Abstract
Saline-alkali stress is a major abiotic stress factor that adversely affects the growth, development, and yield of crops by disrupting ion homeostasis, osmotic balance, and metabolic processes. This study was designed to explore the alleviating effect of melatonin on the growth and development of tomato plants under saline-alkali stress conditions and to screen for optimal concentrations to alleviate the stress. Tomato variety ‘Condine Red’ was used as the test material, and a total of six treatments were designed including no saline-alkali stress and no melatonin spray as control (CK), and foliar spraying of 0, 50, 100, 150, and 200 µmol·L− 1 melatonin under saline-alkali stress (75 mmol·L− 1), which were used to determine the growth and photosynthetic characteristics of tomato plants. The results showed that saline-alkali stress significantly inhibited plant height, stem diameter, root activity and biomass accumulation, significantly reduced the chlorophyll content of tomato leaves and the efficiency of photosynthetic electron transfer from primary quinone receptor QA to secondary quinone receptor QB, and caused significant deformation of the fast chlorophyll fluorescence induced kinetic curve (OJIP), inhibiting photosynthesis. Exogenous melatonin could improve tomato tolerance to saline-alkali stress, and the effect depended on the concentration. In this experiment, treatment with 100 µmol·L− 1 melatonin showed the strongest positive effect on the growth of tomato plants under saline-alkali stress according to the comprehensive evaluation of principal components. In addition, changes in photosynthetic chlorophyll fluorescence parameters and chlorophyll fluorescence induction curves after melatonin treatment highlighted that melatonin could improve the response of the photosynthetic system to saline-alkali stress by enhancing quenching of excess excitation energy and protecting the photosynthetic electron transport system. Collectively, exogenous melatonin pretreatment increased root activity, chlorophyll content and improved photosystem processes, thereby alleviating tomato growth under saline-alkali stress. The results of this study lay the foundation for the practical application of melatonin in saline-alkali stress.
Similar content being viewed by others
Introduction
Tomato (Solanum lycopersicum L.) is an important nutritional and commercial crop, and it is also a model plant for research in genetics, fruit development, and stress tolerance1. However, in addition to moderate salt sensitivity, tomatoes are also vulnerable to abiotic stresses such as salt and alkali, which hinder seed germination, plant growth and fruit yield2,3. With the increase of population and the deterioration of natural environment, soil salinization has become an increasingly serious major limiting factor for global agricultural crop production4,5. According to statistics, currently about 7% (over 900 million hectares) of the world’s land and 33% of arable land are suffering from salinization6, and a large amount of unreasonable agricultural management and environmental pollution are exacerbating the trend of soil salinization in arable land. Generally speaking, the stress mainly caused by neutral salts such as NaCl and Na2SO4 is referred to as salt stress, while the stress caused by alkaline salts such as NaHCO3 and Na2CO3 is referred to as alkaline stress7. Previous studies have shown that soil problems caused by alkaline salts are more severe than those caused by neutral salts, resulting in greater damage to plants8,9. Under natural conditions, salt stress and alkali stress often coexist, and the synergistic effect of the two stresses constitutes saline-alkali stress10. Therefore, its harm to plants is far greater than any single stress. The harm of saline-alkali stress to plants mainly focuses on ion toxicity11, osmotic stress12, oxidative stress13, and high pH stress14. Under the interaction of multiple hazards, the external morphological and internal physiological characteristics of plants will change15, inhibiting plant growth16and ultimately leading to a decrease in crop yield and quality17,18,19. Therefore, finding new strategies for saline-alkali tolerance is crucial for protecting the normal growth and development of tomato plants.
Plant hormones, as important small molecule substances in plants, play a crucial role in their growth, development, and response to environmental stress20, applying plant growth regulators to strengthen the resistance of tomato plant to combined saline-alkali stress is one of the fastest and more effective solutions. Currently, it has been reported that various plant hormones such as abscisic acid, brassinolide, methyl jasmonate, and salicylic acid play key roles in plant response to salt stress21,22,23,24. Melatonin is an indole tryptamine widely distributed in various tissues and organs in plants. It is closely related to plant life activities such as seed germination25, photosynthesis26, root development27, and leaf senescence28, and plays an important role in regulating plant response to environmental stress29. Melatonin has high lipophilicity, so it can freely pass through plasma membranes such as cell membranes, chloroplast membranes, and mitochondria. During the clearance of reactive oxygen species, it can move freely and quickly to areas where a large amount of reactive oxygen species are produced to exert antioxidant effects30. Previous studies have shown that exogenous melatonin could enhance the antioxidant capacity and photosynthesis of maize seedlings, thereby increasing their tolerance to salt stress31. The application of exogenous melatonin can improve the salt tolerance of cotton by coordinating other plant hormone signaling pathways32. In addition, exogenous melatonin alleviates the inhibitory effect of salt stress on cucumber seeds by inducing the expression of genes related to abscisic acid and gibberellin synthesis33. It can be seen that melatonin can act as an antioxidant to eliminate free radicals, thereby reducing oxidative damage, and can act as a signaling molecule to activate stress response gene expression, thereby enhancing abiotic stress resistance.
At present, research on saline-alkali stress mostly focuses on the damage to plants caused by salt stress, while there is relatively little research on the physiological responses, regulatory mechanisms, and signal transduction pathways of plants in response to saline-alkali stress, ignoring the actual situation of saline-alkali land. Saline-alkali stress is relatively more complex than salt stress and alkali stress, and the mechanism of melatonin’s response to saline-alkali stress in tomato is still unclear. Therefore, in this study, tomato seedlings were used as experimental materials to explore the effect of melatonin on plant growth under saline-alkali stress by spraying exogenous melatonin. Our results may provide theoretical significance and practical value for the practical application of melatonin in saline-alkali stress and for guiding stress-resistant cultivation.
Materials and methods
Plant materials and growth conditions experimental design
This study used tomato variety CR (Solanum lycopersicum cv. Condine Red) as the experimental material, and melatonin was purchased from Shanghai Yuanye Biotechnology Co., Ltd. Tomato seeds were soaked in warm water at 55 °C for 15 min and then placed on a shaker at 28 °C and 200 rpm for 6 h to promote germination. Afterwards, germination was carried out in the dark, and the seeds were cultured in an artificial climate chamber after they turned white. When the first true leaf of tomato seedlings is not fully unfolded, the roots were cleaned and transplanted them into 10 L hydroponic boxes, with 11 plants per box. The hydroponic nutrient solution is Hoagland nutrient solution, which is changed every two days. Environment settings inside the incubator: the temperature of 28 °C/18 °C (day/night) and the photoperiod of 12 h/12 h (day/night), 20,000 Lx light for 12 h, relative humidity of 70%. When tomato seedlings grow to four leaves and one center, select healthy seedlings with consistent growth for experimental treatment.
