Abstract
Cancer-associated fibroblasts (CAFs) are a crucial component in the tumor microenvironment (TME) of peritoneal metastasis (PM), where they contribute to tumor progression and metastasis via secretion of interleukin-6 (IL-6). Here, we investigated the role of IL-6 in PM of gastric cancer (GC) and assessed whether anti-IL-6 receptor antibody (anti-IL-6R Ab) could inhibit PM of GC. We conducted immunohistochemical analysis of IL-6 and α-smooth muscle (α-SMA) expressions in clinical samples of GC and PM, and investigated the interactions between CAFs and GC cells in vitro. Anti-tumor effects of anti-IL-6R Ab on PM of GC were investigated in an orthotopic murine PM model. IL-6 expression was significantly correlated with α-SMA expression in clinical samples of GC, and higher IL-6 expression in the primary tumor was associated with poor prognosis of GC. Higher IL-6 and α-SMA expressions were also observed in PM of GC. In vitro, differentiation of fibroblasts into CAFs and chemoresistance were observed in GC cells cocultured with fibroblasts. Anti-IL-6R Ab inhibited the progression of PM in GC cells cocultured with fibroblasts in the orthotopic mouse model but could not inhibit the progression of PM consisting of GC cells alone. IL-6 expression in the TME was associated with poor prognosis of GC, and CAFs were associated with establishment and progression of PM via IL-6. Anti-IL-6R Ab could inhibit PM of GC by the blockade of IL-6 secreted by CAFs, which suggests its therapeutic potential for PM of GC.
Similar content being viewed by others
Introduction
Systemic combination chemotherapy with multiple molecular-targeted therapeutic agents and immunotherapy is a standard treatment for gastric cancer (GC) patients who have distant metastasis and recurrence1,2. Peritoneal metastasis (PM) is one of the most common forms of distant metastasis and recurrence of GC, and curative treatment options remain difficult to find because the unique intraperitoneal tumor microenvironment (TME) and peritoneal–plasma barrier impedes the efficacy of conventional systemic chemotherapy3,4,5. The TME comprises extracellular matrix, endothelial cells, cancer-associated fibroblasts (CAFs), and immune cells, and their interactions are associated with tumor progression and metastasis6,7. CAFs are crucial components of the TME, and as they are related to tumor progression and metastasis, including PM, they are expected to be potential therapeutic targets8,9,10. We have recently described important roles of CAFs in the establishment and progression of PM in GC9, reported that interleukin-6 (IL-6) secreted by CAFs in the TME was associated with tumor immunosuppression, and also that neutralizing IL-6 in the TME suppressed tumor progression11,12. Moreover, we have shown that intraperitoneal IL-6 concentration was significantly elevated in GC patients with PM compared to those without PM, and that among Stage IV GC patients, those with higher intraperitoneal IL-6 concentration had a significantly worse prognosis than those with lower IL-6 concentration13. Therefore, we hypothesize that IL-6 has potential as a therapeutic target in PM of GC.
IL-6 is a multifunctional cytokine involved in inflammation that accompanies injury, infection and immune disease, as well as cancers14,15. IL-6 in the TME is secreted by cancer cells, inflammatory cells, and CAFs, whereas extracellular IL-6 binds to the cell surface receptor glycoprotein (gp130) after binding to its IL-6 receptor. Phosphorylating JAKs then activate several cell-survival pathways, such as the JAK-STAT3 pathway, contributing to the promotion of several malignant phenotypes in various cancers15,16,17.
Tocilizumab (TCZ) is a humanized monoclonal antibody against IL-6 receptor (IL-6R). It blocks the intracellular IL-6 pathway by binding to both soluble and membrane-bound IL-6R, and is an FDA-approved drug for rheumatic arthritis and Crohn’s disease18,19. Although the anti-tumor effects of anti-IL-6R have been investigated in various types of cancer models with the aim of blocking IL-6 related to tumor progression, TCZ has shown no significant benefits as a novel cancer treatment20,21,22. Here, we have demonstrated the efficacy of anti-IL-6R treatment in treatment of a PM model of GC.
In the present study, higher expressions of IL-6 and α-smooth muscle (α-SMA), which is a marker of CAFs, were confirmed in immunohistochemical analysis of clinical samples of primary tumor and PM of GC. Higher IL-6 expression was correlated with α-SMA expression and also associated with poor prognosis of GC. We also investigated the interactions between cancer cells and CAFs via IL-6 in vitro, and whether intraperitoneal administration of anti-IL-6R could suppress tumor progression in a vivo allograft PM model.
