Abstract
A novel, environmentally friendly HPLC-FL method has been developed to simultaneously quantify tamsulosin hydrochloride (TAM) and tolterodine tartrate (TTD) in pharmaceutical formulations. The method effectively separates the drugs using an ODS column and gradient elution at a flow rate of 1.0 mL/min. Fluorescence detection was used to measure the relative fluorescence intensity (RFI) of the analytes, with excitation and emission wavelengths set at 280 nm and 350 nm, respectively. The retention times for TAM and TTD were determined to be 5.66 and 7.26 min, respectively. The method’s accuracy and precision were validated according to ICH guidelines. Linear calibration curves were obtained over the concentration ranges of 0.1–1.5 µg mL-1 for TAM and 1–15 µg mL-1 for TTD, with correlation coefficients exceeding 0.9995. The calculated LOD values for TAM and TTD were 0.03 and 0.30 µg mL-1, respectively. The corresponding LOQ values were 0.10 and 0.92 µg mL-1, respectively. The method’s applicability was demonstrated by analyzing pharmaceutical dosage forms and spiked plasma and urine samples. The observed mean recoveries percent were 99.31-100.97% for TAM and 100.56-101.79% for TTD in human plasma, while in human urine, they were 98.71-101.55% for TAM and 99.53-101.84% for TTD. To evaluate the method’s greenness, GAPI and AGREE tools were employed. The results indicated significant adherence to green chemistry principles.
Similar content being viewed by others
Introduction
The improvement of analytical methods has a significant impact on pharmaceutical analysis. Drug substances and impurities in pharmaceutical ingredients, formulations, and biological matrices must be identified and quantified for numerous applications1,2. Because of its ease of use, high sensitivity, and specificity, HPLC has recently drawn a lot of interest from the pharmaceutical and biological fluid industries. For assaying biological matrices, HPLC in conjunction with various detection techniques like ultraviolet (UV), fluorescence, and mass spectrometry is currently the recommended approach3,4,5.
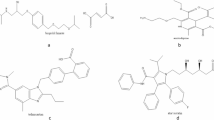
Overactive bladder (OAB) is a common disorder among men over 45, harmfully impacting their quality of life. To manage OAB associated with benign prostatic hyperplasia (BPH), healthcare providers often prescribe alpha-blockers6. However, recent studies suggest that combining antimuscarinics with alpha-1 blockers may provide more effective relief for BPH-related OAB than using alpha-blockers alone7. Tamsulosin hydrochloride (TAM) is a selective alpha-1-blocker used to treat BPH. The chemical structure is illustrated in Fig. 1a. Tolterodine Tartrate (TTD), depicted in Fig. 1b, is an antimuscarinic medication for urinary incontinence. The development of innovative analytical methods is crucial to analyze novel drug combinations like TAM and TTD, used to treat BPH and OAB. TAM is an approved drug in both the United States Pharmacopeia (USP) and the British Pharmacopoeia (BP)8,9. The USP describes an HPLC method for analyzing TAM content in capsules8. While the BP outlines a potentiometric titration method for quantifying TAM in bulk powder9. A comprehensive literature review reveals various techniques for assessing TAM, including spectrophotometry10, spectrofluorimetry11, HPLC12, and voltammetry13. TTD is officially recognized as a pharmaceutical substance solely within the BP. The BP details a potentiometric titration method for assessing TTD in bulk form9. Various techniques have been reported for analyzing TTD, including spectrophotometry14, spectrofluorimetry15, HPLC16, HPTLC17, and voltammetry18,19. However, methods for the simultaneous analysis of TAM and TTD are relatively limited, with spectrophotometry20, spectrofluorimetry21, HPLC-UV22,23, and HPTLC24,25,26 approaches for their analysis in pharmaceutical formulations.