Experimental design
After the fourth leaf of the ‘Condine Red’ tomato seedling was fully unfolded, the leaves of the tomato seedlings were sprayed with melatonin solution of corresponding concentrations and ddH2O respectively for two consecutive days, with 5 mL sprayed on each tomato seedling (spray both the front and back of the leaves). A solution of 75 mmol·L− 1 composite saline-alkali (NaCl: Na2SO4: NaHCO3: Na2CO3 = 1:9:9:1, molar content ratio, pH = 8.6 ± 0.1) was selected for saline-alkali treatment34,35. The experiment set up 6 treatments (Table 1), with 3 replicates per treatment and 11 plants per replicate. Measure relevant indicators after 5 days of processing (Fig. 1).
Determination of tomato growth indicators
On the fifth day of treatment, the plant height and stem thickness of tomato seedlings in each treatment were determined using a straightedge and vernier calipers, The aboveground part of the plant and the fresh weight of the underground part of the plant that was dried with filter paper for surface moisture were weighed with a one in ten thousand scale balance, and then each part was killed in a blower drying oven at 105 °C for 30 min, followed by drying at 80 °C until a constant mass was achieved, at which point the dry weight was measured.
Detection of root activity and relative water content of leaves in tomatoe plants
The root vitality was measured using the 2,3,5-triphenyltetrazolium chloride (TTC) method. Firstly, weigh 0.5 g of roots and add 10 mL of 0.4% TTC and 0.1 mol·L− 1 (pH = 7.5) phosphate buffered saline solution (PBS) separately. Incubate at 37 °C for 1 h, then add 2 mL of 1.0 mol·L− 1 H2SO4. Remove the roots and dry them, then add 3–5 mL of ethyl acetate and a small amount of quartz sand, grind and filter. Measure OD value at 485 nm. Calculate root activity according to the following formula:
Root activity/(µg·g·h− 1) = TTC reduction amount/(root quality×time).
Select fully developed functional leaves, weigh them (FW), and fully hydrated for 24 h by complete immersion in self-sealing bags filled with distilled water. Then, the excess water on the surface was gently blotted to obtain their hydration weight (TW). Finally, the leaves were dried to constant weight and weighed again (DW). The relative water content (RWC) of the leaves was calculated using the formula:
RWC = (FW-DW)/(TW-DW) × 100%.
Determination of chlorophyll and carotenoid content
Using 95% ethanol extraction method for pigment extraction: Extract leaves with 95% ethanol and test in the dark. The absorbance of the extraction solution was measured at 665, 649 nm, and 470 nm, and the content of chlorophyll a, chlorophyll b, chlorophyll (a + b), and carotenoids was calculated using the method proposed by Yan et al.36. The calculation formula is as follows:
Chlorophyll a concentration (Ca) = 13.95A665-6.80A649.
Chlorophyll b concentration (Cb) = 24.96A649-7.32A665.
Chlorophyll a + b concentration (Ca+b) = 18.16A649 + 6.63A665.
Carotenoid concentration =(1000A470-2.05Ca-114.8Cb)/248.
Chlorophyll content (mg·g− 1 FW) = (C × V) / (1000 × m).
In the formula, C is the pigment concentration (mg·L− 1), V is the volume of the extraction solution (L), m is the fresh weight of the sample (g).
Measurement of photosynthetic gas exchange parameters
Using the Ciras-2 (PP System Inc., Amesbury, MA01913, USA) portable photosynthesis analyzer, the photosynthetic gas exchange parameters of tomato leaves were measured from 9:00 to 11:00 in the morning, including net photosynthetic rate (Pn), stomatal conductance (Gs), transpiration rate (Tr), and intercellular CO2 concentration (Ci).
Measurement of chlorophyll fluorescence parameters
We measured chlorophyll fluorescence parameters by referring to the method of Liu et al.37. and using a chlorophyll fluorescence imager (Walz, Effeltrich, Germany). Three plants were randomly selected for each treatment, and after 30 min of dark adaptation, three fully unfolded functional leaves were cut, flattened, and fixed on the measuring table of the fluorescence analyzer.
Determination of kinetic parameters induced by rapid chlorophyll fluorescence
The rapid fluorescence induction parameters of chlorophyll were determined using a plant efficiency analyzer Handy PEA (Hansatech Instruments Ltd.)38, and the calculation formula is shown in Table 2. Measure the sufficient dark adaptation of the front leaves for 30 min, followed by induction with 3000 µmol·m− 2·s− 1 red light for 2 s. Measure the rapid chlorophyll fluorescence induction kinetics curve (O-J-I-P fluorescence induction curve). Measure three plants each time, and quickly induce the kinetic curve based on the measured chlorophyll fluorescence.
Statistical analysis
Use Microsoft Excel 2010 (Microsoft Inc., Red-mond, WA, United States) to organize data and create tables and all figures were made using Origin 2024 (Origin Lab Co., United States). Data ANOVA and principal component analysis were performed using SPSS software (version 26.0; SPSS Institute Inc., Chicago, IL, USA). The significant differences in means among different treatments were evaluated by Duncan’s multiple range test (p < 0.05). All data were presented as mean ± SE.
Results
Effects of melatonin treatment on the growth of tomato seedlings under saline-alkali stress
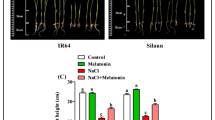
When plants are exposed to stress, one of the earliest responses is a reduction in growth rate. In this experiment, after 5 days of treatment, there was a significant difference in the phenotypes of tomato seedlings (Fig. 2A), which showed a significant decrease in plant height (Fig. 2B) and stem diameter (Fig. 2C), resulting in inhibition of plant growth. However, under saline-alkali stress, melatonin treatment significantly increased plant height and stem diameter compared to untreated plants. The growth-promoting effect of melatonin diminished significantly when the concentration exceeded 100 µmol·L⁻¹. Thus, applying 100 µmol·L⁻¹ exogenous melatonin notably enhanced tomato resistance to saline-alkali stress.
Effects of melatonin on the growth of tomato seedlings under saline-alkali stress. (A) Phenotypes. (B) Plant height. (C) Stem diameter. The results in the figure are means ± SE of three independent replications. Different letters in the figure represent significant differences between treatments (p < 0.05).
