in vivo11,12. The only Hsp90 inhibitor to be approved for clinical use has been TAS-116, which was recently approved in Japan for the treatment of gastrointestinal cancer and has been shown to avoid ocular-toxicity in vivo namely due to a lack of ocular accumulation as compared to other Hsp90 inhibitors11. Despite the potential to avoid Hsp90 inhibition-induced ocular-toxicity, nearly all of the clinical trials involving Hsp90 inhibitors have cited incidents of photopsia, night blindness, flashing lights, blurred vision, or other visual complications13,14,15,16,17,18,19,20.
Cardio-toxicity has been another major concern as a consequence of dose-limiting on-target toxicity. Hsp90 inhibitors were found to disrupt human ether-a-go-go (hERG) channel maturation and IKr function dose-dependently without disrupting other voltage-gated potassium channels, such as IKs. The hERG channel is the α subunit of the cardiac potassium current IKr channel, which plays a critical role in maintaining cardiac action potential and has been found to be dependent on Hsp70 and Hsp90 for assembly into the fully glycosylated and active form21. It was later determined that hERG maturation is solely dependent upon the Hsp90α isoform22. For the most part, cardiac issues associated with the clinical investigation of Hsp90 pan-inhibitors have resulted in grade 1–2 severity. Examples of reported effects include temporary QTcF of greater than 450 ms with normal electrocardiogram recordings, increase in cardiac troponins, cardiac flutter, and Tachycardia13,14,15,16,17,18,19,20. Termination of treatment returned cardiac function to basal levels with no observable permanent damage.
In contrast to pan-Hsp90 inhibitors, the development of Hsp90β-selective inhibitors should avoid the complications associated with Hsp90α inhibition and avoid induction of the heat shock response, which causes the need for dose escalation and dose-limiting toxicities. Hsp90α is the inducible isoform, and cancers commonly overexpress Hsp90α to adapt to the stress of tumorigenesis23,24. In contrast, Hsp90β is constitutively expressed and acts as a “housekeeping” chaperone to ensure essential functions25. The rationale that made Hsp90 a promising drug target was aimed at inhibiting Hsp90α’s role in aiding cancer progression, however, Hsp90α and Hsp90β share a significant number of client proteins, which appear to serve an intrinsic redundancy. Therefore, Hsp90β-selective inhibitors may inhibit the shared pathways regulated by both Hsp90α and Hsp90β (e.g. response to stimuli, protein phosphorylation, cell communication) as well as the distinct processes uniquely regulated by Hsp90β (e.g. protein ubiquitination, cytoskeleton organization, and protein catabolism)25.
A structure-based molecular design and optimization approach was employed by Blagg and colleagues to develop Hsp90β-selective inhibitors26,27. The best Hsp90β-selective inhibitor at the time of this study was NDNB1182, which exhibits an affinity of 65 nM for Hsp90β and > 10 µM for Hsp90α (> 150-fold selective). NDNB1182 was observed to enhance immune checkpoint blockade therapy in vivo, resulting in a significant reduction in tumor volume and an increase in survival rate without significant effects on body weight28. Furthermore, NDNB1182 treatment had no discernable histological differences in the liver or the spleen, suggesting that efficacy is achievable at non-toxic doses. Therefore, the purpose of this study is to determine whether Hsp90β-selective inhibitors (NDNB1182) avoid the cardio and ocular-toxicity exemplified by Hsp90 pan-inhibitors in vitro, which can provide a mechanistic understanding for future in vivo studies.
Results
Clyde Bioscience’s Human Induced Pluripotent Stem Cell (hiPSC)-Derived Cardiomyocytes Voltage Sensitive Dye System and the CellOPTIQ platform was used to collect contraction and electrical activity parameters; cycle length, action potential duration of 30% (APD30), 50% (APD50), or 90% (APD90), rise time (TRise from 10 to 90% of action potential), triangulation, diastolic interval, contraction amplitude (CtAmp), contraction rise time (CtTrise), and contraction duration at 50% amplitude (CtD50). In addition to the Hsp90β-selective inhibitor NDNB1182 and the Hsp90 pan-inhibitor 17-AAG, Dofetilide was used as an FDA-approved anti-arrhythmia positive control, and Doxorubicin was used as an FDA-approved anti-cancer negative control. Four doses of the Hsp90 inhibitors and one dose of the control compounds were tested at 0.5, 24, and 48 h to elucidate time-dependent effects on cardiomyocyte function.
Human Induced Pluripotent Stem Cell (hiPSC)-Derived Cardiomyocytes Voltage Sensitive Dye System contraction and electrical activity parameters after a 30 min incubation with (A) NDNB1182, (B) 17-AAG, (C) Dofetilide, or (D) Doxorubicin. Data are presented as mean ± SEM (N = 8). Statistical data are based on differences between absolute values and recorded as: *p ≤ 0.05, **p ≤ 0.01, ***p ≤ 0.001.
After a 30 min incubation with NDNB1182 (Fig. 1A), minor but noteworthy effects were detected. A slight increase in APD90 suggests mild IKr inhibition between 0.25 and 2.5 µM NDNB1182. An insignificant change in APD30 and APD50 supports this hypothesis and likely explains the observed increase in triangulation. However, the change in APD90 is less than a 20% increase from basal levels, which suggests there is a low/low-moderate risk of torsades de pointes (TdP). A minor decrease in contraction amplitude (CtAmp) and prolongation of contraction rise time (CtTrise) were observed. However, there was no effect on contraction duration (CtD50) even at 50 µM NDNB1182. This may be explained by effects on cellular contractility or adhesion to the matrix. In contrast, potential complications of Hsp90 pan-inhibition by 17-AAG was apparent even at 30 min (Fig. 1B). Minor changes in APD30 and ADP90 suggest a similar mild IKr inhibition at 2.5 µM 17-AAG. However, a progressive increase in rise time (TRise) and quiescence (8/8 wells) at 10 µM 17-AAG suggests INa inhibition > 2.5 µM 17-AAG and a potential concern. 3 nM Dofetilide produced characteristic changes in APD due to selective IKr inhibition and subsequent early after depolarization (EAD) events (5/8 wells) (Fig. 1C). 0.3 µM Doxorubicin largely had no effect on electrophysiology apart from electrical quiescence in 1/8 wells, a decreased contraction amplitude (CtAmp), and a moderately impaired contraction rise time (CtTrise) (Fig. 1D).
