Abstract
This study investigated the presence of 32 antibiotic residues in drinking water, their potential association with antibiotic concentrations in children’s urine samples, and anthropometric indicators. Water samples were collected from the primary water sources supplying Ilo, Peru (Pacocha and Pampa), and urine samples were analysed from children aged 2 to 10 years using liquid chromatography coupled to tandem mass spectrometry with triple quadrupole. Five antibiotics were detected in drinking water, with three of these representing a health risk due to high concentrations and risk quotients (RQ): Doxycycline (2.30), Sulfamethoxazole (1.04), and Metronidazole (25.68). Furthermore, there was no correlation between the antibiotics found in drinking water and those detected in urine samples, as the types and quantities of antibiotics differed. In urine samples, 21 antibiotics were detected in children from Pacocha and 19 from Pampa. The antibiotics and anthropometric variables did not show any significant correlation. Principal component analysis revealed that antibiotic profiles were highly similar across both areas, suggesting a shared source of contamination independent of drinking water. It is concerning that 100% of the children have at least three antibiotics in their urine, which could affect their health now and later in life.
Similar content being viewed by others
Introduction
Contaminants present in aquatic ecosystems pose a risk to the environment and human health1,2. Among these, antibiotic pollution, originating from wastewater, livestock farming, aquaculture, and other sources, stands out3,4. This is problematic, as these resources are essential for drinking water supply5,6, irrigation7,8, aquaculture6,8,9, and livestock farming6.
Prolonged consumption of antibiotic-contaminated water, through both drinking water and food, can disrupt the human microbiome, impacting children’s growth and development and contributing to diseases such as psoriasis, diabetes, cardiovascular disease, and colorectal cancer10, as well as antibiotic resistance and allergic reactions11. Specifically, Norfloxacin is known to be carcinogenic when intake exceeds the acceptable daily intake (ADI) of 24 ng/kg-bw/day12, representing a significant health risk. Consequently, various authors have assessed the risk quotient associated with the presence of antibiotics in drinking water1,3,5,8,11,13 and food products12. Unfortunately, in numerous cases, the antibiotics found in drinking water represented a high risk due to the detected concentrations3,5,14,15.
Children are more susceptible than adults to antibiotic contamination, as low doses during growth and development periods can be harmful, affecting their immediate and long-term health10,16. Previous studies have demonstrated that children exposed to antibiotics may have these compounds in their urine, with up to 93.92% of children evaluated showing antibiotic presence17. This exposure has also been associated with excessive weight gain and a rise in cases of childhood obesity17,18, as antibiotics are known to influence adipogenesis, enhancing this process17,19. Furthermore, antibiotics could impact children’s growth and development. For this reason, anthropometric parameters such as weight and height are frequently assessed in studies investigating the toxicity of environmental contaminants2,18,20, to determine whether these contaminants impair normal growth and development.
Scientific evidence demonstrates antibiotic contamination in aquatic ecosystems in Peru7,9,21,22, including in drinking water and food of animal origin9. However, no studies have been conducted on the potential impact of this contamination on the human population, especially children, who are more vulnerable to the adverse effects of these compounds. Although some studies in different parts of the world have assessed the presence of antibiotics in children’s urine17,19,23,24, there are no previous reports for Latin America, highlighting the need for research in this region to understand the associated risks better.
In this study, we aimed to evaluate the relationship between antibiotic concentrations in water samples from the primary sources supplying the city of Ilo and antibiotic levels in urine samples from children aged 2 to 10 years. Additionally, we sought to determine whether the concentrations detected in the water represent a potential risk to the population through a risk analysis. An additional objective was to investigate possible associations between antibiotic residues and parameters of children’s growth and development, such as weight, height, and head circumference.
Results and discussions
Determining the presence of antibiotics in drinking water
Analyses for 32 antibiotics were carried out in the Pampa and Pacocha areas; overall, five antibiotic residues were found (Table 1), considering the rainy and dry seasons. The analyses reveal the presence of a total of five antibiotics. Of these, the water source for Pacocha contains only one antibiotic during the dry season and two during the rainy season. In contrast, the Pampa area shows the presence of all five antibiotics in the dry season and three during the rainy season. This indicates that the water source for the Pampa area is the most contaminated by antibiotics. Figure 1 illustrates the presence of various antibiotics in the water samples.
It is also worth noting that antibiotics have been reported in drinking water worldwide. For instance, five antibiotics and other pharmaceuticals were detected in drinking water in Malaysia15. The exact number was in Shandong province8 and the United States3. In contrast, ten antibiotics were detected in drinking water in China’s rural Zhejiang and Jiangsu provinces5. In comparison, another area in China reported 13 antibiotics in winter and 17 in summer6. Finally, 21 antibiotics were found in yet another province of China, evidencing that many regions are affected by wastewater discharge into aquatic ecosystems, which impacts even drinking water1. Frequently used antibiotics, such as Ciprofloxacin and Sulfamethoxazole, were also found in other studies15 (Table 1). These reports indicate that global populations are being exposed to antibiotics through drinking water, posing a high risk due to prolonged exposure to low doses of antibiotics11.
