Abstract
This study reports the concomitant contraction pattern of the diaphragm and sternocleidomastoid (SCM) muscles at various inspiratory pressure loads in patients after stroke. Thirty-six participants (stroke duration: 3.6 ± 2.9 months) performed in random order, sets of 10 breaths at inspiratory loads of 30, 40, 50, 60, 70 and 80%, maximum inspiratory pressure (MIP). Bilateral muscle activity of the SCMs and diaphragm thickness were recorded simultaneously using surface electromyography (sEMG) and ultrasonography, respectively. Diaphragmatic thickness was significantly lower on the hemiplegic side compared to the non-affected side. The magnitude of diaphragmatic contraction, reflected by the calculated thickening fraction (DTf) for both hemidiaphragms, increased with inspiratory load and peaked at 50% MIP, but then decreased with any further increase in inspiratory pressure. SCM recruitment continued to increase bilaterally with increasing inspiratory pressure and was highest at 80% MIP, with recruitment activity significantly higher on the hemiplegic side compared to the non-affected side. Our results suggest that inspiratory load demands above 50%MIP are primarily met by increased SCM activity without any increase in diaphragmatic contraction. Adopting training intensities greater than 50%MIP in clinical inspiratory muscle training (IMT) programs needs to be re-considered.
Similar content being viewed by others
Introduction
The diaphragm is the principal muscle for active inspiration and induces approximately 75% of the airflow into the lungs1. However, most people after stroke are affected by contralateral hemidiaphragmatic weakness due to the loss of central motor control2. Diaphragmatic weakness was found to be associated with decreased lung function, higher risk of pneumonia, poor functional outcomes, prolonged hospitalization, and even an increased risk of mortality3,4.
Inspiratory muscle training (IMT) is an intervention which has been deployed in people after stroke to improve inspiratory muscle strength and endurance5. Positive efficacy of IMT on diaphragm function, spirometry lung function, and exercise capacity in the stroke population has also been reported5. IMT intensities in the stroke population reportedly range between 30 and 80% of maximum inspiratory pressure (MIP)6,7,8. A higher MIP however may not necessarily result in an effective diaphragm muscle contraction. As illustrated in studies involving healthy adults, surface electromyography (sEMG) signals demonstrated that an inspiratory effort above 60%MIP was associated with a dramatic increase in the sternocleidomastoid (SCM) muscle activity9,10. Excessive activation of accessory muscles implies an increase in the work of breathing, and may result in fatigue and a decrease in exercise capacity11,12. Ideally, in an IMT program, the inspiratory load intensity adopted should generate maximal diaphragmatic contraction but without causing fatigue or inducing excessive activation of the accessory muscles of respiration. The ‘ideal’ inspiratory load intensity (%MIP) is yet to be determined.
Superficial muscle activity is commonly assessed by sEMG13, and sEMG has been used to evaluate the impact of inspiratory pressure on diaphragm contraction in healthy adults9,14. However, due to signal interference from nearby superficial muscles, the reliability of sEMG for assessment of deep muscles including the diaphragm remains questionable15. On the other hand, measurement of diaphragm thickness by ultrasonography, a portable, noninvasive emerging technique provides a reliable and rapid assessment of diaphragmatic function in people after stroke16,17.
Assessment of simultaneous recruitment of the diaphragm and SCM muscles, by ultrasonography and sEMG respectively, under various inspiratory load pressures (%MIP), could provide useful information on the relationship between inspiratory load and respiratory muscle recruitment patterns. Therefore, the aims of this study were to explore (1) the pattern of simultaneous activities of the diaphragm and SCM muscles (by ultrasonography and sEMG, respectively) under different inspiratory pressure loads, and (2) the differences in recruitment pattern of the diaphragm and SCM muscles between the hemiplegic and non-affected sides, in people after stroke.
Methods
This cross-sectional observational study has received approval from the Institutional Review Board of the Hong Kong Metropolitan University (Ethics approval number: HE-OT2023/13) and Shenzhen Second People’s Hospital, China (Ethics approval number: 2023-274-01PJ). The study protocol was registered on the website of ClinicalTrials.gov, with the registration number NCT06267768. All procedures were performed in accordance with the guidelines and regulations of the hospital and university involved.
