Abstract
Post-discharge management of coronary artery disease (CAD) remains clinically challenging, with digital healthcare’s efficacy underexplored. This study analyzed 16,797 CAD patients enrolled in the HeartMed Digital Management System (June 2018–September 2022), comparing outcomes between a digital management (DM, n = 4,713) and conventional management (CM, n = 12,084) cohort over 12 months. Cox models adjusted for confounders revealed significantly reduced all-cause mortality in the DM group (1.6% vs. 2.7%; HR 0.58, 95% CI 0.45–0.75, p < 0.001) and lower risks for major adverse cardiovascular events (MACCE: 6.4% vs. 9.2%; HR 0.67, 0.59–0.77, p < 0.001), cardiovascular death (HR 0.70, 0.51–0.95), myocardial infarction (HR 0.38, 0.29–0.50), recurrent angina (HR 0.75, 0.65–0.87), revascularization (HR 0.84, 0.71–0.99), and readmissions (HR 0.76, 0.68–0.84) (p < 0.05 for all). Digital healthcare demonstrates superior post-discharge optimization of CAD outcomes, significantly attenuating mortality and morbidity.
Similar content being viewed by others
Introduction
Global data underscore that coronary artery disease (CAD) remains a leading cause of mortality worldwide1. While advances in medical care and preventive strategies that have improved post-event survival, neglecting risk factor control, unhealthy behaviors, poor drug compliance and ineffective self-management continue to pose significant challenges affecting the prognosis of patients with CAD2. These factors have been extensively reported in multiple studies as independent predictors of mortality in CAD patients3. Therefore, the life-long management of patients with CAD is very essential. It is well recognized that the traditional outpatient follow-up management mode, reliant on regular hospital visits, has certain limitations. Physicians are often unable to provide comprehensive and real-time supervision of non-pharmacological treatments, such as lifestyle modifications and psychological support. Additionally, treatment compliance can vary significantly among patients due to individual factors such as culture, habits, geographic distance, and economic circumstances4. These phenomena have a detrimental effect on disease prognosis and treatment efficacy, including patients with CAD. Research data reveals that a significant number of CAD patients in developing countries do not receive adequate health guidance. Among these patients, 50% continue to have persistent risk factors, 35% make no changes to their dietary habits, and medication adherence rates are generally around 12%5,6. Therefore, it is essential to implement a convenient, user-friendly, efficient, and widely accessible intelligent out-of-hospital management system to address the current shortcomings in secondary prevention for CAD patients.
With the rapid development of information technology, digital healthcare has started to play an increasingly crucial role in the management of chronic diseases7. Digital healthcare platforms, leveraging mobile media, intelligent software, and wearable devices, have been reported to play a positive role in enhancing the self-management behaviors of CAD patients8,9. Digital healthcare has shown significant potential in the management of chronic diseases, yet its effectiveness among patients with CAD remains under-researched and insufficiently evaluated. This study, therefore, aims to evaluate the role of digital healthcare management in patients with CAD, with a focus on its improvement on medications adherence, risk factors, lifestyle behaviors, and long-term outcomes.
Results
Baseline characteristics
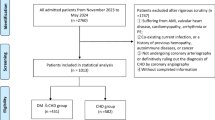
A total of 18565 patients with cardiovascular disease were collected from the “HeartMed Digital Management System” database between June 2018 and September 2022. After excluding patients with coronary artery stenosis of less than 50% or met other exclusion criterias (n = 1768), a final count of 16,797 patients with CAD were included in the study. The mean age of the patients was 63.4 years, with males comprising 69.7% and females 30.3%. All patients were divided into two groups based on their participation in the digital management system: 4713 patients in the DM group and 12,084 in the CM group. The proportion of lost follow-up was not significantly different between the two groups (1.5% vs. 1.6%).
Compared to patients in the CM group, patients in the DM group were older (63.9 ± 11.9 vs. 63.2 ± 11.5 years; p = 0.002) and had a higher mean BMI (26.45 ± 4.60 vs. 25.67 ± 4.25 kg/m2; p < 0.001). A significantly greater proportion of patients in the DM group had a history of chronic heart failure (17.7% vs. 15.8%; p = 0.002) or peptic ulcer (18.7% vs. 16.2%; p < 0.001), and a higher percentage of them also developed hyperlipidemia (29.2% vs. 27.2%; p = 0.008). No significant differences were observed in the other variables.
Patients in the DM group had significantly higher HRs compared to the CM group (73.2 ± 10.4 vs. 67.7 ± 10.2bpm; p < 0.001) and exhibited lower SBP levels (126.1 ± 15.6 vs. 130.2 ± 12.8 mmHg; p < 0.001). Moreover, echocardiographic evaluations revealed a slightly lower LVEF in the DM group (54.8 ± 11.5% vs. 55.3 ± 11.5%; p = 0.016). In terms of laboratory data, these patients also demonstrated significantly higher levels of LDL-c (2.89 ± 1.43 vs. 2.78 ± 1.42 mmol/L; p < 0.001), but lower levels of HbA1c (6.19 ± 1.98%vs. 6.29 ± 1.76%; p = 0.033). Additionally, coronary angiography during hospitalization indicated a higher prevalence of multivessel coronary lesions in the DM group (40.2% vs. 35.0%; p < 0.001), and a greater number of patients in the DM group underwent interventional therapy compared to the CM group (62.4% vs. 55.9%; p < 0.001), yet there were no significant differences in the length of hospitalization between the two groups (p = 0.148). Detailed information is presented in Table 1.
