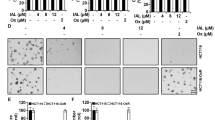
Abstract
Oxaliplatin (OXA) is widely used for colorectal cancer (CRC) as a first-line chemotherapy. However, drug resistance and peripheral neurotoxicity prevail in colorectal cancer therapy. Salinomycin (SAL) makes cancer cells sensitive to ionizing radiation and chemotherapeutic drugs. Chemotherapy regimens that combine more than two drugs can improve the outcome of patients. In the present study, we detected apoptosis and mitochondrial function in CRC cells through MTT assays, Annexin V-FITC/PI staining, colony-forming assays, intracellular reactive oxygen species (ROS) measurements, western blotting and so on. We used CompuSyn software to calculate combination index (CI). The effect of SAL and OXA was synergistic. The combination treatment inhibited cell proliferation, migration and colony formation but increased the expression of proapoptotic proteins and promoted cell apoptosis of CRC cells. In vitro experiments demonstrated that the SAL and OXA cotreatment increased intracellular ROS levels in CRC cell lines, decreased the MMP and activated the mitogen-activated protein kinase (MAPK) pathway, thus inhibiting the proliferation of CRC cells and promoting the apoptosis of CRC cells. Pretreatment with N-acetylcysteine (NAC) reversed this effect. Cotreatment with SAL and OXA increases the apoptotic effects in OXA-treated CRC cell lines. In vivo, combined treatment of SAL and OXA markedly inhibited the tumor growth compared to either drug alone. SAL enhances OXA-induced antitumor effects in CRC both in vitro and in vivo by ROS-mediated mitochondrial apoptosis and activation of the MAPK pathway. These results may provide a rationale for combining SAL with OXA for CRC treatment.
Similar content being viewed by others
Introduction
Colorectal cancer (CRC) is the third most frequent cancer (1.9 million new cases) and the second leading cause of cancer death (935,000 deaths) worldwide in 2020, representing approximately one in 10 cancer diagnosed (10.0%) and deaths (9.4%)1. It is anticipated almost 2.2 million new diagnosed and 1.1 million deaths in 20302. Although early diagnosis and treatment have improved, about 20% of patients have metastasis at the time of diagnosis, up to 50% of nonmetastatic colorectal cancers have metastases at a later stage and approximately one-third of patients relapse after treatment3.
Patients with advanced CRC should undergo a comprehensive treatment plan with surgery as the mainstay and chemotherapy as the supplement. FOLFOX and the CAPOX or XELOX regimen are listed as the same preferred chemotherapy regimen, both chemotherapy regimens contain oxaliplatin (OXA)4. OXA is a first-line anti-colorectal cancer chemotherapy drug and has a high application advantage in the treatment of metastatic CRC. Due to the application of oxaliplatin, the overall treatment of CRC has been significantly improved, increasing the disease-free survival rate of CRC by 20%5. However, nearly half of the patients have a reduced survival rate due to chemotherapy resistance6. Due to the development of chemotherapy resistance and acute or persistent nerve damage, the overall success of oxaliplatin-based treatment options is limited. Therefore, new drug treatments are needed to improve the chemosensitivity of OXA and other original chemotherapeutic drugs to sensitize cancer cells and to obtain higher curative effects while minimizing adverse side effects and improving the treatment of CRC. The new treatments must be efficient, extend the disease-free survival of patients and improve the prognosis7.
Salinomycin (SAL) is a highly efficient, broad-spectrum, low-resistance, rapid excretion and low-residue monocarboxylic acid polyether antibiotic isolated from a strain of Streptomyces albicans. SAL sensitizes the resistance of tumor cells to chemotherapy drugs and significantly promotes the apoptosis of drug-resistant cancer cells8,9,10. SAL has little effect on normal cells but kills drug- resistant cancer cells and tumor stem cells, and it reduces the invasion and of cancer cells11. SAL has been identified as a powerful chemosensitizer, and it induces apoptosis of multidrug-resistant tumor cells. SAL has been demonstrated to target and kill a variety of cancer stem cells12,13.
In the present study, a model of SAL combined with OXA chemotherapy was designed. The combined effect of SAL and OXA on the survival rate of CRC cells was compared to the use of SAL and OXA alone. In vitro experiments were used to study the effects of SAL and OXA on cell growth, cell morphology, cell cycle, cell apoptosis and cell migration ability as well as to explore its related mechanism. In vivo experiments on subcutaneous xenograft tumors in nude mice were performed for further verification.
Results
SAL increased the growth inhibition of OXA in human CRC cells
CRC cells were treated with different doses of SAL and OXA for 24 h, 48 h and 72 h (Fig. 1A, B). SAL or OXA increased the inhibition of CRC cells in a dose- and time-dependent manner.
Evaluation of CRC cell growth inhibition induced by salinomycin and/or oxaliplatin by MTT assay. (A and B) CRC cells were treated with different concentrations of salinomycin and oxaliplatin for 24 h, 48 h and 72 h. (C, D and G) A fraction affected (Fa)-CI plot and isobologram analysis were used to determine whether there were interactions between the two drugs when combined. The CI values for salinomycin and oxaliplatin were calculated according to Chou-Talalay’s method by CompuSyn software at the 24 h time point and plotted with the percent of cell growth inhibition as the fraction affected (Fa) cells. Each symbol was assigned a CI value for each Fa at 15 different combination ratios with CI values < 1 indicating drug synergism, CI values = 1 indicating an additive effect and CI values > 1 indicating antagonism. (E, F and G) Normalized isobologram for the combination of salinomycin and oxaliplatin. A combination data point below the line indicates a synergistic effect, and a combination data point above the line indicates an antagonistic effect. In addition, a combination data point on the line indicates an additive effect. (H) Cells were treated with 2.5 μM salinomycin, 20 μM oxaliplatin or a combination of 2.5 μM salinomycin and 20 μM oxaliplatin for 24 h. (***P < 0.001 versus control group, &&& P < 0.001 versus salinomycin group and $$$ P < 0.001 versus oxaliplatin group).