Effects of melatonin treatment on the biomass of tomato seedlings under saline-alkali stress
Biomass accumulation is a critical indicator of plant growth, development, and productivity. The experiment revealed that saline-alkali stress significantly reduced the dry weight of both aboveground and root parts of tomato plants compared to the control. The inhibitory effects on fresh weight were even more pronounced (Table 3). The application of 50–150 µmol·L⁻¹ melatonin significantly mitigated these negative effects, with the most pronounced improvement observed at 100 µmol·L⁻¹.
Effects of melatonin treatment on root activity and leaf relative water content of tomato seedlings under saline-alkali stress
Root activity is a key indicator of plant health and a predictor of potential yields. After 5 days of treatment, saline-alkali stress significantly reduced the root activity of tomato seedlings (Fig. 3A). This reduction in root activity was paralleled by a significant decrease in leaf relative water content (Fig. 3B). Exogenous melatonin significantly alleviated the effects of saline-alkali stress on root activity and leaf relative water content in a concentration-dependent manner, with 100 µmol·L⁻¹ providing the most effective mitigation.
Effects of melatonin treatment on the content of photosynthetic pigments of tomato seedlings under saline-alkali stress
Chloroplasts, the organelles responsible for photosynthesis, are the primary sites for chlorophyll and carotenoid synthesis. Our results showed that saline-alkali stress significantly reduced chlorophyll a, chlorophyll b, total chlorophyll, and carotenoid content (Fig. 4), disrupting pigment formation and inhibiting photosynthesis in tomato plants. Exogenous melatonin application effectively promoted chlorophyll synthesis, particularly chlorophyll b (Fig. 4B), with the greatest effect observed at 100 µmol·L⁻¹. Additionally, carotenoid content, known for its antioxidant properties and protective role in plants, was also increased (Fig. 4D). These findings suggest that melatonin, within a specific concentration range, promotes the formation of photosynthetic pigments.
Effects of melatonin on the photosynthetic pigment content of tomato leaves under saline-alkali stress. (A) Chl-a, Chlorophyll a content. (B) Chl-b, Chlorophyll b content. (C) Chl-total, Total chlorophyll content. (D) Carotenoid content. The results in the figure are means ± SE of three independent replications.
Effects of melatonin treatment on photosynthetic parameters of tomato leaves under saline-alkali stress
Saline-alkali stress markedly reduced the net photosynthetic rate (Pn), stomatal conductance (Gs), and transpiration rate (Tr) in tomato seedling leaves, while significantly increasing intercellular CO₂ concentration (Ci), thus inhibiting leaf photosynthesis (Fig. 5A-D). However, melatonin pretreatment alleviated the reduction in photosynthetic parameters caused by saline-alkali stress in tomato leaves. Compared to saline-alkali stress alone, applying 100 µmol·L⁻¹ melatonin increased Pn, Gs, and Tr by 59.15%, 47.67%, and 110.85%, respectively. These results suggest that exogenous melatonin effectively enhances photosynthesis in tomato seedlings under saline-alkali stress.
Effects of melatonin on photosynthetic parameters of tomato leaves under saline-alkali stress. (A) Net photosynthetic rate (Pn). (B) Intercellular CO2 concentration (Ci). (C) Stomatal conductance (Gs). (D) Transpiration rate (Tr). The results in the figure are means ± SE of three independent replications.
Effects of melatonin treatment on chlorophyll fluorescence parameters of tomato leaves under saline-alkali stress
Figure 6 shows fluorescence imaging of tomato leaves under saline-alkali stress treated with different concentrations of melatonin. Saline-alkali stress significantly decreased Fm, Fv/Fm, Y(II), and qP, while increasing qN. Melatonin application reduced the degree of damage in a concentration-dependent manner, with the 100 µmol·L⁻¹ treatment showing the greatest alleviation.
Saline-alkali stress significantly reduced Fv/Fm (Fig. 7A), qP (Fig. 7B), and Y(II) (Fig. 7D), but exogenous melatonin application markedly slowed this decline. Saline-alkali stress also reduced the electron transport rate (ETR) (Fig. 7F), while exogenous melatonin increased ETR compared to the T0 group. Furthermore, saline-alkali stress significantly increased qN (Fig. 7C), 1-qP (Fig. 7E), (1-qP)/NPQ (Fig. 7G), and NPQ (Fig. 7H), while 50–150 µmol·L⁻¹ melatonin treatment significantly reduced 1-qP and NPQ compared to the T0 group.
Effects of melatonin on chlorophyll fluorescence parameters of tomato leaves under saline-alkali stress. (A) Maximum photosynthetic efficiency of PSII (Fv/Fm). (B) Photochemical quenching coefficient (qP). (C) Non-photochemical quenching coefficient (qN). (D) Actual photosynthetic efficiency of PSII (Y(II)). (E) Excitation pressure of PSΠ (1-qP). (F) The electron transport rates (ETR). (G) Excess excitation energy ((1-qP)/NPQ). (H) Non-photochemical quenching (NPQ). The results in the figure are means ± SE of three independent replications. Different letters in the figure represent significant differences between treatments (p < 0.05).
Effects of melatonin treatment on the chlorophyll fluorescence kinetics (OJIP) curve and JIP-test parameters of tomato leaves under saline-alkali stress
To understand how melatonin alleviates PSII photoinhibition caused by saline-alkali stress, the chlorophyll fluorescence kinetics (OJIP curve) were measured for each treatment (Fig. 8). Results indicated that, compared to the control (CK), the OJIP curve of tomato leaves under saline-alkali stress exhibited significant deformation, with reduced overall fluorescence intensity and marked decreases in the O, J, I, and P phases. Compared to the T0 treatment, melatonin at varying concentrations partially restored the OJIP curve under saline-alkali stress, improving the O, J, I, and P phases. Among the treatments, the 100 µmol·L⁻¹ melatonin group showed the greatest recovery of the OJIP curve, approaching that of the control group. Exogenous melatonin application under saline-alkali stress alleviated the inhibition of photosynthetic electron transfer from QA to QB in tomato leaves.
Fluorescence parameters such as VJ, ψEo, VI, dV/dto, and PI(ABS) were derived from JIP-test analysis of the measured OJIP curve (Table 4). Comparative analysis revealed that saline-alkali stress increased VI, VJ, and dV/dto, while significantly reducing ψEo and PI(ABS). Exogenous melatonin application mitigated the extent of these changes. These results suggest that saline-alkali stress disrupts primary photochemical reactions in tomato leaves and inhibits electron transfer on the PSII receptor side, while melatonin treatment effectively alleviates this inhibition.
