Abstract
Metal contamination in water bodies globally poses risks to ecosystems and human health. In Pakistan, water sources are impacted by toxic metals and microbial pollutants, affecting Nile Tilapia, a widely consumed fish in Punjab. This study assessed heavy metal and microbiological contamination in Nile tilapia from the Ravi and Chenab Rivers and nearby farms (Head Muhammad Wala, Shorkot, and Rangpur). Heavy metals, including arsenic, chromium, cadmium, mercury and lead, were analyzed using Atomic Absorption Spectroscopy, Arsenic ranged lowest from 0.15 mg/kg (scales) to highest 1.38 mg/kg (Liver), while Chromium was found to be lowest 73 mg/kg in (scales) and highest 321.83 mg/kg (Liver) in Riverine Fish, whereas in farms fish As ranged lowest from 0.05 mg/kg (flesh) to highest 0.92 mg/kg (gills),while Chromium was found to be lowest 92 mg/kg in (skin) and highest 166.67 mg/kg (Liver). Riverine fish showed elevated heavy metal levels as compared to farmed fish. Moreover, Ravi River fish also showed the highest microbial loads, including total plate counts of 15,335 cfu/g, Salmonella at 373.11 cfu/25 g, and E. coli at 76.55 cfu/g, while Chenab River fish had a significantly higher coliform count (p < 0.05). These findings suggest that farmed fish are less contaminated due to reduced exposure to industrial effluents, emphasizing the need for further research on other commonly consumed fish species in high-industrial regions.
Similar content being viewed by others
Introduction
Oreochromis niloticus commonly referred to as Nile tilapia originally belonged to central, western, and eastern African regions. This species is also native to some parts of the Middle East which includes Jordan Valley1,2 Tilapias have been a vital part of inland fisheries and aquaculture in the continent for well over 50 years. It was first cultured in Kenya and now is an important part of the dietary pattern of individuals in more than 90 nations worldwide. In recent years, an increasing trend of consumption of several tilapia species has been observed in almost all Asian States3 The cultivation of this didn’t remain in Africa and now is globally cultured in tropical and subtropical regions of the world2,4. After being introduced to the Asian and Pacific regions, tilapia quickly established self-sustaining populations. Aquaculture brought tilapia fish from Indonesia, Egypt, and Thailand to Pakistan between 1951 and 19545 The increasing popularity of this fish species in the last few decades in Asia is due to the multiple intensive farming techn iques being introduced in the region. Now, this species is a popular fish in the region owing to its availability6
Oreochromis niloticus commonly referred to as Nile tilapia originally belonged to central, western, and eastern African regions. This species is also native to some parts of the Middle East which includes Jordan Valley2. Tilapias have been a vital part of inland fisheries and aquaculture in the continent for well over 50 years. It was first cultured in Kenya and now is an important part of the dietary pattern of individuals in more than 90 nations worldwide. In recent years, an increasing trend of consumption of several tilapia species has been observed in almost all Asian States3. The cultivation of this didn’t remain in Africa and now is globally cultured in tropical and subtropical regions of the world4. After being introduced to the Asian and Pacific regions, tilapia quickly established self-sustaining populations. Aquaculture brought tilapia fish from Indonesia, Egypt, and Thailand to Pakistan between 1951 and 19545. The increasing popularity of this fish species in the last few decades in Asia is due to the multiple intensive farming techniques being introduced in the region. Now, this species is a popular fish in the region owing to its availability6.
Food obtained from the water bodies is heavily contaminated these days. These issues have been intensified due to widespread river water pollution7. Heavy metals and other chemical pollutants, particularly those caused by industrial and agricultural expansion and urbanization, are constantly released into rivers8. Due to industrialization, heavy metal contamination in aquatic habitats is a severe pollution issue9,10. Heavy metals are highly toxic, their large amounts and ability to be part of the ecosystem for a longer time make heavy metal contamination a global issue11
Similarly, these metals pose a huge threat to the environment because of their bio-accumulative nature12 When fish from this intensely polluted environment enter the human food chain and are consumed by people, it imparts toxic impacts on human health. Such deleterious compounds can pass the cell membrane and disturb the function of the body’s organs, including the liver, muscle, kidney, gills, skin, and intestine13 The toxicity of metal compounds is evaluated by their potency to be carcinogenic and non-carcinogenic behavior14. Although metals are essential for the proper growth and development of organisms above certain levels these elements become toxins. Continuous exposure of these heavy metals to living beings is a significant threat to their health15.