Patient characteristics of post-discharge follow-up
Post-discharge follow-up data of participants are presented in Tables 2–5. For risk factor management (Table 2), the DM group showed more significant improvements compared to the CM group. The smoking rate decreased more markedly in the DM group at both the 3rd month (31.3% vs. 38.3%; p < 0.001) and the 12th month (28.2% vs. 39.1%; p < 0.001). These intergroup differences mirrored intragroup trends: the DM group achieved sustained smoking reductions from baseline to the 12th month (41.9% vs. 28.2%; p < 0.001), while CM exhibited only a transient decline from baseline to the 3rd month (41.3% vs. 38.3%; p < 0.001), with no further reduction observed thereafter up to the 12th month (38.3% vs. 39.1%; p = 0.197). Similarly, BMI reductions were greater in the DM group at the 3rd month (25.64 ± 4.61 vs. 25.47 ± 4.26 kg/m2; p = 0.031) and the 12th month (24.95 ± 4.70 vs. 25.45 ± 4.27 kg/m2; p < 0.001), driven by progressive weight loss in DM from baseline to the 12th month (26.45 ± 4.60 vs. 24.95 ± 4.70 kg/m2; p < 0.001) versus minimal CM changes (25.67 ± 4.25 vs. 25.45 ± 4.27 kg/m2; p < 0.001). LDL-c levels also showed a more significant decline in the DM group at the 3rd month (2.77 ± 1.27 vs. 2.69 ± 1.31 mmol/L; p = 0.001), although no significant difference was observed by the 12th month (2.56 ± 1.05 vs. 2.54 ± 1.06 mmol/L; p = 0.311). Longitudinal analysis revealed greater LDL-c reductions in the DM group from baseline to the 12th month (2.89 ± 1.43 vs. 2.56 ± 1.05 mmol/L; p < 0.001) compared to CM (2.78 ± 1.42 vs. 2.54 ± 1.06 mmol/L; p < 0.001). Glycemic control followed a parallel pattern, with DM showing lower HbA1c at the 3rd month (5.75 ± 1.70% vs. 5.93 ± 1.75%; p < 0.001) and sustained glycemic advantage from baseline to the 12th month (6.19 ± 1.98% vs. 5.43 ± 1.78%; p < 0.001) relative to CM (6.29 ± 1.76% vs. 5.46 ± 1.79%; p < 0.001), notwithstanding equivalent intergroup values at the 12th month (5.43 ± 1.78% vs. 5.46 ± 1.79%; p = 0.285).
Hemodynamically, DM demonstrated sustained reductions in SBP (126.1 ± 15.6 vs. 124.7 ± 12.6 mmHg; p < 0.001) and HR (73.2 ± 10.4 vs. 71.0 ± 9.4 bpm; p < 0.001) from baseline to the 12th month, whereas the CM group exhibited increased SBP (130.2 ± 12.8 vs. 130.9 ± 11.5 mmHg; p < 0.001) and HR (67.7 ± 10.2 vs. 69.0 ± 11.1 bpm; p < 0.001) over the same period.
At the 3rd month post-discharge, medication adherence (Table 3) in the DM group was significantly higher compared to the CM group for aspirin/indopuphin (96.3% vs. 89.5%; p < 0.001), clopidogrel/ticagrelor (88.9% vs. 85.9%; p < 0.001), β-blockers (70.1% vs. 66.0%; p < 0.001), ACEI/ARB (61.2% vs. 57.4%; p < 0.001), and statins (96.2% vs. 91.5%; p < 0.001). At the 12th months, the adherence remained higher in the DM group for aspirin/indopuphin (89.4% vs. 78.4%; p < 0.001), clopidogrel/ticagrelor (87.4% vs. 73.3%; p < 0.001), β-blockers (69.2% vs. 58.9%; p < 0.001), ACEI/ARB (59.7% vs. 51.9%; p < 0.001), and statins (94.8% vs. 84.1%; p < 0.001). Ezetimibe/hibomeibe was the exception, with adherence increasing in both groups.
Endpoints
After 12 months, the incidence of adverse events was significantly lower in the DM group compared to the CM group (Table 5). Specifically, the rates of all-cause death (1.6% vs. 2.7%; p < 0.001), MACCE events (6.4% vs. 9.2%; p < 0.001), cardiovascular death (1.1% vs. 1.6%; p = 0.021), myocardial infarction (1.2% vs. 3.0%; p < 0.001), recurrent angina (4.8% vs. 6.4%; p < 0.001), revascularization (3.9% vs. 4.6%; p = 0.039), and readmission (9.2% vs. 11.9%; p < 0.001) were all significantly lower in the DM group. Additionally, the composite endpoint of death, non-fatal myocardial infarction, non-fatal stroke, and re-hospitalization occurred at a significantly lower rate in the DM group (10.6% vs. 13.6%; p < 0.001). No significant differences were observed in the rates of stroke (0.7% vs. 0.8%; p = 0.483) or heart failure (1.0% vs. 1.2%; p = 0.260) between the two groups.
Figure 1 presents the Kaplan–Meier analysis of adverse clinical events, comparing the two groups. The univariable Cox regression analysis demonstrated a significant survival advantage for the DM group regarding primary outcomes—all-cause death, with a HR of 0.582 (95% CI: 0.451–0.750; p < 0.001). Significant differences were also observed in several secondary outcomes, including MACCE events (HR: 0.674, 95% CI: 0.593–0.766; p < 0.001), cardiovascular mortality (HR: 0.697, 95% CI: 0.511–0.949; p = 0.023), myocardial infarction (HR: 0.379, 95% CI: 0.285–0.504; p < 0.001), recurrent angina (HR: 0.751, 95% CI: 0.647–0.871; p < 0.001), and revascularization (HR: 0.836, 95% CI: 0.707–0.989; p = 0.037), yet no significant differences were observed in stroke (HR: 0.870, 95% CI: 0.590–1.284; p = 0.484) or heart failure (HR: 0.829, 95% CI: 0.599–1.148; p = 0.259). After adjusting for confounding factors such as age, gender, BMI, smoking, hypertension, diabetes, heart failure, stroke, systolic blood pressure, heart rate, creatinine, LDL-c, EF, and percutaneous coronary intervention, the multivariable Cox regression analysis confirmed that participation in the DM group remained significantly associated with a reduction in these adverse events compared to the CM group. Notably, heart failure also showed a significant reduction after adjustment (HR: 0.695, 95% CI: 0.490–0.987; p = 0.042). The detailed results are presented in Table 6.
