Abstract
To explore the risk factors of moderate to severe ovarian hyperstimulation syndrome (Ovarian hyperstimulation syndrome, OHSS) in patients using the early-follicular phase long-acting gonadotropin-releasing hormone agonist long protocol (EFLL) group in fresh cycles, and to establish a nomogram model to predict the risk of moderate to severe OHSS. We retrospectively analyzed clinical data from 4,204 patients who receiving in vitro fertilization/intracytoplasmic sperm injection and embryo transfer (IVF/ICSI-ET) at the Reproductive Medicine Department of the Second Affiliated Hospital of Zhengzhou University from January 2015 to August 2024. A total of 4204 cases using EFLL protocol were included. The clinical cases were randomly divided into a modeling group (2,942 cases) and a verification group (1,262 cases) at a ratio of 7:3. Logistic regression analysis was used to identify the independent risk factors associated with the occurrence of moderate to severe OHSS in fresh cycles, Based on the selected independent risk factors and correlated regression coefficients, we established a nomogram model to predict the probability of moderate to severe OHSS in this patients, and the predictive accuracy of the model was measured using the area under the receiver operating characteristic (ROC) curve, calibration curve, and decision curve analysis. Univariate and multivariate logistic regression analyses showed that Antal follicle count (AFC) (OR, 1.04; 95%CI, 1.02–1.07; P = 0.002), estrogen levels on the day of hCG injection (OR, 1.01; 95%CI, 1.01–1.01; P<0.01), progesterone levels on the day of hCG injection (OR, 1.18; 95%CI, 1.04–1.34; P = 0.011),, whether the patient had a hypothyroidism (OR, 3.62; 95%CI, 2.10–6.23; P < 0.001), and infertility type (OR, 0.59; 95%CI, 0.35–0.99; P = 0.048) are the independent risk factors for the occurrence of moderate to severe OHSS in fresh cycles. The ROC curve (AUC) being 0.83 (95% CI: 0.78–0.88) for the modeling group and 0.84 (95% CI: 0.78–0.90) for the validation group. The calibration curve and decision curve demonstrated good consistency between the predicted rates of moderate to severe OHSS and the actual incidence. AFC, estrogen levels on the day of hCG injection, progesterone levels on the day of hCG injection, whether the patient had a hypothyroidism, and infertility type are the independent risk factors for moderate to moderate to severe OHSS in fresh cycles. The established nomogram model has proven to be a novel tool that can intuitively predict the incidence of moderate to severe OHSS in the patients that receiving EFLL protocol in fresh cycles, and this nomogram model developed in this study showed better net benefit, have a good clinical applicability for decision-making and could help the clinician to set up a better clinical management strategies for conducting a precise personal therapy.
Similar content being viewed by others
Introduction
With the development of society and the increasing number of infertility couples, the demand for assisted reproductive technologies is also growing. Currently, more than 1 million ART cycles are performed in China every year1. The complications that happen during controlled ovarian hyperstimulation (COH) have begun to attract people’s attention gradually, among which Ovarian Hyperstimulation Syndrome (OHSS) is an entirely iatrogenic complication2. It is primarily characterized by ovarian enlargement, abdominal discomfort, ascites, hemoconcentration, and hypercoagulability, and in severe cases, it can lead to electrolyte disorders, liver and kidney failure, and thrombosis. It can even be life-threatening3. OHSS is commonly classified into mild, moderate, and severe based on clinical symptoms. Mild OHSS is generally recommended to be treated on an outpatient basis, but moderate to severe cases may lead to shock or even death, often requiring hospitalization4,5,6,7,8. Despite the incidence of moderate to severe OHSS gradually decreasing because of the advancements in medical technology, a retrospective study in 2017 focused on hospitalized patients in China indicated that the incidence of moderate to severe OHSS is approximately 1.14%9, and aroused concern about moderate to severe OHSS based on the given number of undergoing ART cycles annually in China. As there are no effective techniques or methods to prevent the occurrence of moderate to severe OHSS during ovulation induction currently, and the prevention of moderate to severe OHSS is a multi-stage process and the first is to recognize the high-risk factors in advance before initiating controlled ovarian stimulation (COS)10.
The EFLL protocol is a widely adopted ovulation induction strategy in clinical practice. Studies have shown that compared to short-acting long protocols and agonist protocols, although there is no significant difference in cumulative pregnancy rates, the EFLL protocol has a higher success rate in fresh cycle transfers, this study indicated that the EFLL protocol has advantages in fresh embryo transfers11. However, to prevent the occurrence of moderate to severe OHSS, a strategy of freezing all embryos is commonly used in clinical practice7,8,10, which diminishes the apparent advantages of the EFLL protocol. Therefore, this study aims to analyze the influencing factors related to the occurrence of moderate to severe OHSS of fresh cycle in patients undergoing the EFLL protocol, to construct a binary logistic regression model to predict the risks of moderate to severe OHSS, and to visualize the results through nomograms, allowing clinicians to assess the risk of moderate to severe OHSS in patients receiving treatment with the EFLL protocol during the early follicular phase, and enhancing the safety of assisted reproductive therapies and providing a basis for individualized treatment for patients.