Regarding the Risk Quotient, Metronidazole represents the highest human risk (25.68), followed by Doxycycline (2.3), with both being prevalent in the Pampa area (Table 2). This is concerning, as some antibiotics may be quite toxic when ingested in drinking water8, and prolonged exposure to low concentrations of antibiotics through drinking water poses a risk to human health10,11. These antibiotics are frequently found in hospital wastewater26. Metronidazole has antibacterial, antiviral, and even antiprotozoal properties27. Therefore, its widespread use leads to the presence of its residues globally28, directly impacting children18, as it is known to have a carcinogenic, genotoxic, and mutagenic potential28.
Doxycycline is also associated with Metronidazole, as it is often prescribed in combination with Metronidazole to treat gynecological conditions27. Consequently, it is commonly found in environmental samples4, drinking water6,13, and even food sources12, which may contribute to detecting this antibiotic in children’s urine worldwide23,24. While only a few antibiotics (five) were identified in this study, three were found in high concentrations. Prolonged exposure to these could readily increase their accumulation in children’s bodies.
Determine the presence of antibiotics in urine samples from children in the city of Ilo
Figure 2 presents the concentrations of antibiotics in children’s urine samples from two localities, Pampa and Pacocha. The analytical method for antibiotics in urine could be validated for 26 out of the 32 compounds selected in this work (see “Analytical procedure” and S.I. for more information). From these, 21 were detected in Pacocha and 19 in Pampa, although fewer antibiotics (2) were found in Pacocha’s drinking water compared to Pampa’s (5). The most prominent antibiotics included Azithromycin, Ciprofloxacin, Doxycycline, Metronidazole, Nalidixic Acid, and Trimethoprim.
Antibiotics identified corresponded to seven types: fluoroquinolones: NAL-ACID (Nalidixic Acid), CIP (Ciprofloxacin), NOR (Norfloxacin), MOXI (Moxifloxacin), LEVO (Levofloxacin), OXO-ACID (Oxalinic Acid), FLU (Flumequine). Tetracyclines: DOXI (Doxycycline), OXI (Oxytetracycline), TET (Tetracycline). Macrolides: AZT—Azithromycin, ERIT—Erythromycin, ROXI—Roxithromycin, CLA (Clarithromycin). Sulphonamides: STZ (Sulfathiazole), SUL (Sulfadiazine), SMT (Sulfamethoxazole), SPD (Sulphapyridine). Diaminopyrimidines: TRIM (Trimethoprim). Nitroimidazoles: MET (Metronidazole) Lincosamides: CLI (Clindamycin). Specifically, more antibiotics were recorded in Pacocha’s children (21) than in Pampa’s (19). Among the notable antibiotics, Metronidazole concentrations in Pacocha were exceptionally high compared to Pampa (around 4227 ng/L versus 72 ng/L); however, this antibiotic was not found in Pacocha’s drinking water, though it was detected in Pampa’s, suggesting an alternative source of contamination. As for Nalidixic Acid, both regions showed similar concentrations (around 3000 ng/L in Pampa and close to 2700 ng/L in Pacocha). Ciprofloxacin concentrations were higher in Pampa than in Pacocha (815.6 ng/L and 58.49 ng/L, respectively) (Fig. 2). Tetracycline and Trimethoprim concentrations were similar across both areas (see supplementary material).
Additionally, 52.6% of children in Pacocha exhibited between five to eight antibiotics, while in Pampa, it was 52.4%. In both areas, 100% of the children had at least three antibiotics in their urine samples, raising concerns. Even at low concentrations, antibiotics have adverse effects on children16. The elevated levels of Metronidazole found in this study are particularly worrying, as numerous children presented high concentrations. This may not be an isolated case, as these children may have been medicated; Metronidazole is commonly used to treat both anaerobic and aerobic bacterial infections27. Indeed, another study analysing urine samples from 256 children detected Metronidazole in 17% of the samples24, though only non-medicated children were selected in that study.
The observed differences in the presence of antibiotics may be associated with multiple factors, including hygiene conditions8, access to healthcare services29, and sociodemographic characteristics. The antibiotics did not show significance in their concentration.
These conditions are more precarious in areas with lower household incomes and limited parental education18,20, linked to restricted access to safe drinking water20 and inadequate wastewater treatment30. In such regions, self-medication and exposure to contaminated food and water significantly increase antibiotic exposure25. However, urban settings present a different scenario. Studies conducted in Chinese cities found no direct correlation between antibiotics in children’s urine and socioeconomic status. This suggests that exposure in these contexts is primarily driven by environmental sources, particularly contaminated water and food17,18. Unfortunately, regardless of the origin, it is children who bear the most significant impact, with reports indicating that in some regions, up to 100% of children have been reported to have antibiotics in their urine samples31,32. Children may also be exposed to antibiotics through other sources, such as food10,12,19. Reports indicate that 77.4% of the children evaluated had between one and four antibiotics in their urine samples, with higher concentrations found in food than in urine10. This is concerning due to potential long-term consequences, as this issue is becoming widespread, with similar findings reported globally10,23,33,34,35.