Effects of melatonin treatment on energy allocation per unit cross-sectional area of tomato leaves under saline-alkali stress
Analysis of energy allocation per unit cross-sectional area of tomato leaves under saline-alkali stress with different melatonin concentrations (Table 5) showed that, compared to the control, saline-alkali stress significantly reduced the absorbed light energy (ABS/CSm), electron transfer efficiency (ETo/CSm), and captured light energy (TRo/CSm) per unit area, while significantly increasing DIo/CSm (Table 5). Compared to saline-alkali stress alone, melatonin treatment significantly increased ABS/CSm, TRo/CSm, and ETo/CSm, while significantly reducing DIo/CSm, with the 100 µmol·L⁻¹ treatment showing the most pronounced effect.
Effects of melatonin treatment on the specific activity parameters of tomato leaves under saline-alkali stress
We further examined the specific activity parameters of tomato leaves subjected to saline-alkali stress and treated with varying concentrations of melatonin (Table 6). Following saline-alkali stress, the energy dissipated by the unit reaction center (DIo/RC) increased, suggesting that partial inactivation of reaction centers (RCs) enhanced the energy dissipation efficiency of the remaining active RCs, thereby increasing their load (Table 6). However, melatonin application significantly alleviated this burden. Moreover, saline-alkali stress diminished light energy absorption (ABS/RC) by the unit reaction center in tomato leaves, as well as the excitation energy used for QA reduction (TRo/RC) and the electron transfer capacity (ETo/RC) of individual RCs. Exogenous melatonin significantly mitigated the decline in ETo/RC, facilitating greater energy entry into the electron transfer pathway, which supports the normal functioning of PSII linear electron transfer (Table 6). Based on these findings, we conclude that saline-alkali stress increases PSII energy consumption (DIo/CSm, DIo/RC), inhibits electron transfer flux per unit cross-section and per reaction center (ETo/CSm, ETo/RC), and reduces PSII energy absorption efficiency. Spraying melatonin effectively enhances plant growth under saline-alkali stress.
Comprehensive evaluation of melatonin treatment on tomato seedling growth under saline-alkali stress
Principal component analysis
Principal component analysis (PCA) is a statistical method for dimensionality reduction, aiming to use fewer variables to capture the maximum information of the original dataset while minimizing information loss39. In this experiment, PCA was performed on 37 variables, such as plant height and stem thickness, to derive eigenvalues, contribution rates, and cumulative contribution rates (Table 7). Three principal components were selected based on a cumulative contribution rate exceeding 85% and eigenvalues greater than 0.5. The first principal component had an eigenvalue of 33.210, accounting for 89.758% of the variance among the 37 variables. The second principal component had an eigenvalue of 2.363, explaining 6.386% of the variance. The third principal component had an eigenvalue of 0.817, accounting for 2.208% of the variance. The first three principal components accounted for 98.352% of the cumulative variance, indicating that they captured 98% of the original variables’ information. Thus, the first three principal components were used as substitutes for the original 37 variables to assess the impact of exogenous melatonin on tomato seedling growth under saline-alkali stress. This approach reduced the evaluation indicators from 37 to three independent principal components, achieving effective dimensionality reduction.
Comprehensive evaluation
After rotating the principal component load matrix, the load coefficients become closer to 1 or 0, enhancing the interpretability of the principal components for the variables. Considering the cumulative contribution rate of the first three principal components, these components are suitable for constructing an analytical model. As shown in Table 8, the loadings of each variable are divided by the square root of their respective principal component eigenvalues to derive the coefficients or eigenvectors for each variable in the three principal components. The expression functions of the three principal components are formulated using the eigenvectors as weights:
In the four function expressions above, x1 represents plant height, x2 represents stem diameter, x3 represents root activity, x4 represents leaf relative water content, x5 represents aboveground fresh weight, x6 represents underground fresh weight, x7 represents aboveground dry weight, x8 represents underground dry weight, x9 represents chlorophyll a content, x10 represents chlorophyll b content, x11 represents total chlorophyll content, x12 represents carotenoid content, x13∼x37 represent Pn, Tr, Gs, Ci, Fv/Fm, qP, qN, Y(II), 1-qP, ETR, NPQ, (1-qP)/NPQ, ψEo, VJ, VI, dV/dto, PI(ABS), ABS/RC, DIo/RC, TRo/RC, ETo/RC, ABS/CSm, DIo/CSm, TRo/CSm and ETo/CSm, respectively. The comprehensive evaluation function was derived by linearly weighting the principal component scores using their respective variance contribution rates (comprehensive score: Y = 0.898Y1 + 0.064Y2 + 0.022Y3). Using the principal component analysis model, comprehensive scores and rankings of tomato growth under saline-alkali stress were determined for various melatonin treatment concentrations (Table 9). The comprehensive scores for each treatment were ranked as follows: CK > T2 > T3 > T1 > T4 > T0.
Discussion
Saline-alkali stress is a major abiotic factor that limits plant growth and global agricultural productivity40. Thus, enhancing the saline-alkali tolerance of tomatoes is crucial for the tomato industry. Saline-alkali stress severely disrupts plant growth, development, and physiological and biochemical processes41,42. In this study, the compound saline-alkali solution significantly inhibited tomato growth, reducing plant height, stem diameter, fresh weight, and dry weight. In contrast, foliar application of an appropriate concentration of melatonin effectively mitigated growth inhibition caused by severe stress, significantly increasing plant height, stem diameter, fresh weight, and dry weight. Additionally, saline-alkali stress markedly reduced root activity and leaf relative water content, while exogenous melatonin clearly alleviated salt damage symptoms. These results suggest that saline-alkali stress disrupts the resources required for seedling growth, thereby inhibiting above-ground growth and biomass accumulation in tomatoes. Melatonin enhances tomato adaptation to saline-alkali stress by promoting growth and biomass accumulation, thereby improving stress tolerance. This aligns with previous findings on the positive effects of exogenous melatonin on the growth of pepper43, oat44, and rice seedlings45 under saline-alkali stress.
Chloroplasts, the organelles responsible for photosynthesis, are among the most sensitive to salt stress in plant cells46. Chlorophyll, the primary pigment for photosynthesis, degrades more rapidly under abiotic stress47. Exogenous melatonin has been shown to increase chlorophyll content in banana seedlings under salt stress48 and enhance chlorophyll synthesis in tomatoes under cadmium stress49. Additionally, melatonin reduced chlorophyll loss and delayed senescence in cabbage by downregulating the expression of chlorophyll catabolic genes (BrPAO and BrSGR1) and senescence-associated genes (BrSAG12 and BrSEN4)50. In this study, saline-alkali stress significantly reduced the chlorophyll a, chlorophyll b, and total chlorophyll content in tomato leaves. However, treatment with optimal concentrations of melatonin significantly alleviated this reduction, suggesting that melatonin may promote chlorophyll accumulation and maintain photosynthetic efficiency by inhibiting the activity of chlorophyll-degrading enzymes in tomato plants. Thus, maintaining chlorophyll stability may be a key mechanism through which melatonin aids plants in adapting to saline-alkali stress.