Results
Higher IL-6 expression secreted by CAFs is a poor prognostic factor and promotes PM
We performed immunohistochemical (IHC) analysis to evaluate the clinical impacts of the expression of IL-6 and αSMA (used as a CAF marker in primary tumor) in GC.
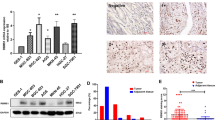
Enrolled in the study were 168 consecutive GC patients who received curative gastrectomy with lymph node dissection at Okayama University Hospital between February 2002 and July 2007. Table 1 lists the patients’ demographics and clinical characteristics. The expression of IL-6 was significantly correlated with expression of αSMA (r = 0.54, p < 0.001) (Fig. 1a). When patients were divided into high and low IL-6 expression groups based on the median value (5.19) of IL-6 area index, patients with high IL-6 expression showed significantly shorter overall survival (OS) and disease-free survival (DFS) than those with low expression (Fig. 1b). To investigate the relationship between IL-6 secreted by CAF and PM, we conducted IHC of surgically resected peritoneal disseminated nodules from 15 GC patients. Similarly, non-metastatic peritoneal (NMP) tissue was investigated as the basal level of IL-6 and αSMA. The mean IL-6 and αSMA area index of 5 NMP tissues were 1.56 ± 1.00% and 0.52 ± 0.29%, which were considerably lower than PM (Fig. 1c).
Correlation of IL-6 and CAFs in primary tumor of gastric cancer (GC) and peritoneal metastasis (PM). (a) Representative microscopic images of H&E, IL-6, and α-SMA at middle and high magnification. Area index for each staining was evaluated by Image J. Scale bar, 500 μm (upper) and 50 μm (lower). Correlation between IL-6 and α-SMA is shown in the scatter plot (Spearman’s correlation coefficient). The correlation coefficient (cc) was 0.54 and p < 0.0001. (b) Overall survival (OS) and disease-free survival (DFS) curves according to IL-6 expression (high or low) in the primary tumor of GC. (c) IL-6 and α-SMA expression in PM resected from 15 GC patients and non-metastatic parts (NMP) of peritoneal tissue. IL-6 expression (blue bars) and α-SMA expression (green bars) are shown for each of the 15 cases. The value of NMP indicates the mean IL-6 and α-SMA expression of 5 NMP tissues.
Fibroblasts promote IL-6 secretion and chemo-resistance in cooperation with cancer cells
We quantified IL-6 concentration by ELISA in supernatants from mouse GC (T3-2D) and mouse fibroblast (MEF) cells and from co-cultures of both cells to investigate the interactions between GC cells and fibroblasts via IL-6 in the TME. Although the IL-6 concentration was quite low when the T3-2D or MEF cells were cultured alone, co-cultures of both cells secreted significantly higher IL-6 concentrations (Fig. 2a). To investigate whether IL-6 could differentiate normal fibroblasts into CAFs, we evaluated αSMA expression in the various conditions by western blot analysis. Conditioned medium (CM) from GC cells and TGF-β, which is known as a CAF-inducing factor, increased the expression of αSMA. Similarly, IL-6 treatment increased the expression of αSMA, which suggests that IL-6 can differentiate normal fibroblasts into CAFs (Fig. 2b).
Interaction between cancer cells and fibroblasts. (a) GC cells (T3-2D) and normal fibroblasts (MEF) secrete IL-6 in a seeding-density-dependent manner (×104 cells). IL-6 secretion is significantly increased under the condition of co-culture of T3-2D and MEF. Data are shown as mean ± SD (n = 3). (b) Whole-cell lysates of MEF and NGF cells collected 72 h after IL-6 (ng/ml) or TGF-β or conditioned medium of GC cells subjected to western blot analysis of α-SMA and β-actin expression. (c) CAFs induce resistance to chemotherapeutic agents in GC cells. T3-2D cells or T3-2D cells co-cultured with MEF were treated with 5-FU and oxaliplatin.
To investigate the effects of CAFs on the resistance of GC to chemotherapy, cell viability against 5-FU and oxaliplatin was compared between T3-2D cells alone and co-cultures of T3-2D and MEF. T3-2D cells co-cultured with MEF had significantly increased resistance to 5-FU compared to T3-2D cells alone. And a similar trend was observed in oxaliplatin treatment (Fig. 2c).