Unfortunately, diverse chemicals are dumped into water bodies in poor nations like Pakistan without adequate screening and treatment, endangering the aquatic ecology and the neighboring aquatic creatures that are consumed by humans as food16. Heavy metal such as Cadmium (Cd) accumulates in the mucus lining of fish gills at extremely high concentrations (parts per thousand). A key characteristic of lead (Pb) toxicity is bioaccumulation, whose exposure leads to toxic effects that disrupt the biochemical and physiological processes in fish. Metals of natural or anthropogenic origin are ubiquitous in the aquatic environment and it is of considerable socio-economic relevance to understand their behavior and interaction with aquatic animals, notably fishes, a key source of protein for human consumption13 Understanding the bioaccumulation of heavy metals in fish is crucial for determining the risks associated with their use in the human diet and for creating pollution prevention methods17,18 Likewise, microbiological contamination from various sources has also severely impacted the environment and ultimately human life19.
The selection of heavy metals in this study was based on significant pollution levels in rivers across Punjab, Pakistan, where contamination from heavy metals and microbial sources is widespread. Due to their high toxicity, persistence, and bioaccumulation potential, these metals pose health risks, particularly in Nile tilapia (Oreochromis niloticus), commonly found in this region”.
The study aimed to assess the contamination levels of significant heavy metals (cadmium, lead, chromium, arsenic, and mercury) in both riverine and farmed Fish (Nile tilapia) across selected regions of the Punjab Pakistan along with evaluation of their microbiological quality. Quantifying heavy metal concentrations and conducting microbial analyses, including total plate count, coliform enumeration, and Escherichia coli detection, to provide insights into potential health risks associated with the consumption of this fish specie was the focus of the study.
Materials and methods
Study area
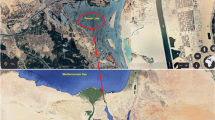
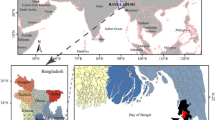
The study was designed to investigate heavy metal bioaccumulation in various organs (liver, flesh gills, scales, and skin) of Tilapia fish species collected from five different locations in winter season (three farms and two rivers) in Punjab, Pakistan. The Chenab River is located at 31°11′12.00″ N 72°11′34.20″E, and the Ravi River at 30°34′59.99″ N 71°48′59.99″E while farms are situated near these rivers.
Sampling of Nile tilapia
The sampling procedure was carefully done. Fish body pieces were packed in polyethylene bags, labelled, and transferred to the laboratory for identification and chemical analysis. There were 75 samples collected in total. In the laboratory, the fish was dissected with a corrosion-resistant stainless-steel knife and separate knives were used for individual fish dissection. After dissecting each specimen, knives were discarded. Organs were placed in a plastic bag and labelled with the date, location, and body organ name. Before digestion of organs, sample bags were stored at 4 °C.
Sample preparation for digestion of fish organs
In a volumetric flask, one gram of each body part (flesh, liver, gills, skin, and scales) was digested with 10 mL of HNO3 and 5 mL of HClO4. On a hotplate (200 to 250 °C), digestion was carried out until a transparent liquid (about 1–2 mL) was formed and the volume was raised to 50 ml. To avoid contamination and evaporation, each diluted digested sample was filtered through Whatman filter paper, and then again double filtration was carried out using 0.42 m filter paper, raised to 50 ml, and placed into a plastic sealed bottle. The digested samples were stored at room temperature for heavy metal tests using an atomic absorption spectrophotometer (240FS AA, Agilent, Santa Clara, Malaysia)20,21
Chemicals and reagents
All dilutions were made using double-deionized water. HNO3 and HClO4 were of superior quality were used for digestion of the organs. Before use, all glassware was cleaned and rinsed with deionized water.
Heavy metal analysis and method validation
Triplicate samples were analyzed for five major heavy metals detected in fish, i.e., As, Cd, Cr, Pb, and Hg using an Atomic Absorption Spectrophotometer (iCE 3000 Series AA Spectrometer)22 The fish samples, as well as the blanks and calibration standard solutions, were analyzed in the same way. The concentration of metals in the fish samples was measured in milligrams per kilogram of wet weight.
Microbiological analysis
The samples of Nile tilapia fish were collected from rivers (Chenab and Ravi) and farms (HMW, Shorkot, Rangpur) and stored at freezing temperatures (− 1 to − 4℃) in ice boxes to prevent deterioration.
Salmonella
1 ml homogenized sample of fish meat in distilled water was cultured on selenite F-broth medium and incubated for 24 h at 37℃. Cultures on Selenite F-Broth established on SSA were incubated for 24 h at 37 °C. The bacteria were allowed to develop to a detectable size during this time. Once gram staining was completed, the colonies’ characteristics were evaluated23.