Materials and methods
4,204 patients received IVF/ICSI treatment at the Reproductive Medicine Department of the Second Affiliated Hospital of Zhengzhou University from January 2015 to August 2024. The EFLL protocol was used to stimulate follicles in these patients. The study was retrospective, these cases were randomly divided into a modeling group (2,942 cases) and a verification group (1,262 cases) at a ratio of 7:3, and the clinical data, such as treatment processes, laboratory data, and pregnancy outcomes of patients was collected and analyzed. All patients signed informed consent before receiving the assisted reproduction treatment. This study complies with the basic principles of the Declaration of Helsinki and has been approved by the Ethics Committee of the Second Affiliated Hospital of Zhengzhou University (KY2024218).
The inclusion criteria were the patients receiving assisted reproductive treatment and received oocyte retrieval with the EFLL protocol in the fresh cycle. Patients with the following exclusion criteria were included IVF/ICSI contraindications, uterine malformations, chromosomal abnormalities, rheumatic immune system diseases, untreated endometrial lesions, drug allergies, severe mental system diseases, pituitary tumors, congenital diseases, abnormalities of blood glucose, blood pressure, prolactin, and so on.
According to the guidelines established by the American National Medical Association in 202410, excluding other causes of abdominal pain and fever, the diagnosis is primarily based on clinical standards. The severity is classified as ① Mild: abdominal distension, discomfort, diarrhea, mild nausea and vomiting, mild dyspnea, ovarian enlargement, and no significant changes in laboratory indicators; ② Moderate: mild clinical features with ultrasound confirming the presence of ascites; ③ Severe: mild and moderate features, clinical evidence of ascites, pleural effusion, severe dyspnea, oliguria, anuria, refractory ascites, vomiting, etc., along with laboratory characteristics: severe hemoconcentration (HCT > 0.55), WBC > 25*109/ml, creatinine clearance rate (CrCl) < 50 ml/min or creatinine (Cr) > 141.4 µmol/L, Na+ < 135 mmol/L or K+ > 5 mmol/L, elevated liver enzymes. ④ Extremely Severe: Severe symptoms accompanied by hypotension/central venous pressure, rapid weight gain (> 1 kg in 24 h), syncope, severe abdominal pain, venous thrombosis, anuria/acute renal failure, arrhythmias/thromboembolism/pericardial effusion, massive pleural effusion, arterial thrombosis, acute respiratory distress syndrome, sepsis, with further elevated laboratory indicators.
On the 2nd–3rd days of menstruation, patients are given a subcutaneous injection of Long-acting GnRH-a (Bayi, Shanghai Li zhu) at 3.75 mg for downregulation. Twenty-eight days later, the COH was initiated when downregulation standards were met (B ultrasound shows no functional cysts in both ovaries, antral follicle diameter ≤ 5 mm, serum estradiol < 40 ng/L, serum luteinizing hormone (LH) < 5 IU/L, serum follicle-stimulating hormone (FSH) < 5 IU/L), gonadotropin (Gn) (Gonal-F, Merck Switzerland or Puregon, MSD Netherlands) is administered at 75–225 IU/d based on the individual patient’s condition, such as patient age, anti-Mullerian hormone level, antral follicle count, body mass index, monitoring follicular development and adjusting Gn dosage according to hormone levels. When at least 3 follicles reach a diameter of ≥ 18 mm, human chorionic gonadotropin (Ovidrel, Merck Switzerland) at 250 µg was used to trigger injection, 36 h later, oocytes were collected under vaginal ultrasound guidance.