The Kaplan–Meier curves for adverse clinical events comparing the Digital Management group and the Conventional Management group, including all-cause death (a), MACCE (b), cardiovascular death (c), myocardial infarction (d), recurrent angina (e) revascularization (f), stroke (g), and heart failure (h). The red line is the Digital Management group, and the green line is the Conventional Management group. * MACCE composite endpoint event of all-cause death, myocardial infarction, revascularization, and stroke.
The subgroup analysis results show that the effect of DM on improving the MACCE endpoint remains consistent across different age groups, genders, and types of CAD (p-interaction > 0.05). However, it is important to note that in the non-ACS population, the effect of DM on the MACCE endpoint lost statistical significance (HR 0.51 [95% CI 0.23–1.16], p = 0.109), although there was a trend toward improvement (Fig. 2).
Discussion
Digital medical management after discharge represents a new approach to chronic disease management, leveraging technologies such as the Internet, mobile terminals, and intelligent cloud platforms10. Compared with the conventional outpatient follow-up mode, this approach has emerged as a cost-effective strategy with expanding utilization across therapeutic applications and is likely to solve many problems such as the low completion rate of secondary prevention of CAD and cardiac rehabilitation treatment11,12. Our research demonstrated that digital healthcare systems can significantly enhance post-discharge management of CAD by reducing the incidence of major adverse cardiac events and ultimately lowering patient mortality through improving lifestyle modifications, controlling risk factors, monitoring medication, etc.
Post-discharge management of CAD typically involves lifestyle improvement, risk factor control, application of secondary preventive drugs and symptom management13. There remain several challenges with the current international application of digital healthcare in the long-term management of CAD. Firstly, only a limited number of mobile health-based interventions have managed multiple risk factors related to CAD, and about 80% only focus on one of the lifestyles, such as diet, exercise, or smoking14,15. The digital management system needs to comprehensively regulate multiple risk factors for patients, including complications, comorbidities, systemic metabolism, psychology, nutrition, exercise, sleep, and other aspects. Only comprehensive intervention can yield greater benefits16. Secondly, since risk factors and conditions vary among patients, personalized management plans, including tailored rehabilitation frequency, intensity, and specific metrics, are essential. However, many digital healthcare solutions still employ a ‘one-size-fits-all’ approach, where all patients follow a similar programme17, and rarely reconstruct personalized content through real-time feedback18. In addition, most digital healthcare trials have focused on patients with acute coronary syndrome or those who have undergone coronary artery procedures12,19, while their application in patients with stable angina, who are often recommended for cardiac rehabilitation, remains understudied. To enhance the overall effectiveness of CAD post-discharge management, future studies should include these patient populations. Finally, most published studies have relied on surrogate endpoints, such as peak exercise capacity and physical activity20, while seldom reporting on more critical outcomes like morbidity, readmission, or mortality17. Consequently, there is a need for more robust data on these reliable endpoints to better demonstrate the effectiveness and value of digital healthcare interventions.
Our investigation has systematically incorporated and optimized the following dimensions to advance post-hospitalization care quality, ultimately demonstrating marked efficacy in mitigating cardiovascular morbidity and mortality rates: 1. Personalization: Recognizing the heterogeneous nature of cardiovascular risk profiles and pathophysiological presentations, we implemented precision medicine principles. Digital health enables highly personalized post-discharge management for CAD patients by integrating real-time data, predictive analytics, and patient engagement tools, with protocol adjustments facilitated through iterative feedback mechanisms. 2. Sustained Longitudinal Care Continuum: Post-discharge management for CAD patients is a prolonged and complex process. However, many studies only cover the first few months post-discharge, failing to provide support throughout the entire follow-up period. This limited coverage often leads to decreased patient adherence and loss of critical medical guidance. Our study implemented a 12-month long-term management plan for CAD patients, continuously tracking disease progression and treatment efficacy. This paradigm ensured uninterrupted clinical oversight, counteracting therapeutic non-compliance and guidance discontinuity prevalent in prior models. 3. Multimodal Patient-Centric Education Framework: Moving beyond conventional SMS-based health communication limitations, which impose excessive reliance on patient autonomy and inadequately convey complex clinical information, we deployed a multi-channel health literacy strategy. Integration of mHealth platforms, dedicated patient portals, social media interfaces, and voice-activated AI systems was complemented by on-demand telecardiology consultations, thereby optimizing therapeutic comprehension and behavioral adherence. 4. Dynamic Monitoring and Feedback: Given the rapid progression of CAD, in addition to active symptom reporting, we utilized daily and professional monitoring devices to track patients’ vital signs in real-time, dynamically monitoring health status. By continuously observing these parameters and promptly alerting medical professionals to abnormalities, potential crises can be anticipated, and adverse events mitigated. Notably, the DM group had more risk factors and comorbidities at enrollment compared to the CM group, as well as worse cardiac function and a higher rate of coronary interventions. Despite these disadvantages, patients in the DM group demonstrated a better prognosis.
Regarding lifestyle improvements, the smoking cessation rate in the DM group of our study was significantly higher than that of the CM group. The smoking rate in the DM group showed a sustained decline throughout the 12-month intervention. In contrast, the CM group exhibited a reduction in smoking rate during the first 3 months, but a slight rebound was observed by the 12th month. At the 12-month follow-up, smoking rates in the DM group decreased by 13.7%, compared to a 2.2% decrease in the CM group. This indicates that the risk factor management in the CM group was less effective in maintaining the reduction in smoking rate over the long term. Moreover, BMI was also better managed in the DM group, with a significantly greater reduction compared to the CM group by the 3rd month, highlighting the early effectiveness of the DM group in weight management. And the BMI in the DM group decreased from 26.45 to 24.95 (mean) over 12 months, while the CM group’s BMI remained almost unchanged. These findings align with the conclusions of previous studies. Smoking is a major modifiable risk factor that should be targeted as part of every primary and secondary CAD prevention programme21. A Meta-analysis on the effectiveness of Internet-based smoking cessation interventions reported that participants receiving the text-messaging programs were almost twice as likely to quit smoking at the 6th month compared with those who did not receive the programme22. Besides, a systematic review evaluating the effectiveness of health apps in managing patients with CAD showed improvements in BMI, waist circumference, and physical activity among app users23.
