Abstract
In hemodialysis patients, it remains unclear whether patient characteristics influence the clinical impacts of changes in serum mineral metabolism parameters on mortality. In this 9-year cohort study, we investigated the associations between the changes in calcium/phosphate levels and all-cause mortality using a time-dependent approach after adjustment for potential confounders in groups stratified by performance status (PS), a history of atherosclerotic cardiovascular disease (ACVD), or diabetic nephropathy (DN). In patients with baseline serum calcium levels of 9.5–<10.0 mg/dL, increases in serum calcium levels were associated with higher mortality exclusively in patients with PS Grade 0. In the same baseline calcium range, a significant association was observed between reduced serum calcium levels and lower mortality only in patients with a history of ACVD or DN. Similarly, in patients with baseline serum phosphate levels of 5.0–<5.5 mg/dL, reduced serum phosphate levels were associated with lower mortality only in those with PS Grade 0, a history of ACVD or DN. These findings indicate that PS should be considered in treating mild hypercalcemia or hyperphosphatemia in hemodialysis patients. Moreover, stringent management of hypercalcemia and hyperphosphatemia in patients with a history of ACVD or DN might be associated with a better prognosis.
Similar content being viewed by others
Introduction
Numerous observational studies have demonstrated an association between elevated serum calcium/phosphate levels and an enhanced risk of mortality in patients undergoing hemodialysis1,2,3. Increased calcium and phosphate load have been linked to accelerated vascular and cardiac valve calcification in hemodialysis patients4,5,6, thereby indicating hypercalcemia and hyperphosphatemia to be significant risk factors for mortality in this group of patients. The Kidney Disease: Improving Global Outcome (KDIGO) guidelines have suggested that elevated levels of calcium and phosphate should be lowered toward the normal ranges, based on this evidence7. Although a few previous observational studies have demonstrated that a reduction in these parameters is associated with a lower mortality risk8,9, the evidence is limited. Randomized controlled trials can prove the clinical impacts of reducing serum calcium or phosphate levels; however, currently there is limited evidence available10,11.
In patients undergoing hemodialysis, the association between serum phosphate levels and mortality depends on patient characteristics12,13,14. The performance status (PS) of the patients undergoing hemodialysis modified the association between serum phosphate levels and mortality12. Our previous study has demonstrated that hemodialysis patients with a history of atherosclerotic cardiovascular disease (ACVD) or diabetic nephropathy (DN) are more likely to benefit from reduced serum phosphate levels13. Therefore, the association of changes in phosphate levels with mortality may differ across groups stratified by PS or a history of ACVD or DN.
This study used the database of the Japanese Society for Dialysis Therapy Renal Data Registry (JRDR) to examine the association between changes in serum calcium/phosphate levels and mortality in hemodialysis patients using a time-dependent approach, stratified by PS, a history of ACVD, or a renal etiology of DN.
Methods
Data source, study population, and study design
Data from our previous studies2,13 was used in this 9-year cohort study. The JRDR is a nationwide registry, its data has been constituted through questionnaires distributed to all dialysis facilities in Japan by the Japanese Society for Dialysis Therapy (JSDT), the response rate for which was 95%. A more detailed description of the survey protocol can be found elsewhere15.
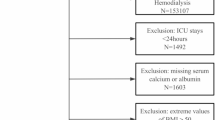
Patients undergoing maintenance hemodialysis or hemodiafiltration three times per week at the end of 2009 constituted the study population. Inclusion criteria for the study were: patients aged 18 years or older; treatment durations of 3–6 h per session; a dialysis vintage of one year or more; having data on calcium, phosphate, intact or whole parathyroid hormone (PTH), and albumin at the end of 2009 (to serve as baseline); and having at least one or more data on calcium, phosphate, intact or whole PTH, and albumin that had been collected after 2010. Patients receiving combined therapy with PD and those with implausible outcome data were excluded from the study (Fig. S1). The Ethics Committee of the JSDT approved the study protocol and waived the need for informed consent since the data were anonymized prior to being transferred to the investigators (approval number 54). Patients were provided with the option to opt out of participation. The study was conducted in accordance with the principles of the Declaration of Helsinki.
