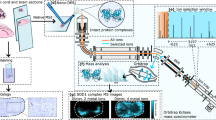
Abstract
Copper/zinc superoxide dismutase (SOD1) is a crucial metalloenzyme that mitigates oxidative stress by scavenging superoxide anion radicals. Mutations and aggregation of SOD1 are closely linked to the pathogenesis of amyotrophic lateral sclerosis (ALS). Targeting pathogenic SOD1 with nanobodies presents a promising therapeutic approach. We report the first high-resolution crystal structures of SOD1 in complex with three distinct nanobodies (Nb1, Nb2, and Nb3) and their multimeric assemblies (1:2 and 1:3 stoichiometries), revealing distinct binding epitopes primarily mediated by their complementarity determining regions (CDRs) through hydrogen bonds, salt bridges, and hydrophobic interactions. Structural and biophysical analyses using isothermal titration calorimetry (ITC) and fluorescence-detection size-exclusion chromatography (FSEC) demonstrated that all three nanobodies bind SOD1 simultaneously with nanomolar affinities (KD values ranging from 23.2 nM to 529 nM). Notably, engineered multimeric tandem nanobodies (Nb1-Nb2-Nb3) achieved higher affinity (KD = 4.39 nM) compared to single nanobodies, as validated by ITC. Characterization via dynamic light scattering (DLS) further revealed colloidal stability of SOD1-nanobody complexes. These results provide the first atomic-resolution insights into multi-nanobody targeting of SOD1 without steric interference, establishing a foundation for developing high-affinity tools to detect and manipulate SOD1 in ALS and related neurodegenerative diseases.

Similar content being viewed by others
Introduction
Copper/zinc superoxide dismutase (SOD1) is an essential intracellular antioxidant enzyme present in nearly all cell types. Under normal physiological conditions, SOD1 plays a crucial role in preventing oxidative injury by catalyzing the dismutation of superoxide into H2O21,2. It also modulates reactive oxygen species signaling pathways3,4,5. In 1993, SOD1 gene mutations were linked to familial ALS6, a fatal neurodegenerative disorder affecting both upper and lower motor neurons in the brain and spinal cord. Over 180 SOD1 mutations have been associated with ALS7,8, and the clinical and pathological features of ALS have been replicated in SOD1-based mouse models, such as SOD1G93A9,10 and SOD1G37R11. Additionally, some studies suggest that wild-type SOD1 contributes to ALS pathology due to its misfolding and abnormal conformations12,13. Various SOD1-targeting strategies have been tested in humans and animals14, including antisense oligonucleotides15, RNA interference16, and CRISPR/Cas9 techniques17. However, these approaches have common drawbacks such as immunogenic side effects, off-target effects, and biosafety concerns18. Additionally, treatment strategies involving small molecules19,20,21, peptides22,23, and monoclonal antibodies24,25,26 are under investigation, but they also struggle with low specificity and limited tissue accessibility, highlighting the need for novel modalities.
With the development of advanced antibody technologies, we are focusing on amyotrophic lateral sclerosis (ALS) therapies utilizing nanobodies. Nanobodies, named for their nanometer size, contain only a heavy chain variable region27,28.
Compared to conventional antibodies, nanobodies are significantly smaller, with a molecular weight about one-tenth that of IgG. Each nanobody consists of four framework regions (FR) and three complementarity-determining regions (CDRs), sharing some similarities with conventional antibodies but with notable differences. In the hypervariable regions, the enlarged H1 loop and extended H3 loop provide a large contact area for antigen–nanobody interaction. In FR2, four highly conserved hydrophobic amino acids are mutated to hydrophilic ones29.
The unique structure of nanobodies offers several biochemical advantages30. They exhibit excellent solubility and are resistant to aggregation31. Nanobodies maintain high conformational stability and unfolding reversibility under extreme conditions such as high pH, temperature, denaturants, and proteases32. Their long, flexible CDRs and loops contribute to high specificity and affinity33. Additionally, nanobodies have low immunogenicity and can be humanized34. They also demonstrate improved permeability for deep tissue penetration35. However, challenges such as short half-life and rapid blood clearance can be addressed by adding polyethylene glycol, conjugation to albumin, or fusion with the Fc fragment of antibodies31. Nowadays, nanobodies have significant potential in various applications, including drug research and development, diagnosis, and treatment. Despite these properties, structural insights into SOD1-nanobody interactions remain limited.
A patented study previously identified a panel of 14 SOD1-targeting nanobodies isolated from immune libraries of dromedary or alpaca origin36. Sequence alignment classified these nanobodies into four distinct groups (Nb1, Nb2, Nb3, and Nb4), with members within each group exhibiting high sequence homology. Based on reported functional data indicating that Nb1, Nb2, and Nb3 (but not Nb4) inhibit SOD1 fibril formation, we selected these three as representatives of their respective sequence families for high-resolution structural analysis.
Here, we bridge this gap by resolving high-resolution structures of SOD1 bound to three nanobodies (Nb1, Nb2, Nb3) individually and in combination. Using orthogonal biophysical methods, we characterize their binding mechanisms, thermodynamic profiles, and functional impacts on SOD1 activity. We further design multimeric nanobodies with sub-nanomolar affinity, demonstrating their potential as tools for SOD1 detection, aggregate inhibition, and therapeutic modulation in ALS.
Results
Characterization of SOD1 and three SOD1-specific nanobodies
We synthesized three SOD1-specific nanobodies (Nb1, Nb2, and Nb3) via whole gene synthesis and expressed the corresponding proteins in E. coli. The proteins were purified using Ni-NTA and size-exclusion chromatography (SEC). Purity and concentration were initially analyzed using SDS-PAGE (Fig. S1). We determined the molecular weight of the recombinant proteins using matrix-assisted laser desorption ionization time-of-flight mass spectrometry (MALDI-TOF). The results showed prominent signal peaks within the theoretical molecular weight range for fully metallated, disulfide-intact SOD1 and the three nanobodies (Nb1, Nb2, and Nb3) (Fig. 1A–D and Table S1).
To obtain stable SOD1-nanobody complexes, we separately mixed SOD1 with excess amounts of Nb1, Nb2, and Nb3, and then separated the complexes by SEC. This allowed us to distinguish the relatively low molecular weight (approximately 13 kD) excess nanobody from the SOD1-nanobody complex (approximately 29 kD~57kD) (Fig. S2). The resulting complex samples were further analyzed using MALDI-TOF, confirming that the SOD1-nanobody complexes separated into two protein monomers with molecular weights corresponding to theoretical values (Fig. 1E–G).
The overall crystal structure of the SOD1-nanobody complexes
To elucidate the mechanism of action of the SOD1-Nb1, SOD1-Nb2, and SOD1-Nb3 complexes at atomic resolution, we resolved their structures through crystallization and X-ray diffraction. Using high-throughput crystallization conditions, we obtained well-formed crystals of the SOD1-nanobody complexes (Fig. S3A–C). The crystal structures were then determined by X-ray diffraction, with crystallographic data summarized in Table 1, providing a comprehensive overview of the crystal structures of the SOD1-nanobody complexes at a 1:1 stoichiometry.