Human Induced Pluripotent Stem Cell (hiPSC)-Derived Cardiomyocytes Voltage Sensitive Dye System contraction and electrical activity parameters after a 24 h incubation with (A) NDNB1182, (B) 17-AAG, (C) Dofetilide, or (D) Doxorubicin. Data are presented as mean ± SEM (N = 8). Statistical data are based on differences between absolute values and recorded as: *p ≤ 0.05, **p ≤ 0.01, ***p ≤ 0.001.
Markedly differential responses between compounds were observed after incubation for 24 h, highlighting the previously disclosed cardio-toxicity manifested by Hsp90 pan-inhibitors. The contraction and electrical activity remained largely unchanged in response to NDNB1182 after 24 h (Fig. 2A). A slight increase of APD90 and an increase in triangulation were again observed with the addition of a slight increase in diastolic intervals. Therefore, there is a low/low-moderate risk of TdP. Criteria for assessing the degree of TdP risk followed the recommendations of Blinova et al.29. Of note, a significantly higher contraction amplitude (CtAmp) was observed at 50 µM NDNB1182. However, contraction rise time (CtTrise) and contraction duration (CtD50) were unaffected. Therefore, the increased contraction may be related to changes in monolayer structure or possibly NDNB1182 solubility issues (based on prior data) at 50 µM affecting contractility video software. Overall, the results do not indicate a significant risk of cardio-toxicity at 10 µM NDNB1182 and potentially up to 50 µM, at which point, complications could be attributed to a lack of solubility. In contrast, severe dysregulation of cardiomyocyte function was observed in response to 17-AAG after 24 h (Fig. 2B). A marked decrease in spontaneous cycle length with an increase in spontaneous frequency was observed and measured by nearly every parameter collected. Additionally, 17-AAG induced quiescence beginning at 2.5 µM (1/8 wells) and complete quiescence (8/8 wells) at 10 µM and 50 µM. A progressive induction of quiescence with a significant decrease in diastolic interval and contraction duration (CtD50) at 2.5 µM 17-AAG are in agreement with APD shortening. 3 nM Dofetilide produced characteristic increased potency of IKr block after 24 h with early after depolarization (EAD) events (8/8 wells) (Fig. 2C). 0.3 µM Doxorubicin largely had no effect on electrophysiology after 24 h and no notable effects on contraction, consistent with its approval as a safe FDA-approved chemotherapeutic (Fig. 2D).
Human Induced Pluripotent Stem Cell (hiPSC)-Derived Cardiomyocytes Voltage Sensitive Dye System contraction and electrical activity parameters after a 48 h incubation with (A) NDNB1182, (B) 17-AAG, (C) Dofetilide, or (D) Doxorubicin. Data are presented as mean ± SEM (N = 8). Statistical data are based on differences between absolute values and recorded as: *p ≤ 0.05, **p ≤ 0.01, ***p ≤ 0.001.
Results were consistent between 24- and 48-hour treatments for all compounds. NDNB1182 had functionally the same effect after 48 h as described above after 24 h (Fig. 3A). The negative effects in response to 17-AAG were present and slightly more exacerbated after 48 h. For example, 17-AAG induced quiescence beginning at 0.25 µM (1/8 wells), an increased rate at 2.5 µM (6/8 wells) and complete quiescence (8/8 wells) at 10 µM and 50 µM (Fig. 3B). All other effects were similar to the 24-hour treatment with the exception of a larger decrease in contraction duration (CtD50). 3 nM Dofetilide produced characteristic effects of IKr inhibition after 48 h with less depolarization (EAD) events (1/8 wells) (Fig. 3C). A decreased cycle length, APD30, and APD50 were observed in response to 0.3 µM Doxorubicin after 48 h, although there was a lesser effect on APD90 (Fig. 3D). Accounting for the decreased cycle length suggests that there may be an underlying mild inhibition of IKr or a potential combination of IKr and ICa, L block by Doxorubicin. The only notable effect on contraction by Doxorubicin was a decreased contraction duration (CtD50).
Voltage Sensitive Dye System contraction and electrical activity parameter summary using CellOPTIQ software after (A) 30 min, (B) 24 h, or (C) 48 h. Example cardiac action potential plots are shown after 48 h treatment with (D) 0.25 µM or (E) 2.5 µM NDNB1182 and 17-AAG. Data are presented as mean ± SEM (N = 8).
Summary data from the Cardiomyocytes Voltage Sensitive Dye System and results from analysis using CellOPTIQ software can be viewed in Fig. 4A-C. Criteria for assessing the degree of TdP risk followed the recommendations of Blinova et al.29 Consolidation of the data highlights that 17-AAG exhibits a significantly high Excitability score and concern for toxicity/dysregulation of cardiac function, which is dose and time-dependent. In contrast, NDNB1182 caused very few and minor changes in activity even at 50 µM. Dofetilide produced no significant Excitability or cause for concern but a significant TdP Score, which is characteristic. Doxorubicin produced near baseline activity, supporting its avoidance of adverse effects specifically on contraction and electrical activity parameters. In conclusion, selective inhibition of Hsp90β and avoidance of Hsp90α inhibition appears to avoid the unintended cardiotoxicity associated with Hsp90 pan-inhibitors as evidenced by 17-AAG. This is further exemplified by example cardiac action potential plots of cardiomyocytes treated with 0.25 µM 17-AAG and NDNB1182 (Fig. 4D) and 2.5 µM 17-AAG and NDNB1182 (Fig. 4E) after 48 h. Although the data is promising, two things remain unclear from the results: Does NDNB1182 affect the cell monolayer (especially at 50 µM), and is NDNB1182 cytotoxic to cardiomyocytes?
