Abstract
The content of terpenoids in tobacco can alter its resistance to TMV. NtSPS1, a pivotal structural gene in tobacco, is capable to regulate the terpenoid content. In this study, we investigated the effect of NtSPS1 knockout in HD on the content of terpenoids and the anti-TMV activity of this mutant using gene editing, widely targeted metabolomics, network pharmacology, and molecular docking. 48 terpenoids (six up-regulated and five down-regulated) in NtSPS1 knockout tobacco compared with WT leaves. Notably, solanesol was remarkable downregulation which was lowered by fourfold and compounds 1 (log2FC = 18.2), 8 (log2FC = 16.7) were significant upregulation between the mutants and wild-type line leaves. The 46 terpenoid’s target network encompassed 150 nodes, 509 edges and their underlying mechanisms in the therapeutic management of TMV are discussed. Furthermore, the network pharmacology and molecular docking revealed that compounds 16, 18, 23, 27, and 36 exhibited significant affinity in their respective interactions. Ultimately, five compounds were assayed for their anti-TMV effects, noteworthily, compounds 36 showed potential anti-TMV activity. Above all, we adopted a multifaceted approach to gain a comprehensive understanding of the terpenoid content and anti-TMV properties in NtSPS1 knockout HD. It enlightens the therapeutic potential of NtSPS1 knockout tobacco and it is helpful to find undescribed anti-TMV activity inhibitors, as well as searching for new anti-TMV candidates from the mutants.
Similar content being viewed by others
Introduction
Nicotiana tabacum (N. tabacum) is well known for its medicinal value purposes1,2,3. Previous phytochemical findings on N. tabacum showed terpenoids play a crucial role in multiple plant functions, for instance response to against biotic and abiotic stresses4,5,6. Tobacco mosaic virus (TMV) has a wide host range and naturally causes extensive damage in tobacco (N. tabacum)7,8,9,10, tomato (S. lycopersicum)11, and pepper (C. annuum)12,13. Plant metabolites, particularly terpenoids, have demonstrated the ability to counter TMV infection14,15,16,17. Such as solanesol, a non-cyclic trisesquiterpenoid alcohol, is mainly found in solanaceous plants: tobacco18,19,20, potato21,22, and tomato23, which might participate in the immune response to TMV infection24,25. NtSPS1 functions as a key enzyme in the biosynthetic pathway of terpenoids, especially solanesol26,27,28. After NtSPS1 overexpression in N. tabacum the total content of solanesol increase significantly29,30. The specific accumulation patterns and regulatory processes of terpenoids in NtSPS1-deficient Hong Hua Da Jin Yuan tobacco (Nicotiana tabacum L., HD) are still unclear. Additionally, there have been no prior studies on the terpenoid levels and anti-TMV effectiveness in this variety following NtSPS1 gene knockout. The present study aimed to systematically compare the terpenoid content and anti-TMV activities of NtSPS1 knockout HD and WT lines by multiple methods. NtSPS1 knockout HD resulted in 48 terpenoids: six up-regulated, five down-regulated, especially remarkable downregulation of solanesol which was lowered by fourfold and significantly upregulation of compound 1 and 8 in mutants compared with the wild-type lines.
The 48 terpenoids were divided into five classes, involving eight monoterpenes (two up-regulated metabolites), 23 sesquiterpenes (four up-regulated, one down-regulated metabolites), ten diterpenoids, six triterpenoids (three down-regulated metabolites) and one trisesquiterpenoid (down-regulated metabolites) between the mutants and wild-type lines. Network pharmacology provides a comprehensive framework for examining the complex relationships between terpenoids and TMV, thus pioneering a new approach to decipher the therapeutic principles31. Guided by network pharmacology and molecular docking, we selected compounds 16, 18, 23, 27, and 36 from our laboratory for testing their anti-TMV activities. The results showed that compound 36 exhibited a protective response against HD, with inhibiting rates of 36.7% (IC50 = 32.1 µM).
Materials and methods
Plant material
The methods employed for construction of NtSPS1 knockout Nicotiana tabacum L. variety Hong Hua Da Jin Yuan (HD) and growth conditions were the same as those described by Zhang et al. (Supplementary Method S1).
