Abstract
Aging affects the 12-lead electrocardiogram (ECG) and correlates with cardiovascular disease (CVD). AI-ECG models estimate aging effects as a novel biomarker but have only been evaluated on single ECGs—without utilizing longitudinal data. We validated an AI-ECG model, originally trained on Brazilian data, using a German cohort with over 20 years of follow-up, demonstrating similar performance (r2 = 0.70) to the original study (0.71). Incorporating longitudinal ECGs revealed a stronger association with cardiovascular risk, increasing the hazard ratio for mortality from 1.43 to 1.65. Moreover, aging effects were associated with higher odds ratios for atrial fibrillation, heart failure, and mortality. Using explainable AI methods revealed that the model aligns with clinical knowledge by focusing on ECG features known to reflect aging. Our study suggests that aging effects in longitudinal ECGs can be applied on population level as a novel biomarker to identify patients at risk early.
Similar content being viewed by others
Introduction
Aging is a universal biological process that is characterized by the gradual decline in physiological functions, increasing the vulnerability to cardiovascular diseases (CVD) and thus mortality. Therefore, advanced “chronological age,” i.e., the years passed since birth, is a major component of clinical risk scores1,2,3,4. In contrast, the concept of “biological age” aims to include individual aging effects5 based on the underlying hypothesis that aging is decelerated in a beneficial environment and accelerated in challenging conditions. Multiple biomarkers such as blood samples6,7 or cardiac ultrasound8 have been proposed to detect patients at risk for pronounced aging effects; however, they did not find their way to routine check-ups. In this paper, we study aging effects in the electrocardiogram (ECG) as a novel biomarker for cardiovascular risk including atrial fibrillation (AF), heart failure (HF), myocardial infarction (MI), and mortality.
ECG measures the heart’s electrical activity, offering clues not only about acute cardiac conditions but also the subtle changes associated with chronic diseases and aging. Changes in ECG patterns over time can reflect alterations in cardiac structure and function9,10,11. During aging, there are two main adaptations that are visible on the ECG. The first is ventricular hypertrophy, which describes a degree of cardiac remodeling12 necessary to compensate for the stiffness of aged vessels13, especially of the aortic arch. The second is fibrosis, which is the molecular consequence of remodeling in which functional tissue is replaced by connective tissue to limit dilatation by holding the enlarged myocardium together14,15. These adoptions manifest slowly over a long period of time and are influenced by the presence and absence of cardiovascular diseases16,17. This underlines the potential for serial ECGs, i.e., recordings from the same subject at different points in time, to reveal aging effects. Using serial instead of single ECGs, for instance, increases the performance in detecting HF9, malignant ventricular arrhythmias18, or MI19. For example, in MI, diagnostic ECG signs—associated with poor outcomes in single ECGs—when still present on follow-up ECGs were shown to increase mortality by a HR of 1.47 (95% CI: 1.10–2.13)19.
Recently, artificial intelligence-based electrocardiography models (AI-ECGs) became available which process 12-lead ECGs and predict a patient’s biological age20,21, denoted as ECG-age. The difference between the predicted ECG-age, i.e., the biological age of the myocardium, and the actual chronological age shows the potential to serve as a novel biomarker for cardiovascular risk: Patients whose ECG-age exceeded their chronological age by more than 8 years exhibited a higher risk of mortality or CVD, while those with an ECG-age at least 8 years less than their chronological age showed a reduced risk20,21.
Despite the reported high accuracy of the AI-ECG20,21 model, its decision-making process and influence on standard ECG parameters are still unclear. Here we provide a thorough analysis of aging effects by means of serial ECGs as well as long-term follow-ups for decades, contributing to novel insights into the AI-ECG mechanisms. However, our primary aim is not to predict mortality directly but to explore the use of ECG-based aging effects for subtle subclinical changes to identify patients at increased cardiovascular risk early. Overall, in this work, we (1) associate the ECG aging effects with increased cardiovascular risk in a population-based cohort study with long follow-ups, (2) investigate to what extent serial ECGs provide additional information compared with single ECGs, and (3) explain the aging effects that the AI-ECG model learned.
Results
Validation of AI-ECG
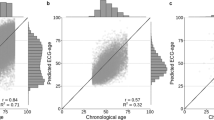
Figure 1 shows the individual predictions of the AI-ECG in the SHIP START subjects and study phases. The differences of the chronological age at ECG recording and predicted age (δage = predicted age − chronological age) were computed from each ECG and classified as either “Overestimation” (δage > 8 years), “Correct Prediction” (−8 years ≤ δage ≤ 8 years), or “Underestimation” (δage < −8 years). The MAE of age prediction was reported in the original CODE dataset as 8.3820 and is 8.15 in the SHIP cohort, which leads to well-distributed group sizes. The correlation coefficient r and the coefficient of determination r2 are with 0.84 and 0.71 at baseline very similar to the results reported in the original publication20 with 0.84 and 0.70, respectively. In the follow-up, this is decreasing with increasing mean age of the cohorts which can be seen in Table 1. The baseline in “START-0” has a mean age of 49.74 years which is very similar to the mean age of the CODE dataset of 51 years. The mean age is increasing over the follow-ups until 60.12 in “START-3” with decreasing r2. Moreover, there are subtle indications of an S-curve pattern, particularly at the edges of the age distribution, where especially predictions for younger subjects (under 40 years) appear to show slightly more deviation.