Finally, a comparison was made with antibiotics found in other studies (Table 3), which highlights that this issue is prevalent worldwide. The most commonly detected antibiotics were Azithromycin, Ciprofloxacin, Sulfamethoxazole, and Trimethoprim. This aligns with the antibiotics found in wastewater from Peru’s treatment plants7 and globally30,35,36. However, several antibiotics detected in this study were not found in others. These include Clindamycin, Levofloxacin, Moxifloxacin, Nalidixic Acid, Flumequine, and Sulphapyridine, which may reflect regional preferences for certain antibiotics or their primary use in veterinary rather than human medicine. For example, Sulphapyridine is used in aquaculture and livestock farming37. Consequently, it is still detected in environmental samples, even in drinking water1,8. Nalidixic acid and Flumequine have also been found in wastewater from other regions of Peru7, suggesting that these antibiotics may originate not only from wastewater but also from different sources, such as livestock and food irrigated or fertilised with these resources.
Relationship between urinary antibiotic concentration and anthropometric variables
Figure 3A and B show no significant relationship between urine serum antibiotic concentrations and children’s anthropometric variables. This may be because the detected concentrations are too low to produce a measurable effect. However, scientific evidence suggests prolonged exposure to trace antibiotics may be associated with weight gain in children17,18,19.
Further research is needed to understand this issue better, as previous studies have shown that prolonged antibiotic exposure in children increases the risk of being overweight, particularly at high concentrations of Deoxytetracycline and quinolones18. Additionally, antibiotic residues have been detected in children as young as three days old, suggesting possible transplacental transfer, which exposes infants even before birth16. This early exposure can disrupt the intestinal microbiota in newborns, potentially leading to long-term consequences. Moreover, studies indicate that early antibiotic exposure affects adipogenesis, which may contribute to the development of overweight and obesity later in life19.
This lack of correlation is likely due to the low concentrations of these compounds, which may require a more extended observation period to detect any significant effects, as these impacts often emerge later. For example, a controlled study on one-year-old children exposed daily to Sulphamethoxazole and Trimethoprim for two years found no significant differences in weight gain compared to a placebo group32. This suggests that extended study periods are crucial for accurately assessing the long-term anthropometric effects of antibiotic exposure. Moreover, other factors beyond antibiotics may influence anthropometric variables38. Therefore, further research is needed to understand better the long-term impacts of antibiotic exposure on children’s growth and development.
Relationship of antibiotics in children’s urine in two areas of the city of Ilo
A principal component analysis (PCA) was conducted to identify patterns in antibiotic presence among children (Fig. 4). For this analysis, Component 1 (31.3%) and Component 2 (26%) were selected, together explaining 57.3% of the total data variability. Data from Pacocha, represented by blue points, are dispersed along Axis 2, with a slight skew towards the opposing end of Axis 1. Conversely, data from Pampa, represented by orange points, are more concentrated near the origin, with a subtle spread along Axis 1. Each point in the plot represents an individual urine sample from the children included in the study.
In Dimension 1, Doxycycline and Ciprofloxacin stand out with correlations of 0.94 and 0.92, respectively, indicating their substantial contribution to this dimension. Their grouping may be attributed to their high water solubility, as both have low LogKow values (Doxy: − 0.02, Cipro: 0.28), which enhances their dispersion in aquatic environments1,3, even leading to their residues being detected in drinking water (Table 1). Both compounds have a bioconcentration factor (log BCF < 0.5), indicating a low bioaccumulation potential in aquatic species3. Trimethoprim and Nalidixic Acid are more closely associated with Axis 2, each displaying correlation values 0.86. Their grouping may also be linked to their moderate Log Kow values (0.91 and 1.41, respectively)39, which influence their ionization and mobility in aquatic environments, representing antibiotics with higher lipophilicity. Although Nalidixic Acid has been rarely studied, reports of its presence have increased worldwide in recent years40. Furthermore, both antibiotics are widely used in gynecological medicine, suggesting a potential co-occurrence in environments contaminated by the hospital or domestic wastewater31. Finally, Tetracycline and Erythromycin are positioned near Axes 1 and 2’s origin, with correlation values below 0.1. This difference in their log Kow values, with − 1.37 and 3.6 for Tetracycline and Erythromycin, respectively3, could influence their behavior in the environment. With its lower log Kow, Tetracycline has a greater affinity for soil particles and organic matter, affecting its mobility and bioavailability4. On the other hand, Erythromycin, with its higher log Kow value, is a lipophilic compound, which increases its likelihood of bioaccumulation4.