The final structures of SOD1-Nb1, SOD1-Nb2, and SOD1-Nb3 were refined to resolutions of 2.13 Å, 2.74 Å, and 2.30 Å, respectively. We observed well-defined binding interfaces between SOD1 and the CDRs 1–3 of the nanobodies. SOD1-Nb1 crystallized in the space group P1211. SOD1-Nb2 crystallized in the space group P6, and SOD1-Nb3 crystallized in the space group P212121 (Table 1).
The structure of SOD1 comprises eight antiparallel β-sheets, forming an unfolded β-barrel structure with long external loops. The copper ion is located at the rims of the molecule, while the zinc ion is buried within its interior. A short segment of α-helix is present in the middle region of the loop. SOD1 naturally forms stable dimers in physiological environments. The binding surfaces of the three nanobodies (Nb1, Nb2, and Nb3) with SOD1 dimer are formed through their CDRs 1-3, each recognizing distinct binding epitopes while preserving the integrity of the dimeric interface (Fig. 2A–C).
A The structure of the SOD1-Nb1 complex (PDB ID: 8K33). B The structure of the SOD1-Nb2 complex (PDB ID: 8K3A). C The structure of the SOD1-Nb3 complex (PDB ID: 8K3L). D Structural superimposition of the three nanobodies, with SOD1, Nb1, Nb2, and Nb3 shown in cyan, gray-white, yellow, and purple, respectively (Only depicted Nb1, Nb2, and Nb3 bound to one SOD1 monomer). E–G Surface electrostatic potentials of the SOD1-Nb1, SOD1-Nb2, and SOD1-Nb3 binary complexes (positive in blue, negative in red, and neutral in white). SOD1 is shown in cyan, with Cu and Zn atoms depicted as blue and orange spheres. Nb1, Nb2, and Nb3 are shown in gray-white, yellow, and purple, respectively. CDRs 1-3 of the nanobodies are highlighted in blue, green, and red, respectively.
Due to the asymmetrical nature of SOD1’s β-barrel, it can be divided into two sides. Nb2 and Nb3 both bind to the same side of the β-barrel, whereas Nb1 binds to the α-helix (VII loop) and the opposite side of the β-barrel, specifically the fifth and sixth β-sheets (Fig. 2A). Nb2 binds close to the side of the β-barrel formed by the third and fourth β-sheets and interacts with loops I, III, and V (Fig. 2B). Nb3 binds near the C-terminal of the fourth β-sheet and interacts with loops II, IV, and VI (Fig. 2C). In physiological conditions, SOD1 exists as a stable dimer, and the crystal structures presented here confirm this. Each monomer is symmetrically positioned and forming 1:1 interactions with nanobodies (Nb1, Nb2, and Nb3) in their dimeric state. For clarity, although the SOD1 dimer is shown in Fig. 2, we have depicted Nb1, Nb2, and Nb3 bound to one SOD1 monomer.
Structural superimposition and sequence alignment reveal high similarity in the overall structures and amino acid sequences of the three nanobodies (Fig. 2D). While CDR1 and CDR2 lengths vary by only 1–3 amino acids, CDR3 is notably shorter in Nb1 (7 residues) compared to Nb2 and Nb3 (18 residues). The root mean square deviation (RMSD) values of 0.61–0.72 Å further confirm their structural similarity.
Analysis of the surface electrostatic potentials of the three SOD1-nanobody complexes revealed that the distribution of partial charges near the contact surfaces includes both positively and negatively charged areas (Fig. 2E–G). These electrostatic interactions likely play a role in stabilizing the structure of the complexes.
Details of the binding sites of the three nanobodies to SOD1
Due to its short CDR3 loop, Nb1 binds to SOD1 primarily through its CDR2. Numerous hydrogen bonds were observed in the CDR2 region, with only three hydrogen bonds formed in the CDR1 and CDR3 regions (Fig. 3A). In CDR1, hydrogen bonds were formed between Leu32 of Nb1 and Leu42 of SOD1 (2.9 Å), Ser31 of Nb1 and Glu40 of SOD1 (3.5 Å) (Fig. 3A). Binding at the CDR2 region involved hydrogen bonds between Ser53 of Nb1 and Ala123 of SOD1 (3.3 Å), Asn61 of Nb1 and Asn131 (2.9 Å) / Asn139 (3.0 Å) of SOD1, Tyr62 of Nb1 and Asn131 of SOD1 (3.0 Å), Asp64 of Nb1 and Gly130 of SOD1 (3.1 Å), Asp64 of Nb1 and H2O (2.7 Å), Glu132 of SOD1 and H2O (2.9 Å) (Fig. 3A). In CDR3, a hydrogen bond was formed between Gly102 of Nb1 in CDR3 and Asn86 of SOD1 (2.8 Å) (Fig. 3A). Additionally, a hydrophobic interaction was observed among Ala34 of Nb1, Leu42 and Ala123 of SOD1.
SOD1 is shown in cyan, Nb1, Nb2, and Nb3 shown in gray-white, yellow, and purple, respectively with CDRs 1-3 of Nb1/Nb2/Nb3 shown in blue, green, and red, respectively. Residues involved in the interactions are depicted as sticks, with yellow dotted lines indicating hydrogen bonds and salt bridges. A Overall view and detailed views of the binding interface between SOD1 and complementarity determining regions (CDRs) of Nb1. B Overall view and detailed views of the binding interface between SOD1 and CDRs of Nb2. C Overall view and detailed views of the binding interface between SOD1 and CDRs of Nb3.
Nb2’s binding affinity to SOD1 is mainly contributed by hydrogen bonds in CDR2 and a hydrophobic interaction in the CDR1 region, with CDR3 making minimal direct contributions to the interface (Fig. 3B). The primary interactions were derived from hydrogen bonds between Tyr55 of Nb2 in CDR2 and Leu38 of SOD1 (2.8 Å), and Gly57 of Nb2 in CDR2 and Glu40 of SOD1 (2.6 Å). The hydrophobic interaction was formed between Tyr32 of Nb2 in CDR1 and Leu144 of SOD1.
In the SOD1-Nb3 complex, the primary interactions are mediated by hydrogen bonds between CDR3 and SOD1 (Fig. 3C). Specifically, hydrogen bonds were observed between His100 of Nb3 and Ser102, Asp76 of SOD1 ( ~ 3.0 Å), between Tyr104 of Nb3 and Lys30 (3.4 Å)/ Ser98 (2.9 Å) / Glu100 (2.7 Å) of SOD1 (Fig. 3C). One hydrogen bond was observed between Asn52 of CDR2 and Glu100 of SOD1 (3.1 Å) (Fig. 3C). A salt bridge was formed between Arg30 of Nb3 in CDR1 and Glu24 of SOD1 (3.6 Å). Additionally, two hydrophobic interactions were observed between Ala53 of Nb3 in CDR2 and Pro28 of SOD1, Phe101 of Nb3 in CDR3 and Pro74 of SOD1.