Photosynthesis provides the materials and energy necessary for plant growth and is fundamental to crop yield formation, playing a critical role in plant growth and development51,52. Understanding how saline-alkali stress influences and limits photosynthesis, along with the adaptive mechanisms of photosynthesis under saline-alkali conditions, is crucial. The net photosynthetic rate is a highly sensitive indicator of plant responses to saline-alkali stress53. Stomata, as channels for water and CO2 exchange, play a critical role in regulating photosynthesis54. Under saline-alkali stress, leaf stomata typically close to varying degrees. Photosynthesis inhibition under saline-alkali stress results from multiple interacting factors, which can be classified into stomatal limitations and non-stomatal limitations55. Farquhar et al.56 highlighted that under water stress, limited stomatal conductance restricts intercellular CO2 concentration, leading to stomatal limitations of photosynthesis. Conversely, non-stomatal limitations arise from decreased activity of chloroplasts and ribulose-1,5-bisphosphate carboxylase, along with reduced regeneration capacity of ribulose-1,5-bisphosphate. This study found that compared to normal conditions, the compound saline-alkali solution significantly reduced the net photosynthetic rate, stomatal conductance, and transpiration rate of tomato leaves, while increasing intercellular CO2 concentration, ultimately inhibiting photosynthesis. These results align with the findings of Xian et al.57. It is suggested that exogenous melatonin alleviates photosynthesis inhibition in tomato leaves under saline-alkali stress by modulating non-stomatal factors.
Chlorophyll fluorescence closely correlates with photosynthetic efficiency. PSII, a key component of the photosynthetic system, is highly vulnerable to stress-induced damage and plays a critical role in light energy conversion and electron transfer during photosynthesis58,59. Light energy absorbed by chlorophyll a in the PSII antenna pigment complex dissipates primarily through three pathways: photosynthetic electron transfer, chlorophyll fluorescence emission, and thermal dissipation60. Chlorophyll fluorescence parameters serve as indicators of photosynthetic activity. Analyzing changes in chlorophyll fluorescence parameters helps identify the sites and severity of damage to the photosynthetic apparatus under stress61. This study found that saline-alkali stress decreased Fv/Fm and Y(II) in tomato leaves, indicating photoinhibition and reduced light energy capture efficiency. qP, the photochemical quenching coefficient, reflects the proportion of light energy used in photochemical electron transfer by PSII antenna pigments62,63. ETR represents the electron transfer rate64,65. Results showed that saline-alkali stress significantly reduced qP and ETR, while increasing NPQ in tomato leaves compared to normal conditions. Additionally, under salt stress, plants dissipate excess excitation energy as heat, and NPQ correlates with energy dissipation. When stress exceeds the capacity of the protective mechanisms, it damages the photosynthetic apparatus66. The increase in qP is generally attributed to an enhanced capacity of QA to transfer electrons downstream in the electron transport chain67. Saline-alkali stress reduces qP, indicating inhibition of electron flow from PSII oxidation to the reaction center, thereby reducing photochemical electron transfer and light energy utilization efficiency. The reduction in energy available for photochemical reactions leads to inhibited photochemical activity, consistent with the findings of Yuan et al.68 in cucumber seedlings. The increase in NPQ further supports this conclusion. Saline-alkali stress also increased 1-qP, indicating elevated excitation energy pressure on the PSII reaction center, blocked electron transfer, and accumulation of excess excitation energy. Saline-alkali stress also increased qN and heat dissipation rates, suggesting that tomatoes release excess energy absorbed by PSII through non-radiative heat dissipation under stress, thereby reducing light energy available for photosynthesis and decreasing photosynthetic capacity. This study demonstrated that exogenous melatonin application under saline-alkali stress increased Fv/Fm, Y(II), qP, and ETR in tomato leaves, while reducing qN, NPQ, and 1-qP. These findings suggest that exogenous melatonin alleviates photoinhibition caused by saline-alkali stress, enhances photochemical electron transfer efficiency, and increases photochemical energy formation. This is reflected in increased electron flow during photochemical reactions and reduced energy dissipation through heat, effectively protecting the photosynthetic apparatus of tomatoes and maintaining PSII function. This may result from the application of optimal melatonin concentrations, which maximize light energy use for photosynthesis in tomato plants under saline-alkali stress, balance light energy distribution, reduce photoinhibition, and enhance energy absorption for carbon fixation.
To better understand the damage to the photosynthetic structure of tomatoes caused by saline-alkali stress and the mechanism by which exogenous melatonin enhances photosynthetic function, this study employed rapid chlorophyll fluorescence analysis. It assessed changes in the OJIP curve, JIP test parameters, PSII unit cross-sectional energy distribution, and single reaction center energy allocation under saline-alkali stress. The OJIP curve provides detailed information about the photosynthetic electron transfer processes in PSII69. The results showed that saline-alkali stress caused significant deformation of the OJIP curve in tomato leaves, with notable decreases in the O, J, I, and P phases. Exogenous melatonin gradually restored the OJIP curve under salinity stress. The J-I segment of the OJIP curve corresponds to the reduction of QA, QB, and PQ70. A decrease in the J-I amplitude indicates that NaCl stress inhibits electron transfer from QA to PQ. Furthermore, a decrease in the I-P stage of the OJIP curve suggests that NaCl stress impairs PSI reduction, while a decrease in the P point results from the degradation or denaturation of PSI electron transport proteins on the receptor side71. This implies that saline-alkali stress inhibits lateral electron transfer at the receptor side of PSII, consistent with observations in spinach72 and sorghum73. VJ and VI values indicate the variable fluorescence intensities of the J-phase and I-phase, respectively. A higher VJ value suggests lower electron transfer efficiency from QA to QB. An increase in VI indicates reduced electron acceptance by the PQ pool, while dV/dto represents the maximum reduction rate of QA during photosynthetic electron transfer74. Saline-alkali stress significantly increased VJ, VI, and dV/dto, while exogenous melatonin application reduced these values under stress conditions. PI(ABS) is a performance index based on light absorption, accurately reflects the overall condition of plant photosynthetic systems and is highly sensitive to stress responses75. This study found that saline-alkali stress significantly reduced PI(ABS), whereas exogenous melatonin application increased PI(ABS) in tomato leaves, indicating an enhancement of photosynthetic capacity by melatonin under stress conditions. Additionally, we analyzed changes in energy partitioning per unit cross-sectional area of leaves under different treatments. Results showed that under saline-alkali stress, ABS/CSm, TRo/CSm, and ETo/CSm decreased, while DIo/CSm increased, indicating a higher proportion of heat dissipation. Exogenous melatonin application increased ABS/CSm, TRo/CSm, and ETo/CSm under saline-alkali stress while reducing DIo/CSm, indicating decreased heat dissipation per unit area. These findings suggest that under saline-alkali stress, melatonin protects PSII reaction center activity in tomato leaves, enhances energy absorption and electron transfer, and reduces energy dissipation. Furthermore, we analyzed specific activity parameters of tomato leaves under different treatments of saline-alkali stress. These values accurately reflect the absorption, conversion, and dissipation of light energy by photosynthetic organs76. The results showed that exogenous melatonin treatment reduced DIo/RC and increased ETo/RC, indicating reduced heat dissipation and enhanced electron transfer efficiency. This improvement in photochemical process efficiency facilitated smoother photosynthesis in tomatoes under saline-alkali stress. This finding aligns with Wei et al.77, who reported similar fluorescence characteristics in Ginkgo leaves under drought stress. These findings suggest that the enhanced photosynthetic defense provided by melatonin may result from improved resistance of the photosynthetic process to saline-alkali stress and more efficient conversion of excess energy into transport electrons.