Participants
People diagnosed with stroke and receiving medical and rehabilitation treatment in the stroke rehabilitation ward at the involved hospital, between 2nd March 2024 and 17th August 2024, were screened for inclusion and exclusion criteria (see below) by a physiotherapist investigator (FL). Participants meeting the inclusion criteria were invited to participate in this study.
Inclusion criteria: (1) age ≥ 18 years; (2) breathing spontaneously; (2) clinically diagnosed with ischemic and/or hemorrhagic stroke; (3) a duration of stroke from onset between 1 and 12 months after diagnosis; (4) no limitation to labial occlusion; (5) no thoracic or abdominal surgery within the last 6 months; (6) able to understand and follow verbal instructions; (7) stable cardiovascular function, and (8) no history of chronic respiratory illness.
Exclusion criteria: (1) acute myocardial infarction or acute heart failure; (2) acute pain in any part of the body; (3) positive clinical signs of impaired respiratory function (such as shortness of breath, hypoxemia, chronic cough and sputum retention); (4) presence of a nasal feeding tube, tracheal tube and/or any condition that impeded measurement and implementation of the study procedure.
Sample size
The sample size calculation was based on the use of repeated-measures analysis of variance (ANOVA) and was conducted using G*Power 3.1.9.7. The findings from our pilot study with stroke patients were used for this calculation. Considering an effect size of 0.20, an alpha error probability of 0.05, and a power of 0.80, the estimated sample size was determined to be 28. An additional 20% was added to account for potential attrition, resulting in a final required sample size of at least 34.
Procedures
The nature of the study was explained to all participants, and written informed consent obtained before data collection. Demographic data for each participant included age, gender, height, weight, body mass index, and smoking history were recorded. The stroke type (cerebral infarction, intracerebral hemorrhage), medical history and comorbidities were recorded. Scores of the National Institutes of Health Stroke Scale, Modified Rankin Scale, and Barthel index were obtained from the patient’s medical records.
Participants were invited to attend the cardiopulmonary laboratory at Shenzhen Second People’s Hospital at least 2 h after a light meal to participate in the measurement. All participants were also requested to avoid consumption of caffeine-containing products, nicotine, alcohol, and vigorous exercise for at least 12 h before the assessment.
All participants undertook a standard spirometry lung function test. Forced vital capacity (FVC), forced expiratory volume in one second (FEV1), and MIP data were recorded.
Participants were then instructed to breathe using a pressure threshold inspiratory load device (POWERbreathe, KH2, UK). A nose clip was used to prevent air leakage. Participants were asked to inspire through the device, in random order (load intensity randomization was achieved by placing 6 notes inscribed with each load intensity inside an envelope. Upon arrival of each participant, a physiotherapist withdrew each of the 6 notes from the envelop one after another, thereby designating the load intensity sequence for that participant), for 10 breaths, at each specific inspiratory load intensities of 30%, 40%, 50%, 60%, 70%, or 80% of their MIP. Instructions on diaphragmatic breathing (focused inspiration of air to the lower part of the chest in the epigastric region) were given to facilitate diaphragmatic recruitment18. A rest period of 15 min was allowed after each set of 10 breaths.
Bilateral diaphragmatic thickness at the end of each loading breath was recorded by ultrasonography and simultaneously, the bilateral activity in the SCM muscles were recorded using sEMG. Self-perceived exertion level for each inspiratory pressure load was recorded using the modified Borg’s Rating Scale of Perceived Exertion19 at the end of each 10-breath set.
Measurements
Spirometry lung function
Spirometry lung data, including FVC and FEV1, were measured using a spirometer (X1, XEEK, China). MIP was measured using another spirometer (POWER breathe, KH2, UK). All lung function tests were conducted following the American Thoracic Society guidelines20. All tests were repeated 3 times, with no variation exceeding 5% or 100 ml between tests, and the highest value adopted for final analysis21.
Diaphragm contraction
Two researchers were involved in ultrasonography measurement. Both had received previous training from a radiologist specialized in ultrasonography. The inter-rater reliability of these two researchers was previously established prior to data collection. The intraclass correlation coefficients (ICCs) were all above 0.8 (see S1 Appendix).