Chemical profiles
LC-MS/MS methods are employed to determine the total terpenoid content (Supplementary Method S2). Extraction of solanesol from mature leaves of NtSPS1 knockout and WT HD was performed according to the method described by Zhao et al. (Supplementary Method S2.1.1, S2.2)32. The LC-MS/MS-based widely targeted metabolomics analysis was used to analyze the terpenoid content in NtSPS1 knockout HD, as reported by Li et al. (Supplementary Method S2.1.2, S2.3)33. The T1 plants (T1 mutant HD), which were free of transgenes and carried a homozygous mutation resulting in premature stop codons, were allowed to self-fertilize for further inheritance in the T2 generation (T2 mutant HD). To determine the effect of NtSPS1 knockout on HD, T2 ntsps1 mutants (T2 mutant HD) and wild-type (WT) HD were cultivated in a glasshouse. The terpenoid content in the leaves of each HD at maturity stage were determined by analyzing their MS fragmentations and/or comparing them with reference samples (Supplementary Tables S1, S2). The PCA scatter plots were generated for all the samples to identify potential biomarkers after data normalization using Principal Component Analysis (PCA). This analysis provides an overview of the chemical relationship between leaves of NtSPS1 knockout and WT HD.
Statistical analysis
The orthogonal partial least squares discriminant analysis (OPLS-DA) and principal component analysis (PCA) were carried out on all the samples to identify the putative biomarkers after data normalization. Unsupervised PCA (principal component analysis) was performed by statistics function prcomp within R (www.r-project.org). The data was unit variance scaled before unsupervised PCA. VIP values were extracted from OPLS-DA result, which also contain score plots and permutation plots, was generated using R package MetaboAnalystR. The data was log transform and mean centering before OPLS-DA. In order to avoid overfitting, a permutation test (200 permutations) was performed. The HCA (hierarchical cluster analysis) results of samples and metabolites were presented as heatmaps with dendrograms, while pearson correlation coefficients (PCC) between samples were caculated by the cor function in R and presented as only heatmaps. Both HCA and PCC were carried out by R package ComplexHeatmap. For HCA, normalized signal intensities of metabolites (unit variance scaling) are visualized as a color spectrum. For two-group analysis, differential metabolites were determined by VIP (VIP > 1), P-value (P-value < 0.05, Student’s t test) and absolute Log2FC (|Log2FC| ≥ 0.58).
Network pharmacological analysis
Screening natural product and targets
The database of Swiss Target Prediction (http://www.swisstargetprediction.ch/) (accessed on 1 October 2024) was utilized to search for the compounds and their respective targets of N. tabacum. Subsequently, the molecules and their corresponding target proteins of N. tabacum were determined. By accessing the Uniprot database (https://www.uniprot.org) (accessed on 1 October 2024), the gene names linked to these protein targets were efficiently obtained.
Construction of compound-target network
Cytoscape is a powerful implement for visualizing network pharmacology and analyzing associated results34,35. By employing Cytoscape 3.10.0 (https://github.com/cytoscape/cytoscape/releases/3.10.0/), we successfully integrated the terpenoids of N. tabacum with their corresponding targets, thereby robustly constructing a compound-target network for this medicinal plant. The integrated target network is constructed with nodes representing either compounds or targets, and the interactions between them are illustrated by lines.
Identification of predicted targets of tobacco mosaic virus
The disease targets of tobacco mosaic virus were retrieved in the Genecards databases (https://www.genecards.org/) (accessed on 1 October 2024)36, and OMIM databases (https://www.omim.org/)(accessed on 1 October 2024)37. The procured targets from the two databases were merged, removing any duplicate entries in the process for further investigation.
Construction of PPI network
We imported the target data of those molecules as well as for TMV, into the Venn Diagram (http://bioinformatics.psb.ugent.be/webtools/Venn/) (accessed on 1 October 2024). This program was utilized to pinpoint the relationship between the compound targets and the TMV targets. The overlapping parts, which are involved in both the disease pathology of TMV and the molecular pharmacodynamics, can be tentatively identified as the effective therapeutic targets where the medicinal impact of drugs is genuinely manifested. We constructed an integrated network of molecules and disease targets to identify the interactions between the compounds and their associated therapeutic targets. Subsequently, he integrated network was uploaded to the STRING database (https://string-db.org/) (accessed on 1 October 2024) to obtain information on the protein-protein interaction network38. The application of this method is relatively rare in TMV, hence no core targets were identified. So, we utilized the previously identified targets for the subsequent work39,40,41.