CAFs promote establishment and progression of PM of GC
We inoculated cancer cells (T3-2D) or co-inoculated T3-2D and fibroblasts (MEF) into the peritoneal cavity of C57BL/6J mice and compared the total number of peritoneal tumors between these groups to assess whether CAFs promote tumor growth in the peritoneal cavity. The total number of tumors was significantly higher in the co-inoculated T3-2D and MEF group than in the T3-2D group (Fig. 3a). In addition, the expression of αSMA in PM was significantly higher in the co-inoculated T3-2D and MEF group than in the T3-2D group (Fig. 3b), which suggests that CAF-rich tumor was established by co-inoculation of fibroblasts. Furthermore, intraperitoneal IL-6 concentration was significantly higher in the co-inoculation group than in the T3-2D group (Fig. 3c).
CAFs promote the establishment and progression of PM of GC. (a) T3-2D cells (5 × 104 cells) alone or co-inoculated with MEF (1 × 105 cells) were inoculated into the abdominal cavity of C57BL/6J mice. Total numbers of PM were measured 14 days after tumor inoculation. Data are shown as the mean ± SD (n = 5). (b) Immunohistological analysis of α-SMA expression in PM. Tumor tissues were harvested 14 days after tumor inoculation. Scale bar, 100 μm. Areas of α-SMA expression in the peritoneal tumors were evaluated using an area index calculated by Image J. Data are shown as the mean ± SD (n = 3). Statistical significance was defined as p < 0.05 (*). (c) Intraperitoneal IL-6 concentration in peritoneal lavage was quantified by ELISA. Data are shown as the mean ± SD (n = 3). Statistical significance was defined as p < 0.05 (*).
MR16-1 suppresses PM consisting of CAF-rich tumors
The anti-tumor effect of MR16-1 to PM was evaluated in an orthotopic mouse model established from T3-2D alone and from co-inoculated T3-2D and MEF (Fig. 4a). Although MR16-1 did not suppress PM established from T3-2D cells alone, it significantly suppressed PM established from co-inoculated T3-2D and MEF (Fig. 4b, c), suggesting an anti-tumor effect of MR16-1 was confirmed in CAF-mixed tumor.
Anti-IL-6R Ab suppressed the progression of PM in CAF-mixed allograft model. T3-2D cells or T3-2D cells with MEF cells were intraperitoneally inoculated into C57BL/6J mice. Mice were treated with intraperitoneal administration of PBS or 500 µL of solution containing MR16-1 (200 µg/body) every 3 days for a total of 3 doses. (A) Schematic of the treatment schedule. Green arrows show the timing of treatment with PBS or MR16-1 and the vertical black line indicates sacrifice. (B) Orthotopic PM model established from T3-2D cells. The total number of tumors in the peritoneal cavity was measured 14 days after tumor inoculation. Data are shown as the mean ± SD (n = 5). There was no difference in total tumor numbers between the groups. (C) Orthotopic PM model established from T3-2D cells and MEF cells. The total number of tumors and tumor weight in the peritoneal cavity were measured 14 days after tumor inoculation. Data are shown as the mean ± SD (n = 5). Statistical significance was defined as p < 0.05 (*).
Discussion
We demonstrated that IL-6 was secreted mainly by CAFs in primary GC tumor and PM, and that higher expression of IL-6 was associated with poor prognosis of GC. Interaction between cancer cells and fibroblasts enhanced the differentiation of fibroblasts into CAFs and the chemo-resistance of GC via IL-6. Furthermore, we showed that intraperitoneal administration of MR16-1, a rodent analog of anti-IL-6R Ab, suppressed PM of GC in a CAF-abundant allograft mouse model. These findings suggest the therapeutic potential of anti-IL-6R Ab for PM of GC.
