Abstract
The prevention of osteoarthritis through controlling body measurements has received increasing attention in recent years, but the relationship between lean body mass (LBM) and osteoarthritis remains unclear. Hence, we explored this association through the data from the National Health and Nutrition Examination Survey (2007–2018). The present study enrolled 31,172 participants. To explore the correlation between LBM and osteoarthritis, we utilized logistic regression equations to explore associations between covariates, exposures, and outcomes. We used multivariate regression models to further control confounding factors. Restricted cubic splines were employed to investigate non-linear relationships. And the inflection point was identified by recursive algorithm. Furthermore, stratified analyses of gender and age were conducted. Osteoarthritis was negatively correlated with LBM [0.74 (0.67, 0.80)] in the model adjusting for all confounders. Based on the restricted cubic spline curve, an inflection point of 52.26 kg was found to confirm the non-linear relationship. LBM was negatively correlated with osteoarthritis at 0.56 (0.48, 0.64) before the inflection point, and the association was not statistically significant afterward. This large-scale study revealed that LBM was non-linearly correlated with osteoarthritis in the general American population. Differences in age and gender were further identified.
Similar content being viewed by others
Introduction
Among the elderly, osteoarthritis is the most common joint disease and a leading cause of disability1. Its clinical features include joint pain, stiffness, and restricted mobility, which in severe cases can lead to joint deformity and loss of function2. The trends of an aging population and increasing obesity are likely to compound this3. The prevalence of osteoarthritis is 10% in males and 18% in females aged over 604. In various high-income countries, the medical cost of osteoarthritis is estimated to account for between 1% and 2.5% of the country’s gross domestic product5. The etiology of osteoarthritis is complex and includes factors such as aging, obesity, genetic predisposition, joint injuries, overuse of joints, hormonal changes, metabolic disorders, occupational factors, and environmental influences6,7,8.
Obesity is a major risk factor for osteoarthritis. A longitudinal cohort study has demonstrated that a high body mass index (BMI) significantly increased the incidence of knee osteoarthritis and was positively associated with the risk of severe osteoarthritis9. Additionally, a Mendelian randomization study supported a causal relationship between BMI and osteoarthritis, which used genetic variants as instrumental variables to minimize the impact of confounding factors, thereby more clearly delineating the direct effects of BMI on osteoarthritis10. However, recent studies have questioned the efficacy of BMI as an indicator for assessing obesity11,12. While BMI is widely used to evaluate an individual’s weight status, its limitations lie in that it only reflects the ratio of weight to height and does not accurately distinguish between the distribution of body fat and lean body13,14. Therefore, future research and clinical evaluations should place greater emphasis on comprehensive measurements of body composition to more accurately assess the impact of obesity on osteoarthritis. This not only aids in a better understanding of the etiology of osteoarthritis but also provides a scientific basis for developing more effective prevention and intervention strategies.
Lean body mass (LBM) serves as a crucial indicator of body composition, more accurately reflecting the proportion of lean mass in the human body15. It is the sum of all non-fat parts of a person’s body and is used clinically to help physicians assess nutritional status, metabolic health, exercise capacity and disease prevention14,16. LBM is a better indicator of actual health than mass alone and is an important part of many health assessments and treatment plans. Higher levels of LBM are typically associated with better metabolic health and physical activity capacity17. Recent studies have discovered significant associations between LBM and various metabolic diseases, such as cardiovascular disease, lung cancer, and diabetes18,19. The use of certain progressive resistance training, adequate sleep, and protein intake may help maintain and increase LBM20,21,22.
As a disease closely related to obesity and lean mass, osteoarthritis has been widely explored for its association with LBM. However, the results of the available evidence are mostly inconsistent. Therefore, there is an urgent need for studies with larger samples to explore the relationship between LBM and osteoarthritis for better prevention of the disease through weight management.
Therefore, we used the data from the National Health and Nutrition Examination Survey (NHANES) to examine the potential association between LBM and osteoarthritis. Our goal is to provide new evidence to support comprehensive strategies focused on weight management and body composition optimization to reduce the risk of osteoarthritis and to establish a scientific foundation for developing personalized prevention and intervention measures in the future.