Molecular docking
We accessed ChemDraw to obtain the molecule structures, the 3D structural files were converted by Chem3D version 18.0. The RSK structure, identified by the PDB code 2OM3, was chosen and prepared for further analysis. Hydrogen was removed, and polar hydrogen atoms were introduced using AutoDock version 4.2.6 (https://autodock.scripps.edu/download-autodock4/) tools. Active sites were constructed, and each one was subsequently docked with the molecule. Active pockets were built, and each active site was docked with the molecule42.
Anti-tobacco mosaic virus activity assays
Using half-leaf method detecte the anti-TMV activities and using ningnanmycin as a positive control (Supplementary Method S2.4). The antiviral inhibition rates of molecules 16, 18, 23, 27, and 36 at 20.0 µM are shown in Table 1.
Results
NtSPS1 mutants obtained by using CRISPR/Cas9 gene editing
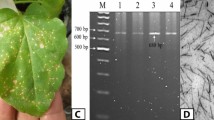
The HD plants with NtSPS1 gene knockout were successfully generated using CRISPR/Cas9 technology. An optimized gene editing vector for tobacco, developed by our group, was used to drive the expression of the NtSPS1 sgRNA and the 2 × 35 S promoter was used to drive the expression of Streptococcus pyogenes Cas9 (SpCas9)43. NPTII, neomycin phosphotransferase gene; LB, left border; RB, right border. (Fig. 1A–E). The sequencing data revealed that two distinct lines exhibited two different types of mutation sites, which deletion of 2 bases (Fig. 1F).
Authentic NtSPS1 of tobacco mutants and wild-type HD. (A) Schematic diagram of pOREU3TR vectors, (B–E) The phenotype of T0 mutant plants (T0 mutant HD) and wild type HD in different periods. WT, wild-type HD. ntsps1, NtSPS1 editing HD. ntsps1-Ros1, a transgenic plant carrying a red marker. (F) Mutations for the 56 green tobacco in the T1 generation (T1 mutant HD). The protospacer adjacent motif (PAM) site “AGG” needed for Cas9 fragmentation is marked in blue. Hom homozygous. #, related plant. No. number.
No off-target effects were observed for the three genes in the ntlht1 mutant strains, including Ntab0818090 and the NtLHT1 gene (Fig. 2A–C). Based on the results, NtSPS1 mutants were successfully obtained, and a dark-red marker was introduced to facilitate the generation of transgene-free plants in the T1 generation and obtained enough homozygous T2 population. The phenotypes of the NtSPS1 mutants in the T2 generation were same as those of WT HD (Fig. S2).
Chemical profiles
Comparison of the metabolic profiles between HDK and HDS group based on widely targeted metabolomics
The overall metabolic profiles of T2 mutant plants (HDK) and WT plants (HDS) at the mature stage show a clear separation tendency, indicating that the terpenoids in the tobacco leaves changed after the knockout of the NtSPS1 gene (Fig. 3A). And the largest principal component PC1 contributes 43.67%, and PC2 and PC3 make the contributions of 12.10% and 10.70%, respectively. The cumulative contribution rate of TOP3 principal components is greater than 60%, which indicated the overall explanation ability of the variance of original variables was acceptable.
The largest principal component, pathway enrichment, heat map analysis of HDS and HDK based on untargeted metabolomics. (A) The largest principal component plot of HDS (in green) and HDK (in red). (B) Pathway enrichment bubble plot related to HDS. The bubbles’ size and darkness pf the color represented the count and level of the enrichment for terpenoids. The mutation types (HDK) and wild types (HDS) at the mature stage.
Functional pathway analysis related to NtSPS1 knockout
The pathway enrichment analysis of 11 metabolic pathways including biosynthesis of flavonoids, alkaloids and terpenoids metabolism, etc. based on metabolic were performed (Fig. 3B). NtSPS1 knockout leads to a significant decrease in the content of downstream terpenoids such as solanesol. Due to limitations like incomplete database coverage and insufficient terpenoid concentrations leading to a low detection rate, the terpenoids enrichment factor is lower than that of flavonoid and alkaloid. Nevertheless, the enriched result the terpenoid pathway ranked TOP3 as its p-value was 0.053 (almost significant) and the FDR is lower than 0.2, demonstrating the regulation of terpenoid pathway after the NtSPS1 knockout. Moreover, since there are currently few reports on the study of terpenoids and related pathways, further study was carried out.
