Abstract
Artificial Intelligence (AI) is revolutionizing biotechnology by accelerating advancements in drug discovery, genomics, medical imaging, and personalized medicine, thereby enhancing efficiency and reducing healthcare costs. This review emphasizes the transformative potential of multimodal AI—systems that integrate diverse data types such as genomic, clinical, and imaging data—to deliver more accurate and holistic biomedical insights. We explore AI’s economic impact, role in driving innovation, and implications for both researchers and policymakers. Additionally, the review addresses key challenges, including data quality, algorithmic transparency, and ethical concerns, highlighting the urgent need for explainable AI models, robust regulatory frameworks, and equitable implementation to ensure responsible and impactful adoption across global healthcare systems.

Similar content being viewed by others
Introduction
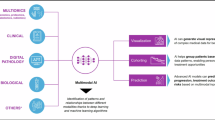
In recent years, the pharmaceutical and biotechnology industries have experienced a significant surge in data digitization. This digital transformation, while promising, presents challenges in collecting, analyzing, and applying vast and complex datasets to solve intricate clinical and biological problems1. Although AI has transformed sectors such as finance and transportation, its full potential in life sciences is only beginning to be realized. According to the McKinsey Global Institute, rapid advancements in AI-driven technologies are poised to reshape the pharmaceutical and biotech landscape2,3. AI and biotechnology, though distinct domains, are converging to unlock transformative opportunities. Artificial Intelligence (AI) involves developing computer systems capable of tasks traditionally requiring human intelligence—such as learning, reasoning, and decision-making—while biotechnology utilizes living organisms or biological systems to develop applications in healthcare, agriculture, and industry. At their intersection lies the opportunity to accelerate innovation, streamline research, and personalize treatment4. Central to this convergence is the rise of multimodal AI—an approach that integrates multiple types of biomedical data, such as genomic sequences, clinical records, medical imaging, and molecular structures. By combining these diverse data streams, multimodal AI enhances the predictive power and contextual understanding of biological systems, enabling more comprehensive insights than any single data type alone.
AI-driven systems, particularly those based on machine learning (ML), have demonstrated the ability to analyze massive datasets to uncover hidden patterns in genetic sequences, protein structures, and patient health data5,6. These capabilities are already being applied across biotech workflows, from hypothesis generation to drug candidate screening. A notable example includes AI’s role in expediting COVID-19 vaccine development—reducing a decade-long timeline to under a year7,8. In another case, AI identified a novel liver cancer drug candidate in just 30 days9. Cloud-based platforms and AI-powered tools are also being introduced to automate labor-intensive processes in drug discovery, underscoring the growing role of technology firms in advancing biomedical research. Today, multimodal AI is considered a transformative force in biotechnology, driving breakthroughs in drug design, diagnostics, and personalized medicine10,11,12,13. The economic momentum behind this trend is substantial. In 2024, the global AI market was valued at USD 233.46 billion and is projected to grow to USD 1771.62 billion by 2032, reflecting a compound annual growth rate (CAGR) of 29.2%. North America alone led the global AI market in 2024, accounting for a significant 32.93% share, according to a research report published by Fortune Business Insight in Jan 202514. Within the pharmaceutical and biotechnology sector specifically, the AI market was valued at USD 1.8 billion in 2023 and is projected to reach USD 13.1 billion by 2034, growing at a CAGR of 18.8%. As demand for faster, cost-effective drug development intensifies and genome-editing technologies mature, AI is increasingly used to simulate clinical trials, reduce R&D timelines, and optimize manufacturing. By 2030, it is anticipated that over half of newly developed drugs will involve AI-based design and production methods.
This review explores the evolving integration of AI in biotechnology and digital medicine, with a focus on multimodal AI, its applications, economic impact, challenges, and future prospects. We aim to provide a comprehensive perspective for researchers, clinicians, and policymakers navigating this rapidly advancing field.
Methods
To ensure a comprehensive and systematic overview of the intersection between AI and biotechnology, we conducted a literature and patent review using multiple academic and industrial databases. The databases searched included PubMed, Google Scholar, and SciFinder. The search covered publications and patents from January 2010 to April 2025, reflecting the recent surge in AI-driven innovations. Keywords used in the search strategy included: “Artificial Intelligence,” “Machine Learning,” “Deep Learning,” “Biotechnology,” “Drug Discovery,” “Medical Imaging,” “Genomics,” and “Precision Medicine.” Inclusion criteria focused on peer-reviewed journal articles, reviews, relevant industry white papers, web articles and patent filings that addressed AI applications in biotechnology. While we included selected gray literature sources such as government reports and industry white papers, we recognize that our inclusion criteria may still be subject to publication bias, particularly toward successful or well-documented AI applications. The implications of this potential bias are further explored later in the manuscript. For bibliometric and trend analyses, data were exported from SciFinder in structured formats and analyzed using Microsoft Excel to generate heatmaps and co-authorship networks. Patent analyses included keyword frequency and assignee data derived from SciFinder. This methodology ensures transparency, reproducibility, and alignment with current bibliometric standards, while enabling an integrated view of both scientific and commercial developments in the field.
Applications of AI in biotechnology
Drug discovery and development
Drug discovery, the process of identifying and developing new medications, is traditionally a complex and time-consuming endeavor, often relying on labor-intensive techniques like trial-and-error experimentation and high-throughput screening. However, AI techniques such as ML and natural language processing can accelerate and enhance this process by enabling more efficient and accurate analysis of large datasets15,16,17,18. With continuous advancement of ML algorithms, AI has become increasingly integral to various stages of drug discovery. Recently, drug design and discovery have entered the big data era, with ML evolving into deep learning techniques that offer powerful generalization capabilities and more effective handling of large datasets19,20. This evolution has further enhanced the integration of AI with computer-assisted drug discovery, accelerating the development of new therapeutics. Figure 1a primarily highlights the key differences between AI-driven and traditional drug development, emphasizing improvements in efficiency, cost, and personalization, while Fig. 1b highlights the progression of AI in drug discovery.
a Comparison between traditional drug development approaches and artificial intelligence (AI)-driven methods, highlighting differences in time, cost, efficiency, and decision-making. b AI technologies across various stages of modern drug development, including target identification, drug design, clinical trials, and post-market surveillance.
The integration of AI in the development of pharmaceutical products, from initial research to patient care, is highly conceivable21. Multimodal AI can facilitate rational drug design, support decision-making, identify optimal therapies for individual patients (including personalized medicine), and manage clinical data, for use in future research22,23. AI has the potential to streamline complex drug development procedures, reducing development time and costs, ultimately accelerating the delivery of new treatments to reach patients. In the pharmaceutical and biotech industries, AI can enhance drug discovery in four key areas:
Accessing new biological targets
Assigning the correct target is crucial for developing an effective pharmacological molecule for therapy. AI can significantly aid in structure-based drug discovery by predicting the three-dimensional structure of target proteins. AI involves the use of ML algorithms to analyze vast datasets to identify potential drug targets by recognizing patterns and associations that might be missed by human researchers24,25. Predictive modeling allows AI to simulate biological processes and interactions, further pinpointing key targets. Additionally, natural language processing techniques can scan and analyze scientific literature, patents, and clinical trial data to extract valuable insights and identify emerging targets. AI significantly enhances the discovery of new biological targets by integrating and analyzing vast amounts of biological data from genomics, proteomics, and metabolomics and also optimizes high-throughput screening processes, making the identification of promising targets from large compound libraries faster and more efficient26,27. Furthermore, AI enhances gene editing techniques, such as CRISPR, by predicting the most effective gene targets for therapeutic intervention28,29,30. These capabilities enable AI to accelerate the discovery of new biological targets, fostering the development of innovative therapies, while also addressing safety considerations prior to synthesis or clinical testing31.
Improving or creating novel compounds
Accurately predicting a drug’s interaction with a receptor or protein is essential for assessing its efficacy, enabling drug repurposing, and preventing undesirable polypharmacology32,33. AI is revolutionizing the development of novel compounds in drug discovery through several cutting-edge methodologies34. ML algorithms can analyze vast chemical datasets to identify patterns and relationships that help in the design of drugs with optimal efficacy and minimal side effects. Generative models, such as variational autoencoders (VAEs)35 and generative adversarial networks (GANs)36,37, can create novel molecular structures with desired properties by learning from existing chemical data38. AI-driven virtual screening processes significantly expedite the identification of potential drug candidates by predicting the binding affinity of compounds to target proteins. Additionally, AI algorithms can optimize chemical synthesis pathways, making the production of novel compounds more efficient and cost-effective. Combined with AI-driven virtual screening, these models predict ligand-protein interactions, expediting candidate selection and improving accuracy39,40.
