Abstract
The gradual increase in ultraviolet B (UVB) health hazards to human skin, coupled with the irritation associated with existing sunscreen products, underscores the critical need for the development of natural sunscreens to combat UVB-induced photoaging. Chuanxiong oil (CXO) and hyaluronic acid (HA) possess excellent antioxidant and anti-apoptotic properties, which are closely linked to the mechanisms of photoaging. In this study, a composite nano-system (HA-CXO-Lip) comprising chuanxiong oil (CXO) and hyaluronic acid (HA) was initially fabricated. Subsequently, both in vitro HaCaT cell models and in vivo murine photoaging models were established to systematically evaluate the therapeutic efficacy and mechanistic actions of HA-CXO-Lip against photoaging under controlled experimental conditions. The investigation encompassed comprehensive assessments of its pharmacological effects and underlying molecular mechanisms through multimodal experimental approaches. Vitro experiments showed HA-CXO-Lip significantly reduced intracellular reactive oxygen species (ROS) levels and senescence-associated β-galactosidase (SA-β-Gal) activity. Furthermore, HA-CXO-Lip restored the levels of antioxidant enzymes, including superoxide dismutase (SOD), glutathione peroxidase (GSH-Px), catalase (CAT), and hydroxyproline (HYP), while also decreasing the levels of lipid metabolites such as 4-hydroxynonenal (4-HNE) and malondialdehyde (MDA). These findings indicate that HA-CXO-Lip effectively inhibits excessive oxidative stress. Additionally, HA-CXO-Lip inhibited apoptosis by reducing Bax levels and enhancing Bcl-2 expression in HaCaT cells. In vivo studies demonstrated that HA-CXO-Lip significantly reduced UVB irradiation-induced erythema and epidermal thickening in the backs of mice. It restored the orderly arrangement of collagen fibers and inhibited the activation of the core senescence pathway, AKT/mTOR, along with the downstream expression of matrix metalloproteinase 9 (MMP9), resulting in a decrease in collagen I disassembly. Additionally, HA-CXO-Lip was shown to significantly decrease the number of apoptotic cells, as indicated by the expression of the apoptosis marker cleaved cysteine aspartic protease-3 (C-Caspase-3) and the surface type I transmembrane glycoprotein (CD44), thereby further inhibiting apoptosis. The findings of this study suggest that HA-CXO-Lip can exert anti-photoaging effects through its antioxidant and anti-apoptotic properties, highlighting the synergistic efficacy of CXO and HA, which holds promise for the prevention and treatment of photoaging.
Similar content being viewed by others
Introduction
The health of the skin, as the body’s primary defense against external threats, is influenced by various factors, among which ultraviolet B (UVB) radiation is a major external risk1. The incidence of skin photodamage is on the rise as UVB radiation exposure increases, which poses a significant threat to human health. The World Health Organization (WHO) has reported that long-term UVB exposure not only accelerates skin photoaging but also may contribute to the development of skin cancer, which accounts for one-third of global cancer cases2. UVB penetrates the epidermis and reaches the basal layer, where it is absorbed by pigments in skin cells, generating large amounts of reactive oxygen species (ROS). These ROS then induce lipid peroxidation and apoptosis, disrupting cellular function3. Lipid peroxidation includes both enzymatic and non-enzymatic processes, with ROS activating phospholipases to release α- and β-unsaturated aldehydes, such as malondialdehyde (MDA) and 4-hydroxyalkenal (4-HNE). These aldehydes disturb lipid asymmetry, decrease the hydrophobicity of the lipid membrane interior, and lead to membrane depolarization. This disruption directly impairs the normal function of the cell membrane, resulting in cellular dysfunction and ultimately leading to apoptosis. Concurrently, ROS accumulation also alters the composition of the extracellular matrix (ECM), where collagen— the most critical component—plays a key role in maintaining skin firmness and elasticity. Compared with healthy skin, UVB-exposed skin exhibits elevated levels of metalloproteinase 9 (MMP9), a gelatinase. The increased expression of MMP9, in turn, leads to the breakdown of the major collagen type I (Collagen I), contributing to irreversible skin aging4.
In light of the harmful effects of UVB, sunscreens are widely used to protect the skin. However, some studies have shown that zinc oxide nanoparticles, which are commonly used in sunscreen products, may induce skin toxicity5,6. As a result, it has become increasingly important to study and develop new, safe, and effective photoprotective strategies. In recent years, natural herbal extracts have garnered significant attention due to their diverse active ingredients and high safety profile. For instance, Eman reported that frankincense essential oil could restore the antioxidant capacity of UVB-irradiated rats by modulating the MAPK pathway7. Additionally, several plant extracts, including Artemisia sieversiana Ehrhart essential oil, Angelica pubescens oil, and Magnolia sieboldii essential oils, have demonstrated anti-UVB radiation potential8. One of the more recently studied components is chuanxiong oil (CXO), an extract derived from the rhizome of Ligusticum chuanxiong hort., a medicinal plant in the Umbelliferae family, which is widely used in southwestern China and Korea as a vegetable and has a high safety profile9. CXO exhibits significant antioxidant properties, and studies have shown that treatment with CXO in LPS-induced neuroinflammation can significantly reduce ROS levels. Additionally, CXO has been found to increase SOD activity in the brain and reduce MDA levels in the treatment of insomnia. In studies examining the effects of CXO on proliferative scar fibroblasts (HSF), CXO was shown to inhibit MMP levels and activate caspase-3-induced HSF apoptosis10,11,12. These findings suggest that CXO plays a critical role in oxidative stress and apoptosis regulation. However, the molecular mechanisms through which CXO impacts photoaging induced by UVB irradiation remain unclear.Hyaluronic acid (HA) is an excellent biocompatible polysaccharide found in the extracellular matrix (ECM) of various tissues. It promotes cell proliferation, tissue regeneration, and prevents collagen degradation and oxidative stress in skin tissues. These properties make HA biomaterials a popular choice in cosmetic dermatology13,14,15. Studies have shown that combining HA with antioxidants can maximize anti-aging effects without causing side effects16,17. In previous studies, researchers have combined hyaluronic acid (HA) with epicatechin to suppress the expression of inflammatory factors and oxidative stress. Additionally, other studies have demonstrated that co-administration of HA and trehalose can exert synergistic anti-photoaging effects by leveraging the complementary properties of both agents18,19. Therefore, we hypothesized that combining CXO with HA could reduce oxidative stress and apoptosis, resulting dual anti-aging effects. However, the poor water solubility and unstable nature of CXO limit its broader application.
Liposomes are nanoformulations with a phospholipid bilayer structure similar to that of the skin, enabling them to efficiently encapsulate drugs, enhance their stability, and improve bioavailability20. Moreover, liposomes have been widely utilized in dermatology, as they significantly enhance drug efficacy and are biodegradable, making them non-immunogenic carriers21,22. Therefore, the use of liposome-encapsulated CXO in combination with HA offers an optimal solution for treating photoaging induced by UVB irradiation.
In this study, we propose combining CXO with HA to create a composite system (HA-CXO-Lip) through liposome encapsulation technology. This approach aims to enhance the stability and bioavailability of CXO and investigate the synergistic effects of HA in protecting against UVB radiation. We established an in vitro model using HaCaT cells exposed to UVB radiation, alongside an ICR mouse model, to evaluate the photoaging protective effects of HA-CXO-Lip and explore its underlying mechanism of action. The goal is to provide scientific evidence and experimental support for the development of safe and effective natural sunscreen products.
Materials and methods
Materials
Ligusticum chuanxiong Hort.( Wawushan Pharmaceutical Co., Ltd., Hehuachi Medicinal Materials Market, Chengdu ,batch number 250202, part used: dried root)have been verified with MPNS (http://mpns.kew.org).
and identified by Li Nan Professor of Chengdu University of Traditional Chinese Medicine; Cholesterol (Shanghai Aweituo Pharmaceutical Technology Co., Ltd., batch number C20392), hyaluronic acid (HA) (8290 Da, Bloomage Biotechnology Co., Ltd., batch number J201220279); CCK8 kit (Baoguang Biotechnology Co., Ltd., batch number BG0025); Cell culture medium (DMEM, lot number 11965092), cell buffer (PBS, lot number 10010023) purchased from Gibco, USA; Glutathione reductase (GSH-PX), lipid oxidation (MDA) detection kit, total SOD activity detection kit (Nanjing Jiancheng Institute of Bioengineering, batch numbers A005-1, A003-1-2, A001-3-2); 4-Hydroxynonenoic acid (4-HNE) (Eliretech Biotechnology LLC, lot number E-EL-0128); Cell senescence β-galactosidase staining kit (Biyuntian Biotechnology Co., Ltd., batch number C0602); ROS reactive oxygen species kit (Yiyi Landi Biotechnology Co., Ltd., batch number D1002); BCA protein concentration assay kit (Lanjieke Technology Co., Ltd., batch number BL521B); AKT, mTOR, MMP9 and other antibodies (Zhengneng Biotechnology Co., Ltd., Sichuan, China).