In all three SOD1-Nb complexes, CDRs 1-3 contribute to binding, though to varying extents. Nb1’s binding specificity arises from its extended CDR2 and shorter CDR3, while Nb2’s binding specificity is attributed to the hydrogen bonds in CDR2. In contrast, Nb3’s binding specificity is influenced by its extended CDR3. Additionally, Hydrophobic interactions involving residues in CDRs 1-3 of three nanobodies further stabilize these complexes.
Our results indicate that the binding epitopes for SOD1 with Nb1, Nb2, and Nb3 are distinct. Consequently, we attempted to obtain crystal structures of SOD1 bound to two or three nanobodies simultaneously. We successfully obtained crystals of the SOD1-Nb1-Nb2 and SOD1-Nb1-Nb2-Nb3 ternary and quaternary complexes (Fig. S3D, E). However, due to the more relaxed packing resulting from multiple nanobody binding, the final resolutions of these complex structures were relatively low, at 3.28 Å and 3.94 Å (Table S2), respectively. Despite this, at these resolutions, the positions of SOD1 and the nanobodies are still clearly distinguishable. All three nanobodies simultaneously bind distinct epitopes on SOD1 while preserving its native dimeric conformation. Structural alignment results indicate that the binding sites of each nanobody in the complexes are consistent with those observed in single nanobody-SOD1 interactions (Fig. 4).
A The overall structure of SOD1, Nb1 and Nb2 complex (PDB ID: 8YAF). B The surface view of the SOD1, Nb1 and Nb2 complex. C The overall structure of SOD1, Nb1, Nb2 and Nb3 complex (PDB ID: 8YAT). D The surface view of SOD1, Nb1, Nb2 and Nb3 complex. E The structure comparison of SOD1, Nb1 and Nb2 complex with SOD1 fibril core. F The surface view of the comparison between SOD1, Nb1 and Nb2 complex with SOD1 fibril core. G The structure comparison of SOD1, Nb1, Nb2 and Nb3 complex with SOD1 fibril core. H The surface view of the comparison between SOD1, Nb1, Nb2 and Nb3 complex with SOD1 fibril core.
Verification of binding and thermodynamics of three nanobodies to SOD1
While crystal structures reveal non-overlapping epitopes for SOD1-nanobody binding, physiological validation was conducted using fluorescence-detection size-exclusion chromatography (FSEC). FSEC enables visualization of macromolecular interactions under physiological conditions. By comparing elution times of SOD1-nanobody mixtures to SOD1 alone, we confirmed the formation of binary and larger complexes, validating nanobody binding characteristics.
To assess stable binary complex formation, labeled SOD1 (fully metallated, disulfide-intact) was mixed with excess nanobodies, and elution times were compared. Stable complexes were confirmed for SOD1-Nb1, SOD1-Nb2, and SOD1-Nb3 (Fig. 5A, B). Elution times were 22.07 min (SOD1), 21.81 min (SOD1-Nb1), 20.31 min (SOD1-Nb2), and 20.56 min (SOD1-Nb3). The distinct shift for SOD1-Nb1 likely results from its shorter CDR3, lower molecular weight, and structural constraints induced by Nb1 binding to the SOD1 α-helix. Progressive leftward peak shifts with additional nanobodies (Fig. 5C–F) confirmed higher molecular weight complexes and non-overlapping epitopes for Nb1, Nb2, and Nb3.
A, B Comparison of SOD1 and SOD1-nanobody binary complexes. C, D Detection of epitope binding with two nanobodies. E, F Detection of epitope binding with three nanobodies. The left panels display the full chromatogram, while the right panels provide an enlarged view of the elution peaks. SOD1, SOD1-Nb1, SOD1-Nb2, and SOD1-Nb3 are represented in black, red, blue, and green, respectively. SOD1-Nb1-Nb2, SOD1-Nb1-Nb3, SOD1-Nb2-Nb3, and SOD1-Nb1-Nb2-Nb3 are shown in purple, orange, light blue, and brown, respectively.
To determine the binding affinity and thermodynamic parameters of the interactions between fully metallated, disulfide-intact SOD1 and the nanobodies, isothermal titration calorimetry (ITC) assays were conducted, as they are considered the gold standard for such measurements. The results showed that Nb2 and Nb3 exhibited high binding affinity to SOD1, with affinity constant (KD) values of 23.2 nM and 48.9 nM, respectively (Fig. 6E, I). However, Nb1 bound to SOD1 with a significantly weaker affinity of 529 nM (Fig. 6A). The binding of Nb2 and Nb3 resulted in similar Gibbs free energy changes (ΔG) of -9.43 and -9.98 kcal/mol, respectively. In contrast, the binding of Nb1 to SOD1 resulted in a ΔG of -8.56 kcal/mol, with an affinity lower by an order of magnitude (Table 2).
The binding of Nb1 and Nb2 was primarily enthalpy-driven, dominated by specific interactions such as hydrogen bonds, electrostatic forces, and hydrophobic contacts, highlighting their potential for further development. In contrast, Nb3 binding was driven by both enthalpy and entropy, with entropy playing a larger role, suggesting reliance on nonspecific interactions and posing optimization challenges. Considering binding affinity and mechanism, Nb2 emerges as the most promising candidate for development.
We further validated the binding interactions through titration experiments with SOD1-nanobody complexes and various nanobodies. The ITC results for ternary and quaternary complexes revealed nanomolar binding affinities, indicating that all three nanobodies can bind to SOD1 simultaneously. However, the binding of one nanobody may affect the binding of others. Nb2 demonstrated a ~ 15 nM affinity for both SOD1-Nb1 and SOD1-Nb1-Nb3, showing significantly improved potency compared to SOD1 alone (Fig. 6F, H). This suggests that Nb1 binding might induce a conformation that enhances Nb2 binding. In contrast, the KD value of Nb2 for SOD1-Nb3 was lower than its affinity for SOD1 alone (KD = 120 nM) (Fig. 6G). Structural comparisons between the SOD1-Nb1 and SOD1-Nb3 complexes revealed a minor RMSD value of 0.226, indicating slight structural changes. For Nb3, the affinity for SOD1-Nb1 was five times lower (KD = 282 nM) compared to SOD1 (Fig. 6J), while the affinity for SOD1-Nb2 was nine times higher (KD = 5.38 nM) (Fig. 6K). Notably, the affinity of Nb1 for SOD1-Nb2-Nb3 increased approximately 4-fold (KD = 127 nM) compared to SOD1 alone (Fig. 6D). These observations may suggest an allosteric mechanism, wherein the binding of one nanobody induces conformational changes that modulate the affinities of other nanobodies37,38.