The confidence ellipses’ positions and shapes suggest a slight distinction between the analyzed areas. However, due to the substantial overlap between the Pacocha and Pampa regions, differences in antibiotic presence between the two zones do not appear to be markedly significant.
A Mann–Whitney U test was conducted for the most common antibiotics in both areas (Table 4). The results indicate no significant difference between the antibiotics analyzed (p > 0.05). A graphical representation can be found in Supplementary Material S2.
The similarity of antibiotics found in the children’s urine samples suggests that the contamination source might be similar or even the same. This rules out drinking water as the primary source, as it contains only five antibiotics compared to the 21 identified in the children. Additionally, medication was ruled out, as the selected children were not under previous or current treatment, which was confirmed through a survey of their parents. Therefore, another possible source of exposure could be food. As previously documented, there is substantial evidence that crops grown with wastewater and animals treated with antibiotics tend to retain residues in their tissues, ultimately reaching children.
Materials and methods
Study area
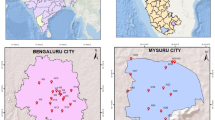
Sampling was conducted at the catchment points of the drinking water treatment plants of Ilo’s two primary water sources: Pacocha and Pampa (Fig. 5). Additionally, samples were taken from two households in each area, covering both dry and wet seasons to provide a more comprehensive representation of the evaluated compounds. In Pacocha, the gender distribution was 63% male and 37% female, while in Pampa, 33% were male and 67% female. This variation in gender representation between the two areas highlights potential regional differences that could influence the results. The study was conducted in two places due to their different drinking water sources. In Pacocha, the water comes from an uncontaminated area, while in La Pampa, the water comes from the Osmore River, where antibiotic residues have been recorded due to wastewater discharge. Mining activity in La Pampa contributes to the local economy. Still, families who access the public healthcare system are generally of low income and distrust the collection of samples from their children, fearing the results may be manipulated due to mining contamination. This distrust hindered sample collection, leading to a change in the study approach. Initially, the study focused on children aged 0 to 5 years, but it wasn’t easy to reach the parents. Most of the mothers attending the healthcare system have secondary education, although high levels of poverty persist. Many of these mothers are young and primarily engaged in informal trade.
Location map of the drinking water sampling sites. Map produced in ArcGIS Pro 3.x using open access resources: political-administrative boundaries from geoBoundaries (https://www.geoboundaries.org/), satellite imagery from Google Earth Pro, and housing locations obtained with a GPS receiver.
Sample collection
Samples of 50 mL of water and 10 mL of urine were collected and stored in a cooler at 4 °C to be transported to the laboratory. Then, they were frozen at − 18 °C until analysis for antibiotics. Although stability of compounds under freezing conditions was not assessed in this study, several papers have reported that most antibiotics are stable under these conditions41,42. Parent compounds have been reported to be less affected by freezing storage than the metabolites, with β-lactams and clindamycin being the most-affected compounds and poorly recovered from the starting concentrations. By contrast, Sulfonamides, macrolides, quinolones and azoles were generally stable under freezing conditions42. Amoxicillin and other Penicillins were stable for at least 30 days at − 23 °C41. All methods performed in this study followed the relevant ethical guidelines and regulations. The study was approved by the Ethics Committee of the National University of Moquegua, known as the Ethics and Research Committee FACIA (Approval Code: INFORME 001-2023). Written informed consent was obtained from all participants, parents, legal guardians, and the children.
Reagents and chemicals
Thirty-two antibiotics were selected as target analytes, including compounds from several families, such as macrolides, β-lactam, fluoroquinolones lincosamides, tetracyclines nitroimidazoles, sulfonamides, and diaminopyrimidines (Supplementary material, for the list of compounds studied, Table 1). Stock solutions of each compound were prepared at around 1000 mg L−1 in methanol or acetonitrile, depending on compound stability, and stored at < − 20 °C in amber glass bottles. Stock solutions of the β-lactam group were prepared in water:acetonitrile 25:75 (v:v) as they are unstable in methanol41. Considering the stability of the different antibiotics in organic solvents, the mixed solutions at 5 mg L−1 were prepared in acetonitrile for β-lactams and in methanol for the remaining antibiotics. Isotopically-labeled analogs were used as internal standards (ILIS) for each selected antibiotic, except Cefditoren, Furaltadone, Lincomycin, Oxacillin, Pipemidic Acid, Sulfamethazine, Sulfamethazine-N-Acetyl, and Sulfathiazole. The final working mix solution containing all compounds and the ILIS mix solution was prepared daily in water. All the analytical reference standards were purchased from LGC (Teddington, UK) and Merck (Darmstadt, Germany).
LC–MS-grade water was obtained by purifying demineralized water using a Milli-Q system from Millipore (Bedford, MA, USA). Methanol and acetonitrile (LC–MS grade), formic acid (> 98%), and ammonium acetate (> 98%) were purchased from Scharlau (Scharlab, Barcelona, Spain).