Conclusion
In summary, saline-alkali stress negatively impacts the growth, photosynthesis, PSII electron transfer, and energy utilization of tomato seedlings. Exogenous melatonin application increases photosynthetic pigment content in tomato leaves, enhances the efficiency of the PSII electron transfer chain, and improves the utilization efficiency of photosynthetic resources. It also reduces heat dissipation in PSII reaction centers, alleviating saline-alkali stress, improving photosynthetic performance, and enhancing photosynthesis in tomato leaves. This promotes dry matter accumulation and root development, increases nutrient absorption efficiency, and supports the growth and development of tomato seedlings, ultimately enhancing their saline-alkali tolerance (Fig. 9). The comprehensive evaluation shows that T2 treatment achieved the highest score among melatonin treatments, followed by T3, T1, and T4, with T0 scoring the lowest. These results suggest that applying 100 µmol·L− 1 melatonin under saline-alkali stress is most effective in improving tomato stress resistance. This provides a theoretical foundation for further research on the physiological, biochemical, and molecular mechanisms underlying tomato resistance to saline-alkali conditions. This study proposes an effective method for enhancing the saline-alkali tolerance of tomato seedlings, offering both theoretical significance and practical value for guiding stress-resistant cultivation and vegetable breeding.
A model of adaptive strategy of tomato photosystem under saline-alkali after melatonin application. PSI, photosystem I; PSII, photosystem II; QA, primary acceptor plastoquinone A; QB, the secondary acceptor plastoquinone B; Cyt b6f, the cytochrome b6f complex; PC, cytochrome and plastocyanin; PH, plant height; SD, stem diameter; RA, root activity; RWC, relative water content; DM, dry matter.
Data availability
All data generated or analysed during this study are included in this published article.
References
Rothan, C., Diouf, I. & Causse, M. Trait discovery and editing in tomato. Plant. J. 97, 73–90 (2019).
Altaf, M. A. et al. Melatonin: first-line soldier in tomato under abiotic stress current and future perspective. Plant. Physiol. Biochem. 185, 188–197 (2022).
C., Y. et al. Silencing of SlMYB50 affects tolerance to drought and salt stress in tomato. Plant. Physiol. Biochem. 193, 139–152 (2022).
Zhang, Y. et al. Characterization of soil salinization and its driving factors in a typical irrigation area of Northwest China. Sci. Total Environ. 837, (2022).
Hassani, A., Azapagic, A. & Shokri, N. Global predictions of primary soil salinization under changing climate in the 21st century. Nat. Commun. 12, (2021).
Chele, K. H., Tinte, M. M., Piater, L. A., Dubery, I. A. & Tugizimana, F. Soil salinity, a serious environmental issue and plant responses: a metabolomics perspective. Metabolites 11, (2021).
Yang, J. Y., Zheng, W., Tian, Y., Wu, Y. & Zhou, D. W. Effects of various mixed salt-alkaline stresses on growth, photosynthesis, and photosynthetic pigment concentrations of Medicago ruthenica seedlings. Photosynthetica 49, 275–284 (2011).
Jia, X. et al. Ionomic and metabolomic analyses reveal the resistance response mechanism to saline-alkali stress in Malus halliana seedlings. Plant. Physiol. Biochem. 147, 77–90 (2020).
Liu, N. et al. Sodic alkaline stress mitigation with exogenous melatonin involves reactive oxygen metabolism and ion homeostasis in tomato. Sci. Hortic. 181, 18–25 (2015).
Shang, C. et al. SlWRKY80-mediated jasmonic acid pathway positively regulates tomato resistance to saline-alkali stress by enhancing spermidine content and stabilizing Na+/K+ homeostasis. Hortic. Res. 11, uhae028 (2024).
Fan, Y., Shen, W. Y., Vanessa, P. & Cheng, F. Q. Synergistic effect of Si and K in improving the growth, ion distribution and partitioning of Lolium perenne L. under saline-alkali stress. J. Integr. Agric. 20, 1660–1673 (2021).
Hongna, C. et al. Exogenous spermidine priming mitigates the osmotic damage in germinating seeds of leymus chinensis under salt-alkali stress. Front. Plant. Sci. 12, (2021).
Xu, Z., Wang, F., Ma, Y., Dang, H. & Hu, X. Transcription factor SlAREB1 is involved in the antioxidant regulation under saline-alkaline stress in tomato. Antioxidants 11, (2022).
Wang, Y. et al. Physiological and comparative transcriptome analysis of leaf response and physiological adaption to saline alkali stress across pH values in alfalfa (Medicago sativa). Plant. Physiol. Biochem. 167, 140–152 (2021).
Sharma, M. et al. Inroads into saline-alkaline stress response in plants: unravelling morphological, physiological, biochemical, and molecular mechanisms. Planta 259, 130 (2024).
Xu, J. et al. Polyamines are involved in GABA-regulated salinity-alkalinity stress tolerance in muskmelon. Environ. Exp. Bot. 164, 181–189 (2019).
Akrami, M. & Arzani, A. Inheritance of fruit yield and quality in melon (Cucumis melo L.) grown under field salinity stress. Sci. Rep. 9, (2019).