Definitions
The following formula was used for correcting the serum calcium levels: Corrected calcium = serum calcium (mg/dL) + [4 - serum albumin (g/dL)], for serum albumin levels, which were less than 4 g/dL16. If whole PTH assays were used, the whole PTH levels were multiplied by 1.7 to obtain equivalent values for the intact PTH assay17. The PS was classified in accordance with the Eastern Cooperative Oncology Group Performance Status classification18.
Statistical analysis
Continuous variables with normal distributions have been expressed in terms of mean and standard deviation, whereas the skewed distributions have been expressed as median and interquartile range (IQR), and the categorical variables as number and percentage.
The relationship between changes in serum calcium/phosphate levels and all-cause mortality was investigated using the multivariate Cox proportional hazard models. Changes in serum calcium and phosphate levels were calculated by deducting the baseline values from the mean serum values of each subsequent year, in accordance with a previous study (Fig. S2A)8. The analyses were stratified based on baseline serum calcium or phosphate levels (calcium: <8.4, 8.4–<9.0, 9.0–<9.5, 9.5–<10.0, or ≥ 10.0 mg/dL; phosphate: <3.5, 3.5–<5.0, 5.0–<5.5, 5.5–<6.0, or ≥ 6.0 mg/dL), given that the association between the changes in serum calcium/phosphate levels and mortality were subject to variations based on baseline8,9. The cut-off values were determined based on the distribution of participants and the recommended ranges according to the Japanese guidelines17. We investigated the association in the whole patients subjected for analyses and subgroups stratified by PS (Grade 0, Grade 1, or Grade 2–4), a history of ACVD, or a renal etiology of DN. Moreover, the proportion of all-cause death and cardiovascular disease (CVD) death, the relationship of TA phosphate and calcium with mortality due to heart failure, and the correlation between changes of serum phosphate levels and those of serum albumin levels in groups stratified by PS were investigated to better understand the mechanism of variations across groups stratified by PS. The same method as that used for calculating the changes in calcium or phosphate was used to calculate the changes in serum albumin levels.
The association of the changes in serum intact PTH levels with all-cause mortality were also investigated. Changes in serum intact PTH levels were determined as indicated in a previous study, by dividing the mean serum value of each year by the baseline values (Fig. S2B)19. Since patients with PTH levels ≥ 600 pg/ml accounted for only 1.9% of the total enrolled patients, the analyses were stratified based on the baseline serum intact PTH levels (< 60, 60–<120, 120–<180, 180–<240, or 240–<600 pg/mL).
Restricted cubic spline models were used to examine the nonlinear associations between changes in serum calcium, phosphate, and intact PTH levels and mortality. In order to mitigate the influence of outliers, values above the 99th percentile and below the 1st percentile of changes in serum calcium, phosphate, and intact PTH levels were excluded. The Akaike Information Criterion was applied for determining the number of knots in each model.