Identification and confirmation of terpenoids based on LC-MS/MS analysis
HDK and HDS were measured by LC-MS/MS based on widely targeted metabolomics analysis in positive ion mode. The results demonstrated that the mutants showed obvious regulation of terpenoid content. The assignment of the peaks for 47 terpenoids were shown in Fig. 4 and Table S1. These molecules including 8 monoterpenes (1, 5, 6, 7, 11, 13, 22, 29), 23 sesquiterpenes (2, 3, 4, 8, 9, 10, 12, 14, 15, 16, 17, 18, 20, 23, 25, 26, 27, 30, 31, 32, 33, 34, 36), 10 diterpenoids (19, 21, 24, 28, 35, 38, 41, 42, 43, 44), 6 triterpenoids (37, 39, 40, 45, 46, 47) were characterized based on MSn fragmentation and reference materials (Fig. 4A and Tables S1, S2, Fig. S2).
Untargeted metabolomics analysis. (A) LC-MS/MS-base multiple reaction monitoring mode (MRM) of one NtSPS1 knockout HD (HDK) in positive ion mode. (B) PCA loading plot of the 47 terpenoids. HDS, the wild types. Numbers are the IDs of 47 terpenoids. (C) Heatmap analysis was conducted on 10 terpenoids which change significantly between HDS and HDK, the content of terpenoids were normalized and visualized where the red and green color represented up- and down-regulation. (D) The variation in terpenoid content (Log2FC) between HDS and HDK. For each test, three specialized replicates were managed. At least 6 tobacco were evaluated in each sample. Data interpreted are mean ± SE of three specialty tests.
The principal component analysis (PCA) loading plot based on all terpenoids was shown in Fig. 4B. NtSPS1 knockout leads to a significant decrease in the content of downstream terpenoids such as solanesol. We have examined solanesol content using the method reported by Zhang et al. (see Supplementary Method S2.1.1), and we found that solanesol content has decreased by fourfold. There was a clear distinction between the HDK and HDS groups, with the replicate samples of each cluster closely grouped together. This suggests that the variations in terpenoids were consistent across the samples within each group and strongly associated with the NtSPS1 knockout of the HDK group.
Metabolic changes in HDS and HDK
The changes of 48 terpenoids before and after NtSPS1 knockout in HD are presented. Among them, 10 terpenoids were differential between HDS and HDK (|Fold change| ≥ 2 and VIP ≥ 1), 6 terpenoids were upregulated and 5 were downregulated, 37 did not show significant changes (Fig. 4C, and D, Supplementary Method S2.2).
Network pharmacological analysis
Compound-target network and analysis
We have created a detailed network of component-target interactions for these molecules, considering their unique structural features and specific receptors as identified by Swiss target prediction. As shown in Fig. 5A, a total of 150 nodes and 509 edges were assigned, with green ellipses indicating potential targets for anti-TMV. We obtained 36 compounds and their respective targets for N. tabacum from the Swiss target prediction database.
PPI network construction and analysis
After screening the active targets of TMV through the disease database and eliminating duplicate values, we identified 390 disease targets. Subsequently, we employed this data to create a Venn chart (Fig. 5B), comparing the active target compounds associated with the disease to those found in the compound formulations. The overlap between these sets revealed 115 common targets between the molecules and TMV. To clarify the complex interactions between pharmaceutical constituents and TMV targets, we established an extensive network linking compounds to disease targets. To depict these shared targets, we constructed a PPI network featuring 7 nodes, yet without any connecting edges. So, we utilized the previously identified targets for the subsequent work.
Molecular docking
Utilizing network pharmacology, we have previously pinpointed five essential active ingredients: compounds 16, 18, 23, 27 and 36. Subsequently, we performed molecular docking analysis of these compounds with RSK protein (Fig. 6). This analysis sought to confirm the critical function of the compounds in treating TMV. Following that, we conducted a detailed examination of the docking outcomes for proteins and ligands individually. Subsequently, we scrutinized the docking results of proteins and ligands separately, molecular docking revealed that 16, 18, 23, 27, and 36 were well located within the bonding site of anti-TMV by contacting ARG92, ILE93, SER15, VAL11, SER14, PHE10, GLY85, ASP116, and ALA86 residues. Our results show that the compounds have a strong affinity for binding to the RSK protein. Additionally, the interactions between each ligand-macromolecule complex are facilitated by hydrogen bonds, ensuring the stability of these interactions.