As shown in Fig. 1G, there were 15 different combination ratios which were selected based on the cytotoxic effects of the drugs. Fa-CI plots which were generated by CompuSyn software indicated that SAL and OXA synergistically inhibited the viability of CRC cells (Fig. 1C, D). Figure 1E and F show the drug synergism as represented by an isobologram. The dose points of drug combinations in CRC Caco-2 and SW480 cells inside the isobologram indicate drug synergism. We selected 2.5 µM SAL and 20 µM OXA for the following experiments. Combined treatment of SAL and OXA for 24 h showed greater inhibitory effects on cell viability than SAL and OXA alone (Fig. 1H). Combined treatment of SAL and OXA for 24 h and 48 h inhibited cancerous cells (Caco-2 and SW480) proliferation but to a lesser extent in noncancerous cells (NCM460 and BV-2), which showed in Fig. S1.
Next, the role of SAL combined with OXA was assessed using the colony-forming assay. The colony formation ability of the combined group was significantly reduced compared with the other three groups (Fig. 2A, B). These results indicated that SAL combined with OXA may inhibit the proliferation of CRC cells.
Salinomycin and oxaliplatin combination treatment efficiently inhibits proliferation and induces apoptosis in CRC cells. (A) After treatment of Caco-2 and SW480 cells with DMSO, salinomycin, oxaliplatin and their combination for 48 h, cells were cultured for 2 weeks and analyzed by colony formation assay. A representative image of colony formation from three independent experiments is presented. (B) The number of colonies of various groups presented in the graphs in A were quantified. (C) CRC cells were treated for 48 h with DMSO and drugs as previously described. Detection of the apoptosis rate of CRC cells via Annexin V-FITC/PI double staining. (D) Quantitative analysis of apoptotic cells in C. (E-H) CRC cells were exposed to medium containing salinomycin and oxaliplatin for 48 h, and cell cycle arrest was detected by flow cytometry. The cell cycle arrest of various groups was quantified. (* P < 0.05, ** P < 0.01 and *** P < 0.001 versus control group; &&& P < 0.001 versus salinomycin group and $$$ P < 0.001 versus oxaliplatin group).
SAL combined with OXA induced apoptosis and cell phase arrest in CRC cells
The apoptosis was quantified by Annexin V-FITC/PI staining (Fig. 2C, D). The cotreatment group contained more apoptotic cells than the control group, SAL group and OXA group.
We determined SAL- and OXA-mediated cell phase arrest by PI staining using a flow cytometric approach (Fig. 2E-H). The number of cells in the G0/G1 phase of the combination treatment group increased significantly, while the number of cells in the G2/M phase and S phase decreased compared to the control group. These data demonstrated that SAL combined with OXA induced G0/G1 phase arrest.
Morphological observation by light microscopy
As shown in Fig. 3A, B, CRC cells after treatment were observed by a bright field phase contrast microscope. The morphology of Caco-2 and SW480 cells in the SAL and OXA combination treatment group indicating that they cells were unhealthy compared to the untreated or drug-treated groups. The combination treatment group showed the following characteristics: decreased cell number; decreased volume; deformed cells; disappearance of the connection between cells; and separation of cells from surrounding cells. As the time of drug treatment increased, these changes became more noticeable.
Salinomycin and oxaliplatin combination treatment decreases wound- healing rates and cell migration. (A and B) Caco-2 and SW480 cells were treated for 48 h with DMSO and drugs as previously described. Morphological examination of characteristic features of CRC cell death (× 100). (C) SW480 cells were treated under different conditions as previously described for 48 h, and their cell migratory ability was assessed by a wound-healing assay (magnification, × 40). (D) The wound-healing rates of various groups presented in the graphs in C were quantified. The statistical significance of the results was analyzed by twoway ANOVA. (E) A total of 1 × 105 Caco-2 or SW480 cells in 200 µl of serumfree medium with DMSO treatment, monotherapy treatment or combined treatment for 48 h were seeded in the upper chamber, and cell migration was assessed by Transwell assay. Representative images are presented (magnification, × 200). (F) The number of cells per field of various groups in E was quantified. (** P < 0.01 and *** P < 0.001 versus control group; & P < 0.05 and &&& P < 0.001 versus salinomycin group; and $ P < 0.05 and $$$ P < 0.001 versus oxaliplatin group).
SAL combined with OXA inhibited the motility of CRC cells
To assess the motility and migration capacity, wound-healing and Transwell assays were used to detect and analyze the changes in the Caco-2 and SW480 cells. The wound-healing assay showed the migratory ability of CRC cells was significant reduced in the cotreatment group compared to the other groups (Fig. 3C, D). As shown in Fig. 3E, F, the number of Caco-2 and SW480 cells migrating into the lower chamber was the lowest among the groups. These results demonstrated that the metastasis of CRC cells is significantly inhibited when cells are treated with both SAL and OXA.