Direct spectrophotometric methods have limited sensitivity, while direct spectrofluorimetric methods offer high sensitivity. However, both lack the selectivity of chromatographic methods27,28,29,30. HPLC with fluorescence detection provides both high sensitivity and selectivity. Consequently, there was a need to create a simple, dependable, rapid, green significantly more sensitive, and selective HPLC with fluorescence detection method for the concurrent analysis of TAM with TTD. Also, the technique would be used to assay them in spiked human plasma and urine. So, it could be used in monitoring the drug’s quality to ensure patient safety31,32. The suggested approach was determined to meet the standards of the International Council for Harmonization (ICH)33.
Experimental
Instrumentations
The HPLC system employed in the experiment consisted of a Sykam S 1130 quaternary pump (Sykam GmbH, Gewerbering, Germany) equipped with an integrated vacuum degasser. A Wakopack Handy-ODS (Wako Pure Chemicals, Osaka, Japan) column (150 × 4.6 mm, 5 μm particle size) was used for chromatographic separation. Fluorescence detection was performed using an RF-20 A fluorescence detector (Sykam GmbH, Gewerbering, Germany). Double-distilled water was used in the experiment, and the pH of the phosphate buffer was adjusted using a Jenway 3510 pH meter (Staffordshire, UK). A Mettler Toledo 5-digit balance (Greifensee, Switzerland) was used for weighing.
Materials and reagents
The reference standards for TAM (purity of 99.55%) and TTD (purity of 99.70%) were generously provided by Amoun Pharmaceutical Company (El-Obour City, Egypt) and Adwia Pharmaceuticals Company (10th of Ramadan, Sharqia, Egypt), respectively. Tamsul® tablets (0.4 mg TAM) and Incont L.A.® tablets (4 mg TTD), produced by Amoun Pharmaceutical Company and Adwia Pharmaceuticals Company, respectively, were purchased from a local pharmacy. The chemicals used in the experiment, including HPLC-grade methanol, HPLC-grade acetonitrile, disodium hydrogen phosphate, and phosphoric acid were supplied by Merck (Darmstadt, Germany).
Human plasma was generously donated by Sohag University Hospital Blood Bank (Sohag, Egypt). It was kept frozen at −20 °C until the analysis was done.
Mobile phase and chromatographic conditions
The mobile phase for the HPLC analysis consisted of three solvents: acetonitrile (A), water (B), and phosphate buffer (10 mM, pH 3.0) (C). A gradient elution system was employed, with the specific gradient profile listed in Table 1. All chromatographic experiments were conducted at room temperature with a mobile phase flow rate of 1.0 mL/min. Fluorescence detection was used to measure the relative fluorescence intensity (RFI) of the analytes, with excitation and emission wavelengths set at 280 nm and 350 nm, respectively. The phosphate buffer was prepared by dissolving 1.42 g of disodium hydrogen phosphate in 1.0 L of double-distilled water and adjusting the pH to 3.0 with phosphoric acid. Before injecting samples into the HPLC system, the column was allowed to stabilize for at least 30 min with the mobile phase. An injection volume of 20 µL was used for all experiments.
Preparation of standard solutions
Stock standard solutions of TAM and TTD, each at a concentration of 200 µg mL[-1, were prepared by dissolving 20 mg of each drug in 100 mL of methanol. These stock solutions were stored at 4 °C. The calibration curve was prepared by further diluting the stock solutions with methanol to obtain the desired concentration ranges, as shown in Fig. 2.
Procedure for TAM and TTD synthetic pharmaceutical formulation
Due to the unavailability of the combined pharmaceutical tablet containing TAM and TTD in local Egyptian pharmacies, a synthetic pharmaceutical formulation was prepared in the laboratory. The process involved weighing and grinding ten Tamsul® tablets, each containing 0.4 mg of TAM, to determine their average weight. Simultaneously, the contents of ten Incont L.A.® tablets, each containing 4 mg of TTD, were weighed and ground to determine the average weight of TTD. A specific amount of powdered tablet, equivalent to 1 mg of TAM and 10 mg of TTD, was dissolved in 70 mL of methanol. The resulting mixture was then transferred to a 100 mL volumetric flask and thoroughly mixed. After 30 min of sonication, the volume of the solution in the flask was adjusted to the mark using methanol. The solution was then filtered, and the initial portion of the filtrate was discarded. Subsequently, 1 mL of the filtered solution was transferred to a 10 mL volumetric flask and diluted to volume with methanol. A representative chromatogram of the sample is shown in Fig. 3.