Covariates in these analyses included age, sex, dialysis vintage, dialysis time, dialysis modality (hemodialysis or hemodiafiltration), body mass index (BMI), Kt/V, normalized protein catabolic rate (nPCR), primary cause of renal failure (glomerulonephritis, DN, hypertension, polycystic kidney disease, others, or unknown), history of myocardial infarction, cerebral hemorrhage, cerebral infarction, amputation, hip fracture, parathyroidectomy (PTx), or percutaneous ethanol injection therapy (PEIT), albumin, creatinine, C-reactive protein (CRP), hemoglobin, magnesium, alkaline phosphatase (ALP), chronic kidney disease-mineral and bone disorder (CKD-MBD) related medications (calcium carbonate, sevelamer chloride, lanthanum carbonate, other phosphate binders, oral vitamin D receptor activator (VDRA), intravenous VDRA, and cinacalcet), dialysate calcium concentration (< 2.75, 2.75 to < 3.0, ≥ 3.0 mEq/L, and acetate-free), and PS (0–4). Among these variables, the covariates including age, dialysis time, BMI, Kt/V, PCR, history of myocardial infarction, cerebral hemorrhage, cerebral infarction, amputation, albumin, creatinine, CRP, and hemoglobin were considered to be the time-varying covariates. For calcium, phosphate, and intact PTH, the analyses were adjusted for the baseline values of the targeted parameter and the time-varying covariates of the other two parameters when analyzing the associations for one parameter. When the number of hemodialysis sessions per week was changed to something other than three, the time-varying covariates were treated as missing values. CRP was log-transformed to normalize its distribution. Based on the U-shaped associations between the variables, serum calcium, phosphate, magnesium, and intact PTH levels and mortality, serum calcium, phosphate, and intact PTH levels were divided into deciles, whereas serum magnesium levels were divided into quintiles2,20.
P value < 0.05 was considered statistically significant. In the comparison of each baseline variable between two groups, standardized differences ≥ 10% indicate an imbalance between variables. The nonparametric test for trend developed by Cuzick was used for assessing the trends across the three groups. The Stata/MP 14.2 software for Windows (Stata, College Station, TX, USA) was used for performing all statistical analyses.
Results
The study population included a total of 68,354 hemodialysis patients. Patients with poor PS were generally older, predominantly female, had shorter dialysis durations, lower BMI and nPCR, reduced hemodiafiltration, and less frequent CKD-MBD-related medication and high-calcium dialysate use (Table 1). They were more likely to have a history of DN, CVD, and fractures, along with lower levels of serum phosphate, PTH, albumin, creatinine, hemoglobin, and magnesium, and higher levels of serum calcium, CRP, and ALP. Patients with ACVD were older, predominantly male, had shorter dialysis durations, lower Kt/V and nPCR, and lesser use of calcium carbonate, sevelamer, lanthanum carbonate, and cinacalcet (Table 2). They were also characterized by a greater prevalence of DN, cerebral hemorrhage, and fractures, and exhibited reduced serum phosphate, albumin, creatinine, and magnesium levels, elevated CRP levels and poorer PS. Patients with DN were older, predominantly male, and the other characters included shorter dialysis durations, lower Kt/V, higher BMI, less hemodiafiltration, along with reduced sevelamer, intravenous VDRA, and cinacalcet usage. These patients also had a higher prevalence of ACVD and lower serum calcium, PTH, and creatinine levels.
A total of 26,364 deaths occurred during the median observational period of 108 (IQR 55–108) months. In restricted cubic spline models, patients with normal or high calcium levels at baseline (≥ 8.4 mg/dL) were associated with a higher mortality risk following an increase in serum calcium levels from baseline values, whereas patients with low baseline calcium levels exhibited no such associations (Fig. S3). In patients with baseline serum calcium levels ≥ 9.5 mg/dL, significant associations were found between decreased serum calcium levels and lower mortality.
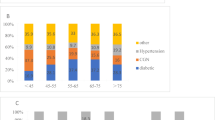
In analyses stratified by PS, similar trends were observed among patients with baseline serum calcium levels of 9.0–<9.5 or ≥ 10.0 mg/dL. In patients with serum calcium levels ranging from 9.5–<10.0 mg/dL, elevated serum calcium levels were associated with mortality risk in patients with PS Grade 0, whereas the association was attenuated in patients with higher PS Grade (Fig. 1).
The association between changes in calcium levels and mortality was modified by a history of ACVD or renal etiology of DN. In patients with baseline serum calcium levels of 9.5–<10.0 mg/dL, patients with ACVD or DN exhibited lower mortality due to decreased calcium levels, whereas no such associations were observed in patients without ACVD or DN (Figs. 2, 3).