Increasing success rates
Accurately estimating the probability of success (POS) in clinical trials is crucial for researchers and biopharma investors. Prudent resource allocation and decision-making depend on timely risk assessments. Without current POS estimates, investors may misjudge risks and values, resulting in lost opportunities for both investors and patients41,42. AI is markedly enhancing success rates in drug development by optimizing various stages of the process (Fig. 2). ML models can predict the efficacy and safety of drug candidates early in the development cycle, significantly reducing the likelihood of late-stage failures. AI algorithms analyze vast datasets from preclinical and clinical studies to identify biomarkers and patient subpopulations most likely to respond to treatments, thus increasing the precision of clinical trials. Moreover, AI-driven predictive analytics can identify potential side effects and toxicity issues before clinical testing, ensuring higher safety profiles for drug candidates. By integrating multi-omics data (genomics, proteomics, metabolomics), AI provides a comprehensive understanding of disease mechanisms, facilitating the identification of more effective therapeutic targets. Additionally, AI enhances the design and execution of clinical trials through adaptive trial designs and real-time data monitoring, leading to more efficient and successful outcomes. These AI-driven innovations collectively contribute to higher success rates in drug development, expediting the delivery of safe and effective therapies to patients20,43.
Accelerating and reducing the costs of discovery methods
AI accelerates discovery and reduces costs by analyzing vast data to identify promising candidates more efficiently than traditional approaches44,45. AI-driven virtual screening processes reduce the need for extensive physical testing by predicting how compounds will interact with target proteins. Optimization of synthesis routes and clinical trial designs reduces resource waste and shortens development timelines35,36,37. Early prediction of efficacy and toxicity helps avoid costly failures, while streamlined data management and trial operations contribute to faster regulatory approvals.
Barriers to the effective application of AI in drug discovery
Despite these advances, various challenges and limitations must still be addressed46,47. Figure 3 outlines several key barriers to the successful integration of AI into biotechnology, including data availability, ethical considerations, data augmentation, and explainability. AI models require large volumes of high-quality, representative training data; however, in biomedical research, available datasets are often limited, noisy, or biased, which can undermine model reliability and generalizability. In addition, many advanced AI systems—particularly deep learning models—operate as “black boxes,” making it difficult to interpret how predictions are generated. This lack of transparency complicates model validation, particularly in complex biological systems where experimental verification is essential. There are also concerns about algorithmic bias, where training data may reflect existing disparities, leading to skewed or unfair outcomes. Ensuring ethical AI use requires active efforts to identify and mitigate these biases. Furthermore, validating AI predictions in biological contexts remains a challenge due to the inherent variability of biological systems and the limitations of current experimental models. Overcoming these challenges is essential for the trustworthy and effective application of AI in drug discovery.
This figure summarizes critical limitations in applying AI to biotechnology, including the need for large, high-quality datasets (Data Availability), risks of algorithmic bias and fairness (Ethical Considerations), the role of synthetic data generation to overcome data scarcity (Data Augmentation), and the necessity for transparent and interpretable models (Explainable AI). These factors represent significant technical and ethical hurdles, particularly in high-stakes domains such as drug discovery and biomedical research. Addressing these issues is essential to ensure model reliability, generalizability, and real-world validation in biological systems.
Industry applications and collaborations in AI-driven drug discovery
Besides these challenges, most large pharma companies like AZ, Bayer, GSK, and Roche are heavily investing billions of $s in AI/ML to boost R&D productivity and drug discovery. Table 1 outlines major collaborations between biopharmaceutical companies and AI platforms, showcasing how industry leaders such as BenevolentAI, Insilico Medicine, DeepMind, and PathAI are transforming various aspects of drug development and clinical management. Tools such as AlphaFold9 have further accelerated protein structure prediction, reinforcing the value of open-source AI tools in biotechnology. GSK employs over 230 individuals in AI/ML roles, focusing on streamlining target identification and drug discovery through machine learning. Roche, through Genentech, is leveraging machine learning for informatics in drug discovery and investing in new technologies to tackle complex biological problems, with strong organizational strengths in data science. Moderna is concentrating on using AI as a tool for clinical development rather than for broad enterprise productivity48. AI enhances decision-making by analyzing vast datasets and identifying patterns and trends that may elude human observation49,50. This capability is particularly valuable in drug development, where accurate predictions and diagnoses can have profound, life-changing implications. Now the question arises, is AI the replacement of human researchers?. So, the answer is: While AI holds immense potential, it is not a standalone solution. AI systems necessitate human input and oversight to function effectively, particularly in making decisions in ambiguous situations, the best example is development of the COVID-19 vaccine. Ultimately, AI is not a substitute for human intelligence; it is a tool that aids in achieving our goals. However, it is crucial to ensure its use is both responsible and ethical51,52. If successful, AI and ML could herald a new era of faster, cheaper, and more effective drug development53.
Genomics and precision medicine
AI has become a transformative force in genomics and precision medicine, driving significant advancements in these fields. Before discussing the importance of AI in genomic and precision medicine, let’s introduce what they are. Precision medicine is a rapidly advancing branch of therapeutics based on an individual’s genetic makeup, lifestyle, gene expression, and environment54,55. Researchers utilize this approach to tailor prevention and treatment by identifying characteristics that predispose people to specific diseases and characterizing the primary biological pathways responsible for these disorders. This field represents one of the most exciting and promising advancements in modern medicine, shifting healthcare from a one-size-fits-all model to one that is individualized and data-driven. This transformation allows for more efficient resource use and improved patient outcomes56. To personalize care, precision medicine needs access to massive amounts of data and the right tech capabilities to process it. This is where AI plays the role to process extremely large and complex datasets57. Genomic medicine is an emerging medical specialty that leverages genetic information to inform diagnosis, treatment, and associated health outcomes, along with policy implications58. It holds significant potential for transforming fields such as oncology, pharmacology, rare and undiagnosed disorders, and infectious diseases.
AI can process vast amounts of genomic data more efficiently than traditional methods. This capability is crucial given the sheer volume of data generated by next-generation sequencing technologies. ML algorithms excel at identifying patterns and correlations within complex datasets, which can lead to the discovery of new genetic markers and the understanding of disease mechanisms. Figure 4 designs a simplified overview of how AI can analyze genetic information to identify early signs of diseases, such as cancer, allowing for earlier and more accurate diagnoses.
By analyzing genomic data, AI can predict an individual’s risk of developing certain diseases, enabling proactive healthcare interventions and helps in developing personalized treatment plans based on an individual’s genetic makeup, lifestyle, and environmental factors59. This approach increases the efficacy of treatments, minimizes adverse effects and accelerates the process of drug discovery by identifying potential drug candidates and predicting their effectiveness, thus reducing the time and cost associated with bringing new drugs to market. AI-driven tools facilitate the discovery of new genes, variants, and their associations with diseases, driving innovation in genomics research, enabling better collaboration among researchers by providing platforms for data sharing and collective analysis, which can lead to more comprehensive studies and breakthroughs60,61,62. It can automate routine tasks such as data entry and preliminary analysis, allowing researchers and clinicians to focus on more complex and creative aspects of their work. By predicting patient responses to treatments, AI can help in better resource allocation and management within healthcare systems, improving overall efficiency and patient outcomes. AI’s integration into genomics and precision medicine is revolutionizing how diseases are diagnosed, treated, and understood. Its ability to handle large datasets, recognize patterns, and provide personalized insights is paving the way for more effective and tailored healthcare solutions5. Real-world applications of AI in genomics are already taking shape. For instance, companies like Deep Genomics and Tempus are leveraging AI to discover gene-disease relationships and deliver personalized oncology treatments, respectively (Table 1). These developments reflect how AI is not only theoretical but is being actively deployed to enhance genomics research and clinical outcomes. As multimodal AI technologies continue to evolve, their impact on genomics and precision medicine is expected to grow, leading to more significant breakthroughs and improved patient care. As multimodal AI technologies continue to evolve, their impact on genomics and precision medicine is expected to grow, leading to more significant breakthroughs and improved patient care.