Data stems from the SHIP-START cohort study, which includes serial ECGs taken 5–6 years apart for the same individuals. The chronological age of subjects is plotted against the predicted ECG-age to assess the accuracy of the AI-ECG predictions. The color code represents the prediction accuracy: “Correct Prediction” (blue) is defined as a predicted ECG-age within ±8 years of the chronological age. “Overestimation” (orange) indicates a predicted ECG-age more than 8 years higher, while “Underestimation” (green) refers to a predicted ECG-age more than 8 years lower. Histograms display the frequency distributions of chronological and predicted ECG-ages. The correlation coefficient r and the coefficient of determination r2 show a decreasing trend over time.
Figure 2 depicts the survival curves of the three defined groups. The subjects classified as “Overestimation,” i.e., more pronounced aging effects, have the lowest survival rate over the whole follow-up period. Subjects classified as “Underestimation,” i.e., with less pronounced aging effects, have the highest survival rates whereas “Correct Prediction” is between both. The sex distribution differs between the groups with 40% females in “Overestimation,” 53% in “Correct Prediction,” and 66% in “Underestimation.” However, age distribution is similar with 56.80 ± 9.53 for “Overestimation,” 57.16 ± 10.25 for “Correct Prediction,” and 58.14 ± 9.92 for “Underestimation,” indicating a balanced comparison of mortality outcomes w.r.t. aging effects.
Tables 2 and 3 show the association between aging effects categories (“Overestimation” or “Underestimation,” while “Correct Prediction” serving as the reference category) and cardiovascular events, adjusted for patient demographics and relevant comorbidities.
In Table 2, “Overestimation” was strongly linked to higher odds of AF diagnosis (2.72 (2.02–3.65), p < 0.001) or developing AF in future (1.69 (1.20–2.38), p = 0.003), while “Underestimation” significantly lowered the odds of AF diagnosis (0.22 (0.10–0.48), p < 0.001) or developing AF (0.37 (0.19–0.72), p = 0.004).
For HF, “Overestimation” increased the likelihood of both HF diagnosis (1.39 (1.20–1.62), p < 0.001) and future HF (1.40 (1.11–1.78), p = 0.005). “Underestimation,” however, was linked to reduced odds of having HF (0.78 (0.65–0.95), p = 0.011), while no significant effect was found for future HF (1.00 (0.75–1.33), p = 0.997).
In the case of MI, “Overestimation” was tied to a higher chance of a prior MI diagnosis (1.27 (1.04–1.56), p = 0.022), though it had no significant effect on future MI events (1.10 (0.73–1.65), p = 0.653). “Underestimation” corresponded to lower odds of having MI (0.60 (0.44–0.83), p = 0.002), with no notable impact on developing MI in future (1.24 (0.89–1.75), p = 0.209).
For mortality, “Overestimation” was associated with an elevated risk of death within the study (1.47 (1.29–1.67), p < 0.001), while “Underestimation” was linked to a decreased risk of death (0.55 (0.46–0.65), p < 0.001).
When separated by sex, the associations between the defined aging effect groups and cardiovascular outcomes were generally more pronounced in females compared to males (Table 3). For instance, females with “Overestimation” had notably higher odds of having AF (4.19 (2.41–7.28), p < 0.001) than males (2.30 (1.62–3.27), p < 0.001), and developing AF (2.06 (1.19–3.54), p = 0.009) was significant in females only. Similar trends were observed for HF and mortality, where “Overestimation” was linked to elevated odds in females, while the associations were generally weaker and non-significant in males. “Underestimation” showed a protective effect for both sexes, but this effect was also stronger in females, particularly for AF and mortality. Interestingly, MI diagnosis was significantly correlated with lower OR in males only (0.57 (0.39–0.84), p = 0.005).
The results for HR regarding mortality risk are shown in Table 4. “Overestimation” was significantly associated with increased mortality risk, with a HR of 1.41 ((1.27–1.57), p < 0.001) after adjusting for age and sex, and an HR of 1.39 ((1.24–1.55), p < 0.001) with full adjustment. Conversely, “Underestimation” was associated with a reduced mortality risk, with HRs of 0.64 ((0.54–0.75), p < 0.001) and 0.65 ((0.55–0.76), p < 0.001) for the age and sex-adjusted and fully adjusted models, respectively.
Impact of serial ECGs
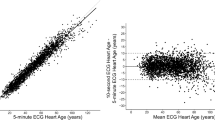
Figure 3 depicts results comparing HRs for overall mortality using different amounts of the SHIP ECG data. We compare “Overestimation” using only baseline ECGs (“baseline”), only the first follow-up (“follow-up”), and across both baseline and follow-up periods (“serial ECG - consistent prediction”). When only a single ECG is considered, the mortality risk at baseline and follow-up show a HR of 1.44 (95% CI: 1.16–1.80, p = 0.001) and 1.43 (1.14–1.80, p = 0.002), respectively. Serial ECG adds novel information to mortality prediction by increasing the HR for subjects with pronounced aging effects to 1.65 (95% CI: 1.25–2.17, p < 0.001). The complete results for serial ECG subgroups are shown in Table 5.
The first row presents data exclusively from a single ECG at baseline examination, illustrating initial risk association. The second row focuses on single ECG from the first follow-up examinations conducted after 5–6 years. The third row includes “Serial ECGs—Consistent Predictions” that showed “Overestimation” in both, baseline and follow-up ECGs. To analyze the influence of serial ECGs, “Follow-up” is compared to “serial ECG,” with both having a follow-up period of up to 16 years w.r.t. mortality data. “Baseline” shows the influence of a longer follow-up period w.r.t. mortality data of up to 21 years in the same individuals.