SAL combined with OXA induced CRC cell apoptosis via the mitochondria-related apoptosis pathway
It has been reported that SAL increases ROS generation in CRC cells. Therefore, we measured the levels of ROS and MMP after the combination treatment. Figure 4A-C showed that the combination treatment increased the intracellular ROS levels compared to the untreated cells as determined by fluorescence microscopy, in which ROS levels were reflected by the enhanced green fluorescence. We then pretreated CRC cells with the NAC (ROS inhibitor) for 2 h. Pretreatment with NAC significantly reduced the production of intracellular ROS in CRC cells induced by the combination of SAL and OXA (Fig. S2A, C & E). We also examined superoxide anion (one of the ROS) production with DHE probes to further emphasize the role of ROS. As shown in Fig. S2B, D. It was found that the detection results of DHE probe were similar to that of DCFH-DA probe, that is, the combination of SAL and OXA showed an enhanced production of superoxide anion, but the addition of NAC could effectively inhibit the production of superoxide anion.
Salinomycin combined with oxaliplatin induces apoptosis of CRC cells via the mitochondria-related apoptosis pathway. (A and B) Cells were treated with DMSO, SAL or/and co-treated with oxaliplatin for 24 h. ROS production was detected and imaged (×100). (C) Quantitative analysis of ROS in A and B. (D) Caco-2 cells were treated with DMSO, salinomycin, oxaliplatin or their combination for 24 h, and fluorescence microscopy was used to detect ΔΨm after staining cells with JC-1. Red fluorescence indicates normal MMP, and green fluorescence reflects MMP loss (×100). (E) Quantitative analysis of MMP in D. (F) CRC cells were treated with DMSO, SAL or/and co-treated with oxaliplatin for 48 h. The expression of proteins in the mitochondrial- related apoptosis pathway was measured. The immunoreactivity of these proteins was normalized to the expression of βactin. (* P < 0.05 versus control group; & P < 0.05 versus salinomycin group; and $ P < 0.05 versus oxaliplatin group).
To clarify whether SAL- and OXA-induced apoptosis is dependent on mitochondria, we used JC-1 dye to examine alterations in MMP (Fig. 4D). The green fluorescence which reflects MMP loss was more significant in the cotreatment group than in the other groups. Data are presented as the ratio of red: green fluorescence (Fig. 4E).
To clarify the underlying mechanisms of apoptosis induced by the SAL or/and OXA, mitochondrial apoptosis-related proteins were measured. Western blot analysis confirmed that the expression of cleaved PARP, Bax, cleaved caspase 3, cleaved caspase 9 and cytochrome C was significantly increased in the cotreatment group (Fig. 4F), however, the expression of Bcl-2 was decreased in the cotreatment group.
We then pretreated CRC cells with the NAC for 2 h. Pretreatment with NAC reversed apoptosis in SW480 and Caco-2 cells induced by the combination of SAL and OXA (Fig. 5A, B).
MAPK signaling is involved in cell death induced by salinomycin and oxaliplatin cotreatment. (A) CRC cells were divided into the control group (DMSO), SAL + OXA group and NAC + combination treatment group. After 48 h of treatment with the corresponding drugs, flow cytometry was used to detect cell apoptosis in each group. (B) Quantitative analysis of apoptotic cells in A. (C) Western blot analysis of the MAPK signaling pathway after CRC cells were subjected to the control group (DMSO), salinomycin treatment, oxaliplatin treatment, combination treatment and combination treatment plus NAC (pretreated) for 48 h. The levels of p-ERK, p-JNK and p-p38 expression were elevated in the combination treatment group. (D-F) The p-ERK, p-JNK and p-p38 expression was normalized by dividing phosphorylated protein expression by total expression. ERK, extracellular signal-regulated kinase; p-ERK, phosphorylated ERK; JNK, c-Jun N-terminal kinase; p-JNK, phosphorylated JNK; p38, p38 kinase. (* P < 0.05, ** P < 0.01 and *** P < 0.001 versus control group; && P < 0.01 versus salinomycin group; $ P < 0.05 and $$ P < 0.01 versus oxaliplatin group; # P < 0.05, ## P < 0.01 and ### P < 0.001 represents the significance between the combination treatment group and the combination treatment plus NAC group). The results are expressed as the mean ± SD (n = 3).
The SAL and OXA combination activated the MAPK pathway in CRC cells
The MAPK pathway plays an important role in regulating cell growth and death. When CRC cells were cotreated with SAL and OXA, p-ERK, p-JNK and p-p38 levels were effectively elevated. After pretreatment with NAC, the expression levels of p-ERK, p-p38 and p-JNK decreased to a great extent in the NAC + combination treatment group compared to the combination treatment group (Fig. 5C-F). These findings indicated that NAC treatment reversed the effect of the drug combination on MAPK.
Antitumor effects of SAL and OXA on CRC cells in vivo
The in vivo growth inhibitory effect of combined treatment of SAL or/and OXA was investigated. As shown in Fig. 6A, B, the tumors were harvested and progressively grew. At the end of the treatment, compared with the other three groups, the combined treatment group greatly inhibited tumor volume growth and showed significant tumor weights loss, (Fig. 6C, D). Subsequently, tumor tissues from the mice were processed for immunohistochemistry (IHC) and H&E (not shown) staining. The combined treatment of SAL and OXA group showed increased expression of p-ERK and decreased expression of Ki-67 compared with the other three groups (Fig. 6E). H&E staining results showed that the liver, kidney, heart, lung and spleen in each treatment group were indistinguishable from normal mice (Fig. S3). The results showed that its application was safe.