In terms of risk factors, the systolic and diastolic blood pressure levels in the DM group were significantly lower than those in the CM group at both the 3rd and the 12th month. This underscores the efficacy of digital management in achieving optimal blood pressure control, a critical determinant in mitigating cardiovascular risk. Although the heart rate in the DM group remained elevated compared to the CM group throughout the study period, it demonstrated a consistent downward trend. In contrast, the CM group exhibited a mild rebound in heart rate. This pattern suggests that the DM group’s management strategy may offer greater benefits for long-term CAD management. At baseline, the LDL-c levels in the DM group were significantly higher than those in the CM group. However, the DM group showed a progressive decline in LDL-c levels at both the 3rd and the 12th months. By the 12th month, LDL-c levels in both groups showed no statistically significant difference, indicating that the DM group achieved a more pronounced reduction in LDL-c levels over time. The digital management approach in the DM group also resulted in a significant improvement in glycemic control during the short term. Although the improvement in glycemic control was similar between the DM and the CM groups by the 12th month, the DM group achieved faster attainment of glycemic targets compared to the CM group. This finding suggests that early and rapid optimization of risk factors represents a more effective strategy for CAD management. In addition, the DM group demonstrated significant improvements in both renal function, as assessed by estimated glomerular filtration rate (eGFR), and cardiac function, as measured by LVEF. These findings highlight the comprehensive efficacy of the digital management in addressing multiple risk factors associated with CAD. The observed improvements underscore the potential of digital management to provide holistic cardiovascular risk reduction, enhancing both patient outcomes and long-term prognosis. Consistent with our findings, a recent 3-month digital medical-guided cardiac rehabilitation program reported an average reduction in LDL-c levels of 11 mg/dl24. Meanwhile, the HERB-DH1 pivotal study affirmed the potential of digital management to reduce blood pressure through non-pharmacologic lifestyle changes in untreated patients with primary hypertension25. A previous study on patients with diabetes also demonstrated that digital management tools are effective in improving glycemic control and reducing the risk of cardiovascular events, and have been recommended by the European Society of Cardiology (ESC) guidelines for Diabetes and Cardiovascular Disease26. As for medication adherence, our study also obtained positive results, aligning with the conclusions of several previous studies27,28.
This study is the largest cohort study on long-term management of CAD patients by digital healthcare system, and it is the first article to discuss the clinical benefits of digital post-discharge management on long-term outcomes. Our DM group adopted comprehensive, real-time follow-up approaches that encompassed lifestyle improvements, tighter control of risk factors, increased adherence to secondary preventive medications, and effective symptom management. These interventions ultimately contributed to improved clinical outcomes.
Our analysis demonstrated that the all-cause mortality and cardiovascular mortality in the DM group were significantly lower than those in the CM group, with the effect being more pronounced after adjusting for confounding factors. In the multivariable analysis, the all-cause mortality risk in the DM group was further reduced by approximately 73% compared to the CM group, with a 41% reduction in the risk of MACCE events and a 65% reduction in the risk of cardiovascular disease mortality. Meanwhile, a similar trend was observed in other adverse events, including myocardial infarction, angina, revascularization, and rehospitalization, where digital management effectively reduced the risk of these events. The digital management consistently reduced MACCE risk across all analyzed subgroups, with no statistically significant heterogeneity detected. These findings reinforce its utility as a scalable strategy for secondary prevention. Our findings are consistent with other research findings both domestically and internationally. The TELE-ACS study showed that the approach of telemedicine to manage patients following acute coronary syndrome can reduce hospital readmissions, emergency department visits, unplanned coronary vascularization, and symptom episodes29. A study of patients with chronic heart failure found that remote monitoring can significantly reduce all-cause mortality and hospitalization rate30. The Meta-analysis by Cruz-Cobo suggests that mHealth has a positive impact on CAD patients in activity capacity, medication adherence, readmission, physical and mental quality of life31. These studies support our view that digital healthcare has significant value in the management of chronic diseases. Moreover, there are some potential benefits of our program that are difficult to quantify. For instance, features such as campaign reminders, risk stratification, two-way information exchange, and video education content likely contribute to enhancing the quality of post-discharge management. Nevertheless, some studies have held different attitudes towards digital healthcare management, arguing that it has little significance to the improvement of long-term cardiovascular events and mortality32,33,34. However, factors such as insufficient sample size, short follow-up duration, and inadequate management may have hindered the overall improvement in these studies.
Digital health management has demonstrated efficacy in managing various chronic conditions35,36 such as diabetes, obesity, and chronic obstructive pulmonary disease (COPD), with its application in CAD offering unique advantages. In CAD management, digital health platforms facilitate continuous remote monitoring of vital signs and symptoms, enabling early detection of exacerbations and timely intervention. This real-time data collection enhances risk stratification and personalized treatment plans, improving patient outcomes. Moreover, digital tools promote medication adherence through reminders and educational content, crucial for secondary prevention in CAD. They also support lifestyle modifications by tracking physical activity, diet, and weight, integral components of cardiac rehabilitation. The integration of telemedicine allows for regular virtual consultations, reducing the need for hospital visits and enabling healthcare providers to adjust therapies promptly. Data analytics and artificial intelligence further refine CAD management by predicting potential adverse events and optimizing resource allocation. Patient engagement is bolstered through interactive platforms that encourage self-management and provide psychosocial support, addressing the holistic needs of individuals with CAD. Interventional cardiologists can leverage digital health technologies to enhance patient management by optimizing post-procedural care and delaying the progression of arterial restenosis, thereby reducing the necessity for repeat interventional procedures. Through continuous remote monitoring and timely early intervention, they effectively minimize hospital readmissions caused by disease exacerbation. This proactive approach enhances the utilization of healthcare resources, ensuring that they are allocated efficiently and effectively to improve patient outcomes and streamline the healthcare delivery process. In essence, digital health management in CAD offers a comprehensive, patient-centered approach that enhances disease monitoring, treatment adherence, lifestyle intervention, and predictive analytics, ultimately leading to improved quality of life and reduced healthcare costs. Given its convenience and scalability, further promotion and integration of digital medical technologies in clinical practice have great potential to optimize long-term CAD management.