XAI analysis
Regarding XAI analysis of the AI-ECG model, the precordial leads of two subjects are exemplarily plotted in Fig. 4. Pseudo-colors in green and orange depict positive relevances computed by the XAI method. On the left, XAI results for a 51-year-old woman who is predicted as being 72 years old are depicted. Relevance is mainly focused on the QRS-complex, but also on P- and T-waves. However, early R progression as a sign of right ventricular hypertrophy indicated by a dominant R wave is highlighted by relevances on lead V3. On the right, XAI results for a 52-year-old woman predicted as being 41 years old is shown. The relevances are on similar leads and waveforms compared to the ECG shown on the right, whereas the R progression is not highlighted. The full 12-lead ECGs of both examples can be seen in Supplementary Figs. 1 and 2.
The left side depicts the precordial leads of a 51-year-old woman who is predicted as 72 years old. Relevances are highlighted in orange. The right side shows the precordial leads of a 52-year-old woman who is predicted as 41 years old. Relevances are highlighted in green. Both ECG show regular sinus rhythm whereas the first shows early R progression, which is caused by ventricular hypertrophy and partial atrial fibrosis; all findings are typical for “cor pulmonale.” The depicted grid is identical to standard ECG showing 10 mm/mV and 25 mm/s.
To further investigate the leads and waveforms that mostly contributed to the AI-ECG’s decision-making, lead importance is shown in Fig. 5. As can be seen, precordial leads V1 and V4 contribute mostly to the detection of the aging effect by the AI-ECG. Furthermore, there seems to be a subtle tendency for the “Overestimation” class to focus more on the right-sided leads V1-V3, whereas the “Underestimation” class leans slightly toward the left-sided leads V4-V6.
Results are categorized by predicted aging effects. Each boxplot shows how relevant each precordial lead was in determining whether an ECG was classified as “Overestimation,” “Correct Prediction,” or “Underestimation” based on the AI's aging predictions. This visualization helps to understand which specific leads contributed most to the AI's assessment of aging effects in the ECG recordings.
Based on the lead importance results, we selected leads V1 and V4 to investigate which part of the P-QRS-T-waveform mostly contributes to the AI-ECG’s decision-making. Figure 6 shows results for lead V1 at the top and lead V4 at the bottom. Positive and negative relevances in AI-based ECG analysis highlight parts of the waveform that influence age prediction. Positive relevances indicate regions of the ECG where aging effects are more pronounced, leading to a higher predicted age. Negative relevances, on the other hand, to regions that correspond to reduced aging effects, resulting in a younger predicted age. These relevances help to pinpoint which waveform the AI-ECG considers important in assessing biological age. In general, we observe that all parts of the ECG waveforms influence age prediction whereas both leads focus on different aspects. In lead V1, the P-wave and the QRS-complex have a major influence where the whole P-wave indicates pronounced aging effects and 30 ms before and after the R-peak indicates reduced aging effects. In contrast, the following 60 ms of the QRS-complex show positive relevances pointing to pronounced aging effects. In lead V4, one can observe mainly positive relevances for the R-peak and T-waves that indicate that these waveforms are important to detect pronounced aging effects and little emphasis on the P-wave.
The relevance values are normalized between −1 and 1 and aligned by the detected R peaks (dashed line) to obtain an average relevance over all subjects and heartbeats. Standard ECG parameters such as P-wave or QRS-complex are defined via physiological boundaries. Positive relevances indicating more pronounced aging effects resulting in a higher age prediction whereas negative relevances result in the prediction of younger age.
In Fig. 7, concordance of the AI-ECG with clinical standard parameters that are known from the literature to change with the myocardial aging processes22 can be seen. P duration, PR interval, QRS duration and QTc interval are plotted w.r.t. the investigated groups. All parameters are highly significant differing when grouped by the AI-ECG’s detected aging effects. Despite this statistical significance, one can observe that over all boxplots, there is a high overlap, i.e., both mean and standard deviation differ only slightly. This indicates that there have to be further minor effects which are highly important for the AI-ECG model.
Shown are P duration, PR interval, QRS duration, and QTc interval22. Each feature is positively correlated with higher age groups. Boxplots display significant differences across age groups, as indicated by the asterisks (***), denoting a highly significant statistical difference (p < 0.001) calculated with a two-sided t-test.
Discussion
Our results indicate that the AI-ECG model is able to associate aging effects with increased mortality risk given by a HR of 1.41 (1.27–1.57, p < 0.001) for “Overestimation,” i.e., more pronounced aging effects, over the 21 years follow-up period of the SHIP study. “Underestimation,” i.e., reduced aging effects, results in a HR of 0.64 (0.54–0.75, p < 0.001). Hence, we could reproduce the findings of the initial study20 that showed an increase of 1.53 (1.30–1.80, p < 0.001) for “Overestimation” and 0.66 (0.57–0.76, p < 0.001) for “Underestimation” with a follow-up of 3.4 ± 1.5 years. Another work based on the population-based Framingham heart study21 reported an HR for mortality of 1.37 (1.25–1.50, p < 0.001) for “Overestimation” and HR 0.79 (95% CI: 0.73–0.86, p < 0.001) for “Underestimation” with a follow-up of 17 ± 8 years.