Antitumor effects of the combination of salinomycin and oxaliplatin in vivo and schematic representation of the mechanism of anticancer action of salinomycin and oxaliplatin cotreatment against CRC cells. (A) Xenograft tumors in each group were harvested and extracted after the mice were sacrificed. (B) Each time point represents the mean tumor volume for each group. (C) Tumor volume was calculated at the end of the experiment. (D) Tumor weight was obtained at the end of the experiment. (E) Immunohistochemical detection of Ki-67 and p-ERK expression in tumor tissues. (*** P < 0.001 versus the control group; && P < 0.01 and &&& P < 0.001 versus the salinomycin group; and $$ P < 0.01 and $$$ P < 0.001 versus the oxaliplatin group).
Discussion
SAL enhances the sensitivity of CRC cells to a variety of chemotherapeutic drugs8,14,15,16,17 and can kill multidrug-resistant cells and tumor stem cells. Thus, SAL is a powerful chemosensitizer. In the present study, we combined SAL with OXA (the classic chemotherapeutic drug) and the results confirmed that SAL enhanced the antitumor effect of OXA in vivo and in vitro. The two drugs had a synergistic effect, which can increase the sensitization of cancer cells and reduce drug dosage and toxicity. Side effects are of great significance and provide a theoretical basis for their further clinical application.
Firstly, we analyzed the effect of each drug on CRC cells. Cytotoxicity studies indicated that SAL and OXA had anti-proliferative effects in a time- and dose-dependent manner. We used CompuSyn software to analyze the interaction between the drugs, which was represented by the CI value and an isobologram (DRI) diagram. The results showed that the two drugs were synergistic (CI value was less than 1). The DRI plot showed that the dose points of drug combinations in CRC cells inside the isobologram indicated drug synergism18. We found that combined treatment of SAL and OXA significantly inhibited the activity of CRC cells, and SW480 cells were more sensitive.
Next, we selected a low-dose combination of SAL and OXA for further experiments. We used a colony formation assay to measure the effects of cotreatment on the proliferation ability of CRC cells, and the colony formation assay results were similar with the MTT assay.
Apoptosis is closely related to proliferation. Flow cytometry was used to analyze apoptosis. The results showed that combined treatment of SAL and OXA induced apoptosis more significantly than either monotherapy or the control. Because cell cycle arrest can inhibit tumor growth, we next analyzed the cell cycle. The combination treatment caused a significant increase in the number of cells in the G1 phase and a decrease in the number of cells in the G2/M and S phases. The results indicated that the combined treatment induced G1 phase arrest in CRC cells.
Cell migration is one of the essential steps in the invasion and metastasis of malignant tumors. Approximately 90% of tumor patients die from metastasis-related complications19. We next used Transwell and scratch wound healing assays to detect the migration ability of the cells. Combined treatment of SAL and OXA resulted in significantly inhibiting the migration of the CRC cells.
Apoptosis can be triggered by a variety of intrinsic and extrinsic signals, including different stresses, such as ROS, viral infection, DNA-damaging agents (e.g., radiation), serum deprivation, heat shock and hypoxia20. Abnormally elevated ROS in cancer can activate antitumor signal transduction, leading to oxidative stress- induced cancer cell death15,20.
The apoptotic pathways discovered thus far are the death receptor apoptosis pathway, mitochondrial apoptosis pathway and endoplasmic reticulum pathway. Various apoptotic signals activate cysteine-containing aspartate-specific protease (caspase) through transmission. Caspases change cell characteristics by hydrolyzing different proteins, thereby inducing cell apoptosis. Caspases are the executors of cell apoptosis. The upstream/priming type of caspases (caspases 2, 8, 9, 10 and 12) are located upstream of the apoptosis signal transmission pathway. These caspases are first activated under the stimulation of apoptosis signals and then hydrolyzed, further activating downstream/executive type caspases (caspase 3, 6 and 7), in which caspase 3 plays the most important role. Caspase 3 is downstream of the apoptosis signal transmission pathway, and it is activated by upstream caspases and directly hydrolyzes the substrate proteins related to apoptosis, causing cell morphology changes, biochemical changes and DNA damage. Caspase substrates are cytoskeleton proteins and DNA damage repair-related proteins and enzymes that prevent the repair of DNA damage. Poly (ADP-ribose) polymerase (PARP) cleavage is an important indicator of apoptosis and caspase 3 activation21,22.
Bcl-2 protein mainly exists on the outer mitochondrial membrane, the endoplasmic reticulum membrane and outer nuclear membrane. The main function of Bcl-2 is to antagonize proapoptotic proteins. Most proapoptotic proteins are mainly located in the cytoplasm. Once cells are induced by apoptotic factors, the proapoptotic proteins translocate to the mitochondria and form transmembrane channels through oligomerization in the outer mitochondrial membrane, leading to release of apoptotic factors such as cytochrome c release from the mitochondria. Then the formation of the apoptosome leads to the activation of caspase 9. Finally, activated caspase 9 initiates apoptosis through cleavage, which activates caspases, leading to apoptosis. These are the processes of intrinsic or mitochondrial apoptosis pathway23,24.