Medical imaging and diagnostics: the transformative role of deep learning in image analysis and biomarker discovery
Multimodal AI approaches are also driving broader innovation in healthcare, as evidenced by the U.S. Food and Drug Administration (FDA), which has compiled a list of AI/ML medical algorithms cleared for market use63. Some of these algorithms, categorized by their medical specialty, are highlighted in Fig. 5. So far, there are over 500 that have been cleared and the majority of these relate to medical imaging, for example, in diagnostics. Medical imaging and diagnostics have been profoundly impacted by the advent of deep learning, a subset of multimodal AI that leverages complex neural networks to analyze vast amounts of data with unparalleled accuracy. Among the most significant advancements within this field is the application of deep learning models to the analysis of medical images, such as magnetic resonance imaging (MRI), computed tomography (CT) scans, and other radiological modalities.
Convolutional Neural Networks (CNNs) are commonly used for image classification and lesion detection tasks due to their ability to automatically extract hierarchical image features. U-Net architectures, which combine an encoder-decoder structure with skip connections, are particularly effective for medical image segmentation, such as delineating tumor boundaries64,65,66. Transformer-based models are also gaining attention for their capacity to capture long-range spatial dependencies and contextual relationships, which are critical for interpreting complex anatomical structures. These advanced models not only improve diagnostic accuracy but also reduce the need for manual annotation, supporting more efficient and standardized clinical workflows. Collectively, these deep learning techniques are enhancing the precision and scalability of medical image analysis across a range of applications.
These models are not only revolutionizing the way images are interpreted but also are paving the way for early diagnosis and more precise treatment planning, thereby improving patient outcomes and reducing the burden on healthcare systems67. Real-Time Monitoring: Wearable devices and sensors equipped with AI algorithms enable continuous health monitoring. These devices can detect abnormalities and alert healthcare providers and patients, enabling timely intervention68.
Deep learning algorithms excel in processing medical images, often surpassing human capabilities in speed and accuracy. Unlike traditional methods that rely on radiologists to painstakingly review scans, AI models analyze vast datasets of labeled images, recognizing patterns and features that may be imperceptible to the human eye43,67,69. This enables them to identify and highlight potential concerns like tumors or lesions with precision, significantly enhancing the diagnostic process. One of the most promising applications of AI in medical imaging is in the early detection of diseases. For instance, in oncology, the ability to detect tumors at an early stage is crucial for successful treatment. AI models can be trained to identify minute changes in tissue structure or density that may indicate the presence of a tumor, even before symptoms manifest. This early detection capability is particularly valuable in cancers such as lung, breast, and prostate cancer, where early intervention can drastically improve survival rates. Moreover, these models can be continuously updated and refined as new data becomes available, ensuring that they remain at the cutting edge of diagnostic accuracy70,71.
Beyond mere image analysis, multimodal AI is also revolutionizing the field of biomarker discovery. Biomarkers are biological indicators that can be used to detect or monitor a disease, predict its progression, or assess the response to treatment. Traditionally, biomarkers have been identified through laboratory-based techniques that analyze biological samples such as blood or tissue. However, AI offers a novel approach to biomarker discovery by integrating imaging data with omics data—such as genomics, proteomics, and metabolomics—to identify new biomarkers that may not be apparent through conventional methods72,73. The integration of imaging and omics data is particularly powerful because it allows for a more comprehensive understanding of the biological processes underlying a disease. For example, in cancer research, AI models can analyze imaging data to identify phenotypic changes in tumors, while simultaneously analyzing genomic data to uncover the genetic mutations driving these changes74,75. By correlating these datasets, AI can identify novel biomarkers that are associated with specific disease subtypes or treatment responses. This information can then be used to develop more personalized and targeted therapies, thereby improving the efficacy of treatments and reducing the risk of adverse effects. Multimodal AI excels in biomarker discovery by effectively handling vast, complex datasets from modern imaging and omics technologies76. Unlike traditional methods, multimodal AI can manage nonlinear relationships and multiple interacting factors, uncovering subtle patterns and associations that may otherwise go unnoticed, enhancing the precision of biomarker identification77.
Furthermore, AI’s deep learning models can be used to identify biomarkers that are predictive of disease progression or response to treatment. For example, in the context of neurodegenerative diseases such as Alzheimer’s, these models can analyze longitudinal imaging data to identify early biomarkers of disease progression78,79. These biomarkers can then be used to develop predictive models that can inform treatment decisions and improve patient outcomes. Similarly, in oncology, AI models can be used to identify biomarkers that predict how a patient will respond to a particular therapy, allowing for more personalized and effective treatment plans80,81.
The impact of AI on medical imaging and diagnostics extends beyond the laboratory and into the clinic. For instance, AI-driven tools are being integrated into radiology workflows to assist clinicians in making faster and more accurate diagnoses. These tools can automatically analyze images, flag potential abnormalities, and even suggest possible diagnoses based on the patterns detected in the data. This not only speeds up the diagnostic process but also reduces the likelihood of missed diagnoses, particularly in busy clinical settings where radiologists are often required to review large numbers of scans in a short amount of time82,83. Moreover, AI-driven imaging techniques are being developed to enhance the resolution of MRI and CT scans, allowing for more precise visualization of tissues and structures. These enhanced imaging techniques can provide clinicians with a clearer picture of the underlying pathology, leading to more accurate diagnoses and better-informed treatment decisions84.
As the field of medical imaging and diagnostics continues to evolve, the role of AI is likely to expand even further. Ongoing research is focused on developing more sophisticated models that can analyze multiple types of data simultaneously, such as combining imaging data with electronic health records, clinical notes, and other sources of patient information. These multimodal models have the potential to provide a more holistic view of a patient’s health, enabling more comprehensive and accurate diagnoses85,86. In conclusion, the application of AI in medical imaging and diagnostics is transforming the way diseases are detected, diagnosed, and treated. By enhancing the accuracy of image analysis and facilitating the discovery of novel biomarkers, AI is not only improving patient outcomes but also driving the development of more personalized and effective therapies. As these technologies continue to advance, they hold the promise of ushering in a new era of precision medicine, where treatments are tailored to the individual characteristics of each patient, leading to better health outcomes for all.
As we have explored the pivotal role of AI in revolutionizing biotech, it becomes evident that these technological advancements are not only transforming scientific processes but also have profound implications for the broader economy. By enhancing efficiency, reducing costs, and accelerating innovation, AI-driven innovation is poised to create significant economic value. Now, we will delve into the economic impact of these developments, examining how they influence market dynamics, drive new business opportunities, and shape the future of the biotech industry.
AI research and patent landscape
In the rapidly advancing field of biotechnology, the integration of AI represents a pivotal innovation, offering unprecedented opportunities for enhancing research, development, and application. AI has rapidly transformed multiple industries, driving innovation across various fields. This section of the review analyzes the trends in AI-related publications and patents, explores the global distribution of AI activity, and highlights key collaborations among leading institutions and companies. The insights are based on a heatmap of publication and patent trends covering the period from 2010 to 2025 (Fig. 6). A total of 98,744 documents were retrieved from 2010 to 2025. However, the figure primarily utilizes data from 2010 to 2024 to enhance visualization and interpretability.
a Comprehensive visualization of publication trends. b The figure illustrates the number of publications across five languages, c Heatmap illustrates the trends in AI-related publications and patents across 24 key subfields. The color gradient represents the number of publications or patents, with darker red indicating higher values and blue indicating lower values.
Figure 6a provides a comprehensive visualization of publication trends. The pie chart illustrates the overall distribution of publications, where journal articles dominate (75.8%), followed by patents (16.1%) and reviews (8.1%). The bar chart further breaks this down across three time periods (2010–2015, 2016–2020, and 2021–2024), showing a significant rise in journal articles, reviews and patents in recent years. The line chart highlights an increasing trend in annual publications, The publication trend remained relatively stable until 2018, after which a notable acceleration occurred, particularly between 2019–2024. The period from 2019 to 2024 witnessed a dramatic increase in publication activity, nearly equaling the total output of the previous decade in just 6 years. This surge suggests a 4- to 6-fold increase in research output, highlighting a significantly intensified focus on AI applications. This trend is indicative of growing interest and substantial investments from academic institutions, biotech companies, and the pharmaceutical industry.
Figure 6b illustrates the number of publications across five languages—English, Chinese, Japanese, Korean, and Russian—between 2010 and 2024. English consistently dominates, experiencing a steady rise from 1497 publications in 2010 to 20,353 in 2024, reflecting a 13-fold growth in research output. Chinese follows as the second most published language, growing from 292 to 1260 publications over the same period. Japanese, Korean, and Russian exhibit similar upward trends, though at lower volumes. The overall increase in publications across all languages suggests a growing global research output, with English maintaining its role as the predominant language of scientific communication.