Escherichia coli & coliform
Buffered peptone water (BPW) was prepared by combining 1000 mL of distilled water with 5 g of buffered peptone and 5 g of sodium chloride. A portion of the fish meat sample was taken out and 9 mL of Buffered Peptone Water (BPW) was utilized to homogenize it. Each of the ten tubes, containing 9 mL of BPW, was supplemented with 1 mL of the homogenized material. 0.1 mL of the BPW from each of the 10 tubes was added to the well of an Eosin methylene blue (EMB) agar plate. The plates were then incubated at 37 °C for 24 h. On the plates, the presence of E. coli was detected by the growth of colonies that had a distinct colour. The most likely number of E. coli colonies per gram of fish meat was determined using the most probable number (MPN) table, which involved counting the positive wells on the EMB agar plate. This strategy employed the MPN method to identify the presence of E. coli in fish meat by quantifying the number of E. coli colonies in a sample. E. coli was employed to differentiate itself from other gram-negative bacteria on the EMB agar by its ability to produce distinct dark purple colonies24 An identical methodology was employed to identify the presence of coliform bacteria.
Total plate count
Homogenizing a representative fish meat sample created a homogenous solution. The homogenized material was diluted with buffered peptone water to achieve a plate count within detection limits. Autoclaving sterilized Petri plates 0.1 mL of nutritious agar growth media was inoculated. Infected plates were incubated at the right temperature and time for the intended bacterium 30–35 °C for 24–48 h was common for fish meat plate counts. Post-incubation colonies were counted. Colony counters or hand counts were used. The plate count was the dilution factor times the colony count25.
Results
Highly significant (p < 0.05) statistical results were obtained for the heavy metals present in the various body parts of Nile tilapia collected from Pakistan’s Chenab and Ravi rivers (Table 1). The findings showed that the liver of Chenab Nile tilapia had the greatest levels of arsenic, chromium, and cadmium, at 0.80 mg/kg/w.w, 321.83 mg/kg/w.w, and 13.59 mg/kg/w.w, respectively. While the lowest concentrations of these heavy metals at 0.15 mg/kg/w.w, 173.33 mg/kg/w.w, and 6.22 mg/kg/w.w respectively were found in the scales.The result depicted that the highest concentration of Chromium ranged 321.83 mg/kg in liver while its lowest concentration was found in the scales which ranged 173 mg/kg. On the other hand, lead concentrations were found to be lowest in the scales (11.01 mg/kg/w.w) and highest in the gills (33.79 mg/kg/w.w). The findings made it clear that all other body organs contained these heavy metals in intermediate concentrations. The analysis of the Ravi Nile tilapia produced results that were comparable to those of the Chenab Nile tilapia, having the lowest levels of the heavy metals in the scales (0.41 mg/kg/w.w, 73.33 mg/kg/w.w, 2.46 mg/kg/w.w, and 0.75 mg/kg/w.w, respectively) and the highest levels of arsenic, chromium, cadmium, and lead in the liver region of the body. The liver was found to have the highest quantities of these heavy metals possibly because of the liver’s role in detoxifying the body’s harmful chemicals and making them water soluble resulting in excreting these metals through the kidney. Scales, on the other hand, had the lowest quantities since they are the inner skin’s protective coverings and do not have much capacity for absorbing or binding harmful substances or heavy metals. In Table 2, highest concentrations of chromium in the HMW Farm samples were found in gills (152.67 mg/kg/w.w), followed by the liver (166.67 mg/kg/w.w). The lowest concentration of chromium was found in the scales (105.83 mg/kg/w.w). Meanwhile, the highest concentrations of lead were found in the liver (16.57 mg/kg/w.w) and the lowest concentration was found in the skin (3.82 mg/kg/w.w). At Shorkot Farm, the highest concentrations of chromium were found in the gills (127 mg/kg) and the liver (129.5 mg/kg). The liver had the highest concentrations of lead (6.73 mg/kg/w.w) while the lowest concentrations were found in the scales (1.33 mg/kg/w.w). On the other hand, in the Rangpur Farm samples, the highest concentrations of chromium were found in the liver (130 mg/kg/w.w) and the gills (126.3 mg/kg/w.w). The highest concentrations of lead were found in the gills (17.71 mg/kg/w.w) and the lowest concentrations were found in the scales (3.25 mg/kg/w.w).
Moreover, in Table 3, the data suggested that mercury concentrations differ across the two rivers and among fish tissues. Fish liver contained the highest concentrations of mercury in both rivers; however, the concentration in the Ravi River was 0.08 mg/kg/w.w higher than that in the Chenab River in which it was just 0.01 mg/kg/w.w. Fish from the Ravi River had three times as much mercury per kilogram as that from the Chenab River (0.01 mg/kg/w.w). Fish caught in the Chenab River also had greater mercury levels in their gills (0.05 mg/kg/w.w) than those caught in the Ravi River (0.02 mg/kg/w.w). Mercury concentrations were lowest in the skin and scales of fish from both rivers, ranging from 0.00 to 0.02 mg/kg/w.w. Table 4 shows the mercury levels in various fish tissues from three farms in the South Punjab region of Pakistan, i.e., HMW, Shorkot, and Rangpur. The data are provided as mercury milligrams per kilogram of fish (mg/kg/w.w). The study suggested that mercury concentrations differed between farms. All three farms’ fish gills contained the greatest levels of mercury, with HMW having the most at 0.11 mg/kg/w.w and Shorkot being too low to detect. The muscle of fish from Rangpur was not detected with heavy contamination, indicating that this farm has the least contaminants. The study further showed that mercury levels in the liver were greater in the HMW and the Shorkot farms (0.08 mg/kg/w.w and 0.07 mg/kg/w.w,) than in the muscle of fish. Liver from fish raised at the farm in Rangpur, on the other hand, had mercury concentrations of only 0.01 mg/kg/w.w. Mercury concentrations in the scales and skin of fish from all three farms were extremely low, at 0.00 to 0.01 mg/kg/w.w (Fig. 1).