Cui, R. et al. Effects of salt stress on grain quality and starch properties of high-quality rice cultivars. Agronomy-Basel 14, (2024).
Li, Y. et al. Rice yield penalty and quality deterioration is associated with failure of nitrogen uptake from regreening to panicle initiation stage under salinity. Front. Plant. Sci. 14, (2023).
Zheng, Y. et al. Phytohormones regulate the abiotic stress: an overview of physiological, biochemical, and molecular responses in horticultural crops. Front. Plant. Sci. 13, 1095363 (2022).
Yang, Y. et al. Exogenously applied methyl Jasmonate induces early defense related genes in response to Phytophthora infestans infection in potato plants. Hortic. Plant. J. 8, 511–526 (2022).
Hu, E. et al. Relationship between melatonin and abscisic acid in response to salt stress of tomato. Sci. Hortic. 285, (2021).
Tanveer, M., Shahzad, B., Sharma, A., Biju, S. & Bhardwaj, R. 24-Epibrassinolide; an active brassinolide and its role in salt stress tolerance in plants: a review. Plant. Physiol. Biochem. 130, 69–79 (2018).
Yang, W., Zhou, Z. & Chu, Z. Emerging roles of salicylic acid in plant saline stress tolerance. Int. J. Mol. Sci. 24, (2023).
Castanares, J. L. & Bouzo, C. A. Effect of exogenous melatonin on seed germination and seedling growth in melon (Cucumis melo L.) under salt stress. Hortic. Plant. J. 5, 79–87 (2019).
Li, J. et al. Melatonin enhances the low-temperature combined low-light tolerance of pepper (Capsicum annuum L.) seedlings by regulating photosynthesis, carotenoid, and hormone metabolism. Environ. Exp. Bot. 199, (2022).
Pelagio-Flores, R., Munoz-Parra, E., Ortiz-Castro, R. & Lopez-Bucio, J. Melatonin regulates Arabidopsis root system architecture likely acting independently of auxin signaling. J. Pineal Res. 53, 279–288 (2012).
Zhao, Y. et al. Melatonin: a potential agent in delaying leaf senescence. Crit. Rev. Plant. Sci. 40, 1–22 (2021).
Debnath, B. et al. Melatonin mediates enhancement of stress tolerance in plants. Int. J. Mol. Sci. 20, (2019).
Ahammed, G. J. et al. Reactive oxygen species signaling in melatonin-mediated plant stress response. Plant. Physiol. Biochem. 207, (2024).
Er, C. Y. et al. Exogenous melatonin enhances salt stress tolerance in maize seedlings by improving antioxidant and photosynthetic capacity. Physiol. Plant. 164, 349–363 (2018).
Zhang, Y. et al. Seed priming with melatonin improves salt tolerance in cotton through regulating photosynthesis, scavenging reactive oxygen species and coordinating with phytohormone signal pathways. Ind. Crop Prod. 169, (2021).
Zhang, H. et al. Melatonin promotes seed germination under high salinity by regulating antioxidant systems, ABA and GA4 interaction in cucumber (Cucumis sativus L). J. Pineal Res. 57, 269–279 (2014).
Li, J., Hu, L., Zhang, L., Pan, X. & Hu, X. Exogenous spermidine is enhancing tomato tolerance to salinity-alkalinity stress by regulating chloroplast antioxidant system and chlorophyll metabolism. BMC Plant. Biol. 15, (2015).
Hu, X. et al. Effect of exogenous spermidine on polyamine content and metabolism in tomato exposed to salinity-alkalinity mixed stress. Plant. Physiol. Biochem. 57, 200–209 (2012).
Yan, Y. et al. A study of methods for the determination of chlorophyll. J. China Agric. Univer. 02, 53–67 (1982).
Liu, Z. et al. Effects of Xanthomonas campestris Pv. Campestris on the photosynthesis of cabbage in the early stage of infection. Sci. Hortic. 324, (2024).
Zhang, Z., Lan, M., Han, X., Wu, J. & Wang-Pruski, G. Response of ornamental pepper to high-temperature stress and role of exogenous salicylic acid in mitigating high temperature. J. Plant. Growth Regul. 39, 133–146 (2020).
Liu, R. X., Kuang, J., Gong, Q. & Hou, X. L. Principal component regression analysis with SPSS. Comput. Methods Programs Biomed. 71, 141–147 (2003).
Chen, S. et al. Responsive mechanism of hemerocallis citrina baroni to complex saline-alkali stress revealed by photosynthetic characteristics and antioxidant regulation. Plant. Cell. Rep. 43, (2024).
Guo, R. et al. Metabolomic and physiological analysis of alfalfa (Medicago sativa L.) in response to saline and alkaline stress. Plant. Physiol. Biochem. 207, (2024).
Li, Z., Chen, H., Guan, Q., Li, L. & Xuan, Y. H. Gibberellic acid signaling promotes resistance to saline-alkaline stress by increasing the uptake of ammonium in rice. Plant. Physiol. Biochem. 207, (2024).
Usman, S. et al. Melatonin and arginine combined supplementation alleviate salt stress through physiochemical adjustments and improved antioxidant enzymes activity in Capsicum annuum L. Sci. Hortic. 321, (2023).
Wang, Q. et al. The physiological mechanism of melatonin enhancing the tolerance of oat seedlings under saline-alkali stress. Agronomy-Basel 13, (2023).
Lu, X. et al. Exogenous melatonin alleviates alkaline stress by removing reactive oxygen species and promoting antioxidant defence in rice seedlings. Front. Plant. Sci. 13, (2022).
Zahra, N. et al. Regulation of photosynthesis under salt stress and associated tolerance mechanisms. Plant. Physiol. Biochem. 178, 55–69 (2022).
Fan, J. et al. Alleviation of cold damage to photosystem II and metabolisms by melatonin in Bermudagrass. Front. Plant. Sci. 6, (2015).
Wei, J. et al. Melatonin-induced physiology and transcriptome changes in banana seedlings under salt stress conditions. Front. Plant. Sci. 13, (2022).
Qin, C., Lian, H., Alqahtani, F. M. & Ahanger, M. A. Chromium mediated damaging effects on growth, nitrogen metabolism and chlorophyll synthesis in tomato can be alleviated by foliar application of melatonin and jasmonic acid priming. Sci. Hortic. 323, (2024).
Tan, X. et al. Melatonin delays leaf senescence of postharvest Chinese flowering cabbage through ROS homeostasis. Food Res. Int. 138, (2020).