Regarding phosphate, in patients with baseline serum phosphate levels ≥ 3.5 mg/dL, elevated serum phosphate levels from baseline were associated with higher mortality (Fig. S4). In patients with baseline serum phosphate levels < 3.5 mg/dL, phosphate level increases less than 0.5 mg/dL were associated with lower mortality, whereas increases > 1.2 mg/dL were associated with higher mortality. In patients with baseline serum phosphate levels ≥ 5.0 mg/dL, decreased serum phosphate levels were associated with lower mortality, whereas in patients with baseline serum phosphate levels < 3.5 mg/dL, decreased phosphate levels were associated with higher mortality.
The association between changes in serum phosphate levels and all-cause mortality was modified by PS (Fig. 4). Increased phosphate levels were associated with higher mortality and decreased phosphate levels were associated with lower mortality in PS Grade 0 patients, with baseline serum phosphate levels of 5.0–<6.0 mg/dL. However, the associations became weaker in patients with poorer PS.
With regards to patients with a history of ACVD or renal etiology of DN, a lower mortality risk was observed following reductions in serum phosphate levels in patients with baseline serum phosphate levels ≥ 5.0 mg/dL; however, in patients without a history of ACVD or DN, and with baseline serum phosphate levels ≥ 5.5 mg/dL a significant association was observed between decreased serum phosphate levels and a lower mortality (Figs. 5, 6).
The proportion of CVD deaths was similar among the groups stratified by PS. Worse PS was associated with a higher proportion of heart failure related deaths (Fig. S5). With regards to the association of serum time-averaged (TA) calcium or phosphate levels with mortality due to heart failure, patients with PS Grade 0 demonstrated significant positive linear associations. However, with poorer PS, the associations were attenuated (Fig. S6). In patients with PS Grade 0, a reduction in serum phosphate levels was not associated with a reduction in serum albumin levels; however, in those with poor PS and baseline serum phosphate levels of 5.0–<6.0 mg/dL, reduced phosphate levels were associated with reduction in albumin levels (Fig. S7). In the case of patients with PS Grade 2–4 and baseline serum phosphate levels ≥ 6.0 mg/dL, decreases in phosphate levels > 1.0 mg/dL were associated with decreases in albumin.
In patients with serum PTH levels ≥ 180 pg/mL at baseline, PTH increases were associated with a higher mortality risk (Fig. S8). In patients with serum PTH levels < 60 pg/mL, 60–<120 pg/mL, and 120–<180 pg/mL, a higher mortality risk was observed, corresponding to 3.2-, 2.1-, and 1.5-fold increases from baseline values, respectively. In patients with baseline serum PTH levels < 240 pg/mL, decreases in serum PTH levels were not significantly associated with mortality. However, reductions in serum PTH levels were associated with a lower risk of mortality in patients with serum PTH levels 240–<600 pg/mL.
Discussion
In this large cohort study based on a nationwide database, in patients with baseline calcium levels ≥ 10.0 mg/dL or baseline phosphate levels ≥ 6.0 mg/dL, decreases in serum calcium or phosphate levels were associated with a lower mortality risk, regardless of PS or a history of ACVD or DN. However, among patients with baseline calcium levels of 9.5–<10.0 mg/dL or baseline phosphate levels of 5.0–<6.0 mg/dL, the association between changes in serum calcium or phosphate levels and all-cause mortality was weaker in those with poor PS compared to those with good PS. Moreover, in patients with a history of ACVD or a renal etiology of DN and baseline calcium levels of 9.5–<10.0 mg/dL or phosphate levels of 5.0–<5.5 mg/dL, lower all-cause mortality associated with reduced serum calcium or phosphate levels was observed. In contrast, in patients without a history of ACVD or DN, reductions in serum calcium or phosphate levels were not associated with mortality at these baseline calcium or phosphate levels.