The heatmap in Fig. 6c visualizes the growth of AI publications and patents across 24 key subfields over time, from 2010 to 2024. Each AI subfield, including Drug Discovery and Development, Precision Medicine, Genomics and Proteomics, Synthetic Biology, Clinical Trials, Healthcare, Bioinformatics, Digital Biomarkers and more, shows increasing trends in AI-related publications and patents over the years. From 2010 to 2015, research activity remained minimal, but a sharp increase began post-2018, reflecting advancements in AI-driven biotech solutions. Drug Discovery saw the most significant rise, reaching 275 in 2024, followed by Precision Medicine (150) and Genomics (106). This surge indicates the growing reliance on AI for pharmaceutical R&D, personalized treatment approaches, and genomic analysis. The early projections for 2025 suggest continued expansion, emphasizing AI’s critical role in shaping the future of biotechnology.
This steady increase in activity across AI subfields indicates the growing interest and investment in AI technologies, particularly in Biomarkers, proteomics, genomics, drug discovery and precision medicine systems. Subfields such as biomarkers, COVID-19 and drug discovery have seen rapid growth due to advancements in AI-driven applications, while other subfields are anticipated to see further expansion in the coming years.
To further elucidate the distribution of patent activity, an additional tool, Patent Lens, was used to provide a comprehensive analysis of AI-related patent activity across various jurisdictions. The top-left graph in Fig. 7 illustrates the increasing trend of patent filings over time, with a notable rise in patent applications and granted patents, particularly in recent years. This reflects growing innovation and investment in AI technologies. The world map on the top-right highlights the leading jurisdictions in AI patent filings, with the United States emerging as the dominant region. The color gradient represents the density of patents filed, with darker shades indicating higher concentrations.
The bottom-left bar chart categorizes the top applicants by jurisdiction, showing key contributors from the United States, WIPO (World Intellectual Property Organization), and the European Patent Office. Institutions such as the Broad Institute, Harvard College, and major corporations like Bayer AG are prominent players in AI-related patents.
The bottom-right bar graph details the legal status of patents across different jurisdictions. It categorizes patents into active, pending, patented, expired, discontinued, inactive, and unknown statuses. The data suggests that a significant proportion of patents remain active or pending, indicating ongoing innovation and commercialization in the AI domain. Overall, Fig. 7 highlights the global landscape of AI patenting, emphasizing the concentration of intellectual property in key regions and organizations.
These findings provide critical insights into the trajectory of AI in biotechnology, underscoring the rapid advancements and growing influence of AI technologies in this domain. The increasing volume of research and patent activity signals a robust and expanding interest in harnessing AI to address complex challenges in biotechnology. As AI continues to drive innovation, it is poised to revolutionize various biotechnological processes, leading to more effective and efficient solutions in areas such as healthcare, agriculture, and environmental sustainability. The convergence of AI and biotechnology not only enhances the scientific landscape but also promises to deliver transformative impacts across multiple sectors.
The AI landscape is evolving rapidly, with increasing activity in both research and patents across multiple subfields. The heatmap, geographic map, and network graph provide a comprehensive view of AI’s global development, revealing trends, geographic clusters, and key collaborations that shape the field. As AI technologies continue to advance, the intersection of research, innovation, and collaboration will play a crucial role in determining the future direction of AI. The visualizations presented here underscore the dynamic growth of AI, not only as a field of academic study but also as a driver of innovation and industry transformation. Continued investment, global collaboration, and strategic partnerships will be essential in unlocking AI’s full potential.
We acknowledge that our analysis may be subject to publication bias, as studies reporting significant or positive outcomes in AI applications are more likely to be published and indexed in the databases we surveyed. This can lead to an overrepresentation of successful implementations and underreporting of negative or inconclusive results, potentially skewing the perceived effectiveness of multimodal AI in biotechnology. To partially mitigate this, we complemented peer-reviewed literature with selected gray literature sources, including government reports and industry white papers, where feasible. However, we recognize that access to unpublished data and proprietary outcomes remains limited. To enhance the robustness of future analyses, we suggest incorporating systematic gray literature reviews, as well as qualitative inputs such as expert interviews, failure case studies, and internal reports where available. These methods would help triangulate evidence and provide a more balanced view of both the promises and limitations of AI adoption. Additionally, researchers could explore bias-aware bibliometric techniques that weigh the influence of citation practices and research funding dynamics on the visibility of published outcomes.
Economic impact
The technical advancements in AI outlined in the previous sections—such as AI-driven target identification, generative molecule design, and automated diagnostics—are not only transforming biotechnology workflows but also reshaping the industry’s economic landscape. These innovations significantly reduce R&D timelines, improve trial efficiency, and lower operational costs, generating substantial economic value. As a result, AI is now being leveraged across the biotechnology spectrum—from early-stage startups in private markets to major pharmaceutical firms in public markets. For example, cost reductions in early-phase drug development through AI-led target discovery have fueled investor interest in platforms using generative models like VAEs and GANs for virtual screening35,36,37. Likewise, the rising market valuation of AI in medical imaging reflects the broad clinical adoption of CNNs for radiological diagnostics—evidenced by more than 500 FDA-cleared AI/ML-enabled imaging devices64,65. These economic signals are not merely indicative of market enthusiasm; they are grounded in demonstrable advancements in algorithmic performance, regulatory approval, and real-world clinical impact. By tying economic trends to specific technical innovations, we provide a more integrated and meaningful understanding of AI’s value in transforming biopharmaceutical R&D and healthcare delivery. In this section, we examine capital deployment trends and deal activity to map where the greatest opportunities lie for investment, innovation, and further research.
Venture financing and partnerships with early stage companies
The AI vertical as a whole has seen an explosion in venture financing over the past decade, with 2021 marking a peak with over $130 billion in committed capital, up from just $3 billion in 201287. Venture capital funding in AI-biotech startups increased by approximately 23% compared to 2019, reaching nearly $1.9 billion. This amount surpassed the combined investments of 2015, 2016, and 2017. AI-related biotech and healthcare startups secured $12.5 billion in venture capital, marking a record year for investments in this sector. The biotech industry in the U.S. and Europe raised nearly $19 billion in venture capital. While this figure encompasses the broader biotech sector, a substantial portion was directed toward AI-driven initiatives. Funding rebounded to $6.7 billion for AI-related biotech and healthcare startups through early December, indicating renewed investor interest. These data points illustrate a robust upward trajectory in venture capital investments in AI-focused biotech and pharmaceutical companies over the past decade. The cumulative investment from 2015 to 2025 is substantial, reflecting the growing confidence in AI’s potential to revolutionize the biotech and pharma industries. Phase 2 trial data is expected to continue driving biopharma VC investments in 2025. In biotech, AI startups and growth companies offer unique value propositions in numerous sub-verticals, including drug discovery, personalized medicine, and healthcare data management. 25 out of the 30 largest AI biotech venture financing deals have occurred after the beginning of 2020, reflecting a broader sense of investor excitement behind artificial intelligence technologies across domains. Recent data indicate a surge in funding, with large rounds reported for AI-focused biotech firms, especially those developing AI-enabled platforms for drug discovery, gene therapy, and precision medicine. For example, companies like Formation Bio and AQEMIA have raised hundreds of millions in the first quarter of 2024 alone, demonstrating continued investor confidence in AI biotech innovations88.
The increasing trend of large-scale investments aligns with the growing importance of AI in streamlining drug development processes, improving patient recruitment, and enhancing therapeutic discovery. As we look at 2024, more biotech firms are incorporating AI tools, leading to continued strong funding. The ongoing venture interest highlights the pivotal role AI plays in transforming the biotech landscape, with substantial contributions to the growth of personalized medicine and other high-impact medical fields89.
However, despite the peak surge in investor excitement in 2021, 2022 and 2023 saw reduced venture deal count and capital commitments, both in AI biotech companies as well as in venture as a whole. This trend can be attributed to various factors, including the macroeconomic environment impacting deal-making and shifting sentiments regarding the business models of startups. In the US, rising inflation and interest rate hikes increased the cost of borrowing, making investors more cautious. Market volatility and geopolitical tensions, such as the Russia-Ukraine war, further eroded investor confidence. Additionally, decreased IPO activity and increased regulatory scrutiny led to a more conservative investment environment, affecting venture investments across verticals, including the AI biotech industry90.
Moreover, while AI offers significant potential for leveraging big data and transforming the industry, investor confidence in the business models of many companies is evolving. Feedback from venture capitalists indicates that investors are increasingly emphasizing that AI on its own cannot deliver the bulk of value to be created. They seek concrete outcomes, such as the successful delivery of new drugs to the market, rather than being impressed by technological potential alone. This shift reflects a “show me” market mentality, where demonstrable achievements are prioritized over visionary claims.