Methods
Study population
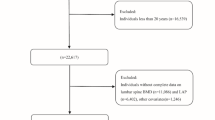
This study used NHANES dataset, a continuous health and nutrition survey conducted by the Centers for Disease Control and Prevention (CDC). To ensure national representativeness, stratified multistage probability samples were used. Its protocols were all approved by the National Center for Health Statistics Research Ethics Review Board, and the informed consents were provided by all participants. All researchers have free access to the data via the NHANES official website (https://wwwn.cdc.gov/nchs/nhanes). Based on NHANES data from six cycles (2007–2018), we conducted our study. The total number of study participants who finally completed demographic surveys, laboratory tests, and health status questionnaires was 59,842. The following are the exclusion criteria: (1) Age less than 20 years (n = 25,072); (2) Missing LBM data (n = 3534); (3) Missing osteoarthritis data (n = 64). Ultimately, a total of 31,172 participants were included (Fig. 1).
Assessment of lean body mass
Using Lee’s anthropometric prediction equations, the LBM was calculated23. They collected dual-energy radiographic data on 15,065 men and 16,107 women and measured the LBM by dual-energy radiographic data. Furthermore, they used multiple linear regression analysis and estimated LBM using sex, age, weight (kg), height (cm), and waist circumference (cm) as predictor variables, and the linear regression model had the highest consistency [LBM (females: R2 = 0.85; males: R2 = 0.91)]. The specific assessment formula is as follows:
Men:
Women:
Diagnosis of osteoarthritis
Osteoarthritis diagnostic data were obtained from the NHANES questionnaire, which assesses arthritis through the question in its codebook: “ Have you ever been told by a doctor or other health care professional that you suffered from arthritis?” The response options were “yes” or “no”, and if they answered “yes”, the staff member would further ask “What kind of arthritis”, with options of “osteoarthritis”, “rheumatoid arthritis”, “psoriatic arthritis”, “other”, “don’t know”, and “refused”. If the participant answered “osteoarthritis”, then he/she was diagnosed with osteoarthritis24,25.
Covariates
Based on previous literature26,27, we used covariates including demographic data, laboratory data, examination data, and questionnaire data to ensure the validity and robustness of the findings. Demographic information included age, sex (men or women), race (Mexican American, White, Black, or Other), education level (less than high school, high school, or more than high school), marital status (married/living with partner, divorced/separated/widowed or never married), and income-to-poverty ratio. Anthropometric data included BMI, and waist circumference. The BMI was calculated as weight divided by the square of height. Laboratory data included c-reactive protein (CRP), and uric acid. Smoking status and alcohol use were categorized as “current” or "never," while physical activity, stroke, cardiovascular disease, hypertension, diabetes, and cancer were defined as “yes” or “no”.
Statistical analysis
According to the NCHS analysis guidelines, we weighted the data using sample weights in our analysis. Statistical analyses in this investigation were conducted using R software(Version 4.3.2). Significance was determined at a p-value of less than 0.05, utilizing a two-tailed test. The interpolation of missing data was performed using predictive mean matching for numeric variables and logistic regression for binary variables28. The representation of continuous variables was in the form of mean values (standard error), while categorical variables were reported in numbers (%). For the comparison of the differences between the four groups, the weighted linear regression model was used for continuous variables, and weighted chi-square tests for categorical variables29. The relationship between LBM and osteoarthritis was quantified using odds ratios (OR) and their 95% confidence intervals (CI). Multivariable logistic regression models were systematically developed to explore the independent influence of LBM on osteoarthritis. Model 1 did not adjust for any covariates, model 2 adjusted only for age, sex, and race, while model 3 adjusted for all covariates. Moreover, the restricted cubic splines (RCS) were employed to examine the non-linear associations between LBM and osteoarthritis, further investigating the dose–response relationship between them. Upon detecting a nonlinear relationship, we employed a recursive algorithm to calculate inflection points and created a two-part binary logistic regression model on either side of the inflection point30. In addition, we performed subgroup analyses and interaction tests to explore whether there were subgroup population differences.
