Abstract
Promoting cardiomyocyte proliferation is a highly promising strategy to repair the damaged myocardium and treat myocardial infarction (MI). DNA damage is a key factor leading to cell cycle arrest in cardiomyocytes, and DNA repair is required to relieve the restriction of proliferation. However, the potential of natural small-molecule compounds to enhance DNA repair and proliferation in cardiomyocytes has not been fully explored. Through screening of natural products, we found imperatorin could stimulate cardiomyocyte DNA repair and proliferation both in vitro and in vivo, which resulted in a significant improvement in cardiac function. By virtual prediction of pharmacological targets, we found that the target protein of imperatorin was the DNA repair protein tyrosyl-DNA phosphodiesterase 1 (TDP1). In terms of mechanism, imperatorin enhanced the phosphorylation of TDP1 at serine 81 by facilitating the proximity-mediated interaction between TDP1 and DNA-dependent protein kinase catalytic subunit (DNA-PKcs). Collectively, these findings suggest that imperatorin is a promising lead for the development of cardiac regeneration agents, and TDP1 is a hitherto unrecognized potential therapeutic target for MI treatment.
Similar content being viewed by others
Introduction
Myocardial infarction (MI) and subsequent heart failure continue to be a prominent cause of mortality on a global scale1. Timely restoration of blood flow and the use of medication can decrease morbidity and mortality, but the development of changes in the structure and function of the heart remains permanent, mainly due to the loss of cardiomyocytes2. It is now acknowledged that the adult mammalian heart has the ability to regenerate itself; however, this ability is restricted3. Emerging evidence from fate-mapping research suggests that the regeneration of the heart is predominantly driven by pre-existing cardiomyocytes, both in healthy and injured hearts4. Therefore, promoting cardiomyocyte proliferation is a highly promising strategy to repair the damaged myocardium and treat MI5.
Cardiac regeneration is significantly influenced by DNA damage and repair mechanisms6,7. The birth of a mammal involves a transition from a low-oxygen environment inside the uterus to a high-oxygen environment outside the uterus8. This transition is accompanied by an increase in cardiac mitochondria and reactive oxygen species (ROS) following delivery8. The accumulation of DNA damage caused by ROS is crucial in causing cell-cycle arrest in cardiomyocytes9. DNA repair is a self-feedback system that responds to DNA damage, resulting in a significant reduction in the extent of DNA damage10. Nevertheless, the search for small-molecule drugs that might enhance DNA repair and proliferation in cardiomyocytes and have potential for use in clinical settings is still ongoing.
Natural products (NPs) have been refined through evolution to effectively fulfill certain biological roles, making them a valuable resource for the development of new drugs11. NPs have served as the inspiration or source for almost 50% of the small-molecule pharmaceuticals approved by the FDA thus far12. Additionally, traditional Chinese medicine has played a significant role in the process of discovering new drugs13. A recent study has screened 722 small molecules isolated from herbs used in traditional Chinese medicine and scored their efficiency of homologous recombination (HR) and non-homologous end joining (NHEJ)14. Imperatorin, a furocoumarin found naturally in Angelica dahurica, demonstrates the most potent DNA repair activity and provides protective benefits on the central nervous system and cardiovascular system15. In the present study, we found imperatorin could stimulate cardiomyocyte DNA repair and proliferation both in vitro and in vivo. Through virtual prediction of pharmacological targets, we have shown that the target protein of imperatorin is the DNA repair protein tyrosyl-DNA phosphodiesterase 1 (TDP1). Imperatorin enhanced the phosphorylation of TDP1 by facilitating the proximity-mediated interaction between TDP1 and DNA-dependent protein kinase catalytic subunit (DNA-PKcs). Collectively, the prominent potential and mechanism of imperatorin in cardiomyocyte DNA repair and proliferation manifest it as a promising lead for the development of cardiac regeneration agents, and TDP1 is a hitherto unrecognized potential therapeutic target for MI treatment.
Results
The screen of DNA repair enhancers identified that imperatorin promoted cardiomyocyte proliferation and inhibited DNA damage
A recent study has created an HR-NHEJ dual fluorescent reporter system in the CLZ3 cell line, and successful HR repair results in functional tdTomato, which causes cells to turn red, while NHEJ restores active GFP, which causes cells to turn green (Fig. 1A)14. After screening 722 small molecules isolated from herbs used in traditional Chinese medicine and FACS (fluorescence-activated cell sorting) analysis to quantify DNA double-strand breaks (DSBs) repair efficiency, the top 10 small molecules with the strongest ability to repair DNA have been identified (Fig. 1B)14. DNA damage increases in cardiomyocytes postnatally and plays a key role in postnatal cell-cycle arrest, as proved by a significant increase in DNA damage foci at P7 compared to P1 hearts8. To show the effect of DNA repair enhancers on cardiomyocyte proliferation, NMVMs or NRVMs were isolated from P1 and P7 neonates and treated with these candidates (except for CPU-0530 due to unavailability). The results showed that in P1 cardiomyocytes, there were no candidates with a significant effect on cardiomyocyte proliferation, while in P7 cardiomyocytes, imperatorin significantly increased the rate of Ki67-positive and pH3-positive cardiomyocytes (Fig. 1C–F and Supplementary Fig. 1A–D). Furthermore, in human induced pluripotent stem cell (iPSC)-derived cardiomyocytes (hiPSC-CMs), imperatorin also significantly increased the rate of Ki67-positive and pH3-positive cardiomyocytes (Supplementary Fig. 1E, F). The cell cycle markers, including Ccna1, Ccnb1, Ccnd1, Ccne1, Cdc20, and Pcna, were upregulated after imperatorin treatment in P7 NMVMs (Supplementary Fig. 1G). Imperatorin is a naturally occurring furocoumarin (Fig. 1G). DNA repair by NHEJ is essential for the cell cycle re-entry of cardiomyocytes by inhibiting DNA damage7. A linear DNA substrate of approximately 4000 bp was used in DNA end-joining processes to examine the role of imperatorin in NHEJ, and imperatorin significantly increased the efficiency of end-joining in P7 NMVMs (Fig. 1H). When DNA damage occurs, ATM kinase is quickly autophosphorylated on serine 1981; this causes an inactive ATM dimer to dissociate into a catalytically active monomeric form, and it also phosphorylates H2AX on serine 139 (γH2AX) at the locations of DSBs16,17,18. We evaluated DNA damage through the expression and intensity of p-ATM and γH2AX. The results revealed that imperatorin significantly inhibited DNA damage in cardiomyocytes (Fig. 1I–L). The effect of imperatorin on cultured cardiomyocyte proliferation was further examined using phosphorylated histone 3 (pH3, a marker of mitosis) and Aurora B kinase (Aurkb, a marker of cytokinesis). Imperatorin could significantly increase P7 cardiomyocyte mitosis and cytokinesis, as determined by immunostainings of Ki67, pH3, and Aurkb (Fig. 1M–O). These data suggested the enhancement effect of imperatorin on DNA repair and proliferation of cardiomyocytes in vitro.