Anti-tobacco mosaic virus activity assays
Research indicates that solanesol could be involved in the resistance anti-TMV, but upon TMV infection, mutants (solanesol content has decreased by fourfold) and wild-type plants exhibit comparable characteristics (Fig. 8A and B). Other substances might be responsible for anti-TMV effects; to verify this hypothesis, guided by network pharmacology and molecular docking, the anti-TMV properties of compounds 16, 18, 23, 27, and 36 were evaluated (Fig. 7). The HD was pre-treated with 20.0 µM solutions of the compounds or a DMSO solution for 6 h prior to inoculation with TMV. The consequences clearly show that 36 offered protection against TMV, with an IC50 value of 32.1 µM (Table 1). For the practical application of our lab findings to tobacco cultivation, we chose mutant tobacco at the 7–8 leaf stage to evaluate the therapeutic efficacy of 36 against TMV with ningnanmycin as positive control. The negative control group showed a severe infection of TMV, the lesions were clearly visible, the growth rate was slow, and the leaves exhibited noticeable deformities (Fig. 8B), this may substantially affect the tobacco leaf yield and quality. However, after therapy with 36 (compared with ningnanmycin, Fig. 8C), the lesions showed a decrease compared to that of ningnanmycin (Fig. 8D). This result demonstrates that 36 exerts a significant therapeutic effect on HDK infected with TMV, and it can substantially alleviate the detrimental effects of TMV on the host plants.
The curative effect of compound 36 on TMV after Photos were taken 20 days after treatment (down). (A) Normal mutations, (B) HDK and HDS at the 7–8 leaf stage infected with TMV to be treated, (C) HDK infection of TMV treated with 100.0 µg/mL ningnanmycin (NNM), (D) HDK infection of TMV treated with compound 36.
Discussion
We identified 48 terpenoid metabolites and categorized them into five distinct classes: monoterpenoids, sesquiterpenes, diterpenoids, triterpenoids, and polyisoprenoid alcohol. Among these, compounds 1, 8, 9, 11, 25, 32, 33, 39, 45, 46, and solanesol exhibited differential accumulation between the mature leaves of the NtSPS1 knockout plants and wild-type plants (Tables S1, S2, and S3). Knockdown of NtSPS1 may inhibit the synthesis of compounds 9, 39, 45, 46, and solanesol, potentially leading to the accumulation of compounds 1, 8, 11, 25, 32, and 33, which could consequently result in the observed effects. The PCA scatter plots summarize the chemical connection between mutants and WT plants at the mature stage. Meanwhile, it can be observed from Fig. 4B that HDK samples were clustered together, while HDS samples were grouped together, which indicated that our analysis was stable and repeatable.
Additionally, we investigated the potential healing properties of the 36 metabolites for anti-TMV infections. These discoveries highlight the key role of terpenoids from N. tabacum in managing TMV. By extracting TMV-related targets from the Genecards database, we pinpointed 115 potential targets that overlapped with the compounds. The analysis of docking poses indicated that compounds 16, 18, 23, 27, and 36 exhibited strong interactions with the catalytic pocket of TMV (Fig. 6). Compound 16, the oxygen of the carbonyl group formed hydrogen bond with ARG92 and ILE93 residues (Fig. 6A). Compound 18, the hydroxyl groups on the ligand backbone and sugar formed hydrogen bonds with SER15 and VAL11 residues (Fig. 6B). Compound 23, the hydroxyl groups on the ligand backbone formed hydrogen bonds with SER14 and PHE10 residues (Fig. 6C). Compound 27, the hydroxyl groups on the ligand backbone and sugar formed hydrogen bonds with GLY85, ASP116, and ALA86 residues (Fig. 6D). Compound 36, the hydroxyl group on the ligand backbone formed hydrogen bond with ALA86 residue (Fig. 6E).