The female partner undergoes oocyte retrieval while the male partner provides sperm, which is then optimized. Based on sperm quality and previous assisted reproduction outcomes, the IVF or ICSI fertilization method for patients, and fertilized oocytes with two visible pronuclei (2PN) are selected for further culture. On day 3 post-retrieval, embryos are scored according to the Peter scoring system. In our center, embryos derived from 2PN with 7 to 12 blastomeres and less than 20% fragmentation, with good cell morphology and homogeneity, are rated as high-quality embryos. On days 5–6 post-retrieval, according to the Gardner scoring standard, blastocysts at stage 3 or above with inner cell mass and trophectoderm free of grade C are rated as high-quality blastocysts. On the day of transfer, basic endocrine and ultrasound examinations are conducted. If there are no contraindications for transfer (e.g. low-quality embryo grade, unsuitable endometrial conditions for transfer, elevated hormone levels, mild OHSS that the patient cannot tolerate, moderate to severe OHSS, infection after oocyte retrieval, bleeding, or other untreated complications unsuitable for transfer), fresh cycle transfer will be performed, followed by luteal support. Blood serum hCG is measured 12–14 days later; if negative, luteal support is stopped; if positive, luteal support continues. A transvaginal ultrasound is performed 30 days after transfer; detection of a gestational sac indicates clinical pregnancy and luteal support is continued until 12 weeks.
Randomly divided the samples into modeling group and validation group at a ratio of 7:3. Based on the occurrence of moderate to severe OHSS, classified into moderate to severe OHSS group and non-OHSS group. Normally distributed quantitative data were described as mean ± standard deviation (Mean ± SD). After conducting the Kolmogorov-Smirnov test for normality, non-normally distributed quantitative data were expressed as median (25th percentile, 75th percentile) [M (Q1, Q3)]; categorical variables were represented as percentages (%). Differences in baseline information and clinical characteristics between the two groups were compared using the Mann-Whitney U test and χ2 test. Logistic regression was used for variable selection, and the effect of factors on outcomes was evaluated by odds ratio (OR) and 95% confidence interval (CI). Factors with P < 0.05 were included in the multivariate analysis, and a nomogram constructed a predictive model. The model’s predictive ability was assessed using the area under the receiver operating characteristic curve (AUC), calibration curves were used to evaluate the calibration of the model, and decision curves assessed the clinical applicability of the model. All analyses were performed using Empower Stats 4.0 based on R language, with P < 0.05 considered statistically significant.
Results
A total of 4204 cycles were included. Using random sampling, patients were divided into the modeling group (2942 cases) and the validation group (1262 cases) in a 7:3 ratio. After comparing the two groups various indicators such as age, body mass index (BMI), AFC, anti-Müllerian hormone (AMH), baseline estradiol levels, baseline follicle-stimulating hormone (FSH) levels, baseline luteinizing hormone (LH) levels, progesterone (P) levels, estradiol (E2) levels on the day of hCG injection, follicle-stimulating hormone levels on the day of hCG injection, luteinizing hormone levels on the day of hCG injection, progesterone levels on the day of hCG injection, endometrial thickness on the day of hCG injection, number of oocytes retrieved, whether the patient had hypothyroidism (HP), assisted reproductive techniques used, whether the patient had polycystic ovary syndrome (PCOS), GnRH agonist starting dose, GnRH agonist usage days, and total GnRH agonist dosage, the results showed there were no statistically significant differences in general data between the two groups (P > 0.05). Among them, 117 patients experienced moderate to severe OHSS, with 87 cases occurring in the modeling group and 33 cases in the validation group, of which 69 underwent fresh embryo transfer and 64 achieved clinical pregnancy. The results of the comparison of the baseline clinical and laboratory data are listed in Tables 1 and 2.
Based on the clinical characteristics and laboratory analysis, 117 patients were classified into the moderate to moderate to severe OHSS group, and 3,365 patients were in the non-OHSS group. The results indicated that female age, male age, AFC, AMH, FSH, LH, E2 on the day of hCG injection, P on the day of hCG injection, total Gn usage, whether the patient had HP, whether the patient had PCOS, and infertility type were statistically different between the two groups (P < 0.05; Table 3).
Multiple univariate logistic regression analysis demonstrated that AFC (OR, 1.04; 95%CI, 1.02–1.07; P = 0.002), estrogen levels on the day of hCG injection (OR, 1.01; 95%CI, 1.01–1.01; P<0.01), progesterone levels on the day of hCG injection (OR, 1.18; 95%CI, 1.04–1.34; P = 0.011), whether the patient had a hypothyroidism (OR, 3.62; 95%CI, 2.10–6.23; P < 0.001), and infertility type (OR, 0.59; 95%CI, 0.35–0.99; P = 0.048) were independent risk factors affecting moderate to severe OHSS in patients who receiving EFLL protocol in fresh cycles (P < 0.05; Table 4).