A Diagram of the HR-NHEJ dual fluorescent reporter. Successful repair by HR leads to functional tdTomato, therefore turning cells red, while NHEJ restores active GFP, turning cells green. B The top 10 small molecules with the strongest ability to repair DNA have been screened and identified. C Representative images and quantification of Ki67+ cardiomyocytes in NMVMs from P1 mice treated with small molecules. Scale bar, 40 μm. N = 6 repeats/group, ~500 cells/repeat. D Representative images and quantification of Ki67+ cardiomyocytes in NMVMs from P7 mice treated with small molecules. Scale bar, 40 μm. N = 6 repeats/group, ~500 cells/repeat. *p < 0.05 vs. Control. E Representative images and quantification of pH3+ cardiomyocytes in NMVMs from P1 mice treated with small molecules. Scale bar, 40 μm. N = 6 repeats/group, ~500 cells/repeat. F Representative images and quantification of pH3+ cardiomyocytes in NMVMs from P7 mice treated with small molecules. Scale bar, 40 μm. N = 6 repeats/group, ~500 cells/repeat. *p < 0.05 vs. Control. G Chemical structure of imperatorin. H DNA end-joining assays after imperatorin treatment. I Representative images and quantification of γH2AX protein expression after imperatorin treatment, analyzed by western blotting. Histone H2AX was used as a control. N = 6 repeats/group. *p < 0.05 vs. Control. J Representative images and quantification of p-ATM protein expression after imperatorin treatment, analyzed by western blotting. ATM was used as a control. N = 6 repeats/group. *p < 0.05 vs. Control. K Representative images and quantification of γH2AX intensity after imperatorin treatment. Scale bar, 20 μm. N = 6 repeats/group, ~50 cells/repeat. *p < 0.05 vs. Control. L Representative images and quantification of p-ATM intensity after imperatorin treatment. Scale bar, 20 μm. N = 6 repeats/group, ~50 cells/repeat. *p < 0.05 vs. Control. M Representative images and quantification of Ki67+ cardiomyocytes after imperatorin treatment. Scale bar, 40 μm. N = 5 repeats/group, ~500 cells/repeat. *p < 0.05 vs. Control. N Representative images and quantification of pH3+ cardiomyocytes after imperatorin treatment. Scale bar, 40 μm. N = 6 repeats/group, ~500 cells/repeat. *p < 0.05 vs. Control. O Representative images and quantification of Aurkb+ cardiomyocytes after imperatorin treatment. Scale bar, 40 μm. N = 6 repeats/group, ~500 cells/repeat. *p < 0.05 vs. Control.
Imperatorin promoted cardiomyocyte proliferation after apical resection (AR)
To determine the function of imperatorin on heart development, we treated P1 mice with imperatorin for 7 days, and P7 hearts were harvested when most cardiomyocytes had become post-mitotic (Fig. 2A)19. The results revealed that there were no differences between the control and imperatorin groups in the anatomical characteristics of heart weight-to-body weight ratio, heart weight-to-tibia length ratio, or cardiac function (Fig. 2B–E). Moreover, cardiomyocyte size and proliferative ability were unaffected by imperatorin (Fig. 2F–H), suggesting imperatorin did not influence cardiac development. Next, we assessed the effect of imperatorin on P8 (postnatal 8-day-old) mice with AR injury when the heart has lost complete regenerative capacity (Fig. 2I)20. The resection area and weight of different groups were comparable (Fig. 2J). Echocardiography revealed that the cardiac function of imperatorin-treated hearts was significantly recovered at 21 days post-resection (DPR) (Fig. 2K). The imperatorin-treated hearts showed a significant decrease in scar size compared with control hearts (Fig. 2L). WGA staining showed that cardiomyocyte size was significantly decreased in the imperatorin group (Fig. 2M), and we found a striking increase in the number of Ki67-positive and pH3-positive cardiomyocytes on imperatorin-treated myocardium (Fig. 2N, O). In addition, imperatorin significantly inhibited DNA damage in cardiomyocytes (Fig. 2P, Q). Collectively, these data suggested that the hearts treated with imperatorin did not affect normal heart development but were sufficient to promote cardiomyocyte proliferation in response to injury.
A Schematic diagram of the experimental design for imperatorin treatment in P1 neonates. B Hematoxylin-eosin staining showing the anatomical structure after imperatorin treatment at P7. Scale bar, 1 mm. C Heart weight to body weight ratios of hearts after imperatorin treatment at P7. N = 6 mice/group. D Heart weight to tibia length ratios of hearts after imperatorin treatment at P7. N = 6 mice/group. E Cardiac function in P7 mice after imperatorin treatment was measured by ejection fraction (EF%). N = 10 mice/group. F Wheat germ agglutinin (WGA) staining and quantification of cardiomyocyte size of hearts after imperatorin treatment at P7. Scale bar, 40 µm. N = 5 mice/group. G Representative images and quantification of Ki67+ cardiomyocytes of hearts after imperatorin treatment at P7. Scale bar, 40 µm. N = 10 mice/group. H Representative images and quantification of pH3+ cardiomyocytes of hearts after imperatorin treatment at P7. Scale bar, 40 µm. N = 10 mice/group. I AR was performed on P8 mice, and hearts were harvested at 7 and 21 days post-resection. J Representative images and weights of resected apexes at P8. Scale bar, 1 mm. N = 10-12 mice/group. K Cardiac function in P29 mice after imperatorin treatment was measured by EF% and fraction shortening (FS%). N = 10 mice/group. *p < 0.05 vs. Control. L Representative images and quantification of scar size after imperatorin treatment at P29. Scale bar, 160 µm. N = 8 mice/group. *p < 0.05 vs. Control. M WGA staining and quantification of cardiomyocyte size of hearts after imperatorin treatment at P15. Scale bar, 40 µm. N = 5-8 mice/group. *p < 0.05 vs. Control. N Representative images and quantification of Ki67+ cardiomyocytes of hearts after imperatorin treatment at P15. Scale bar, 40 µm. N = 5-8 mice/group. *p < 0.05 vs. Control. O Representative images and quantification of pH3+ cardiomyocytes of hearts after imperatorin treatment at P15. Scale bar, 40 µm. N = 5-8 mice/group. *p < 0.05 vs. Control. P Representative images and quantification of γH2AX foci in cardiomyocytes after imperatorin treatment at P15. Scale bar, 40 µm. N = 6-7 mice/group. *p < 0.05 vs. Control. Q Representative images and quantification of p-ATM foci in cardiomyocytes after imperatorin treatment at P15. Scale bar, 40 µm. N = 6-7 mice/group. *p < 0.05 vs. Control.
Imperatorin promoted cardiomyocyte proliferation after MI in adult mice
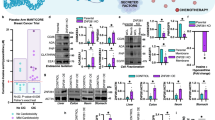
The effects of imperatorin on cardiac remodeling and regeneration following MI were then evaluated. Imperatorin was injected into eight-week-old mice with a permanent ligation of left anterior descending (LAD) coronary artery (Fig. 3A), as shown by increased plasma cTnT levels (Fig. 3B), and the treatment of imperatorin significantly improved survival probability after MI injury (Fig. 3C). A baseline echocardiographic study was conducted at the age of 8 weeks, as well as 24 hours, 14 days, and 28 days after MI (Fig. 3A). Lower ejection fraction (EF) is an indicator of impaired cardiac function following MI; the treatment with imperatorin, however, resulted in a significant improvement in cardiac function at 28 days after MI (Fig. 3D). A significant reduction in infarct sizes was also observed in the imperatorin group 28 days post-MI (Fig. 3E). Furthermore, imperatorin treatment produced significantly more isolated cardiomyocytes compared to control mice (Fig. 3F). We then examined EdU incorporation into cardiomyocyte nuclei to indicate DNA replication, and cardiomyocytes in the imperatorin group showed a significant increase of EdU incorporation in the infarct zone, border zone, and remote zone (Fig. 3G). Furthermore, we quantified the percentage of cardiomyocytes that showed positive Ki67 and pH3, the markers of mitosis, and found that there were significantly increased Ki67- and pH3-positive cardiomyocytes after imperatorin treatment (Fig. 3H, I). Additionally, we discovered that imperatorin-treated mice had much higher localization of the cytokinesis marker Aurkb to the cardiomyocyte cleavage furrow (Fig. 3J). According to WGA staining, cardiomyocyte size decreased at the infarct border and remote zones (Fig. 4A, B). Moreover, imperatorin significantly inhibited DNA damage of myocardium after MI (Fig. 4C–F). To examine multi-nucleation, we isolated cardiomyocytes 2 weeks after MI and found that imperatorin significantly increased the proportion of mono-nucleated cardiomyocytes and significantly reduced the proportion of bi-nucleated cardiomyocytes (Fig. 4G). Using MADMGT/TG; α-MHC-MerCreMer (MADM; MCM) transgenic mice, cytokinesis events in cardiomyocytes were evaluated by lineage tracing in order to confirm that completed and authentic cytokinesis had actually occurred (Fig. 4H). We injected adult MADM; MCM mice with imperatorin every day for 14 days, and the tamoxifen injections were used concomitantly to induce Cre-Lox recombination for 15 days, beginning the day before imperatorin therapy. After 14 days of MI injury, the hearts of imperatorin-treated MADM; MCM mice showed a significant increase in the proportion of both RFP-exclusive and GFP-exclusive cardiomyocytes (Fig. 4I). Moreover, imperatorin treatment did not influence cardiomyocyte apoptosis (Supplementary Fig. 2). These results demonstrated that imperatorin promoted authentic cardiomyocyte proliferation and resulted in new cardiomyocyte formation, which contributed to the myocardium repair in adult MI injuries.