CAF is an important component associated with the establishment and progression of PM of GC in the TME6,7,9. It has been reported that IL-6 is significantly upregulated in CAFs compared to normal fibroblasts in GC9,23. In the present study, expression of IL-6 showed a significant correlation with the expression of α-SMA, one of the CAF markers in the primary GC tumor, suggesting that IL-6 is secreted mainly by CAFs in GC tumors. Previous analysis of The Cancer Genome Atlas data has also shown that IL-6 within GC tissues was mainly co-expressed with stromal-related genes, although IL-6 was also secreted by cancer cells and immune cells such as tumor-associated macrophages (TAMs)9,11,12,23,24. Genetic and epigenetic alteration in CAF contributes to its cancer-supportive properties in several cancers25,26. We have previously shown that alteration of p53 phosphorylation CAFs in GC contributed to its cancer-supportive properties; and have reported that higher CAF expression in GC was associated with poor prognosis of GC9 and that higher IL-6 expression in esophageal cancer was also associated with poor prognosis12. Similarly, the present study revealed that higher IL-6 expression in primary GC tumors was associated with poor prognosis of GC, and that higher IL-6 expression was observed in the PM tissues of GC. Previous studies have reported that serum IL-6 level was a poor prognostic marker in several cancers, including GC27,28,29. We have previously shown that serum IL-6 level was significantly higher in co-inoculated cancer cells and fibroblasts group than in cancer cells alone group in mouse model using colon cancer. And serum IL-6 level was highly correlated with αSMA expression in TME, which suggests serum IL-6 level is correlated with IL-6 expression in TME12. Furthermore, we have previously reported that intraperitoneal IL-6 level was a poor prognostic marker in GC patients with PM13. Therefore, IL-6 secreted by CAFs is one of the key mediators associated with the establishment and progression of PM, and might therefore be a therapeutic target for PM.
Although IL-6 is known to be a multifunctional cytokine involved in the immune and inflammatory responses30, it also plays a critical role in the progression and metastasis of tumors. IL-6 promotes metastasis by inducing epithelial mesenchymal transition in various types of cancers in addition to GC, and activates the JAK/STAT3 pathway after binding with the IL-6 receptor of cancer cells, thus contributing to development of resistance to chemotherapy16,23,31,32. In the present study, cancer cells stimulated the differentiation of normal fibroblasts into CAFs and triggered IL-6 secretion from CAFs, which further increased the secretion of IL-6 and differentiation into CAFs. Furthermore, GC cells co-cultured with fibroblasts developed chemoresistance, although we did not demonstrate the precise mechanism related to chemoresistance. We have previously reported that IL-6 secretion from CAFs was increased under hypoxic conditions in the TME, and that IL-6 induced tumor immunosuppression by decreasing cytotoxic T cells and increasing regulatory T cells and TAMs via hypoxia-pseudohypoxia-mediated hypoxia-inducible factor 1α11,12. Intraperitoneal TAMs induced by IL-6 were strongly associated with tumor immunosuppression and promoted PM of GC13,33. Furthermore, intraperitoneal TME bearing PM is known to be a hypoxic condition34, which might enhance tumor immunosuppression via IL-6. Therefore, targeting IL-6 could be a promising treatment option for PM of GC.
In addition to its use in conventional inflammation-related diseases such as rheumatic arthritis and Crohn’s disease, TCZ has recently been applied to the treatment of patients with severe or critical coronavirus disease 2019 (COVID-19) and to alleviate the symptoms of toxicity induced by chimeric antigen receptor T cell therapy35,36. As IL-6 is well known as a tumor-progressing cytokine, TCZ monotherapy has been also attempted for treatment of several types of cancers, but showed no significant anti-tumor effects20,21,22. However, TCZ in combination with conventional chemotherapeutic agents has been reported to improve chemotherapeutic resistance in the treatment of GC23. In the present study, CAF-mixed tumors showed increased secretion of IL-6 in the peritoneal cavity compared to the cancer-cell-alone model. Furthermore, TCZ suppressed the progression of PM in CAF-mixed tumors although it could not in the cancer-cell-alone model. TCZ monotherapy might have clinical potential for the treatment of PM because analysis revealed that CAFs were highly expressed in clinical samples of PM. However, TCZ monotherapy might not be sufficient to overcome PM of GC, and combination therapy with conventional cytotoxic agents or immune therapy would be more effective.
This study has some limitations. First, although we demonstrated that higher IL-6 expression of primary GC tumor was correlated with αSMA expression and also associated with poor prognosis of GC, IL-6 expression in PM was analyzed in only 15 patients and it is unclear whether IL-6 expression in PM is associated with poor prognosis of GC. Second, we did not show a direct mechanism for the anti-tumor effects of anti-IL-6R Ab for PM, although IL-6 secreted from CAFs has been reported to contribute to promoting several malignant phenotypes and progression of tumors via activation of the JAK/STAT3 pathway. Finally, MR16-1 was used to evaluate the anti-tumor effect of anti-IL-6 Ab for PM. Future clinical trials using TCZ are needed to evaluate its efficacy and safety for patients with PM.
In conclusion, we have demonstrated that higher IL-6 expression secreted by CAFs was associated with poor prognosis of GC, and that IL-6 might contribute to the establishment and progression of PM. Furthermore, anti-IL-6R Ab treatment suppressed the progression of PM in a CAF-mixed allograft model. Anti-IL-6R Ab shows promise as a novel treatment strategy against PM of GC.