Our experiments showed that the combination of drugs greatly increased ROS levels in CRC cells and decreased the mitochondrial membrane potential. In order to further evaluate the role of ROS, we pretreated CRC cells with NAC followed by combined treatment of SAL and OXA. Then we detected the production of ROS and superoxide anions. We found that the total ROS and superoxide anions were significantly reduced after adding NAC. These results further evaluated the role of ROS. However, ROS levels were slightly increased by SAL or OXA treatment alone. Mitochondrial membrane potential was the same result. We also examined the expression of mitochondrial apoptosis pathway-related proteins using western blot. Combined treatment of SAL and OXA significantly increased the expression of Bax, cytochrome c, cleaved caspase 9, cleaved caspase 3 and cleaved PARP. While the expression of the antiapoptotic protein, Bcl-2, was significantly reduced in the cotreatment group. Therefore, these findings indicated that the combined treatment of SAL and OXA promotes the accumulation of ROS in CRC cells, which alters the mitochondrial membrane potential, ultimately leading to cell apoptosis.
ROS trigger the activation of mitogen-activated protein kinases (MAPKs)25,26. MAPK cascades play an important role in cell growth, cell cycle, cell death and so on27. Among them, the research on ERK-1/2, JNK and p38 MAPK is the most extensive. The MAPK pathway regulates apoptosis via transcriptional and posttranscriptional mechanisms. ERK1/2, JNK and p38 MAPK have a dual role in cell apoptosis28,29,30,31,32,33,34,35, and these kinases regulate many transcription factors to increase proapoptotic proteins and decrease antiapoptotic proteins to promote cell apoptosis. Stress signals such as Ca2+, ROS, or death receptors activate the MAPK cascade, which can form a positive feedback to achieve higher MAPK activation. When the threshold of MAPK activation is exceeded, its downstream targets are activated, promoting cytochrome c release and caspase activation36.
In the present study, MAPK pathway-related proteins were detected by western blot analysis. we found that p-ERK, p-JNK and p-p38 were activated and the expression of them increased after the combined treatment of SAL and OXA. ROS inducers and inhibitors are usually identified and tested by NAC (an antioxidant)37. We pretreated CRC cells with NAC followed by combined treatment of SAL and OXA, and we found that the expression of the above proteins and apoptosis decreased. These results further indicated that ROS activates the MAPK pathway, which induces apoptosis. Our experiment also revealed that the combination treatment of SAL and OXA displays enhanced growth inhibition activities against CRC cell in vivo. We used immunohistochemistry to confirm that Ki-67 expression in the tumors in the cotreatment group was reduced compared to the other three groups.
In conclusion, the overall findings revealed that the induction of apoptosis in CRC cells by the SAL and OXA combination treatment involved ROS-mediated mitochondrial apoptosis and the MAPK pathway (Fig. 7). Based on the above study, it can be shown that SAL is a promising intervention drug for CRC. These results may contribute to the development of OXA-based chemotherapy for cancer patients receiving SAL treatment.
The new combined treatment of SAL and OXA for CRC still needs further research to clarify the specific mechanism of its inhibitory effect on cell migration and invasion.
Schematic representation of the mechanism of anticancer action of salinomycin and oxaliplatin cotreatment against CRC cells. Salinomycin and oxaliplatin cotreatment synergistically enhances ROS generation in CRC cells, which disrupts mitochondrial membrane potential, thereby increasing the release of cytochrome c, caspase activation and PARP cleavage. Cotreatment also modulates the MAPK pathway and induces apoptosis in CRC cells.
Materials and methods
Drugs and antibodies
Oxaliplatin (O9512), salinomycin (S4526), dissolution in dimethyl sulfoxide (DMSO, D2650) and thiazolyl blue tetrazolium bromide (MTT, M2128) were obtained from Sigma-Aldrich. Stocks of SAL and OXA were prepared via dissolution in dimethyl sulfoxide at concentrations of 50 mM and 50 mM respectively, and the solutions were stored at − 20 °C. The final concentration of DMSO was less than 0.1%.
N-acetylcysteine was obtained from Santa Cruz Biotechnology. Antibodies against GAPDH (#5174), Ki-67 (#9449), cleaved PARP (#5625), cleaved caspase 9 (#7237), cleaved caspase 3 (#9664), phosphorylated‑p38 (#4511) and p38 (#8690) were obtained from Cell Signaling Technology (CST, MA, USA), while anti‑phosphorylated‑JNK, anti‑JNK and anti‑ERK (ab184699) antibodies were purchased from Abcam. The anti‑phosphorylated‑ERK antibody (MA5‑15173) was obtained from Invitrogen. Antibodies against cytochrome c (sc-13561), Bcl-2 (sc-509) and Bax (sc-20067) were obtained from Santa Cruz. An Annexin V‑fluorescein isothiocyanate (FITC)/propidium iodide (PI) apoptosis detection kit (556547) was purchased from BD Biosciences. A Reactive Oxygen Species Assay Kit (S0033S) and a mitochondrial membrane potential assay kit with JC-1 (C2006) were purchased from Beyotime (Beijing, China).
Cell lines and culture conditions
Human CRC cells (Caco-2 and SW480) were obtained from Shanghai Zhong Qiao Xin Zhou Biotechnology Co., Ltd. Caco-2 and BV-2 mouse microglial cells were routinely grown in DMEM, and SW480 and NCM460 (normal human colon cells) cells were routinely grown in 1640, respectively, containing 10% fetal bovine serum (FBS) and 1% antibiotic solution (100 µg/ml streptomycin and 100 U/ml penicillin). The cells were incubated at 37 °C in a humidified atmosphere containing 5% CO2.