This research inevitably has some inherent limitations. First, the chemical and pharmacological basis for NtSPS1 knockout on tobacco is still to be fully understood. In addition, the leaves of N. tabacum are also not fully used due to the absence of chemical and pharmacological supports. In this investigation, NtSPS1 silencing significantly downregulated solanesol content (decreased to 25% compared with wild type control) in tobacco leaves. As well as we used LC-MS/MS-based widely targeted metabolomics to understand terpenoid content of mutant and WT plants at the mature stage. But the precision and currency of database information need to be enhanced, given that the predictive results of network pharmacology heavily depend on comprehensive bioinformatics data. Moreover, although network pharmacology mainly concentrates on investigating the interactions between natural products and biological systems, it does not completely account for the intricate and holistic nature of N. tabacum. In addition, molecular docking is primarily used to simulate drug-target interactions, but N. tabacum often involve multiple targets and therapeutic pathways. Moreover, our study has only identified the key targets of terpenoids from N. tabacum in TMV treatment, hinting at the biological mechanisms and important pathways without further exploration.
Conclusion
48 terpenoids, including six up-regulated and five down-regulated terpenoids compared with the wild type leaves control. We carried out an extensive investigation into the use of terpenoids for anti-TMV infections. Employing a combination of network pharmacology and molecular docking techniques, this exhaustive study sought to thoroughly comprehend the medicinal properties of N. tabacum. In the future, guided by network pharmacology and molecular docking, compounds 16, 18, 23, and 27 were found to have moderate anti-TMV activities. Furthermore, compound 36 exhibited potential anti-TMV activity, with inhibition rates in the range of 36.7% (IC50 = 32.1 µM, Table 1). This detailed analysis of gene knockout mutants and LC-MS/MS analysis will offer new insights into the chemical differences between the mutants and the wild type (WT), as well as their extensive application.
In this study, we investigated the effect of NtSPS1 knockout in HD on the content of terpenoids and the anti-TMV activity of this mutant. This provides valuable insight into the regulation of terpenoids, specifically solanesol, biosynthesis in tobacco. In recent years, research on terpenoid metabolism has been accelerated by advancements in synthetic biology. Advancements have led to engineering platforms for high-value terpenoid production, but engineering terpenoids in HD still face challenges due to complex metabolic and regulatory networks. Due to the limited number of standard substances, we did not obtain more comprehensive information about the correlation between NTSPS1 and other terpenoid content. In further research, we will isolate and identify more compounds from NtSPS1 knockout HD to search for relevant connections of the knockout and WT plants.
Data availability
The datasets generated and/or analyzed during the current study are available in the PDB data of 2OM3 (https://www.rcsb.org/structure/2OM3), Full wwPDB EM Validation Report of 2OM3 (https://files.rcsb.org/pub/pdb/validation_reports/om/2om3/2om3_full_validation.pdf). The datasets used and/or analyzed during the present study are available from the corresponding author on reasonable request.
Abbreviations
- NtSPS1 :
-
A solanesyl diphosphate synthases gene
- HD:
-
Nicotiana tabacum L. variety Hong Hua Da Jin Yuan
- TMV:
-
Tobacco mosaic virus
- WT:
-
Wild-type
References
Yan, B. W. et al. Extraction, purification and anti-TMV effects of α (β)-2,7,11-cembratriene-4,6-diol from tobacco leaves. Ind. Crop Prod. 174, 114197 (2021).
Nagai, A., Yamamoto, T. & Wariishi, H. Identification of fructo- and malto-oligosaccharides in cured tobacco leaves (Nicotiana tabacum). J. Agric. Food Chem. 60, 6606–6612 (2012).
Yang, G. Y. et al. Isoindolin-1-ones from the stems of Nicotiana tabacum and their antiviral activities. Arch. Pharm. Res. 45, 572–583 (2022).
Häkkinen, S. T. et al. Differential patterns of dehydroabietic acid biotransformation by Nicotiana tabacum and Catharanthus roseus cells. J. Biotechnol. 157, 287–294 (2012).
Jassbi, A. R., Zare, S., Asadollahi, M. & Schuman, M. C. Ecological roles and biological activities of specialized metabolites from the genus Nicotiana. Chem. Rev. 117, 12227–12280 (2017).
Dewey, R. E. & Xie, J. H. Molecular genetics of alkaloid biosynthesis in Nicotiana tabacum. Phytochemistry 94, 10–27 (2013).
Dai, J. M. et al. The anti-TMV potency of the tobacco-derived fungus Aspergillus versicolor and its active alkaloids, as anti-TMV activity inhibitors. Phytochemistry 205, 113485 (2023).