Activity of diaphragmatic contraction at each inspiratory pressure loading was represented by the diaphragmatic thickening fraction (DTf)22. DTf is calculated by the difference between mean diaphragmatic thickness at the end of each specific inspiratory pressure load (DTi) and mean diaphragmatic thickness at end-tidal expiration (DTe), and divided by the diaphragmatic thickness at end-tidal expiration22, i.e. [(DTi-DTe)/DTe].
Two identical ultrasound machines (Mindray M9, Shenzhen, China) were used for simultaneous measurement of hemidiaphragm thickness. Participants adopted an upright sitting position on a chair throughout the measurement process. Two linear array transducer probes, operating on B mode with a bandwidth of 4–12 MHz were placed on either side of the chest, between the anterior-axillary and mid-axillary lines, at the level of 8th and 9th intercostal space, with a 45° cephalic tilt at the abdominal wall surface.
To enhance the precision and continuity of diaphragmatic thickness measurements for each inspiratory pressure load, the changes in diaphragmatic thickness for each 10-breath set were video recorded. The mean diaphragmatic thickness at the end of each breath was manually identified and measured at a recording review. Hemi diaphragmatic thickness data harvested from each 10-breath set were averaged and used to calculate the DTf for each inspiratory pressure load, for each hemi diaphragm.
Measurement of muscle activity of SCM
Prior to electrode application, the skin surface over the SCM was cleaned with alcohol to ensure optimal electrode impedance. To measure SCM muscle activity, 2 self-adhesive, disposable, surface Ag/AgCl electrodes (3 M™ Red Dot™, USA) were placed bilaterally on the skin of the neck, 2 cm apart, in alignment with the muscle fibers, and midway between the mastoid process and the medial end of the clavicle23. Participants were instructed to contract the SCM muscles by turning their head against slight resistance to verify proper electrode placement and validate the acquisition of electrical signals.
A Noraxon Ultium wireless multichannel sEMG system (Noraxon USA, Inc., Scottsdale, AZ, USA) was employed to concurrently monitor the muscle activity of the SCM muscles bilaterally. The system was configured with a common-mode rejection ratio of > 100 dB, and an input impedance of > 100 MΩ. Processing of the recorded sEMG signal was conducted by the MyoMotion system 3.8 MR (Noraxon Ltd., USA), with sensor sampling rate at 2000 Hz. Bandpass filtering between 20 and 500 Hz was applied for initial filtering followed by signal smoothing and rectification24. Root mean square (RMS) values were calculated using a moving average window of 100 ms24. The peak EMG amplitudes were determined for each SCM muscle from 3 maximal voluntary inspiratory maneuvers during the MIP measurement. The peak EMG value for each SCM muscle was used for normalization18. Bilateral activity of the SCM muscle at each inspiratory pressure load was determined by averaging the peak EMG amplitude over each 10-breath set and expressed as a percentage of the MIP amplitude.
Maximum EMG was defined as the highest level of EMG observed from maximal voluntary inspiratory maneuvers (sniff, MIP, and inspiratory capacity maneuvers) and during IMT. Regardless of the maneuver, the highest EMG value was used to represent the maximum EMG for normalization purposes.
Statistical analysis
IBM SPSS Statistics for Windows Version 25.0 (Armonk, NY: IBM Corp) was utilized for all data analysis. Demographic data and clinical characteristics for all participants were summarized using descriptive statistics. The intraclass correlation coefficient (ICC2,1) was employed to determine the inter-rater reliability of the two ultrasonic operators25. The standard error of measurement (SEM) was calculated as the square root of the mean square error of repeated measurements (MSE)25. Changes in DTf and SCM muscle activity under different inspiratory pressure loads between the hemiplegic and non-affected sides, and within each side, were analyzed using repeated-measures ANOVA. Post-hoc multiple comparisons were adjusted using the Bonferroni correction. The level of significance for all statistical tests was set at 0.05. Differences in perceived exertion levels were compared using the Wilcoxon signed-rank test for the six comparisons with a Bonferroni correction of α set at < 0.05/6 = 0.0083.