Despite mortality, the authors also reported CVDs: For AF incidence, an HR of 1.44 (1.23–1.69, p < 0.001) was reported for “Overestimation” and 0.89 (0.75–1.05, p = 0.16) for “Underestimation”21. While we have similar results for “Overestimation” (OR: 1.69 (1.20–2.38, p = 0.003)), we achieved different results for “Underestimation” (OR: 0.37 (0.19–0.72, p = 0.004)). The tendency for HF incidence was similar with reported HR of 1.75 (1.45–2.12, p < 0.001) for “Overestimation” and 0.73 (0.56–0.88, p = 0.002) for “Underestimation”21, while our OR were 1.40 (1.11–1.78, p = 0.005) for “Overestimation” and 0.78 (0.65–0.95, p = 0.011) for “Underestimation. Interestingly, the results of MI incidence were not significant in the Framingham heart study21 as well as in our study data. Since MI is caused by an occluded coronary vessel23, no significant correlation to structural heart changes like fibrosis or remodeling—that we hypothesize are learned by the AI-ECG model—seems logical. Furthermore, we found for the prevalence of MI an OR of 0.60 (0.44–0.83, p = 0.002) for “Underestimation,” which could indicate that structural changes of the myocardium after the MI event can be detected.
Overall, our results suggest that the AI-ECG model effectively captures fundamental aging-related changes in cardiac function that are generalizable across different geographic locations, healthcare systems, and patient populations as the ECGs were acquired in different cohorts (USA, Brazil, Germany) and stem from population and clinical studies. Previous studies have developed AI models that directly predict individual mortality from ECG data24,25. In contrast, our indirect approach—using aging effect as cardiovascular biomarker—is associated not only with mortality but also with the incidence of specific cardiovascular diseases, such as AF and HF. This indicates that aging effects may reflect subclinical pathologies in an early stage. Individuals at risk might benefit from early interventions before cardiovascular events like diseases or mortality occur. We hypothesize that the difference between the AI-predicted ECG-age and the chronological age captures important information about cardiovascular health that may not be fully reflected in direct mortality prediction models. However, comparing direct mortality prediction to the indirect approach using aging effects is an avenue of future work. Additionally, our analysis revealed a potential sex bias in the OR, particularly in the detection of AF incidence and mortality, suggesting an area for future investigation. Since ECG parameters are known to vary between sexes22,26, it may be beneficial to develop AI-ECG models that account for aging effects separately for males and females7 to avoid unfair predictions.
In this work, serial ECGs were used for the first time for ECG-age prediction, enabling to follow the trajectory of multiple thousand patients over time. We observed that the additional information gathered in the serial ECG improves the HR for mortality from 1.43 (95% CI: 1.16–1.80) with a single ECG from follow-up to 1.65 (95% CI: 1.25–2.17) when using both ECGs from baseline and follow-up examinations.
To gather which aging effects the AI-ECG model learned over all subjects, leads, and waveforms, we extended an established XAI workflow27. Analyzing individual ECG signals for their relevance distributions (Fig. 6) clearly demonstrates that relevances are aggregated at regular positions across the P-QRS-T patterns. For example, in right-sided lead V1, the AI-ECG focuses on the atrial activity (P-wave), while in left-sided lead V4, the ventricular activity (R-peak and T-wave) is of importance.
Comparing differences between leads (Fig. 5) demonstrates the importance of lead configuration. Leads V1 and V4 were of high importance while all other leads also contributed to the AI-ECG’s decision-making. Hence, the entire 12-lead ECG is important for the AI-ECG when determining aging effects. Generally, the right-sided leads V1–V3 have a tendency to more pronounced aging effects whereas the left-sided leads V4–V6 are slightly more important to predict reduced aging effects. While acknowledging the importance of all leads, we concentrated our detailed waveform analysis on leads V1 and V4 due to their specific clinical relevance. Lead V1 provides insights into atrial activity, whereas lead V4 offers information on ventricular activity. This focus allowed us to explore how the AI model captures aging effects in both atrial and ventricular regions, providing clearer insights into its predictive mechanisms. Additional analyses of all leads, including similar relevant leads I and V5, are provided in the Supplementary Figs. 3–7. Comparing our findings to established clinical ECG features known for longer durations and intervals in older individuals22 shows that these conventional features are not sufficient to distinguish between the groups (Fig. 7). This may be explained by the fact that the model’s decisions are based on a multifactor decision with features distributed across the heartbeats and leads as demonstrated in Fig. 6.
Regarding clinical use, our findings indicate that aging effects in ECG learned by the AI-ECG model can be linked to increased cardiovascular risk. We presume they are based on both physiological and pathological changes in cardiac structure like fibrosis and remodeling. Its decisions are based on a multifactorial process including numerous standard ECG parameters like enlarged P amplitude, prolonged PR intervals and R progression on the front wall, indicating right-sided cardiac remodeling. Since the effects are minor but significant, they can be effectively detected by the AI-ECG model. This eventually could be used to detect pronounced aging effects as subclinical indicators in common screening programs. Subsequently, cardiac remodeling could be diagnosed via cardiac ultrasound as hypertrophic myocardium, i.e., the heart muscle appears enlarged and thickened8,12. The gold standard to quantify cardiac fibrosis is the Late Gadolinium Enhancement, in which a contrast agent accumulates in the micro scars during a cardiac magnetic resonance imaging (MRI)28. However, both are not part of routine check-ups as they are expensive and time-consuming. Moreover, regularly there is no clinical consequence after this investigation since the level of remodeling and fibrosis is too advanced and cannot be reversed29,30. This highlights the urgent need for novel methods to quantify the level of both cardiac remodeling and fibrosis in early stages to prevent the irreversible manifestations in the myocardium15,31,32,33,34,35. Since the ECG is the most frequent diagnostic tool36, quantification of aging effects by AI-ECG could be a suitable tool to identify patient at risk that could benefit from additional medical attention like cardiac ultrasound or MRI.