Materials and methods
Patients and immunohistochemistry
A total of 168 GC patients who received gastrectomy with lymph node dissection at Okayama University Hospital between February 2002 and July 2007 were retrospectively reviewed and investigated. The clinical and pathological stage classification and diagnosis were performed based on the Japanese Classification of Gastric Carcinoma37. In 15 patients with small white nodules on the peritoneum, the nodules were resected and IHC was performed on those that were diagnosed histologically as PM of GC. Sectioned tissues were incubated with mouse anti-IL-6 monoclonal antibody (mAb) (Abcam, Cambridge, MA, USA), anti-α-SMA mAb (Sigma-Aldrich, St. Louis, MO, USA), rabbit anti-IL-6 receptor polyclonal antibody (pAb) (Abcam, Cambridge, MA, USA) for immunohistochemistry after the presence of tumor was confirmed using hematoxylin and eosin staining. The expression levels of IL-6 and α-SMA were evaluated using area index, calculated at low magnification (× 40), by image J software (http://rsb.info.nih.gov/ij/), as described previously9,11,12. Area index was evaluated for three carefully selected fields per sample that contained the cancerous area and were well stained. Similarly, four non-NMP tissues were evaluated as a control. The mean value obtained for each sectioned tissue was defined as the area index of α-SMA and IL-6. All evaluations were performed by an independent pathologist who was blinded to the clinical information. Immunoreactive signals were visualized with 3,3’-diaminobenzidine tetrahydrochloride solution, and nuclei were counterstained with hematoxylin. Sections were observed under light microscopy (BX50; Olympus, Tokyo, Japan).
Cell lines
The human GC cell line MKN45 was purchased from the Japanese Collection of Research Bioresources Cell Bank and maintained in RPMI-1640 medium supplemented with 10% heat-inactivated fetal bovine serum (FBS) (Sigma-Aldrich). T3-2D is a mouse gastric cancer cell line established by Dr. Ohki and Dr. Ohtsuka (National Cancer Center Research Institute, Tokyo, Japan)38 and kindly provided and maintained in DMEM supplemented with 10% FBS and 1% penicillin-streptomycin (100 U/mL). The murine fibroblast (MEF) cell line was purchased from American Type Culture Collection (ATCC, Manassas, VA, USA). Normal gastric fibroblasts (NGFs) were established from non-tumoral gastric wall tissue that had been surgically excised, as reported previously9. NGFs were maintained in DMEM with 10% FBS and 0.5 mM sodium pyruvate. All media were supplemented with 100 U/mL penicillin and 100 µg/mL streptomycin. Cells were routinely maintained at 37 °C in a humidified atmosphere with 5% CO2.
Reagents and chemotherapeutic reagents
Recombinant mouse and human IL-6/IL-6 receptor-α protein were obtained from R&D Systems (Minneapolis, MN, USA), and these agents were used in a 1:5 ratio. Recombinant human transforming growth factor β1 (TGF-β1) was obtained from Sigma-Aldrich (St. Louis, MO, USA). Oxaliplatin and 5-fluorouracil (5-FU) were purchased from Nippon Kayaku (Tokyo, Japan) and dissolved in PBS. A rat anti-mouse-IL-6 receptor antibody, MR16-1, was kindly provided by Chugai Pharmaceutical Co., Ltd. (Tokyo, Japan).
Cell viability assay
T3-2D cells were seeded onto 96-well plates at a density of 1 × 103 cells/well and cultured or co-cultured with MEF (1 × 103 cells/well) for 24 h before the administration of chemotherapeutic agents. Cell viability was examined 72 h after cell seeding using the Cell Proliferation Kit II (Roche Molecular Biochemicals, Indianapolis, IN, USA), which is based on the sodium 3’-[1-(phenylaminocarbonyl)-3,4-tetrazolium]-bis(4-methoxy-6-nitro) benzene sulfonic acid hydrate (XTT) assay, in accordance with the protocol from the manufacturer.