Procedure for TAM and TTD in biological human samples
Procedure for TAM and TTD in spiked human plasma
2.0 mL of stored plasma was mixed with 1.0 mL of a standard solution containing TAM and TTD (concentration ranges: 1–15 µg mL[-1for TAM and 10–150 µg mL[-1 for TTD) and 6.0 mL of methanol was finally added to precipitate plasma proteins. The mixture was vortex-mixed for 60 s and then centrifuged at 4000 rpm for 10 min to separate the precipitated proteins. The clear supernatant, which fell within the linear range of the proposed chromatographic method, was injected into the HPLC system. A blank sample was prepared similarly using drug-free plasma (Fig. 4).
Procedure for TAM and TTD in spiked human urine
A portion of 2.0 mL of urine was mixed with 1.0 mL of a standard solution containing TAM and TTD (concentration ranges: 1–15 µg mL[-1for TAM and 10–150 µg mL[-1 for TTD) and 6.0 mL of methanol. The mixture was vortex-mixed for 60 s and centrifuged at 4000 rpm for 10 min. The clear supernatant, which fell within the linear range of the proposed chromatographic method, was injected into the HPLC system. A blank sample was prepared similarly using drug-free urine, as shown in Fig. 5.
Evaluation of system suitability
Six 20 µL injections of each standard solution mixture were made, and the resulting chromatograms were recorded. Key system suitability parameters, including column efficiency, plate number, retention time, and %RSD, were calculated. The results indicated high column efficiency and low standard deviations for the retention times of both TAM and TTD. The %RSD for the standard solution was found to be less than 2%. These findings are summarized in Table 2.
Results
Method development
To achieve optimal separation of the drugs with short analysis time and symmetrical peak shapes, the composition of the mobile phase was carefully optimized. Various combinations of mobile phases (specifically the following ratios, 90:10, 80:20, 60:40, 50:50, 40:60, 20:80, and 10:90) were explored, including acetonitrile-water, methanol-water, acetonitrile-citrate buffer, methanol-citrate buffer, acetonitrile-phosphate buffer, and methanol-phosphate buffer in both isocratic and gradient modes. The influence of organic modifiers on separation efficiency was evaluated while keeping the buffer concentration and pH constant at 10 mM and 3.0, respectively. Other chromatographic parameters, such as column type (C18 and C8) and flow rate (1.0, 1.5, and 2.0 mL/min), were also investigated. It was found that gradient elution mode with the specific profile listed in Table 1 and consisted of three solvents: acetonitrile (A), water (B), and phosphate buffer (10 mM, pH 3.0) (C) gave the best separation parameters on C18 column with a flow rate of 1.0 mL/min. Fluorescence detection was used to measure RFI of the analytes, with excitation and emission wavelengths set at 280 nm and 350 nm, respectively.
Method validation
ICH guidelines were followed to validate the suggested method33.
Linearity and range
To assess the linearity of the method, different concentration combinations, ranged from 0.1 to 1.5 and 1–15 for TAM and TTD, respectively, were injected in the gradient elution HPLC, with the specific gradient profile listed in Table 1. A linear relationship was observed between the area under the curve and the concentration of each drug. Linearity graphs for TAM and TTD were represented in Fig. 1S. The statistical data presented in Table 3, including low values for standard deviations of residuals, intercept, and slope, as well as high correlation coefficients, further confirms the linearity of the proposed method.