Despite the promising results for association with increased cardiovascular risk, the current AI-ECG model has certain limitations regarding (1) age distribution, (2) sex differences, and (3) availability of serial ECG data.
First, the age range during the development of the model was unbalanced with respect to younger and older subjects since the CODE dataset is based on clinical ECG examinations from a telehealth network. Although the age range was addressed during development, effects can still be seen, especially in subject under 40 years, indicating that a more balanced age dataset could possibly further improve the robust detection of ECG-based aging effects.
Second, differences w.r.t sex were expected due to anatomical differences that are also present in other ECG-based diagnoses, such as the diagnosis of myocardial infarction23. Therefore, the development of sex-adjusted AI-ECG models with mitigated bias should be addressed in future work to make AI transparent and non-discriminatory37.
Third, regarding the follow-up period, our analysis was limited to only two serial ECGs due to the available follow-up data of the second cohort in the SHIP study. This limitation restricts the assessment of longitudinal changes in ECG patterns over time. Our study serves as a proof-of-concept highlighting the potential of serial ECGs for risk classification. Future research incorporating more ECGs across longer follow-up periods would allow for even more comprehensive insights.
As the global demographic shifts towards an older population, understanding the dynamics between aging and diseases becomes crucial. Our results underline the potential of using the standard 12-lead ECG as biomarker due to the high correlations between aging effects, disease occurrence and mortality. Our XAI results confirm that the ECG-AI model makes use of already-known ECG features—representing the underlying biological effects of cardiac remodeling—while also using a complex decision-making process with different weights for each lead. The performance of the AI-ECG model for aging effects was shown to be robust over different healthcare system (Brazil, USA, Germany) and can be improved by additional follow-up examinations (serial ECG). Since the 12-lead ECG is the most commonly used exam in regular screening programs, this method could be implemented with only minor additional effort and costs in these programs to detect cardiac remodeling early with the aim to prevent cardiovascular events before their manifestation. However, our study also shows the need for careful adjustment of the AI-ECG model, including a broader age range and different predictions depending on patient sex, to address potential bias.
Methods
Dataset
The Study of Health in Pomerania (SHIP) is a population-based study conducted in Western Pomerania, Germany. It encompasses two independent cohorts with follow-up examinations: SHIP-START and SHIP-TREND. The baseline examinations are designated SHIP-START-0, SHIP-TREND-0, and the follow-up examinations at 5–6 year intervals have running numbers, e.g., SHIP-START-1, SHIP-START-2. All subjects received examinations adhering to the SHIP protocol, including 12-lead ECG acquisition. Descriptive statistics for all subjects can be found in Table 1 and prevalence for CVD in Table 6. The prevalence for the CVDs was collected during the baseline and follow-up examination. Mortality data, including overall death and cardiovascular death, was extracted from the subjects’ health insurance records including a longer time period than the follow-up examinations.
To further assess the influence of CVD like diabetes and ECG-altering medications on ECG signals, we extracted relevant data using “Anatomical Therapeutic Chemical” (ATC) codes (see Table 7). Specifically, we mapped each medication category to its respective ATC codes: Metformin and other oral antidiabetics (“A10BA”), Insulins (“A10A”), and antihypertensives (“C02,” “C03,” “C07,” “C08,” and “C09”). For medications impacting the ECG, we identified beta blockers (“C07AA,” e.g., metoprolol and bisoprolol), calcium channel blockers (“C08CA,” e.g., verapamil), and antiarrhythmic drugs (“C01AA,” e.g., amiodarone).
Ethical considerations
The SHIP studies adhered to the ethical guidelines of the 1964 Helsinki Declaration. The Ethics Committee at the University Medicine Greifswald approved the SHIP studies (approval number BB 39/08). All participants provided written informed consent before participation in the study. Data for this work was acquired via the Transfer Unit for Data and Biomaterials of the Institute of Community Medicine at the University Medicine Greifswald.
AI-ECG model
We use a freely available pre-trained ResNet (available at: https://github.com/antonior92/ecg-age-prediction) trained on clinical ECG exams of the Brazilian TeleHealth Network of Minas Gerais20,38,39. The model processes 12-lead ECGs and predicts the age of the patient in an end-to-end fashion, without any intermediate steps—such as filtering or feature extraction—defined by a human. The model was trained on ECGs from 1,558,415 patients.
Signal quality
To assess the quality of the ECG signals, we calculated the Signal-to-Noise Ratio (SNR) for each recording of the SHIP study. Following the methodology described in previous work40, the SNR was computed based on the ratio of signal power to noise power in the ECG frequency spectrum. We used the Fourier Transform to separate the signal frequencies (0.66 to 2.5 Hz, corresponding to 40–150 beats per minute) from the noise frequencies and expressed the SNR values on a logarithmic decibel scale.
To evaluate the impact of signal quality on our model’s predictions, we investigated the relationship between SNR and the predicted ECG-age. Pearson correlation analysis revealed no significant linear relationship, with a correlation coefficient of r = −0.0016 and a p-value of 0.857. This indicates that the AI-ECG model is robust to variations in signal quality. In a previous work, we benchmarked the impact of noise on deep learning40 and similarly showed that the influence of noise is minimal for this model architecture due to its ability to filter noise in the initial layers. Additionally, during the development of the AI-ECG, raw data were used, and similar observations were made regarding signal quality20.