In fact, an analysis of the largest venture financing events in the past 10 years reflects several key trends and preferences among investors. Companies “prioritizing” the combination of high growth potential, proprietary technological innovation, scalability, and market demand have been attracting the greatest amounts of capital.
Many of these companies are turning profits and growing, but we are still very early and many valuations have been forward-looking. The next 5–10 years will be crucial in identifying the companies that can truly deliver on their value proposition. Regardless, venture financing remains a leading catalyst in the incubation of ideas and technologies in the AI biotech space, as evidenced by a surge in investment over the past decade. The coming decade will be pivotal as investors closely monitor the evolution of companies in this sector, carefully assessing both successful strategies and areas for improvement.
Initial Public Offering (IPO) market
The IPO market serves as a critical gateway for private companies to access public capital, enabling them to expand their operations, increase visibility, and provide liquidity to early investors. Following the COVID-19 pandemic, the biotech industry shined, helping to develop vaccines and drugs that would save countless lives. As a result, IPOs in the biotech space launched at a record pace, giving early backers massive payouts. However, momentum stalled beginning in 2021, and the number of new IPOs nosedived in 2022 and 2023. AI-focused startups in biotech have followed a similar trajectory. Despite the success of companies like Tempus AI, which went public in June 2024 and has since generated significant investor enthusiasm from major players such as Google and SoftBank91 Biotech IPOs in 2024 are on track to roughly match the number of offerings priced in each of the last 2 years [Table 2]92.
Partnerships and private M&A deals
With the slowdown of the IPO market, private M&A deals and partnerships have gained prominence as alternative strategies for companies to achieve growth and access new technologies. With a majority of the 2024 IPO class trading at share prices well below their debut value, private M&A offers a strong alternative. With the looming patent cliff, pharma companies have strong incentives to find new drugs this decade; at the same time, the slowdown in IPO activity over the past few years has led to an accumulation of biotech companies that have not yet gone public, creating the perfect conditions for a robust private M&A market92.
For firms looking for a more risk-averse approach, partnerships are a great strategy. Early-stage biotech companies today are often partnering with large pharmaceutical companies to access more capital and share both risks and rewards93. For example, GlaxoSmithKline inked a partnership with In Silico Medicine in 2017 to explore how the latter’s artificial intelligence technology could aid in the drug discovery process. At the time of the partnership announcement in 2017, InSilico was just raising its Series A round. As of 2024, the drug developer has raised around $400 million, marked $51.18 million in yearly revenue, and is turning to the Hong Kong stock market for an IPO funding boost.
Regulatory and ethical Issues
The rapid advancement of AI technologies in biotechnology introduces complex regulatory and ethical challenges that must be proactively addressed to ensure safe, equitable, and responsible innovation. Regulatory frameworks currently struggle to keep pace with the evolving nature of AI, particularly in areas such as data privacy, algorithmic transparency, and accountability. For example, the use of large-scale biomedical data in AI models raises significant concerns around patient consent and confidentiality, which are governed by regulations such as the General Data Protection Regulation (GDPR) in Europe and the Health Insurance Portability and Accountability Act (HIPAA) in the United States94,95.
Ethical considerations are equally critical. AI systems may perpetuate or even amplify existing biases if trained on unrepresentative datasets, leading to inequitable outcomes in healthcare and biotechnology applications96, such as dermatology algorithms trained predominantly on lighter skin tones leading to reduced diagnostic accuracy for patients with darker skin67 and risk prediction tools in cardiology that underrepresent minority populations, thereby perpetuating healthcare disparities97. Furthermore, the “black-box” nature of many AI algorithms challenges transparency, complicating clinical decision-making and undermining trust among practitioners and patients98. There are also concerns regarding the fair distribution of AI-driven biotechnological benefits, particularly in low-resource settings, raising issues of global health equity99. Efforts such as the WHO’s deployment of AI-supported diagnostic tools in underserved regions and initiatives like OpenMRS, an open-source medical record system100 used in over 40 developing countries, highlight steps being taken to address this imbalance. To address these challenges, multidisciplinary collaboration among policymakers, ethicists, scientists, and industry stakeholders is essential. Recent efforts include the development of AI ethics guidelines by organizations such as the World Health Organization (WHO) 2021101 and the European Commission, 2020102, which emphasize principles of fairness, accountability, and transparency. Moving forward, regulatory bodies need to implement adaptive and context-specific frameworks that promote innovation while safeguarding public interest. Continuous ethical oversight, stakeholder engagement, and transparent reporting are crucial for building public trust and ensuring that AI applications in biotechnology contribute positively and equitably to society. Future research should prioritize not only technical improvements but also the integration of ethical considerations into the development and deployment of AI systems in this field. To support responsible and trustworthy AI, one key area of focus is explainability, which plays a central role in both ethical AI design and regulatory compliance.
One of the most promising technical approaches to addressing ethical and regulatory challenges is as AI systems increasingly inform critical decisions in healthcare and life sciences, the need for transparency, fairness, and accountability becomes paramount. Regulatory bodies such as the U.S. Food and Drug Administration (FDA) have begun implementing tailored frameworks—such as the proposed regulatory approach for AI/ML-based Software as a Medical Device—that emphasize algorithm interpretability, continuous validation, and real-world performance monitoring. For example, FDA-approved systems such as IDx-DR for diabetic retinopathy provide interpretable outputs, while imaging tools often use saliency maps to highlight areas relevant to diagnosis103. Despite progress, adoption barriers persist. These include challenges in integrating heterogeneous data, lack of standardized regulatory pathways, limited interpretability in complex models, and infrastructural or resource constraints in clinical settings. User trust remains a critical issue, particularly when AI decisions affect patient outcomes99,104. To address fairness, tools such as fairness-aware algorithms105 that adjust for underrepresented groups in clinical trial data, and case studies like IBM’s AI Fairness 360 toolkit106, which provides transparency and bias-detection tools for healthcare applications. Overcoming these barriers requires a multi-stakeholder approach involving industry, regulators, healthcare providers, and researchers. Emphasizing transparency through XAI, establishing standardized data protocols, and fostering trust, and aiding compliance with evolving legal and ethical standards will be essential to ensuring that AI technologies can be adopted safely and effectively in real-world biotechnology applications.
Future prospects
The future of AI in biotechnology is promising, with key advancements expected to overcome current challenges and broaden its applications107,108. A significant development will be the integration of AI with emerging technologies like blockchain and the Internet of Things (IoT). This combination can enhance data security and enable real-time monitoring of biotechnological processes. For instance, IoT devices could continuously collect data from bioreactors, which AI could analyze to optimize conditions, improving efficiency and product quality. Notably, AI is already being deployed to optimize key bioprocess parameters such as pH, oxygen levels, and nutrient flow in industrial biotechnology settings, enabling improved efficiency and predictive maintenance109,110. However, further development is needed to enhance the scalability, transparency, and interoperability of these systems—particularly as the field moves toward integrating AI with emerging technologies like IoT and blockchain. Real-time data from bioreactors, when combined with secure and decentralized platforms, could enable more adaptive and autonomous manufacturing environments.
Another crucial advancement is the development of explainable AI. As multimodal AI systems become more complex, their decision-making processes often become opaque, creating a “black box” effect. This lack of transparency poses challenges for regulatory approval, especially in biotechnology and healthcare111,112,113. Developing AI models that provide clear, interpretable results will foster trust among regulators and clinicians, enabling broader AI adoption. For example, an explainable AI model in drug discovery could show how it predicts the efficacy of a new compound, enhancing clinical and regulatory acceptance114. Collaborative platforms will also play a vital role in the future of AI in biotechnology115. These platforms, which bring together AI developers, biotechnologists, and healthcare professionals, will drive innovation by ensuring that AI applications are practically relevant and technically sophisticated. Moreover, global collaboration will be essential for pooling resources and addressing global health challenges. International partnerships can enhance the accuracy and generalizability of AI models by leveraging diverse data, leading to more effective biotechnological solutions.