Extraction of chuanxiong oil
To prevent skin irritation caused by residual organic reagents, a steam distillation method was employed to extract Ligusticum chuanxiong oil (CXO). A total of 150 g of dried Ligusticum chuanxiong rhizome powder was weighed and mixed with 1200 mL of distilled water. An ultrasonic machine was set up at 200 W to extract the mixture for 30 min, followed by steam distillation for 8 h. The CXO was then collected, dried over anhydrous sodium sulfate, and stored at 4 °C for future use.
Gas chromatography–mass spectrometry analysis of CXO
50μLCXO was diluted to 1 mL with hexane and water was removed with anhydrous sodium sulfate. Subsequently, impurities were removed using a 0.22 μm microporous filter membrane. Gas chromatography-mass spectrometry (GC–MS) analysis was used for the determination. The analytical parameters were as follows: the carrier gas was helium; the volume flow rate was 1.0 mL/min; the temperature was programmed to increase, the initial temperature was 50 °C, and kept for 2 min; the rate of 10 °C/min was increased to 120 °C, and kept for 8 min; the rate of 5 °C/min was increased to 180 °C, and kept for 8 min; the rate of 20 °C/min was increased to 260 °C, and kept for 4 min, and the sample was injected in shunt, and the shunt ratio was 1:60; the injection volume was 1uL; the temperature of the injection port was 280 °C.
Preparation of HA-CXO-Lip
Liposomes were prepared using the film dispersion method. An appropriate amount of lecithin and cholesterol was weighed, and chloroform:ether (1:2, v/v) was added to dissolve the mixture. This solution was placed on a rotary evaporator, where the pressure was reduced and the water bath temperature maintained at 40 °C, allowing for the formation of a thin film on the walls of a pear-shaped bottle. The CXO was then mixed with Tween-80 and added to a pre-dissolved hyaluronic acid (HA)-containing PBS solution, ensuring thorough mixing. This mixture was subsequently added to the thin film formed in the pear-shaped bottle. The solution was rotated in a water bath at atmospheric pressure to create a homogeneous white emulsion. Following this, the emulsion was ultrasonicated using a cell crusher (JRA-650X,650W,6 mm) for 5 min, alternating between 5 s of operation and 5 s of rest. Finally, the product was filtered through a 0.22 μm microporous filter membrane and stored at 4 °C to obtain HA-CXO-Lip.
The response surface-center composite design optimizes the preparation process
To determine the optimal conditions for liposome preparation, the three factors with the greatest influence on the encapsulation rate (EE) and drug loading (DL) of HA-CXO-Lip were screened based on the one-factor results: the amount of CXO (A), the amount of HA (B), and the volume of PBS hydration (C). The preparation of the formulation was optimized using a three-factor, three-level central composite design. The ranges of the three parameters are shown in Table 1.
Characterization of HA-CXO-Lip
The EE, DL, transmission electron microscopy (TEM), particle size and polydispersity index (PDI) of the prepared nanopreparation HA-CXO-Lip were investigated.
Examination of HA-CXO-Lip release in vitro
2 mL samples (CXO, HA-CXO, HA-CXO-Lip) were placed in a pretreated dialysis bag (MD 34-14000D) and dialyzed with 400 mL of pH 7.4 PBS solution (PBS: Tween-80, 400:1, v/v) as dialysis medium, and the conditions of intelligent dissolution apparatus (ZRS-8G) were set to 37 °C ± 0.5 °C, 200 r/min. 4.0 mL of dissolution medium was taken after 0.5, 1, 2, 3, 4, 6, 8, 10, 24, 48 and 72 h, respectively, and supplemented with the same volume and temperature of PBS. Absorbance was measured by UV spectrophotometry and cumulative release was calculated. Parallel manipulation experiments were performed 3 times.
Franz proliferation
Remove the subcutaneous fat and connective tissue from the back skin of mice, wash it with PBS and store it in the refrigerator at − 80 °C. In the experiment, the thawed mice skin with the stratum corneum facing upward and the dermis facing downward was fixed between the donor pool and the receiver pool in the Franz diffusion cell (diffusion area of 0.785 cm2). PBS7.4 solution (PBS: Tween-80, 400:1, v/v) was added to the receiving pool. In the supply cell, 2 mL of drug was added and the temperature of the water bath was maintained at 37 °C ± 0.5 °C at a speed of 200 r/min. Samples were taken at 0.5, 1, 2, 3, 4, 6, 8, 10, and 24 h, respectively, to calculate the permeability of the drug per unit area.
Evaluation of free radical scavenging capacity
DPPH free radical scavenging
Antioxidant activity was evaluated using 1,1-diphenyl-2-picrylhydrazyl (DPPH). A series of samples containing CXO at concentrations (0.2, 0.5, 1, 2, and 4 mg/mL) were prepared for testing. 2 mL of the samples were mixed with 2 mL of 0.04 mmol/L DPPH and allowed to stand at room temperature and protected from light for 30 min, after which the samples were centrifuged at 5000 r/min for 10 min. The absorbance of the resulting supernatant at 517 nm was measured.
ABTS free radical scavenging
Prepare a stock solution of 2,2'-Azino-bis(3-ethylbenzothiazoline-6-sulfonic acid) diammonium salt (ABTS) with a concentration of 7 mmol/L and a K2S2O8 solution at a concentration of 2.45 mmol/L. After mixing in equal proportions, let it stand in the dark for 12 h to obtain the ABTS solution. Dilute the ABTS solution with PBS to an absorbance of 0.70 ± 0.02 at 734 nm, take 100 μL of the sample solution containing different concentrations of CXO (0.2, 0.5, 1, 2 and 4 mg/mL) in a 96-well plate, quickly add 200 μL of ABTS solution and mix well, react at 25 °C in the dark for 6 min, and measure the absorbance at a wavelength of 734 nm.
HaCaT cell culture
Human immortalized keratinocytes (HaCaT) were obtained from Gineo Biotechnology Ltd (Guangzhou, China). High sugar DMEM containing 10% FBS, 1% penicillin–streptomycin was used to culture the cells. When the cell growth density reached 70%-80%, they were used for passaging or subsequent experiments.
Establishment and grouping of the HaCaT photoaging model
UVB irradiation (313 nm, G8T5E UVB-313 EL) was employed to establish a model of photoaging in HaCaT cells. Briefly, HaCaT cells that had been passaged for 2–3 generations were seeded in appropriate well plates and irradiated with UVB at a distance greater than 50 cm from the plates. The total radiation doses administered were 0 mJ/cm2, 20 mJ/cm2, 40 mJ/cm2, 60 mJ/cm2, 80 mJ/cm2, and 100 mJ/cm2. The HaCaT cells were categorized into four groups: the control group (non-irradiated cells), the UVB group (cells treated with UVB only), and the drug groups (CXO, CXO-Lip, HA-CXO-Lip, Ligustilide). The drug intervention was applied 1 h prior to irradiation.
CCK8 assay for cell viability
HaCaT cells (1 × 105 cells/well) were inoculated into 96-well plates and incubated in a 5% CO2, 37 °C incubator (Thermo-3111) for 24 h. Subsequently, the cells were treated with different concentrations of drug versus radiation doses, respectively, by adding 10% (v/v) CCK8 solution and continuing the incubation for 2 h. Then, the viability of the cells was calculated by using an enzyme labeling instrument (Multiskan FC) 450 nm to measure the absorbance value of the solution and calculate the cell viability.
Wound scratch assay
To evaluate the effect of HA-CXO-Lip on the migration ability of cells, HaCaT cells were inoculated in 6-well plates at a density of 4 × 105/well, and when the cells grew to the bottom of the well plates were spread out, the tip of the 200 μL sterile tip of the gun was used to produce linear scratches, and PBS was washed to remove the cellular debris, and serum-free medium diluted with the drug was used for culture. Photographs were observed under the microscope at 6,12and 24 h. Quantitative analysis was performed using (Fiji Is Just) ImageJ).
SA-β-gal assay
HaCaT cell SA-β-gal activity was assessed using the Cellular Senescence β-Galactosidase Staining Kit according to the manufacturer’s protocol. HaCaT cells were inoculated in 6-well plates when the relative density of the cells was 70–80%, and were pretreated with the drug 1 h prior to UVB irradiation. Incubation was continued for 6 h after UVB irradiation, washed once with PBS, and 1 mL of β-galactosidase staining fixative was added for 15 min at room temperature. The cell fixative was aspirated, and the cells were incubated overnight at 37 °C. Glucosidase staining fixative was fixed at room temperature for 15 min. the cell fixative was aspirated, washed three times with PBS, 1 mL of working solution was added to each well, and incubated overnight at 37 °C. The cells were observed and photographed under a light microscope. The cells were observed and photographed under a light microscope.
ROS detection
ROS levels in cells were determined using a 2,7-dichlorodihydrofluorescein diacetate (DCFH-DA) fluorescent probe. HaCaT cells were inoculated in 6-well plates, incubated for 24 h, and drug-treated as described above. The cell supernatant was discarded, and 1 mL of DCFH-DA solution with a final concentration of 0.01 mol/L was added, and the incubation was continued for 30 min and washed with PBS. Photographs were taken and observed in a fluorescence microscope (TSC SP8 STED).