Results
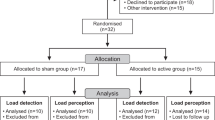
Thirty-six people after stroke, with a mean duration of 3.6 ± 2.9 months, were included for data analysis (Fig. 1). Among all participants, sixteen participants (44%) exhibited left-side hemiplegia. The demographic data and clinical characteristics of these individuals are summarized in Table 1.
Changes in diaphragmatic thickness and DTf
The thickness of the diaphragm on the hemiplegic side was significantly thinner than the non-affected side across the respiratory cycle, irrespective of the increasing inspiratory load (Table 2; Fig. 2). However, the changes in DTf at different inspiratory loads followed a similar pattern on both the hemiplegic and non-affected side. DTf in both the non-affected and hemiplegic sides increased from 42 to 84%, and 43 to 83% respectively when the inspiratory load was increased from 30% MIP to 50% MIP; DTf peaked at 50% MIP, but then decreased with any further increase in inspiratory load, down to only 27 and 31% respectively at 80% MIP (Tables 2 and 3; Fig. 3). The mean reduction in DTF between 80% MIP and 30% MIP was 15% (P < 0.001, 95% CI − 24 to − 5) on the non-affected side and 12% (P = 0.003, 95% CI − 22 to − 2) on the hemiplegic side. At any loading intensity, there was no statistical difference in the calculated DTf between the two hemidiaphragms.
Diaphragmatic thickening fraction (DTf) on both hemi diaphragms under different inspiratory pressure loads. DTf: Diaphragm thickening fraction; MIP: Maximum inspiratory pressure; Inserted numbers are mean DTf% ± SD. Data of the hemiplegic side are in italics and bold. \(< - - - - >\)Between intensity load comparison on hemiplegic side. \(\longleftrightarrow\) Between intensity load comparison on non-affected side. *Denotes p<0.05. **denotes p<0.001 (mean difference values refer to Table 3).
Changes in SCM muscle activity
The activity of the SCM muscles on both sides increased with increasing inspiratory pressure loading, reaching the highest sEMG signal at 80% MIP. As the inspiratory pressure load increased from 30% MIP to 80% MIP, the SCM muscle activity increased from 60 to 106% on the non-affected side and from 72 to 129% (nearly 60% increase) on the hemiplegic side (Tables 2 and 3; Fig. 4). The change in muscle activity, whether an increase or decrease, with each 10% incremental change in MIP, was statistically significant (Table 3). At all inspiratory pressure loads, the muscle activity of the SCM on the hemiplegic side was significantly higher than the non-affected side (Table 2; Fig. 4).
Changes in sEMG activity of both sides of sternocleidomastoid under different inspiratory pressure loads. RMS Root mean square, sEMG Surface electromyography, MIP Maximum inspiratory pressure; Inserted numbers are mean sEMG ± SD. Data of the hemiplegic side are in italics. Comparison between hemiplegic and non-affected sides at different inspiratory pressure loads denoted by *p < 0.05 or **denotes p < 0.001.
Changes in self-perceived exertion level
The recorded Borg score was 2 at 30% MIP and increased with increasing inspiratory loads, peaking at 9 at the maximal inspiratory load of 80% MIP. Participants demonstrated high exertion levels when the inspiratory load exceeded 60% MIP, with Borg scores of 8 at 70% MIP and 9 at 80% MIP (Table 2).
Discussion
This is the first study to examine simultaneous diaphragmatic contraction and SCM muscle activity under various inspiratory pressure loads in people after stroke. In accord with previous reports, the diaphragm thickness on the hemiplegic side of an individual with stroke was thinner than that on the non-affected side3,26. This observed difference in diaphragmatic thickness is likely attributable to brain cortex injury after stroke, affecting the regulation of the contraction of the contralateral diaphragm27,28.