This study has several limitations. First, this research is an observational study, which inherently carries limitations. Observational studies are prone to confounding factors and cannot establish causality. The lack of randomization may result in unmeasured variables influencing the outcomes, thereby limiting the generalizability and robustness of the findings. Secondly, from the perspective of baseline characteristics, there is a potential for selection bias. Participants in the DM group were older, had more comorbidities, and exhibited poorer baseline cardiac function. These factors may have driven a greater urgency for post-discharge management guidance and a stronger willingness to participate. In contrast, the CM group consisted of younger patients who may have had insufficient awareness of disease management, limited time and energy, or concerns regarding privacy. These disparities in baseline characteristics could skew the results, as the DM group might inherently benefited more from structured interventions. Therefore, in light of the above, future studies should consider designing and conducting randomized controlled trials (RCTs) to minimize bias and unify baseline differences, which could provide a more comprehensive understanding of the true impact of digital health management in CAD patients. Thirdly, the digital management system used in this study requires further enhancement, such as integrating more wearable devices for comprehensive monitoring. With the rapid advancement of AI, a more sophisticated management system is expected to achieve better outcomes. Finally, this study is primarily based on patient data from China, and the results may not be fully generalizable to patients in other countries and regions. Therefore, future research should be conducted across different populations and regions37 to validate the effectiveness of digital healthcare.
In conclusion, this study indicates that digital healthcare offers significant advantages over conventional follow-up mode in the post-discharge management of CAD patients, effectively optimizing lifestyle, reducing risk factors, and improving patient prognosis. Our study highlights the significant reduction in all-cause mortality in patients with CAD. These findings underscore the potential value of digital healthcare in enhancing outcomes for CAD patients. Future research should continue to explore the impact of digital healthcare across diverse patient groups and regions to further validate its role and value in the management of chronic diseases.
Methods
Study population and data collection
The data of this study were obtained from “HeartMed Digital Management System”, a comprehensive system for collecting, analyzing, and monitoring multidimensional clinical data designed to enable discharge management of CAD patients. Figure 3 provides a brief summary of the main functions of the HeartMed system. Our research employs retrospective analysis of real-world clinical implementation data collected from patients with cardiovascular disease enrolled between June 2018 and September 2022 (n = 18,565). Continuous follow-up was conducted for 12 months via the digital system or telephone, with all information recorded in the system. Inclusion criteria were as follows: (1) All patients aged 18 years or above, with a definitive diagnosis of CAD. This diagnosis encompasses myocardial infarction, as well as unstable or stable angina. The diagnosis38 was confirmed either by coronary angiography showing stenosis of 50% or greater, regardless of whether the patient required or did not require revascularization procedures; or by coronary computed tomography angiography (CCTA) demonstrating stenosis of 50% or more in at least one coronary artery during their index admission. (2) Patients who provided informed consent to enroll in the HeartMed Digital Management System. We excluded women who were pregnant at the time of the clinical examination and individuals who were hemodynamically unstable, had no Internet access at their place of residence, or pre-existing comorbid disease with a life expectancy of less than 1 year. Figure 4 shows the flow diagram of patient screening.
“Heartmed medical digital management system” integrates smartphones, wearable devices, and medical data platforms to achieve secondary prevention of coronary heart disease. Through bidirectional data transmission between participants and caregivers, the system facilitates the transmission of educational knowledge, real-time assessment of risk factors, personalized reminders for lifestyle changes and rehabilitation exercises, recording of medication usage, and reminders for follow-up appointments. The system simultaneously adjusts the health management status based on the risk stratification and providing personalized disease management plans. *CAD coronary artery disease, GDMT guideline-directed medical therapy; LDL low-density lipoprotein.
The clinical data from the 12-month follow-up were collected from the system, including diagnosis, personal basic information, risk factors, comorbidities, admission status, post-discharge health data, laboratory tests, and medication.
Ethics review
This study has undergone a rigorous review process by the Ethics Committee of Fuwai Hospital, Chinese Academy of Medical Sciences, which has granted approval for the conduct of the study (Approval Number: 2023-2210). The study has been designed and conducted in accordance with the principles outlined in the Declaration of Helsinki and other relevant ethical guidelines.
Intervention and enrollment process
Patients were divided into the DM group and the CM group based on whether patients enrolled in the digital management system. Both groups of patients participated voluntarily, without any charges or compensation. In the DM group, a refined post-discharge management approach will be implemented by focusing on the following aspects, with specific management standards adjusted in accordance with the latest relevant guidelines39,40,41,42,43,44,45,46:
-
1.
Symptom management: After enrollment, each patient was provided with a chip-implanted sphygmomanometer to monitor their blood pressure and pulse in real time. Structured health education and symptom management interventions were systematically delivered at standardized post-discharge intervals (1-, 3-, 6-, and 12-month timepoints) following a predefined clinical pathway. These interventions were delivered through a combination of automated digital platforms, including system-integrated mobile application notifications and WeChat-based communications. Health education contents were presented in multimodal formats, such as text-based materials, infographics, and short video modules, optimized for patient engagement and comprehension. According to the specific conditions of patients (such as age, gender, disease type and risk factors, etc.) and needs, targeted health education plans were formulated to provide personalized guidance and services. Symptom management encompassed symptom consultation, clinical assessment and medication reconciliation; patients could report symptoms through WeChat mini-program or designated system telephone. In addition to routine follow-up, patients could also report symptoms to health managers at any time according to the needs of the condition and generate alerts to health managers. Through internal clinical decision-making algorithms, the telemedicine team would conduct clinical evaluations and seek decision guidance from clinical cardiologists if necessary. Supervisors of symptom management included 142 cardiovascular specialists and 36 licensed physician assistants.