To determine the size distributions of fully metallated, disulfide-intact SOD1 and its complexes with nanobodies, we employed dynamic light scattering (DLS) as an orthogonal validation method alongside size-exclusion chromatography to ensure robust confirmation of SOD1’s native conformation. The results indicated that the proteins were nearly monodisperse and exhibited good homogeneity. SOD1 had an approximate diameter of 4.0 nm align with expectations for the dimeric SOD1 ( ~ 4.5 nm theoretical value for a 50 kDa dimer), confirming that the protein remained in its native dimeric form across all experiments. Binary complexes formed with nanobodies showed increased sizes, ranging from 4.6 to 5.2 nm (Fig. 7A), likely due to the different binding epitopes recognized by the nanobodies. Specifically, Nb2 and Nb3, which bind to the side of the β-barrel, exhibited greater flexibility compared to Nb1. For ternary and quaternary complexes, the sizes further increased to approximately 5.0 nm (SOD1-Nb1-Nb3), 6.0 nm (SOD1-Nb1-Nb2), and 7.0 nm (SOD1-Nb2-Nb3). When all three nanobodies were bound simultaneously, the diameter of the SOD1-Nb1-Nb2-Nb3 complex increased to about 8.0 nm (Fig. 7B).
A, B Particle size distributions of SOD1 and its nanobody complexes by dynamic light scattering. A Size distributions for SOD1 (black), SOD1-Nb1 (red), SOD1-Nb2 (blue), and SOD1-Nb3 (green); B Size distributions for SOD1 (black), SOD1-Nb1-Nb2 (red), SOD1-Nb1-Nb3 (blue), SOD1-Nb2-Nb3 (green), and SOD1-Nb1-Nb2-Nb3 (purple). C Measurement of SOD1 enzyme activity using the Kit-WST. The activities of SOD1, SOD1-Nb1, SOD1-Nb2, and SOD1-Nb3 are shown in blue, purple, pink, and green, respectively. Error bars represent the standard error from three independent experiments (n = 3). D–G Hydrogen bond distances between Asp124 and metal-binding histidines (His46/His71) in different SOD1 conformational states. D WT SOD1, E Nb1 binding SOD1, F Nb2 binding SOD1, G Nb3 binding SOD1. H Thioflavin T (ThT) fluorescence intensities representing the filament formation of SOD1 and nanobodies binding SOD1. Error bars represent the standard error from three independent experiments (n = 3).
Assessment of enzyme activity of SOD1 and its nanobody complexes
Previous studies have reported that SOD1 knockout (SOD1 KO) can result in several adverse phenotypes, including oxidative stress in peripheral nerves and mitochondrial loss at motor nerve terminals39,40. Consequently, for the newly developed nanobodies targeting SOD1, it is essential to ensure that they do not compromise the intrinsic enzymatic activity of SOD1. Enzyme activity of fully metallated, disulfide-intact SOD1 and its nanobody complexes was assessed using the SOD1 assay Kit-WST, which measures superoxide anion reduction via WST-1 conversion to formazan dye, inhibited by SOD1 (Fig. 7C). The SOD1-nanobody complexes exhibited higher activity than SOD1 alone, likely due to nanobody-induced conformational changes.
In SOD1, Asp124 structurally couples the electrostatic and zinc loops by forming hydrogen bonds with the non-ligating imidazole nitrogens of His46 (Cu2+ ligand) and His71 (Zn2+ ligand), thereby stabilizing both metal-binding sites, as reported41. In wild-type SOD1 (WT-SOD1), Asp124 forms hydrogen bonds with His46 at 2.7 Å and with His71 at 3.1 Å and 2.7 Å (Fig. 7D), respectively. Nanobody binding induced distinct structural modulations: while Nb2 binding maintained these hydrogen bond distances essentially unchanged (Fig. 7F), Nb3 binding significantly shortened the Asp124-His46 distance to 2.5 Å without altering the Asp124-His71 distances (Fig. 7G). Notably, Nb1 binding established the shortest hydrogen bond distances (Asp124- His46: 2.5 Å, Asp124- His71: 2.8/2.6 Å) (Fig. 7E), creating an optimized hydrogen-bonding network that markedly enhanced the stability of the metal-binding sites. This structural optimization correlated well with the observed enzymatic activity hierarchy: Nb1-SOD1 > Nb3-SOD1 > Nb2-SOD1 ≈ WT-SOD1, demonstrating a clear structure-function relationship.
Inhibition of SOD1 fibril formation by nanobodies
The Thioflavin T (ThT) fluorescence assay reliably monitored SOD1 (fully metallated, disulfide-intact) amyloid fibril formation, where enhanced fluorescence emission specifically reflects ThT binding to the cross-β-sheet structure of amyloid fibrils42. Under reducing conditions with continuous shaking, prolonged incubation led to progressive increases in ThT signal, indicating time-dependent accumulation of SOD1 fibril. However, nanobody treatment significantly attenuated this fibrillization process. After 96 hours of fibrillation, samples containing Nb1 or Nb3 exhibited approximately 70% inhibition of fibril formation, while Nb2 showed weaker suppression (~30%). Notably, combinatorial application of multiple nanobodies synergistically enhanced the inhibitory effect to 90% (Fig. 7H), demonstrating their simultaneous binding to SOD1 without steric hindrance. This cooperative action suggests that distinct nanobody epitopes collectively stabilize SOD1’s native conformation, thereby more effectively preventing pathogenic fibril assembly.
Design and verification of multimeric tandem nanobodies targeting SOD1
Based on the structural and binding assay data of SOD1’s nanobodies (Fig. 2A–C), Nb1, Nb2, and Nb3 bind at different sites, suggesting their potential for development into tandem nanobodies to enhance selectivity and affinity for fully metallated, disulfide-intact SOD1. First, we measured the distances between the amino and carboxyl ends of the three nanobodies based on crystal structures to design tandem nanobodies. The groups with the closest distances were Nb1C-Nb2N at 5.7 nm, Nb2C-Nb3N at 7.6 nm, and Nb3C-Nb1N at 3.6 nm. Therefore, we selected several combinations, including Nb1-Nb2, Nb2-Nb3, and Nb1-Nb2-Nb3, for the design of tandem nanobodies. Given that the peptide chain length formed by each 5 repeated small amino acids (GGGGS) is approximately 17 Å43,44, and 4 GGGGS exceeds 60 Å, we used (GGGGS)4 to link them. The ITC results (Fig. 8 and Table S3) demonstrated that the tandem nanobodies retained high affinity for SOD1, with a lower ΔH value, indicating stronger binding specificity.
Discussion
The nanobody sequences used in this study were primarily sourced from patent US 9862777 B236, which describes a total of 14 nanobodies (Nb1, 4S10, 4S-37, 4SP-4, 4SP-9, 4SP-19, 4S16, Nb2, 4 SD34, Nb3, 4SD-29, 4SD-7, RSO-R2-14, Nb4). With the exception of Nb4, which has no closely related sequences, all other nanobodies possess highly homologous counterparts: Nb1 is highly similar to 4S10, 4S-37, 4SP-4, 4SP-9, 4SP-19, and 4S16; Nb2 shares an extremely high sequence identity with 4 SD34; and Nb3 shows strong similarity to 4SD-29, 4SD-7, and RSO-R2-14. This high degree of sequence conservation suggests that nanobodies within the same group are unlikely to exhibit significant differences in their structural features or SOD1-binding properties.
