Abstract
The phenomenon of partial synchronization has attracted significant interest in the field of nonlinear dynamics, largely due to observations of related phenomena in real-world situations. In particular, the coexistence of synchronized and desynchronized phases, known as a chimera state, has been the subject of intense study. In this work, we experimentally demonstrate that networks of identical photonic spiking neurons based on coupled degenerate optical parametric oscillators can exhibit various chimera states in which, depending on their local synchronization and desynchronization, different kinds of spiking dynamics can develop in a self-organized manner. Even when only a static interaction is implemented, the spiking mode of photonic neurons can be spontaneously and adaptively changed between the Class-I and Class-II modes classified by A. L. Hodgkin. This spontaneous spiking-mode shift induces a significant change in the spiking frequency despite all neurons having the same natural spiking frequency, which encourages the generation of chimera states.
Similar content being viewed by others
Introduction
Chimera states in networks of coupled oscillators have been the subject of considerable interest in complex nonlinear systems1,2. Kuramoto et al. pointed out the counterintuitive fact that identical oscillators with homogeneous interactions can achieve an inhomogeneous state in which the phases of some of them are in synchronization while those of the others are out of synchronization1. This ‘mixed’ state of synchronized and desynchronized oscillators has been named the chimera state2. In fact, chimera-like behavior has been observed in the real world3, such as in a national power grid4 and in condensed matter5,6. Neuromorphic and biological systems also show chimera-like phenomena, including social systems7,8, neuronal bump states9, contractions of heart cells10, unihemispheric sleep11, and diseases related to the brain12,13,14. In response to these findings, the trend of theoretical studies on neural networks has been changing to the spiking neural network (SNN) models15,16 composed of the Fitzhugh-Nagumo17,18,19, Hindmarsh-Rose20,21,22,23, Morris-Lecar24, Wilson-Cowan25, and other models26,27.
It was initially thought that the occurrence of chimera states requires a very strict set of conditions to be imposed on the networked oscillators, such as in the Kuramoto model1. For example, it was believed that non-local interactions were required to induce chimera states. However, recent research on SNNs, e.g., with the Hindmarsh-Rose model21, has shown that they can occur under a broader range of conditions than believed prior1, e.g., local (nearest-neighbor)28,29 or global (all-to-all) interactions30. Furthermore, it has been proposed that adaptive interactions, namely, dynamically changing ones such as biological systems with Hebbian rules, stabilize chimera states in a self-organized manner18. In addition, the discovery of amplitude chimeras has led to a broadening of the definition of chimera states31, and a scheme to phenomenologically categorize chimeras has been established that can be widely applied to simple oscillatory and SNN systems as well as to experimental results32. However, while theoretical studies on chimera states in various systems, in particular, SNN systems15,16,17,18,19,20,21,22,23,24,25,26,27, have become widespread, so far, there has been little experimental research on chimera states in SNN-like systems mimicked by electronic circuits33, even though various oscillatory networks34,35,36,37 and chaotic optical systems38,39,40,41,42 have been experimentally studied. Experiments on SNNs would allow us to simulate real-world chimera-like phenomena7,8,9,10,11,12,13,14 and may lead to progress on, for example, understanding brain diseases.
In this paper, we report on various chimera states that were experimentally observed in a photonic SNN through control of the experimental parameters and propose an experimental platform to research chimera states in the SNN. Our neuromorphic neurons each consist of a pair of degenerate optical parametric oscillators (DOPOs). Pairs of DOPOs can exhibit two spiking modes from Hodgkin’s classification43, which allows our optical system to mimic neuronal dynamics in real biological systems. Furthermore, synchronization of the networked neurons causes a spontaneous spiking-mode shift accompanied by a large frequency change44. This unique property stabilizes the chimera states and causes their remarkable moving behavior.