Results
Baseline characterization
Among the 59,842 individuals who completed the initial survey, 25,072 did not meet the age requirement and were excluded from analysis, and a total of 3598 individuals with missing exposure and outcome data were excluded, resulting in 31,172 participants being included in the study (Fig. 1). The demographic characteristics of the study population based on LBM quartiles can be seen in Table 1. The mean age of the study population was 47.19 (0.22) years, of which 48.33% were males and 51.67% were females. People who possessed higher LBM had a higher likelihood of being male, married or living with a partner. Moreover, LBM was positively associated with physical activity, BMI, waist circumference, uric acid, and CRP, and negatively associated with age, cancer and osteoarthritis.
Univariate analysis
The association of covariates with osteoarthritis in the study is shown in Table 2. Age, gender, education level, marital status, physical activity, income-to-poverty ratio, hypertension, cardiovascular disease, stroke, diabetes, cancer, waist circumference, BMI,CRP, and uric acid were statistically associated with osteoarthritis. Compared to men, women were more likely to develop osteoarthritis [1.78 (1.65, 1.92)]. Osteoarthritis is more likely to occur in older individuals, those with higher levels of education, those who are divorced, separated, or widowed, and those with cancer, diabetes, hypertension, cardiovascular disease, or stroke. In addition, the development of osteoarthritis was positively associated with waist circumference, BMI, CRP, uric acid, and income-to-poverty ratio.
Relationship between lean body mass and osteoarthritis
We developed multivariable logistic regression models to explore the association between LBM and osteoarthritis (Table 3). The OR (95% CI) was 0.89 (0.86, 0.93) and 1.00 (0.96, 1.04) in Model 1 and Model 2. After correcting for all covariates, a negative relationship was found [0.74 (0.67, 0.80)], which indicated that the odds of developing osteoarthritis were reduced by 26% when LBM increased by one unit.
The RCS was developed to better elucidate the dose–response relationship of LBM with osteoarthritis, and a non-linear correlation was observed between LBM and osteoarthritis (Fig. 2). We then calculated the inflection point 52.26 kg by recursive algorithm (Table 4). The OR (95% CI) of the relationship was 0.56 (0.48, 0.64) before the inflection point. After the inflection point, the difference was not statistically significant[0.90 (0.80, 1.01)]. In addition, we explored if there were similar associations existed in subgroups. After plotting stratified RCS, the non-linear association, consistent with the overall trend, was also evident among women and those over 60 years of age (Figs. 3, 4). The final subgroup analysis revealed no significant differences among the population examined (Fig. 5).
Association between lean body mass and osteoarthritis among US adult population. Age, sex, race, education level, income-to-poverty ratio, marital status, smoking status, alcohol use, vigorous recreational activity, BMI, waist circumference, stroke, diabetes, CVD, cancer, hypertension, uric acid, and CRP were adjusted.
Discussion
This study investigated the association between LBM and osteoarthritis among US adult populations. In regression models adjusted for all covariates, we observed a non-linear relationship between LBM and osteoarthritis, with an inflection point of 52.26 kg. Before the inflection point, LBM was negatively associated with osteoarthritis, whereas after the inflection point, the association was not statistically significant. In addition, stratified analysis by gender and age revealed that this non-linear relationship was also present among women and those over 60 years of age.
This study revealed that the association between LBM and osteoarthritis was more significant when LBM was lower than 52.26 kg, which may be manifested as a significant correlation between an increase in LBM and a decrease in the risk of osteoarthritis. This suggested that the higher LBM levels might play a protective role against osteoarthritis. When LBM exceeded 52.26 kg, the effect of LBM on osteoarthritis was no longer significant. This indicated that there may be a saturation point for the effect of LBM. After reaching this level, a further increase in LBM may no longer have a significant benefit in reducing the risk of osteoarthritis. The above results showed that for people with LBM below 52.26 kg, especially those at high risk of osteoarthritis, increasing LBM may be effective in reducing the risk of osteoarthritis. In contrast, for those with already high LBM, further interventions may not be of significant benefit. In clinical practice, priority intervention groups can be identified based on the inflection point value, thus providing a reference for intervention in high-risk groups.
Although the relationship between LBM and osteoarthritis has been explored, their results are not consistent and their small sample sizes have lacked further analysis of stratification. A meta-analysis of osteoarthritis and body composition suggested that LBM was positively associated with osteoarthritis, while lean mass percentage seemed to be a protective factor31. Contradictory to this, Studies from Sweden showed that patients with osteoarthritis of the knee or hip both phenotypically exhibited a low proportion of whole-body LBM32,33. A study of Japanese women revealed that reduced LBM was associated with knee osteoarthritis34. Whereas, a study of middle-aged and older Korean adults showed that lower extremity LBM was negatively correlated with radiographic osteoarthritis of the knee35. Differing from all of the above findings, the study from Vietnam did not find a significant association between LBM and osteoarthritis36. In contrast, our study, with the largest sample size, found a non-linear relationship and a negative correlation before the inflection point.