Limit of detection (LOD) and limit of quantification (LOQ)
LOD) and LOQ were calculated using the following equations: LOD = 3.3×Sa/ b and LOQ = 10×Sa/ b. where Sa is the standard deviation of the intercept and Sb is the slope of the calibration curve. The calculated LOD values for TAM and TTD were 0.03 and 0.30 µg mL[-1, respectively. The corresponding LOQ values were 0.10 and 0.92 µg mL[-1, respectively. These results are summarized in Table 3. These findings illustrate that the proposed method demonstrates heightened sensitivity in the analysis of TAM and TTD.
Precision and accuracy
To evaluate the precision of the method, the intra-day assay precision was assessed at three different concentration levels for both TAM and TTD. The concentrations tested were 0.15, 0.80, and 1.50 µg mL[-1for TAM and 1.50, 8.00, and 15.00 µg mL[-1 for TTD. The relative standard deviations (RSD) were calculated for each concentration level to quantify the precision of the method. These results are typically presented in Table 1S. To further evaluate the precision of the method, inter-day assay precision was evaluated at three different concentration levels for both TAM and TTD. The concentrations tested were 0.15, 0.80, and 1.50 µg mL[-1for TAM and 1.50, 8.00, and 15.00 µg mL[-1 for TTD. These analyses were conducted over three consecutive days to account for day-to-day variability. RSD was calculated for each concentration level and day to quantify the inter-day precision of the method. The results, typically presented in Table 1S, should indicate an RSD below 2%, signifying good inter-day precision.
To evaluate the accuracy of the method, a standard addition method was employed at three different concentration levels. Known amounts of TAM and TTD were added to samples, and the recovery of the added analytes was determined. The results, expressed as a percentage recovery, were documented in Table 2S. SD below 2% indicates that the method is accurate and reliable.
Robustness
To assess the robustness of the proposed method, small deliberate changes were made to the experimental parameters. Specifically, the flow rate of the mobile phase, excitation and emission wavelengths were slightly altered, and the impact on the method’s sensitivity was evaluated. The results of this robustness testing are presented in Table 3S.
Discussions
TAM and TTD displayed indistinguishable fluorescence spectra, revealing emission peaks following excitation at 280 nm15,21. So, the fluorometric properties of both drugs were used to establish sensitive HPLC with fluorescence detection to separate and assay TAM and TTD. The chromatographic conditions were carefully optimized to achieve optimal separation of TAM and TTD, resulting in well-defined, symmetrical peaks with high theoretical plate numbers and resolution. The mobile phase composition, buffer type, and pH were adjusted to separate TAM and TTD within 5.66 and 7.26 min, respectively. This short analysis time makes the method suitable for routine analysis. The high accuracy and precision demonstrated by the method ensure reliable and reproducible results, making it suitable for quality control applications. Additionally, the robustness testing confirms the reliability of the method under slight variations in experimental conditions. Also, the selectivity of chromatographic methods enabled assaying of TAM and TTD in spiked human plasma and urine without any interference from plasma and urine components.
Method application
Pharmaceutical application
The developed method was successfully applied to analyze synthetic pharmaceutical formulations containing TAM and TTD. Statistical comparisons, including t-tests and F-tests, were conducted at a 95% confidence level to compare the results obtained from the proposed method with those of a previously reported method21. The calculated t-tests values were 0.80 and 0.42 for TAM and TTD, respectively. The calculated F-tests values were 1.67 and 2.77 for TAM and TTD, respectively. These results indicate that the proposed method is capable of accurately determining the drugs in pharmaceutical formulations, with no significant differences observed between the two methods. Table 4. summarizes the results of the statistical comparisons.