Western blot analysis
MEF/NGF cells were seeded in a 100-mm dish at a density of 2 × 105 cells/dish. Supernatants were exchanged to the conditioned medium from T3-2D/MKN45 cells or the normal medium with or without IL-6 or TGF-β administration at 24 h after seeding. After a further 72 h of cell culture, whole-cell lysates were prepared in PBS containing a phosphatase inhibitor cocktail (PhosSTOP; Roche Applied Science, Mannheim, Germany). Proteins were separated by SDS-PAGE and transferred to polyvinylidene difluoride membranes (Hybond P; GE Healthcare, Chicago, IL, USA). Membranes were blocked with Blocking One (Nacalai Tesque, Kyoto, Japan) at room temperature for 30 min and then incubated overnight at 4 °C with rabbit anti-α-SMA mAb (Sigma-Aldrich) and mouse anti-β-actin mAb (Sigma-Aldrich). Immunoreactive bands on blots were visualized using enhanced chemiluminescence substrates (ECL Plus; GE Healthcare).
ELISA
Cells were seeded at the indicated density and supernatants were exchanged 24 h after cell seeding. After a further 48 h of cell culture, IL-6 levels in cell culture supernatants were assessed using a Mouse IL-6 Quantikine ELISA Kit (R&D Systems), according to the manufacturer’s protocol.
Animal experiments
T3-2D cells (5 × 104 cells) were inoculated into the peritoneal cavity of 6- to 8-week-old female C57BL/6J mice that were purchased from CLEA (Tokyo, Japan) as models of peritoneal dissemination of GC. In the co-injection model, both T3-2D (5 × 104 cells) and MEF (1 × 105 cells) were inoculated into the peritoneal cavity. In the experiment with MR16-1 treatment, T3-2D cells (3 × 104 cells) or T3-2D cells (1 × 104 cells) co-injected with MEF (2 × 104 cells) were inoculated into the peritoneal cavity, and PBS or 500 µL of solution containing MR16-1 (200 µg/body) was injected into the intraperitoneal cavity every 3 days for a total of 3 doses. Five mice were used for each group. All tumor nodules in the peritoneal cavity were resected and total weights were measured on day 14. Animals were excluded from the experiments only if tumors did not form or if health concerns were reported. For all animal experiments, mice were randomly grouped and the measurements of tumor numbers or weight were carried out blind for the groups.
Anti-α-SMA mAb (Sigma-Aldrich) and rabbit anti-IL-6 receptor pAb (Abcam) were used for immunohistochemical analysis of peritoneal tumor nodules. Immunoreactive signals were visualized with a 3,39-diaminobenzidine tetrahydrochloride solution, and nuclei were counterstained with hematoxylin. Sections were viewed under light microscopy (BX50; Olympus).
Statistical analysis
For the area index of IL-6, cut-off was defined using the median value of the high and low groups. Spearman’s correlation was used to assess relationships between IL-6 and α-SMA. Overall survival (OS) was calculated using the Kaplan–Meier method, with the log rank test used for comparisons between subgroups. Student’s t test was used to identify significant differences between groups. All data are expressed as mean ± SD. Values of p < 0.05 were considered statistically significant. Statistical analysis was performed using JMP version 12.2 (SAS Institute, Cary, NC, USA).
Study approval
This study was conducted in accordance with the ethical standards of the Declaration of Helsinki and the ethical guidelines for medical and health research involving human subjects. Studies using clinical samples were approved and reviewed by the Institutional Review Board of Okayama University Hospital (approval nos. 1707-022 and 2307-012). All cases were de-identified and details were removed from the case descriptions to ensure anonymity. Information about the aim of this retrospective study was posted on the website of the Department of Gastroenterological Surgery, Okayama University Hospital, and potential participants could decline to participate or opt out at any time. Owing to its retrospective nature, we requested and received permission to waive informed consent from the Ethics Committee of Okayama University Hospital. All animal experimental protocols were approved by the Ethics Review Committee for Animal Experiments of Okayama University (approval no. OKU-2020167). All animal experiments were conducted in accordance with the guidelines and regulations of the committee.
ARRIVE guidelines
The study is reported in accordance with the ARRIVE guidelines.
Data availability
All data generated or analyzed during this study are included in this article and its supplementary material files. Further enquiries can be directed to the corresponding author.
References
Japanese Gastric Cancer Association. Japanese Gastric Cancer Treatment Guidelines 2021 (6th edition). Gastric Cancer. 26, 1–25. (2023).
Janjigian, Y. Y. et al. First-line nivolumab plus chemotherapy versus chemotherapy alone for advanced gastric, gastro-oesophageal junction, and oesophageal adenocarcinoma (CheckMate 649): a randomised, open-label, phase 3 trial. Lancet 398, 27–40 (2021).
Verstegen, M. H. et al. Metastatic pattern in esophageal and gastric cancer: influenced by site and histology. World J. Gastroenterol. 26, 6037–6046 (2020).