Yang, G. Y. et al. Cyclopiazonic acid type Indole alkaloids from Nicotiana tabacum-derived fungus Aspergillus versicolor and their anti-tobacco mosaic virus activities. Phytochemistry 198, 113137 (2022).
Yang, F. X. et al. Hu, Q. F. Isochromenes from the Nicotiana tabacum-derived endophytic fungus Aspergillus versicolor and their anti-tobacco mosaic virus activities. Nat. Prod. Res. 37, 1608–1616 (2023).
Ohnishi, J. et al. The coat protein of tomato mosaic virus L11Y is associated with virus-induced chlorosis on infected tobacco plants. J. Gen. Plant. Pathol. 75, 297–306 (2009).
Abdelkhalek, A., Al-Askar, A. A., Arishi, A. A. & Behiry, S. I. Trichoderma Hamatum strain Th23 promotes tomato growth and induces systemic resistance against tobacco mosaic virus. J. Fungi. 8, 228 (2022).
Scholthof, K. B. G. et al. Top 10 plant viruses in molecular plant pathology. Mol. Plant. Pathol. 12, 938–954 (2011).
Shin, R. et al. Isolation of pepper mRNAs differentially expressed during the hypersensitive response to tobacco mosaic virus and characterization of a proteinase inhibitor gene. Plant. Sci. 161, 727–737 (2001).
Zhao, L. H. et al. Anti-TMV activity and functional mechanisms of two sesquiterpenoids isolated from Tithonia diversifolia. Pestic Biochem. Phys. 140, 24–29 (2017).
Xu, J. et al. Characterization of diterpenoids from Caesalpinia decapetala and their anti-TMV activities. Fitoterapia 113, 144–150 (2016).
Shang, S. Z. et al. Antiviral sesquiterpenes from leaves of Nicotiana tabacum. Fitoterapia 108, 1–4 (2016).
Ma, Y. et al. Diterpene alkaloids and diterpenes from Spiraea japonica and their anti-tobacco mosaic virus activity. Fitoterapia 109, 8–13 (2016).
Yan, N. et al. Solanesol Biosynth. Plants Mol. 22, 510 (2017).
Yan, N. et al. Bioactivities and medicinal value of Solanesol and its accumulation, extraction technology, and determination methods. Biomolecules 2019(9), 334 (2019).
Yan, N. et al. Solanesol: a review of its resources, derivatives, bioactivities, medicinal applications, and biosynthesis. Phytochem Rev. 14, 403–417 (2015).
Lan, T. et al. A simple and standardized method for the determination of total Solanesol in potato leaves and its extracts based on HPLC-MS. J. AOAC Int. 104, 479–484 (2021).
Chen, T. et al. Optimization of microwave-assisted extraction of Solanesol from potato leaves and stems. Med. Chem. Res. 19, 732–742 (2010).
Taylor, M. A., Fraser, P. D. & Solanesol Added value from Solanaceous waste. Phytochemistry 72, 1323–1327 (2011).
Akinyemi, I. A., Wang, F., Zhou, B. G., Qi, S. S. & Wu Q. F. Ecogenomic survey of plant viruses infecting tobacco by next generation sequencing. Virol. J. 13, 181 (2016).
Bailey, J. A., Carter, G. A., Burden, R. S. & Wain, R. L. Control of rust diseases by diterpenes from Nicotiana glutinosa. Nature 255, 328–329 (1975).
Campbell, R., Freitag, S., Bryan, G. J., Stewart, D. & Taylor, M. A. Environmental and genetic factors associated with Solanesol accumulation in potato leaves. Front. Plant. Sci. 7, 1263 (2016).
Xiang, D. H. et al. Analysis on Solanesol content and genetic diversity of Chinese flue-cured tobacco (Nicotiana tabacum L). Crop Sci. 57, 847–855 (2017).
Yan, N. et al. RNA sequencing provides insights into the regulation of Solanesol biosynthesis in Nicotiana tabacum induced by moderately high temperature. Biomolecules 8, 165 (2018).
Yan, N. et al. Effects of NtSPS1 overexpression on Solanesol content, plant growth, photosynthesis, and metabolome of Nicotiana tabacum. Plants 9, 518 (2020).
Yan, N. et al. Organ- and growing stage-specific expression of Solanesol biosynthesis genes in Nicotiana tabacum reveals their association with Solanesol content. Molecules 21, 1536 (2016).