In terms of coliform concentration, fish samples from the Ravi River showed the highest concentration (455.83 CFU/g), followed by the Chenab River (561.33 CFU/g). The lowest coliform concentrations were found in fish samples from HMW Farm (115.99 CFU/g), Rangpur Farm (53.49 CFU/g), and Shorkot Farm (76.25 CFU/g). Fish samples from the Ravi River had the highest E. coli concentration (76.55 CFU/g), followed by the Chenab River (70.66 CFU/g). The lowest E. coli concentrations, as shown in Fig. 3, were found in fish samples from HMW Farm (50.0 ± 2.0 CFU/g) and Shorkot Farm (0.00 ± 0 CFU/g). The highest Salmonella concentrations were found in fish samples from the Ravi River (373.11 CFU/g) and Rangpur Farm (293.78 CFU/ ± ± g). The lowest Salmonella concentrations were found in fish samples from HMW Farm (0.00 ± 0 CFU/g) and Shorkot Farm (0.00 ± 0 CFU/g). Table 5 shows the permissible limits of heavy metals for humans as defined by the WHO and FAO, our findings indicate greater values than the allowable limits. This could be due to the presence of industrial and agricultural activities in the area, which could be contaminating the water and affecting the quality of fish in the river. Figures from 2, 3, 4 and 5 show the concentration of total plate count (TPC), coliform, E. coli, and Salmonella in fish samples collected from the five different locations: the Chenab River, Ravi River, HMW Farm, Shorkot Farm, and Rangpur Farm. As shown in Fig. 5, the highest TPC concentration was found in fish samples from the Ravi River (15,335 CFU/g), followed by the Chenab River (13,730 CFU/g) and Shorkot Farm (12,615 CFU/g). On the other hand, the lowest TPC concentrations were found in fish samples from the HMW Farm (5,048.4 CFU/g) and Rangpur Farm (6,616.7 CFU/g). In terms of coliform concentration, fish samples from the Ravi River showed the highest concentration (455.83 CFU/g), followed by the Chenab River (561.33 CFU/g), as shown in Fig. 3. The lowest coliform concentrations were found in fish samples from the HMW Farm (115.99 CFU/g), Rangpur Farm (53.49 CFU/g), and Shorkot Farm (76.25 CFU/g). Figure 3 shows that fish samples from the Ravi River had the highest E. coli concentration (76.55 CFU/g), followed by the Chenab River (70.66 CFU/g). The lowest E. coli concentrations were found in fish samples from the HMW Farm (50.0 CFU/g) and Shorkot Farm (0.00 CFU/g). As shown in Fig. 2, the highest Salmonella concentrations were found in fish samples from the Ravi River (373.11 CFU/g) and Rangpur Farm (293.78 CFU/g). The lowest Salmonella concentrations were found in fish samples from the HMW Farm (0.00 CFU/g) and Shorkot Farm (0.00 CFU/g).
Discussion
The results indicate that the concentrations of heavy metals in fish from different locations vary from each other. This may be due to difference in the sources of water and feeding habits. The concentration of heavy metals in the different body parts of fish also varies, with the gills and liver having higher concentrations compared to the flesh, skin, and scales. All other body components, including the meat, skin, and gills, had intermediate levels of the above mentioned heavy metals, demonstrating that these organs have a lower capacity to bind heavy metals than the liver but a higher capacity than scales29. Lead poisoning from repeated exposure can induce oxidative stress, allergies, dyslexia, weight loss, hyperactivity, paralysis, muscle weakness, brain damage, kidney damage, and even death30.
The farm fishes of Bangladesh found lowest levels of chromium heavy metal in the range of 1.054–1.349 mg/kg as compared to the current study results for riverine and farm tilapia fish. Similarly,31 found results (49.00–6.00 mg/g) comparable with the current study for the chromium concentration in fishes collected from the Turkey’s Head Qadirabad Area. However,32 measured the highest quantities of chromium (524.00 to 53.00 mg/kg) in fishes collected from cultured fish farms in India. The use of tannery, industrial waste, and household wastage in the Chenab and Ravi rivers is most likely to blame for the increased levels of Cr seen in fish from these rivers. Fish that swim close to the wastewater disposal locations have been shown to have the highest rates of chromium toxicity.