Two assays were performed to assess the monolayer and toxicity concerns of NDNB1182. First, an assessment of the cardiomyocyte monolayer maintenance was determined and termed “Cell health index” (Fig. 5A). Compared to 17-AAG, NDNB1182 manifested a significantly less destructive effect to the cell monolayer and produced small-moderate holes at ≥ 10 µM after 48 h. However, Doxorubicin produced greater monolayer damage at both 24 and 48 h. Secondly, to determine whether NDNB1182’s effect on the monolayer was a concern for toxicity, an extracellular LDH assay was performed after a 48-hour treatment. Compared to 17-AAG, which was highly dose-dependently toxic, NDNB1182 resulted in only a slight increase in extracellular LDH, even at 50 µM, providing evidence of an avoidance of dose-escalating toxicity. This data suggests that NDNB1182 is no more disruptive to the cardiomyocyte monolayer nor toxic than Doxorubicin. Additionally, these results suggest that the previously mentioned increased contraction in response to 50 µM NDNB1182 at 24 and 48 h was likely due to solubility issues.
As previously discussed, maturation of the hERG channel is dependent upon the Hsp90α isoform22. The effects observed in cardiomyocytes support that NDNB1182 avoids the disruption of hERG channel function, except potentially at 50 µM. Therefore, a hERG fluorescence polarization (FP) assay was performed to determine whether NDNB1182 inhibits the hERG channel directly (Fig. 6). NDNB1182 manifested a 41.47 µM IC50 against the hERG channel, which agrees with the cardiomyocyte data. In contrast, 17-AAG exhibited a 30.86 µM hERG IC50, which is significantly higher than the doses that cause cardio-toxicity and disrupt IKr function in the cardiomyocyte experiments. These results indicate that Hsp90β-selective inhibitors avoid disruption of hERG function except at concentrations where the hERG channel is directly inhibited, as opposed to pan-inhibitor 17-AAG, which has been shown to disrupt hERG channel maturation at much lower concentrations30. Therefore, direct inhibition of the hERG channel may be a more limiting factor for Hsp90β-selective inhibitors than toxicity to cardiac cells, although the hERG IC50 is ~ 100-fold higher than the therapeutic concentration in vitro.
A hERG fluorescence polarization assay was performed in a 1:3 serial dilution in quadruplicate wells. The top was constrained to the untreated (tracer fully bound) negative control wells and the bottom to the 30 µM E-4031 (fully displaced) positive control wells represented as dashed lines. Polarization values are reported as the Avg ± SD with N = 4 technical replicates.
As previously mentioned, another unintended side-effect of Hsp90 pan-inhibition is ocular-toxicity. ARPE-19 human retinal cells were used to assess whether Hsp90β-selective inhibitors avoid the ocular-toxicity exhibited by Hsp90 pan-inhibitors. ARPE-19 cells were treated for 48 h, and cell viability (SI) and growth (Fig. 7) were determined by an MTS assay. Hsp90 pan-inhibitors NVP AUY922 and 17-AAG both exhibited potent anti-proliferation with IC50 values < 0.01 µM and 0.02 µM, respectively. In contrast, Hsp90α and Hsp90β-selective TAS-116 resulted in an IC50 of 0.444 µM. Hsp90β-selective NDNB1182 was comparable to TAS-116 with an IC50 of about 0.458 µM. Although the results suggest that all the inhibitors exhibit some ocular-toxicity in vitro, the results are still promising. Compared to previous Hsp90 pan-inhibitors, TAS-116 was found to avoid any ocular toxicities in vivo11. Since NDNB1182 had nearly identical effects on retinal viability and growth as compared to TAS-116 in vitro, the data suggest NDNB1182 would similarly avoid the unintended ocular-toxicity observed in response to Hsp90 pan-inhibition. However, it is worth noting that the study by Ohkubo et al. attributed the avoidance of TAS-116 ocular-toxicity primarily to a lack of accumulation within the eye11. The results obtained in this in vitro study provide encouragement for the avoidance of one of the primary concerns of Hsp90 inhibitors.
MTS cell viability assay to assess toxicity against ARPE-19 human retinal cells via measurement of cell growth after 48 h. An example bar graph is shown with the Avg ± SD from N = 3 replicate wells from one of the N = 3 biological replicate assays. IC50 values are reported as the Avg ± SD from N = 3 biological replicates.
Another advantage of avoiding Hsp90α inhibition is the lack of Hsp90 induction, which has been found to confer resistance to Hsp90 pan-inhibitors31. It has been previously shown that Hsp90β-selective inhibitors do not induce Hsp90 levels. In addition, HSF1 and Hsp27 levels were not induced in response to Hsp90β-selective inhibition, but rather dose-dependently decreased against HCT-116 colorectal cancer cells27. NDNB1182 similarly avoided HSF induction in a MC3 mouse colorectal cancer cell line28. Hsp90α and Hsp70 levels were measured in response to NDNB1182 treatment against normal MCF-10A cells and triple-negative breast cancer cell lines MDA-MD-231 and MDA-MB-468 (Fig. 8).
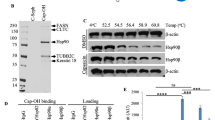
Western blots of MCF-10A, MDA-MB-231, and MDA-MB-468 cells after 24-hour treatment with NDNB1182. 100 nM pan-inhibitor AUY922 and 0.1% DMSO were used as positive and negative controls. Hsp90β-selective inhibitor NDNB1182 induces expression of Hsp70 in normal (MCF-10A) and triple-negative breast cancer (MDA-MB-231, MDA-MB-468). However, a significant induction of Hsp90α was only observed in the normal MCF-10A cells and not in either of the breast cancer cell lines. Bands were derived from the same experiment and duplicate blots that were run in parallel.