Another promising direction is the use of federated learning, a decentralized AI approach that allows models to be trained across multiple datasets without transferring sensitive data between institutions. This technique preserves data privacy and ownership—critical in clinical and biotechnological contexts—while enabling large-scale collaborative learning across geographically distributed sources115,116. In the field of synthetic biology, AI is expanding beyond gene editing to include the automated design and optimization of genetic circuits, metabolic pathway engineering biomanufacturing, bioprocess optimization and the simulation of synthetic ecosystems117. Machine learning models can predict biological behavior and interactions at a systems level, enabling more sophisticated and scalable design of synthetic organisms for applications in healthcare, agriculture, and bioenergy. Equally important is the growth of open-source tools and platforms that are democratizing access to AI technologies. Initiatives such as AlphaFold9 by DeepMind, DeepChem, and BioJupies offer scalable, accessible pipelines for protein structure prediction, molecular modeling, and omics data analysis. These platforms not only foster reproducibility and transparency but also accelerate cross-disciplinary collaboration. The open-source ecosystem is poised to be a critical enabler for innovation, particularly for under-resourced institutions and researchers globally. In summary, the future of AI in biotechnology will be shaped by its integration with other technologies, the development of explainable models, collaborative efforts, and global partnerships118,119. These advancements will expand AI’s capabilities, leading to more efficient and accessible biotechnological innovations.
The review of AI in the biotechnology landscape reveals a transformative influence on the industry, driving economic growth and presenting both opportunities and challenges. AI’s integration into biotechnology has accelerated drug discovery, personalized medicine, and synthetic biology, leading to reduced costs and increased efficiency. Economically, this has fostered innovation, attracted significant investments, and created new job opportunities in AI-driven biotech companies. However, the rapid adoption of AI also presents challenges, including ethical concerns, data privacy issues, and the potential for job displacement due to automation. The socioeconomic impact is profound, as AI-driven advancements in biotechnology promise to address global health challenges, improve agricultural productivity, and contribute to environmental sustainability120,121. However, these benefits are not evenly distributed, with disparities in access to AI technologies exacerbating existing inequalities between developed and developing regions. The high costs associated with implementing AI and the need for specialized skills can create barriers for smaller companies and under-resourced communities122,123.
Furthermore, the reliance on large datasets raises concerns about data ownership, privacy, and the potential misuse of information, leading to societal debates on the ethical implications of AI in biotechnology. The challenge lies in balancing the economic benefits with the need for responsible AI deployment that considers ethical, legal, and social implications. Addressing these challenges requires a collaborative approach involving policymakers, industry leaders, and the scientific community to ensure that AI’s integration into biotechnology not only drives economic growth but also promotes equitable and sustainable development124. In conclusion, while AI presents significant opportunities for the biotechnology sector, careful consideration of its broader impacts is essential to harness its full potential responsibly.
Conclusion
AI is poised to revolutionize the biotechnology landscape, offering unprecedented opportunities for advancements in drug discovery, genomics, medical imaging, and synthetic biology. While challenges remain, the economic benefits of AI—cost reduction, increased productivity, market growth, job creation, and healthcare savings—are driving rapid adoption and development. As technology continues to evolve, the integration of AI in biotechnology promises to unlock new frontiers in biological research and healthcare, ultimately improving human health and well-being while contributing to economic growth. Addressing the challenges and ensuring ethical practices will be key to realizing the full potential of AI in biotechnology.
Data availability
No datasets were generated or analyzed during the current study.
References
Ramesh, A. Artificial intelligence in medicine. Ann. R. Coll. Surg. Engl. 86, 334–338 (2004).
Smith, R. G. & Farquhar, A. The road ahead for knowledge management: an AI perspective. AI Mag. 21, 17–17 (2000).
Lamberti, M. J. A study on the application and use of artificial intelligence to support drug development. Clin. Ther. 41, 1414–1426 (2019).
Holzinger, A., Keiblinger, K., Holub, P., Zatloukal, K. & Müller, H. AI for life: trends in artificial intelligence for biotechnology. N. Biotechnol. 74, 16–24 (2023).
Quazi, S. Artificial intelligence and machine learning in precision and genomic medicine. Med. Oncol. 39, 120 (2022).
Das, S. et al. Applications of artificial intelligence in machine learning: review and prospect. Int. J. Comput. Appl. 115, 9 (2015).
Sharma, A. et al. Artificial intelligence-based data-driven strategy to accelerate research, development, and clinical trials of COVID vaccine. Biomed. Res. Int. 2022, 7205241 (2022).
Alimadadi, A. et al. Artificial intelligence and machine learning to fight COVID-19. Physiol. Genom. 52, 200–202 (2020).
Ren, F. et al. AlphaFold accelerates artificial intelligence-powered drug discovery: efficient discovery of a novel CDK20 small molecule inhibitor. Chem. Sci. 14. https://doi.org/10.1039/D3SC00722J (2023).
Shen, C. et al. DrugFlow: an AI-driven one-stop platform for innovative drug discovery. J. Chem. Inf. Model. 64, 5381–5391 (2024).
Liu, Z. et al. AI-based language models powering drug discovery and development. Drug Discov. Today 26, 2593–2607 (2021).
Tripathi, A. et al. Artificial intelligence in accelerating drug discovery and development. Recent Pat. Biotechnol. 17, 9–23 (2023).
Kalidindi, V. R. et al. Revolutionizing medicine: unleashing the power of real-world data and AI in advancing clinical trials. Braz. J. Pharm. Sci. 60, e23980 (2024).
Artificial Intelligence Market Size and Future Outlook. https://www.fortunebusinessinsights.com/industry-reports/artificial-intelligence-market-100114
Xu, Y. et al. Artificial intelligence: a powerful paradigm for scientific research. Innovation 2, 100179 (2021).
Nagarhalli, T. P. et al. The review of natural language processing applications with emphasis on machine learning implementations. In: Proc.International Conference on Electronics and Renewable Systems (ICEARS) (IEEE, 2022).
Ofori-Boateng, R. et al. Towards the automation of systematic reviews using natural language processing, machine learning, and deep learning: a comprehensive review. Artif. Intell. Rev. 57, 1–60 (2024).
Alwahedi, F. et al. Machine learning techniques for IoT security: current research and future vision with generative AI and large language models. Internet Things Cyber-Phys. Syst. 4, 167–185 (2024).
Smalley, E. AI-powered drug discovery captures pharma interest. Nat. Biotechnol. 35, 604–606 (2017).
Mak, K. K. & Pichika, M. R. Artificial intelligence in drug development: present status and future prospects. Drug Discov. Today 24, 773–780 (2019).
Rehman, A. U. et al. Role of artificial intelligence in revolutionizing drug discovery. Fundam. Res. https://doi.org/10.1016/j.fmre.2024.04.021 (2024).
Paul, D. et al. Artificial intelligence in drug discovery and development. Drug Discov. Today 26, 80–93 (2021).
Blanco-González, A. et al. The role of AI in drug discovery: challenges, opportunities, and strategies. Pharmaceuticals 16, 891 (2023).
Yadav, B. S. & Tripathi, V. Recent advances in the system biology-based target identification and drug discovery. Curr. Top. Med. Chem. 18, 1737–1744 (2018).
Sharma, V. et al. Role of Artificial Intelligence in Drug Discovery and Target Identification in Cancer. Curr. Drug Deliv. 21, 870–886 (2024).
Bifarin, O. O. & Fernández, F. M. Automated machine learning and explainable AI (AutoML-XAI) for metabolomics: improving cancer diagnostics. J. Am. Soc. Mass Spectrom. 35, 1089–1100 (2024).
Chi, J. et al. Artificial intelligence in metabolomics: a current review. Trends Anal. Chem. 117852 (2024).
Umar, T. P. Artificial intelligence-enhanced application of CRISPR-Cas13a for cancer gene therapy: a breakthrough concept. Experimed 14, 61–62 (2024).
Dara, M. et al. Convergence of CRISPR and artificial intelligence: a paradigm shift in biotechnology. Hum. Gene 41, 201297 (2024).
Ibrahim, U. et al. CRISPR biosensing and AI-driven tools for detection and prediction of COVID-19. J. Exp. Theor. Artif. Intell. 35, 489–505 (2023).
Nantasenamat, C., Isarankura-Na-Ayudhya, C. & Prachayasittikul, V. Advances in computational methods to predict the biological activity of compounds. Expert Opin. Drug Discov. 5, 633–654 (2010).
Akter, T. & Rahman, M. M. Computational analysis of common gene and design protein–drug interaction network for the target diseases based on protein–protein interaction network in bioinformatics. Inform. Med. Unlocked 42, 101357 (2023).
Cava, C., Bertoli, G. & Castiglioni, I. A protein interaction map identifies existing drugs targeting SARS-CoV-2. BMC Pharmacol. Toxicol. 21, 1–11 (2020).
Schneider, G. Automating drug discovery. Nat. Rev. Drug Discov. 17, 97–113 (2018).