Some fishes had a Cd concentration of 0.003 mg/kg wet weight, and L. rohita and P. pangasius had a Cd concentration of 0.001 mg/kg wet weight in cultured fish from Bangladesh. The flesh of A. testudeneus in the market fish of Dhaka city had the highest overall content of Cd, which was 0.775 mg/kg33. Heavy metal concentration and its toxicity assessment in some market fishes of Dhaka city34. According to Paudel et al.35, fish from the Kathmandu Valley in Nepal contained 0.88–0.35 g of Cd per g of dry weight. These two pieces of literature data both indicate lower concentrations than the study we conducted in five locations. This shows that the low quality and poor water management system has adverse effects on aquatic life and ultimately on humans.
As has been employed for isotope labeling in cancer research in place of radioactive elements36. Human arsenic poisoning is associated with mining and ore smelting for industrial uses, although the main source of exposure is from natural sources, such as contaminated water.
Biological and metabolic processes in the body may be disrupted if heavy metals are consumed in food at hazardous concentrations stomatitis, tremors, diarrhea, hemoglobinuria, paralysis, vomiting, convulsions, and depression are the hallmarks of these illnesses37. A study by Arulkumar et al. discovered that fish samples had various concentrations of Cd, Pb, Cu, and Zn metals38. The levels of dangerous heavy metals were lower than those deemed safe for human consumption by the World Health Organization, the European Union, and the Food and Agriculture Organization.
Another study showed that the levels of heavy metals were found to be highest in the liver as compared to other body parts of the fish. These heavy metals include copper and nickel. Lead was found in highest concentrations in muscle tissues39. The levels of heavy metals increased greatly with industrialization and urbanization in global river and lake water bodies. They were relatively low in the 1970s and 1980s and high in the 1990s, 2000s, and 2010s, indicating that heavy metal loadings have increased from 1972 to 201740. In Africa’s Niger River, the mean Cd concentration was 0.00 μg L −1 in the 1980s, 50.0 μg L −1 in the 2000s, and 19.14 μg L −1 in the 2010s41.
Mercury pollution in fish meat is a worldwide problem that has received a lot of attention in recent years. Fish meat in Australia, Canada, and the United States contained elevated amounts of mercury as well42. In a study conducted by Alina et al. (2012) the range of heavy metals in wet samples were 1.0–3–6.5–3 μg/ g for Hg, 0.5–2–47–2 μg/ g for Cd, 0.01–0.39 μg/ g for Pb, and 0.14–6.57 μg/g for As.
In one investigation, a total of 384 fish and fish product samples were gathered. Mercury standard solutions for atomic absorption spectroscopy were employed, with a wavelength of 254 nm. Nowadays, the majority of nations and international organisations impose a maximum mercury concentration of roughly 0.5 mg.kg −1. Overall, 184 samples (50.52% of all fish samples examined) had mercury levels below the limit allowed by the European Commission’s fish mercury regulations43.
Selected tissue samples from 30 common carp and 51 cormorants were obtained for another study. It was discovered that the cormorant had the capacity to store mercury in the food chain; the total amount of mercury in the cormorant’s muscle was approximately 35 times greater than that of the carp, which it consumes. The kidney and liver of cormorants had an overall mercury concentration that was statistically considerably greater (p < 0.01) than the other tissues that were investigated (2.23, 2.12 mg/kg)44,45. The mean concentration of mercury in the water of tilapia farms was 0.07 ± 0.009 ppm in another study by Bawuro et al. (2018), whereas the mean amounts of mercury in fish samples’ gills, liver, kidney, and muscles were 0.04, 0.055, 0.020 ± 0.005, and 3.50 ppm, respectively. We did not detect mercury levels surpassing international safety criteria for Nile tilapia, while overall environmental studies in Pakistan do show the prevalence of mercury contamination in some places.
The concentration of microorganisms in fish samples gathered from various places revealed significant differences (p < 0.05) showing that the concentration of TPC, coliform, E. coli, and Salmonella in fish samples from the Ravi River were higher compared to the other locations. This could be due to the presence of industrial and agricultural activities in the area, which could be contaminating the water and affecting the quality of fish in the river. Figures 2, 3, 4 and 5 show the concentration of total plate count (TPC), coliform, E. coli, and Salmonella in fish samples collected from the five different locations: the Chenab River, Ravi River, HMW Farm, Shorkot Farm, and Rangpur Farm. As shown in Fig. 4, the highest TPC concentration was found in fish samples from the Ravi River (15,335 CFU/g), followed by the Chenab River (13,730 CFU/g) and Shorkot Farm (12,615 CFU/g). On the other hand, the lowest TPC concentrations were found in fish samples from HMW Farm (5048.4 CFU/g) and Rangpur Farm (6616.7 CFU/g).