To streamline the structural characterization of nanobody-SOD1 interactions, we selected one representative nanobody from each homology group for structural analysis. Nb4 was excluded from further investigation since the patent reported no detectable binding to SOD1 and no inhibitory effect on SOD1 fibril formation.
The patent WO 2016/12451245 also describes three nanobodies (cb0987, cb0989, and cb0991) capable of binding to SOD1. We also expressed and purified these nanobodies and attempted to form complexes by co-incubation with SOD1. However, size-exclusion chromatography experiments did not yield stable complexes.
Our analysis of the interaction interfaces in the SOD1-nanobody complexes, using PDBePISA46, revealed interface areas ranging from 599.1 to 761.7 Ų (Table S4). Notably, the interface area and number of hydrogen bonds between Nb1 and SOD1 were relatively smaller, which likely accounts for its weaker binding affinity compared to Nb2 and Nb3.
Distinct binding epitopes were identified for each nanobody, with unique residues interacting with SOD1 as determined by crystal structure data. Structural alignment of the SOD1-nanobody complexes with the human wild-type SOD1 homodimer (PDB: 2C9V)2 showed that the nanobody binding sites were located far from the homodimer interface (Fig. S4). The SOD1 homodimer is stabilized by inter-subunit hydrogen bonds involving conserved residues such as Gly51, Gly114, and Ile151, which do not overlap with the nanobody binding sites. Consequently, nanobody binding is unlikely to interfere with dimer formation while enabling the rational design of multivalent constructs (e.g., tandem nanobodies) for enhanced avidity.
The molecular mechanism of SOD1 fibril formation begins with the destabilization and misfolding of SOD1 monomers47,48. This process can be triggered by mutations, oxidative stress, or metal ion imbalances, which lead to partial unfolding of the SOD1 protein. Once destabilized, the monomers expose hydrophobic regions and aggregation-prone sequences that are typically hidden in the native structure. These exposed regions promote the nucleation phase, where misfolded SOD1 monomers aggregate into small, unstable oligomers. During the elongation phase, these oligomers act as seeds for additional SOD1 monomers, which undergo conformational changes to incorporate into the growing fibril.
A comparison between the apo SOD1 dimer and the SOD1 fibrils reveals a major conformational transition from a β-sheet-rich, antiparallel β-barrel structure in the immature SOD1 to a distinct β-sheet-rich fibrillar structure, characterized by in-register, parallel β-strands49. This transition is associated with a diminished capacity of the protein to bind Cu2+ and Zn2+ ions, as well as the reduction of the disulfide bond between Cys57 and Cys146, which contributes to the disorder of loops IV and VII50. This shift underscores the transition from the native, stable dimeric form of SOD1 to its aggregation-prone fibrillar state during the pathogenesis of amyotrophic lateral sclerosis (ALS). Understanding the fibril structure will provide key insights into the molecular mechanisms driving SOD1 misfolding and aggregation, as well as the structural polymorphism of different SOD1 strains and their potential link to ALS progression.
The mechanism by which Nb1, Nb2, and Nb3 prevent SOD1 fibril formation may involves their specific binding to distinct epitopes on the SOD1 monomer. For Nb1, its binding on the VII loop of SOD1, which is crucial for the binding of Cu2+ and Zn2+ ions. In the case of Nb3, its binding on the IV loop of SOD1, where this region plays a key role in protecting the disulfide bond between Cys57 and Cys146 in SOD150. Although Nb2 does not bind to the VII or IV loops of SOD1, it exhibits a strong interaction with the V loop. Destabilization of this region can result in the separation of the β5 and β6 strands, leading to the opening of the β-strand and, consequently, promoting aggregation51. By binding to these sites, the nanobodies stabilize the native conformation of SOD1, preventing the exposure of hydrophobic regions and aggregation-prone sequences that trigger fibrillation. During the elongation phase, nanobodies bound to the surface of SOD1 sterically hinder the addition of monomers to the growing fibril ends. This steric hindrance inhibits the efficient assembly of the cross-β-sheet structures required for fibril growth.
Previous studies by Robberecht et al. demonstrated that the nanobodies Nb54 and Nb61 bind to both wild-type and mutant SOD152. These nanobodies inhibit the fibril formation and aggregation of SOD1A4V and SOD1G93A in various cell lines. Furthermore, they have been shown to rescue axonopathy induced by SOD1A4V mRNA in zebrafish models. In a SOD1G93A mouse model of ALS, nanobodies delayed disease onset, extended lifespan, and improved survival. These findings highlight the potential of nanobodies in modulating SOD1 fibril formation and emphasize their therapeutic relevance in neurodegenerative diseases.
The properties of three SOD1-specific nanobodies, Nb1, Nb2, and Nb3, were characterized through a series of in vitro experiments, demonstrating good homogeneity, high purity, and a stable monomeric state. Although nanobodies exhibit greater stability than traditional antibodies, further optimization is necessary to enhance their stability for future applications.
The identification of diverse binding modes and epitopes for these nanobodies provides a promising foundation for developing tools to modulate SOD1 activity. Our structural studies on SOD1-nanobody complexes offer molecular insights into the specific recognition mechanisms of these nanobodies. Detailed analysis of the interaction sites and thermodynamic properties provides valuable guidance for the modification and optimization of these nanobodies. Among the three nanobodies tested, Nb2 demonstrates the most potential for further development.
Unlike full-length antibodies, nanobodies offer exceptional modularity and design flexibility, our findings reveal that the binding epitopes of Nb1, Nb2, and Nb3 are entirely distinct, allowing for simultaneous binding to SOD1. This suggests the possibility of developing novel multimeric nanobodies with improved affinity, which could enhance therapeutic efficacy. These nanobodies can also be leveraged to develop homogeneous assay tools for high-throughput applications, such as ELISA and TR-FRET. By using one SOD1-specific nanobody to capture SOD1 in solution and conjugating another with a fluorescent or chemiluminescent group, highly sensitive detection systems can be established. Moreover, designing ultrasensitive detection systems by utilizing nanobodies that recognize different binding epitopes in tandem can further increase the binding affinity and specificity for SOD1. Additionally, fusing nanobodies with functional proteins, such as those involved in post-translational modifications or specific degradation pathways, would allow for precise manipulation of SOD1 under various conditions.
In conclusion, our multiple high-resolution structures of SOD1-nanobody complexes provide a robust foundation for both fundamental research and potential clinical applications.