Huang, Z., Chen, S. & Yu, L. Predicting new drug indications based on double variational autoencoders. Comput. Biol. Med. 164, 107261 (2023).
Tripathi, S. et al. Recent advances and application of generative adversarial networks in drug discovery, development, and targeting. Artif. Intell. Life Sci. 2, 100045 (2022).
Abbasi, M. et al. Designing optimized drug candidates with Generative Adversarial Network. J. Cheminform. 14, 40 (2022).
Zhavoronkov, A. Artificial intelligence for drug discovery, biomarker development, and generation of novel chemistry. Mol. Pharm. 15, 4317–4324 (2018).
Schapin, N. et al. On machine learning approaches for protein-ligand binding affinity prediction. https://doi.org/10.48550/arXiv.2407.19073 (2024).
Kairys, V. et al. Recent advances in computational and experimental protein-ligand affinity determination techniques. Expert Opin. Drug Discov. 19, 649–670 (2024).
DiMasi, J. A., Feldman, L., Seckler, A. & Wilson, A. Trends in risks associated with new drug development: success rates for investigational drugs. Clin. Pharmacol. Ther. 87, 272–277 (2010).
Wong, C. H., Siah, K. W. & Lo, A. W. Estimation of clinical trial success rates and related parameters. Biostatistics 20, 273–286 (2019).
Topol, E. J. High-performance medicine: the convergence of human and artificial intelligence. Nat. Med. 25, 44–56 (2019).
Walters, W. P. & Murcko, M. A. Artificial intelligence in drug discovery and development. Curr. Opin. Biotechnol. 65, 34–42 (2020).
Ekins, S. et al. Exploiting machine learning for end-to-end drug discovery and development. Nat. Mater. 18, 435–441 (2019).
Udegbe, F. C. et al. Machine learning in drug discovery: a critical review of applications and challenges. Comput. Sci. IT Res. J. 5, 892–902 (2024).
Schneider, P. et al. Rethinking drug design in the artificial intelligence era. Nat. Rev. Drug Discov. 19, 353–364 (2020).
Kassekert, R. et al. Industry perspective on artificial intelligence/machine learning in pharmacovigilance. Drug Saf. 45, 439–448 (2022).
Jarrahi, M. H. Artificial intelligence and the future of work: human-AI symbiosis in organizational decision making. Bus. Horiz. 61, 577–586 (2018).
Wang, L. et al. Artificial intelligence facilitates drug design in the big data era. Chemom. Intell. Lab. Syst. 194, 103850 (2019).
Naik, N. et al. Legal and ethical consideration in artificial intelligence in healthcare: who takes responsibility?. Front. Surg. 9, 266 (2022).
Karimian, G., Petelos, E. & Evers, S. M. A. A. The ethical issues of the application of artificial intelligence in healthcare: a systematic scoping review. AI Ethics 2, 539–551 (2022).
Vamathevan, J. et al. Applications of machine learning in drug discovery and development. Nat. Rev. Drug Discov. 18, 463–477 (2019).
Aronson, S. J. & Rehm, H. L. Building the foundation for genomics in precision medicine. Nature 526, 336–342 (2015).
What is precision medicine? [Internet]. Genetics Home Reference. https://ghr.nlm.nih.gov/primer/precisionmedicine/definition (2018).
Parmar, P. et al. Personalized medicine: TIME for one person, one medicine. Int. J. Pharm. Drug Anal. 9, 86–92 (2021).
Singhania, K. & Reddy, A. Improving preventative care and health outcomes for patients with chronic diseases using big data-driven insights and predictive modeling. Int. J. Appl. Health Care Anal. 9, 1–14 (2024).
Wirth, T., Parker, N. & Ylä-Herttuala, S. History of gene therapy. Gene 525, 162–169 (2013).
Maqsood, K., Hagras, H. & Zabet, N. R. An overview of artificial intelligence in the field of genomics. Discov. Artif. Intell. 4, 9 (2024).
Guo, K. et al. Artificial intelligence-driven biomedical genomics. Knowl.-Based Syst 279, 110937 (2023).
Melo, M. C. R., Maasch, J. R. M. A. & de la Fuente-Nunez, C. Accelerating antibiotic discovery through artificial intelligence. Commun. Biol. 4, 1050 (2021).
Boniolo, F. et al. Artificial intelligence in early drug discovery enabling precision medicine. Expert Opin. Drug Discov. 16, 991–1007 (2021).
U.S. Food and Drug Administration. Artificial Intelligence and Machine Learning (AI/ML)-Enabled Medical Devices. https://www.fda.gov/medical-devices/software-medical-device-samd/artificial-intelligence-and-machine-learning-aiml-enabled-medical-devices
Litjens, G. et al. A survey on deep learning in medical image analysis. Med. Image Anal. 42, 60–88 (2017).
LeCun, Y., Bottou, L., Bengio, Y. & Haffner, P. Gradient-based learning applied to document recognition. Proc. IEEE 86, 2278–2324 (1998).
Ronneberger, O., Fischer, P., Brox, T. U-Net: convolutional networks for biomedical image segmentation. In: Navab, N., Hornegger, J., Wells, W., Frangi, A. (eds) Medical Image Computing and Computer-Assisted Intervention—MICCAI 2015. Lecture Notes in Computer Science, 9351. https://doi.org/10.1007/978-3-319-24574-4_28 (Springer, 2015).
Esteva, A. et al. Dermatologist-level classification of skin cancer with deep neural networks. Nature 542, 115–118 (2017).
Shen, D., Wu, G. & Suk, H. I. Deep learning in medical image analysis. Annu. Rev. Biomed. Eng. 19, 221–248 (2017).
Wang, S. & Summers, R. M. Machine learning and radiology. Med. Image Anal. 16, 933–951 (2012).
Zhou, S. K. et al. A review of deep learning in medical imaging: Imaging traits, technology trends, case studies with progress highlights, and future promises. Proc. IEEE 109, 820–838 (2021).
Jiang, F. et al. Artificial intelligence in healthcare: past, present, and future. Stroke Vasc. Neurol. 2, 230–243 (2017).
Chen, R. J. et al. Synthetic data in machine learning for medicine and healthcare. Nat. Biomed. Eng. 5, 493–497 (2021).
Bhinder, B. et al. Artificial intelligence in cancer research and precision medicine. Cancer Discov. 11, 900–915 (2021).
Capobianco, E. High-dimensional role of AI and machine learning in cancer research. Br. J. Cancer 126, 523–532 (2022).
Mann, M. et al. Artificial intelligence for proteomics and biomarker discovery. Cell Syst. 12, 759–770 (2021).
Lazaris, A. et al. Predictive biomarker discovery in cancer using a unique AI model based on set theory. Inform. Med. Unlocked 46, 101481 (2024).
Fabrizio, C. et al. Artificial intelligence for Alzheimer’s disease: promise or challenge?. Diagnostics 11, 1473 (2021).
Khan, S., Barve, K. H. & Kumar, M. S. Recent advancements in pathogenesis, diagnostics and treatment of Alzheimer’s disease. Curr. Neuropharmacol. 18, 1106–1125 (2020).
Hunter, B., Hindocha, S. & Lee, R. W. The role of artificial intelligence in early cancer diagnosis. Cancers 14, 1524 (2022).
Arefin, S. IDMap: leveraging AI and data technologies for early cancer detection. Valley Int. J. Digit. Libr. 1138–1145 (2024).
Najjar, R. Redefining radiology: a review of artificial intelligence integration in medical imaging. Diagnostics 13, 2760 (2023).
Bouchareb, Y. et al. Artificial intelligence-driven assessment of radiological images for COVID-19. Comput. Biol. Med. 136, 104665 (2021).
Khalifa, M. & Albadawy, M. AI in diagnostic imaging: revolutionising accuracy and efficiency. Comput. Methods Programs Biomed. Update, 5, 100146 (2024).
Soenksen, L. R. et al. Integrated multimodal artificial intelligence framework for healthcare applications. NPJ Digit. Med. 5, 149 (2022).
Acosta, J. N. et al. Multimodal biomedical AI. Nat. Med. 28, 1773–1784 (2022).
Montanaro, B., Croce, A. & Ughetto, E. Venture capital investments in artificial intelligence. J. Evol. Econ. 34, 1–28 (2024).
Review Report. Artificial intelligence (AI) startup funding worldwide from 2011 to 2023 (in billion U.S. dollars, by quarter). https://reviewreport.co/artificial-intelligence-ai-startup-funding-worldwide-from-2011-to-2023-in-billion-u-s-dollars-by-quarter/ (2024).