MTT assay
Cell viability was evaluated by MTT assay. In Brief, cells were seeded in 96-well plate (5 × 103 cells/well) and incubated overnight for adhere. Then cells were treated with various concentrations of SAL (0, 2.5, 5, 10, 20, 40 and 80 µM) or OXA (0, 10, 20, 40, 80, 160 and 320 µM) for 24, 48 and 72 h. In addition, some cells were processed together for 24 h and 48 h in the combination of the two drugs shown in Fig. 1G. Some cells were treated with DMSO, 2.5 µM SAL and 20 µM OXA for 24 h and 48 h. After treatment, MTT reagent (20 µl/well) was added to every well, and the plates were incubated for 4 h in dark at 37 °C. Then, medium was removed, and DMSO (100 µL/well) was added into every well. The intensity of the solvent was measured by the microplate reader (Tecan Group Ltd.) at 550 nm. The effect of SAL and OXA was analyzed by Calcusyn software38,39,40,41.
Colony-forming assay
Cells (700 cells/well) were seeded into 6-well plates and treated with DMSO, 2.5 µM SAL, 20 µM OXA or a combination of SAL and OXA for 48 h. After replacing the normal medium, cells were cultured for 2 weeks. Cells were then fixed with methanol and stained with crystal violet staining solution. Finally, cells were washed with PBS and clusters (≥ 50 cells) were photographed with a camera, the number of which was counted by Image J software.
Analysis of apoptosis and cell cycle distribution
Caco-2 and SW480 cells (1 × 106 cells/dish) were seeded in 6-cm dishes and treated for 48 h with DMSO and drugs as previously described. The cell morphology of apoptosis or cell death were observed by Nikon phase contrast microscope, and images were acquired. Cells were collected in 1× Binding Buffer and incubated with 5 µl of Annexin V-FITC and 5 µl of PI solution for 15 min in darkness. Then, 1×Binding Buffer (400 µl/tube) was added to every tube. The co-stained cells were analyzed with a FACSCalibur flow cytometer (BD Biosciences). Cells were harvested and fixed with 70% ethanol for 18 h at 4 °C. After that, the fixed cells were washed with PBS and stained for 30 min using 5 µl of PI working solution in the dark. Finally, cell cycle distribution was analyzed by a flow cytometer as previously described. Cell cycle distribution and apoptosis were analyzed with Modfit Software.
Cell migration and invasion assays
Wound-healing assays were very useful to evaluate cell migration. Cells after treatment as previously described for 48 h, and were seeded in 6-well plates (8 × 105 cells/well). Cells were incubated in complete medium overnight until formation of a monolayer, which was scratched with a 10-µl pipette tip in a straight line. The gap width needs to be consistent. Then cell monolayer was washed gently, and cells were incubated in serum-free medium. Finally, the cells were washed with PBS, and the wounded area was calculated using ImageJ software.
Transwell chambers (8.0 μm pore size; Costar; Corning, Inc.) were used to assess the migration and invasion abilities of CRC cells. After the indicated treatments as previously described for 2 days, CRC cells were suspended in FBS-free medium and seeded (1 × 105cells/well) in the upper chamber. The lower chambers were filled with medium containing with 10% FBS. After 24 h of incubation, the migrated cells were fixed with methanol and stained with crystal violet42. Migrated cells were imaged by a light microscope (5 random fields/well).
Detection of intracellular ROS
Intracellular ROS levels were detected by a DCFH-DA probe (Beyotime, China). Intracellular ROS can convert the nonfluorescent moiety into fluorescent dichlorofluorescein (DCF). Cells (6 × 105 cells/well) were seeded in 6-well plates and treated with DMSO, SAL or/and co-treated with oxaliplatin for 24 h. After treatment, cells were washed with and treated with DCFH-DA for 20 min at 37 °C in the dark. Cells were then washed with PBS, and ROS in CRC cells were observed by a fluorescence microscopy (Nikon, Japan).
Cells were treated with DMSO, NAC or/and co-treated with combination for 24 h. Experiments were performed according to the manufacturer’s instructions. The fluorescence intensity was measured using a microplate reader (Tecan Group Ltd.) and a fluorescence microscopy (Nikon, Japan).
Detection of mitochondrial membrane potential
Mitochondrial Membrane Potential Assay Kit (Beyotime) with JC-1 was used to assess Mitochondrial membrane potential loss. Cells (6 × 104 cells/well) were seeded in 24-well plates and treated with DMSO, SAL and OXA either alone or in combination for 24 h. Then cells were washed with PBS and incubated with JC-1 for 20 min at 37 °C in darkness. Finally, cells were washed and observed by a fluorescence microscope (Nikon, Japan).
Immunoblotting
After treatment of Caco-2 and SW480 cells with the indicated treatments for 2 days, cells were lysed with RIPA buffer, and protein concentrations were detected by a BCA Protein Assay Kit. Equal amount of each protein sample (30 µg) was separated by SDS-PAGE (12% resolving gel and 4% stacking gel). After electrophoresis, separated proteins transferred from the gel onto polyvinylidene difluoride (PVDF) membranes (Millipore). Then the PVDF Membranes were blocked with 5% non-fat dry milk in TBS for 1 h, washed in TBST and incubated with the first antibodies at 4 °C overnight. After washing with TBST to clean the unbound first antibodies, the membranes were treated with secondary antibodies for 1 h at room temperature. The membranes were then washed with TBST and developed using enhanced chemiluminescent detection reagent. GAPDH was used as an internal control. The densitometry of the blots was quantified by ImageJ software.