A Schematic diagram of the experimental design for imperatorin treatment in mice with MI. Echocardiographic analysis was performed at the age of 8 weeks as a baseline and 24 h, 14 d, and 28 d after MI. Daily imperatorin treatment was performed 0-28 days after MI. EdU was intraperitoneally injected 7-14 days after MI. B Quantification of plasma cTnT in imperatorin treatment mice 24 h after Sham or MI. N = 8 mice/group. *p < 0.05 vs. Sham. C The probability of survival in imperatorin treatment mice after MI. N = 30 mice/group. *p < 0.05 vs. Control. D Cardiac function in adult MI mice after imperatorin treatment was measured by ejection fraction (EF%). N = 6-10 mice/group. *p < 0.05 vs. Control. E Serial sectioning was performed at 500 μm intervals from the ligation area to the apex. Representative images and quantification of infarct/fibrotic length by Masson’s trichrome staining 28 d after MI. Scale bar, 2 mm. N = 5 mice/group. *p < 0.05 vs. Control. F Representative images and quantification of cardiomyocyte numbers isolated from imperatorin treatment hearts 14 days after MI. N = 5-6 mice/group. Scale bar, 100 µm. *p < 0.05 vs. Control. G Representative images and quantification of EdU+ cardiomyocytes of control or imperatorin-treated hearts in infarct (G2), infarct border (G3), and remote zones (G4) at 14 days after MI. Scale bar, 40 μm. N = 6-8 mice/group. *p < 0.05 vs. Control. Representative images and quantification of Ki67+(H), pH3+(I), and Aurkb+(J) cardiomyocytes of control or imperatorin-treated hearts at 14 d after MI. Scale bar, 40 μm. N = 6-8 mice/group. *p < 0.05 vs. Control.
Representative images and quantification of cardiomyocyte size of control or imperatorin-treated hearts in the infarct border (A) and remote zones (B) at 14 days after MI. Scale bar, 40 μm. N = 6-8 mice/group. *p < 0.05 vs. Control. C Representative images and quantification of γH2AX foci in cardiomyocytes of control or imperatorin-treated hearts at 14 days after MI. Scale bar, 40 μm. N = 7-9 mice/group. *p < 0.05 vs. Control. D Representative images and quantification of p-ATM foci in cardiomyocytes of control or imperatorin-treated hearts at 14 days after MI. Scale bar, 40 μm. N = 7-9 mice/group. *p < 0.05 vs. Control. E Representative images and quantification of γH2AX protein expression after imperatorin treatment at 14 days after MI, analyzed by western blotting. Histone H2AX was used as a control. N = 6 mice/group. *p < 0.05 vs. Control. F Representative images and quantification of p-ATM protein expression after imperatorin treatment at 14 days after MI, analyzed by western blotting. ATM was used as a control. N = 6 mice/group. *p < 0.05 vs. Control. G Representative images and quantification of mono-nuclear, bi-nuclear, and multi-nuclear cardiomyocytes after imperatorin treatment at 14 days after MI. N = 5-6 mice/group. *p < 0.05 vs. Control. H MADM (mosaic analysis with double markers) recombination in a parent cardiomyocyte in α-MHC-expressing cells leads to RFP+ and GFP+ single-labeled daughter cardiomyocytes. Schematic diagram of the experimental design for the imperatorin treatment in MADM mice. Tamoxifen (TM) was injected intraperitoneally into mice for 15 days, beginning the day before imperatorin therapy. I Representative images and quantification of the percentage of single-colored cells to total labeled cells in MADM mouse hearts treated with control or imperatorin at 14 days after MI. Scale bar, 40 μm. N = 6-8 mice/group. *p < 0.05 vs. Control.
Imperatorin promoted DNA repair and cardiomyocyte proliferation by regulating the phosphorylation of TDP1
To probe the mechanism of imperatorin on cardiomyocyte DNA repair and proliferation, we applied the SuperPred 3.0 database to predict the molecular target of imperatorin. A DNA repair protein, TDP1, was found to exhibit the highest probability of interaction (Fig. 5A and Supplementary Data 1). The phosphorylation of TDP1 is critical for the focal accumulation of TDP1 proteins at DNA damage ends and repairing DSBs21. Moreover, we found that imperatorin treatment markedly increased the phosphorylation levels of TDP1 without influencing the protein and mRNA levels of TDP1 (Fig. 5B–D). The serine or threonine residues followed by glutamine or proline (SQ, TQ, or SP motif) are target sites for ATM or DNA-PK, which are key regulators in the DSB response and DNA repair pathway21. The human TDP1 phosphorylation on S81 is both ATM and DNA-PK-dependent, which results in enhancing the mobilization of TDP1 to DNA damage sites21. The SQ motif of human TDP1 on S81 is replaced by the SP motif in rat or mouse TDP1 on S81, and the SP motif is also a target site for DNA-PK (Fig. 5E)21. There were two conserved TQ motifs of rat and mouse on T101 and T294 (Fig. 5F); therefore, wild-type Tdp1 (Tdp1-WT) and mutated Tdp1 variants (Tdp1-S81A, Tdp1-T101A, and Tdp1-T294A) were transfected into P7 NMVMs. We found that the enhanced effect of TDP1 phosphorylation by imperatorin was reversed by Tdp1-S81A overexpression, rather than Tdp1-T101A and Tdp1-T294A expression (Fig. 5G).
A The results of the molecular target of imperatorin predicted by the SuperPred 3.0 database. B Quantification of TDP1, analyzed by qPCR, in P7 NMVMs after imperatorin treatment. Gapdh was used as a control. N = 8 repeats/group. C Representative images and quantification of TDP1 protein expression after imperatorin treatment, analyzed by western blotting. N = 5 repeats/group. D Representative images and quantification of TDP1 protein and phosphorylated TDP1 expression after imperatorin treatment. N = 6 repeats/group. *p < 0.05 vs. Control. E, F Amino acid sequence alignment of the TDP1 protein in different species. G Representative images and quantification of TDP1 protein and phosphorylated TDP1 expression after imperatorin treatment and adenovirus transfection. N = 5 repeats/group. *p < 0.05 vs. Control. H DNA end-joining assays after imperatorin treatment and adenovirus transfection. Representative images and quantification of Ki67+ (I) and pH3+ (J) cardiomyocytes after imperatorin treatment and TDP1-S81A transfection. Scale bar, 40 μm. N = 5 repeats/group, ~500 cells/repeat. *p < 0.05 vs. TDP1-WT, #p < 0.05 vs. TDP1-WT+Imperatorin. Representative images and quantification of γH2AX (K) and p-ATM (L) intensity after imperatorin treatment and TDP1-S81A transfection. Scale bar, 40 μm. N = 6-7 repeats/group, ~50 cells/repeat. *p < 0.05 vs. TDP1-WT, #p < 0.05 vs. TDP1-WT+Imperatorin.