Analytical procedure
The analytical methodology for the determination of antibiotic residues in water was carefully evaluated and validated in real-world water samples4,41,43,44. In addition, quality control (QC) samples were prepared throughout the batch and injected along with the analysis samples to ensure the reliability of the analytical method45.
The analytical methodology for determining antibiotic residues in water was carefully evaluated and validated in real-world water samples4,41,43,44. In addition, quality control (QC) samples were prepared throughout the batch and injected along with the analysis samples to ensure the reliability of the analytical method43.
The excellent sensitivity of modern LC–MS/MS instrumentation allowed direct injection (DI) of samples without any preconcentration steps44,45,46. Direct injection, in combination with the use of a notable number of isotope-labeled internal standards (ILIS), is a fast, no-sample handling alternative to SPE for the LC–MS/MS determination of pharmaceuticals in different water matrices, as recently reported46. In this work, 2 mL of surface water (SW) sample was centrifuged at 12,000 rpm for 10 min, and then 960 μL of supernatant were transferred into a glass vial, and 40 μL of the ILIS solution of 5 μg L−1 (200 ng L−1 in the injection vial) were added. Finally, 100 μL of the final solution was injected into the LC–MS/MS system. In the case of urine, 2 mL of sample was centrifuged, and then 100 μL was taken, adding 40 μL of ILIS mix at 5 μg L−1 and adjusting the final volume to 1 mL with ultrapure water. Finally, 100 μL were injected into the LC–MS/MS system.
Quality control (QC) consisted of SW samples selected among the water samples under study, which were spiked with the target antibiotics at two concentrations: 0.1 and 1 μg L−1. For this purpose, 910 μL of the sample were taken, and 50 μL of the mix standard solution (2 μg L−1 or 20 μg L−1) and 40 μL of the mix ILIS solution of 5 μg L−1 were added.
The method for urine analysis was validated for 26 out of the 32 compounds selected for this study. Validation was made at two concentration levels (0.2 and 1 μg L−1) (n = 5) with satisfactory recoveries, between 70 and 120%, for the vast majority of antibiotics (see S.I. for more information). Additionally, QCs were prepared by spiking a pool of urine samples with the target antibiotics at two concentrations: 0. 2 and 1 μg L−1 (final concentration in the injection vial 0.02 μg L−1 and 0.1 μg L−1) Preparation of QCs for urine was as follows: aliquots of 100 μL of the sample were taken, and 810 μL of Milli-Q water, 50 μL of the mix standard solution (0.4 μg L−1 and 2 μg L−1), and finally 40 μL of mixed ILIS of 5 μg L−1 were added.
Quantification of analytes was made using the quantification transition (Q) and external calibration with standards in a solvent containing the same ILIS concentration as the samples. For most compounds, the analyte ILIS was available (24 out of 32 compounds analyzed); then, relative areas were used for quantification. This way, potential matrix effects were corrected, as supported by the acceptable QC recoveries.
The reliable identification of compounds in the samples was carried out by calculating the ion ratios between the confirmation (q1 and q2) and the quantification (Q) transitions. To this aim, three MS/MS transitions were acquired, and thus, two intensity ion ratios were available to confirm the identity (q1/Q and q2/Q). The finding was considered positive when at least one experimental ion ratio and the compound’s retention time in the sample were within the tolerance ranges (± 30% for ion ratio, ± 0.1 min for retention time) compared with the reference standards injected in the calibration47.
Instrumentation
Acquity UPLC™ H-Class liquid chromatography system (Waters Corp., Milford, MA, USA) interfaced to a triple quadrupole mass spectrometer Xevo TQ-STM equipped with an orthogonal Z-Spray electrospray ionization interface (ESI) (Waters Corp, Manchester, UK) was used for sample analysis. The UHPLC separation was performed using an Atlantis T3 analytical column (3.0 × 150 mm, 3 μm particle size, Waters Corp.) maintained at 40 °C. The mobile phases were (A) water and (B) methanol, both with 2 mM ammonium acetate and 0.1% formic acid, delivered at a flow rate of 0.4 mL min−1. The mobile phase gradient was: 0 min, 10% B; 6 min, 99% B; 8 min, 99%; 8.10 min, 10% B; and maintained until 10 min for column re-equilibration. Injection volume was 100 μL. ESI was operated in positive ionization mode (ESI+) using a capillary voltage of 1 kV. Nitrogen desolvation gas flow was set to 1200 L h−1 and cone gas to 250 L h−1, while source temperature was set to 150 °C, and desolvation temperature was 650 °C. Cone voltage and collision energies were optimized for each compound using argon (99.995%, Nippon Gases) as collision gas. Four selected reaction monitoring (SRM) transitions were acquired per compound (Q, quantification transition; q1 and q2, confirmation transitions). Dwell times were automatically chosen to obtain 12 points/peak, with at least 14 ms per transition. The UHPLC-MS/MS parameters for the selected antibiotics and their corresponding ILIS are shown in S.I. (Supplementary material, Table 1). The lowest calibration level was taken as the estimated limit of quantification, which by default was established at 10 ng L−1 for water samples and 20 ng L−1 for urine samples (corresponding to 2 ng L−1 in the calibration curve). Data were acquired and processed using MassLynx 4.1 software and quantified with the TargetLynx application (Waters Corp, Manchester, UK).