Results
DOPO spiking neuron
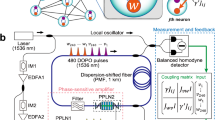
A photonic SNN can be represented as a network of paired DOPOs. Each single DOPO is a nonlinear oscillator generated by using a phase-sensitive amplifier (PSA) based on the parametric down-conversion process in a \({\chi }^{2}\) nonlinear material. Since degenerate parametric amplification is phase sensitive, a DOPO can oscillate only in two-phase states of 0 or π above the threshold pump power of the oscillator45,46. Optical coherence between these bistable states is essential to represent the membrane potential of the neuron. By introducing energy transfer between paired DOPOs, named \(v\)- and \(w\)-DOPOs, through antisymmetric coupling, as shown in Fig. 1a, the spiking dynamics can be implemented with optical amplitudes \({v}_{i}\) and \({w}_{i}\) of the ith pair of DOPOs that can be described as follows:
where \({v}_{i}\) and \({w}_{i}\) represent the optical amplitude of the in-phase component of each DOPO where the quadrature components become negligible due to the PSA above the oscillation threshold. \({v}_{i}\) and \({w}_{i}\) are renormalized by the saturated photon number after the oscillation. The magnitude of the pump amplitude is renormalized as \({p}_{i}=1\) to be the oscillation threshold value of the DOPO. The nonlinear terms, \({v}_{i}^{3}\) and \({w}_{i}^{3}\), result from the second-order nonlinearity and gain saturation of the PSA module45. The coupling strengths between the DOPOs represented by \({J}_{{vw}}\), \({J}_{{wv}}\), and \({J}_{{ij}}\). The intra- and inter-neuron interactions are implemented by using a measurement feedback (MFB) method based on an optical and electronic hybrid system44, which allows us to arbitrarily control them: namely, arbitrary network structures and weights of signed 8-bit integers can be implemented. Here, we introduce parallel and cross-type inter-neuron interactions with weights \(\alpha\) and \(\beta\), as shown in Fig. 1b, c. Without these \(\alpha =\beta =0\), by changing the operating parameters consisting of the \({P}_{i}=-1+{p}_{i}\) and \({\omega }_{0}=\sqrt{-{J}_{{vw}}{J}_{{wv}}}\), a single DOPO neuron exhibits the spiking modes of Class-I and Class-II neurons, as originally classified by A.L. Hodgkin43. One of the differences between these classes is that Class-I (-II) neurons show a (dis-) continuous change in spiking frequency over a wide (narrow) range as a \({P}_{i}/{{{{{{\rm{\omega }}}}}}}_{0}\) changes (see Supplementary Note 1). The methods section provides more explanations about the bifurcation models of our DOPO neurons.
a Single photonic neuron composed of coupled degenerate optical parametric oscillators (DOPOs). Coupling strengths between the DOPOs represented by \({J}_{{vw}}\) and \({J}_{{wv}}\). b Parallel-type coupling between i and jth DOPO neurons with weight \(\alpha\). The network structure of DOPO neurons is represented by \({J}_{{ij}}\). c Cross-type coupling with weight \(\beta\). d Schematic diagram of a photonic spiking neural network with 256 DOPO neurons. PPLN periodically poled lithium-niobate, PMF polarization-maintaining fiber, IM intensity modulator, EDFA erbium-doped-fiber amplifier, FPGA field-programmable gate array.
Spontaneous mode change
Our previous study with parallel-type couplings (\(\alpha =1\) and \(\beta =0\)) showed that the spiking modes of synchronized neurons spontaneously shift from Class-II to Class-I44. In the present study, we used cross-type coupling with \(\alpha =0\) and \(\beta =1\), which induces a spiking-mode shift from Class-I to Class-II after synchronization, in contrast to the parallel-type coupling case. This behavior can be understood from the fact that the order parameter of the synchronization effectively renormalizes the pump \({P}_{i}\) and basic frequency \({\omega }_{0}\) for parallel and cross-type interactions, respectively (see Method). Note that the renormalized parameter \(P/{\omega }_{0}\) effectively determines the class of the synchronized neurons, where a small (large) \(P/{\omega }_{0}\) induces Class-II (-I) type neurons. This function of the self-organized spiking-mode shift can assist various synchronous phenomena because the spiking frequencies can be adjusted.