IMT is used to improve diaphragmatic strength and endurance5, implying a higher inspiratory resistance load will induce a stronger diaphragm contraction. However, our study showed that the maximum diaphragmatic contraction occurred at the intensity of 50% MIP, and any further increase in inspiratory resistance was associated with a decline of diaphragm contraction as reflected by a decrease in DTf. Accessory muscles (e.g., SCM) are typically activated when inspiratory demand is increased29. As predicted, our results demonstrated that activity of the SCM muscle increased with increasing inspiratory loads, peaking at the highest inspiratory resistance (80%MIP). The result of our simultaneous recording of the diaphragm and SCM muscles suggest that, in patients after stroke, when the inspiratory resistance is greater than 50%MIP, further inspiratory demand is mainly met by contraction of the accessory muscles and not the diaphragm. Jung and Kim measured recruitment of SCM and the diaphragm in healthy subjects by EMG and found SCM activity continued to increase with increases in inspiratory load from 40 to 80% but the diaphragmatic activity decreased when the load was increased from 40–60%9. Our recent study of healthy adults30 (adopting a similar measurement methodology) also demonstrated that diaphragmatic muscle recruitment followed a non-linear pattern, peaking at 50% MIP, but SCM recruitment continued to increase as the inspiratory load increased to 80% MIP30. This suggests that the recruitment pattern of the diaphragm and SCM during increasing inspiratory load intensity appeared similar between healthy cohorts and people after stroke.
Simultaneous recording of activity of the SCM muscle illustrated in this current study showed that irrespective of the inspiratory resistance load, activity of the SCM muscle was higher on the hemiplegic side compared to the non-affected side. Although the diaphragm muscle was found to be thinner on the hemiplegic side in our stroke cohort, the diaphragm contraction activity, as reflected by DTf, was the same for both the hemiplegic and non-affected hemidiaphragms. This suggests that in people with stroke, the increased demand of an inspiratory task on the hemiplegic hemi diaphragm are contributed to by a significant contraction of the SCM muscle.
A study employing phrenic nerve stimulation showed that an inspiratory resistance of 60% MIP was associated with diaphragm fatigue in healthy adults31. Excessive activation of accessory muscles during contraction is not recommended in clinical practice, as it can lead to dyspnoea and fatigue in healthy adults32. Our findings affirmed that our cohort of participants with stroke exhibited a very high level of exertion at high resistance levels, particularly, at 70% MIP and 80% MIP. Our findings suggest that IMT at intensity levels greater than 50% MIP may be ineffective for diaphragmatic training, as the training stimulus then appears to promote the contribution of the accessory muscles to meet the additional work of breathing requirements.
Limitation
Our study only investigated the effects of a single set of 10 breaths at each inspiratory pressure load. IMT programs commonly involve 5 sets of 8 to 10 breaths, with 1 or 2-min rest intervals33. However, requiring participants in this study to complete 5 sets of 10 breaths at each testing load would likely increase the risk of muscle fatigue and was therefore considered impractical.
Stroke duration, side of hemiplegia, and comorbidities may influence respiratory muscle recruitment during inspiratory resistance loading. A systematic review on the effect of IMT on patients after stroke showed that the improvement in MIP was greater in patients with chronic stroke (stroke duration > 6 months), compared to the improvement in MIP in the sub-acute cohort (duration of stroke < 6 months)34. However, this inference is confounded by the fact that the training intensity adopted in IMT programs for patients with chronic stroke was higher than the training intensity reported in patients with sub-acute stroke. In our study, the stroke duration in the majority of our cohort was between 1 and 6 months; less than a third were less than 1 month and only 5 patients were over 6 months. With such a small sample size, we were unable to conduct any meaningful sub-group analysis to determine whether stroke duration might have an impact on the SCM and diaphragm recruitment pattern during increased inspiratory resistance loads. This aspect of our research warrants further investigation.
In conclusion, this study elucidates the simultaneous, bilateral muscle activity of the diaphragm and sternocleidomastoid under various inspiratory loads in people after stroke. In our post-stroke cohort, an inspiratory load of 50% MIP was associated with the highest diaphragmatic contraction bilaterally. Increasing the inspiratory load beyond this intensity did not further enhance diaphragmatic contraction but resulted in further recruitment of the sternocleidomastoid muscle, particularly on the hemiplegic side. Further RCTs exploring the holistic effects of IMT programs at training intensity of 50% MIP in the stroke population are warranted.