Flexas, J. & Carriqui, M. Photosynthesis and photosynthetic efficiencies along the terrestrial plant’s phylogeny: lessons for improving crop photosynthesis. Plant. J. 101, 964–978 (2020).
Huang, G., Peng, S. & Li, Y. Variation of photosynthesis during plant evolution and domestication: implications for improving crop photosynthesis. J. Exp. Bot. 73, 4886–4896 (2022).
Munns, R. Comparative physiology of salt and water stress. Plant. Cell. Environ. 25, 239–250 (2002).
Lawson, T. & Vialet-Chabrand, S. Speedy stomata, photosynthesis and plant water use efficiency. New. Phytol. 221, 93–98 (2019).
Jiao, L., Wang, L., Zhou, Q. & Huang, X. Stomatal and non-stomatal factors regulated the photosynthesis of soybean seedlings in the present of exogenous bisphenol A. Ecotoxicol. Environ. Saf. 145, 150–160 (2017).
Farquhar, G. D. & Sharkey, T. D. Stomatal conductance and photosynthesis. Annu. Rev. Plant Physiol. 33, 317–345 (1982).
Xian, X. et al. Exogenous melatonin strengthens saline-alkali stress tolerance in apple rootstock M9-T337 seedlings by initiating a variety of physiological and biochemical pathways. Chem. Biol. Technol. Agric. 11, (2024).
Spaniol, B. et al. Complexome profiling on the Chlamydomonas lpa2 mutant reveals insights into PSII biogenesis and new PSII associated proteins. J. Exp. Bot. 73, 245–262 (2022).
Anket, S. et al. Photosynthetic response of plants under different abiotic stresses: a review. J. Plant. Growth Regul. 39, 509–531 (2019).
Li, X., Wang, H. & Jin, H. Light signaling-dependent regulation of PSII biogenesis and functional maintenance. Plant. Physiol. 183, 1855–1868 (2020).
Recchia, I., Sparla, F. & Pupillo, P. Photosynthetic properties of spring geophytes assessed by chlorophyll fluorescence analysis. Plant. Physiol. Biochem. 118, 510–518 (2017).
Nie, R., Wei, X., Jin, N., Su, S. & Chen, X. Response of photosynthetic pigments, gas exchange and chlorophyll fluorescence parameters to light quality in Phoebe Bournei seedlings. Plant. Growth Regul. 103, 675–687 (2024).
Lin, H. et al. Comparisons between yellow and green leaves of sweet potato cultivars in chlorophyll fluorescence during various temperature regimes under high light intensities. Sci. Hortic. 288, (2021).
Liu, J., Hou, H., Zhao, L., Sun, Z. & Li, H. Protective effect of foliar application of sulfur on photosynthesis and antioxidative defense system of rice under the stress of cd. Sci. Total Environ. 710, (2020).
He, L., Yu, L., Li, B., Du, N. & Guo, S. The effect of exogenous calcium on cucumber fruit quality, photosynthesis, chlorophyll fluorescence, and fast chlorophyll fluorescence during the fruiting period under hypoxic stress. BMC Plant. Biol. 18, (2018).
Ikeuchi, M., Sato, F. & Endo, T. Allocation of absorbed light energy in Photosystem II in NPQ mutants of arabidopsis. Plant. Cell. Physiol. 57, 1484–1494 (2016).
Altaf, M. A. et al. Melatonin affects the photosynthetic performance of pepper (Capsicum annuum L.) seedlings under cold stress. Antioxidants 11, (2022).
Yuan, Y. et al. Effects of exogenous putrescine on chlorophyll fluorescence imaging and heat dissipation capacity in cucumber (Cucumis sativus L.) under salt stress. J. Plant. Growth Regul. 33, 798–808 (2014).
Chen, H., Li, K., Xue, C. & Wang, Q. A novel method for non-invasive estimation of primary productivity in aquatic ecosystems using a chlorophyll fluorescence-induced dynamic curve. Front. Microbiol. 12, 682250 (2021).
Kalaji, H. M. et al. Chlorophyll a fluorescence as a tool to monitor physiological status of plants under abiotic stress conditions. Acta Physiol. Plant. 38, (2016).
Kuepper, H. et al. Analysis of OJIP Chlorophyll fluorescence kinetics and QA reoxidation kinetics by direct fast imaging. Plant. Physiol. 179, 369–381 (2019).
Zharmukhamedov, S. K. et al. Probing the influence of novel organometallic copper (II) complexes on spinach PSII photochemistry using OJIP fluorescence transient measurements. Biomolecules 13, (2023).
Stefanov, M. A. A. et al. Protective effects of sodium nitroprusside on photosynthetic performance of Sorghum bicolor L. under salt stress. Plants 12, (2023).
Yin, Z. et al. Mapping quantitative trait loci associated with chlorophyll a fluorescence parameters in soybean (Glycine max (L.) Merr). Planta 231, 875–885 (2010).
Rodriguez, A. A. et al. Field and genetic evidence support the photosynthetic performance index (PIABS) as an indicator of rice grain yield. Plant. Physiol. Biochem. 201, 107897 (2023).
Cao, Y. et al. The effects of different nitrogen forms on chlorophyll fluorescence and photosystem II in Lonicera japonica. J. Plant. Growth Regul. 42, 4106–4117 (2023).
Wei, X. et al. Effects of drought on fluorescence characteristics of photosystem II in leaves of Ginkgo biloba. Acta Ecol. Sin. 32, 7492–7500 (2012).
Acknowledgements
The authors thank the projects and funds for the support and sponsorship of this experiment.
Funding
This research was funded by the Science and Technology Innovation Fund of Gansu Agricultural University (GAUKYQD-2019-19), Modern Silk Road Cold and Dry Agricultural Science and Technology Support Project (GSLK-2021-6), Major Science and Technology Special Projects in Gansu Province (23ZDNA008) and Gansu Top Leading Talent Plan (GSBJLJ-2021-14).
Author information
Authors and Affiliations
Contributions
Z.T. and J.Y. conceived and designed the research. J.D. executed the experiment. J.D. and G.W. analyzed the date and prepared the figures and tables. J.D. wrote the manuscript. Z.T. read the manuscript and checked the language grammar. W.A., Y.Z. and Q.Y. read the manuscript and made valuable inputs. All authors contributed to the articles and approved the submitted version.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Dou, J., Tang, Z., Yu, J. et al. Effects of exogenous melatonin on the growth and photosynthetic characteristics of tomato seedlings under saline-alkali stress. Sci Rep 15, 5172 (2025). https://doi.org/10.1038/s41598-025-88565-5
Received:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s41598-025-88565-5