-
2.
Clinical evaluation: The system stratified patients by risk levels. For high-risk patients, the system intensified health education tailored to the patient’s risk factors and recommended increasing the frequency of symptom reporting and indicator monitoring (blood pressure, heart rate, blood lipids, etc.). It also adjusted medication based on corresponding risk factors. The digital management system automatically transmitted abnormal data to a doctor’s assistant, with early detection of life-threatening complications (such as bleeding) and critical conditions. The assistant would then contact the patient and address the issue promptly, either through the system or by phone.
-
3.
Lifestyle intervention: The lifestyle intervention protocol encompassed comprehensive management of dietary patterns, physical activity regimens, body weight parameters, tobacco usage, alcohol consumption, sleep hygiene, and psychological status. Personalized dietary plans, exercise recommendations, weight management strategies, and smoking cessation/alcohol moderation advice were delivered based on individual patient characteristics. Drawing on the Dietary Guidelines for Chinese Residents 2022 issued by the National Health Commission of China, an artificial intelligence (AI) algorithm integrated with Constraint Satisfaction Problem (CSP) reasoning algorithm generated individualized daily recipes. This computational model dynamically adapted to patients’ geolocation data, seasonal variations, and comorbid conditions (e.g., hyperlipidemia, diabetes mellitus), ensuring nutritional adequacy while addressing specific metabolic requirements. Based on each patient’s physical activity levels and disease status, a tailored daily-exercise program was provided. Psychological assessments utilized the Patient Health Questionnaire-9 (PHQ-9) and Generalized Anxiety Disorder-7 (GAD-7) scales to evaluate anxiety and depression status, with recommendations for psychological interventions based on results. Sleep quality was assessed via the Pittsburgh Sleep Quality Index (PSQI), informing decisions regarding sleep interventions.
Post-discharge evaluations and guidance above were scheduled at standardized intervals (1-, 3-, 6-, and 12-month timepoints) following discharge, primarily delivered through a WeChat mini-program. If no patient feedback was received after three consecutive notifications, manual phone calls were initiated.
-
4.
Risk factors control: For patients who smoked, the system offered smoking cessation guidance and promoted relevant educational contents. Meanwhile, the system implemented an image-based scanning interface enabling patients to digitize laboratory reports (e.g., blood glucose, lipid profiles) via mobile application upload. Integrated optical character recognition (OCR) and clinical data extraction modules automatically parsed quantitative biomarkers, which were subsequently routed to clinical teams for evidence-based clinical guidance. Concurrently, the platform employed adaptive optimization algorithms to dynamically update personalized nutrition regimens and exercise prescriptions in real-time. This closed-loop feedback mechanism fostered enhanced patient activation through gamified health engagement metrics, thereby promoting sustained self-management adherence and data-driven behavioral modifications.
-
5.
Medication management: The system checked the type, name, and dosage of drugs medication in each period of the day, regularly sent reminders to patients every day, urged patients to take medication correctly, recorded medication usage, reported feasible possible adverse drug reactions, and made timely adjustments.
By contrast, the CM group received routine outpatient visits in accordance with discharge instructions and personal medical needs. Doctors provided the following medical services to patients: educating patients on secondary prevention of CAD, guiding them on proper medication use, and ensuring timely follow-ups. Besides, patient medication, blood pressure, heart rate, and follow-up examinations were also recorded. The management of patients by physicians was strictly conducted in accordance with the international guidelines for secondary prevention of CAD39,40,41,42,43,44,45,46. All participating physicians underwent standardized training to ensure uniformity in the application of these guidelines.
Clinical outcome evaluation
All patients were followed up as planned for 12 months. The primary endpoint was all-cause death. Secondary endpoints included Major Adverse Cardiac and Cerebrovascular Events (MACCE, composite endpoint event of all-cause death, myocardial infarction, revascularization, and stroke47), as well as cardiovascular disease death, myocardial infarction, recurrent angina, revascularization, stroke, heart failure, and readmission.
Statistical analysis
The continuous variables with a normal distribution were statistically described by means ± standard deviation (SD) and then compared between groups and within groups by the t-test or t’-test depending on their equal or unequal variances, respectively, while the non-normally distributed continuous variables were statistically described by medians (interquartile range [IQR]) and compared between groups by the Mann-Whitney U test. Count variables, described by number and percentage (n, %), of two groups were compared by the chi-square or Fisher test. The threshold for retaining covariates with missing data was set at 5% to ensure stability and accuracy in the analysis. Missing values were handled using mode imputation for categorical variables and mean or median imputation for continuous variables. The Kaplan–Meier survival curve and the log-rank test were used to reveal and compare the 12-month cumulative adverse events rates of both groups. The 12-month risk of adverse events was analyzed through Cox regression modeling, with results expressed as hazard ratios (HRs) and 95% confidence intervals (CIs) in both univariable and multivariable analyses (adjusted for potential influencing factors including age, gender, body mass index (BMI), smoking, hypertension, diabetes, heart failure, stroke, systolic blood pressure (SBP), heart rate (HR), creatinine, the low-density lipoprotein-cholesterol (LDL-c), left ventricular ejection fraction (LVEF) and percutaneous coronary intervention (PCI)). To further explore the impact of DM on different populations, multiplicative interaction analyses were conducted in three key subgroups—age, sex, and coronary artery disease type—to assess the impacts of DM on MACCE events across different demographic and clinical characteristics. All tests for statistical significance were two-sided and variables with differences of p < 0.05 were considered statistically significant; All analyses were performed using SPSS (V.25.0).