A previous study had demonstrated that poor PS attenuated the effect of hyperphosphatemia on all-cause mortality12. In our study, PS influenced the association of alterations in the serum phosphate as well as calcium levels with all-cause mortality. The precise mechanisms underlying this modification caused by PS remain unclear; however, the current study has demonstrated that the associations between serum calcium/phosphate levels and deaths caused by heart failure were weaker in patients with poor PS compared with patients with good PS. Moreover, patients with poor PS exhibit a greater proportion of deaths due to heart failure. Various factors are responsible for causing heart failure in patients with chronic kidney disease21. These findings suggest that factors other than calcium or phosphate could influence heart failure in hemodialysis patients with poor PS.
In this observational study, the factors responsible for reduced serum phosphate levels included either reduction due to interventions such as phosphate binders or non-intervention such as worsening nutritional status. A previous observational study had demonstrated that a worse prognosis was observed in hemodialysis patients with decreased protein intake and serum phosphate levels, contrary to patients with an increase in both parameters22. Among patients with poor PS (PS Grade ≥ 1) in our study, and in patients with baseline phosphate levels of 5.0–<6.0 mg/dL, decreased phosphate levels correlated with decreased albumin levels and were not associated with lower mortality; however, in patients with baseline phosphate levels ≥ 6.0 mg/dL, decrease in serum phosphate levels by 1.0 mg/dL, were not correlated with decreased albumin levels and were associated with lower mortality. These findings suggest that reduced serum phosphate levels without worsening nutrition might be associated with a lower risk of all-cause mortality even in patients with poor PS.
In this study, it was observed that hemodialysis patients with a history of ACVD or DN―particularly patients with lower baseline values―benefited to a greater extent from serum phosphate level reductions compared to those without a history of ACVD or DN. Our previous study had demonstrated linear associations between TA serum phosphate levels and all-cause mortality in hemodialysis patients with a history of ACVD or DN, whereas, the associations plateaued at serum phosphate levels below 5.0 mg/dL in patients without these histories13. In another study investigating non-dialysis patients, a stronger association was observed between elevated plasma phosphate levels and all-cause mortality in individuals with type 2 diabetes compared to those without a history of diabetes23. In vitro studies had demonstrated that compared with high-phosphate and normal-glucose medium, high-phosphate and high-glucose medium induced more severe calcium deposits in vascular smooth muscle cells, potentially through upregulation of a phosphate transporter or cellular-senescence-related factors24,25,26. In an animal study conducted on rats fed with high-phosphate diets, more severe vascular calcification was observed in rats with chronic kidney disease and diabetes mellitus as compared with rats having only chronic kidney disease27. Although fewer studies have examined the impact of hyperphosphatemia in groups stratified by a history of ACVD, individuals with the history of ACVD, characterized by more severe vascular calcification at baseline28, might tend to experience progression of vascular calcification, similar to diabetic patients, compared with those without ACVD. These findings suggest the significance of a stricter management of serum phosphate levels in hemodialysis patients with a history of ACVD or DN.
Similarly, for patients with serum calcium levels of 9.5–<10.0 mg/dL, only those with a history of ACVD or DN displayed an association between reduced calcium levels and a reduced mortality risk. Unfortunately, the association between serum calcium levels and mortality in hemodialysis patients stratified by a history of ACVD or DN has been seldom evaluated. The COSMOS study reported no significant effect modification by diabetes on the association between serum calcium levels and mortality, which is inconsistent with our findings29. The exact reason for this discrepancy is not clear. Since the sample size used in the current study had been larger compared with the COSMOS study, it might have made the detection of effect modification based on a history of ACVD or DN more feasible. High serum calcium levels are a well-established risk factor for vascular calcification in patients undergoing hemodialysis4. Therefore, stricter control of serum calcium levels, similar to phosphate levels, might confer greater benefits on hemodialysis patients with a history of ACVD or DN. Stricter calcium control can be achieved by reducing the use of calcium-based phosphate binders, optimizing VDRA therapy, and switching to a low-calcium dialysate.