NDNB1182 was shown to induce Hsp70 in all three cell lines tested. However, an induction of Hsp90α was only observed in normalized MCF-10A cells and not in either breast cancer cell line. This suggests that normal cells may confer an innate resistance to Hsp90β inhibitors32. Furthermore, Hsp90β is not stress-inducible, so dose-escalation is likely not required for effectiveness. These results are consistent with previous findings and highlight that Hsp90β-selective inhibitors exhibit a differential effect on the HSR as compared to Hsp90 pan-inhibitors26,27. The current hypothesis is that Hsp90 ATP-competitive inhibitors disrupt the Hsp90-HSF1 interaction, HSF1 is activated, inducing the expression of heat shock proteins such as Hsp90α and Hsp70, and then Hsp90α represses HSF1 through a negative feedback loop33,34. The field’s current understanding of the underlying mechanisms of the HSR requires further investigation, as it is likely a more complicated regulation of the HSR potentially involving HSF2 or the fact that HSF1 exhibits a higher relative affinity for Hsp90α than Hsp90β33,35. An examination of this mechanism is outside the scope of this study.
Discussion
Hsp90β-selective inhibitors have arisen as an alternative NTD-targeted strategy to avoid the complications that ATP-competitive Hsp90 pan-inhibitors faced in the clinical setting, which have primarily been attributed to the inhibition of Hsp90α. Inhibition of other Hsp90 domains such as the use of Hsp90 C-terminal inhibitors (e.g. KU-174) and Hsp90 protein-protein interaction inhibitors (e.g. Conglobatin, Celastrol) are alternatives that avoid activation of the HSR36,37. The Hsp90β-selective inhibitor NDNB1182 was used to assess whether the two most reported on-target safety concerns, cardio- and ocular-toxicity, would be prevalent despite a lack of Hsp90α inhibition. A Human Induced Pluripotent Stem Cell (hiPSC)-Derived Cardiomyocytes Voltage Sensitive Dye System provided contraction and electrical activity data that demonstrates NDNB1182 avoids severe dysregulation of cardiomyocyte function when compared to Hsp90 pan-inhibitor, 17-AAG. Furthermore, and in contrast to 17-AAG, NDNB1182 did not impact maintenance of the cardiomyocyte cell monolayer or induce toxicity as determined by an extracellular LDH assay. The cardiomyocyte data suggested that a potential mild inhibition of IKr may result at 50 µM NDNB1182. As maturation of the hERG potassium ion channel has been found to rely solely upon Hsp90α, NDNB1182’s direct inhibition of hERG was assessed via an FP assay. NDNB1182 exhibited an IC50 of 41.47 µM for the hERG channel, suggesting that direct hERG inhibition could be a limiting factor for this scaffold. The potential for ocular-toxicity was assessed using an MTS cell viability assay in ARPE-19 human retinal cells. NDNB1182 exhibited an IC50 of 0.458 ± 0.095 µM, which was comparable to TAS-116 (0.444 ± 0.15 µM) and > 20-fold and > 40-fold higher than Hsp90 pan-inhibitors 17-AAG and AUY922, respectively. Furthermore, TAS-116, an Hsp90α and β-selective inhibitor, has manifested fewer ocular detriments in the clinic38. Therefore, Hsp90β-selective inhibitors are expected to avoid ocular-toxicity in vivo due to their comparable activity. NDNB1182 also avoids activation of the heat shock response in agreement with previous Hsp90β-selective inhibitors, thereby avoiding the necessity for dose-escalation and subsequently unintended off-target effects. In conclusion, this study provides promising in vitro support for Hsp90β-selective inhibitors as a safer alternative to Hsp90 pan-inhibitors and encourages further advancement of Hsp90β-selective inhibitors for the treatment of various diseases.
Experimental methods
Cell cultures
ARPE-19, MCF-10A, MDA-MB-231, and MDA-MB-468 cells were grown in a water jacketed incubator at 37 °C with 5% CO2. ARPE-19 cells were cultured in DMEM-F-12 media supplemented with 10% HI FBS (Gibco, 10438-026) and 1% Pen-Strep (VWR, K952-100ML). MDA-MB-231 and MDA-MB-468 cells were cultured in DMEM media with the same HI FBS and Pen-Strep supplements. MCF-10A cells were cultured in DMEM/F-12 K + GluMAX, 5% Horse Serum, 1% Pen-Strep, 20 ng/mL EGF, 0.5 mg/mL hydrocortisone, 100 ng/mL cholera toxin, and 10 µg/mL insulin (All growth factors were ordered from Gibco).
Cell growth assay
ARPE-19 cells were plated in clear flat bottom 96-well plates at a density of 3,000 cells per well. After an overnight attachment period, cells were treated either with 1% DMSO (vehicle control) or compound. The number of viable cells was determined at 48 h using CellTiter 96 Aqueous One Solution Cell Proliferation assay (Promega Corp., Madison, WI). 20 µl CellTiter 96 solution was added to each well, and the plates were incubated for 3 h at 37 °C after which the absorbance of each well was measured at a wavelength of 490 nm using a microplate spectrophotometer (BioTek Epoch). All assay wells were performed in triplicate, and each assay was repeated three times. Cell growth of treated plates was calculated relative to an untreated time zero plate measured at the time of treatment. Statistical analysis was performed using GraphPad Prism V9.