Determination of MDA, SOD, CAT, HYP, 4-HNE and GSH-Px in oxidative stress-related markers in cells
HaCaT cells were seeded in 6-well plates and incubated for 24 h following drug treatment and exposure to UVB light. Subsequently, the cells were collected, and the levels of antioxidant enzymes, including SOD, CAT, HYP, and GSH-Px, as well as the lipid oxidation products MDA and 4-HNE, were measured in accordance with the manufacturer’s instructions.
Apoptotic protein Bax, Bcl-2 detection
HaCaT cells were seeded in six-well plates containing round coverslip at the bottom and were treated with UVB and a drug after the cells had adhered. Following incubation, the cells were washed three times with pre-chilled PBS and fixed with 4% paraformaldehyde. Serum was blocked, and the primary antibody was added dropwise, followed by an overnight incubation at 4 °C. The cells were then washed three times with PBS for 5 min each; subsequently, they were incubated at 37 °C for 30 min. After another set of three PBS washes for 5 min each, DAPI was added dropwise and incubated at room temperature for 10 min. The cells were washed three more times with PBS for 5 min each, and the slides were mounted with an anti-fluorescence attenuation mountant before imaging under a microscope.
Animal grouping and modeling of photoaging
All animal experiments were conducted in accordance with the Guidelines for the Care and Use of Laboratory Animals and approved by the Ethics Committee of Chengdu University of Traditional Chinese Medicine (Sichuan, China, 2020–27). This study is reported in compliance with the ARRIVE guidelines (https://arriveguidelines.org). Male ICR mice, aged 3–4 weeks, were purchased from Sparfoam Ltd (SCXK 2024-0001, Beijing, China). As showed in Fig. 1, the mice were housed at a temperature of 25 °C ± 0.5 with 50% humidity and were fed ad libitum under a 12-h light/dark cycle. After a 1-week acclimatization period, the mice were dorsally depilated to expose an area of 4 cm × 4 cm of skin. The mice were then divided into six groups: the control group, which was neither exposed to UV light nor subjected to any treatments; the UVB group, which received only UVB irradiation; and the drug group, comprising CXO, CXO-Lip, HA-CXO-Lip, and Ligustilide, in which 200 μl of a drug was applied to the skin on the back of the mice 30 min prior to UVB exposure. Ultimately, the mice were anesthetized with an injection of 1% pentobarbital sodium, concluding the experiment. Subsequently, euthanasia was performed on the rats through cervical dislocation.
Histopathological testing
For histological observation, dorsal skin tissue specimens were taken from mice, fixed in 4% paraformaldehyde, and the fixed tissues were dehydrated by an automatic dehydrator, trimmed, embedded, sectioned, stained with Hematoxylin Eosin (HE) staining and Masson staining, respectively, and sealed. The epidermal thickness was quantified.
Measurement of skin water content
Skin water content was measured by direct drying method. Briefly, about 0.2 g of mice dorsal skin were clipped, weighed, quickly placed in an oven at 105 °C, and dried to a constant weight. The skin water content (%) was calculated.
Organ immunity index
The organ immunity index reflects to a certain extent the ability of the organism to defend itself against external harmful factors. The spleen and thymus of mice were taken to measure the immunity index.
Measurement of MDA, SOD, CAT, HYP, 4-HNE and GSH-Px content in skin tissue
Mice dorsal skin tissues were incubated with Enhanced RAPI Lysis Buffer at 4 °C for 30 min. Following incubation, the tissues were homogenized and centrifuged at 1500 rpm for 10 min using a tissue homogenizer, after which the supernatant was collected. The levels of oxidative stress-related factors were measured according to the instructions provided with the commercial assay kit, and absorbance values were recorded using an enzyme meter.
Tunel assays apoptosis
After the dehydration of paraffin sections, citric acid (pH 6.0) was introduced into the repair box for tissue repair. Following this, a proteinase K working solution and Tunel reaction solution were added dropwise in accordance with the instructions provided by the terminal deoxynucleotidyl transferase-mediated dUTP nick end labeling (Tunel) kit, using fluorescein-labeled dUTP solution as a negative control. After washing, the nuclei were stained with DAPI, and the samples were mounted in glycerol for observation under fluorescence microscopy.
Immunohistochemical analysis
The paraffin sections were subjected to dehydration and antigen retrieval using an EDTA solution at pH 9.0. Endogenous peroxidase activity was blocked with 3% hydrogen peroxide. Following this, the tissue was subjected to a series of steps including blocking, incubation with a secondary antibody after an overnight incubation with the primary antibody, and dropwise addition of DAB chromogenic solution for color development. Hematoxylin was then used for nuclear staining, and the samples were observed and photographed under a microscope after mounting.
Immunofluorescence assay
Immunofluorescence was employed to assess the expression levels of AKT, p-AKT, p-mTOR, MMP9, and Collagen I in the dorsal skin tissues of mice across all experimental groups. The paraffin-embedded skin sections underwent deparaffinization, followed by antigen retrieval using 1 × citric acid (pH 6.0). Endogenous enzyme activity was blocked with 3% H2O2. Subsequently, the sections were incubated with the primary antibody overnight at 4 °C, washed three times, and then incubated with the secondary antibody for 1 h prior to imaging with a confocal microscope.
Western blot analysis
The dorsal skin tissue of the mice was homogenized, and the supernatant was collected following centrifugation. Protein concentration was determined using the BCA Protein Assay Kit. After performing polyacrylamide gel electrophoresis, the PVDF membrane was transferred and sealed with 5% skimmed milk powder for 2 h. Primary antibodies p-AKT (1:1000), AKT, p-mTOR (1:1000), mTOR, and MMP9 (1:1000) were then added and incubated overnight at 4 °C. Following three washes with TBST, secondary antibody incubation was conducted. Finally, the enhanced chemiluminescent western blot substrate was applied, and a film-based chemiluminescence imager (Touch Imager Pro) was utilized for the visualization of protein bands.
Statistical analysis of data
Statistical analysis was performed using GraphPad Prism 9.5. Results were expressed as mean ± SD. Analysis of variance (ANOVA) was used for multiple comparisons of means. p-values less than 0.05 were considered statistically significant. The significance level is represented by the following equation *p < 0.05, ***p < 0.01, **** p < 0.001, ****p < 0.0001; ns, not significant.
Results
GC–MS compositional analysis of Ligusticum chuanxiong essential oil
The corresponding graphical and mass spectral data were obtained through GC–MS analysis. The relative percentage content of each component was determined by identifying the peaks in the NIST14 database and combining this information with relevant graphical analysis using peak area normalization. A total of 46 compounds were identified via GC–MS analysis (Fig. 2, Fig. S1 and Table S1). The most prominent components included cis-ligustilide (37.2%), alpha-selinene (9.97%), allyl phenoxyacetate (5.65%), cedrene (4.20%), (Z)-4-ethyl-3-nonen-5-yne (3.03%), terebene (2.89%), and moslene (2.58%), among others. We speculate that cis-ligustilide may be one of the key components in chuanxiong that exerts medicinal effects; therefore, it was subsequently included in this study.
Optimal preparation process of HA-CXO-Lip
The three factors- CXO (mg) dosage, HA (mg) dosage, and PBS (mL) hydration volume, exerted the greatest influence on the EE and DL of HA-CXO-Lipin one-way experiments. Based on these factors, the best raw material ratios were obtained by response surface optimization experiments (Table 2).
Dseign-Expert 12 was used to analyze the influencing factors in Table 2, and a model was fitted with EE (Y1) and DL (Y2) as the dependent variables, and the degree of fit was judged based on the correlation coefficient (R2) and analysis of variance (ANOVA). The regression model for EE and DL was as follows:
Fitting Y1 mathematical model p < 0.0001, equation fitting correlation coefficient R2 = 0.9976, AdjR2 = 0.9946. misfit term p = 0.3083 (P > 0.05); Y2 mathematical model p < 0.0001, equation fitting correlation coefficient R2 = 0.9939, AdjR2 = 0.9862, misfit term p = 0.0622 (p > 0.05), so we can exclude the interference of unknown factors. Therefore, the quadratic multivariate mathematical model of EE and DL can be used to predict the relationship between the response values EE and DL and the independent variables.3D surface plot (Fig. 3) responds to the interaction of factors. From Fig. 3A, it was found that the EE% value of HA-CXO-Lip was maximum at the dosage of 90–100 mg of CXO, 4.5–5.5 mg of HA, and 7 -8 mL of PBS. From Fig. 3B, it was found that the DL value of HA-CXO-Lip was maximum at CXO dosage of 80–90 mg, HA dosage of 5.5–6.5 mg, and PBS dosage of 7–8 mL. This may be related to the liposome phospholipid bilayer structure. The final response surface optimization designed the optimal preparation conditions of HA-CXO-Lip as CXO (99.49 mg), HA (5.60 mg), and PBS (7.93 mL). Validation experiments were performed according to the optimal conditions, and the EE of HA-CXO-Lip prepared in three batches was 90.08% ± 0.27, and the DL was 0.97 mg ± 0.03. The results showed that the quadratic term equation was well fitted with a high confidence level (Table 3).