Traditionally, excessive PTH suppression has been considered a cause of adynamic bone disease, leading to the progression of vascular calcification30,31. Therefore, the KDIGO guidelines have suggested a lower limit for PTH levels7. However, recent observational studies have reported that PTH levels below the suggested lower limit are not associated with an increased mortality risk in hemodialysis patients2,19,32. In this study, a decrease in serum PTH levels was not significantly associated with a higher mortality risk in patients whose baseline serum PTH levels were < 60 pg/mL. These findings suggest that we should reconsider whether a lower limit for serum PTH levels is necessary in these patients.
Our study found that decreases in serum PTH levels were associated with a lower mortality risk in patients with baseline PTH levels of 240–<600 pg/mL. The upper limit for PTH levels recommended in the Japanese guidelines (240 pg/mL) is lower than that specified in the KDIGO guidelines (nine times the upper normal limit for assay, i.e., 585 pg/mL)7,17. Although the optimal target range remains uncertain due to limited evidence, our findings suggest that lowering the upper limit of the KDIGO target range might be beneficial. Further research is needed to determine the most appropriate upper limit for serum PTH levels in patients undergoing hemodialysis.
The current Japanese guidelines, published in 2012, recommend a targeted range for serum phosphate levels between 3.5 and 6.0 mg/dL17, which is higher than that recommended by the KDIGO guidelines (the normal range)7. However, our study found that reduced serum phosphate levels were associated with a lower mortality risk in patients with baseline serum phosphate levels of 5.0–<6.0 mg/dL. A recent clinical trial demonstrated that stricter phosphate control (target: 3.5–<4.5 mg/dL) slowed the progression of vascular calcification compared with standard phosphate control (target: 5.0–<6.0 mg/dL)11. These findings suggest that lowering the upper limit of the target range for serum phosphate levels in the Japanese guidelines might be more beneficial, particularly for patients with good PS or a history of ACVD or DN. The primary strategies for achieving stricter phosphate control include increasing phosphate removal through dialysis, restricting dietary phosphate intake, using phosphate-lowering medications, and administering calcimimetics for patients with high PTH levels.
This study has several limitations. First, being an observational study, it could not establish causal relationships. Second, residual confounding may still be present despite adjusting for significant confounders. Third, the study population was limited to hemodialysis patients in Japan, and as such the findings may not be generalizable to other countries. Fourth, blood collection of most hemodialysis patients was performed at the beginning of the week in Japan33. Therefore, if blood is collected in the middle of the week, the results may be different. However, the large sample size and extended follow-up period are the strengths of the study, since they enabled the stratification of the patients into distinct ranges for serum calcium and phosphate levels.
In conclusion, our findings suggest the consideration of PS in treating mild hypercalcemia (Ca 9.5–<10.0 mg/dL) or hyperphosphatemia (P 5.0–<6.0 mg/dL) in hemodialysis patients. Furthermore, stricter management of hypercalcemia and hyperphosphatemia might lead to better prognosis in patients with a history of ACVD or DN.
Data availability
The data underlying this article were provided by the JSDT by permission. Data will be shared on request to the corresponding author with permission of the JSDT.
References
Taniguchi, M. et al. Serum phosphate and calcium should be primarily and consistently controlled in prevalent Hemodialysis patients. Ther. Apher Dial. 17, 221–228 (2013).
Goto, S. et al. Hypocalcemia and cardiovascular mortality in Cinacalcet users. Nephrol. Dial Transpl. 39, 637–647 (2024).
Block, G. A. et al. Mineral metabolism, mortality, and morbidity in maintenance Hemodialysis. J. Am. Soc. Nephrol. 15, 2208–2218 (2004).
Shroff, R. C. et al. Chronic mineral dysregulation promotes vascular smooth muscle cell adaptation and extracellular matrix calcification. J. Am. Soc. Nephrol. 21, 103–112 (2010).
Sasakawa, Y. et al. Factors associated with aortic valve stenosis in Japanese patients with end-stage kidney disease. BMC Nephrol. 23, 129 (2022).