Western blot analysis
Cells were seeded at 600,000 cells/well in 6-well plates (VWR, 10861-696). Once cells had reached ~ 80% confluency, the media was aspirated and replaced with 2 mL of media containing compound or vehicle (0.25% DMSO) and incubated for a 24-hour treatment time. After 24 h, cells were washed with ice cold PBS and then lysed with cell lysis buffer (130 mM NaCl, 1% Triton X-100, 1 mM EDTA, 0.1% SDS, 10 mM Tris-Cl pH 8.0 in water + freshly added 1 mM Protease Cocktail 2, 1 mM Protease Cocktail 3, 1 mM Phosphatase inhibitor, and 1 mM PMSF). Cell lysates were obtained by centrifugation at 10, 000 g for 10 min at 4 °C. Protein concentrations were determined using the Pierce BCA assay kit following the manufacturer’s instructions. Then, 40 ug of each normalized protein lysate was electrophoresed on 8% SDS-polyacrylamide gels and transferred onto PVDF membranes. Membranes were washed in Tris-buffered saline containing Tween (TBST: 10 mM Tris-HCl, 150 mM NaCl, pH 7.2, and 0.1% Tween 20), incubated in blocking buffer (5% non-fat milk in TBST), and then incubated with primary antibodies at 4 °C overnight. After washing with TBST, membranes were incubated in their respective secondary antibodies for 1 h at room temperature. The blots were developed using Clarity Max Western ECL Blotting Substrates (Bio-Rad). The following primary antibodies were obtained from Cell Signaling Technology (Danvers, MA): β-Actin (8H10D10).The following primary antibodies were obtained from Enzo Life Sciences (Farmingdale, NY): Hsp70/Hsp72 (C92F3A-5), Hsp90α (9D2). All primary antibodies were used at 1:1000 dilutions, and all secondary antibodies at 1:2000 dilutions. Horseradish peroxidase-conjugated secondary goat anti-mouse IgG and goat anti-rat IgG antibodies were purchased from Southern Biotech (Birmingham, AL). Densitometry was performed using ImageJ and statistical analysis was performed with GraphPad Prism V9.
Human-induced pluripotent stem cell–derived cardiomyocyte (hiPSC-CM) cell culture iCell2
Cardiomyocytes (FUJIFILM Cellular Dynamics, Madison, WI, USA) were kept at − 190 °C and prepared for culture as per the manufacturer’s instructions. Cell donor (01434) was registered with the ethics committee for research uses (NICHD-NIH, USA, with approval number N-01-HD-4-2865). Standard fibronectin well coating: The cells were cultured using in a humidified incubator at 37 °C with 5% CO2. The 96-well glass-bottomed plates (MatTek, Ashland, MA) were coated with fibronectin (10 mg/mL in PBS supplemented with Ca2+ and Mg2+; Sigma, St. Louis, MO, USA) (defining FM). Cells were introduced at a density of 50,000 cells/well to allow the formation of a confluent monolayer in each well. The maintenance protocols followed the manufacturer’s instructions and used the iCell2 Cardiomyocytes Maintenance media for media changes every 2 days. Experiments were performed between days 5 and 6, as recommended by the manufacturers. Before beginning an experiment, cells were washed in serum-free media (SF media) (Fluorobrite DMEM, Gibco, Thermo Fisher Scientific, Horsham, UK).
Drug treatment
The drug powders were dissolved in DMSO, and four stock concentrations at 1000× the final target concentration were prepared in DMSO. For the experiment, an intermediate concentration 2× the target solution was prepared in SF media. The addition of drugs to the cells was accomplished by replacing 50% of the well volume with the intermediate 2× solution. The same procedure was done for vehicle control using DMSO. The experimental number n = 8/concentration/testing and for each positive or negative control were considered. Light protection precautions were taken during stock drug preparation, such as the use of amber vials and silver foil covers. All the drugs (stocks and target concentrations) were prepared in glass containers to minimize drug adsorption to and/or absorption from plastic.
Membrane potential signals from hiPSC-CM using voltage sensitive dyes
Cultures of hiPSC-CMs (5–6 days post-plating) were treated with FluoVolt dye (1:1000, Invitrogen, Cat# F10488) in SF media and incubated for 20 min at 37 °C with 5% CO2 and 80% humidity. Multi-well plates were placed in the environmentally controlled stage incubator (37 °C, 5% CO2, water-saturated air atmosphere) (Okolab Inc. Burlingame, CA) of the CellOPTIQ platform (Clyde Biosciences Ltd., Glasgow, Scotland). The FluoVolt signal was recorded from a 0.2 mm × 0.2 mm area using a 40× (numerical aperture: NA 0.6) Excitation wavelength was 470 ± 10 nm using a light-emitting diode (LED), and emitted light was collected by a photomultiplier (PMTs) at 510–560 nm (channel 1). LED, PMT, and associated power supplies and amplifiers were supplied by Cairn Research Ltd. (Kent, UK) and digitized at 10 kHz and stored on a computer hard drive. In the case of FluoVolt-based action potential recordings, the short wavelength (channel 1) was analyzed for action potential features.
Voltage recordings
The dye FluoVolt is loaded into the surface membrane (outer leaflet), and the dye remains within the membrane for many days, allowing intermittent voltage measurements over the experimental period. In this study, measurements were restricted to within 30 min of application of the drug. The measurements were made on the same area before/after drug/DMSO addition. As reported in other studies, there were minimal effects of the DMSO vehicle (0.1%) on the function of all electrophysiological and contractility parameters. Baseline spontaneous electrical activity and the associated contractility signal were recorded by capturing 10 s segments of fluorescent signal and video from each well prior to compound addition. Drugs were tested at four concentrations in n = 8 independent replicates (i.e., 8 wells from a single plating) at each concentration. A vehicle control was included for each drug. A 10 s recording was then taken 30 min after exposure to the drug or vehicle with only one concentration applied/well. Offline analysis was performed using proprietary software (CellOPTIQ). The following (averaged) parameters were obtained from the AP recordings: cycle length (CL, ms); rise time (TRise, ms) between 10 and 90% of the AP upstroke; and AP durations (APD, ms) from 10 to 90% repolarization at 10% intervals.