In addition, TDP1 phosphorylation induced by imperatorin promoted the binding of TDP1 and XRCC1, which was consistent with a previous study (Supplementary Fig. 3)21. A recent study has revealed that the phosphorylation of TDP1 at serine 61 is associated with mitosis in human embryonic kidney origin (HEK293) and human breast cancer (MCF7) cell lines, with the ability of immortalized proliferation22. However, in P7 NMVMs with limited proliferative capacity, Tdp1-S61A overexpression could not reverse the enhanced effect of TDP1 phosphorylation by imperatorin (Supplementary Fig. 4). Moreover, imperatorin treatment alone significantly increased the efficiency of end-joining, which was reversed by Tdp1-S81A overexpression (Fig. 5H). We also assessed cardiomyocyte proliferation by Ki67 and pH3 immunofluorescent staining, and DNA damage by γH2AX and p-ATM intensity or expression. The results revealed that imperatorin treatment alone significantly increased cardiomyocyte proliferation and inhibited DNA damage, while Tdp1-S81A overexpression could reverse the effect of imperatorin on cardiomyocyte proliferation and DNA damage (Fig. 5I–L). Collectively, these results suggested imperatorin promoted DNA repair and cardiomyocyte proliferation by enhancing the phosphorylation of TDP1 on S81.
Imperatorin promoted TDP1 phosphorylation via the proximity-mediated effect between TDP1 and DNA-PKcs
It has been proven that the phosphorylation of human TDP1 on S81 is both ATM- and DNA-PK- dependent21. Through the SwissTargetPrediction database, we predicted that human and mouse DNA-PKcs, encoded by PRKDC and Prkdc, were also possible targets of imperatorin (Supplementary Data 2 and 3). DNA-PK mediates the phosphorylation of the TDP1 protein on S81, and its key component is DNA-PKcs23. Therefore, molecular docking analysis was performed, and imperatorin was predicted to form a proximity-mediated effect between TDP1 and DNA-PKcs (Fig. 6A, B). Imperatorin treatment did not affect the protein levels of DNA-PKcs, TDP1, and ATM in P1 and P7 NMVMs (Supplementary Fig. 5). However, imperatorin treatment significantly increased the binding of DNA-PKcs and TDP1 in P7 cardiomyocytes (Fig. 6C and Supplementary Fig. 6), and the enhanced effect of TDP1 phosphorylation and end-joining by imperatorin was reversed by DNA-PKcs silencing (Fig. 6D, E). Imperatorin treatment alone significantly increased cardiomyocyte proliferation and inhibited DNA damage, while DNA-PKcs silencing could reverse the effect of imperatorin on cardiomyocyte proliferation and DNA damage in P7 cardiomyocytes (Fig. 6F–I).
A, B Molecular docking of imperatorin, TDP1, and DNA-PKcs in two and three dimensions. C Representative images and quantification of the binding of TDP1 and DNA-PKcs proteins after imperatorin treatment. N = 6 repeats/group. *p < 0.05 vs. Control. D Representative images and quantification of phosphorylated TDP1 expression after imperatorin treatment and siRNA-DNA-PKcs transfection. N = 6 repeats/group. *p < 0.05 vs. Control. E DNA end-joining assays after imperatorin treatment and siRNA-DNA-PKcs transfection. Representative images and quantification of Ki67+ (F) and pH3+ (G) cardiomyocytes after imperatorin treatment and siRNA-DNA-PKcs transfection. Scale bar, 40 μm. N = 6 repeats/group, ~500 cells/repeat. *p < 0.05 vs. siRNA-scramble, #p < 0.05 vs. siRNA-scramble+Imperatorin. Representative images and quantification of γH2AX (H) and p-ATM (I) intensity after imperatorin treatment and siRNA-DNA-PKcs transfection. Scale bar, 40 μm. N = 6 repeats/group, ~50 cells/repeat. *p < 0.05 vs. siRNA-scramble, #p < 0.05 vs. siRNA-scramble+Imperatorin.
Imperatorin promoted the DNA repair and proliferation of hiPSC-CMs
To assess the translational potential of imperatorin, we treated hiPSC-CMs with imperatorin. The results showed a significant increase in TDP1 phosphorylation and end-joining. Imperatorin treatment also significantly increased hiPSC-CMs proliferation and inhibited DNA damage, which were reversed by TDP1-S81A overexpression (Fig. 7A–F). Moreover, DNA-PKcs silencing could reverse the effect of imperatorin on TDP1 phosphorylation, end-joining, proliferation, and DNA damage in hiPSC-CMs (Fig. 7G–L). These findings further emphasize the potential of imperatorin as a therapeutic candidate for enhancing cardiac repair.
A Representative images and quantification of phosphorylated TDP1 expression after imperatorin treatment and TDP1-S81A transfection in hiPSC-CMs. N = 6 repeats/group. *p < 0.05 vs. Control. B DNA end-joining assays after imperatorin treatment and TDP1-S81A transfection in hiPSC-CMs. Representative images and quantification of Ki67+ (C) and pH3+ (D) cardiomyocytes after imperatorin treatment and TDP1-S81A transfection in hiPSC-CMs. Scale bar, 40 μm. N = 6 repeats/group, ~900 cells/repeat. *p < 0.05 vs. TDP1-WT, #p < 0.05 vs. TDP-WT+Imperatorin. Representative images and quantification of γH2AX (E) and p-ATM (F) intensity after imperatorin treatment and TDP1-S81A transfection in hiPSC-CMs. Scale bar, 10 μm. N = 6-7 repeats/group, ~50 cells/repeat. *p < 0.05 vs. TDP1-WT, #p < 0.05 vs. TDP-WT+Imperatorin. G Representative images and quantification of phosphorylated TDP1 expression after imperatorin treatment and siRNA-DNA-PKcs transfection in hiPSC-CMs. N = 6 repeats/group. *p < 0.05 vs. Control. H DNA end-joining assays after imperatorin treatment and siRNA-DNA-PKcs transfection in hiPSC-CMs. Representative images and quantification of Ki67+ (I) and pH3+ (J) cardiomyocytes after imperatorin treatment and siRNA-DNA-PKcs transfection in hiPSC-CMs. Scale bar, 40 μm. N = 6 repeats/group, ~900 cells/repeat. *p < 0.05 vs. siRNA-scramble, #p < 0.05 vs. siRNA-scramble+Imperatorin. Representative images and quantification of γH2AX (K) and p-ATM (L) intensity after imperatorin treatment and siRNA-DNA-PKcs transfection in hiPSC-CMs. Scale bar, 10 μm. N = 6 repeats/group, ~50 cells/repeat. *p < 0.05 vs. siRNA-scramble, #p < 0.05 vs. siRNA-scramble+Imperatorin.
Discussion
DNA damage is a key factor leading to cell cycle arrest in cardiomyocytes, and DNA repair is required to relieve the restriction of proliferation7,8. However, the potential of natural small-molecule compounds to enhance DNA repair and proliferation in cardiomyocytes has not been fully explored. The major type of DNA damage that causes cell cycle arrest in cardiomyocytes is DSBs8. Upon DSB induction, two different DSB repair mechanisms are available to repair the lesion, including HR and NHEJ24. Template NHEJ is the major pathway for mending the broken ends in mammalian cells in all cell cycle stages, whereas HR requires an undamaged homologous sequence to serve as a template for repair of both broken strands and occurs primarily in the S and G2 phases of the cell cycle24. Therefore, it is speculated that the primary function of NHEJ may be to safeguard the genomic integrity of cardiomyocytes and facilitate their transition into the cell cycle, after which DNA repair could be carried out precisely by HR. The NHEJ process might play a crucial role in the initial phase of cardiomyocyte proliferation, while HR could subsequently complement this process to ensure the precise and complete preservation of the daughter cardiomyocytes’ genome.