Cong, S., Feng, Y. & Tang, H. P. Network Pharmacology and molecular Docking to explore the potential mechanism of urolithin A in combined allergic rhinitis and asthma syndrome. Naunyn-Schmiedeberg’s Arch. Pharmacol. 396, 2165–2177 (2023).
Zhao, C. J., Li, C. Y. & Zu, Y. G. Rapid and quantitative determination of Solanesol in Nicotiana tabacum by liquid chromatography–tandem mass spectrometry. J. Pharm. Biomed. Anal. 44, 35–40 (2007).
Li, S. P. et al. Metabolic and transcriptomic analyses reveal different metabolite biosynthesis profiles between leaf buds and mature leaves in Ziziphus jujuba mill. Food Chem. 347, 129005 (2021).
Paul Shannon; Andrew Markiel; Owen Ozier, Nitin, S., Baliga; Jonathan, T. & Wang Daniel Ramage; Nada Amin; Benno Schwikowski; Ideker, T. Cytoscape: A software environment for integrated models of biomolecular interaction networks. Genome Res. 13, 2498–2504 (2003).
Otasek, D., Morris, J. H., Bouças, J., Pico, A. R. & Demchak, B. Cytoscape automation: empowering workflow-based network analysis. Genome Biol. 20, 185 (2019).
Stelzer, G. et al. The genecards suite: from gene data mining to disease genome sequence analyses. Curr. Protoc. Bioinf. 54, 1301–13033 (2016).
Amberger, J. S. & Hamosh, A. Searching online mendelian inheritance in man (OMIM): A knowledgebase of human genes and genetic phenotypes. Curr. Protoc. Bioinf. 58, 1.2.1–1.2.12 (2017).
Szklarczyk, D. et al. The STRING database in 2017: quality-controlled protein–protein association networks, made broadly accessible. Nucleic Acids Res. 45, D362–D368 (2017).
Yang, F. X. et al. Extraction and characterization of anti-virus anthraquinones from Nicotiana tabacum-derived Aspergillus oryzae YNCA1220. Pestic Biochem. Physiol. 196, 105613 (2023).
Ma, Y. Y. et al. X. Indole alkaloids isolated from the Nicotiana tabacum-derived Aspergillus fumigatus 0338 as potential inhibitors for tobacco powdery mildew and their mode of actions. Pestic Biochem. Physiol. 200, 105814 (2024).
Liu, H. Y. et al. Characterization of anti-TMV Indole alkaloid and isocoumarin derivatives from Aspergillus versicolor YNCA0363. Chem. Biol. Technol. Agric. 10, 138 (2023).
Trott, O., Olson, A. J., AutoDock & Vina Improving the speed and accuracy of Docking with a new scoring function, efficient optimization, and multithreading. J. Comput. Chem. 31, 455–461 (2010).
Zhang, J. D. et al. Highly efficient transgene-free genome editing in tobacco using an optimized CRISPR/Cas9 system, pOREU3TR. Plant. Sci. 326, 111523 (2023).
Funding
The authors are grateful to the project of Yunnan Daguan Laboratory (No. YNDG202301YZ04 and No. YNDG202401YZ01), the key projects of China Tobacco Yunnan Industrial (No. 2022JY02 and No. 2023YJ05) and the Project from Yunnan Key Laboratory of Tobacco Chemistry (No. 2021539200340239) for the financial support. The authors declare that they have no competing interests with the funding company.
Author information
Authors and Affiliations
Contributions
JL, MLC, and QFH conceived and designed the project; JMD performed the experiment of T0 mutant plants; XL, LFZ, JDZ, and JW performed the experiment of T1 and T2 mutant plants; YX and JL performed the experiment of LC-MS/MS analysis; GYY completed the biological evaluation; JMD, XL, JDZ, JW and GYY analyzed the experimental data; JMD wrote the initial draft; All the authors discussed the results and contributed to the preparation of the final paper.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Dai, JM., Zhang, JD., Liu, X. et al. Gene editing, metabolomics, network pharmacology strategies to explore terpenoid content and anti-TMV activity in NtSPS1 knockout Nicotiana tabacum. Sci Rep 15, 14581 (2025). https://doi.org/10.1038/s41598-025-98745-y
Received:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s41598-025-98745-y