Contractility measurements using video imaging
Contractility was recorded via HCImage Live (Hamamatsu Corporation, Bridgewater, NJ, USA), which controlled a Hamamatsu ORCA-Flash4.0 V3 Digital CMOS camera at 100 fps (1024 × 2048-pixel resolution). Short periods (10–15 s) of video were analyzed using the ContractilityTool application (developed by F.L. Burton), which is an implementation of the MUSCLEMOTION algorithm. This application extracts several parameters from the resultant motion signal, including the average spontaneous frequency and amplitude, the duration at 50% of the contraction transient amplitude (CD50), the time to contract (from 10 to 90% of the contraction amplitude), and the time to relax (from 90 to 10% of the contraction amplitude).
Data analysis and statistics
Statistical analysis was performed using paired t-tests for differences between absolute values, with P-values adjusted for multiple comparisons using Benjamini-Hochberg procedure. Statistical significance was designated as * p < 0.05.
Extracellular lactate dehydrogenase (LDH) toxicity assay
The hiPSC-CMs that were used in method 2.1 (50,000 cells/well) were identically seeded, treated, and assessed for toxicity via a Lactate Dehydrogenase Activity Assay Kit (Sigma-Aldrich MAK066). The LDH assay measures release of the cytoplasmic protein LDH due to loss of membrane function and therefore its presence in the extracellular space is an indicator of cell death. At the 48-hour time point, after contraction and electrical activity measurements were taken, 30 µL samples were collected from the conditioned media of a replicate 96-well plate. The assay proceeded according to the manufacturer’s protocol. Statistical software analysis was performed in GraphPad Prism to produce the LDH activity (mu/mL) bar graph (Fig. 5B) with N = 8 technical replicates and values reported as the Avg ± SD. Values taken from wells with no cells (Blank) were subtracted as background from every reading.
Cell health index cell monolayer assessment
The replicate plate cells seeded in method 3.1 were monitored for the maintenance of their cell monolayer in parallel with the LDH assay. At t = 0, 30 min, 24 h, and 48 h, the status of the monolayer was monitored by visual inspection using white light and a 40x objective. An operator blinded to the drug/concentration reported the following metric of each well: 0 = intact monolayer; 1 = some small holes appearing; 2 = larger numerous holes in monolayer; 3 = peeling of monolayer/large areas without cells. The average cell health index rating of the N = 8 replicate wells was reported in Fig. 5A as a qualitative assessment of the cardiomyocyte cell monolayer health.
hERG fluorescence polarization assay
The Predictor hERG Fluorescence Polarization Assay was followed according to the manufacturer’s protocol (Invitrogen PV5365). A 570 nm dichroic mirror (Agilent 7137570) was used in a BioTek Synergy 2 plate reader. The excitation and emission wavelength filters were 530/25 nm (7082223) and 590/35 nm (7082224). Untreated, black, round-bottom 384 well low volume microplates were used for this assay (Corning 4511). All assays were run in a total volume of 20 uL with a final concentration of 1% DMSO. Controls included tracer background without membrane, fully bound tracer with membrane, positive control 30 µM E-4031 (fully displaced tracer), and negative control DMSO treated (fully bound tracer). Every well was repeated in 4X replicates for each sample. The polarization values were constrained to the negative (DMSO) and positive (E-4031) control values as upper and lower bounds and reported as Avg ± SD N = 4. IC50 values were calculated using GraphPad Prism V9.
NDNB1182 characterization

Synthesis was performed according to the procedure described previously27. Yield 65%, 1H NMR (400 MHz, DMSO) δ 11.13 (s, 1H), 9.56 (d, J = 6.5 Hz, 1H), 6.78 (d, J = 1.9 Hz, 1H), 6.49 (d, J = 1.9 Hz, 1H), 6.30 (d, J = 1.8 Hz, 1H), 4.17 (dp, J = 6.7, 3.3 Hz, 1H), 3.93 (dd, J = 9.0, 5.4 Hz, 1H), 3.86 (q, J = 7.6 Hz, 1H), 3.77 (td, J = 8.3, 5.2 Hz, 1H), 3.63 (dd, J = 9.0, 3.0 Hz, 1H), 2.98 (s, 2 H), 2.50 (p, J = 1.9 Hz, 4 H), 2.41 (d, J = 2.9 Hz, 5 H), 2.34 (s, 2 H), 2.28 (dt, J = 12.6, 7.5 Hz, 1H), 1.83 (dtd, J = 12.6, 6.1, 3.6 Hz, 1H), 1.63 (h, J = 7.4 Hz, 2 H), 1.02 (s, 6 H), 0.91 (t, J = 7.3 Hz, 3 H). 13C NMR (101 MHz, DMSO) δ 193.37, 165.42, 151.09, 150.16, 148.74, 143.69, 142.72, 142.11, 116.93, 107.71, 105.58, 104.13, 99.84, 73.17, 66.89, 52.76, 52.28, 37.02, 35.85, 34.34, 33.20, 28.22, 21.73, 13.83, 13.64. LRMS (ESI) m/z [M + H] calculated for C26H32N4O3, 448.2, found 449.2.
Data availability
All data generated or analyzed during this study are included in this published article (and its supplementary information files).