Imperatorin demonstrated superior DNA repair efficiency among 722 small-molecule compounds, with high and well-balanced scores for NHEJ and HR, resulting in the highest overall score. Out of the top 10 small-molecule compounds, imperatorin was the sole drug capable of enhancing P7 cardiomyocyte proliferation. Imperatorin exhibits a diverse range of protective effects on the cardiovascular system, including its ability to reduce hypertension, prevent cardiac hypertrophy, and inhibit fibrosis. Imperatorin exerts its anti-hypertensive action by decreasing the activity of NADPH oxidase and blocking the MAPK pathway25. Although studies have identified sustained MAPK pathway activation as a hallmark of tumor cell proliferation26, inhibition of this pathway in cardiomyocytes has been shown to promote cardiomyocyte proliferation27. Imperatorin has also been discovered to exhibit cardiac activities that inhibit hypertrophy and fibrosis through the phosphorylation of eNOS15,28. And imperatorin exerts an anti-tumor effect by suppressing HIF-1α synthesis29, while its appropriate expression promotes cardiomyocyte proliferation6. These opposing effects likely arise from divergent proliferative demands between tumor cells and cardiomyocytes. In our study, we augmented the impact of imperatorin by promoting DNA repair, mitigating DNA damage, and stimulating cardiomyocyte proliferation and cardiac regeneration. Moreover, imperatorin was established by clinical research to be an admirable safety drug when given along with the daily diet15. Additionally, we observed that imperatorin had a positive impact on enhancing the DNA repair and proliferation of hiPSC-CMs. Based on these findings, imperatorin could be a highly promising therapeutic treatment for patients suffering from MI or heart failure.
To investigate the impact of imperatorin on signaling pathways, we performed molecular target prediction and docking analysis. Our findings revealed that imperatorin can induce a proximity-mediated effect between TDP1 and DNA-PKcs. TDP1 is required for efficient NHEJ in human cells and plays a significant role in removing topoisomerase 1-associated DNA breaks in the nucleus and mitochondria by hydrolyzing the 3’-phosphotyrosine bond30. Previous investigations have indicated that the ability of TDP1 to repair DSBs is dependent on the phosphorylation of serine 81 in TDP121. Furthermore, the kinase responsible for phosphorylating TDP1 at serine 81 is DNA-dependent protein kinase (DNA-PK)21. DNA-PK is a molecular assembly consisting of a large catalytic subunit called DNA-PKcs and a pair of Ku proteins (Ku70/80) that form a heterodimer23. The PRKDC gene encodes DNA-PKcs, which is also a potential binding target of imperatorin. Molecular docking simulation revealed that imperatorin’s structure was positioned between the two proteins. Hence, we hypothesized that imperatorin may establish a bridging connection akin to TDP1 and DNA-PKcs, and experimental results confirmed that imperatorin could promote the binding of the two proteins and phosphorylation of TDP1. This approach has been utilized in the domain of creating artificial small-molecule compounds with targeted functionalities. It involves the development of phosphorylation-inducing chimeric small molecules by connecting small-molecule binders of the kinase and the target protein31. By employing this approach, a prior investigation has developed small chemical compounds that effectively trigger both native and neo-phosphorylations of BRD4 through the activation of AMPK or PKC31. Similarly, another emerging technology based on proteolysis-targeting chimaeras that exploit cellular quality control machinery to degrade target proteins selectively is attracting considerable attention, which increases the effective molarity of the ubiquitin ligase around the target protein, triggering ubiquitination even when the target protein is otherwise not a substrate of the given ligase32. Imperatorin employs a similar strategy to these two technologies in order to facilitate the spatial connection of two protein molecules. However, the key distinction is that imperatorin is a naturally small molecule, as opposed to an artificially engineered chimeric molecule. Imperatorin may possess a superior clinical safety profile in this specific aspect, but it lacks the flexibility of chimeric small molecules.
Nevertheless, our study is subject to certain limitations. Prior to practical translation, it is necessary to confirm the regeneration effectiveness and safety of imperatorin in large animals, as their larger hearts would have greater therapeutic relevance. Furthermore, imperatorin exhibits protective effects on the cardiovascular system, including anti-hypertensive, anti-fibrotic, and anti-hypertrophic properties15. Therefore, the therapeutic impact of imperatorin on MI is attributed not only to its ability to induce cardiac regeneration but also to its combined protective effects, which require further investigation. Furthermore, the Cre-recombinase-dependent MADM lineage tracking system underestimates cytokinesis events, and the actual proportion of cardiomyocyte cytokinesis should be higher than the statistical value33.
In conclusion, the investigation into the DNA repair properties of herbs used in traditional Chinese medicine, along with the identification of their potential to stimulate cardiomyocyte proliferation, has led to the discovery of imperatorin, which promotes DNA repair and cell cycle re-entry in cardiomyocytes. The mechanism of this benefit stems from imperatorin inducing the steric association of TDP1 and DNA-PKcs, leading to the phosphorylation of TDP1 (Fig. 8). This makes imperatorin a prospective treatment for MI injury.
Methods
Experimental animals
Similar to the criteria of the previous studies7,34, C57BL/6 J mice were used as experimental animals in this study. The experimental protocol was approved by the Institutional Animal Care and Use Committee at Jilin University. Every surgical procedure was carried out under anesthesia, and care was taken to reduce the pain of the animals. Every experiment was conducted in compliance with the guidelines provided by the National Institutes of Health’s Guide for the Care and Use of Laboratory Animals.
Isolation and treatment of neonatal mouse ventricular myocytes (NMVMs) and neonatal rat ventricular myocytes (NRVMs)
According to the previously described methods34, on postnatal day 1 (P1) or 7 (P7), we isolated NMVMs from C57BL/6 J mice or NRVMs from Sprague-Dawley rats. After being removed, the ventricles were put in a cold D-Hank’s solution (Solarbio, H1045). The tissues were then divided into pieces and digested with collagenase II (1.0 mg/ml) and 1.25 mg/ml trypsin (Worthington, LS003707). The cell suspension was centrifuged using low- and high-density Percoll solutions following an hour of pre-plating to exclude fibroblasts35. At the intersection of the low- and high-density gradients were purified live cardiomyocytes35. Following a wash, the cardiomyocytes were cultured in 89% DMEM containing 10% FBS and 1% penicillin-streptomycin (Gibco, 15140122).
NMVMs or NRVMs were treated with different small molecules (Imperatorin, Bavachinin, Zingerone, Shanzhiside methylester, Apiin, Hesperidin, Stevioside, Dendrobine, and Farrerol) at a concentration of 5 μM. Adenoviruses encoding Tdp1-wild type and Tdp1-mutant (S61A, S81A, T101A, and T294A) were constructed. The multiplicity of infection for cell transfection was set at 100 in accordance with the manufacturer’s recommendations. The sequences of siRNAs are shown in Supplementary Table 1, and a scrambled sequence was used as a negative control. The siRNA-DNA-PKcs was transfected into NMVMs using RNAiMAX (Invitrogen, 13778030), following the manufacturer’s instructions.
Culture and transfection of human induced pluripotent stem cell (iPSC)-derived cardiomyocytes (hiPSC-CMs)
Commercial hiPSC-CMs were purchased from Saibei Biotechnology (CA2201106, Beijing, China) and cultured in the maintenance medium (Saibei Biotechnology, CA2013100). Commercial hiPSC-CMs were treated with different small molecules (Imperatorin, Bavachinin, Zingerone, Shanzhiside methylester, Apiin, Hesperidin, Stevioside, Dendrobine, and Farrerol) at a concentration of 5 μM. Adenoviruses encoding TDP1-wild-type and TDP1-mutant (S81A) were constructed. The multiplicity of infection for cell transfection was set at 100 in accordance with the manufacturer’s recommendations. The sequences of siRNAs are shown in Supplementary Table 1, and a scrambled sequence was used as a negative control. The siRNA-DNA-PKcs was transfected into hiPSC-CMs using RNAiMAX (Invitrogen, 13778030) following the manufacturer’s instructions.