The exact mechanism of the current relationship between LBM and osteoarthritis is unclear and may be related to several biological mechanisms. The first is oxidative stress. LBM gain enhances the body’s antioxidant defense mechanisms, which in turn reduces damage to articular cartilage from oxidative stress37. Studies have shown that oxidative stress is one of the main causes of cartilage matrix degradation and chondrocyte apoptosis, whereas the secretion of antioxidant enzymes such as superoxide dismutase (SOD) and glutathione peroxidase (GPx) in muscle tissues is increased during exercise and these enzymes are effective in scavenging reactive oxygen species (ROS) in the body, protecting articular cartilage from oxidative stress damage38,39. The second is inflammation. LBM gain may improve joint health by reducing systemic inflammation (e.g., lowering levels of CRP, leukocyte-mediated inflammation)40. However, beyond a certain level, the suppressive effect of further increases in muscle mass on inflammation levels is probably not evident anymore. The third is the balance between joint loading and muscle support. LBM primarily reflects muscle mass, which protects the joints by enhancing joint stability and absorbing mechanical stress41. When LBM exceeds a certain level, the mechanical load protection of the joints may have reached an optimal state, and the mechanical support effect on the joints by further increasing muscle mass tends to be saturated. In addition, the increase in LBM may be associated with an increase in total body weight, especially since excess body weight imposes greater mechanical loads on the joints42. Beyond the inflection point, the increased joint loading associated with increased LBM may counteract its protective effects.
In this study, we found a significant gender difference, with LBM showing a linear association with osteoarthritis in men and a non-linear association in women. The reason for the difference may be related to the regulation of sex hormones. Androgens, which have anti-inflammatory and pro-muscle building effects in men, may have a sustained protective effect on joint health by enhancing muscle mass and the stability of periarticular structures43. This may explain the linear negative correlation between LBM and osteoarthritis in men. In addition, testosterone inhibits inflammatory factors associated with cartilage degradation, further reducing the risk of osteoarthritis44,45. Estrogens are protective of articular cartilage in the premenopausal period, but significant decreases in estrogen levels in the postmenopausal period may lead to cartilage degradation and an increased risk of osteoarthritis46. The non-linear relationship in women may reflect the fact that changes in estrogen levels play a dominant role in low LBM populations, whereas when LBM reaches a certain inflection point, the role of estrogen may diminish, and other factors (e.g., metabolic or mechanical loading) become the main influences.
There were several advantages of the present study over previous studies. First, this study included 31,172 individuals, which was the largest sample size to date. Second, NHANES included a representative population of non-institutionalized American civilians, so our findings would be broadly applicable to the entire American population. Third, NHANES has comprehensive health data collection, enabling researchers to conduct more sophisticated analyses of outcomes and associations among various factors. There are also some limitations to this study. First, some self-reported data may be subject to recall bias. Second, NHANES is a cross-sectional survey, and the data were collected at a specific point in time, limiting the ability to make causal inferences. Third, the dynamics of population health and nutritional status over time may not be adequately captured by cross-sectional data. Fourth, although we have made a relatively comprehensive adjustment for covariates, the effects of covariates not included may be unavoidable due to database limitations.
In conclusion, we found a non-linear association between LBM and osteoarthritis in this cross-sectional study of the general U.S. adults. Differences in age and gender were further explored. Our study might provide information for public health policy makers and clinicians to intervene in osteoarthritis by controlling body measurements. Further randomized controlled trials are needed to verify this finding.
Data availability
The datasets used and/or analyzed during the current study are publicly available from the NHANES database, https://wwwn.cdc.gov/nchs/nhanes.
References
Hunter, D. J. & Bierma-Zeinstra, S. Osteoarthritis. Lancet 393, 1745–1759. https://doi.org/10.1016/S0140-6736(19)30417-9 (2019).