Spiked human plasma and urine application
Due to the high sensitivity and selectivity of the proposed chromatographic method, TAM and TTD levels in spiked human plasma and urine at various concentrations within the predetermined range could be assayed. The concentrations of TAM and TTD were determined using their respective regression equations: (RFI = 14763.62X − 273.90 for TAM and RFI = 5249.30X − 1792.92 for TTD). The observed mean recovery rates were (99.31–100.97%) ± (0.96–1.53) for TAM and (100.56–101.79%) ± (0.78–1.53) for TTD in human plasma, while in human urine, they were (98.71–101.55%) ± (0.58–1.44) for TAM and (99.53–101.84%) ± (0.34–1.31) for TTD (Table 5). These results demonstrate the method’s efficiency for quantifying TAM and TTD in spiked human plasma and urine samples.
Evaluation of method of greenness
Analysts play a crucial role in protecting human health and the environment by ensuring the safe handling and disposal of harmful substances, particularly in industries like chemicals and pharmaceuticals34. Green analytical chemistry (GAC), which incorporates energy-efficient equipment and minimal waste generation, assists in lowering the quantities of hazardous chemicals and reagents. Current trends in the development of analytical methods include using fewer or non-toxic solvents, minimizing the use of devices for sample preparation, and using solventless extraction procedures35,36. To assess the environmental impact of analytical methods, tools like the Green Analytical Procedure Index (GAPI)37, and the Analytical Greenness Calculator (AGREE)38approaches are employed39,40,41. It is advised to compare analytical methodologies using a variety of evaluation tools in order to determine the ecological impact of each. GAPI provides a qualitative assessment of the environmental impact of each step of an analytical process, using a color-coded system (green, yellow, and red) to indicate low, medium, and high impact, respectively. The GAPI assessment of the proposed chromatographic method revealed a predominantly green profile, with 8 green, 3 yellow, and 4 red-shaded areas. This indicates a relatively low environmental impact (Fig. 6A).
AGREE is a quantitative tool that provides a numerical score and a visual representation of the method’s greenness. A higher score indicates a greener method. The AGREE assessment of the proposed method yielded a score close to 1 (0.66), indicating a high level of greenness. The pictogram also shows a predominantly green color, further emphasizing the method’s environmental friendliness (Fig. 6B). By employing both GAPI and AGREE, a comprehensive evaluation of the method’s greenness can be achieved. This information is valuable for selecting environmentally friendly analytical methods and minimizing the overall environmental impact of laboratory activities.
Comparison with reported methods
The proposed chromatographic method was compared to several previously reported methods in terms of linear range, application, mobile phase, solvent, LOD, LOQ, and greenness evaluation. Table 6 provides a detailed comparison. It is evident that the proposed method, while having a relatively lower linear range compared to some other methods, is unique in its application to spiked human plasma and urine samples. Additionally, it is the only method that has been evaluated for its greenness, highlighting its environmental friendliness.
Conclusion
A novel, green, sensitive, and accurate reversed-phase chromatographic method with fluorescence detection has been developed for the simultaneous determination of TAM and TTD. The method was validated according to ICH. Linear calibration curves were attained over the concentration ranges of 0.1–1.5 µg mL[-1for TAM and 1–15 µg mL[-1for TTD. The calculated LOD values for TAM and TTD were 0.03 and 0.30 µg mL[-1, respectively. The corresponding LOQ values were 0.10 and 0.92 µg mL[-1, respectively. The method was successfully applied to analyze TAM and TTD in pharmaceutical formulations and spiked human plasma and urine samples. The observed mean recovery percent were 99.31–100.97% for TAM and 100.56–101.79% for TTD in human plasma, while in human urine, they were 98.71–101.55% for TAM and 99.53–101.84% for TTD. The high accuracy and precision demonstrated by the method make it suitable for quality control applications. By incorporating green analytical chemistry principles, the method was assessed using the GAPI and AGREE tools, confirming its environmental friendliness. The main limitation of the proposed HPLC method is the need for proficient personnel.
Data availability
All data generated or analyzed during this study are included in this published article.