Characterization of HA-CXO-Lip
The external morphology of HA-CXO-Lip was characterized. The results presented in Fig. 4A indicate that the HA-CXO-Lip solution is a homogeneous, semi-transparent solution. Additionally, the transmission electron microscopy (TEM) images of HA-CXO-Lip shown in Fig. 4B reveal that the liposomes are nearly spherical or elliptical in shape, exhibiting homogeneous characteristics and good dispersibility. Furthermore, Fig. 4C demonstrates that the average particle size of the liposomes was 154 ± 6.03 nm, with a polydispersity index (PDI) of 0.19 ± 0.004.
As illustrated in Fig. 5, the FT-IR spectrum of CXO displays distinct vibrational absorption peaks for C–H at 2925 cm−1 and 2863 cm−1. Additionally, there are notable vibrational absorption peaks for C=O and C–O at 1764 cm−1 and 1463 cm−1, respectively, which may be attributed to the presence of esters and ketones in the volatile oil. HA exhibited a symmetric telescopic vibrational peak for –COOH at 1629 cm−1 (νC = O) and a characteristic absorption peak for –OH at 3461 cm−1. Additionally, it displays asymmetric and symmetric telescopic vibrational absorption peaks for –COOH near 1737 cm−1 and 1463 cm−1, respectively. The bending vibrational absorption peaks for C–H are observed near 1450 cm−1 and 1375 cm−1. Furthermore, HA has characteristic absorption peaks for the sugar ring structure at 1025 cm−1 and 1110 cm−1. Lip exhibited C–H stretching vibrational absorption peaks at approximately 2856 cm−1 and 2927 cm−1, as well as C=O and C–O stretching vibrational absorption peaks around 1737 cm−1 and 1467 cm−1. These peaks may be attributed to the presence of esters in its liposomes. The IR spectrum of CXO-HA, as a physical mixture, may exhibit a simple superposition of the characteristic absorption peaks of HA and CXO. In contrast, the IR spectrum of HA-CXO-Lip displayed distinct characteristic absorption peaks that differed from those of HA and CXO alone. This observation suggests that the chemical environments of HA and CXO were altered during the formation of the liposomes. Furthermore, the IR spectra of the blank Lip and HA-CXO-Lip were highly similar, indicating that HA and CXO were effectively encapsulated within the Lip, resulting in the masking of their characteristic absorption peaks. This confirms the successful preparation of HA-CXO-Lip.
Stability of HA-CXO-Lip
After 90 d, the EE, DL, and average particle size of HA-CXO-Lip stored at 4 °C were evaluated in Table 4. The resultsdemonstrates that HA-CXO-Lip maintained stability,with no precipitation orparticle aggregation observed throughout the storage period.
Sustained release and transdermal effects of HA-CXO-Lip
The in vitro release results for CXO, CXO-Lip, and HA-CXO-Lip solutions are presented in Fig. 6A. The findings indicate that CXO exhibited the fastest release rate, while the release rates of CXO-Lip and HA-CXO-Lip were comparable and not significantly different during the first 12 h. At the 12-h mark, the release rate of CXO-Lip was 25.71%, whereas that of HA-CXO-Lip was 29.76%. This difference may be attributed to the presence of osmotically promoting small molecule HA in HA-CXO-Lip. Notably, at the 10-h point, the CXO release rate began to stabilize, showing no significant changes, whereas CXO-Lip and HA-CXO-Lip continued to release slowly over time. These results suggest that the prepared liposomes exhibit a prolonged and sustained release profile. The Franz transdermal diffusion results presented in Fig. 6B indicate that the cumulative release of CXO was the lowest, with a transdermal amount of only 117.95 μg/cm2 at 48 h. This suggests that CXO does not permeate and absorb effectively. In contrast to the in vitro release pattern, the cumulative release of CXO-Lip was greater than that of HA-CXO-Lip during Franz transdermal diffusion. Specifically, at 24 h, the cumulative transdermal amount of CXO-Lip was 870.62 μg/cm2, which increased to 1126.48 μg/cm2 at 48 h. Meanwhile, the cumulative transdermal amounts of HA-CXO-Lip were 841.11 μg/cm2 and 975.20 μg/cm2, respectively, at the same time points. This phenomenon can be attributed to the complex structure of the skin, where hyaluronic acid (HA) is present in both the epidermal and dermal layers. Compared with CXO-Lip, HA-CXO-Lip exhibited significantly enhanced skin retention, thereby improving its anti-aging effects on the skin.
Antioxidant effect of HA-CXO-Lip
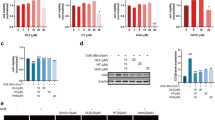
The free radical scavenging activities of CXO, HA-CXO-Lip, and HA-HA-CXO-Lip with the main component (Ligustilide) in CXO were further evaluated by DPPH and ABTS methods. As shown in Fig. 6C, all four antioxidants, CXO, HA-CXO-Lip, HA-HA-CXO-Lip with Ligustilide, were able to exert their antioxidant capacity better, and the DPPH radical scavenging capacity was concentration-dependent. At 4 mg/mL, CXO scavenging capacity was 75.02%, CXO-Lip scavenging capacity was 77.46%, while HA-CXO-Lip scavenging rate was as high as 86.05%, and Ligustilide scavenging rate was similar to that of HA-CXO-Lip, and DPPH radical scavenging rate was 80.26%. Therefore, the DPPH radical scavenging capacity was HA-CXO-Lip > Ligustilide > CXO-Lip > CXO. Interestingly, the ABTS radical scavenging assay found that the ABTS radical scavenging rate followed a similar pattern to the DPPH radical scavenging rate (Fig. 6D), with HA-CXO-Lip scavenging rate having the greatest capacity of up to 86.04% at 4 mg/mL, and the CXO-Lip scavenging rate at the same concentration was 81.75%, and the ABTS radical scavenging rate of Ligustilide was 83.09%, while the CXO scavenging rate was only 77.79%. This suggests that CXO has significant antioxidant activity and improved free radical scavenging ability after preparation into liposomes, which may be related to the improved CXO stability by liposomes. Similarly, HA was also found to play an antioxidant role in liposomes, which preliminarily confirmed that Ligustilide is an important component in CXO that plays an antioxidant role.
Viability of HaCaT cells
To assess drug safety, the CCK8 method was employed to determine the optimal concentration for drug administration. The results of the CCK8 assay are presented in Fig. 7A.The viability of HaCaT cells exhibited a dose-dependent decrease following co-incubation with CXO, CXO-Lip, HA-CXO-Lip, and Ligustilide. Notably, cell viability remained above 90% at drug concentrations ranging from 3.125 to 25 μg/mL. Furthermore, at a concentration of 25 μg/mL, the drug demonstrated a promoting effect on cell viability. Consequently, we selected 25 μg/mL as the administered dose. Moreover, we screened the UVB radiation dose of 0 -100 mJ/cm2 (Fig. 7B), and based on the calculation of the IC50 value of cell viability, we chose the radiation dose of 60 mJ/cm2 for the subsequent experiments.
Drug doseand radiation dose screening, HaCaT cell migration capacity. (A) Effect of the drug on the viability of HaCaT cells at different concentrations. (B) Effect of UVB dose of 0–100 mJ/cm2 on HaCaT cell viability. (C–D) Effects ofCXO,CXO-Lip, HA-CXO-Lip, and Ligustilide on cell migration at 6, 12 and 24 h. *p < 0.05, **p < 0.01, ***p < 0.001, ****p < 0.0001 compared with the control group; #p < 0.05, ##p < 0.01, ###p < 0.001, ####p < 0.0001 compared with the UVB group; ^p < 0.05, ^^p < 0.01, ^^^p < 0.001, ^^^^p < 0.0001 compared with the CXO group.
In addition, the process of cell migration is usually in response to extracellular signals. To the best of our knowledge, there are no reports discussing the potential effects of CXO on wound healing capacity. The effect on cell migration was investigated using the cell scratch method. As demonstrated in Fig. 7C, CXO, CXO-Lip, HA-CXO-Lip and Ligustilide significantly enhancd cell migration compare to the UVB-irradiated at 6, 12, and 24 h. Surprisingly, as shown in Fig. 7D, the HA-CXO-Lip group reached 49.50% migration at 6 h, while the cell migration was 28.58% in the CXO group, 36.48% in the CXO-Lip group, and 42.84% in the Ligustilide group, while the cell migration was only 24.35% in the UVB group without any drug treatment. By 24 h posr-scratch, the HA-CXO-Lip group was basically completely healed, and the Ligustilide group also reached 92.47%; the UVB group reached 72.71%, and the migration rate of the CXO group reached 74.84%, with no significant difference between the two groups. Thesefidings confirm that CXO, CXO-Lip, HA-CXO-Lip, and Ligustilide promote cell migration and skin repair post-external stress, while UVB radiation suppresses migratory capacity. The co-encapsulation of HA and CXO in liposomes synergistically maximized skin repair efficacy.