Abbreviations
- Hsp90:
-
Heat shock protein 90
- Grp94:
-
Glucose–regulated protein 94
- Trap1:
-
Tumor necrosis factor receptor–associated protein 1
- NTD:
-
N–terminal domain
- HSF1:
-
Heat shock factor 1
- TRPM1:
-
Transient Receptor Potential cation channel subfamily M member 1
- hERG:
-
Human ether–à–gogo–related gene
- QTcF:
-
Fridericia corrected QT interval
- hiPSC:
-
Human induced pluripotent stem cell
- APD:
-
Action potential duration
- TRise:
-
Rise time
- CtAmp:
-
Contraction amplitude
- CtTrise:
-
Contraction rise time
- CtD50 :
-
Contraction duration at 50% amplitude
- TdP:
-
Torsades de pointes
- EAD:
-
Early after depolarization
- LDH:
-
Lactate dehydrogenase
- FP:
-
Fluorescence polarization
References
Hartl, F. U., Bracher, A. & Hayer-Hartl, M. Molecular chaperones in protein folding and proteostasis. Nature 475(7356), 324–332. https://doi.org/10.1038/nature10317 (2011).
Saibil, H. Chaperone machines for protein folding, unfolding and disaggregation. Nat. Rev. Mol. Cell Biol. 14(10), 630–642. https://doi.org/10.1038/nrm3658 (2013).
DeZwaan, D. C. & Freeman, B. C. HSP90 manages the ends. Trends Biochem. Sci. 35(7), 384–391. https://doi.org/10.1016/j.tibs.2010.02.005 (2010).
Hanahan, D. & Weinberg, R. A. Hallmarks of cancer: the next generation. Cell 144(5), 646–674. https://doi.org/10.1016/j.cell.2011.02.013 (2011).
Rastogi, S. et al. An update on the status of HSP90 inhibitors in cancer clinical trials. Cell. Stress Chaperones. 29(4), 519–539. https://doi.org/10.1016/j.cstres.2024.05.005 (2024).
Bagatell, R. et al. Induction of a heat shock factor 1-dependent stress response alters the cytotoxic activity of hsp90-binding agents. Clin. Cancer Res. 6(8), 3312–3318 (2000).
Wu, W. C. et al. Geldanamycin and its analog induce cytotoxicity in cultured human retinal pigment epithelial cells. Exp. Eye Res. 91(2), 211–219. https://doi.org/10.1016/j.exer.2010.05.005 (2010).
Kanamaru, C. et al. Retinal toxicity induced by small-molecule Hsp90 inhibitors in beagle dogs. J. Toxicol. Sci. 39(1), 59–69. https://doi.org/10.2131/jts.39.59 (2014).
Shen, C. H. et al. AUY922 induces retinal toxicity through attenuating TRPM1. J. Biomed. Sci. 28(1), 55. https://doi.org/10.1186/s12929-021-00751-5 (2021).
Zhou, D. et al. A rat retinal damage model predicts for potential clinical visual disturbances induced by Hsp90 inhibitors. Toxicol. Appl. Pharmacol. 273(2), 401–409. https://doi.org/10.1016/j.taap.2013.09.018 (2013).
Ohkubo, S. et al. TAS-116, a highly selective inhibitor of heat shock protein 90α and β, demonstrates potent antitumor activity and minimal ocular toxicity in preclinical models. Mol. Cancer Ther. 14(1), 14–22. https://doi.org/10.1158/1535-7163.MCT-14-0219 (2015).
Ying, W. et al. Ganetespib, a unique triazolone-containing Hsp90 inhibitor, exhibits potent antitumor activity and a superior safety profile for cancer therapy. Mol. Cancer Ther. 11(2), 475–484. https://doi.org/10.1158/1535-7163.MCT-11-0755 (2012).
Goldman, J. W. et al. A first in human, safety, pharmacokinetics, and clinical activity phase I study of once weekly administration of the Hsp90 inhibitor ganetespib (STA-9090) in patients with solid malignancies. BMC Cancer. 13, 152. https://doi.org/10.1186/1471-2407-13-152 (2013).
Rajan, A. et al. A phase I study of PF-04929113 (SNX-5422), an orally bioavailable heat shock protein 90 inhibitor, in patients with refractory solid tumor malignancies and lymphomas. Clin. Cancer Res. 17(21), 6831–6839. https://doi.org/10.1158/1078-0432.CCR-11-0821 (2011).
Pacey, S. et al. A phase I study of the heat shock protein 90 inhibitor alvespimycin (17-DMAG) given intravenously to patients with advanced solid tumors. Clin. Cancer Res. 17(6), 1561–1570. https://doi.org/10.1158/1078-0432.CCR-10-1927 (2011).
Sessa, C. et al. First-in-human phase I dose-escalation study of the HSP90 inhibitor AUY922 in patients with advanced solid tumors. Clin. Cancer Res. 19(13), 3671–3680. https://doi.org/10.1158/1078-0432.CCR-12-3404 (2013).
Slovin, S. et al. Pharmacodynamic and clinical results from a phase I/II study of the HSP90 inhibitor onalespib in combination with abiraterone acetate in prostate cancer. Clin. Cancer Res. 25(15), 4624–4633. https://doi.org/10.1158/1078-0432.CCR-18-3212 (2019).
Piotrowska, Z. et al. Activity of the Hsp90 inhibitor luminespib among non-small-cell lung cancers harboring EGFR exon 20 insertions. Ann. Oncol. 29(10), 2092–2097. https://doi.org/10.1093/annonc/mdy336 (2018).
Goyal, L. et al. A phase 2 clinical trial of the heat shock protein 90 (HSP 90) inhibitor ganetespib in patients with refractory advanced esophagogastric cancer. Invest. New. Drugs. 38(5), 1533–1539. https://doi.org/10.1007/s10637-019-00889-y (2020).
Shimomura, A. et al. First-in-human phase I study of an oral HSP90 inhibitor, TAS-116, in patients with advanced solid tumors. Mol. Cancer Ther. 18(3), 531–540. https://doi.org/10.1158/1535-7163.MCT-18-0831 (2019).