Yu, H., Huang, T., Lu, W. W., Tong, L. & Chen, D. Osteoarthritis pain. Int. J. Mol. Sci. https://doi.org/10.3390/ijms23094642 (2022).
Hunter, D. J., March, L. & Chew, M. Osteoarthritis in 2020 and beyond: A lancet commission. Lancet 396, 1711–1712. https://doi.org/10.1016/S0140-6736(20)32230-3 (2020).
Barnett, R. Osteoarthritis. Lancet 391, 1985. https://doi.org/10.1016/S0140-6736(18)31064-X (2018).
Hunter, D. J., Schofield, D. & Callander, E. The individual and socioeconomic impact of osteoarthritis. Nat. Rev. Rheumatol. 10, 437–441. https://doi.org/10.1038/nrrheum.2014.44 (2014).
Silverwood, V. et al. Current evidence on risk factors for knee osteoarthritis in older adults: A systematic review and meta-analysis. Osteoarthritis Cartilage 23, 507–515. https://doi.org/10.1016/j.joca.2014.11.019 (2015).
Allen, K. D., Thoma, L. M. & Golightly, Y. M. Epidemiology of osteoarthritis. Osteoarthritis Cartilage 30, 184–195. https://doi.org/10.1016/j.joca.2021.04.020 (2022).
Berenbaum, F., Wallace, I. J., Lieberman, D. E. & Felson, D. T. Modern-day environmental factors in the pathogenesis of osteoarthritis. Nat. Rev. Rheumatol. 14, 674–681. https://doi.org/10.1038/s41584-018-0073-x (2018).
Mork, P. J., Holtermann, A. & Nilsen, T. I. Effect of body mass index and physical exercise on risk of knee and hip osteoarthritis: Longitudinal data from the Norwegian HUNT Study. J. Epidemiol. Community Health 66, 678–683. https://doi.org/10.1136/jech-2011-200834 (2012).
He, Y., Zheng, C., He, M. H. & Huang, J. R. The Causal relationship between body mass index and the risk of osteoarthritis. Int. J. Gen. Med. 14, 2227–2237. https://doi.org/10.2147/IJGM.S314180 (2021).
Pischon, T. Commentary: Use of the body mass index to assess the risk of health outcomes: Time to say goodbye?. Int. J. Epidemiol. 39, 528–529. https://doi.org/10.1093/ije/dyp388 (2010).
Javed, A. et al. Diagnostic performance of body mass index to identify obesity as defined by body adiposity in children and adolescents: A systematic review and meta-analysis. Pediatr. Obes. 10, 234–244. https://doi.org/10.1111/ijpo.242 (2015).
Stern, L. et al. The effects of low-carbohydrate versus conventional weight loss diets in severely obese adults: One-year follow-up of a randomized trial. Ann. Intern. Med. 140, 778–785. https://doi.org/10.7326/0003-4819-140-10-200405180-00007 (2004).
Willoughby, D., Hewlings, S. & Kalman, D. Body Composition changes in weight loss: Strategies and supplementation for maintaining lean body mass, a brief review. Nutrients https://doi.org/10.3390/nu10121876 (2018).
Lee, D. H. et al. Predicted lean body mass, fat mass, and all cause and cause specific mortality in men: Prospective US cohort study. BMJ 362, k2575. https://doi.org/10.1136/bmj.k2575 (2018).
Romero-Corral, A. et al. Accuracy of body mass index in diagnosing obesity in the adult general population. Int. J. Obes. 32, 959–966. https://doi.org/10.1038/ijo.2008.11 (2008).
Bann, D. et al. Physical activity across adulthood in relation to fat and lean body mass in early old age: Findings from the Medical Research Council National Survey of Health and Development, 1946–2010. Am. J. Epidemiol. 179, 1197–1207. https://doi.org/10.1093/aje/kwu033 (2014).
Cordoba-Rodriguez, D. P. et al. Fat-free/lean body mass in children with insulin resistance or metabolic syndrome: A systematic review and meta-analysis. BMC Pediatr. 22, 58. https://doi.org/10.1186/s12887-021-03041-z (2022).
Jeong, S. M., Lee, D. H. & Giovannucci, E. L. Predicted lean body mass, fat mass and risk of lung cancer: Prospective US cohort study. Eur. J. Epidemiol. 34, 1151–1160. https://doi.org/10.1007/s10654-019-00587-2 (2019).