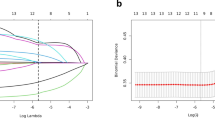
Based on the univariate and multivariate logistic regression analyses we have shown above, a nomogram model was presented (Fig. 1). The model was validated using data from the validation group, and the discriminative ability and calibration of the nomogram predictive model were evaluated using the receiver operating characteristic (ROC) curve, calibration curves, and decision curves. The results showed that the area under the ROC curve (AUC) for the modeling group and validation group were 0.83 (95% CI: 0.78–0.88) and 0.84 (95% CI: 0.78–0.90) (Fig. 2). Respectively, indicating that the prediction model has good conformity, and in calibration curves and decision curves (Fig. 3), we can see the standard curve and the calibration prediction curve fit well, indicating that this nomogram model predicts the incidence of moderate to severe OHSS in fresh cycles with good consistency with the actual incidence.
The nomogram for predicting moderate to severe OHSS with EFLL protocol in fresh single embryo transfer cycle. Points indicate the individual scores corresponding to the five risk factors. Hypothyroid patients (The number 1 represents that the patient has hypothyroidism, The number 0 represents that the patient does not have hypothyroidism); Infertility type (The number 0 represents that primary infertility, The number 1 represents that secondary infertility); Total Points represents the sum of individual scores for the seven risk factors; Risk indicates the probability of incidence corresponding to the total score.
The calibration curves and decision curves between the modeling group and validation group. Figure a represents the decision curve of the modeling group; Figure b represents the decision curve of the validation group. Figure c represents the calibration curve of the modeling group; Figure d represents the calibration curve of the validation group.
Discussion
Ovarian stimulation is a crucial component of assisted reproductive technology, with clinicians using various medications to stimulate the development of multiple follicles within a single cycle12thereby obtaining more oocytes to achieve a maximal live birth rate in fresh treatment13. While a certain degree of ovarian stimulation is beneficial for patients, excessive stimulation often leads to the occurrence of ovarian hyperstimulation syndrome (OHSS)14,15. It is currently believed that OHSS is caused by the action of hCG on the ovaries, leading to the production of inflammatory mediators such as vascular endothelial growth factor, which acts on the ovarian vascular endothelial cells, promoting endothelial cell proliferation and microvascular proliferation, resulting in increased ovarian volume. Additionally, various inflammatory mediators increase vascular permeability, causing plasma to migrate from the vasculature to the third space, leading to symptoms such as pleural effusion, ascites, pericardial effusion, acute respiratory distress syndrome, reduced blood volume, and instability in hemodynamics, which can result in electrolyte disturbances, oliguria, hypotension, hypercoagulability, venous thrombosis, as well as gastrointestinal symptoms like nausea, abdominal pain, and bloating2,4,5. Research has demonstrated that certain medications can reduce the incidence and alleviate the symptoms of moderate to severe OHSS, such as calcium agents, metformin, aspirin, and letrozole; however, the efficacy of these methods is limited7,10.
The primary goal of the assisted reproduction technique is to provide an individualized treatment plan for every patient to maximize the chances of a live birth rate within a single cycle while minimizing iatrogenic risks and reducing cancellation rates16. OHSS is a complication that is entirely induced by medical intervention, and early identification of high-risk patients with OHSS is crucial for preventing it, as clinicians can reduce the incidence of OHSS by adopting appropriate ovarian stimulation protocols and implementing preventive measures in advance6. Further studies have demonstrated that some risk factors were correlated with OHSS, such as age, history of previous OHSS, presence of polycystic ovary syndrome, and estradiol levels on the day of hCG injection17,18,19. However, there is limited research on estimating the incidence of moderate to severe OHSS in assisted reproductive patients through the development of predictive models. Our data indicate that for moderate to severe OHSS, AFC, E2 levels on the day of HCG, P levels on the day of HCG, whether the patient had HP and infertility type are risk factors influencing the occurrence of moderate to severe OHSS in fresh cycles.
AFC refers to the number of antral follicles in the ovaries, which can be measured during the menstrual period through transvaginal ultrasound20. AMH is produced by granulosa cells, antral follicles, and pre-antral follicles, and its primary physiological role is to inhibit the early stages of follicle development21. AMH is secreted into the bloodstream by the ovaries. Serum AMH levels essentially reflect the number of follicles in the follicle pool and are widely used in clinical practice because they are easy to measure22. AFC is closely related to serum AMH levels23. Compared to other known ovarian response biomarkers (such as Inhibin B, FSH, HCG, and estradiol) ART, AFC, and AMH are acknowledged for their superior sensitivity in predicting ovarian response. They are the most sensitive biomarkers of ovarian reserve and often be considered interchangeable16. Studies have shown that an AFC value above 16 predicts ovarian hyper-responsiveness with a sensitivity of 89% and specificity of 92%24. The critical value of AMH for predicting OHSS is 3.36 ng/mL, with a sensitivity of 90.5% and specificity of 81.3%25. Customizing ovulation induction protocols based on AMH levels of patients can reduce cycle cancellation rates (2.3 vs. 6.9%, P < 0.05) compared to standard ovulation induction protocols. It can also decrease clinical costs by 43%25. AMH and AFC levels are associated with OHSS, and ovarian reserve markers can scientifically estimate the pool of follicles that may respond to ovarian stimulation and predict ovarian reactivity22. As a further study on the application of ovarian reserve markers, classifying patients based on ovarian response makers and providing personalized treatment can be beneficial before they accept the assisted reproductive technology treatment24,25.