Determination of potential risks to human health
The Risk quotient (RQ) assessment is used to analyse the health risk of antibiotics found in drinking water on human health5,14,15. A value that exceeds 1 indicates a risk associated with exposure through drinking water; the equation for its calculation is shown below:
where Cdw is the concentration of antibiotics in drinking water, ng/L; IR is the ingestion rate L/D (1.8 L/child/day), EF is the exposure frequency (365), ED is the duration of exposure (5 years), BW is the weight of the child, and AT is the average time of exposure5,6,8. ADI is the acceptable daily intake of antibiotics, µg/kg/day8,12.
Based on characteristics such as the amount of water consumed and the duration of exposure, among others, the risk quotient was calculated. Of the five recorded, two exceeded the safe value (< 1), while values greater than 1 represent an apparent risk to human health.
Statistical analyses
Descriptive statistical analyses were conducted using the antibiotic data from the water samples. Additionally, a Spearman multiple correlation analysis was performed for the areas of Pacocha and Pampa, correlating the most frequently detected antibiotics in the urine samples with the anthropometric parameters of the children. Furthermore, a Mann–Whitney U test was conducted between the most representative antibiotics present in the children. Finally, a Principal Component Analysis (PCA) was performed on the antibiotics present in each area. Statistical analyses were performed in RStudio (version 2024.04.1) using R (version 4.3.3). For correlation analysis and Principal Component Analysis (PCA), the following packages were used: ggcorrplot, FactoMineR, Factoextra, ggplot2, and dplyr, which enabled data manipulation, visualization, and dimensionality reduction, ensuring the reproducibility of the results.
Limitations
It would be advisable to increase the number of samples in drinking water and children, as the current ones may not represent the entire population. Additionally, concerns about mining contamination hindered data collection and required modifications to the study approach. This led to a change in the target population, as the study initially focused on children aged 0 to 5. However, difficulties in reaching parents made it necessary to adjust the strategy, which also resulted in an uneven gender distribution.
Conclusions
The two drinking water sources that supply the city of Ilo contain antibiotics: two in the Pacocha area and five in the Pampa area. However, these do not correlate with the antibiotics detected in the children’s urine samples, which revealed 21 in Pacocha and 19 in Pampa. This suggests that the source of contamination for children in Ilo originates elsewhere. Among the antibiotics found in the water samples, three exceed the antibiotic risk quotient, posing health risks. As for the antibiotics detected in the children’s urine, the most prominent are Azithromycin, Ciprofloxacin, Doxycycline, Metronidazole, Nalidixic Acid, and Trimethoprim. Additionally, anthropometric variables (head circumference, weight, and height) show a moderate positive relationship only with the antibiotics and did not show significance concerning the concentration of the antibiotics. The antibiotics in children’s urine samples are similar in both areas, indicating a likely common source of contamination. These findings highlight the need for further studies to identify other potential sources of contamination, such as meat products and vegetables.
Data availability
The data supporting this study’s findings are available from the corresponding author upon reasonable request.
References
Lyu, J., Chen, Y. & Zhang, L. Antibiotics in drinking water and health risk—China, 2017. China CDC Wkly. 2, 413–417 (2020).
Shin, M. W., Bin, K. H., Kwon, A., Park, M. J. & Kim, S. H. Associations between urinary mercury/cadmium concentrations and anthropometric features in Korean children. Toxics 12, 1–15 (2024).
Schwab, B. W. et al. Human pharmaceuticals in US surface waters: A human health risk assessment. Regul. Toxicol. Pharmacol. 42, 296–312 (2005).
Hernández, F. et al. Occurrence of antibiotics and bacterial resistance in wastewater and sea water from the Antarctic. J. Hazard. Mater. 363, 447–456 (2019).
Wang, Y. et al. Antibiotic residues of drinking-water and its human exposure risk assessment in rural Eastern China. Water Res. 236, 119940 (2023).
Liu, Y. et al. Distribution and potential risk assessment of antibiotic pollution in the main drinking water sources of Nanjing, China. Environ. Sci. Pollut. Res. 27, 21429–21441 (2020).
Nieto-Juárez, J. I., Torres-Palma, R. A., Botero-Coy, A. M. & Hernández, F. Pharmaceuticals and environmental risk assessment in municipal wastewater treatment plants and rivers from Peru. Environ. Int. 155, 106674 (2021).
Hanna, N. et al. Presence of antibiotic residues in various environmental compartments of Shandong province in eastern China: Its potential for resistance development and ecological and human risk. Environ. Int. 114, 131–142 (2018).