Experimental setup
Our photonic spiking neural network consists of a 1-km fiber ring cavity, a PSA module, and an MFB system, as shown in Fig. 1d44,47. A DOPO pulse is generated by degenerate parametric amplification, which is provided by the PSA module based on \({\chi }_{2}\) nonlinearity of periodically poled lithium-niobate (PPLN) waveguides in the cavity48. An optical pulse at a wavelength of 1536 nm is converted into a pump pulse at a wavelength of 768 nm through second harmonic generation in one of the PPLN waveguides (PPLN1). By sending the converted pump pulse into the other PPLN waveguide (PPLN2) in the cavity and setting an optical band-pass filter that has a passband width of 13 GHz and center wavelength of 1536 nm, the DOPO pulse transmitting through the PSA module undergoes degenerate parametric amplification. When the pump amplitude increases above an oscillation threshold, the optical output of the DOPO bifurcates to a 0 or \({{{{{\rm{\pi }}}}}}\) phase state. To generate a large number of DOPO pulses in the 1-km fiber ring cavity, sequential pump pulses with a 60-ps width and 1-GHz repetition frequency are input to the PSA module. The round-trip time of the cavity is about 5 \({{{{{\rm{\mu }}}}}}{{{{{\rm{s}}}}}}\), and thus, 5000 DOPO pulses can be generated with time-domain multiplexing. Arbitrary network structures can be implemented with the MFB system, in which optical couplings between DOPO pulses are achieved by injecting optical feedback pulses into the cavity. In this system, the in-phase component of each DOPO output is measured by a balanced homodyne detector during each round trip. These measured signals are multiplied with a coupling matrix by a field-programmable gate array (FPGA) module. The calculated results of the FPGA module are then converted into optical phases and amplitudes of the optical feedback pulses by an optical modulator. By repeating this procedure in each round trip, DOPO pulses in different time slots can behave as a network. The 512 DOPO pulses can be networked with an arbitrary coupling matrix composed of signed 8-bit integers. Since a single DOPO neuron consists of a pair of DOPOs, this experimental setup can implement photonic SNNs with 256 DOPO neurons, where 56 neurons are used as references to check the experimental conditions.
Stationary chimera state with a dense network
We investigated the chimera states on a ring lattice structure composed of 100 neurons. The neurons within the ring were made to be identical by setting \({P}_{i}=P\) for all \(i\). First, we used a constant long-range interaction \({J}_{{ij}}=K\varGamma (r)\) with a distance \(r=|i-j|\) and \(\varGamma \left(r\right)=1\) for \(0\, < \,r \le {d}_{\max }\) and \(0\) otherwise, as illustrated in Fig. 2a. Here, we set the interaction length \({d}_{\max }=35\), pump amplitude \(P/{\omega }_{0}=2.4\), and coupling strength \(K/{\omega }_{0}=0.067\). To prepare the initial states of the DOPO neurons in the experiment, in the beginning, we increased the pump amplitudes under a randomized bias field without encoding the interactions between the DOPOs including \({J}_{{vw}}\) and \({J}_{{wv}}\). As a result, the uncoupled \(v\)- and \(w\)-DOPOs reached the saturation amplitude and randomly chose 0 or π phases with equal probability due to the phase-sensitive amplification. Thus, the initial state of the DOPO neuron, namely, a pair of \(v\)- and \(w\)-DOPOs, were randomly distributed with four phase degrees of freedom (0, 0), (0, π), (π, 0) and (π, π) with similar optical amplitudes. Thus, we can say that the initial state was based on limited random phase distributions. After this initialization, the bias field was removed, and each pair of DOPOs was coupled as a neuron by introducing the intra-neuron couplings \({J}_{{vw}}\) and \({J}_{{wv}}\). At the same time, inter-neuron couplings \({J}_{{ij}}\) were also introduced. Figure 2b shows the dynamics of the phases of the neurons after the initialization where \({\theta }_{i}={{{\tan }}}^{-1}({w}_{i}/{v}_{i})\), and Fig. 2d shows a snapshot at 20.48 ms in Fig. 2b. These results indicate the coexistence of two kinds of region, synchronized and desynchronized. We experimentally found a double-headed chimera state, which is analogous to the results obtained from numerical calculations based on the Fitzhugh-Nagumo model17. Note that changing the parameters, \({d}_{\max }\), K, and P, complexly affect the properties of the chimera, such as the number and size of the heads, which will be discussed elsewhere. Here we focus on the characteristic meandering movement such as that of the “snake”, which was not observed in the spatial-stable chimeras in ref. 17. This meandering movement shows similar features with alternating chimera19 (see Supplementary Note 3).