Hasovits, C. & Clarke, S. Pharmacokinetics and pharmacodynamics of intraperitoneal cancer chemotherapeutics. Clin. Pharmacokinet. 51, 203–224 (2012).
Takahashi, K. et al. Altered intraperitoneal immune microenvironment in patients with peritoneal metastases from gastric cancer. Front. Immunol. 13 https://doi.org/10.3389/fimmu.2022.969468 (2022).
Quail, D. F. & Joyce, J. A. Microenvironmental regulation of tumor progression and metastasis. Nat. Med. 19, 1423–1437 (2013).
Yasuda, T. et al. Inflammation-driven senescence-associated secretory phenotype in cancer-associated fibroblasts enhances peritoneal dissemination. Cell. Rep. 34 https://doi.org/10.1016/j.celrep.2021.108779 (2021).
Chen, X. & Song, E. Turning foes to friends: targeting cancer-associated fibroblasts. Nat. Rev. Drug Discov. 18, 99–115 (2019).
Ogawa, T. et al. Modulation of p53 expression in cancer-associated fibroblasts prevents peritoneal metastasis of gastric cancer. Mol. Ther. Oncolytics. 25, 249–261 (2022).
Watanabe, S. et al. Photoimmunotherapy for cancer-associated fibroblasts targeting fibroblast activation protein in human esophageal squamous cell carcinoma. Cancer Biol. Ther. 20, 1234–1248 (2019).
Kato, T. et al. Cancer-associated fibroblasts affect intratumoral CD8(+) and FoxP3(+) T cells via IL6 in the tumor microenvironment. Clin. Cancer Res. 24, 4820–4833 (2018).
Nishiwaki, N. et al. Overcoming cancer-associated fibroblast-induced immunosuppression by anti-interleukin-6 receptor antibody. Cancer Immunol. Immunother. 72, 2029–2044 (2023).
Sakamoto, S. et al. Intraperitoneal cancer-immune microenvironment promotes peritoneal dissemination of gastric cancer. Oncoimmunology 8 (12), e1671760. https://doi.org/10.1080/2162402X.2019.1671760 (2019).
Rincon, M. Interleukin-6: from an inflammatory marker to a target for inflammatory diseases. Trends Immunol. 33, 571–577 (2012).
Kumari, N., Dwarakanath, B. S., Das, A. & Bhatt, A. N. Role of interleukin-6 in cancer progression and therapeutic resistance. Tumour Biol. 37, 11553–11572 (2016).
Bharti, R., Dey, G. & Mandal, M. Cancer development, chemoresistance, epithelial to mesenchymal transition and stem cells: a snapshot of IL-6 mediated involvement. Cancer Lett. 375, 51–61 (2016).
So, K. A., Min, K. J., Hong, J. H. & Lee, J. K. Interleukin-6 expression by interactions between gynecologic cancer cells and human mesenchymal stem cells promotes epithelial-mesenchymal transition. Int. J. Oncol. 47, 1451–1459 (2015).
Tanaka, T., Narazaki, M. & Kishimoto, T. Anti-interleukin-6 receptor antibody, tocilizumab, for the treatment of autoimmune diseases. FEBS Lett. 585, 3699–3709 (2011).
Scott, L. J. Tocilizumab: a review in rheumatoid arthritis. Drugs 77, 1865–1879 (2017).
Nagasaki, T. et al. Interleukin-6 released by colon cancer-associated fibroblasts is critical for tumor angiogenesis: anti-interleukin-6 receptor antibody suppressed angiogenesis and inhibited tumor-stroma interaction. Br. J. Cancer. 110, 469–478 (2017).
Noguchi-Sasaki, M., Sasaki, Y., Shimonaka, Y., Mori, K. & Fujimoto-Ouchi, K. Treatment with anti-IL-6 receptor antibody prevented increase in serum hepcidin levels and improved anemia in mice inoculated with IL-6-producing lung carcinomacells. BMC Cancer. 16, 270. https://doi.org/10.1186/s12885-016-2305-2 (2016).
Shinriki, S. et al. Humanized anti-interleukin-6 receptor antibody suppresses tumor angiogenesis and in vivo growth of human oral squamous cell carcinoma. Clin. Cancer Res. 15, 5426–5434 (2009).
Ham, I. H. et al. Targeting interleukin-6 as a strategy to overcome stroma-induced resistance to chemotherapy in gastric cancer. Mol Cancer. 18; (2019). https://doi.org/10.1186/s12943-019-0972-8
Lowe, J. M. et al. p53 and NF-kappaB coregulate proinflammatory gene responses in human macrophages. Cancer Res. 74, 2182–2192 (2014).