Zirena, F. et al. Occurrence of residues of veterinary antibiotics in water, sediment and trout tissue (Oncorhynchus mykiss) in the southern area of Lake Titicaca, Peru. J. Great Lakes Res. 47, 1219–1227 (2021).
Li, N., Ho, K. W. K., Ying, G. G. & Deng, W. J. Veterinary antibiotics in food, drinking water, and the urine of preschool children in Hong Kong. Environ. Int. 108, 246–252 (2017).
Bruce, G. M., Pleus, R. C. & Snyder, S. A. Toxicological relevance of pharmaceuticals in drinking water. Environ. Sci. Technol. 44, 5619–5626 (2010).
Ben, Y. et al. Human daily dietary intakes of antibiotic residues: Dominant sources and health risks. Environ. Res. 212, 113387 (2022).
Liu, L. Occurrence and health risk assessment of antibiotics in drinking water of a city in Southern China. IOP Conf. Ser. Earth Environ. Sci. 657, 1–7 (2021).
Feng, L. et al. Distribution and human health risk assessment of antibiotic residues in large-scale drinking water sources in Chongqing area of the Yangtze River. Environ. Res. 185, 109386 (2020).
Adzima, F., Nasir, M., Mangala, S. & Zaharin, A. Ecotoxicology and Environmental Safety Public awareness level and occurrence of pharmaceutical residues in drinking water with potential health risk: A study from Kajang ( Malaysia ). Ecotoxicol. Environ. Saf. 185, 109681 (2019).
Fan, P. et al. Urinary antibiotics concentrations, their related affecting factors and infant growth in the first 6 months of life: A prospective cohort study. Ecotoxicol. Environ. Saf. 262, 115196 (2023).
Li, J. et al. Antibiotic exposure and risk of overweight/obesity in school children: A multicenter, case-control study from China. Ecotoxicol. Environ. Saf. 240, 113702 (2022).
Wen, J. et al. Urinary antibiotic levels and risk of overweight/obesity in preschool children: A biomonitoring-based study from eastern China. Ecotoxicol. Environ. Saf. 269, 115733 (2024).
Wang, H. et al. Antibiotics detected in urines and adipogenesis in school children. Environ. Int. 89–90, 204–211 (2016).
Oginawati, K., Yapfrine, S. J., Fahimah, N., Salami, I. R. S. & Susetyo, S. H. The associations of heavy metals exposure in water sources to the risk of stunting cases. Emerg. Contam. 9, 100247 (2023).
de Loayza, T. D. C., Maldonado, I. & Vilca, F. Z. Identification and quantification of antibiotic residues and evaluation of microbial resistance to antibiotics in Huatanay river waters in Peru. Pollution 9, 1236–1250 (2023).
Fabregat-Safont, D., Botero-Coy, A. M., Nieto-Juárez, J. I., Torres-Palma, R. A. & Hernández, F. Searching for pharmaceutical ly active products and metabolites in environmental waters of Peru by HRMS-based screening: Proposal for future monitoring and environmental risk assessment. Chemosphere 337, 139375 (2023).
Wang, H. et al. Urinary antibiotic level of school children in Shanghai. East. Environ. po 291, 118167 (2021).
Lerbech, A. M. et al. Antibiotic exposure in a low-income country: Screening urine samples for presence of antibiotics and antibiotic resistance in coagulase negative staphylococcal contaminants. PLoS ONE 9, 1–18 (2014).
Ben, Y. et al. Efficient detection and assessment of human exposure to trace antibiotic residues in drinking water. Water Res. 175, 115699 (2020).
Ajala, O. J., Tijani, J. O., Salau, R. B., Abdulkareem, A. S. & Aremu, O. S. A review of emerging micro-pollutants in hospital wastewater: Environmental fate and remediation options. Results Eng. 16, 100671 (2022).
Freeman, C. D., Klutman, N. E. & Lamp, K. C. Metronidazole. A therapeutic review and update. Drugs 54, 679–708 (1997).
Si, P. et al. Enhanced degradation of Metronidazole by the coupling of photocatalytic and microbial fuel cell: Mechanism and electrochemistry characteristic. J. Environ. Chem. Eng. 11, 109707 (2023).
Sivagami, K., Vignesh, V. J., Srinivasan, R., Divyapriya, G. & Nambi, I. M. Antibiotic usage, residues and resistance genes from food animals to human and environment : An Indian scenario. J. Environ. Chem. Eng. 8, 102221 (2020).
Liu, S. et al. Antibiotics in a general population: Relations with gender, body mass index (BMI) and age and their human health risks. Sci. Total Environ. 599–600, 298–304 (2017).
Han, S. et al. Estimating antibiotics use in major cities in China through wastewater-based epidemiology. Sci. Total Environ. 826, 154116 (2022).