a Network structure of each neuron on a ring lattice structure composed of 100 neurons. Blue lines represent constant long-range interactions \({J}_{{ij}}=K\varGamma (r)\) with \(r=\left|i-j\right|\), where \(\varGamma \left(r\right)=1\) for \(0 < r\le {d}_{\max }=35\) and \(0\) otherwise. b Time evolutions of rotation phases of each neuron \({\theta }_{i}={{{\tan }}}^{-1}({w}_{i}/{v}_{i})\). c Local curvature D. d Phase distribution at t = 20.48 ms. e \({g}_{0}\left(t\right)\) and \({h}_{0}\) indices. Pump power and coupling strength are set to be \(P/{\omega }_{0}=2.4\) and \(K/{\omega }_{0}=0.067\), respectively.
To clarify the properties of this chimera, we calculated the local curvature measure \(D\) introduced in ref. 32, which is the second derivative of the phase of a neuron and its nearest neighbors: \(D=f\left(x-\triangle x\right)-2f\left(x\right)+f(x+\triangle x)\) with \(f(x)={\theta }_{i}\) and \(\Delta x=1\). The local curvature presented in Fig. 2c clearly distinguishes the regions: one is synchronized with \(D\sim 0\) and the other is desynchronized with \(D\ne 0\). In addition, we calculated the probability density \(g\) as a function of \(\left|D\right|\), where the density of the synchronized components, defined as \({g}_{0}\left(t\right)={\int }_{0}^{\delta }g(t,\left|D\right|)d\left|D\right|\), represents the relative size of the synchronized regime at time \(t\)32\(.\) We set the threshold to \(\delta =0.1{D}_{\max }\), where \({D}_{\max }\) is the maximum value of \({|D|}\). The measure \({g}_{0}\left(t\right)\) can identify the chimera state when \(0 < {g}_{0}\left(t\right) < 1\), where \({g}_{0}\left(t\right)=0\) and \(1\) represent asynchronous and synchronous states, respectively. In addition, the time dependence of \({g}_{0}\left(t\right)\) characterizes the spatial fluctuations of the chimera states32. We also analyze density function h of \(|{\rho }_{{ij}}|\) by calculating quantity \({\rho }_{{ij}}=\frac{\langle {({Z}_{i}-{\mu }_{i})}^{* }({Z}_{j}-{\mu }_{j})\rangle }{{\sigma }_{i}{\sigma }_{j}}\), where \({{\langle }} \cdot {{\rangle }}\) is the time average, \({Z}_{i}={{\exp }}i{\theta }_{i}\), \({\mu }_{i}\) and \({\sigma }_{i}\) are means and standard deviations of \({Z}_{i}\), respectively32. Here, the density of the temporary stable component \({h}_{0}\), defined as \(\sqrt{{\int }_{\gamma }^{1}h(|\rho |){d|}\rho |}\) with \({{{{{\rm{\gamma }}}}}}=0.9\), characterizes the temporal fluctuations of the chimera states. By using these indices \({g}_{0}\left(t\right)\) and \({h}_{0}\), the chimera state can be well categorized as follows32. Figure 2e shows \({g}_{0}\left(t\right)\) to be almost constant except for some experimental fluctuations and shows \({h}_{0}\) to be nearly equal to 0, suggesting that the observed chimera state is categorized as a moving-stationary chimera state. Here, “stationary” means that the ratio of the spatial regions of chimera states stays almost constant, and “moving” comes from the meandering movement of the chimera.