Animal experiments
Neonatal mice were operated on at 8 days of age in order to resect the apex of the left ventricle (LV) in accordance with the previously described methods of the AR model7. In brief, the pups were put on an ice bed to induce anesthesia, and a thoracotomy was performed at the fourth intercostal area. The apex of the heart was externalized after the abdomen was gently compressed, and approximately 15% of the left ventricle was carefully removed without causing endocardial damage. As previously mentioned7, we permanently ligated the LAD coronary artery in order to induce MI in mice that were 8 weeks old. Under 2% isoflurane anesthesia, the LAD was promptly sutured with 7-0 silk 2-3 mm distal to the left atrial appendage. The mice were randomly divided into two groups to receive a vehicle or imperatorin (10 mg/kg/d) intraperitoneal treatment.
Adult cardiomyocyte isolation
According to the previously described methods34, mice were injected intraperitoneally with 100 μl of heparin (6.25U/μl) to inhibit clotting in order to isolate mature cardiomyocytes. The mice were given 2% isoflurane anesthesia and had their hearts exposed, with an aortic cannula used to attach them to a Langendorff perfusion system, after a 30-minute break. To remove remaining blood, the hearts were first perfused for five minutes with a perfusion buffer (140 mM NaCl, 4 mM KCl, 1 mM MgCl2, 10 mM HEPES, 10 mM taurine, 10 mM 2,3-butanedione monoxime, and 10 mM glucose at pH 7.3). Following that, the hearts were perfused for 12 to 15 minutes with a digestion solution (perfusion buffer containing 1 mg/ml collagenase II, 0.12 mg/ml trypsin, and 0.02 mM CaCl2) until they softened and collapsed. After being digested, the hearts were then minced. The digestion process was then stopped by immersing the minced hearts in a stop solution (a perfusion buffer containing 0.1 mM CaCl2 and 5 mg/ml bovine serum albumin). As previously mentioned, the separated cells were filtered through a 100 μm strainer and centrifuged for three minutes at 50 g to extract the cardiomyocytes.
Echocardiographic evaluation
According to the previously described methods7,34, utilizing a Vevo 3100 LT system (Visual Sonics), echocardiography was performed to assess the structure and functionality of the heart. An experienced operator conducted the examination, and heart rates were regulated by inhaling 0.5%–3% isoflurane to keep them between 420 and 480 beats per minute. The long-axis view and M-mode imaging were utilized to quantify the left ventricular internal diameter (LVIDs) and diastole (LVIDd). The following formula was used to get the LV ejection fraction (EF): LVEF (%) = {(LVIDd)3 - (LVIDs)3} /(LVIDd)3×100. Additionally, the formula LVFS (%) = (LVIDd - LVIDs) / LVIDd ×100 was used to compute the LV fraction shortening (FS). Before the echocardiographic exams, a random number was given to every mouse to guarantee blindness to a particular therapy.
Measurement of cardiac fibrosis
After being rinsed with PBS, the hearts were fixed overnight at 4°C in 4% paraformaldehyde. The hearts were dehydrated in 15% and 30% sucrose in PBS solution at 4°C overnight after three PBS washes, and then they were embedded in the optimum cutting tissue (OCT, Sakura). Cryosections that were 10-μm thick were obtained and stored at -20°C until use.
Vimentin-immunostaining was used in the AR model to quantify the size of the fibrosis scar. Shortly after being immersed in PBS for 10 minutes to remove OCT, heart sections were permeabilized with 0.1% Triton X-100, followed by being blocked with 2.5% normal donkey serum in PBS for 30 minutes at room temperature. The antibodies against cardiac troponin-I (cTnI, Abcam, ab56357, 1:100) and vimentin (Abcam, ab92547, 1:100) were applied to the heart sections overnight at 4°C. Following three PBS washes, the sections were treated for an hour at 37°C in the dark with Alexa Fluor 555 (Invitrogen, A31572) and 488 (Invitrogen, A11055) secondary antibodies (1:200). Using an Olympus confocal laser scanning microscope, images were captured (FluoView 3000, Japan). The vimentin+/cTnI- area defined the size of the scar.
Using a modified Masson’s trichrome staining kit (Solabio, G1346), the infarct size of fibrosis was assessed in the MI model. Heart sections were stained with Masson trichrome solution in accordance with the manufacturer’s instructions. Between the epicardial and endocardial surfaces, the left ventricle’s myocardial midline was marked in the center. Using the Fiji software, midline lengths of infarct and non-infarct walls in the left ventricle were measured in the digital images that were captured. Infarct length/total length (infarct + non-infarct length) × 100 was used to calculate the percentage of fibrotic length that indicates the infarct ratio7.
Hematoxylin-eosin (HE) staining
According to the previously described methods7 and following the manufacturer’s instructions, mouse tissues were cryosectioned and stained using a HE staining kit (Solarbio, G1120).
Plasma cardiac troponin T (cTnT) detection
According to the previous methods7, heparin was used as an anticoagulant to collect blood samples from the left ventricular cavity after a 24-hour MI. The plasma was separated from the blood samples by centrifuging them for 15 minutes at 1000 g and 4 °C. The mouse cardiac troponin T (cTnT) was measured using an ELISA kit (Kamiya Biomedical Company, KT-58997) in accordance with the manufacturer’s instructions.
Cardiomyocyte proliferation, apoptosis, and DNA damage detection
Animals were treated with 500 μg of EdU intraperitoneally every two days for the pulse-chase experiment using EdU (5-ethynyl-2’-deoxyuridine, Thermo Fisher Scientific, A10044). The hearts were harvested 14 days after the MI. Thermo Fisher Scientific’s Click-iT Plus EdU Alexa Fluor 555 Imaging Kit (C10638) was used to stain the EdU according to the manufacturer’s instructions. TUNEL (TdT-mediated dUTP NickEnd Labeling) staining was performed using a One Step TUNEL Apoptosis Assay Kit (Beyotime Biotechnology), according to the manufacturer’s instructions.
Heart sections were pretreated with 2.5% normal donkey serum in PBS for 30 minutes at room temperature, followed by treatments with antibodies against Ki67 (Thermo Fisher Scientific, MA5-14520, 1:100), pH3 (Thermo Fisher Scientific, PA5-17869, 1:100), Aurkb (Abcam, ab2254, 1:100), and cTnI (Abcam, ab56357, 1:100) overnight at 4°C for immunostaining in vivo. Following three PBS washes, the sections were treated for an hour at 37°C in the dark with Alexa Fluor 555 (Invitrogen, A31572) and 488 (Invitrogen, A11055) secondary antibodies (1:200).
The cells were washed with PBS, fixed with 4% paraformaldehyde, and permeabilized with 0.1% Triton X-100 at room temperature for immunostaining of Ki67, pH3, and Aurkb in vitro. The cells were treated with antibodies against Ki67 (Cell Signaling Technologies, D3B5, 1:100), pH3 (Thermo Fisher Scientific, PA5-17869, 1:100), Aurkb (Abcam, ab2254, 1:100), and α-actinin (Abcam, ab9465, 1:100) overnight at 4°C after being incubated with 2.5% normal donkey serum in PBS for 30 minutes. The cells were then treated for an hour at 37°C in the dark with Alexa Fluor 555 (Invitrogen, A31572) and 488 (Invitrogen, A32723) secondary antibodies (1:200). The Duolink proximity ligation assay (PLA) detection was performed with DuoLink PLA technology probes and reagents (Merck, DUO92101) according to the manufacturer’s protocols.
Heart sections or cells were incubated with antibodies against γH2AX (Abcam, ab81299, 1:100), p-ATM (Abcam, ab81292, 1:100), α-actinin (Abcam, ab9465, 1:100), and cTnI (Abcam, ab56357, 1:100) overnight at 4°C for immunostaining of γH2AX and p-ATM in vivo and in vitro.