Due to the surplus pollutants released by industries and household waste, the microbial load was also high in riverine fish. This analysis demonstrated that the levels of heavy metal and pesticide pollution exceeded the permitted levels at the national and international levels. Furthermore, the amount of contamination with microbiological contaminants in a sizeable fraction of the samples was higher than allowed. In contrast to popular belief, the research findings have shown that wild tilapia had higher levels of contamination than farmed tilapia46, and our findings matched those of the study.
Conclusion
This study underscores the health risks associated with consuming Nile Tilapia from Punjab, Pakistan, where elevated levels of heavy metals including Mercury, arsenic, chromium, cadmium, and lead and pathogenic microbes like Salmonella and E. coli were detected. The bioaccumulation of these toxic metals and the presence of harmful pathogens in fish pose serious risks, not only to consumers but also to other species within the food chain, potentially impacting broader ecological and human health. Continuous intake of such contaminants through fish consumption could lead to severe health issues, including organ damage and infections. Addressing these risks requires urgent action, including rigorous monitoring of fish contamination levels, public awareness initiatives, and the enforcement of food safety regulations to protect consumer health and preserve the integrity of aquatic ecosystems.
Complies with international, national and/or institutional guidelines
Experimental research and field studies on plants (either cultivated or wild), comply with relevant institutional, national, and international guidelines and legislation. Experimental studies were carried out in accordance with relevant institutional, national or international guidelines or regulations.
Permissions or licenses
The experiment was started, after taking permission from Bahauddin Zakariya University, Punjab, Pakistan.
Data availability
All data generated or analysed during this study are included in this published article.
Change history
04 April 2025
A Correction to this paper has been published: https://doi.org/10.1038/s41598-025-95857-3
References
Trewavas E. Tilapiine fishes of the genera Sarotherodon, Oreochromis and Danakilia. British Museum (Natural History). (1983)
Zengeya TA, Kumschick S, Weyl OLF, van Wilgen B. An Evaluation of the Impacts of Alien Species on Biodiversity in South Africa Using Different Assessment Methods. In: van Wilgen BW, Measey J, Richardson DM, Wilson JR, Zengeya TA, editors. Biological Invasions in South Africa. Berlin: Springer: p. 487–512. (2020)
Munguti, J. M. et al. Ogello EO Nile tilapia (Oreochromis niloticus Linnaeus, 1758) culture in Kenya: Emerging production technologies and socio-economic impacts on local livelihoods. Aquacult. Fish Fisheries 2(4), 265–276 (2022).
Zambrano, L., Martínez-Meyer, E., Menezes, N. & Peterson, T. A. Invasive potential of common carp (Cyprinus carpio) and Nile tilapia (Oreochromis niloticus) in American freshwater systems. Can. J. Fish Aquat. Sci. 63, 1903–1910 (2006).
El-Gohary, FA., Zahran, E., Abd El-Gawad EA., El-Gohary AHM., Abdelhamid F., El-Mleeh A., et al Investigation of the prevalence, virulence genes, and antibiogram of motile aeromonads isolated from Nile tilapia fish farms in Egypt and assessment of their water quality. Animals 10:1–16 (2020).
Narasimha Murthy, M. N. Department Of Aquaculture College Of Fisheries, Mangalore. (2011)
Saha, P. & Paul, B. Suitability assessment of surface water quality with reference to drinking, irrigation and fish culture: a human health risk perspective. Bull. Environ. Contamination Toxicol. 101, 262–271 (2018).
Liu, M. et al. Contamination features, geo-accumulation, enrichments and human health risks of toxic heavy metal (loids) from fish consumption collected along swat river. Pakistan. Environ. Technol. Innov. 17, 100554 (2020).
Ali, H. & Khan, E. Environmental chemistry in the twenty-first century. Environ. Chem. Lett. 15(2), 329–346 (2017).
Javed, M. & Usmani, N. Accumulation of heavy metals and human health risk assessment via the consumption of freshwater fish Mastacembelus armatus inhabiting, thermal power plant effluent loaded canal. Springerplus. 5, 776–778 (2016).
Taghinia Hejabi, A., Basavarajappa, H. T., Karbassi, A. R. & Monavari, S. M. Heavy metal pollution in water and sediments in the Kabini River, Karnataka India. Environ. Monit Assess. 182, 1–13 (2011).
Begum, A., Amin, M. N., Kaneco, S. & Ohta, K. Selected elemental composition of the muscle tissue of three species of fish, Tilapia nilotica, Cirrhina mrigala and Clarius batrachus, from the fresh water Dhanmondi Lake in Bangladesh. Food chem. 9(33), 439–443 (2005).