References
Haque, S. K. M. Box–Behnken experimental design for optimizing the HPLC method to determine Hydrochlorothiazide in pharmaceutical formulations and biological fluid. J. Mol. Liq. 352, 118708 (2022).
Ajitkumar Bhaskaran, N., Kumar, L., Reddy, M. S. & Pai, K. An analytical quality by design approach in RP-HPLC method development and validation for reliable and rapid Estimation of Irinotecan in an injectable formulation. Acta Pharm. 71, 57–79 (2021).
Rahman, N., Azmi, S. N. H. & Wu, H. F. The importance of impurity analysis in pharmaceutical products: an integrated approach. Accred. Qual. Assur. 11, 69–74 (2006).
Marques, S. M. & Kumar, L. Quality-by-Design-based development of an eco-friendly HPLC method for the Estimation of nisoldipine in nanoformulations: forced degradation studies and in-vitro release studies. Sustainable Chem. Pharm. 36, 101254 (2023).
Reddy, M. S., Kumar, L., Attari, Z. & Verma, R. Statistical optimization of extraction process for the quantification of Valsartan in rabbit plasma by a HPLC method. Indian J. Pharm. Sci. 79, 16–28 (2017).
Shrivastava, A. & Aggrawal, P. Various analytical methodologies for determination of selective α1A receptor blocker Tamsulosin hydrochloride and its combinations in different matrices. World J. Anal. Chem. 1, 37–48 (2013).
Kaplan, S. A. et al. Solifenacin plus Tamsulosin combination treatment in men with lower urinary tract symptoms and bladder outlet obstruction: a randomized controlled trial. Eur. Urol. 63, 158–165 (2013).
The United States pharmacopeia. Thirty-third Edition, the National Formulary, Twenty-eighth Edition (United States Pharmacopeial Convention, Inc., 2011).
The British Pharmacopoeia. The Stationery Office, London (2016).
Amanlou, M., Moghadam, A. G., Tehrani, M. B. & Souri, E. Validated spectrophtometric method for determination of Tamsulosin in bulk and pharmaceutical dosage forms. Iran. J. Pharm. Research: IJPR. 13, 81 (2014).
Karasakal, A. & Ulu, S. T. Validated spectrofluorimetric method for the determination of Tamsulosin in spiked human urine, pure and pharmaceutical preparations. Luminescence 29, 239–242 (2014).
Macek, J., Klíma, J. & Ptáček, P. Rapid determination of Tamsulosin in human plasma by high-performance liquid chromatography using extraction with Butyl acetate. J. Chromatogr. B. 809, 307–311. https://doi.org/10.1016/j.jchromb.2004.06.043 (2004).
Özkan, S. A., Uslu, B. & Aboul-Enein, H. Y. Voltammetric investigation of Tamsulosin. Talanta 61, 147–156. https://doi.org/10.1016/S0039-9140(03)00248-0 (2003).
Ibrahim, M. & Fraihat, S. Simple spectrophotometric methods for determination of Tolterodine tartrate in pharmaceutical forms. Int. J. ChemTech Res. 8, 665–669 (2015).
Derayea, S. M., Badr El-Din, K. M., Ahmed, S. A., Abdelshakour, M. A. & Oraby, M. An eco-friendly one-pot spectrofluorimetric approach for the facile determination of overactive bladder drug, Tolterodine: application to dosage forms and biological fluids. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 311, 123986. https://doi.org/10.1016/j.saa.2024.123986 (2024).
Zhang, B., Zhang, Z., Tian, Y. & Xu, F. High performance liquid chromatography-electrospray ionization mass spectrometric determination of Tolterodine tartrate in human plasma. J. Chromatogr. B. 824, 92–98 (2005).
Mishra, A. et al. Stability-indicating HPTLC method for analysis of Tolterodine in the bulk drug. JPC–Journal Planar Chromatography–Modern TLC. 24, 150–153 (2011).