In vivo study
BALB/c nude mice (male, 4–6 weeks old) were purchased from Jiangsu GemPharmatech Technology Co. Ltd. (China). We performed all animal procedures and protocols according to the ARRIVE guidelines and the guidelines of the Guide for the Care and Use of Laboratory Animals (Institute of Laboratory Animal Resources, Commission on Life Sciences, National Research Council). This project was approved by the Laboratory Animal Ethical and Welfare Committee of Shandong University Cheeloo College of Medicine (Approval number: 19072). The mice were housed in SPF breeding units and acclimated for 1 week. The collected Caco-2 cells (in 100 µl of serum-free DMEM) were subcutaneously implanted into the dorsal region of nude mice. The collected Caco-2 cells (in 100 µl of serum-free DMEM) were subcutaneously implanted into the right oxter of nude mice. When the tumor was palpable, nude mice were randomized into four groups (n= 4/each group): vehicle control (normal saline), SAL (5 mg/kg), OXA (10 mg/kg) and combination group. Before taking the medicine, there was no difference between the four groups. Mice were injected intraperitoneally every other day. Tumor sizes were measured every two days. Finally, mice were euthanized using 2% isoflurane anesthesia for subsequent experiments. After experiments, tumor specimens were harvested and weighed43. Tumors were measured with the formula: tumor volume (mm3) = 0.5 × D × d2; where D and d are the longest and shortest diameters, respectively. HE staining and immunohistochemical staining were performed.
Statistical analysis
All the data were presented as the mean ± standard deviation of three independent experiments and analyzed using GraphPad Prism version 8.01 for Windows (GraphPad Software, San Diego, USA). Drug synergism and the CI were calculated using CompuSyn software (by Ting-Chao Chou and Nick Martin Biosoft, Cambridge, UK). Comparisons between two groups were performed using unpaired Student’s t test. Comparisons among three or more groups were analyzed by one-way ANOVA. Two-way ANOVA was used in the wound-healing assay. P < 0.05 was considered statistically significant.
Data availability
The original study contributions are detailed in the article/Supplementary Material. For additional inquiries, please contact the corresponding author.
References
Sung, H. et al. Global Cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 0, 1–41 (2021).
Arnold, M. et al. Global patterns and trends in colorectal cancer incidence and mortality. Gut 66 (4), 683–691 (2017).
Zacharakis, M. et al. Predictors of survival in stage IV metastatic colorectal cancer. Anticancer Res. 30, 653–660 (2010).
Al, B. et al. Colon cancer, version 2.2018 featured updates to the NCCN guidelines. J. Natl. Compr. Canc Netw. 16 (4), 359–369 (2018).
Martinez, N. W. et al. Metformin protects from oxaliplatin induced peripheral neuropathy in rats. Neurobiol. Pain. 8, 100048 (2020).
Matsuoka, T. et al. Evaluation of vascular pain in patients with colorectal cancer receiving peripheral venous chemotherapy with or without oxaliplatin. Sci. Rep. 9 (1), 1819 (2019).
Cunningham, D. et al. Colorectal cancer Lancet ; 375:1030–1047. (2010).
Zhou, J. et al. Salinomycin induces apoptosis in cisplatin-resistant colorectal cancer cells by accumulation of reactive oxygen species. Toxicol. Lett. 222 (2), 139–145 (2013).
Parajuli, B. et al. Salinomycin inhibits Akt/NF-kB and induces apoptosis in cisplatin resistant ovarian cancer cells. Cancer Epidemiol. 37 (4), 512–517 (2013).
Daman, Z. et al. Polymeric micelles of PEG-PLA copolymer as a carrier for salinomycin against gemcitabine-resistant pancreatic cancer. Pharm. Res. 32 (11), 3756–3767 (2015).
Dewangan, J., Srivastava, S., Rath, S. K. & Salinomycin A new paradigm in cancer therapy. Tumour Biol. 39 (3), 1–12 (2017).
Zhang, C. et al. Salinomycin inhibits the growth of colorectal carcinoma by targeting tumor stem cells. Oncol. Rep. 34 (5), 2469–2476 (2015).
Gupta, P. B. et al. Identification of selective inhibitors of cancer stem cells by high-throughput screening. Cell 138, 645–659 (2009).
Zhou, Q. et al. DACH1 suppresses epithelial to mesenchymal transition (EMT) through Notch1 pathway and reverses progestin resistance in endometrial carcinoma. Cancer Med. 8 (9), 4380–4388 (2019).
Chung, S. et al. Salinomycin abolished STAT3 and STAT1 interactions and reduced telomerase activity in colorectal cancer cells. Anticancer Res. 37 (2), 445–453 (2017).
Skeberdytė, A. et al. Dichloroacetate and salinomycin exert a synergistic cytotoxic effect in colorectal Cancer cell lines. Sci. Rep. 8 (1), 17744 (2018).
Liu, F. et al. Salinomycin and Sulforaphane exerted synergistic antiproliferative and proapoptotic effects on colorectal Cancer cells by inhibiting the PI3K/Akt signaling pathway in vitro and in vivo. Onco Targets Ther. 13, 4957–4969 (2020).
He, C., Lu, J. & Lin, W. Hybrid nanoparticles for combination therapy of cancer. J. Control Release. 219, 224–236 (2015).
Chaffer, C. L. & Weinberg, R. A. A perspective on cancer cell metastasis. Science 331 (6024), 1559–1564 (2011).
Fulda, S., Gorman, A. M., Hori, O. & Samali, A. Cellular stress responses: cell survival and cell death. Int J Cell Biol ;10 (2010) :1–23. (2010).