Data Availability
The datasets generated and analyzed in the study are not publicly available due to institutional policy and the privacy of study individuals but are available from the corresponding author upon request under the data- sharing agreements between institutions.
Code availability
No code was written by the authors.
References
Stolpe, S., Kowall, B. & Stang, A. Decline of coronary heart disease mortality is strongly effected by changing patterns of underlying causes of death: an analysis of mortality data from 27 countries of the WHO European region 2000 and 2013. Eur. J. Epidemiol. 36, 57–68 (2021).
Magnussen, C. et al. Global effect of modifiable risk factors on cardiovascular disease and mortality. N. Engl. J. Med. 389, 1273–1285 (2023).
Salzwedel, A. et al. Effectiveness of comprehensive cardiac rehabilitation in coronary artery disease patients treated according to contemporary evidence based medicine: update of the cardiac rehabilitation outcome study (CROS-II). Eur. J. Prev. Cardiol. 27, 1756–1774 (2020).
Wang, X. & Luan, W. Research progress on digital health literacy of older adults: a scoping review. Front. Public Health10, 906089 (2022).
Mathes, T., Pieper, D., Antoine, S. L. & Eikermann, M. 50% adherence of patients suffering chronic conditions–where is the evidence?. Ger. Med. Sci.10, Doc16 (2012).
Yusuf, S. et al. Use of secondary prevention drugs for cardiovascular disease in the community in high-income, middle-income, and low-income countries (the PURE Study): a prospective epidemiological survey. Lancet 378, 1231–1243 (2011).
Marvel, F. A. et al. Digital health intervention in acute myocardial infarction. Circ. Cardiovasc. Qual. Outcomes 14, e007741 (2021).
Williams, G. J. et al. Wearable technology and the cardiovascular system: the future of patient assessment. Lancet Digit. Health 5, e467–e476 (2023).
Schukraft, S. et al. Remote blood pressure monitoring with a wearable photoplethysmographic device in patients undergoing coronary angiography: the senbiosys substudy. Blood Press. Monit. 27, 402–407 (2022).
Vardas, P. E., Asselbergs, F. W., van Smeden, M. & Friedman, P. The year in cardiovascular medicine 2021: digital health and innovation. Eur. Heart J. 43, 271–279 (2022).
Ramachandran, H. J., Jiang, Y., Tam, W. W. S., Yeo, T. J. & Wang, W. Effectiveness of home-based cardiac telerehabilitation as an alternative to Phase 2 cardiac rehabilitation of coronary heart disease: a systematic review and meta-analysis. Eur. J. Prev. Cardiol. 29, 1017–1043 (2022).
Batalik, L. et al. The cost-effectiveness of exercise-based cardiac telerehabilitation intervention: a systematic review. Eur. J. Phys. Rehabil. Med. 59, 248–258 (2023).
Sigamani, A. & Gupta, R. Revisiting secondary prevention in coronary heart disease. Indian Heart J. 74, 431–440 (2022).
Xiao, Q., Lu, S., Wang, Y., Sun, L. & Wu, Y. Current status of cardiovascular disease-related smartphone apps downloadable in China. Telemed. J. E-Health 23, 219–225 (2017).
Milne-Ives, M., Lam, C., De Cock, C., Van Velthoven, M. H. & Meinert, E. Mobile apps for health behavior change in physical activity, diet, drug and alcohol use, and mental health: systematic review. JMIR MHealth UHealth 8, e17046 (2020).
Birtcher, K. K. et al. 2022 ACC expert consensus decision pathway for integrating atherosclerotic cardiovascular disease and multimorbidity treatment: a framework for pragmatic, patient-centered care: a report of the American College of Cardiology Solution Set Oversight Committee. J. Am. Coll. Cardiol. 81, 292–317 (2023).
Brouwers, R. W. M., Scherrenberg, M., Kemps, H. M. C., Dendale, P. & Snoek, J. A. Cardiac telerehabilitation: current status and future perspectives. Neth. Heart J. 32, 31–37 (2024).
Scherrenberg, M. et al. Development and internal validation of the digital health readiness questionnaire: prospective single-center survey study. J. Med. Internet Res. 25, e41615 (2023).
Scherrenberg, M., Falter, M. & Dendale, P. Cost-effectiveness of cardiac telerehabilitation in coronary artery disease and heart failure patients: systematic review of randomized controlled trials. Eur. Heart J. Digit. Health 1, 20–29 (2020).
Giggins, O. M. et al. Remotely delivered cardiac rehabilitation exercise for coronary heart disease: nonrandomized feasibility study. JMIR Cardio 7, e40283 (2023).
Yusuf, S. et al. Effect of potentially modifiable risk factors associated with myocardial infarction in 52 countries (the INTERHEART study): case-control study. Lancet 364, 937–952 (2004).
Whittaker, R., McRobbie, H., Bullen, C., Rodgers, A. & Gu, Y. Mobile phone-based interventions for smoking cessation. Cochrane Database Syst. Rev. 4, Cd006611 (2016).
Coorey, G. M., Neubeck, L., Mulley, J. & Redfern, J. Effectiveness, acceptability and usefulness of mobile applications for cardiovascular disease self-management: systematic review with meta-synthesis of quantitative and qualitative data. Eur. J. Prev. Cardiol. 25, 505–521 (2018).
Harzand, A. et al. Effects of a patient-centered digital health intervention in patients referred to cardiac rehabilitation: the Smart HEART clinical trial. BMC Cardiovasc. Disord. 23, 453 (2023).
Kario, K. et al. Efficacy of a digital therapeutics system in the management of essential hypertension: the HERB-DH1 pivotal trial. Eur. Heart J. 42, 4111–4122 (2021).