Garai, P., Banerjee, P., Mondal, P., & Saha, N. C. Effect of heavy metals on fishes: Toxicity and bioaccumulation. J. Clin. Toxicol. S, 18 (2021).
Esmaeilbeigi, M., Kazemi, A., Gholizadeh, M. & Rezaeiyeh, R. D. Microplastics and heavy metals contamination in Atropus atropos and associated health risk assessment in the northwest of the Persian Gulf. Iran. Regional Stud. Mar. Sci. 57, 102750 (2023).
Ganjavi, M., Ezzatpanah, H., Givianrad, M. H. & Shams, A. Effect of canned tuna fish processing steps on lead and cadmium contents of Iranian tuna fish. Food Chem. 118(3), 525–528 (2010).
Afzaal, M. et al. Heavy metals contamination in water, sediments and fish of freshwater ecosystems in Pakistan. Water Pract. Technol. 17, 1253–1272 (2022).
Ali, M. M. et al. Heavy metal concentrations in commercially valuable fishes with health hazard inference from Karnaphuli river Bangladesh. HERA Int. J. 26, 2646–2662 (2020).
Ambreen, F., Javed, M., Batool U et al Tissue specific heavy metals uptake in economically important fish, Cyprinus carpio at acute exposure of metals mixtures. Pak J Zool (2015).
Zhang, S. et al. Interactive effects of polystyrene microplastics and roxithromycin on bioaccumulation and biochemical status in the freshwater fish red tilapia (Oreochromis niloticus). Sci. Total. Environ. 648, 1431–1439 (2019).
Mensoor, M. Said a determination of heavy metals in freshwater fishes of the tigris river in Baghdad. Aust Fish. 3, 23 (2018).
Murtala, B. A., Abdul, W. O. & Akinyemi, A. A. Bioaccumulation of heavy metals in fish (Hydrocynus forskahlii, Hyperopisus bebe occidentalis and Clarias gariepinus) organs in downstream Ogun coastal water. Nigeria. J. Agric. Sci. 4, 11–51 (2012).
Bano, Z., Abdullah, S., Ahmad, W., Zia, M. A. & Hassan, W. Assessment of heavy metals and antioxidant enzyme in different organs of fish from farm, hatchery and Indus river of Pakistan. Pak J Zool. 49, 2227–2233 (2017).
Ekawati ER & Yusmiati., S. N. H. Detection of Salmonella sp., Vibrio sp. and total plate count bacteria on blood cockle (Anadara granosa). In IOP Conference Series: Earth and Environmental Science Vol. 102, No. 1, p. 012086 (2018).
Acharya, A. et al. Isolation and identification of bacteria from meat processing units of Kathmandu Valley. IJOMAS 2016, 2–14 (2016).
Nurmasytha, A., Yuliati ,FN., Prahesti KI. Microbiological analysis of raw chicken meat sold at Maros traditional markets: Total Plate Count and Escherichia coli. In IOP Conference Series: Earth and Environmental Science (Vol. 788, No. 1, p. 012118) (2021).
Bawuro, A. A., Voegborlo, R. B., & Adimado, A. A. Bioaccumulation of heavy metals in some tissues of fish in Lake Geriyo, Adamawa State, Nigeria. J. Environ. Public Health. 2018(1), 1854892 (2018).
Makedonski, L., Peycheva, K., & Stancheva, M. Determination of heavy metals in selected black sea fish species. Food Control. 72, 313-318 (2017).
Hossain. MB., Tanjin. F., Rahman, MS., Yu, J., Akhter S., Noman MA., et al Metals bioaccumulation in 15 commonly consumed fishes from the lower Meghna river and adjacent areas of Bangladesh and associated human health hazards. Toxics. 10, 139 (2022).
Moiseenko TI & Gashkina NA Distribution and bioaccumulation of heavy metals (Hg, Cd and Pb) in fish: Influence of the aquatic environment and climate. Environ. Res. Lett., 15(11), 115013 (2020).
Mohanta, VL., Naz, A., Mishra, BK. Distribution of heavy metals in the water, sediments, and fishes from Damodar river basin at steel city, India: A probabilistic risk assessment. Hum. Ecol. Risk Assessment (2019).
Chatta, A., Khan, M., Mirza, Z. & Ali, A. Heavy metal (cadmium, lead, and chromium) contamination infarmed fish: A potential risk for consumers’ health. Turk J Zool. 40, 248–256 (2016).
Singh, AK., Srivastava, SC., Verma, P., Ansari, A., Verma., A. Hazard assessment of metals in invasive fish species of the Yamuna River, India in relation to bioaccumulation factor and exposure concentration for human health implications. Environ. Monitor. Assess. 186, 3823–3836 (2014).