Data availability
Data and materials are accessible from the corresponding author upon reasonable request.
References
McKenzie, D., Butler, J. & Gandevia, S. Respiratory muscle function and activation in chronic obstructive pulmonary disease. J. Appl. Physiol. 107, 621–629 (2009).
Kumar, S., Reddy, R. & Prabhakar, S. Contralateral diaphragmatic palsy in acute stroke: an interesting observation. Indian J. Crit. Care Med. 13, 28–30 (2009).
Liu, X. et al. Assessment of diaphragm in hemiplegic patients after stroke with ultrasound and its correlation of extremity motor and balance function. Brain Sci. 12, 882 (2022).
Catalá-Ripoll, J., Monsalve-Naharro, J. & Hernández-Fernández, F. Incidence and predictive factors of diaphragmatic dysfunction in acute stroke. BMC Neurol. 20, 79 (2020).
Zhang, X. et al. Can inspiratory muscle training benefit patients after stroke? A systematic review and meta-analysis of randomized controlled trials. Clin. Rehabil. 34, 866–876 (2020).
Jung, J. & Kim, N. The effect of progressive high-intensity inspiratory muscle training and fixed high-intensity inspiratory muscle training on the asymmetry of diaphragm thickness in stroke patients. J. Phys. Ther. Sci. 27, 3267–3269 (2015).
Fabero-Garrido, R. et al. Respiratory muscle training improves exercise tolerance and respiratory muscle function/structure post-stroke at short term: a systematic review and meta-analysis. Ann. Phys. Rehabil Med. 65, 101596 (2021).
Gomes-Neto, M. et al. Effects of respiratory muscle training on respiratory function, respiratory muscle strength, and exercise tolerance in patients poststroke: a systematic review with meta-analysis. Arch. Phys. Med. Rehabil. 97, 1994–2001 (2016).
Jung, J. & Kim, N. Relative activity of respiratory muscles during prescribed inspiratory muscle training in healthy people. J. Phys. Ther. Sci. 28, 1046–1049 (2016).
Chino, K., Ohya, T., Katayama, K. & Suzuki, Y. Diaphragmatic shear modulus at various submaximal inspiratory mouth pressure levels. Respir. Physiol. Neurobiol. 252–253, 52–57. https://doi.org/10.1016/j.resp.2018.03.009 (2018).
Romer, L. & Polkey, M. Exercise-induced respiratory muscle fatigue: implications for performance. J. Appl. Physiol. 104, 879–888. https://doi.org/10.1152/japplphysiol.01157.2007 (2008).
Dominelli, P. et al. Effects of respiratory muscle work on respiratory and locomotor blood flow during exercise. Exp. Physiol. 102, 1535–1547. https://doi.org/10.1113/ep086566 (2017).
Raez, M., Hussain, M. & Mohd-Yasin, F. Techniques of EMG signal analysis: detection, processing, classification and applications. Biol. Proced. Online. 8, 11–35 (2006).
Hawkes, E., Nowicky, A. & McConnell, A. Diaphragm and intercostal surface EMG and muscle performance after acute inspiratory muscle loading. Respir. Physiol. Neurobiol. 155, 213–219. https://doi.org/10.1016/j.resp.2006.06.002 (2007).
Cabral, E. et al. Surface electromyography (sEMG) of extradiaphragm respiratory muscles in healthy subjects: a systematic review. J. Electromyogr. Kinesiol. 42, 123–135. https://doi.org/10.1016/j.jelekin.2018.07.004 (2018).
Chen, Y. et al. Diaphragmatic ultrasound can help evaluate pulmonary dysfunction in patients with stroke. Front. Neurol. 14, 1061003 (2023).
Noh, D., Lee, J. & You, J. Diaphragm breathing movement measurement using ultrasound and radiographic imaging: a concurrent validity. Biomed. Mater. Eng. 24, 947–952. https://doi.org/10.3233/bme-130889 (2014).
Ramsook, A. et al. Diaphragm recruitment increases during a Bout of targeted inspiratory muscle training. Med. Sci. Sports Exerc. 48, 1179–1186. https://doi.org/10.1249/mss.0000000000000881 (2016).