Arandkar, S. et al. Altered p53 functionality in cancer-associated fibroblasts contributes to their cancer-supporting features. Proc. Natl. Acad. Sci. U S A. 115, 6410–6415 (2018).
Costea, D. E. et al. Identification of two distinct carcinoma-associated fibroblast subtypes with differential tumor-promoting abilities in oral squamous cell carcinoma. Cancer Res. 73, 3888–3901 (2013).
Liao, W. C. et al. Serum interleukin-6 level but not genotype predicts survival after resection in stages II and III gastric carcinoma. Clin. Cancer Res. 14, 428–434 (2008).
Chen, I. et al. Serum interleukin-6 as a prognostic biomarker for survival in patients with unresectable pancreatic cancer. Ann. Oncol. 27 https://doi.org/10.1093/annonc/mdw141 (2016).
Nakashima, J. et al. Serum interleukin 6 as a prognostic factor in patients with prostate cancer. Clin. Cancer Res. 6, 2702–2706 (2000).
Grivennikov, S. I., Greten, F. R. & Karin, M. Immunity, inflammation, and cancer. Cell 140, 883–899 (2010).
Wu, X. et al. IL-6 secreted by cancer-associated fibroblasts promotes epithelial-mesenchymal transition and metastasis of gastric cancer via JAK2/STAT3 signaling pathway. Oncotarget 8, 20741–20750 (2017).
Rokavec, M. et al. IL-6R/STAT3/miR-34a feedback loop promotes EMT-mediated colorectal cancer invasion and metastasis. J. Clin. Invest. 124, 1853–1867 (2014).
Tabuchi, M. et al. Functional remodeling of intraperitoneal macrophages by oncolytic adenovirus restores anti-tumor immunity for peritoneal metastasis of gastric cancer. Mol. Ther. Oncol. https://doi.org/10.1016/j.omton.2024.200806 (2024).
Kanda, M. & Kodera, Y. Molecular mechanisms of peritoneal dissemination in gastric cancer. World J. Gastroenterol. 22, 6829–6840 (2016).
Li, G., Hilgenfeld, R., Whitley, R. & De Clercq, E. Therapeutic strategies for COVID-19: progress and lessons learned. Nat. Rev. Drug Discov. 22, 449–475 (2023).
Jain, M. D., Smith, M. & Shah, N. N. How I treat refractory CRS and ICANS after CAR T-cell therapy. Blood 141, 2430–2442 (2023).
Japanese Gastric Cancer Association. Japanese classification of gastric carcinoma: 3rd English edition. Gastric Cancer. 14, 101–112 (2011).
Ohtsuka, J. et al. Functional loss of p53 cooperates with the in vivo microenvironment to promote malignant progression of gastric cancers. Sci Rep. 8; (2018). https://doi.org/10.1038/s41598-018-20572-1
Acknowledgements
This work was supported by JSPS KAKENHI grant number 24K11912 (S.Ki.). We wish to thank Ms. Tomoko Sueishi, Ms. Tae Yamanishi, and Ms. Yuko Hoshijima for their excellent technical assistance.
Funding
This study was supported by JSPS KAKENHI grant no. 24K11912 (S.Ki.).
Author information
Authors and Affiliations
Contributions
SKi concepted and designed the study. EM, SKi, TOk and TF developed the methodology of the experiments. EM, SKi, TOk, YU and NN performed the experiments and acquired the data. RO and JO developed T3-2D cells. EM, SKi, HT, KN and TOh analyzed and interpreted the data. EM and SKi wrote the first draft of the article. TH and TF revised the draft and SKu, KN, and SKa discussed the result and further revised the manuscript. All authors contributed to the final manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Disclosure of potential conflicts of interest
The authors have no real or potential conflicts of interest to declare.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Mitsui, E., Kikuchi, S., Okura, T. et al. Novel treatment strategy targeting interleukin-6 induced by cancer associated fibroblasts for peritoneal metastasis of gastric cancer. Sci Rep 15, 3267 (2025). https://doi.org/10.1038/s41598-025-88033-0
Received:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s41598-025-88033-0
Keywords
This article is cited by
-
Immune profiling identifies the contribution of extracellular vesicles to immune modulation and progression in prostate cancer
Scientific Reports (2025)
-
Research progress on chronic stress stimulation and gastric cancer metastasis
Discover Oncology (2025)