Materials and methods
Cloning, expression, and purification of SOD1 and nanobodies
The amino acid sequences for nanobodies against SOD1 (Nb1, Nb2, Nb3) were obtained from the patent US 9862777 B236. To express the proteins in E. coli, the SOD1, and three nanobody sequences were synthesized by GeneCreate (Wuhan, China) and codon-optimized. The proteins’ open reading frames (ORFs) were amplified, inserted into the pET28a-SUMO vector with a cleavable N-terminal 6xHis tag followed by a SUMO tag, and confirmed by sequencing. The resulting plasmids were transformed into E. coli BL21 (DE3) for protein expression. The cells were grown in LB medium at 37 °C until an OD600 of approximately 0.6, then induced with 0.4 mM isopropyl-D-1-thiogalactopyranoside (IPTG) and incubated at 22 °C for 20 h. To induce the expression of SOD1, CuSO4, and ZnSO4 were added at a final concentration of 0.1 mM. After expression, the proteins were purified using a combination of Ni-NTA affinity chromatography, Ulp1 protease cleavage of the His6-SUMO tag, a second Ni-NTA purification, anion exchange chromatography on a HiTrap Q FF column, and size exclusion chromatography on a Superdex 75 Increase column. The resulting proteins’ purity and approximate molecular weight were determined using SDS-polyacrylamide electrophoresis. The corresponding amino acid sequences can be found in Table S1.
Crystallization and data collection
SOD1 and nanobodies were combined at a mole ratio of 1:1.5 and incubated for 2 hours at 4 °C. To separate SOD1-nanobody complexes from the mixtures and remove excess nanobodies, a Superdex75 Increase column (Cytiva) was used. The SOD1-nanobody complexes were prepared in a buffer containing 20 mM HEPES pH 7.4 and 150 mM NaCl and were then concentrated at 15-25 mg/ml. For initial screening, crystals were obtained at 293 K using sitting-drop vapor diffusion, and hanging-drop vapor diffusion was used for optimization. Figure S3 provides detailed conditions for crystal growth. Before data collection, the crystals were transferred to a cryoprotectant solution by adding glycerol to the reservoir buffer at a concentration of 10%-25% (v/v) and were flash-frozen in liquid nitrogen. The X-ray diffraction data were collected at 100 K on beamlines BL17U1 and BL19U153 at Shanghai Synchrotron Radiation Facility.
Determination and refinement of protein structure
To index, integrate, and scale diffraction data, the HKL2000 suite was employed54. Molecular replacement was used to solve the structures of SOD1-nanobody complexes with the program Phaser from the CCP4 program suit55 using SOD1 (PDB ID: [2C9V]) and a nanobody (PDB ID: [3K1K]) as the search model. Model building was carried out using COOT (Version 0.8.9.1)56, Phenix (Version 1.20.1)57 and Refmac58 were utilized to refine the structures. The Pymol (Version 2.6) was used to create the corresponding structure figures.
Isothermal titration calorimetry (ITC)
Thermodynamic parameters for the interactions between SOD1 and nanobodies were obtained using MicroCal PEAQ-ITC (Malvern) at a constant temperature of 25 °C. All protein samples were in the same buffer. Eighteen injections were administered, each consisting of a 2 μl aliquot of protein sample from a syringe into a sample well containing another reactant at a time interval of 150 s. Detailed information on protein sample concentrations in the cell and syringe is listed in Table S5. Data acquisition was conducted using a reference power of 10.0 µcal/s, an initial delay of 60 s, and a stir speed of 750 rpm. The resulting isotherm was fitted with the one-set-of-sites binding model to obtain affinity constant (KD), stoichiometry (n), enthalpy changes (ΔH), entropy changes (TΔS), and Gibbs free energy change (ΔG) using analysis software.
Fluorescence-detection size-exclusion chromatography (FSEC)
For rapid detection of binding between SOD1 and nanobodies, FSEC was utilized. SOD1 was labeled with Cyanine5-NHS ester iodide (MCE) at a 1:2 molar ratio at 4 °C overnight, and excess red fluorescent dye was removed using Zeba Spin Desalting columns (ThermoFisher). Labeled SOD1 and unlabeled nanobodies were mixed in a 1:5 molar ratio and incubated for 1 h at 4 °C. The final concentrations of SOD1 and nanobodies were 10 μM and 50 μM, respectively. After ultracentrifugation, the supernatants of the SOD1-nanobody mixtures were loaded onto a Superdex 200 Increase column (Cytiva). The excitation and emission wavelengths were set at 646 nm and 662 nm, respectively.
Matrix-assisted laser desorption ionization time-of-flight (MALDI-TOF)
The molecular weight of monomers and SOD1-nanobody binary complexes were accurately determined using MALDI-TOF mass spectrometry (Bruker). Before analysis, protein samples were buffer-exchanged with 10 mM CH3COONH4 via a Superdex 75 Increase column (Cytiva). The analysis was conducted in linear mode, and bovine serum albumin (BSA) was utilized as a control for baseline measurements.
Dynamic light scattering (DLS)
The size distributions of monomers and SOD1-nanobody complexes were measured using DynaPro NanoStar (Wyatt). All protein samples were diluted to 50 µM and analyzed ten times after the baseline stabilized. The scattered light was detected at an angle of 90° and a temperature of 25 °C. While SEC can determine oligomeric states, DLS proved critical for detecting low-concentration solution behavior, where aggregation or conformational heterogeneity might otherwise go undetected.
SOD1 enzyme activity assay
The activity of SOD1 was measured using the SOD1 Assay Kit-WST (Dojindo). WST-1 can react with superoxide anion to produce a water-soluble dye inhibited by SOD1. The reduction rate with superoxide anion is linearly related to the xanthine oxidase activity, allowing the activity of SOD1 to be calculated from the absorbance at 450 nm. SOD1 and SOD1-nanobody binary complexes of various concentrations (1000, 750, 500, 400, 300, 200, 100, 75, 50, and 10 nM) were mixed with WST working solution and enzyme working solution and then incubated at 37°C for 20 min. The OD 450 nm value was obtained by Synergy2 (BioTek). The activity of SOD1 was calculated using the following formula: WST-1 inhibition rate (%) = [(Ablank1−Ablank3)-(Asample−Ablank2)] / (Ablank1−Ablank3) *100%.
SOD1 fibril formation assay
Purified SOD1 protein (50 μM) was incubated in a solution containing 20 mM Tris-HCl (pH 7.4) and 5 mM TCEP at 37 °C with constant shaking at 1000 rpm to induce fibril formation. After agitation, visible flocculent fibrils were observed, which dispersed into a turbid solution upon pipetting. For nanobody-treated groups, nanobodies were added at a 2:1 molar ratio (nanobody: SOD1) to the SOD1 solution prior to agitation under identical fibrillation conditions.
Thioflavin T (ThT) fluorescence assay
Fibril formation was quantified using ThT fluorescence. Briefly, 50 μL of fibril solution was mixed with ThT at a final concentration of 5 μM and incubated at 37 °C with shaking at 1000 rpm for 15 min in the dark. Fluorescence measurements were performed using a FlexStation3 multimode microplate reader with excitation at 450 nm and emission at 485 nm. Blank buffer values were subtracted from all readings, and fluorescence intensities were normalized to the 96-hour SOD1-only control (set as 1). All experiments were performed in triplicate.