Cosentino, F. et al. 2019 ESC Guidelines on diabetes, pre-diabetes, and cardiovascular diseases developed in collaboration with the EASD. Eur. Heart J. 41, 255–323 (2020).
Liu, H., Zhang, H., Qin, Y., Li, C. & Jiao, Y. Study on out-of-hospital management mode of patients with acute coronary syndrome after PCI in rural areas. Int. Heart J. 63, 1026–1033 (2022).
Ni, Z. et al. An mHealth intervention to improve medication adherence and health outcomes among patients with coronary heart disease: randomized controlled trial. J. Med. Internet Res. 24, e27202 (2022).
Alshahrani, N. S. et al. Randomized trial of remote assessment of patients after an acute coronary syndrome. J. Am. Coll. Cardiol. 83, 2250–2259 (2024).
Chen, C. et al. Post-discharge short message service improves short-term clinical outcome and self-care behaviour in chronic heart failure. ESC Heart Fail. 6, 164–173 (2019).
Cruz-Cobo, C., Bernal-Jiménez, M., Vázquez-García, R. & Santi-Cano, M. J. Effectiveness of mHealth Interventions in the Control of Lifestyle and Cardiovascular Risk Factors in Patients After a Coronary Event: Systematic Review and Meta-analysis. JMIR MHealth UHealth 10, e39593 (2022).
Piotrowicz, E. et al. Effects of a 9-week hybrid comprehensive telerehabilitation program on long-term outcomes in patients with heart failure: the telerehabilitation in heart failure patients (TELEREH-HF) randomized clinical trial. JAMA Cardiol. 5, 300–308 (2020).
Yu, C. et al. Smartphone-based application to improve medication adherence in patients after surgical coronary revascularization. Am. Heart J. 228, 17–26 (2020).
Treskes, R. W. et al. Effect of smartphone-enabled health monitoring devices vs regular follow-up on blood pressure control among patients after myocardial infarction: a randomized clinical trial. JAMA Netw. Open 3, e202165 (2020).
Bentley, C. L. et al. The use of a smartphone app and an activity tracker to promote physical activity in the management of chronic obstructive pulmonary disease: randomized controlled feasibility study. JMIR MHealth UHealth 8, e16203 (2020).
Wang, Y. et al. Effectiveness of mobile health interventions on diabetes and obesity treatment and management: systematic review of systematic reviews. JMIR MHealth UHealth 8, e15400 (2020).
Guasti, L. et al. Digital health in older adults for the prevention and management of cardiovascular diseases and frailty. A clinical consensus statement from the ESC Council for Cardiology Practice/Taskforce on Geriatric Cardiology, the ESC Digital Health Committee and the ESC Working Group on e-Cardiology. ESC Heart Fail. 9, 2808–2822 (2022).
Shahjehan, R. D., Sharma, S. & Bhutta, B. S. Coronary artery disease. In StatPearls (StatPearls Publishing Copyright © 2025, StatPearls Publishing LLC., 2025).
Roffi, M. et al. 2015 ESC guidelines for the management of acute coronary syndromes in patients presenting without persistent ST-segment elevation. Rev. Esp. Cardiol. 68, 1125 (2015).
Collet, J. P. et al. 2020 ESC Guidelines for the management of acute coronary syndromes in patients presenting without persistent ST-segment elevation. Rev. Esp. Cardiol. 74, 544 (2021).
Ibanez, B. et al. 2017 ESC Guidelines for the management of acute myocardial infarction in patients presenting with ST-segment elevation. Kardiol. Pol. 76, 229–313 (2018).
Knuuti, J. et al. 2019 ESC Guidelines for the diagnosis and management of chronic coronary syndromes. Eur. Heart J. 41, 407–477 (2020).
Visseren, F. L. J. et al. 2021 ESC Guidelines on cardiovascular disease prevention in clinical practice. Eur. J. Prev. Cardiol. 29, 5–115 (2022).
Catapano, A. L. et al. 2016 ESC/EAS guidelines for the management of dyslipidaemias. Rev. Esp. Cardiol. 70, 115 (2017).
Mach, F. et al. 2019 ESC/EAS Guidelines for the management of dyslipidaemias: lipid modification to reduce cardiovascular risk. Eur. Heart J. 41, 111–188 (2020).
Lawton, J. S. et al. 2021 ACC/AHA/SCAI guideline for coronary artery revascularization: executive summary: a report of the American College of Cardiology/American Heart Association Joint Committee on Clinical Practice Guidelines. Circulation 145, e4–e17 (2022).
Wang, Z. et al. Persistent lipoprotein(a) exposure and its association with clinical outcomes after acute myocardial infarction: a longitudinal cohort study. Ann. Med. 57, 2454975 (2025).
Acknowledgements
This work was supported by the Continuous Improvement Research Project on Evidence-based Healthcare Quality Management (YLZXXZH006), National High-Level Hospital Clinical Research Funding (2024-GSP-GG-4), CAMS Innovation Fund for Medical Sciences (CIFMS) (2024-I2M-C&T-B-040), Noncommunicable Chronic Diseases-National Science and Technology Major Project (2023ZD0504000), Artificial Intelligence and Information Technology Application Fund of Fuwai Hospital and Chinese Academy of Medical Sciences (2024-AI22).
Author information
Authors and Affiliations
Contributions
All authors read and approved the final version of the manuscript. X.G. and Y.W. proposed the idea. L.Y. and Z.W. performed the data analyses and drafted the manuscript. Z.W., S.Z., M.L., Y.Z., and F.H. checked the integrity and plausibility of data analysis. X.G. and Y.W. revised the manuscript and were responsible for the integrity of data acquisition and statistical analyses. L.Y., Z.W., X.G., and Y.W. verified the underlying data.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Yang, L., Wang, Z., Zhao, S. et al. Effectiveness of digital healthcare to improve clinical outcomes in discharged patients with coronary artery disease. npj Digit. Med. 8, 473 (2025). https://doi.org/10.1038/s41746-025-01655-6
Received:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s41746-025-01655-6