Polycystic ovary syndrome (PCOS) and age have traditionally been recognized as risk factors for OHSS in physician’s clinical experience, but it is not precise. Compared to normal women, PCOS patients exhibit an increased number of developing antral follicles26, and the AMH levels in PCO women are approximately 75 times higher than those of normal women27, consequently, AMH levels in PCOS women are found to be 2–3 times higher than in healthy controls28,29. Therefore, we can observe a strong correlation between high AMH levels and PCOS patients, but research has demonstrated that high AMH levels are associated with PCO rather than PCOS, as PCOS encompasses not only the excessive accumulation of antral follicles but also features hyperandrogenism and decreased insulin sensitivity. Given that AMH serves as a diagnostic marker for PCO with relatively high specificity and sensitivity (92% and 67%), therefore it has been suggested that AMH could be incorporated into the diagnosis of PCOS23. However, the reason why clinical doctors used to take PCOS as a sensitive predictor for OHSS is that PCOS represents high AMH levels30. Similar to the factor of PCOS, age is also considered an effective predictor of OHSS, although this influence was not reflected in our study. Many clinicians usually take the patient’s age into consideration when choosing the ovarian stimulation protocol for patients largely because age is a simple and convenient indicator that reflects AMH levels16. Serum estradiol levels are also often regarded as predictive factors for OHSS31, but estradiol is not an essential element in the occurrence of OHSS12. The association between elevated serum estradiol levels and OHSS is considered merely an indicator of granulosa cell activity. Nevertheless, estradiol remains a good visual indicator for OHSS, as high-risk OHSS patients frequently exhibit elevated estradiol levels10.
Hypothyroidism is a common endocrine disorder33. One study identified hypothyroidism as a risk factor for moderate and severe OHSS in non-PCOS patients34, finding that patients with hypothyroidism have a fivefold higher probability of developing OHSS during ovulation induction compared to non-hypothyroid patients17,35. Some researchers suggest that this may be due to the difficulties in medication usage during ovulation induction, leading to decreased FT4 levels and increased TSH levels, thereby exacerbating hypothyroidism36. It is known that TSH, FSH, LH, and HCG share structural similarities, possessing similar alpha subunits and distinct beta subunits37. In patients with hypothyroidism, low thyroid levels negatively feedback to the pituitary, resulting in elevated TSH, FSH, LH, and HCG38. Under normal circumstances, FSH and TSH bind to their respective receptors, FSH-R and TSH-R, but due to the similarity in results between TSH-R and FSH-R, TSH may mistakenly bind to FSH-R, leading to the occurrence of OHSS39. Therefore, some studies propose that stabilizing thyroid function through medication during OHSS can help improve patient symptoms40. Additionally, reproductive medicine specialists should work with endocrinologists to identify patients with hypothyroidism early before ovulation induction begins and improve thyroid function promptly to reduce the risk of OHSS[43].
Ovarian hyperstimulation syndrome (OHSS) is the most common complication in assisted reproductive technology. Once it occurs, a hierarchical management system should be implemented, and corresponding symptomatic supportive treatments should be provided according to the severity of the disease. This not only brings psychological burdens to patients but also causes economic pressure. In the prevention and treatment strategy of OHSS, prevention is far more important than treatment. In the work of assisted reproductive clinics, we should identify high-risk groups in advance, evaluate the risk of OHSS occurrence in a phase, and take effective preventive measures promptly are the key clinical strategies for OHSS management. This study aims to explore the influencing factors of the incidence of moderate to severe OHSS in the population undergoing follicular-phase long-acting long-protocol ovulation induction. Constructing a nomogram model and visualizing, it provides a reference for clinicians to predict the incidence of moderate to severe OHSS in advance when implementing the early follicular-phase long-acting long-protocol ovulation induction for patients. This helps to reduce the incidence of OHSS during assisted reproduction and alleviates the physical burden, economic pressure, and psychological stress that patients may bear during assisted reproductive technology. However, this study has certain limitations. Firstly, it is a single-center retrospective study, which cannot completely eliminate differences in patients’ baseline data. Secondly, the sample size is limited, and the conclusions drawn require validation through large-scale multicenter studies. Finally, they are nearly 50% of patients who underwent fresh cycle transfers developed moderate to severe OHSS in our center, which means that half of the patients experienced moderate to severe OHSS due to the impact of pregnancy because the data of this group has also been included in the statistics, So we cannot exclude the influence of pregnancy on the occurrence of moderate to severe OHSS in this study. However, in our clinical practice, we have established strict operating guidelines and tried to avoid fresh cycle transfers for patients with mild OHSS, besides, we cannot predict which kind of patients will develop moderate to severe OHSS after pregnancy. So it was hard to exclude this portion of data from our statistical analysis, and we can only predict the incidence of moderate to severe OHSS based on pre-pregnancy data. Therefore, our model is constructed based on pre-transfer patient data, which may influence the outcomes, and further research is needed to clarify this. Looking ahead, it is necessary to integrate the data of multiple centers to expand the sample size, conduct prospective studies, incorporate more predictive factors, and consider the impact of OHSS occurrence on the clinical outcomes of patients, so as to further improve the predictive ability and clinical practicability of the model.