Design of multimeric tandem nanobodies
To engineer multimeric nanobodies, we first measured the spatial separation between the N- and C-termini of Nb1, Nb2, and Nb3 using PyMOL based on their crystal structures (PDB: 8K33, 8K3A, 8K3L). To minimize steric hindrance and maintain conformational flexibility, we selected (GGGGS)₄ linkers (20-amino acid repeats, theoretical length ~68 Å) for covalent fusion between nanobodies. Tandem constructs (Nb1-Nb2, Nb2-Nb3, and Nb1-Nb2-Nb3) were then synthesized and verified by sequencing.
Statistics and reproducibility
Prior to experimentation, a power analysis was conducted using PASS 16 to ensure a statistical power >0.8 for each assay. Data are presented as mean ± SEM. Statistical comparisons were performed using unpaired, two-tailed Student’s t-tests for two groups. For comparisons among multiple groups, one-way ANOVA was applied, followed by post hoc tests: Dunnett’s test for comparisons against a single control group and Bonferroni’s test for all pairwise comparisons. For datasets involving two independent variables (e.g., treatments across different time points), two-way ANOVA was used. All analyses were conducted using GraphPad Prism 7 and Microsoft Excel 2021.
Data availability
The atomic coordinates and structure factors have been deposited in the Protein Data Bank under accession codes 8K33, 8K3A, 8K3L, 8YAF, and 8YAT. All data supporting the conclusions of this study are contained within the article and its Supplementary Information. The uncropped SDS-PAGE images are provided in Supplementary Fig. S5, and the original data of Fig. 7C, H can be found in the Excel file named “Supplementary Data”. Any additional information is available from the corresponding author upon reasonable request.
References
Eleutherio, E. C. A. et al. SOD1, more than just an antioxidant. Arch. Biochem. Biophys. 697, 108701 (2021).
Strange, R. W. et al. Variable metallation of human superoxide dismutase: atomic resolution crystal structures of Cu–Zn, Zn–Zn and As-isolated wild-type enzymes. J. Mol. Biol. 356, 1152–1162 (2006).
Fukai, T. & Ushio-Fukai, M. Superoxide dismutases: role in redox signaling, vascular function, and diseases. Antioxid. Redox Signal 15, 1583–1606 (2011).
Wang, Y. et al. Superoxide dismutases: dual roles in controlling ROS damage and regulating ROS signaling. J. Cell Biol. 217, 1915–1928 (2018).
Montllor-Albalate, C. et al. Sod1 integrates oxygen availability to redox regulate NADPH production and the thiol redoxome. Proc. Natl. Acad. Sci. USA 119, e2023328119 (2022).
Rosen, D. R. et al. Mutations in Cu/Zn superoxide dismutase gene are associated with familial amyotrophic lateral sclerosis. Nature 362, 59–62 (1993).
Hayashi, Y., Homma, K. & Ichijo, H. SOD1 in neurotoxicity and its controversial roles in SOD1 mutation-negative ALS. Adv. Biol. Regul. 60, 95–104 (2016).
Li, H. F. & Wu, Z. Y. Genotype-phenotype correlations of amyotrophic lateral sclerosis. Transl. Neurodegener. 5, 3 (2016).
Gurney, M. E. et al. Motor neuron degeneration in mice that express a human Cu,Zn superoxide dismutase mutation. Science 264, 1772–1775 (1994).
Tu, P. H. et al. Transgenic mice carrying a human mutant superoxide dismutase transgene develop neuronal cytoskeletal pathology resembling human amyotrophic lateral sclerosis lesions. Proc. Natl. Acad. Sci. USA 93, 3155–3160 (1996).
Wong, P. C. et al. An adverse property of a familial ALS-linked SOD1 mutation causes motor neuron disease characterized by vacuolar degeneration of mitochondria. Neuron 14, 1105–1116 (1995).
Rotunno, M. S. & Bosco, D. A. An emerging role for misfolded wild-type SOD1 in sporadic ALS pathogenesis. Front. Cell Neurosci. 7, 253 (2013).
Pare, B. et al. Misfolded SOD1 pathology in sporadic amyotrophic lateral sclerosis. Sci. Rep. 8, 14223 (2018).
Franklin, J. P., Azzouz, M. & Shaw, P. J. SOD1-targeting therapies for neurodegenerative diseases: a review of current findings and future potential. Expert Opin. Orphan Drugs 8, 379–392 (2020).
Smith, R. A. et al. Antisense oligonucleotide therapy for neurodegenerative disease. J. Clin. Invest. 116, 2290–2296 (2006).
Ding, H. et al. Selective silencing by RNAi of a dominant allele that causes amyotrophic lateral sclerosis. Aging Cell 2, 209–217 (2003).
Gaj, T. et al. In vivo genome editing improves motor function and extends survival in a mouse model of ALS. Sci. Adv. 3, eaar3952 (2017).
Abati, E. et al. Silence superoxide dismutase 1 (SOD1): a promising therapeutic target for amyotrophic lateral sclerosis (ALS). Expert Opin. Ther. Targets 24, 295–310 (2020).
Benatar, M. et al. Randomized, double-blind, placebo-controlled trial of arimoclomol in rapidly progressive SOD1 ALS. Neurology 90, e565–e574 (2018).
Tsuburaya, N. et al. A small-molecule inhibitor of SOD1-Derlin-1 interaction ameliorates pathology in an ALS mouse model. Nat. Commun. 9, 2668 (2018).
Lange, D. J. et al. Pyrimethamine decreases levels of SOD1 in leukocytes and cerebrospinal fluid of ALS patients: a phase I pilot study. Amyotroph. Lateral Scler. Frontotemporal Degener. 14, 199–204 (2013).
Banerjee, V. et al. Superoxide dismutase 1 (SOD1)-derived peptide inhibits amyloid aggregation of familial amyotrophic lateral sclerosis SOD1 mutants. ACS Chem. Neurosci. 7, 1595–1606 (2016).
Shteinfer-Kuzmine, A. et al. A VDAC1-derived N-terminal peptide inhibits mutant SOD1-VDAC1 interactions and toxicity in the SOD1 model of ALS. Front. Cell Neurosci. 13, 346 (2019).
Gros-Louis, F. et al. Intracerebroventricular infusion of monoclonal antibody or its derived Fab fragment against misfolded forms of SOD1 mutant delays mortality in a mouse model of ALS. J. Neurochem. 113, 1188–1199 (2010).
Maier, M. et al. A human-derived antibody targets misfolded SOD1 and ameliorates motor symptoms in mouse models of amyotrophic lateral sclerosis. Sci. Transl. Med. 10, eaah3924 (2018).
Urushitani, M., Ezzi, S. A. & Julien, J. P. Therapeutic effects of immunization with mutant superoxide dismutase in mice models of amyotrophic lateral sclerosis. Proc. Natl. Acad. Sci. USA 104, 2495–2500 (2007).
Jin, B. K. et al., NANOBODIES(R): a review of diagnostic and therapeutic applications. Int. J. Mol. Sci. 24, 5994 (2023).
Hamers-Casterman, C. et al. Naturally occurring antibodies devoid of light chains. Nature 363, 446–448 (1993).
Padlan, E. A. Anatomy of the antibody molecule. Mol. Immunol. 31, 169–217 (1994).