Li, H. L. et al. VALD-3, a schiff base ligand synthesized from o-vanillin derivatives, induces cell cycle arrest and apoptosis in breast cancer cells by inhibiting the Wnt/β-catenin pathway. Sci. Rep. 11 (1), 14985 (2021).
John, M. & Pascal The comings and goings of PARP-1 in response to DNA damage. DNA Repair. (Amst). 71, 177–182 (2018).
Danial, N. N. BCL-2 family proteins: critical checkpoints of apoptotic cell death. Clin. Cancer Res. 13 (24), 7254–7263 (2007).
McIlwain, D. R., Berger, T. & Mak, T. W. Caspase functions in cell death and disease. Cold Spring Harb Perspect. Biol. 7 (4), a026716 (2015).
Takata, T. & Araki, S. Tsuchiya1 Y. Oxidative stress orchestrates MAPK and Nitric- oxide synthase signal. Int. J. Mol. Sci. 21 (22), 8750 (2020).
Zhang, J. et al. ROS and ROS-Mediated cellular signaling. Oxid. Med. Cell. Longev. 2016, 4350965 (2016).
Rubinfeld, H. & Seger, R. The ERK cascade: A prototype of MAPK signaling. Mol. Biotechnol. 31, 151–174 (2005).
Balmanno, K. & Cook, S. J. Tumour cell survival signalling by the ERK1/2 pathway. Cell. Death Differ. 16, 368–377 (2009).
Cagnol, S. & Chambard, J. C. ERK and cell death: mechanisms of ERK-induced cell death-apoptosis, autophagy and senescence. FEBS J. 277, 2–21 (2010).
Liu, Z. et al. The roles of p38 MAPK and ERK1/2 in coplanar polychlorinated Biphenyls-Induced apoptosis of human extravillous Cytotrophoblast- derived transformed cells. Cell. Physiol. Biochem. 36, 2418–2432 (2015).
Wagner, E. F. & Nebreda, A. R. Signal integration by JNK and p38 MAPK pathways in cancer development. Nat. Rev. Cancer. 9 (8), 537–549 (2009).
Cuenda, A. & Rousseau, S. p38 MAP-kinases pathway regulation, function and role in human diseases. Biochim. Biophys. Acta. 1773, 1358–1375 (2007).
Dhanasekaran, D. N. & Reddy, E. P. JNK signaling in apoptosis. Oncogene 27, 6245–6251 (2008).
Feng, X. et al. Annexin A2 contributes to cisplatin resistance by activation of JNK-p53 pathway in non-small cell lung cancer cells. J. Exp. Clin. Cancer Res. 36 (1), 123 (2017).
Shao, Q. et al. Nur77 inhibits OxLDL induced apoptosis of macrophages via the p38 MAPK signaling pathway. Biochem. Biophys. Res. Commun. 471, 633–638 (2016).
Yue, J. C. & López, J. M. Understanding MAPK signaling pathways in apoptosis. Int. J. Mol. Sci. 21 (7), 2346 (2020).
Halasi, M. et al. ROS inhibitor N-acetyl-L-cysteine antagonizes the activity of proteasome inhibitors. Biochem. J. 454, 201–208 (2013).
He, C., Lu, J. & Lin, W. Hybrid nanoparticles for combination therapy of cancer. J Control Release 2015; 219:224–236. (2015).
Chou, T. C. Drug combination studies and their synergy quantification using the Chou-Talalay method. Cancer Res. 70, 440–446 (2010).
Chou, T. C. & Martin, N. CompuSyn for drug combinations: PC Software and User’s Guide: a computer program for quantitation of synergism and antagonism in drug combinations, and the determination of IC50 and ED50 and LD50 values (ComboSyn, 2005). for video demonstration).http://www.combosyn.com/
Chou, T. C. Theoretical basis, experimental design, and computerized simulation of synergism and antagonism in drug combination studies. Pharmacol. Rev. 58, 621681 (2006).
Wang, Q. et al. Calycosin suppresses TGF-β-induced epithelial-to- mesenchymal transition and migration by upregulating BATF2 to target PAI-1 via the Wnt and PI3K/Akt signaling pathways in colorectal cancer cells. J. Exp. Clin. Cancer Res. 38 (1), 240–252 (2019).
Han, H. et al. Inhibition of cell proliferation and migration through nucleobase-modified polyamidoamine-mediated p53 delivery. Int. J. Nanomed. 13, 1297–1311 (2018).
Funding
The present study was funded by the Natural Science Foundation of Shandong Province, China (ZR2020MH239), Shandong Province Medical Staff Science and Technology Innovation Plan Project (SDYWZGKCJHLH2023095), Taian science and technology innovation and development project (2020 NS160 and 2022 NS319) and Nursery Project of the Affiliated Taian City Central Hospital of Qingdao University (2022MPM03).
Author information
Authors and Affiliations
Contributions
F.L. and R.B.L. contributed equally to this study. M. W. and F.L. conceived and designed the experiments. F.L., R.B.L., X.W.Q., and J. W. performed the experiments and analysed the data. F.L., G.L., J.G., H.P.Y. and X.H.W. involved in writing, review and revision of the paper. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Liu, F., Lv, R., Qiao, X. et al. Salinomycin and oxaliplatin synergistically enhances cytotoxic effect on human colorectal cancer cells in vitro and in vivo. Sci Rep 15, 14056 (2025). https://doi.org/10.1038/s41598-025-98633-5
Received:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s41598-025-98633-5