In summary: AFC, E2 levels on the day of hCG, P levels on the day of hCG, whether the patient has hypothyroidism, and the type of infertility are risk factors influencing the occurrence of moderate to severe OHSS in fresh cycles. The nomogram constructed based on these factors can assist in predicting the incidence of moderate to severe OHSS in fresh cycles when using the EFLL protocol.
Data availability
All data generated during this study are included in this published article [and its supplementary information files].
References
Jiao, J., Geng, C., Zhang, Y. & Cui, L. Research progress on the influencing factors and related mechanisms of birth weight in offspring of assisted reproductive technology. Chin. J. Reprod. Contracept. https://doi.org/10.3760/cma.j.cn101441-20230704-00261 (2024).
Delvigne, A. & Rozenberg, S. Epidemiology and prevention of ovarian hyperstimulation syndrome (OHSS): a review. Hum. Reprod. Update 8, 559–577. https://doi.org/10.1093/humupd/8.6.559 (2002).
Zhu, Q. & Sun, L. Prevention and prevention of ovarian hyperstimulation syndrome. Chin. J. Practical Gynecol. Obstet. https://doi.org/10.19538/j.fk2023100102 (2023).
McClure, N. et al. Vascular endothelial growth factor as capillary permeability agent in ovarian hyperstimulation syndrome. Lancet 344, 235–236. https://doi.org/10.1016/s0140-6736(94)93001-5 (1994).
Namavar Jahromi, B. M. et al. Ovarian hyperstimulation syndrome: a narrative review of its pathophysiology, risk factors, prevention, classification, and management. Iran. J. Med. Sci. 43, 248–260 (2018).
Ji, J. et al. The optimum number of oocytes in IVF treatment: an analysis of 2455 cycles in China. Hum. Reprod. 28, 2728–2734. https://doi.org/10.1093/humrep/det303 (2013).
Nelson, S. M. Prevention and management of ovarian hyperstimulation syndrome. Thromb. Res. 151(Suppl 1), S61–s64. https://doi.org/10.1016/s0049-3848(17)30070-1 (2017).
Gera, P. S., Tatpati, L. L., Allemand, M. C., Wentworth, M. A. & Coddington, C. C. Ovarian hyperstimulation syndrome: steps to maximize success and minimize effect for assisted reproductive outcome. Fertil. Steril. 94, 173–178. https://doi.org/10.1016/j.fertnstert.2009.02.049 (2010).
Zheng, D. et al. The incidence of moderate and severe ovarian hyperstimulation syndrome in hospitalized patients in China. Health Data Sci. 3, 0009. https://doi.org/10.34133/hds.0009 (2023).
Practice Committee of the American Society for Reproductive Medicine. Prevention of moderate and severe ovarian hyperstimulation syndrome: a guideline. Fertil. Steril. 121, 230–245. https://doi.org/10.1016/j.fertnstert.2023.11.013 (2024).
Xu, B. et al. The depot GnRH agonist protocol improves the live birth rate per fresh embryo transfer cycle, but not the cumulative live birth rate in normal responders: a randomized controlled trial and molecular mechanism study. Hum. Reprod. 35, 1306–1318. https://doi.org/10.1093/humrep/deaa086 (2020).
Macklon, N. S., Stouffer, R. L., Giudice, L. C. & Fauser, B. C. The science behind 25 years of ovarian stimulation for in vitro fertilization. Endocr. Rev. 27, 170–207. https://doi.org/10.1210/er.2005-0015 (2006).
Fauser, B. C., Devroey, P. & Macklon, N. S. Multiple birth resulting from ovarian stimulation for subfertility treatment. Lancet 365, 1807–1816. https://doi.org/10.1016/s0140-6736(05)66478-1 (2005).