Romeijn, M. M. et al. The effect of additional protein on lean body mass preservation in post-bariatric surgery patients: A systematic review. Nutr. J. 20, 27. https://doi.org/10.1186/s12937-021-00688-3 (2021).
Morton, R. W. et al. A systematic review, meta-analysis and meta-regression of the effect of protein supplementation on resistance training-induced gains in muscle mass and strength in healthy adults. Br. J. Sports Med. 52, 376–384. https://doi.org/10.1136/bjsports-2017-097608 (2018).
Dattilo, M. et al. Sleep and muscle recovery: Endocrinological and molecular basis for a new and promising hypothesis. Med. Hypotheses 77, 220–222. https://doi.org/10.1016/j.mehy.2011.04.017 (2011).
Lee, D. H. et al. Development and validation of anthropometric prediction equations for lean body mass, fat mass and percent fat in adults using the National Health and Nutrition Examination Survey (NHANES) 1999–2006. Br. J. Nutr. 118, 858–866. https://doi.org/10.1017/S0007114517002665 (2017).
Chen, S., Han, H., Jin, J., Zhou, G. & Li, Z. Osteoarthritis and sarcopenia-related traits: the cross-sectional study from NHANES 2011–2014 and Mendelian randomization study. J. Orthop. Surg. Res. 18, 502. https://doi.org/10.1186/s13018-023-03960-w (2023).
Xiao, Q. et al. L-shaped association of serum 25-hydroxyvitamin D concentrations with cardiovascular and all-cause mortality in individuals with osteoarthritis: Results from the NHANES database prospective cohort study. BMC Med. 20, 308. https://doi.org/10.1186/s12916-022-02510-1 (2022).
Huang, J. et al. Association between lipid accumulation products and osteoarthritis among adults in the United States: A cross-sectional study, NHANES 2017–2020. Prev. Med. 180, 107861. https://doi.org/10.1016/j.ypmed.2024.107861 (2024).
Wang, J. et al. Vitamin D status and risk of all-cause and cause-specific mortality in osteoarthritis patients: results from NHANES III and NHANES 2001–2018. Nutrients https://doi.org/10.3390/nu14214629 (2022).
Liang, W. et al. Development and validation of a clinical risk score to predict the occurrence of critical illness in hospitalized patients with COVID-19. JAMA Intern. Med. 180, 1081–1089. https://doi.org/10.1001/jamainternmed.2020.2033 (2020).
Zhao, L. et al. Relationship between composite dietary antioxidant index and depression among overweight and obese adults. J. Affect. Disord. 341, 358–365. https://doi.org/10.1016/j.jad.2023.08.140 (2023).
Ding, L. et al. The prognostic value of the stress hyperglycemia ratio for all-cause and cardiovascular mortality in patients with diabetes or prediabetes: Insights from NHANES 2005–2018. Cardiovasc. Diabetol. 23, 84. https://doi.org/10.1186/s12933-024-02172-8 (2024).
Long, H. et al. Association between body composition and osteoarthritis: A systematic review and meta-analysis. Int. J. Rheum. Dis. 22, 2108–2118. https://doi.org/10.1111/1756-185X.13719 (2019).
Karlsson, M. K., Magnusson, H., Coster, M., Karlsson, C. & Rosengren, B. E. Patients with knee osteoarthritis have a phenotype with higher bone mass, higher fat mass, and lower lean body mass. Clin. Orthop. Relat. Res. 473, 258–264. https://doi.org/10.1007/s11999-014-3973-3 (2015).
Karlsson, M. K. et al. Patients with hip osteoarthritis have a phenotype with high bone mass and low lean body mass. Clin. Orthop. Relat. Res. 472, 1224–1229. https://doi.org/10.1007/s11999-013-3395-7 (2014).
Segal, N. A. & Toda, Y. Absolute reduction in lower limb lean body mass in Japanese women with knee osteoarthritis. J. Clin. Rheumatol. 11, 245–249. https://doi.org/10.1097/01.rhu.0000182148.74893.3f (2005).
Kim, S. R. et al. Associations between fat mass, lean mass, and knee osteoarthritis: The Fifth Korean National Health and Nutrition Examination Survey (KNHANES V). Calcif. Tissue Int. 99, 598–607. https://doi.org/10.1007/s00223-016-0190-y (2016).