Labiotech. Recent biotech fundings. https://www.labiotech.eu/recent-biotech-fundings/.
Fierce Biotech. Fierce Biotech Fundraising Tracker ‘24. Fierce Biotech. https://www.fiercebiotech.com/biotech/fierce-biotech-fundraising-tracker-24.
Benzinga. Tempus AI soars to new highs, investments from Google and SoftBank boost bullish sentiment. https://www.benzinga.com/trading-ideas/long-ideas/24/08/40419923/tempus-ai-soars-to-new-highs-investments-from-google-and-softbank-boost-bullish-sentimen.
BioPharma Dive. Private biotech M&A surges amid difficult IPO market. https://www.biopharmadive.com/news/biotech-startup-private-acquisitions-pharma-2024/721981/.
Iazzolino, G. & Bozzo, R. Partnership models for R&D in the pharmaceutical industry. In Quantitative Models in Life Science Business (eds Canci, J. K., Mekler, P. & Mu, G.). https://doi.org/10.1007/978-3-031-11814-2_3 (Springer, 2023).
Noble, W. S. What is the future of AI in biotechnology?. Nat. Rev. Genet. 22, 67–68 (2021).
Kumar, A., Sharma, A. & Gupta, R. Data privacy in healthcare: an overview of regulations and technologies. J. Med. Syst. 45, 1–15 (2021).
Price, W. N. & Cohen, I. G. Privacy in the age of medical big data. Nat. Med. 25, 37–43 (2019).
Rudin, C. Stop explaining black box models for high stakes decisions and use interpretable models instead. Nat. Mach. Intell. 1, 206–215 (2019).
Obermeyer, Z., Powers, B., Vogeli, C. & Mullainathan, S. Dissecting racial bias in an algorithm used to manage the health of populations. Science 366, 447–453 (2019).
Char, D. S., Shah, N. H. & Magnus, D. Implementing machine learning in health care — addressing ethical challenges. N. Engl. J. Med. 378, 981–983 (2018).
Vayena, E., Blasimme, A. & Cohen, I. G. Machine learning in medicine: addressing ethical challenges. PLoS Med 15, e1002689 (2018).
Open MRS. OpenMRS: open source enterprise electronic medical record system platform. https://openmrs.org/ (2024).
World Health Organization (WHO). Ethics and Governance of Artificial Intelligence for Health. https://www.who.int/publications/i/item/9789240029200 (2021).
European Commission. Ethics Guidelines for Trustworthy AI. https://ec.europa.eu/digital-strategy/en/news/ethics-guidelines-trustworthy-ai (2020).
Rajpurkar, P. et al. AI in healthcare: the potential and pitfalls. Nat. Med. 28, 31–38 (2022).
Topol, E. Deep Medicine: How Artificial Intelligence Can Make Healthcare Human Again (Basic Books, 2019).
Rajkomar, A. et al. Ensuring fairness in machine learning to advance health equity. Ann. Intern. Med. 169, 866–872 (2018).
Bellamy, R. K. E. et al. AI Fairness 360: An extensible toolkit for detecting, understanding, and mitigating unwanted algorithmic bias. IBM J. Res. Dev. 63, 4/5. https://doi.org/10.1147/JRD.2019.2942287 (2019).
Panch, T., Mattie, H. & Atun, R. Artificial intelligence and algorithmic bias: implications for health systems. J. Glob. Health 9, 020318 (2019).
Wang, L., Alexander, C. A. & Anderson, T. The convergence of AI and IoT in smart healthcare: a survey. IEEE Internet Things J. 7, 4857–4879 (2020).
Zhao, J. et al. Machine learning in bioprocessing: present status and future prospects. Biotechnol. Adv. 44, 107608 (2020).
Möller, J. et al. Real-time prediction of bioprocess variables using recurrent neural networks. Comput. Chem. Eng. 140, 106917 (2020).
Adadi, A. & Berrada, M. Peeking inside the black-box: a survey on explainable artificial intelligence (XAI). IEEE Access 6, 52138–52160 (2018).
Villalobos-Arias, M., Villanueva-Polanco, R. & Ucan-Puc, E. AI and IoT: integration for smart agricultural biotechnology. Agric. Biotechnol. 7, 25–32 (2022).
Buch, V. H., Ahmed, I. & Maruthappu, M. Artificial intelligence in medicine: current trends and future possibilities. Br. J. Gen. Pract. 68, 143–144 (2018).
Izankar, S. V., Kumar, P. & Waghmare, G. Evolution of artificial intelligence in biotechnology: from discovery to ethical and beyond. AIP Conf. Proc. 3188, 1 (2024).
Li, T., Sahu, A. K., Talwalkar, A. & Smith, V. Federated learning: challenges, methods, and future directions. IEEE Signal Process. Mag. 37, 50–60 (2020).
Nielsen, A. A. K. & Voigt, C. A. Deep learning to predict the lab-of-origin of engineered DNA. Nat. Commun. 9, 3135 (2018).
Cheng, F. & Zhao, Z. Machine learning and synthetic biology. Nat. Chem. Biol. 16, 1244–1251 (2020).
Luu, V. P. et al. Challenges of artificial intelligence in precision oncology: public-private partnerships including national health agencies as an asset to make it happen. Ann. Oncol. 35, 154–158 (2024).
Peters, M. A. & Besley, T. The geopolitics of postdigital educational development: from territories to networks to rival World Systems. Postdigit. Sci. Educ. 6, 391–399 (2024).
da Silva, R. G. L. The advancement of artificial intelligence in biomedical research and health innovation: challenges and opportunities in emerging economies. Glob. Health 20, 44 (2024).
Sturdivant, C. W. The impact of emerging technologies. Strength Knowledge: Warrant Officer J. (2024).
Barakat, O., Bouanba, N. The barriers to AI adoption in supply chains: case of Moroccan companies. In: Benadada, Y., Mhada, FZ., Boukachour, J., Ouzayd, F., El Hilali Alaoui, A. (eds.) Proc. 7th International Conference on Logistics Operations Management, GOL'24. GOL 2024 (Springer, 2024).
Bobier, J.-F. et al. Breaking the cost barrier in biomanufacturing. Ind. Biotechnol. 20, 113–124 (2024).
Chakraborty, C. et al. The changing scenario of drug discovery using AI to deep learning: recent advancement, success stories, collaborations, and challenges. Mol. Ther. Nucleic Acids 35, 102295 (2024).
Liu, J. et al. Machine learning and deep learning approaches for enhanced prediction of hERG blockade: a comprehensive QSAR modeling study. Expert Opin. Drug Metab. Toxicol. 20, 665–684 (2024).
Chen, X. et al. Deep learning-assisted high-content screening identifies isoliquiritigenin as an inhibitor of DNA double-strand breaks for preventing doxorubicin-induced cardiotoxicity. Biol. Direct 18, 63 (2023).
Datta, M. The role of industry in changing the landscape of healthcare using artificial intelligence. In Revolutionising Medical Imaging with Computer Vision and Artificial Intelligence 68 (2024).
Mallahan, S. et al. Optimizing clinical trial subject selection: insights from Exigent Research Network and the Tempus AI TIME Program collaboration. AI Precision Oncol 1, 306–314 (2024).
Savage, N. Tapping into the drug discovery potential of AI. https://www.nature.com/articles/d43747-021-00045-7 (2021).
Mohammed, Z. AI-driven drug target identification for therapeutic development. J. Deep Learn. Genom. Data Anal. 4, 94–103 (2024).
Khanna, A. & Jain, S. Personalized drug treatment: transforming healthcare with AI. In Artificial Intelligence and Machine Learning in Drug Design and Development 295–319 (Wiley, 2024).
Rifaioglu, A. S. et al. Recent applications of deep learning and machine intelligence in silico drug discovery: methods, tools and databases. Brief. Bioinform. 20, 1878–1912 (2019).
Harrer, S. et al. Artificial intelligence for clinical trial design. Trends Pharmacol. Sci. 40, 577–591 (2019).
Merolla, P. A. et al. A million spiking-neuron integrated circuit with a scalable communication network and interface. Science 345, 668–673 (2014).
Author information
Authors and Affiliations
Contributions
Conceptualization, A.B.; writing—original draft preparation, A.B., writing—reviewing & editing, A.B. and P.M.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Bhushan, A., Misra, P. Unlocking the potential: multimodal AI in biotechnology and digital medicine—economic impact and ethical challenges. npj Digit. Med. 8, 619 (2025). https://doi.org/10.1038/s41746-025-01992-6
Received:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s41746-025-01992-6