Edmonson, M. B. & Eickhoff, J. C. Weight gain and obesity in infants and young children exposed to prolonged antibiotic prophylaxis. JAMA Pediatr. 171, 150–156 (2017).
Wang, H. et al. Predictors of urinary antibiotics in children of Shanghai and health risk assessment. Environ. Int. 121, 507–514 (2018).
Huang, Y. et al. Antibiotic burden of school children from Tibetan, Hui, and Han groups in the Qinghai-Tibetan Plateau. PLoS ONE 15, 1–16 (2020).
Wang, H. et al. Antibiotic body burden of Chinese school children: A multisite biomonitoring-based study. Environ. Sci. Technol. 49, 5070–5079 (2015).
Wang, W., Zhang, W., Liang, H. & Gao, D. Seasonal distribution characteristics and health risk assessment of typical antibiotics in the Harbin section of the Songhua River basin. Environ. Technol. 40, 2726–2737 (2019).
Mao, F., Liu, X., Wu, K., Zhou, C. & Si, Y. Biodegradation of sulfonamides by Shewanella oneidensis MR-1 and Shewanella sp. strain MR-4. Biodegradation 29, 129–140 (2018).
Jess, T. et al. Antibiotic use during pregnancy and childhood overweight: A population-based nationwide cohort study. Sci. Rep. 9, 1–9 (2019).
Fernandes, M. J. et al. Antibiotics and antidepressants occurrence in surface waters and sediments collected in the north of Portugal. Chemosphere 239, 124729 (2020).
Kümmerer, K. Antibiotics in the aquatic environment—A review—Part I. Chemosphere 75, 417–434 (2009).
Fabregat-Safont, D., Pitarch, E., Bijlsma, L., Matei, I. & Hernández, F. Rapid and sensitive analytical method for the determination of amoxicillin and related compounds in water meeting the requirements of the European union watch list. J. Chromatogr. A 1658, 462605 (2021).
Xu, L. & Kasprzyk-Hordern, B. Assessment of the stability of antimicrobials and resistance genes during short- and long-term storage condition: accounting for uncertainties in bioanalytical workflows. Anal. Bioanal. Chem. 415, 6027–6038 (2023).
Hernández, F., Fabregat-Safont, D., Campos-Maña, M. & Quintana, J. B. Efficient validation strategies in environmental analytical chemistry: A focus on organic micropollutants in water samples. Annu. Rev. Anal. Chem. 16, 401–428 (2023).
Fabregat-Safont, D., Gracia-Marín, E., Ibáñez, M., Pitarch, E. & Hernández, F. Analytical key issues and challenges in the LC-MS/MS determination of antibiotics in wastewater. Anal. Chim. Acta 1239, 340739 (2023).
Botero-Coy, A. M. et al. An investigation into the occurrence and removal of pharmaceuticals in Colombian wastewater. Sci. Total Environ. 642, 842–853 (2018).
Simarro-gimeno, C., Garlito, B. & Pitarch, E. Evaluation of direct sample injection as a fast, no-sample handling, approach for the LC-MS / MS monitoring of pharmaceuticals in different water matrices. Microchem. Jou 193, 108985 (2023).
SANTE. Analytical Quality Control and Method Validation Procedures for Pesticide Residues Analysis in Food and Feed SANTE 11312/2021 v2. (2022).
Acknowledgements
The authors would like to thank the Universidad Nacional de Moquegua and the project “Presence of antibiotic residues in the Osmore River, in drinking water in the cities of Ilo and Moquegua and its relationship with the health of the population” approved by resolution of the organizing committee N° 310-2020-UNAM.
Author information
Authors and Affiliations
Contributions
F Z V: conceptualization, supervision, visualization, funding acquisition, methodology, resources, writing—review and editing. M R B: conceptualization, methodology, writing—original draft. I M: methodology, data curation; formal analysis and writing—original draft. C N C Q: conceptualization, methodology, writing—original draft. F H, M B-C: methodology, data curation, writing—review and editing.
Corresponding author
Ethics declarations
Ethical approval and study design
This study was approved by an ethics committee of the National University of Moquegua, and the necessary permits have been requested from the health entities involved (CARTA 001-002-2023-PROYECTO-UNAM/FZV), in this case, the Pampa and Pacocha health center in the city of Ilo. In addition, all methods used in this study followed existing guidelines and regulations, mainly those governing the university above (INFORME 001-2023).
Informed consent
We hereby declare that written informed consent was obtained from the parents or legal guardians of all participating children before the study was conducted. The consent process included a clear explanation of the study’s purpose and procedures, including the collection of urine samples. All data collected during the survey has been handled with strict confidentiality and used exclusively for research purposes, following ethical guidelines and regulations.
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Zirena Vilca, F., Rojas Barreto, M., Maldonado, I. et al. Presence of antibiotics in children’s urine: a silent risk beyond drinking water. Sci Rep 15, 12078 (2025). https://doi.org/10.1038/s41598-025-94705-8
Received:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s41598-025-94705-8