Effects of HA-CXO-Lip on oxidative stress
Subsequently, we further measured the content of antioxidant enzymes and lipid metabolites in HaCaT cells. As shown in Fig. 5, the concentrations of SOD (Fig. 8A), GSH-Px (Fig. 8B), HYP (Fig. 8C) and CAT (Fig. 8D) in the UVB group decreased to only 3.98 U/mg, 26.60 U/mg, 0.14 μg/mL and 1.91 U/mg after oxidative stress, respectively, while the contents of the four antioxidant enzymes recovered to 12.63 U/mg and 48.97 U/mg after HA-CXO-Lip pretreatment. 0.36 μg/mL, 9.92 U/mg, which was comparable to that of the control group. As shown in Fig. 8E,F, the content of lipid metabolites increased after the cells were stimulated. Figure 8E shows that the concentration of MDA in the cell lysate of the UVB group reached 79.22 μmol/mg, while the concentration in the control group was only 12.89 μmol/mg. Figure 8F shows that the concentration of 4-HNE in the UVB group was 25.44 ng/mL and that in the control group was 1.14 μmol/mg. Interestingly, the expression levels of MDA and 4-HNE decreased after CXO, CXO-Lip, HA-CXO-Lip and Ligustilide treatments, and the expression levels of MDA and 4-HNE decreased to 37.77 μmol/mg and 3.07 ng/mL in the HA-CXO-Lip group, respectively.
Detection of oxidative stress related factors. (A) SOD. (B) GSH-Px. (C) HYP. (D) CAT. (E) MDA. (F) 4-HNE. *p < 0.05, **p < 0.01, ***p < 0.001, ****p < 0.0001 compared with the control group; #p < 0.05, ##p < 0.01, ###p < 0.001, ####p < 0.0001 compared with the UVB group; ^p < 0.05, ^^p < 0.01, ^^^p < 0.001, ^^^^p < 0.0001 compared with the CXO group.
SA-β-gal is a well-recognized biomarker of cellular senescence; therefore, SA-β-gal is staining was performed to assess SA-β-gal activity. HaCaT cells exhibited significant perinuclear SA-β-gal staining following UVB exposure; however, the number of SA-β-gal-positive cells was notably reduced after HA-CXO-Lip treatment (Fig. 9A). The regulatory mechanisms underlying its molecular action remain to be elucidated. As previously demonstrated, HA-CXO-Lip can enhance antioxidant enzyme activities and reduce lipid oxidation levels, thereby exerting a protective effect against photoaging. Our experiments revealed that reactive oxygen species (ROS), indicated by green fluorescence, were significantly elevated in the UVB group (Fig. 9B) compared to the control group, suggesting that UVB exposure leads to excessive ROS accumulation. In contrast, treatments with CXO, CXO-Lip, and HA-CXO-Lip combined with Ligustilide resulted in reduced green fluorescence intensity. The quantitative results, as shown in Fig. 9C, indicate that HA-CXO-Lip treatment effectively restored ROS levels to near normal. The fluorescence intensities were ranked as follows: UVB group > CXO > CXO-Lip > Ligustilide > HA-CXO-Lip.
SA-β-gal Staining and ROS detection. (A) SA-β-gal staining. (B–C) ROS expression profile levels. *p < 0.05, **p < 0.01, ***p < 0.001, ****p < 0.0001 indicates comparison with the control group; #p < 0.05, ##p < 0.01, ###p < 0.001, ####p < 0.0001 indicates comparison with the UVB group; ^p < 0.05, ^^p < 0.01, ^^^p < 0.001, ^^^^^ ^p < 0.0001 indicates comparison with the CXO group.
HA-CXO-Lip reduces HaCaT cell apoptosis
We further investigated the effect of HA-CXO-Lip on apoptosis following oxidative stress. Cellular immunofluorescence results indicated that the pro-apoptotic protein Bax and the anti-apoptotic protein Bcl-2 were localized to the nuclear membrane, the endoplasmic reticulum membrane, and the outer mitochondrial membrane, as evidenced by green fluorescence. After UVB modeling, the expression level of Bax increased (Fig. 10A,C), while the expression of Bcl-2 decreased (Fig. 10B,D) in HaCaT cells. Notably, following the administration of CXO, CXO-Lip, and HA-CXO-Lip, the expression of Bax was reduced, and the Bcl-2 content was partially restored. Additionally, we observed that chuanxiong ligusticum extract exhibited a weaker effect than Ligustilide in mitigating UVB-induced apoptosis. However, its effect was more pronounced compared to the anti-phototoxicity effect of ligustilide when combined with HA and formulated as a nano-preparation, suggesting that the combination of HA and ligustilide yields a stronger anti-phototoxicity effect. This finding indicates a potential synergistic role for HA and CXO.
Cellular immunofluorescence detection of apoptotic proteins. (A, C) Effects of CXO, CXO-Lip, HA-CXO-Lip, and Ligustilide on Bax protein in HaCaT cells. (B, D) Effects of CXO, CXO-Lip, HA-CXO-Lip, and ligustilide on Bcl-2 protein in HaCaT cells. *p < 0.05, **p < 0.01, ***p < 0.001, ****p < 0.0001 indicates comparison with the control group; #p < 0.05, ##p < 0.01, ###p < 0.001, ####p < 0.0001 indicates comparison with the UVB group; ^p < 0.05, ^^p < 0.01, ^^^p < 0.001, ^^^^^ ^p < 0.0001 indicates comparison with the CXO group.
HA-CXO-Lip reduces pathologic skin damage
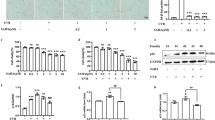
The mice exhibited varying degrees of skin damage following UVB exposure (Fig. 11A). Notably, the mice in the UVB group displayed significant dryness, diminished luster and elasticity of the skin on their backs, as well as deepening of skin lines and the emergence of scabs. Treatments with CXO, CXO-Lip, HA-CXO-Lip, and Ligustilide effectively ameliorated the UVB-induced alterations in skin appearance. Interestingly, UVB irradiation also affected the body weight of the mice, which initially increased before subsequently decreasing, as illustrated in Fig. 11B. Furthermore, UVB exposure led to alterations in the water content of the mice’s skin. As depicted in Fig. 11C, after 4 weeks of UVB irradiation, the skin water content in the UVB group decreased to 57.35%. Importantly, following treatment with CXO, CXO-Lip, HA-CXO-Lip and Ligustilide, the skin water content of the mice recovered to 62.70%, 69.04%, 73.88%, and 70.43%, respectively. Long-term UVB irradiation affects the appetite of mice, which in turn has a significant impact on their immune capabilities. Our findings indicate that the spleen and thymus indices of mice decreased markedly following UVB exposure (Fig. 11D,E), suggesting a reduction in immune function. Fortunately, the topical application of the HA-CXO-Lip preparation mitigated this decline, leading to an improvement in the immune indices of the mice.
Skin appearance of UVB-irradiated mice, changes in body weight, water content, immunity index, HE pathology test with Masson staining. (A) Skin condition of the back of UVB irradiated mice. (B) Body weight changes. (C) Water content changes. (D) Thymus index. (E) Spleen index. (F) HE staining. (H) Epidermal thickness. (G) Masson staining. *p < 0.05, **p < 0.01, ***p < 0.001, ****p < 0.0001 indicates comparison with the control group; #p < 0.05, ##p < 0.01, ###p < 0.001, ####p < 0.0001 indicates comparison with the UVB group; ^p < 0.05, ^^^p < 0.01, ^^^p < 0.001, ^^^^^ ^p < 0.0001 indicates comparison with the CXO group.
HE staining results (Fig. 11F) indicated that the mice in the UVB group exhibited notable scab formation, thickening of the degenerative necrosis of the epidermal layer, infiltration of neutrophils, an increase in fibroblasts with ovoid nuclei, and a substantial presence of necrotic cell fragments. Interestingly, treatment with CXO, CXO-Lip, and HA-CXO-Lip containing Ligustilide ameliorated inflammatory cell infiltration to varying degrees. The epidermal thickness of the mice reached 87.7 μm following UVB irradiation, which was significantly reduced to 35.77 μm after intervention with HA-CXO-Lip (Fig. 11H). To assess changes in collagen fibers, the dorsal skin was stained with Masson’s trichrome stain (Fig. 11G). Post-UVB irradiation, the collagen fibers in the dorsal dermis were significantly reduced and disorganized. However, the degradation of collagen fibers was restored to varying extents in the treated group, with the fiber arrangement gradually returning to a more ordered state.
To further confirm that UVB-induced photoaging is closely related to oxidative stress, we measured the levels of SOD (Fig. 12A), GSH-Px (Fig. 12B),CAT (Fig. 12C), HYP (Fig. 12D), MDA (Fig. 12E), and 4-HNE (Fig. 12F)in mouse skin tissues. The results aligned with our in vitro experiments, indicating that the antioxidant enzyme system was disrupted in the mice. Specifically, the levels of SOD, GSH-Px, HYP, and CAT were significantly decreased following UVB irradiation (p < 0.0001), while the levels of MDA and 4-HNE were significantly increased. Notably, the antioxidant enzyme system was restored through drug treatments, with HA-CXO-Lip demonstrating the most effectiveness in alleviating oxidative stress (p < 0.0001). Thus, the investigated preparation of HA-CXO-Lip exhibited photoprotective properties against skin histochemical damage and oxidative stress in UVB-exposed mice.