Langmaier, J., Skopalová, J., Navrátil, T. & Samec, Z. Detection of antimuscarinic agents Tolterodine and fesoterodine and their metabolite 5-hydroxymethyl Tolterodine by ion transfer voltammetry at a polarized room-temperature ionic liquid membrane. Electrochim. Acta. 304, 54–61 (2019).
Attia, A. K., Frag, E. Y. Z. & Ahmed, H. E. Validated electroanalytical determination of flavoxate hydrochloride and Tolterodine tartrate drugs in bulk, dosage forms and urine using modified carbon paste electrodes. Arab. J. Chem. 11, 483–491 (2018).
Nanda, R. K., Gaikwad, J. & Prakash, A. Estimation of Tamsulosin and Tolterodine in its pharmaceutical dosage form by spectrophotometric method. Int. J. Pharm. Tech. Res. 1, 420–423 (2009).
El-Kimary, E. I., Khamis, E. F., Belal, S. F. & Abdel Moneim, M. M. Enhanced spectrofluorimetric determination of two novel combination therapies for the treatment of benign prostatic hyperplasia containing Tamsulosin hydrochloride. Luminescence 33, 771–779 (2018).
Mhamunkar, S. M., Vyavaharkar, R. Y., Bhoir, S. & I. RP-HPLC method development and validation for the simultaneous Estimation of Tamsulosin HCl and Tolterodine tartrate in pharmaceutical dosage form. Int. J. Pharm. Pharm. Sci. 4, 319–322 (2012).
Bhavsar, H. K., Patel, J. G. & Patel, B. R. Development and validation of stability indicating assay method for Estimation of Tamsulosin hydrochloride and Tolterodine tartrate in capsules. Int. J. Res. Anal. Rev. 6, 89–96 (2019).
Patel, M., Dabhi, B., Jebaliya, H., Singh, B. & Shah, A. Simultaneous Estimation of Tolterodine tartrate and Tamsulosin HCl by validated HPTLC assay method from combination capsule form. J. Chem. Pharm. Res. 7, 81–88 (2015).
El-Kimary, E. I., Khamis, E. F., Belal, S. F. & Abdel Moneim, M. M. Novel validated HPTLC method for the analysis of two binary mixtures containing Tamsulosin hydrochloride with antimuscarinic agents. J. Chromatogr. Sci. 56, 81–91 (2018).
Rizk, M., Mahmoud, Z. M. & Azab, M. M. Simultaneous ultra-sensitive analysis of Tamsulosin hydrochloride and Tolterodine tartrate binary mixture in their dosage form via high-performance thin-layer chromatography with fluorimetric detection. JPC–Journal Planar Chromatography–Modern TLC. 35, 509–517 (2022).
Badr El-Din, K., Ahmed, A., Khorshed, A., Derayea, S. & Oraby, M. Smart spectrophotometric methods based on feasible mathematical processing and classical chemometry for the simultaneous assay of alcaftadine and ketorolac in their recently approved pharmaceutical formulation. Egypt. J. Chem. 65, 167–174 (2022).
Haque, S. M. et al. Application of Box–Behnken design combined response surface methodology to optimize HPLC and spectrophotometric techniques for quantifying febuxostat in pharmaceutical formulations and spiked wastewater samples. Microchem. J. 184, 108191 (2023).
Azmi, S. N. H. et al. Box–Behnken design based development of UV-Reversed phase high performance liquid chromatographic method for determination of ascorbic acid in tablet formulations. Separations 9, 361 (2022).
Manirul Haque, S. K. Validated kinetic spectrophotometric methods to optimize robustness study with Youden factorial combinations to determine repaglinide using response surface methodology via box–behnken design. Arab. J. Sci. Eng. 48, 129–144 (2023).
Haque, S. K. M. Optimized Box–Behnken experimental design based response surface methodology and Youden’s robustness test to develop and validate methods to determine nateglinide using kinetic spectrophotometry. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 268, 120712 (2022).