Ho-Pham, L. T., Lai, T. Q., Mai, L. D., Doan, M. C. & Nguyen, T. V. Body composition in individuals with asymptomatic osteoarthritis of the knee. Calcif. Tissue Int. 98, 165–171. https://doi.org/10.1007/s00223-015-0080-8 (2016).
Dos Santos, J. M. et al. Oxidative stress biomarkers and quality of life are contributing factors of muscle pain and lean body mass in patients with fibromyalgia. Biology https://doi.org/10.3390/biology11060935 (2022).
Henrotin, Y., Kurz, B. & Aigner, T. Oxygen and reactive oxygen species in cartilage degradation: Friends or foes?. Osteoarthritis Cartilage 13, 643–654. https://doi.org/10.1016/j.joca.2005.04.002 (2005).
Riegger, J., Schoppa, A., Ruths, L., Haffner-Luntzer, M. & Ignatius, A. Oxidative stress as a key modulator of cell fate decision in osteoarthritis and osteoporosis: A narrative review. Cell. Mol. Biol. Lett. 28, 76. https://doi.org/10.1186/s11658-023-00489-y (2023).
Park, S. & Shin, B. K. Intermittent fasting with a high-protein diet mitigated osteoarthritis symptoms by increasing lean body mass and reducing inflammation in osteoarthritic rats with Alzheimer’s disease-like dementia. Br. J. Nutr. 127, 55–67. https://doi.org/10.1017/S0007114521000829 (2022).
Velarde-Mejia, Y. et al. Joint damage and low lean body mass in a cohort of peruvian patients with rheumatoid arthritis. J. Clin. Rheumatol. 30, 251–254. https://doi.org/10.1097/RHU.0000000000002115 (2024).
Li, X. et al. Genetic variation in lean body mass, changes of appetite and weight loss in response to diet interventions: The POUNDS Lost trial. Diabetes Obes. Metab. 22, 2305–2315. https://doi.org/10.1111/dom.14155 (2020).
Dubois, V., Laurent, M., Boonen, S., Vanderschueren, D. & Claessens, F. Androgens and skeletal muscle: Cellular and molecular action mechanisms underlying the anabolic actions. Cell. Mol. Life Sci. 69, 1651–1667. https://doi.org/10.1007/s00018-011-0883-3 (2012).
Schmidt, M. et al. Androgen conversion in osteoarthritis and rheumatoid arthritis synoviocytes–androstenedione and testosterone inhibit estrogen formation and favor production of more potent 5alpha-reduced androgens. Arthritis Res. Ther. 7, R938-948. https://doi.org/10.1186/ar1769 (2005).
Freystaetter, G. et al. Total serum testosterone and western ontario and mcmaster universities osteoarthritis index pain and function among older men and women with severe knee osteoarthritis. Arthritis Care Res. 72, 1511–1518. https://doi.org/10.1002/acr.24074 (2020).
Martin-Millan, M. & Castaneda, S. Estrogens, osteoarthritis and inflammation. Joint Bone Spine 80, 368–373. https://doi.org/10.1016/j.jbspin.2012.11.008 (2013).
Acknowledgements
The authors gratefully appreciate the time and effort given by the participants during the NHANES data collection.
Funding
This work was supported by Construction of PhD Research and Innovation Team in Acupuncture and Moxibustion Discipline (grant numbers 2017sit01).
Author information
Authors and Affiliations
Contributions
H.L: Data curation, Writing-original draft, and Formal Analysis. Z.Z and G.W: Methodology, Visualization, and Validation. M.Z and C.Y: Project administration, Writing-editing, and Visualization. D.W: Supervision, Funding acquisition, Writing-review & editing. All authors reviewed the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Ethical approval
The NHANES protocol was approved by the National Center for Health Statistics of the Institutional Review Board.
Informed consent
Informed consent was obtained from all subjects involved in the study.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Lu, H., Zhuang, Z., Wang, G. et al. Association between lean body mass and osteoarthritis: a cross-sectional study from the NHANES 2007–2018. Sci Rep 15, 14726 (2025). https://doi.org/10.1038/s41598-025-98795-2
Received:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s41598-025-98795-2