Ficker, E., Dennis, A. T., Wang, L. & Brown, A. M. Role of the cytosolic chaperones Hsp70 and Hsp90 in maturation of the cardiac potassium channel HERG. Circ. Res. 92(12), e87–100. https://doi.org/10.1161/01.RES.0000079028.31393.15 (2003).
Peterson, L. B., Eskew, J. D., Vielhauer, G. A. & Blagg, B. S. J. The hERG channel is dependent upon the Hsp90 alpha isoform for maturation and trafficking. Mol. Pharm. 9(6), 1841–1846 https://doi.org/10.1021/mp300138n (2012).
Reynolds, T. S. & Blagg, B. S. J. Extracellular heat shock protein 90 alpha (eHsp90α)’s role in cancer progression and the development of therapeutic strategies. Eur. J. Med. Chem. 277, 116736. https://doi.org/10.1016/j.ejmech.2024.116736 (2024).
Trepel, J., Mollapour, M., Giaccone, G. & Neckers, L. Targeting the dynamic HSP90 complex in cancer. Nat. Rev. Cancer. 10(8), 537–549. https://doi.org/10.1038/nrc2887 (2010).
Zuehlke, A. D., Beebe, K., Neckers, L. & Prince, T. Regulation and function of the human HSP90AA1 gene. Gene 570(1), 8–16. https://doi.org/10.1016/j.gene.2015.06.018 (2015).
Khandelwal, A. et al. Structure-guided design of an Hsp90 beta N-terminal isoform-selective inhibitor. Nat. Commun. 9, 7 https://doi.org/10.1038/s41467-017-02013-1 (2018).
Mishra, S. J. et al. The development of Hsp90 beta-selective inhibitors to overcome detriments associated with pan-Hsp90 inhibition. J. Med. Chem. 64(3), 1545–1557. https://doi.org/10.1021/acs.jmedchem.0c01700 (2021).
Rahmy, S., Mishra, S. J., Murphy, S., Blagg, B. S. J. & Lu, X. Hsp90 beta inhibition upregulates interferon response and enhances immune checkpoint blockade therapy in murine tumors. Front. Immunol. 13, 13., Article. https://doi.org/10.3389/fimmu.2022.1005045 (2022).
Blinova, K. et al. International multisite study of human-induced pluripotent stem cell-derived cardiomyocytes for drug proarrhythmic potential assessment. Cell. Rep. 24(13), 3582–3592. https://doi.org/10.1016/j.celrep.2018.08.079 (2018).
Asahi, Y. et al. Electrophysiological evaluation of pentamidine and 17-AAG in human stem cell-derived cardiomyocytes for safety assessment. Eur. J. Pharmacol. 842, 221–230. https://doi.org/10.1016/j.ejphar.2018.10.046 (2019).
Piper, P. W. & Millson, S. H. Mechanisms of resistance to Hsp90 inhibitor drugs: a complex mosaic emerges. Pharmaceuticals (Basel). 4(11), 1400–1422. https://doi.org/10.3390/ph4111400 (2011).
Kamal, A. et al. J. A high-affinity conformation of Hsp90 confers tumour selectivity on Hsp90 inhibitors. Nature 425(6956), 407–410. https://doi.org/10.1038/nature01913 (2003).
Prince, T. L. et al. Client proteins and small molecule inhibitors display distinct binding preferences for constitutive and stress-induced HSP90 isoforms and their conformationally restricted mutants. PLoS One. 10(10), e0141786. https://doi.org/10.1371/journal.pone.0141786 (2015).
Kijima, T. et al. HSP90 inhibitors disrupt a transient HSP90-HSF1 interaction and identify a noncanonical model of HSP90-mediated HSF1 regulation. Sci. Rep. 8(1), 6976. https://doi.org/10.1038/s41598-018-25404-w (2018).
Roos-Mattjus, P. & Sistonen, L. Interplay between mammalian heat shock factors 1 and 2 in physiology and pathology. FEBS J. 289(24), 7710–7725. https://doi.org/10.1111/febs.16178 (2022).
Amatya, E. & Blagg, B. S. J. Recent advances toward the development of Hsp90 C-terminal inhibitors. Bioorg. Med. Chem. Lett. 80, 129111. https://doi.org/10.1016/j.bmcl.2022.129111 (2023).
Siddiqui, F. A. et al. Novel Small Molecule Hsp90/Cdc37 interface inhibitors indirectly target K-Ras-signaling. Cancers (Basel). 13(4). https://doi.org/10.3390/cancers13040927 (2021).
Naoki, K. et al. Cardiovascular safety of pimitespib in patients with advanced solid tumors: an open-label, nonrandomized, phase 1 study. Cancer https://doi.org/10.1002/cncr.35447 (2024).
Acknowledgements
The authors gratefully acknowledge financial support of this work via the National Institutes of Health to B.S.J.B. (CA167079), and the Chemistry, Biochemistry, Biology Interface (CBBI) Program via the NIH training grant to T.S.R. (T32GM145773). Special thanks to Grannus Therapeutics Inc. for conducting in vitro cardiotoxicity studies at Clyde Biosciences and Taylor Watters for providing details of hiPSC-derived cardiomyocyte contraction and electrical activity, extracellular LDH, and cell health index assays.
Author information
Authors and Affiliations
Contributions
TR wrote manuscript and designed/conducted experiments. SM designed and conducted experiments. BB designed experiments and edited manuscript.
Corresponding author
Ethics declarations
Competing interests
SM and BB are co-founders of Grannus Therapeutics Inc., a company that aims to bring Hsp90b-selective inhibitors to the clinic for the treatment of cancer. TR is not associated with Grannus Therapeutics Inc.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Reynolds, T.S., Mishra, S.J. & Blagg, B.S.J. Assessment of Hsp90β-selective inhibitor safety and on-target effects. Sci Rep 15, 3692 (2025). https://doi.org/10.1038/s41598-025-86647-y
Received:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s41598-025-86647-y