Watanabe, K., Fujii, H., Kono, K., Goto, S. & Nishi, S. Comparison of the effects of lanthanum carbonate and calcium carbonate on the progression of cardiac valvular calcification after initiation of hemodialysis. BMC Cardiovasc. Disord. 20, 39 (2020).
Kidney Disease: Improving Global Outcomes (KDIGO) CKD-MBD Update Work Group. KDIGO 2017 clinical practice guideline update for the diagnosis, evaluation, prevention, and treatment of chronic kidney Disease–Mineral and bone disorder (CKD-MBD). Kidney Int. Suppl. 7, 1–59 (2017).
Fernández-Martín, J. L. et al. Improvement of mineral and bone metabolism markers is associated with better survival in haemodialysis patients: the COSMOS study. Nephrol. Dial Transpl. 30, 1542–1551 (2015).
Kato, C. et al. Changes in 3-month mineral and bone disorder patterns were associated with all-cause mortality in prevalent Hemodialysis patients with secondary hyperparathyroidism. BMC Nephrol. 21, 432 (2020).
Isaka, Y. et al. Optimal phosphate control related to coronary artery calcification in dialysis patients. J. Am. Soc. Nephrol. 32, 723–735 (2021).
Bhargava, R. et al. A randomized controlled trial of different serum phosphate ranges in subjects on hemodialysis. BMC Nephrol. 20, 37 (2019).
Wakasugi, M. et al. Functional impairment attenuates the association between high serum phosphate and mortality in dialysis patients: a nationwide cohort study. Nephrol. Dial Transpl. 34, 1207–1216 (2019).
Goto, S. et al. The benefit of reducing serum phosphate levels depends on patient characteristics: a nationwide prospective cohort study. Clin. Kidney J. 17, sfae263 (2024).
Sakaguchi, Y. et al. Magnesium modifies the cardiovascular mortality risk associated with hyperphosphatemia in patients undergoing hemodialysis: a cohort study. PLoS One. 9, e116273 (2014).
Nakai, S. et al. Overview of regular dialysis treatment in Japan (as of 31 December 2009). Ther. Apher Dial. 16, 11–53 (2012).
Payne, R. B., Little, A. J., Williams, R. B. & Milner, J. R. Interpretation of serum calcium in patients with abnormal serum proteins. Br. Med. J. 4, 643–646 (1973).
CKD-MBD Guideline Working Group; Japanese Society for Dialysis Therapy. Clinical practice guideline for the management of chronic kidney disease-mineral and bone disorder. Ther. Apher Dial. 17, 247–288 (2013).
Oken, M. M. et al. Toxicity and response criteria of the Eastern cooperative oncology group. Am. J. Clin. Oncol. 5, 649–655 (1982).
Komaba, H. et al. Lower parathyroid hormone levels are associated with reduced fracture risk in Japanese Hemodialysis patients. Kidney Int. Rep. 9, 2956–2969 (2024).
Sakaguchi, Y. et al. Hypomagnesemia is a significant predictor of cardiovascular and non-cardiovascular mortality in patients undergoing Hemodialysis. Kidney Int. 85, 174–181 (2014).
Tuegel, C. & Bansal, N. Heart failure in patients with kidney disease. Heart 103, 1848–1853 (2017).
Shinaberger, C. S. et al. Is controlling phosphorus by decreasing dietary protein intake beneficial or harmful in persons with chronic kidney disease? Am. J. Clin. Nutr. 88, 1511–1158 (2008).
van der Vaart, A. et al. Plasma phosphate and all-cause mortality in individuals with and without type 2 diabetes: the Dutch population-based lifelines cohort study. Cardiovasc. Diabetol. 21, 61 (2022).
Wang, P., Zhou, P., Chen, W. & Peng, D. Combined effects of hyperphosphatemia and hyperglycemia on the calcification of cultured human aortic smooth muscle cells. Exp. Ther. Med. 17, 863–868 (2019).