In conclusion, no ECGs were excluded from our analyses due to signal quality.
ECG aging effects and cardiovascular risk
As proposed in ref. 20, we use a mean absolute error (MAE) of 8 years as threshold: If the predicted ECG-age of a subject is more than 8 years higher than the real chronological age, we assume “Overestimation,” i.e., more pronounced aging effects. ECG-ages that are more than 8 years lower than the chronological age are classified as “Underestimation,” referring to reduced aging effects. To account for the current limitation of the model to older age groups, we examine a typical age range for population-wide screening programs of 40–75 years, including 12,724 ECG recordings. The lower age limit of 40 years has been set since the age group of subjects under 40 years was underrepresented during the development of the AI-ECG model20. Furthermore, it is anticipated that the majority of this age group will not exhibit pronounced age-related effects. The upper limit of 75 years was set since the age range of the SHIP41 is higher than that of the CODE dataset20,38, especially in the follow-up periods. Information on vital status of all SHIP study participants was requested from local health authorities in regular intervals from the time of enrollment into the study through last contact March 31, 2019. Subjects were censored at death or last contact. The number of years between examination and censoring was used as the follow-up length w.r.t. vital status. To analyze the incidence of CVDs, the prevalence was used to calculate which subjects developed AF, HF or MI between follow-up examinations.
Serial ECG analysis
The Study of Health in Pomerania (SHIP) includes successive ECGs from the same individuals at multiple time points, offering a perspective on how ECGs change over time. We simulate a screening scenario by taking the 3190 subjects who participated in the first two examinations: Serial ECGs were defined as having exactly two ECG recordings for the same individual taken at baseline examination (“START-0,” “TREND-0”) and first follow-up examination (“START-1,” “TREND-1”). In the serial ECG cohort, the mean age is 53.89 years including 51.85% female and an overall mortality rate of 11.25%. We defined consistent predictions as those where participants were categorized into the same risk group across both their baseline and follow-up ECGs. This approach highlights stable patterns in ECG-derived predictions, reinforcing the robustness of classifications over time. To compare the impact of serial ECGs compared to single measurements, we applied three different scenarios:
-
1.
Baseline: Using only the ECGs recorded during the initial recruitment.
-
2.
Follow-up: Using only the first follow-up ECGs.
-
3.
Serial ECG: Using ECGs from both time points.
To prevent label leakage, we compare only “Follow-up” directly with “Serial ECG” when investigating the influence of longitudinal data for aging effects associated with increased mortality risk. The time to mortality is adjusted for both conditions to only start at the time of the “Follow-up” examination. The single ECG at “Baseline” can be compared to “Follow-up” to investigate the association over time and the impact of longer follow-up periods w.r.t. mortality.
XAI analysis
We extend and adapt a previously validated Explainable AI(XAI) approach27 using the posthoc attribution method integrated gradients42 to gain insight into the AI-ECG decision-making. We compute “relevance” values for each input sample of each processed ECG and visualize them on top of the standard 12-lead ECG to highlight the most relevant part for aging effects. Importance of individual leads is calculated by lead-wise addition of all relevance values and subsequent normalization across leads. To investigate which part of the P-QRS-T sequence is most important for AI-ECG decision-making, we first detect the R-peaks in the original ECG recordings using the freely available xQRS43 method. For robust detection, we implemented a majority vote to ensure that the R-peak is simultaneously detected in at least 7 leads with a tolerance of 10 ms; otherwise, the heartbeat is not considered. After splitting the ECGs into individual heartbeats, we extract fixed-length segments of 650 ms (250 ms before and 400 ms after each R-peak), aligning each heartbeat without altering durations. This approach maintains the PQRST fiducial points at their physiological positions within each segment.
To prevent overlap due to tachycardia or premature ventricular activity, we excluded segments where the fixed-length windows around R-peaks would overlap with adjacent segments. This exclusion ensures that each analyzed heartbeat is independent and free from interference caused by overlapping cardiac cycles. We define physiological boundaries around the R-peak for visualization (e.g., P-wave) and average the relevances lead-wise across all subjects and recordings. We selected leads V1 and V4 for detailed analysis in our XAI approach, illustrating how the AI-ECG model detects aging effects in different cardiac regions.
All XAI analyses were conducted using Python and are freely available at https://gitlab.gwdg.de/medinfpub/biosignal-processing-group/detecting-aging-effects-in-serial-ecg.
Clinical features describing ECG aging effects
We use clinical standard ECG parameters which are known to change due to aging22 to compare them to the AI-ECG’s decision. The ECG parameters were taken from the SHIP dataset and were measured during the cardiological investigations, namely P duration, PR interval, QRS duration, and QTc interval. The P duration represents the time required for the atria to depolarize, whereas the PR interval refers to the delay between the atria and ventricles in the atrioventricular (AV) node. The QRS duration shows the time needed for the ventricular depolarization while the QTc interval provides the total time for depolarization and repolarization in the ventricles corrected by the heart frequency.
Statistical analysis
To assess the association between AI-ECG-based aging effects and clinical outcomes, ORs and HRs were calculated using logistic regression and Cox proportional hazards models, respectively.