DAPI was used to label the nuclei (Solabio, C0065). An Olympus confocal laser scanning microscope (FluoView 3000, Japan) was used to capture images, and Fiji software was used to analyze the images.
Measurement of cardiomyocyte size
Wheat germ agglutinin (WGA) staining was used to measure the size of the cardiomyocytes (Invitrogen, W11261). In brief, heart sections were incubated overnight at 4°C with the anti-cTnI antibody (Abcam, ab56357, 1:100). Following three PBS washes, the sections were incubated with Alexa Fluor 555 (Invitrogen, A21432) secondary antibody for one hour at 37°C in the dark. Finally, Alexa Fluor 488-conjugated WGA was added to the heart sections in accordance with the manufacturer’s instructions.
Quantitative real-time polymerase chain reaction (qPCR)
Using the Trizol reagent (Takara, 9109), total RNA was extracted from hearts or cells in accordance with the manufacturer’s instructions. PrimeScript RT Master Mix (Takara, RR036A) was used with random primers to reversely transcribe one μg of RNA from each sample into cDNA. In the CFX96TM Real-Time PCR Detection System, three duplicates using cDNA and TB Green Premix EX TaqTM II (Takara, RR820A) were carried out (Bio-Rad, USA). Within a 10 µl reaction system, PCR was performed. The cDNA was amplified for 39 cycles at 95°C for 10 seconds, 58°C for 20 seconds, and 72°C for 10 seconds after reverse transcription was conducted at 37°C for 15 minutes. In order to determine the relative RNA expression levels, values were standardized to Gapdh. Supplementary Table 1 contains a list of the primer sequences that were utilized to determine mRNA expression.
Western blot analysis and immunoprecipitation
The samples’ total proteins were isolated for western blot examination using standard RIPA lysis buffer, and nuclear fractionation was extracted using a nucleoprotein extraction kit (Solabio, R0050). On SDS-PAGE gels, the protein samples were resolved after being prepared with a 5×sample loading buffer. Transferred nitrocellulose membranes were probed with antibodies against γH2AX (Abcam, ab81299, 1:1000), histone H2AX (Abcam, ab11175, 1:1000), ATM (ataxia-telangiectasia, mutated, Abcam, ab199726, 1:1000), p-ATM (Abcam, ab81292, 1:1000), TDP1 (Invitrogen, PA5-98189, 1:1000), DNA-PKcs (Abcam, ab70250, 1:1000), XRCC1 (Invitrogen, H00007515-B01P, 1:1000), β-actin (Proteintech, 20536-1-AP, 1:1000), Vinculin (Proteintech, 26520-1-AP, 1:1000), and GAPDH (Proteintech, 10494-1-AP, 1:1000).
Native lysis buffer was used to collect the proteins from the samples for immunoprecipitation. After 12 hours of incubation with antibodies against TDP1 (Invitrogen, PA5-98189, 1:200) or DNA-PKcs (Abcam, ab70250, 1:200), the cell lysates were treated for 1 hour at 4°C with protein A/G-agarose. The immunological precipitates were suspended in a 4×sample loading buffer, boiled for 10 minutes, and resolved on SDS-PAGE gels. Transferred nitrocellulose membranes were probed with antibodies against DNA-PKcs (Abcam, ab168854, 1:1000) and phosphoserine/threonine (Invitrogen, MA5-38234, 1:1000) from different host species to eliminate the detection of heavy or light chains. To assess the immunoblots’ specificity, rabbit IgG (Abcam, ab172730) was used as a negative control. The chemiluminescent signals were detected by LiCor Odyssey Fc instrument.
DNA end-joining assay
The nuclei extraction and DNA end-joining assay were performed in compliance with previously published protocols7,36. In summary, after nuclei were recovered by centrifugation using 300 mM sucrose, nuclear pellets were digested and extracted into high-salt buffer (420 mM NaCl, 0.2 mM EDTA, 25% glycerol, 1.5 mM MgCl2, 20 mM Hepes, pH 7.5). The insoluble material was centrifuged out, and the remaining soluble extract was used in the subsequent DNA end-joining experiments. EGFP-C1 plasmids were digested with the restriction enzymes EcoRI and NheI, resulting in the production of linearized DNA fragments with non-homologous ends that weighed approximately 4 kb. In order to repair DNA, 2 µg of nucleus extract and 100 ng of linearized DNA substrate were combined and incubated for 30 minutes at 25 °C in end-joining assay buffer (0.2 mM CaCl2, 10 mM MgCl2, 50 mM KCl, 1.2 mM ATP, 0.5 mM DTT, and 7.5 mM Tris, pH 8.0). After that, 0.5 M EDTA, 0.5% SDS, and 10 mg/mL proteinase K were added for 30 minutes to halt the repair process. Using a 0.7% agarose gel, electrophoretically separated end-joining products were then photographed with a BioRad ChemiDoc imaging system.
Mosaic analysis with double markers (MADM) transgenic mice
Jackson Laboratory provided the MADMGT/TG (stock number 030578) and α-MHC-MerCreMer (stock number 005650). Following LAD ligation, eight-week-old MADM mice were randomized to receive either a vehicle or an intraperitoneal dose of imperatorin (10 mg/kg/d) for a period of 14 days. Starting on the day before imperatorin therapy, mice received intraperitoneal injections of dissolved tamoxifen (TM) (Sigma Aldrich H6278) at a dose of 40 mg/kg for a duration of 15 days. Fourteen days following MI, the hearts were harvested for histological examination. After the nuclei of heart cryosections were labeled with DAPI (Solabio, C0065), the images were captured. The percentage of single-colored cardiomyocytes from the total labeled cardiomyocytes was analyzed.
Virtual prediction of pharmacological targets
The SuperPred 3.0 database (https://prediction.charite.de/subpages/target_prediction.php) and the SwissTargetPrediction database (http://www.swisstargetprediction.ch) were applied to predict the molecular target of imperatorin.
Molecular docking
The cryo-electron microscope structure of human DNA-PKcs used for docking was downloaded from the PDB database (https://www.rcsb.org/structure/5W1R). The AlphaFold predictive structure of human TDP1 used for docking was downloaded from the AlphaFold protein structure database (https://alphafold.ebi.ac.uk/entry/Q9NUW8). The docking structure of DNA-PKcs and TDP1 complexes was predicted by ZDOCK Server (https://zdock.umassmed.edu). The 3D structure of imperatorin was downloaded from the PubChem database (https://pubchem.ncbi.nlm.nih.gov/compound/10212). The docking program was carried out using the Molecular Operating Environment software.
Statistical analysis
According to the previous analysis7,34, GraphPad Prism 9.0 software was utilized to perform statistical analysis. The mean and standard error were chosen to represent all numerical data in our experiments. Using the Shapiro-Wilk test, normality was verified. A two-tailed unpaired Student’s t-test with or without Welch’s correction was used to compare two groups of normally distributed data, depending on the homogeneity of variance. One-way ANOVA was used for statistical analyses when homogeneity of variance was assumed; otherwise, Brown-Forsythe and Welch ANOVA tests with Dunnett’s T3 multiple comparisons were employed for comparisons involving more than two groups. If two factors were present, a two-way ANOVA was employed. Numerical data at various times were analyzed using the multiple t-test. If a result’s p-value was less than 0.05, it was considered statistically significant.
Reporting summary
Further information on research design is available in the Nature Portfolio Reporting Summary linked to this article.
Data availability
All relevant raw data associated with the manuscript are located in the Supplementary Data 4 file. The data underlying this article will be shared on reasonable request to the corresponding author.
References
Bahit, M. C., Kochar, A. & Granger, C. B. Post-myocardial infarction heart failure. JACC Heart Fail. 6, 179–186 (2018).
Frantz, S., Hundertmark, M. J., Schulz-Menger, J., Bengel, F. M. & Bauersachs, J. Left ventricular remodelling post-myocardial infarction: pathophysiology, imaging, and novel therapies. Eur. Heart J. 43, 2549–2561 (2022).