Wang, P., Quan, Z., Luo, D., Chen, W. & Peng, D. Spironolactone dose–dependently alleviates the calcification of aortic rings cultured in hyperphosphatemic medium with or without hyperglycemia by suppressing phenotypic transition of VSMCs through downregulation of Pit–1. Mol. Med. Rep. 19, 3622–3632 (2019).
Zhang, M. et al. Both high glucose and phosphate overload promote senescence-associated calcification of vascular muscle cells. Int. Urol. Nephrol. 54, 2719–2731 (2022).
Watanabe, S. et al. Influence of oxidative stress on vascular calcification in the setting of coexisting chronic kidney disease and diabetes mellitus. Sci. Rep. 10, 20708 (2020).
Ito, M. et al. Association between serum magnesium levels and abdominal aorta calcification in patients with pre-dialysis chronic kidney disease stage 5. PLoS One. 16, e0253592 (2021).
Martín-Carro, B. et al. Mineral and bone metabolism markers and mortality in diabetic patients on haemodialysis. Nephrol. Dial Transpl. 38, 2589–2597 (2023).
London, G. M. et al. Arterial calcifications and bone histomorphometry in end-stage renal disease. J. Am. Soc. Nephrol. 15, 1943–1951 (2004).
Tomiyama, C. et al. Coronary calcification is associated with lower bone formation rate in CKD patients not yet in dialysis treatment. J. Bone Min. Res. 25, 499–504 (2010).
Yamamoto, S. et al. Alkaline phosphatase and parathyroid hormone levels: international variation and associations with clinical outcomes in the DOPPS. Kidney Int. Rep. 9, 863–876 (2024).
Patient Registration Committee, Japanese Society for Dialysis Therapy. An overview of regular dialysis treatment in Japan (as of 31 December 2002). Ther. Apher Dial. 8, 358–382 (2004).
Acknowledgements
We thank the members of the Committee of Renal Data Registry of the JSDT as well as the personnel from the institutions that participated in this survey. The data for this study were provided by the JSDT. The interpretation and reporting of these data are the responsibility of the authors and should not be seen as an official policy or interpretation of the JSDT.
Funding
This research received no specific grant from any funding agency in the public, commercial or not-for-profit sectors.
Author information
Authors and Affiliations
Contributions
S.G. and T.H. were responsible for the research idea and study design, and contributed to the analysis and drafting of the manuscript. H.F., M.T., M.A., K.N. and S.N. contributed to the design, analysis, interpretation of data, and improvement of the draft.
Corresponding author
Ethics declarations
Competing interests
S. G. reports receiving honoraria from Kyowa Kirin, Sanwa Kagaku Kenkyusho, Kissei, Astellas, and Ono and scholarship grants from Kyowa Kirin and Chugai. T. H. reports receiving honoraria from Kyowa Kirin, Ono, Sanwa Kagaku Kenkyusho, Kissei, Astellas, Chugai, and Torii and research funding from Kyowa Kirin, Kissei, Chugai, Sanwa Kagaku Kenkyusho, and Torii. M. T. reports receiving honoraria from Kyowa Kirin, Torii, Kissei, Ono, Bayer, Daiichi Sankyo, Sanwa Kagaku Kenkyusho, Astellas, and Mitsubishi Tanabe. M. A. reports receiving research funding from Kyowa Kirin and Torii. K. N. reports receiving honoraria from Kyowa Kirin and receiving research funding from Kyowa Kirin. S. N. reports receiving honoraria from Kyowa Kirin, Chugai, Bayer Yakuhin, Kissei, Astellas, and Torii, and receiving scholarship grants from Kyowa Kirin and Chugai. H. F. reports receiving speaker fees as honoraria from Kyowa Kirin, Bayer, Kissei, and Astellas and scholarship grants from Kyowa Kirin and Chugai.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Goto, S., Hamano, T., Taniguchi, M. et al. Patient characteristics modify the association between changes in mineral metabolism parameters and mortality in a nationwide hemodialysis cohort study. Sci Rep 15, 8089 (2025). https://doi.org/10.1038/s41598-025-92359-0
Received:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s41598-025-92359-0