In vivo SOD, GSH-Px, CAT, HYP, MDA, and 4-HNE content assays. (A) SOD assay. (B) GSH-Px concentration. (C) CAT concentration. (D) HYP assay. (E) 4-HNE content assay. (F) MDA assay. *p < 0.05, **p < 0.01, ***p < 0.001, ****p < 0.0001 indicates comparison with the control group; #p < 0.05, ##p < 0.01, ###p < 0.001, ####p < 0.0001 indicates comparison with the UVB group; ^p < 0.05, ^^^p < 0.01, ^^^p < 0.001, ^^^^^ ^p < 0.0001 indicates comparison with the CXO group.
HA-CXO-Lip inhibits the activation of the AKT/ mTOR signaling pathway
The substantial accumulation of ROS in the UVB model exacerbates oxidative stress and activates the AKT/mTOR signaling pathway, leading to increased expression of MMP9, which in turn amplifies oxidative stress and collagen degradation. Tissue immunofluorescence analysis (Fig. 13A) demonstrated that the AKT/mTOR signaling pathway was activated following UVB irradiation7,23,24,25,26. Notably, the expression of phosphorylated AKT (p-AKT) (Fig. 13B) was significantly upregulated in mouse skin tissues, alongside increased levels of phosphorylated mTOR (p-mTOR) (Fig. 13C) and MMP9 (Fig. 13D). This is also consistent with previous reports that UVB exposure activates the AKT/mTOR signaling pathway, which in turn regulates MMP9 protein expression and accelerates the process of skin damage27,28,29. In comparison to the UVB group, treatments with CXO, CXO-Lip, HA-CXO-Lip, and Ligustilide significantly reduced the expression levels of p-AKT/AKT, p-mTOR/mTOR, and MMP9 proteins. Furthermore, the fluorescence intensity of these three proteins in the CXO-Lip group was lower than that in the CXO group, indicating that only liposome-encapsulated CXO could enhance the utilization efficiency of CXO (p < 0.001). Additionally, HA-CXO-Lip exhibited a more pronounced inhibitory effect on protein expression compared to CXO-Lip, suggesting that HA may contribute to anti-photoaging effects when combined with CXO.
Immunofluorescence detection of oxidative stress-related protein expression. (A) Fluorescence images of AKT (red), mTOR (yellow) and MMP9 (green) expression. (B) Relative fluorescence intensity of AKT. (C) Relative fluorescence intensity of mTOR. (D) MMP9 relative fluorescence intensity. *p < 0.05, **p < 0.01, ***p < 0.001, ****p < 0.0001 indicates comparison with the control group; #p < 0.05, ##p < 0.01, ###p < 0.001, ####p < 0.0001 indicates comparison with the UVB group; ^p < 0.05, ^^p < 0.01, ^^^p < 0.001, ^^^^^ ^p < 0.0001 indicates comparison with the CXO group.
Similarly, the results of protein blotting analysis further confirmed that UVB irradiation can enhance the phosphorylation levels of AKT and mTOR proteins, as well as increase the expression of MMP9. After treating the mice with different drug groups, the HA-CXO-Lip group demonstrated the greatest ability to inhibit the elevation of inhibitory protein phosphorylation levels, attenuate signaling cascades, and reduce the generation of photodamage (Fig. 14A–D).
Western blotting to verify the expression of AKT, mTOR, and MMP9 in mice skin tissue homogenate of each group. The samples derive from the same experiment and that gels/blots were processed in parallel. *p < 0.05, **p < 0.01, ***p < 0.001, ****p < 0.0001 indicates comparison with the control group; #p < 0.05, ##p < 0.01, ###p < 0.001, ####p < 0.0001 indicates comparison with the UVB group; ^p < 0.05, ^^p < 0.01, ^^^p < 0.001, ^^^^^ ^p < 0.0001 indicates comparison with the CXO group.
HA-CXO-Lip inhibits Collagen 1 degradation
HA regulates the structural properties of collagen, the most abundant extracellular matrix (ECM) protein, which contributes to the fibrous microstructure within the ECM30,31. Figure 15A illustrates the expression of Collagen 1 in the skin, as indicated by green fluorescence; more intense fluorescence corresponds to higher Collagen 1 content. It demonstrates that Collagen 1 expression in the control group is elevated and well-structured. In contrast, the UVB group exhibits thickened epidermis due to crusting and a notable degradation of Collagen 1 in the dermis. Figure 15B indicates that the collagen content in UVB-exposed mice is significantly reduced compared to the control group (p < 0.0001). Following intervention with CXO and CXO-Lip, the collagen content in UVB-irradiated mice increased; though not statistically significant. Notably, the HA-CXO-Lip group exhibited significantly enhanced therapeutic efficacy compared to both the UVB group (p < 0.0001) and the CXO group (p < 0.0001), indicating that HA modulates extracellular matrix (ECM) functionality to promote collagen formation.
Effect of UVB irradiation on collagen 1 in mice skin. *p < 0.05, **p < 0.01, ***p < 0.001, ****p < 0.0001 indicates comparison with the control group; #p < 0.05, ##p < 0.01, ###p < 0.001, ####p < 0.0001 indicates comparison with the UVB group; ^p < 0.05, ^^p < 0.01, ^^^p < 0.001, ^^^^^ ^p < 0.0001 indicates comparison with the CXO group.
HA-CXO-Lip reduces skin tissue apoptosis and aging
To further investigate the protective effects of drugs against apoptosis, this study evaluated the apoptosis of mouse skin cells in each group using TUNEL staining, (n = 6). The results are presented in Figure X. Compared to the normal group, the number of positive (green fluorescent) cells in the skin of UVB-exposed mice was significantly increased (Fig. 16). Conversely, the number of positive cells in the skin of mice treated with CXO, CXO-Lip, HA-CXO-Lip, and Ligustilide was reduced to varying degrees compared to the UVB group. Notably, the number of apoptotic cells in the skin of mice in the HA-CXO-Lip group exhibited levels of apoptosis similar to those of the control group.
Subsequently, we employed histochemistry to evaluate the impact of HA-CXO-Lip on the aging of mice skin tissues, with a specific focus on apoptosis marker CD44 and C-caspase-3. Figure 17 demonstrates a significant increase in the expression of DNA damage marker C-caspase-3 in the dorsal skin of mice subjected to prolonged UVB exposure compared to the control group. Furthermore, Administration of CXO, CXO-Lip, HA-CXO-Lip, and Ligustilide markedly decreased the cleaved caspase-3 (C-caspase-3)-positive area in murine dorsal skin compared to UVB-exposed controls. Mice treated with HA-CXO-Lip and Ligustilide exhibited varying degrees of reduction in C-caspase-3 expression. The N-terminal end of the CD44 peptide chain binds to hyaluronic acid, which is recognized as a receptor for this substance. An increase in CD44 levels is associated with a decrease in autophagy, contributing to the aging process. Our findings (Fig. 18) indicate that CXO-energy reduced CD44 expression, while skin adhesion in the UVB group mice was diminished, leading to an increase in CD44 expression. The positive area of CD44 in the CXO-Lip, HA-CXO-Lip, and Ligustilide groups was less than that in the CXO group, resulting in the following order of expression: UVB > CXO > CXO-Lip > Ligustilide > HA-CXO-Lip. This suggests that CXO has a protective effect against photoaging, and that the combined application of HA and CXO may provide a synergistic effect, thereby confirming our hypothesis.
Discussion
Herein, we successfully developed a novel composite nanosystem (HA-CXO-Lip) for the treatment of skin photoaging. The results demonstrated that HA-CXO-Lip exhibited excellent sustained-release properties and enhanced cutaneous retention, which may be attributed to the incorporation of HA reducing the surface hydrophobicity of the liposomes and thereby inhibiting CXO permeation through the phospholipid bilayer32. Stability studies revealed no significant changes in EE or DL after 30 days of storage at 4 °C. However, following a 90-day long-term stability assessment, HA-CXO-Lip displayed an 18.63% reduction in EE, a 0.33 mg/mL decrease in DL, and a 77 nm increase in average particle size. These findings highlight the necessity for further optimization of the formulation process to improve stability, including incorporating distearoylphosphatidylcholine (DSPC) to enhance membrane rigidity, adding antioxidants such as vitamin E to mitigate degradation, and developing lyophilized powder formulations to prolong shelf life33.