Jain, B. et al. Novel capsule phase microextraction-Gas chromatography-Mass spectrometry for analysis of Pheniramine in a case of its fatal intoxication. Sustainable Chem. Pharm. 36, 101286 (2023).
ICH I. in International Conference on Harmonization, Geneva.
Oraby, M., Ahmed, A. S., Abdel-Lateef, M. A. & Mostafa, M. A. H. Hassan, A. I. Employ FTIR spectroscopic method for determination of certain multiple sclerosis medications in plasma and pharmaceutical formulations. Microchem. J. 167, 106329 (2021).
Haque, S. K. M., Abu-Judeh, A., Jain, R. & Kabir, A. Greener spectrophotometric and HPLC investigation of CNS agent Pregabalin: Taguchi model and box-behnken design for method parameters optimization. J. Mol. Struct. 1317, 139143 (2024).
Haque, S. K. M. et al. Charge transfer based green spectrophotometric method to determine remogliflozin etabonate applying response surface methodology supported with computational studies in pharmaceutical formulations. Sustainable Chem. Pharm. 35, 101193 (2023).
Płotka-Wasylka, J. A new tool for the evaluation of the analytical procedure: green analytical procedure index. Talanta 181, 204–209 (2018).
Pena-Pereira, F., Wojnowski, W. & Tobiszewski, M. AGREE—Analytical greenness metric approach and software. Anal. Chem. 92, 10076–10082 (2020).
Derayea, S. M., Bader El-Din, K. M., Ahmed, A. S., Khorshed, A. A. & Oraby, M. Development of a green synchronous spectrofluorimetric technique for simultaneous determination of Montelukast sodium and Bilastine in pharmaceutical formulations. BMC Chem. 18, 1–13 (2024).
Derayea, S. M., Ahmed, A. S., Abdelshakour, M. A., Oraby, M. & El-Din, K. M. B. Diaryl pyrrolone fluorescent probing strategy for mirabegron determination through condensation with ninhydrin and phenylacetaldehyde: application to dosage forms, human urine and plasma. Spectrochim. Acta Part A Mol. Biomol. Spectrosc., 124515. https://doi.org/10.1016/j.saa.2024.124515. (2024).
Derayea, S. M., Badr El-din, K.M., Ahmed, A. S., Khorshed, A. A. & Oraby, M. Determination of antihistaminic drugs alcaftadine and Olopatadine hydrochloride via ion-pairing with Eosin Y as a spectrofluorimetric and spectrophotometric probe: application to dosage forms. BMC Chem. 18, 40 (2024).
Acknowledgements
Not applicable.
Funding
Open access funding provided by The Science, Technology & Innovation Funding Authority (STDF) in cooperation with The Egyptian Knowledge Bank (EKB).
Author information
Authors and Affiliations
Contributions
A.S.A.: Conceptualization, methodology, data curation, software, and preparation of original draft writing. K.M. B.: Conceptualization and data curation. M.A.A.: investigation, Visualization, and supervision. M.O.: Methodology, validation, reviewing, and writing. S.M. D.: Methodology, validation, reviewing, writing, and editing.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Derayea, S.M., Badr El-Din, K.M., Ahmed, A.S. et al. Green HPLC-Fluorescence detection method for concurrent analysis of Tamsulosin hydrochloride and Tolterodine tartrate in dosage forms and biological fluids. Sci Rep 15, 10615 (2025). https://doi.org/10.1038/s41598-025-92183-6
Received:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s41598-025-92183-6
Keywords
This article is cited by
-
Validation of a green and sensitive spectrofluorimetric method for determination of Bilastine and its application to pharmaceutical preparations, content uniformity test, and spiked human plasma
BMC Chemistry (2025)
-
GLANCE-aided snapshotting for sustainable integration of synchronous spectrofluorimetry and micellar boosting for nanoscale assay of tolterodine binary mixtures in crucial matrices
Scientific Reports (2025)