Nastri, C. O., Ferriani, R. A., Rocha, I. A. & Martins, W. P. Ovarian hyperstimulation syndrome: pathophysiology and prevention. J. Assist. Reprod. Genet. 27, 121–128. https://doi.org/10.1007/s10815-010-9387-6 (2010).
Steward, R. G. et al. Oocyte number as a predictor for ovarian hyperstimulation syndrome and live birth: an analysis of 256,381 in vitro fertilization cycles. Fertil. Steril. 101, 967–973. https://doi.org/10.1016/j.fertnstert.2013.12.026 (2014).
La Marca, A. & Sunkara, S. K. Individualization of controlled ovarian stimulation in IVF using ovarian reserve markers: from theory to practice. Hum. Reprod. Update. 20, 124–140. https://doi.org/10.1093/humupd/dmt037 (2014).
Johnson, M. D. et al. Relationship between human chorionic gonadotropin serum levels and the risk of ovarian hyperstimulation syndrome. Gynecol. Endocrinol. 30, 294–297. https://doi.org/10.3109/09513590.2013.875998 (2014).
Anaya, Y. et al. A novel oocyte maturation trigger using 1500 IU of human chorionic gonadotropin plus 450 IU of follicle-stimulating hormone May decrease ovarian hyperstimulation syndrome across all in vitro fertilization stimulation protocols. J. Assist. Reprod. Genet. 35, 297–307. https://doi.org/10.1007/s10815-017-1074-4 (2018).
Weenen, C. et al. Anti-Müllerian hormone expression pattern in the human ovary: potential implications for initial and Cyclic follicle recruitment. Mol. Hum. Reprod. 10, 77–83. https://doi.org/10.1093/molehr/gah015 (2004).
Themmen, A. P. Anti-Müllerian hormone: its role in follicular growth initiation and survival and as an ovarian reserve marker. J. Natl. Cancer Inst. Monogr. 2005, 18–21. https://doi.org/10.1093/jncimonographs/lgi026 (2005).
La Marca, A. et al. Anti-Mullerian hormone (AMH) as a predictive marker in assisted reproductive technology (ART). Hum. Reprod. Update 16, 113–130. https://doi.org/10.1093/humupd/dmp036 (2010).
Pigny, P., Jonard, S., Robert, Y. & Dewailly, D. Serum anti-Mullerian hormone as a surrogate for antral follicle count for definition of the polycystic ovary syndrome. J. Clin. Endocrinol. Metab. 91, 941–945. https://doi.org/10.1210/jc.2005-2076 (2006).
Aflatoonian, A., Oskouian, H., Ahmadi, S. & Oskouian, L. Prediction of high ovarian response to controlled ovarian hyperstimulation: anti-Müllerian hormone versus small antral follicle count (2–6 mm). J. Assist. Reprod. Genet. 26, 319–325. https://doi.org/10.1007/s10815-009-9319-5 (2009).
Lee, T. H. et al. Serum anti-Müllerian hormone and estradiol levels as predictors of ovarian hyperstimulation syndrome in assisted reproduction technology cycles. Hum. Reprod. 23, 160–167. https://doi.org/10.1093/humrep/dem254 (2008).
Webber, L. J. et al. Formation and early development of follicles in the polycystic ovary. Lancet 362, 1017–1021. https://doi.org/10.1016/s0140-6736(03)14410-8 (2003).
Pellatt, L. et al. Granulosa cell production of anti-Müllerian hormone is increased in polycystic ovaries. J. Clin. Endocrinol. Metab. 92, 240–245. https://doi.org/10.1210/jc.2006-1582 (2007).
Wang, J. G., Nakhuda, G. S., Guarnaccia, M. M., Sauer, M. V. & Lobo, R. A. Müllerian inhibiting substance and disrupted folliculogenesis in polycystic ovary syndrome. Am. J. Obstet. Gynecol. 196, 77e71–77e75. https://doi.org/10.1016/j.ajog.2006.07.046 (2007).
Wachs, D. S., Coffler, M. S., Malcom, P. J. & Chang, R. J. Serum anti-mullerian hormone concentrations are not altered by acute administration of follicle stimulating hormone in polycystic ovary syndrome and normal women. J. Clin. Endocrinol. Metab. 92, 1871–1874. https://doi.org/10.1210/jc.2006-2425 (2007).
Bellver, J. et al. Intravenous albumin does not prevent moderate-severe ovarian hyperstimulation syndrome in high-risk IVF patients: a randomized controlled study. Hum. Reprod. 18, 2283–2288. https://doi.org/10.1093/humrep/deg451 (2003).