It is well-established that UVB radiation is a primary contributor to skin photoaging. By inducing oxidative stress in cutaneous cells through both direct and indirect mechanisms, UVB exposure triggers cellular membrane damage, lipid peroxidation, and apoptosis. These cumulative pathological effects ultimately manifest as hallmark signs of skin aging, including wrinkle formation, reduced elasticity, and tissue laxity34,35,36. This study revealed that UVB-exposed mice developed severe skin scabbing and desquamation. Histopathological analysis via H&E staining, combined with measurements of epidermal thickness and skin hydration levels, further confirmed the detrimental effects of chronic UVB exposure on cutaneous health, including hyperkeratosis and significantly reduced hydration. However, HA-CXO-Lip-treated mice exhibited markedly alleviated photoaging symptoms. In addition, the HA-CXO-Lip group showed a superior alleviating effect compared to the CXO-Lip group, which further confirmed that HA exerts an important synergistic effect in the HA-CXO-Lip to improve the skin’s resistance to UVB radiation. However, the distribution of HA-CXO-Lip in skin structure needs to be further studied and clarified. Previous studies have reported that UVB radiation induces intracellular redox imbalance through both direct and indirect pathways. The resulting accumulation of reactive oxygen species (ROS) disrupts cellular membranes, leading to functional dysregulation37,38,39. Under physiological conditions, the endogenous antioxidant system—comprising superoxide dismutase (SOD), glutathione peroxidase (GPx), catalase (CAT), and hydroxyproline (HYP)—maintains redox equilibrium by neutralizing ROS. However, sustained or high-intensity UVB exposure compromises this defense mechanism, exacerbating oxidative damage and ultimately triggering skin cell apoptosis and senescence40. Our results showed that HA-CXO-Lip could significantly inhibit the accumulation of ROS caused by UVB exposure and restore the activity of antioxidant enzymes. At the same time, it inhibits the accumulation of lipid metabolites such as MDA and 4-HNE, further reducing UVB-induced cell damage. In addition, we found that UVB increased the expression of lysosomal senescence SA-β-gal enzyme in HaCaT cells, which is also the most important marker of senescence progression41. These results suggest that HA-CXO-Lip can inhibit the excessive oxidative stress caused by UVB exposure and exert a protective effect on skin photoaging.
Additionally, HA-CXO-Lip effectively regulated UVB-induced cell apoptosis. Our study demonstrated that under UVB radiation-induced oxidative stress, members of the Bcl-2 family play a critical role. HA-CXO-Lip reduced apoptosis by suppressing Bax protein expression and promoting Bcl-2 expression. Furthermore, immunofluorescence and Western blot (WB) experimental results revealed that the therapeutic mechanism of HA-CXO-Lip against photoaging is closely associated with the AKT/mTOR signaling pathway. AKT, a key signaling molecule for cell survival, typically activates downstream targets such as Caspase-3 through the activation of the AKT/mTOR pathway, thereby initiating the apoptosis process42,43. Furthermore, HA-CXO-Lip effectively mitigated UVB-induced cell apoptosis. Our study demonstrated that members of the Bcl-2 family play a pivotal role in oxidative stress triggered by UVB radiation. HA-CXO-Lip reduced apoptotic activity by suppressing the expression of the pro-apoptotic protein Bax while upregulating the anti-apoptotic protein Bcl-2. Additionally, immunofluorescence staining and Western blot (WB) analyses revealed that the anti-photoaging mechanism of HA-CXO-Lip is closely associated with the AKT/mTOR signaling pathway. As a key regulator of cell survival, activation of the AKT/mTOR pathway typically initiates apoptosis through downstream targets such as Caspase-3. Intriguingly, HA-CXO-Lip attenuated UVB-driven apoptosis by modulating AKT/mTOR phosphorylation dynamics, suggesting its dual role in balancing survival signaling and stress-responsive apoptosis29. It is worth noting that studies have reported CD44, as the primary receptor for HA, plays a significant role in UVB radiation-induced skin aging. As an HA receptor, CD44 can influence the process of skin aging by regulating cellular autophagy and modulating senescence-associated signaling pathways (such as the mTOR pathway). Our study has also demonstrated that HA-CXO-Lip administration significantly reduces CD44 expression in mice. This mechanism may represent a critical component of HA-CXO-Lip’s protective effects against photoaging44,45. In subsequent research, we will focus on exploring the mechanism by which HA-CXO-Lip regulates cellular apoptosis.
Although there are no studies reporting the anti-photoaging properties of ligustilide, existing research indicates that ligustilide alleviates oxidative stress and cellular apoptosis to improve pulmonary injury and renal ischemia–reperfusion injury in rats 46,47. Therefore, to investigate the significance of ligustilide’s pharmacological efficacy, we utilized ligustilide with a purity ≥ 95% for exploring its potential in photoaging treatment. To our knowledge, our study is the first to demonstrate both the anti-photoaging effects of ligustilide and its underlying mechanisms. Results from in vivo and in vitro experiments revealed that ligustilide exhibits antioxidant and anti-apoptotic properties similar to HA-CXO-Lip, albeit slightly less potent than HA-CXO-Lip. This finding suggests that ligustilide may serve as a key functional component of CXO in exerting its therapeutic effects.
To more accurately mimic normal human skin, ICR mice with HaCaT cell model were chosen for this experiment. The results of the in vitro and in vivo experiments showed that HA-CXO- Lip significantly inhibited the progression of UVB-induced photoaging. This is mainly reflected in the fact that HA-CXO- Lip improved cellular repair ability, reduced the body’s ROS level and lipid metabolite level, restored the antioxidant enzyme system, restored the collagen fiber order as well as decreased the expression level of pro-apoptotic proteins, increased the expression level of anti-apoptotic proteins and reduced the aging marker products. These results demonstrated that our prepared HA-CXO- Lip has significant protective effects against UVB-induced photoaging and can be used as a potential material component for anti-photoaging product development. It should be noted that UVB radiation first affects the epidermis of the skin. Therefore, transdermal drug delivery is the best way to prevent or mitigate UVB damage compared with other delivery methods such as oral drug delivery, which can provide higher drug concentration locally, so that the drug directly reaches the target site and enters the circulatory system to play a role through the skin absorption, which greatly improves patient compliance and avoids the first-pass effect48.
In summary, this study provides a new theoretical basis for the first joint application of CXO and HA in skin photoaging. We demonstrated that the HA-CXO-Lip composite system not only enhances the stability and bioavailability of CXO, but also significantly slows down UVB-induced skin aging through multiple mechanisms such as antioxidant and anti-apoptosis. However, due to the physiological differences between mouse and human skin, more human trials are needed to verify its clinical effects. Therefore, how to further optimize the drug-carrying efficiency of liposomes and improve their targeting and persistence in the deeper layers of the skin may be an important direction for future research.
Data availability
All data are provided in the text and can be obtained from the corresponding authors upon reasonable request.
References
Liu, K. et al. Pilose antler protein relieves UVB-induced HaCaT cells and skin damage. Molecules (Basel, Switzerland) 29, 4060 (2024).
Kim, S. H. et al. Protective effects of an electrophilic metabolite of docosahexaenoic acid on UVB-induced oxidative cell death, dermatitis, and carcinogenesis. Redox Biol. 62, 102666 (2023).
Xiang, F., Lucas, R., Hales, S. & Neale, R. Incidence of nonmelanoma skin cancer in relation to ambient UV radiation in white populations, 1978–2012: Empirical relationships. JAMA Dermatol. 150, 1063–1071 (2014).
Park, J. et al. Enzyme-treated caviar prevents UVB irradiation-induced skin photoaging. Mar. Drugs 20, 685 (2022).
Qin, Z., Balimunkwe, R. M. & Quan, T. Age-related reduction of dermal fibroblast size upregulates multiple matrix metalloproteinases as observed in aged human skin in vivo. Br. J. Dermatol. 177, 1337–1348 (2017).
Wang, B. J. et al. Zinc oxide nanoparticles exacerbate skin epithelial cell damage by upregulating pro-inflammatory cytokines and exosome secretion in M1 macrophages following UVB irradiation-induced skin injury. Part. Fibre Toxicol. 21, 9 (2024).
Kotb, E. A. et al. Protective potential of frankincense essential oil and its loaded solid lipid nanoparticles against UVB-induced photodamage in rats via MAPK and PI3K/AKT signaling pathways; A promising anti-aging therapy. PLoS ONE 18, e0294067 (2023).
Zhou, Y., He, L., Zhang, N., Ma, L. & Yao, L. Photoprotective effect of artemisia sieversiana ehrhart essential oil against UVB-induced photoaging in mice. Photochem. Photobiol. 98, 958–968 (2022).
Chen, X. et al. LcSAO1, an unconventional DOXB Clade 2OGD Enzyme from ligusticum chuanxiong catalyzes the biosynthesis of plant-derived natural medicine butylphthalide. Int. J. Mol. Sci. 24, 17417 (2023).
Zuo, J. et al. Essential oil from Ligusticum chuanxiong Hort. Alleviates lipopolysaccharide-induced neuroinflammation: Integrating network pharmacology and molecular mechanism evaluation. J. Ethnopharmacol. 319, 117337 (2024).
Hu, Y. et al. Sedative-hypnotic effect and mechanism of carbon nanofiber loaded with essential oils of Ligusticum chuanxiong (Ligusticum chuanxiong Hort.) and finger citron (Citrus medica L. var. sarcodactylis) on mice models of Insomnia. Biomolecules 14, 1102 (2024).