Borg, G. Psychophysical bases of perceived exertion. Med. Sci. Sports Exerc. 14, 377–381 (1982).
Graham, B. et al. Standardization of spirometry 2019 update. An official American thoracic society and European respiratory society technical statement. Am. J. Respir. Crit. Care. 200, e70–e88 (2019).
American Thoracic Society. ATS/ERS statement on respiratory muscle testing. Am. J. Respir. Crit. Care Med. 166, 518–624 (2002).
Gottesman, E. & McCool, F. Ultrasound evaluation of the paralyzed diaphragm. Am. J. Respir. Crit. Care Med. 155, 1570–1574. https://doi.org/10.1164/ajrccm.155.5.9154859 (1997).
Criswell, E. Cram’s Introduction to Surface Electromyography 2nd Edition (Jones and Bartlett, 2011).
Koizumi, J. & Ohya, T. Effects of high-intensity inspiratory muscle warm-up on inspiratory muscle strength and accessory inspiratory muscle activity. Respir. Physiol. Neurobiol. 313, 104069. https://doi.org/10.1016/j.resp.2023.104069 (2023).
Weir, J. Quantifying test-retest reliability using the intraclass correlation coefficient and the SEM. J. Strength. Cond. Res. 19, 231–240 (2005).
Lee, K., Cho, J., Hwang, D. & Lee, W. Decreased respiratory muscle function is associated with impaired trunk balance among chronic stroke patients: a cross-sectional study. Tohoku J. Exp. Med. 245, 79–88. https://doi.org/10.1620/tjem.245.79 (2018).
Gandevia, S. & Rothwell, J. Activation of the human diaphragm from the motor cortex. J. Physiol. 384, 109–118 (1987).
Rochester, C. & Mohsenin, V. Respiratory complications of stroke. Semin. Respir. Crit. Care Med. 23, 248–260. https://doi.org/10.1055/s-2002-33033 (2002).
Koulouris, N. & Dimitroulis, I. Structure and function of the respiratory muscles. Pneumon 14, 91–108 (2001).
Liu, F., Jones, A., Tsang, R., Yam, T. & Tsang, W. Recruitment of the diaphragm and sternocleidomastoid muscle during increasing inspiratory pressure loads in healthy young adults. Respir. Physiol. Neurobiol. 331, 104365 (2025).
Sheel, A., Derchak, P., Pegelow, D. & Dempsey, J. Threshold effects of respiratory muscle work on limb vascular resistance. Am. J. Physiol. Heart Circ. Physiol. 282, H1732–1738. https://doi.org/10.1152/ajpheart.00798.2001 (2002).
Boyle, K. et al. The effect of diaphragm fatigue on the multidimensional components of dyspnoea and diaphragm electromyography during exercise in healthy males. J. Physiol. 598, 3223–3237. https://doi.org/10.1113/jp279755 (2020).
Marco, E. et al. High-intensity vs. sham inspiratory muscle training in patients with chronic heart failure: a prospective randomized trial. Eur. J. Heart Fail. 15, 892–901. https://doi.org/10.1093/eurjhf/hft035 (2013).
Liu, F. et al. Effects of inspiratory muscle training on pulmonary function, diaphragmatic thickness, balance and exercise capacity in people after stroke: a systematic review and meta-analysis. Disabil. Rehabil. 1–16 (2024).
Funding
This project was supported by the Research Matching Grant Scheme, UGC, Hong Kong SAR (Ref. no. 2023/3007).
Author information
Authors and Affiliations
Contributions
All authors were involved in the study design. F.L., A.J. and W.T. contributed to the conception and design of this study. F.L. and T.Y. contributed to the material preparation and data collection. F.L. and R.T. contributed to the data analysis. F.L. drafted the initial manuscript. All authors reviewed and revised the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Liu, F., Jones, A.Y.M., Tsang, R.C.C. et al. Diaphragm and sternocleidomastoid muscle activity with increasing inspiratory pressure loads in people after stroke. Sci Rep 15, 5856 (2025). https://doi.org/10.1038/s41598-025-90199-6
Received:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s41598-025-90199-6