For OR calculation, logistic regression models were applied with predictors being the defined aging effect groups (“Overestimation” or “Underestimation,” with “Correct Prediction” serving as the reference category) for each clinical condition: AF prevalence (“AF diagnosed”), AF incidence (“Will Develop AF”), HF prevalence (“HF diagnosed”), HF incidence (“Will Develop HF”), MI prevalence (“MI diagnosed”), MI incidence (“Will Develop MI”), and Mortality. Models were adjusted for key confounding factors: chronological age, sex, diagnosed hypertension, hyperlipidemia, diabetes (metformin or insulin application), and medications affecting ECG signals (beta blockers, calcium channel blockers, antiarrhythmics). The full OR data are provided in Supplementary Tables 1–4.
For HRs, Cox proportional hazards models examined the relationship between the defined aging effect groups and mortality. During validation on all SHIP ECG cohorts, models included chronological age and sex, with additional adjustments for hypertension, hyperlipidemia, diabetes, and ECG-altering medications. In the smaller serial ECG cohort (3190 subjects), models were adjusted for chronological age and sex. HRs with 95% confidence intervals were reported for each model. Survival plots for both sex and medication subgroups can be seen in Supplementary Figs. 8 and 9.
Statistical significance was determined for each predictor by evaluating the p-values obtained from model coefficients. Additionally, two-sided t-tests were performed to assess differences in clinical features known to influence aging effects.
Data availability
The data of the SHIP study cannot be made publicly available due to the informed consent of the study participants, but it can be accessed through a data application form available at https://fvcm.med.uni-greifswald.de/ for researchers who meet the criteria for access to confidential data.
Code availability
All scripts for analysis, including parallelization for HPC computation, and for generating the figures are freely available in Python at https://gitlab.gwdg.de/medinfpub/biosignal-processing-group/detecting-aging-effects-in-serial-ecg.
References
Dhingra, R. & Vasan, R. S. Age as a risk factor. Med. Clin. North Am. 96, 87–91 (2012).
Cooney, M. T. et al. Cardiovascular risk age: concepts and practicalities. Heart 98, 941–946 (2012).
Hjalmarsson, C. et al. Impact of age and comorbidity on risk stratification in idiopathic pulmonary arterial hypertension. Eur. Respir. J. 51, 1702310 (2018).
Alonso, A. et al. Simple risk model predicts incidence of atrial fibrillation in a racially and geographically diverse population: the CHARGE-AF consortium. J. Am. Heart Assoc. 2, e000102 (2013).
Ansari, M. Y. et al. Estimating age and gender from electrocardiogram signals: a comprehensive review of the past decade. Artif. Intell. Med. 146, 102690 (2023).
Goyal, P., Maurer, M. S. & Roh, J. Aging in heart failure: embracing biology over chronology: JACC family series. JACC Heart Fail. 12, 795–809 (2024).
Keller, K. M. & Howlett, S. E. Sex differences in the biology and pathology of the aging heart. Can. J. Cardiol. 32, 1065–1073 (2016).
Ganau, A. et al. Echocardiographic heart ageing patterns predict cardiovascular and non-cardiovascular events and reflect biological age: the SardiNIA study. Eur. J. Prev. Cardiol. 31, 677–685 (2024).
Sbrollini, A. et al. Automatic diagnosis of newly emerged heart failure from serial electrocardiography by repeated structuring & learning procedure. Biomed. Signal Process. Control 79, 104185 (2023).
Martens, T. et al. QRS duration during follow-up of tetralogy of Fallot: how valuable is it? Analysis of ECG changes in relation to pulmonary valve implantation. Pediatr. Cardiol. 42, 1488–1495 (2021).
Neyazi, M. et al. Deep learning-based NT-proBNP prediction from the ECG for risk assessment in the community. Clin. Chem. Lab. Med. 62, 740–752 (2024).
Bacharova, L. & Estes, E. H. Left ventricular hypertrophy by the surface ECG. J. Electrocardiol. 50, 906–908 (2017).
Lakatta, E. G. & Levy, D. Arterial and cardiac aging: major shareholders in cardiovascular disease enterprises: Part I: aging arteries: a “set up” for vascular disease. Circulation 107, 139–146 (2003).
Biernacka, A. & Frangogiannis, N. G. Aging and cardiac fibrosis. Aging Dis. 2, 158–173 (2011).
Zhu, L., Wang, Y., Zhao, S. & Lu, M. Detection of myocardial fibrosis: where we stand. Front. Cardiovasc. Med. 9, 926378 (2022).
Tomek, J. & Bub, G. Hypertension-induced remodelling: on the interactions of cardiac risk factors. J. Physiol. 595, 4027–4036 (2017).
Wu, Q.-Q. et al. Mechanisms contributing to cardiac remodelling. Clin. Sci. 131, 2319–2345 (2017).
Kolk, M. Z. et al. Dynamic prediction of malignant ventricular arrhythmias using neural networks in patients with an implantable cardioverter-defibrillator. Ebiomedicine 99, 104937 (2024).
Lehmacher, J. et al. Predictive value of serial ECGs in patients with suspected myocardial infarction. J. Clin. Med. 9, 2303 (2020).
Lima, E. M. et al. Deep neural network-estimated electrocardiographic age as a mortality predictor. Nat. Commun. 12, 5117 (2021).
Brant, L. C. et al. Association between electrocardiographic age and cardiovascular events in community settings: the Framingham heart study. Circ. Cardiovasc. Qual. Outcomes 16, e009821 (2023).
Vicent, L. & Martínez-Sellés, M. Electrocardiogeriatrics: ECG in advanced age. J. Electrocardiol. 50, 698–700 (2017).
Thygesen, K. et al. Fourth universal definition of myocardial infarction. Circulation 138, e618–e651 (2018).