Wu, J. G. et al. Essential oil from rhizomes of Ligusticum chuanxiong induces apoptosis in hypertrophic scar fibroblasts. Pharm. Biol. 49, 86–93 (2011).
Juan, S. H. et al. Tetramethylpyrazine protects rat renal tubular cell apoptosis induced by gentamicin. Nephrol. Dial. Transplant.: Off. Publ. Eur. Dial. Transplant Assoc.—Eur. Renal Assoc. 22, 732–739 (2007).
Wekwejt, M. et al. Hyaluronic acid/tannic acid films for wound healing application. Int. J. Biol. Macromol. 254, 128101 (2024).
Ni, C. et al. Hyaluronic acid and HA-modified cationic liposomes for promoting skin penetration and retention. J. Control. Rel.: Off. J. Controll. Rel. Soc. 357, 432–443 (2023).
Scarano, A. et al. Clinical evaluation of efficacy and tolerance of a skin reconditioning compound for anti-aging. J. Biol. Regul. Homeost. Agents 35, 217–226 (2021).
Wang, S. et al. Clinical efficacy and safety of non-cross-linked hyaluronic acid combined with L-carnosine for Horizontal neck wrinkles treatment. Aesth. Plast. Surg. 45, 2912–2917 (2021).
He, Z. et al. Injectable and tissue adhesive EGCG-laden hyaluronic acid hydrogel depot for treating oxidative stress and inflammation. Carbohyd. Polym. 299, 120180 (2023).
Chmielewski, R. & Lesiak, A. Mitigating glycation and oxidative stress in aesthetic medicine: Hyaluronic acid and trehalose synergy for Anti-AGEs Action in skin aging treatment. Clin. Cosmet. Investig. Dermatol. 17, 2701–2712 (2024).
Ren, C. et al. Palmitoylethanolamide-incorporated elastic nano-liposomes for enhanced transdermal delivery and anti-inflammation. Pharmaceutics 16, 876 (2024).
Wang, Y. et al. Anti-photoaging effects of flexible nanoliposomes encapsulated Moringa oleifera Lam. isothiocyanate in UVB-induced cell damage in HaCaT cells. Drug Del. 29, 871–881 (2022).
Zhang, C. et al. Skin delivery of hyaluronic acid by the combined use of sponge spicules and flexible liposomes. Biomater. Sci. 7, 1299–1310 (2019).
Xiao, Z. et al. A peptide YGDEY from tilapia gelatin hydrolysates inhibits UVB-mediated skin photoaging by regulating MMP-1 and MMP-9 EXPRESSION in HaCaT cells. Photochem. Photobiol. 95, 1424–1432 (2019).
Rashid, Z. A. & Bardaweel, S. K. Novel matrix metalloproteinase-9 (MMP-9) inhibitors in cancer treatment. Int. J. Mol. Sci. 24, 12133 (2023).
Iranpanah, A. et al. The exosome-mediated PI3K/Akt/mTOR signaling pathway in neurological diseases. Pharmaceutics 15, 1006 (2023).
Glaviano, A. et al. PI3K/AKT/mTOR signaling transduction pathway and targeted therapies in cancer. Mol. Cancer 22, 138 (2023).
Wu, H. T. et al. Luteolin suppresses androgen receptor-positive triple-negative breast cancer cell proliferation and metastasis by epigenetic regulation of MMP9 expression via the AKT/mTOR signaling pathway. Phytomed.: Int. J. Phytother. Phytopharmacol. 81, 153437 (2021).
Fang, J. et al. Enhancement of human epidermal cell defense against UVB damage by fermentation of passiflora edulis sims peel with saccharomyces cerevisiae. Nutrients 15, 501 (2023).
Tu, Y. et al. DNA-dependent protein kinase catalytic subunit (DNA-PKcs)-SIN1 association mediates ultraviolet B (UVB)-induced Akt Ser-473 phosphorylation and skin cell survival. Mol. Cancer 12, 172 (2013).
Wu, H. et al. Hyaluronic acid-mediated collagen intrafibrillar mineralization and enhancement of dentin remineralization. Carbohyd. Polym. 319, 121174 (2023).
Hakim, M. H. et al. Investigation of macromolecular transport through tunable collagen hyaluronic acid matrices. Colloids Surfaces B, Biointerfaces 222, 113123 (2023).
Zhou, J. et al. Hyaluronic acid-based dual network hydrogel with sustained release of platelet-rich plasma as a diabetic wound dressing. Carbohyd. Polym. 314, 120924 (2023).
Yu, J. Y., Chuesiang, P., Shin, G. H. & Park, H. J. Post-processing techniques for the improvement of liposome stability. Pharmaceutics 13, 1023 (2021).
Sun, S. et al. Anti-photoaging effect and the mechanism of Coreopsis tinctoria okanin against UVB-induced skin damage in mice. Int. Immunopharmacol. 139, 112657 (2024).
Wacewicz-Muczyńska, M. et al. Antioxidant properties of maqui berry extract (Aristotelia chilensis (Mol) Stuntz) and its potential photoprotective role on human skin fibroblasts. Molecules (Basel, Switzerland) 28, 7802 (2023).
Krutmann, J., Schalka, S., Watson, R. E. B., Wei, L. & Morita, A. Daily photoprotection to prevent photoaging. Photodermatol. Photoimmunol. Photomed. 37, 482–489 (2021).
Yoon, J. H. et al. Tangeretin reduces ultraviolet B (UVB)-induced cyclooxygenase-2 expression in mouse epidermal cells by blocking mitogen-activated protein kinase (MAPK) activation and reactive oxygen species (ROS) generation. J. Agric. Food Chem. 59, 222–228 (2011).
Itoh, T., Fujita, S., Koketsu, M. & Hashizume, T. Citrulluside H and citrulluside T from young watermelon fruit attenuate ultraviolet B radiation-induced matrix metalloproteinase expression through the scavenging of generated reactive oxygen species in human dermal fibroblasts. Photodermatol. Photoimmunol. Photomed. 37, 386–394 (2021).
Chang, W. S. et al. Baicalin scavenged reactive oxygen species and protected human keratinocytes against UVB-induced cytotoxicity. In vivo (Athens, Greece) 30, 605–610 (2016).
Lu, W., Kong, C., Cheng, S., Xu, X. & Zhang, J. Succinoglycan riclin relieves UVB-induced skin injury with anti-oxidant and anti-inflammatory properties. Int. J. Biol. Macromol. 235, 123717 (2023).
Dimri, G. P. et al. A biomarker that identifies senescent human cells in culture and in aging skin in vivo. Proc. Natl. Acad. Sci. U.S.A. 92, 9363–9367 (1995).
Sahoo, G., Samal, D., Khandayataray, P. & Murthy, M. K. A review on caspases: Key regulators of biological activities and apoptosis. Mol. Neurobiol. 60, 5805–5837 (2023).
Huang, R. et al. Protective effect of quercetin on cadmium-induced renal apoptosis through cyt-c/caspase-9/caspase-3 signaling pathway. Front. Pharmacol. 13, 990993 (2022).
Yao, Z., Wu, J. & Fang, Y. Moderate constraint facilitates association and force-dependent dissociation of HA-CD44 complex. Int. J. Mol. Sci. 24, 2243 (2023).
Ouyang, Q., Zhao, Y., Xu, K., He, Y. & Qin, M. Hyaluronic acid receptor-mediated nanomedicines and targeted therapy. Small Methods 8, e2400513 (2024).
Luo, S., Gong, J., Cao, X. & Liu, S. Ligustilide modulates oxidative stress, apoptosis, and immunity to avoid pathological damages in bleomycin induced pulmonary fibrosis rats via inactivating TLR4/MyD88/NF-KB P65. Ann. Transl. Med. 8, 931 (2020).
Xia, K. et al. Ligustilide alleviates oxidative stress during renal ischemia-reperfusion injury through maintaining Sirt3-dependent mitochondrial homeostasis. Phytomed.: Int. J. Phytother. Phytopharmacol. 134, 155975 (2024).
Fan, S., Lopez Llorens, L., Perona Martinez, F. P. & Schirhagl, R. Quantum sensing of free radical generation in mitochondria of human keratinocytes during UVB exposure. ACS Sens. 9, 2440–2446 (2024).
Funding
Details of all funding sources should be provided, including grant numbers if applicable. Please ensure to add all necessary funding information, as after publication this is no longer possible. This study was supported by the Talent Program of the State Administration of Traditional Chinese Medicine (No. T20194828003), the Innovative Research Group Program of Sichuan Provincial Natural Science Foundation (No. 2023NSFSC1997), and the Scientific Research Enhancement Program for the "Apricot Grove Scholars" of Chengdu University of Traditional Chinese Medicine (QJJJ2022014).
Author information
Authors and Affiliations
Contributions
All authors read and approved the final manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
He, X., Hu, Y., Wu, Y. et al. Hyaluronic acid modified chuanxiong oil liposomes as a novel therapeutic agent for photoaging prevention. Sci Rep 15, 12237 (2025). https://doi.org/10.1038/s41598-025-97450-0
Received:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s41598-025-97450-0