Hossain, M. B. et al. Metals bioaccumulation in 15 commonly consumed fishes from the lower Meghna river and adjacent areas of Bangladesh and associated human health hazards. Toxics. 10, 139 (2022).
Hossain, A., Rahman, M. M., Saha, B., Moniruzzaman, M. & Begum, M. Heavy metal concentration and its toxicity assessment in some market fishes of Dhaka city. Int. J. Fish. Aquat. Stud. 4, 523–527 (2016).
Paudel, P. N., Pokhrel, B., Kafle, B. K., & Gyawali, R. Analysis of heavy metals in some commercially important fishes of Kathmandu Valley, Nepal. Int. Food Res. J. 23(3) (2016).
Yang, F. et al. Application of stable isotopes to the bioaccumulation and trophic transfer of arsenic in aquatic organisms around a closed realgar mine. Sci. Total Environ. 15(726), 138550 (2020).
Real, MIH., Azam, HM., Majed., N. Consumption of heavy metal contaminated foods and associated risks in Bangladesh. Environ. Monit Assess. 189:651 (2017).
Arulkumar, A., Paramasivam, S. & Rajaram, R. Toxic heavy metals in commercially important food fishes collected from Palk Bay, southeastern India. Mar. Pollut. Bull. 119, 454–459 (2017).
Sarah R, Tabassum B, Idrees N, Hashem A, Abd_Allah EF. Bioaccumulation of heavy metals in Channa punctatus (Bloch) in river Ramganga (UP), India. Saudi J. Biol. Sci.. 2019;26(5):979-84.
Zhou, X. et al. Construction of multiple electromagnetic loss mechanism for enhanced electromagnetic absorption performance of fish scale-derived biomass absorber. Compos. Part B Eng. 192, 107980 (2020).
Wangboje, O. M. & Ikhuabe, A. J. Heavy metal content in fish and water from river from fish consumption collected along swat river. Pakistan. Environ. Technol. Innov. 17, 100554 (2020).
Jinadasa, BKKK., Jayasinghe, GDTM., Pohl, P., Fowler, SW Mitigating the impact of mercury contaminants in fish and other seafood—a review. Mar Pollut Bull. 171:112710 (2021).
Kimakova, T., Kuzmova, L., Nevolna, Z. & Bencko, V. Fish and fish products as risk factors of mercury exposure. Ann. Agric. Environ. Med. 25, 488–493 (2018).
Grgec, A. S. et al. Potential risks and health benefits of fish in the diet during the childbearing period: focus on trace elements and n-3 fatty acid content in commonly consumed fish species from the Adriatic Sea. Adv. Environ. 8, 100226 (2022).
Kral, T. et al. Accumulation of mercury in the tissues of the great cormorant (Phalacrocorax carbo) from common carp. Bull. Environ. Contam. Toxicol. 98, 167–171 (2017).
FishstatJ FAO. FishStatJ-software for fishery and aquaculture statistical time series. FAO Fish Div Rome. (2020).
Acknowledgements
The authors extend their appreciation to the Researchers Supporting Project number (RSP2025R176) King Saud University, Riyadh, Saud Arabia. The authors are highly thankful to Pioneer Aqua’s Ltd. Company for their generous support in helping with the collection of samples for this study.
Funding
The authors extend their appreciation to the Researchers Supporting Project number (RSP2025R176) King Saud University, Riyadh, Saud Arabia.
Author information
Authors and Affiliations
Contributions
Raheel Suleman, Muhammad Aftab Zahoo, Muhammad Awais Qarni, Weili Rao, Majid Hussain, Tariq Ismail, Shah Saud, Shah Hassan, Taufiq Nawaz and Saeed Akhtar, collected data, visited the study area, analyzed the data, and wrote the Manuscript. Raheel Suleman supervised the whole research and provided guidelines for the conduction of research while Shah Fahad reviewed the manuscript before submission, Muhammad Aftab Zahoo, and Muhammad Awais Qarni designed the material and methods. Sultan Muhammad, Mohammad K. Okla , Ibrahim A. Alaraidh, Ibrahim A. Saleh, Gehad AbdElgayed helped in data collection and provided accessibility to the study area and data analysis. Shah Fahad revised and arranged the tables and figures.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Ethics approval and consent to participate
We all declare that manuscripts reporting studies do not involve any human participants, human data, or human tissue. So, it is not applicable.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
The original online version of this Article was revised: In the original version of this Article, the Conclusion section contained an incorrect statement. Full information regarding the correction made can be found in the correction for this Article.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Suleman, R., Zahoor, M.A., Qarni, M.A. et al. Assessment of heavy metals and microbial loads in Nile tilapia (Oreochromis niloticus) from different farms and rivers. Sci Rep 15, 5055 (2025). https://doi.org/10.1038/s41598-025-87152-y
Received:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s41598-025-87152-y