Sun, W. et al. Towards artificial intelligence-based learning health system for population-level mortality prediction using electrocardiograms. NPJ Digit. Med. 6, 21 (2023).
Raghunath, S. et al. Prediction of mortality from 12-lead electrocardiogram voltage data using a deep neural network. Nat. Med. 26, 886–891 (2020).
Haukilahti, M. A. E. et al. Electrocardiographic risk markers of cardiac death: gender differences in the general population. Front. Physiol. 11, 578059 (2021).
Bender, T. et al. Analysis of a deep learning model for 12-lead ECG classification reveals learned features similar to diagnostic criteria. IEEE J. Biomed. Health Inform. 28, 1848–1859 (2023).
Siebermair, J., Kholmovski, E. G. & Marrouche, N. Assessment of left atrial fibrosis by late gadolinium enhancement magnetic resonance imaging: methodology and clinical implications. JACC Clin. Electrophysiol. 3, 791–802 (2017).
Emmons-Bell, S., Johnson, C. & Roth, G. Prevalence, incidence and survival of heart failure: a systematic review. Heart 108, 1351–1360 (2022).
Authors/Task Force Members. 2021 ESC guidelines for the diagnosis and treatment of acute and chronic heart failure: developed by the task force for the diagnosis and treatment of acute and chronic heart failure of the European Society of Cardiology (ESC). With the special contribution of the Heart Failure Association (HFA) of the ESC. Eur. J. Heart Fail. 24, 4–131 (2022).
Gjesdal, O., Bluemke, D. A. & Lima, J. A. Cardiac remodeling at the population level—risk factors, screening, and outcomes. Nat. Rev. Cardiol. 8, 673–685 (2011).
Power, D. A. et al. Cardiovascular complications of interatrial conduction block: JACC state-of-the-art review. J. Am. Coll. Cardiol. 79, 1199–1211 (2022).
Bayés de Luna, A., Massó-van Roessel, A. & Robledo, L. A. E. The diagnosis and clinical implications of interatrial block. Eur. Cardiol. Rev. 10, 54 (2015).
Bayés de Luna, A., Baranchuk, A., Robledo, L. A. E., Massó van Roessel, A. & Martínez-Sellés, M. Diagnosis of interatrial block. J. Geriatr. Cardiol. 14, 161–165 (2017).
Chung, M. K. et al. Lifestyle and risk factor modification for reduction of atrial fibrillation: a scientific statement from the American Heart Association. Circulation 141, e750–e772 (2020).
Kessen, B. & Williamson, K. The ECG in clinical medicine. Electrocardiogram in Clinical Medicine, 1–11 (Chicago, 2020).
Vokinger, K. N., Feuerriegel, S. & Kesselheim, A. S. Mitigating bias in machine learning for medicine. Commun. Med. 1, 25 (2021).
Ribeiro, A. L. P. et al. Implementation of a telecardiology system in the state of Minas Gerais: the Minas Telecardio project. Arq. Bras. Cardiol. 95, 70–78 (2010).
Alkmim, M. B. et al. Improving patient access to specialized health care: the telehealth network of Minas Gerais, Brazil. Bull. World Health Organ. 90, 373–378 (2012).
Bender, T. et al. Benchmarking the impact of noise on deep learning-based classification of atrial fibrillation in 12-lead ECG. Stud. Health Technol. Inform. 302, 977–981 (2023).
Völzke, H. et al. Cohort profile update: the Study of Health in Pomerania (SHIP). Int. J. Epidemiol. 51, e372–e383 (2022).
Kokhlikyan, N. et al. Captum: a unified and generic model interpretability library for pytorch. Preprint at https://arxiv.org/abs/2009.07896 (2020).
Xie, C. et al. Waveform database software package (WFDB) for Python (version 3.4. 1) Available at https://doi.org/10.13026/gjvw-1m31 (2021).
Acknowledgements
This work was supported by the Lower Saxony “Vorab” of the Volkswagen Foundation and the Ministry for Science and Culture of Lower Saxony under Grant 76211-12-1/21. The Study of Health in Pomerania is part of the Community Medicine Research Network of the University Medicine Greifswald, which was funded by the German Federal Ministry for Education and Research, the Ministry for Education, Research and Cultural Affairs, and the Ministry for Social Affairs of the State Mecklenburg-Western Pomerania (funding numbers 01ZZ9603, 01ZZ0103, and 01ZZ0403). We acknowledge support by the Open Access Publication Funds/transformative agreements of the Göttingen University.
Funding
Open Access funding enabled and organized by Projekt DEAL.
Author information
Authors and Affiliations
Contributions
P.H.: Conceptualization, Methodology, Software, Validation, Formal Analysis, Investigation, Data Curation, Visualization, Writing—Original Draft, Review and Editing. A.H.R.: Methodology, Software, Investigation, Resources, Writing—Review and Editing. M.V.: Resources, Investigation, Data Curation, Writing—Review and Editing. T.B.: Writing—Review and Editing. M.D.: Resources, Writing—Review and Editing. D.K.: Supervision, Funding Acquisition, Writing—Review and Editing. N.S.: Investigation, Supervision, Project Administration, Writing—Review and Editing. All authors have read and approved the final manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Hempel, P., Ribeiro, A.H., Vollmer, M. et al. Explainable AI associates ECG aging effects with increased cardiovascular risk in a longitudinal population study. npj Digit. Med. 8, 25 (2025). https://doi.org/10.1038/s41746-024-01428-7
Received:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s41746-024-01428-7