Zhu, H. & Ding, Y. Nanobodies: from discovery to AI-driven design. Biology 14, 547 (2025).
Bannas, P., Hambach, J. & Koch-Nolte, F. Nanobodies and nanobody-based human heavy chain antibodies as antitumor therapeutics. Front. Immunol. 8, 1603 (2017).
Kunz, P. et al. The structural basis of nanobody unfolding reversibility and thermoresistance. Sci. Rep. 8, 7934 (2018).
Truong, T. T. T. et al. Studying the characteristics of nanobody CDR regions based on sequence analysis in combination with 3D structures. J. Genet. Eng. Biotechnol. 20, 157 (2022).
Sulea, T. Humanization of camelid single-domain antibodies. Methods Mol. Biol. 2446, 299–312 (2022).
Sun, S. et al. Nanobody: a small antibody with big implications for tumor therapeutic strategy. Int. J. Nanomed. 16, 2337–2356 (2021).
Robberecht, W., Rousseau, F. & Schymkowitz, J. Single Domain Antibodies Against SOD1 and Their Use in Medicine (V.I.v.B.V. Katholieke Universiteit Leuven, Life Sciences Research Partners vzw, Editor, 2014).
Nussinov, R. & Tsai, C. J. Allostery in disease and in drug discovery. Cell 153, 293–305 (2013).
Tsai, C. J., Del Sol, A. & Nussinov, R. Protein allostery, signal transmission and dynamics: a classification scheme of allosteric mechanisms. Mol. Biosyst. 5, 207–216 (2009).
Hayes, L. R. et al. Distal denervation in the SOD1 knockout mouse correlates with loss of mitochondria at the motor nerve terminal. Exp. Neurol. 318, 251–257 (2019).
Fischer, L. R. et al. Absence of SOD1 leads to oxidative stress in peripheral nerve and causes a progressive distal motor axonopathy. Exp. Neurol. 233, 163–171 (2012).
Valentine, J. S. & Hart, P. J. Misfolded CuZnSOD and amyotrophic lateral sclerosis. Proc. Natl. Acad. Sci. USA 100, 3617–3622 (2003).
Baek, Y. et al. Structural analysis of the overoxidized Cu/Zn-superoxide dismutase in ROS-induced ALS filament formation. Commun. Biol. 5, 1085 (2022).
Chen, X., Zaro, J. L. & Shen, W. C. Fusion protein linkers: property, design and functionality. Adv. Drug Deliv. Rev. 65, 1357–1369 (2013).
Waldo, G. S. et al. Rapid protein-folding assay using green fluorescent protein. Nat. Biotechnol. 17, 691–695 (1999).
Georg Bauer, M. M. Antigen-binding constructs, namely single domain vhh fragments which bind to and inhibit catalase and/or superoxide dismutase as well as pharmaceutical compositions containing them for tumor therapy, U. Freiburg, Editor. (2016).
Krissinel, E. & Henrick, K. Inference of macromolecular assemblies from crystalline state. J. Mol. Biol. 372, 774–797 (2007).
Rakhit, R. et al. Monomeric Cu,Zn-superoxide dismutase is a common misfolding intermediate in the oxidation models of sporadic and familial amyotrophic lateral sclerosis. J. Biol. Chem. 279, 15499–15504 (2004).
Sen Mojumdar, S. et al. Partially native intermediates mediate misfolding of SOD1 in single-molecule folding trajectories. Nat. Commun. 8, 1881 (2017).
Wang, L. Q. et al. Cryo-EM structure of an amyloid fibril formed by full-length human SOD1 reveals its conformational conversion. Nat. Commun. 13, 3491 (2022).
Furukawa, Y. et al. Conformational disorder of the most immature Cu, Zn-superoxide dismutase leading to amyotrophic lateral sclerosis. J. Biol. Chem. 291, 4144–4155 (2016).
Jahan, I. & Nayeem, S. M. Conformational dynamics of superoxide dismutase (SOD1) in osmolytes: a molecular dynamics simulation study. RSC Adv. 10, 27598–27614 (2020).
Kumar, M. S. et al. Anti-SOD1 nanobodies that stabilize misfolded SOD1 proteins also promote neurite outgrowth in mutant SOD1 human neurons. Int. J. Mol. Sci. 23, 16013 (2022).
Xu, Q. et al. The biosafety level-2 macromolecular crystallography beamline (BL10U2) at the Shanghai Synchrotron Radiation Facility. Nucl. Sci. Tech. 34, 202 (2023).
Otwinowski, Z. & Minor, W. Processing of X-ray diffraction data collected in oscillation mode. Methods Enzymol. 276, 307–326 (1997).
Collaborative Computational Project, N. The CCP4 suite: programs for protein crystallography. Acta Crystallogr. D Biol. Crystallogr. 50, 760–763 (1994).
Emsley, P. et al. Features and development of Coot. Acta Crystallogr. D Biol. Crystallogr. 66, 486–501 (2010).
Adams, P. D. et al. PHENIX: a comprehensive Python-based system for macromolecular structure solution. Acta Crystallogr. D Biol. Crystallogr. 66, 213–221 (2010).
Vagin, A. A. et al. REFMAC5 dictionary: organization of prior chemical knowledge and guidelines for its use. Acta Crystallogr. D Biol. Crystallogr. 60, 2184–2195 (2004).
Acknowledgements
This work was supported by the grant 2021YFA0805200 from the National Key Research and Development Program of China, grants 32471529, 32070939, and 82030106 from the National Natural Science Foundation of China, and grant FudanX24AI032 from AI for Science Foundation of Fudan University. We thank Prof. M. Hattori of Fudan University for providing FSEC-related equipment; Prof. Jixi Li of Fudan University for help with the DLS experiment; the staff members of the Large-scale Protein Preparation System at the National Facility for Protein Science in Shanghai (NFPS), Shanghai Advanced Research Institute, Chinese Academy of Sciences, China, for providing technical support and assistance in data collection and analysis; staff members of beamline BL10U1, BL17U1 and BL19U1 at SSRF for data collection; the staff members of State Key Laboratory of Genetic Engineering, School of Life Sciences, Fudan University for providing technical support.
Author information
Authors and Affiliations
Contributions
Y.D.: conceived and supervised the study. S.C., C.Z., H.Z., K.M., H.J., P.Z., Z.M., X.L., and Z.W.: performed protein purification, verification, crystallization, crystal data analysis, and protein-protein interaction assay. R.L.: performed crystallization and crystal data analysis. S.C., C.Z., and Y.D. wrote the draft. All authors reviewed the manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Communications Biology thanks the anonymous reviewers for their contribution to the peer review of this work. Primary Handling Editors: Dr Ken-ichiro Kamei and Dr Ophelia Bu.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Cheng, S., Zhong, C., Zhu, H. et al. Structural mechanisms and insights on multiple nanobodies binding diverse SOD1 epitopes. Commun Biol 9, 30 (2026). https://doi.org/10.1038/s42003-025-09293-0
Received:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s42003-025-09293-0