Asch, R. H., Li, H. P., Balmaceda, J. P., Weckstein, L. N. & Stone, S. C. Severe ovarian hyperstimulation syndrome in assisted reproductive technology: definition of high risk groups. Hum. Reprod. 6, 1395–1399. https://doi.org/10.1093/oxfordjournals.humrep.a137276 (1991).
Aboulghar, M. A. & Mansour, R. T. Ovarian hyperstimulation syndrome: classifications and critical analysis of preventive measures. Hum. Reprod. Update. 9, 275–289. https://doi.org/10.1093/humupd/dmg018 (2003).
Soares, S. R., Gómez, R., Simón, C., García-Velasco, J. A. & Pellicer, A. Targeting the vascular endothelial growth factor system to prevent ovarian hyperstimulation syndrome. Hum. Reprod. Update. 14, 321–333. https://doi.org/10.1093/humupd/dmn008 (2008).
Bachmakova, N. V. et al. The development of ovarian hyperstimulation syndrome in the implementation of assisted reproductive technology in women with a background of endocrine pathology. Gynecol. Endocrinol. 30 (Suppl 1), 25–29. https://doi.org/10.3109/09513590.2014.945780 (2014).
Busnelli, A., Cirillo, F. & Levi-Setti, P. E. Thyroid function modifications in women undergoing controlled ovarian hyperstimulation for in vitro fertilization: a systematic review and meta-analysis. Fertil. Steril. 116, 218–231. https://doi.org/10.1016/j.fertnstert.2021.01.029 (2021).
Muller, A. F., Verhoeff, A., Mantel, M. J., De Jong, F. H. & Berghout, A. Decrease of free thyroxine levels after controlled ovarian hyperstimulation. J. Clin. Endocrinol. Metab. 85, 545–548. https://doi.org/10.1210/jcem.85.2.6374 (2000).
Cheng, S. Y., Leonard, J. L. & Davis, P. J. Molecular aspects of thyroid hormone actions. Endocr. Rev. 31, 139–170. https://doi.org/10.1210/er.2009-0007 (2010).
Taher, B. M. et al. Spontaneous ovarian hyperstimulation syndrome caused by hypothyroidism in an adult. Eur. J. Obstet. Gynecol. Reprod. Biol. 112, 107–109. https://doi.org/10.1016/s0301-2115(03)00283-5 (2004).
Guvenal, F., Guvenal, T., Timuroglu, Y., Timuroglu, T. & Cetin, M. Spontaneous ovarian hyperstimulation-like reaction caused by primary hypothyroidism. Acta Obstet. Gynecol. Scand. 85, 124–125. https://doi.org/10.1080/00016340500324571 (2006).
Lee, Y. J. et al. Subclinical hypothyroidism diagnosed by thyrotropin-releasing hormone stimulation test in infertile women with basal thyroid-stimulating hormone levels of 2.5 to 5.0 mIU/L. Obstet. Gynecol. Sci. 57, 507–512. https://doi.org/10.5468/ogs.2014.57.6.507 (2014).
Zhou, J. et al. Ovarian hyperstimulation syndrome combined with hypothyroidism: a comprehensive review. J. Ovarian Res. 17, 896. https://doi.org/10.1186/s13048-024-01406-3 (2024).
Acknowledgements
The authors thank the women who participated in this study and all the physicians and nurses at the second Affiliated Hospital of Zhengzhou University.
Funding
No grant from funding agencies in the public, commercial, or not-for-profit sectors was obtained.
Author information
Authors and Affiliations
Contributions
Dan Zhang supervised the entire study, including procedures, conception, design, and completion. Huihui Deng and Qian Dou contributed to the data analysis and manuscript drafting. Peipei Guo, Huanxin Liu , Yungai Xiang , Xujing Geng, and Pengfen Li were responsible for the collection of data and prepared Figs. 1, 2 and 3; Tables 1, 2 and 3. All authors participated in the ultimate interpretation of the study data and in revisions to the article.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Ethics approval and consent to participate
The study protocol was approved by the Ethics Committee of the Second Affiliated Hospital of Zhengzhou University (KY2024218). The study was performed in accordance with the ethical standards as laid out in the 1964 Declaration of Helsinki. Written informed consent was obtained from individual participants or their guardian.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Deng, H., Dou, Q., Guo, P. et al. Nomogram model to predict the risk of moderate to severe ovarian hyperstimulation syndrome of long protocol group in fresh cycle. Sci Rep 15, 9211 (2025). https://doi.org/10.1038/s41598-025-94049-3
Received:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s41598-025-94049-3
Keywords
This article is cited by
-
Risk prediction models for ovarian hyperstimulation syndrome: a systematic review and meta-analysis
BMC Pregnancy and Childbirth (2025)