He, L., Nguyen, N. B., Ardehali, R. & Zhou, B. Heart regeneration by endogenous stem cells and cardiomyocyte proliferation: controversy, fallacy, and progress. Circulation 142, 275–291 (2020).
Li, Y. et al. Genetic lineage tracing of nonmyocyte population by dual recombinases. Circulation 138, 793–805 (2018).
Zhang, J. et al. Basic and translational research in cardiac repair and regeneration: JACC state-of-the-art review. J. Am. Coll. Cardiol. 78, 2092–2105 (2021).
Nakada, Y. et al. Hypoxia induces heart regeneration in adult mice. Nature 541, 222–227 (2017).
Fu, W. et al. Loss of NPPA-AS1 promotes heart regeneration by stabilizing SFPQ-NONO heteromer-induced DNA repair. Basic Res. Cardiol. 117, 10 (2022).
Puente, B. N. et al. The oxygen-rich postnatal environment induces cardiomyocyte cell-cycle arrest through DNA damage response. Cell 157, 565–579 (2014).
Cardoso, A. C. et al. Mitochondrial substrate utilization regulates cardiomyocyte cell cycle progression. Nat. Metab. 2, 167–178 (2020).
Shrivastav, M., De Haro, L. P. & Nickoloff, J. A. Regulation of DNA double-strand break repair pathway choice. Cell Res. 18, 134–147 (2008).
Atanasov, A. G., Zotchev, S. B., Dirsch, V. M. & Supuran, C. T. Natural products in drug discovery: advances and opportunities. Nat. Rev. Drug Discov. 20, 200–216 (2021).
Newman, D. J. & Cragg, G. M. Natural products as sources of new drugs over the nearly four decades from 01/1981 to 09/2019. J. Nat. Prod. 83, 770–803 (2020).
Chen, D. Q., Feng, Y. L., Cao, G. & Zhao, Y. Y. Natural products as a source for antifibrosis therapy. Trends Pharm. Sci. 39, 937–952 (2018).
Zhang, W. et al. A high-throughput small molecule screen identifies farrerol as a potentiator of CRISPR/Cas9-mediated genome editing. Elife 9, e56008 (2020).
Nasser, M. I. et al. Effects of imperatorin in the cardiovascular system and cancer. Biomed. Pharmacother. 120, 109401 (2019).
Bakkenist, C. J. & Kastan, M. B. DNA damage activates ATM through intermolecular autophosphorylation and dimer dissociation. Nature 421, 499–506 (2003).
Kozlov, S. V. et al. Involvement of novel autophosphorylation sites in ATM activation. EMBO J. 25, 3504–3514 (2006).
Scully, R. & Xie, A. Double strand break repair functions of histone H2AX. Mutat. Res. 750, 5–14 (2013).
Porrello, E. R. et al. Regulation of neonatal and adult mammalian heart regeneration by the miR-15 family. Proc. Natl. Acad. Sci. USA 110, 187–192 (2013).
Porrello, E. R. et al. Transient regenerative potential of the neonatal mouse heart. Science 331, 1078–1080 (2011).
Das, B. B. et al. Optimal function of the DNA repair enzyme TDP1 requires its phosphorylation by ATM and/or DNA-PK. EMBO J. 28, 3667–3680 (2009).
Paul Chowdhuri, S. & Das, B. B. TDP1 phosphorylation by CDK1 in mitosis promotes MUS81-dependent repair of trapped Top1-DNA covalent complexes. EMBO J. 43, 3710–3732 (2024).
Blackford, A. N. & Jackson, S. P. ATM, ATR, and DNA-PK: the trinity at the heart of the DNA damage response. Mol. Cell 66, 801–817 (2017).
Symington, L. S. & Gautier, J. Double-strand break end resection and repair pathway choice. Annu. Rev. Genet. 45, 247–271 (2011).
Cao, Y., Zhang, Y., Wang, N. & He, L. Antioxidant effect of imperatorin from Angelica dahurica in hypertension via inhibiting NADPH oxidase activation and MAPK pathway. J. Am. Soc. Hypertens. 8, 527–536 (2014).
Cargnello, M. & Roux, P. P. Activation and function of the MAPKs and their substrates, the MAPK-activated protein kinases. Microbiol. Mol. Biol. Rev. 75, 50–83 (2011).
Tang, L. et al. Reversing metabolic reprogramming by CPT1 inhibition with etomoxir promotes cardiomyocyte proliferation and heart regeneration via DUSP1 ADP-ribosylation-mediated p38 MAPK phosphorylation. Acta Pharm. Sin. B 15, 256–277 (2025).
Zhang, Y., Cao, Y., Duan, H., Wang, H. & He, L. Imperatorin prevents cardiac hypertrophy and the transition to heart failure via NO-dependent mechanisms in mice. Fitoterapia 83, 60–66 (2012).
Mi, C. et al. Imperatorin suppresses proliferation and angiogenesis of human colon cancer cell by targeting HIF-1α via the mTOR/p70S6K/4E-BP1 and MAPK pathways. J. Ethnopharmacol. 203, 27–38 (2017).
Li, J., Summerlin, M., Nitiss, K. C., Nitiss, J. L. & Hanakahi, L. A. TDP1 is required for efficient non-homologous end joining in human cells. DNA Repair 60, 40–49 (2017).
Siriwardena, S. U. et al. Phosphorylation-inducing chimeric small molecules. J. Am. Chem. Soc. 142, 14052–14057 (2020).
Lai, A. C. & Crews, C. M. Induced protein degradation: an emerging drug discovery paradigm. Nat. Rev. Drug Discov. 16, 101–114 (2017).
Ali, S. R. et al. Existing cardiomyocytes generate cardiomyocytes at a low rate after birth in mice. Proc. Natl. Acad. Sci. USA 111, 8850–8855 (2014).
Fu, W. et al. Transient induction of actin cytoskeletal remodeling associated with dedifferentiation, proliferation, and redifferentiation stimulates cardiac regeneration. Acta Pharm. Sin. B 14, 2537–2553 (2024).
Pereira, A. H. M., Cardoso, A. C. & Franchini, K. G. Isolation, culture, and immunostaining of neonatal rat ventricular myocytes. STAR Protoc. 2, 100950 (2021).
de Silva, H. C., Lin, M. Z., Phillips, L., Martin, J. L. & Baxter, R. C. IGFBP-3 interacts with NONO and SFPQ in PARP-dependent DNA damage repair in triple-negative breast cancer. Cell Mol. Life Sci. 76, 2015–2030 (2019).
Acknowledgements
This study was supported by grants from the National Natural Science Foundation of China (82200307, 82070362), by a grant from the National Key Research and Development Program of China (2022YFC3601305), and by grants from Jilin Province Special Project of Medical and Health Talents (JLSWSRCZX2023-9, JLSCZD2019-003). Illustration items in the figures were created with BioRender.com.
Author information
Authors and Affiliations
Contributions
Wenbin Fu: conceptualization, funding acquisition, investigation, data curation, project administration, formal analysis, and writing—original draft. Lulu Hou: data curation, methodology, project administration, and formal analysis. Xinyu Yang: investigation and software. Jingyue Wang: investigation and methodology. Jingyu Jin: investigation and methodology. Qian Tong: conceptualization, funding acquisition, supervision, and writing—review & editing.
Corresponding authors
Ethics declarations
competing interests
The authors declare no competing interests.
Peer review
Peer review information
Communications Biology thanks Benu Brata Das and the other, anonymous, reviewer(s) for their contribution to the peer review of this work. Primary Handling Editors: Joanna Timmins and Joao Valente. A peer review file is available.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Fu, W., Hou, L., Yang, X. et al. Targeting TDP1 phosphorylation by the natural product imperatorin promotes DNA repair and cardiac regeneration. Commun Biol 9, 151 (2026). https://doi.org/10.1038/s42003-025-09429-2
Received:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s42003-025-09429-2