Spiking mode change by synchronization
Here, we discuss the cause of the chimera state meandering. Note that this meandering movement does not originate from noise in the experiments because the numerical simulations without noise reproduce this movement (see Supplementary Note 4). Rather, this behavior originates from the spontaneous change of the spiking modes at the boundary between the desynchronized and synchronized regions. Figure 3a, b shows trajectories of specific neurons in the \(v\)–\(w\) plane and the dynamics of the neuron phase \({\theta }_{i}\), where the left, middle, and right panels respectively show these quantities for neurons #40–45 in the desynchronized region, #65–70 in the synchronized region and #30–35 at the boundary between the two types of regions. Interestingly, the identical neurons clearly show different spiking modes, namely, the stepwise \(\theta\) of Class-I (desynchronized region) and the linear \(\theta\) of Class-II (synchronized region). We found that changes in class occasionally occur at the boundary. The synchronization causes a spontaneous spiking-mode shift from Class-I to Class-II, as mentioned above (see Method). These two classes have clearly different spiking frequencies, as shown in Fig. 3c. The main characteristic of the Class-I spiking mode is the continuous change in spiking frequency from zero. This results in meandering movements because a Class-I neuron at the boundary between synchronized and desynchronized regions can adjust its spike timing (see Fig. 3b right panel) and then chaotically go back and forth between regions depending on the randomness coming from the initial state. This meandering movement occurs even without noise (see Supplementary Note 4).
a Limit cycle in the v–w plane for sampled neurons in Fig. 2b from 12.8 to 16.8 ms. Each color represents the time evolution of a different neuron. (i) neurons #40–45 in the desynchronized (Class-I) region, (ii) #65–70 in the synchronized (Class-II) region, and (iii) #30–35 at the boundary between regions of two classes. b Time evolution of rotation phase. c Mean spiking frequencies from 14 to 16 ms for neurons #40–50 (desynchronized) and #60–70 (synchronized), corresponding to Class-I and Class-II, respectively.
It should be noted that the present system has inversion symmetry between \(v\) and \(w\), meaning that the time scales of two-dimensional variables are intrinsically equivalent to each other. This fact suggests that the slow-fast multiple-time scales, which are usually included in the SNN systems, such as fast-electronic and slow-chemical synaptic dynamics, are not a prerequisite for chimeras to appear in the SNN15. However, in the present system, the change in the spiking mode spontaneously causes a large time-scale difference between synchronized Class-II neurons with high spiking frequencies and desynchronized Class-I neurons with very low frequencies, which may stabilize the chimera states. A similar self-organized mechanism for stabilizing chimeras has been discussed in ref. 18, which considers adaptive interactions, namely, dynamically changing interaction strengths, while the present system only has static interactions. Note that the present system intrinsically contains adaptive controllability of spiking frequencies via synchronization44. In addition to causing the frequency difference, the spiking-mode shift changes the amplitudes in each region, which may also stabilize the chimera states, as in the case of the amplitude chimera found in ref. 31 (see Supplementary Note 7).
Turbulent chimera state with non-local interaction
Using the same ring structure, we were furthermore able to find a different category of chimera state. Here, we used exponentially decreasing local interactions, as discussed in the Kuramoto model1. As shown in Fig. 4a, we set the interaction as \(\varGamma \left(r\right)={2}^{{d}_{\max }-r-1}\) with \({d}_{\max }=6\) for \(0{ < r < d}_{\max }\) and \(\varGamma \left(r\right)=0\) otherwise. The interactions were set to be integer values because of the experimental limitation where \({J}_{{ij}}\) has to be a signed 8-bit integer.
a Network structure of each neuron on a ring lattice structure composed of 100 neurons. The coupling strength between neurons is exponentially decreased depending on a distance \(r=\left|i-j\right|\) as \(\varGamma \left(r\right)={2}^{{d}_{\max }-r-1}\) with \(0{ < r < d}_{\max }=6\) and \(\varGamma \left(r\right)=0\) otherwise. b, c \({g}_{0}\left(t\right)\) and \({h}_{0}\) indices, d, e rotation phase of DOPO neurons, f, g local curvature for weak (\(P/{\omega }_{0}=0.5\)) and strong (\(P/{\omega }_{0}=2.6\)) pump amplitudes with \(K/{\omega }_{0}=0.008\) and \(0.017\), respectively.
Figure 4b, c shows \({g}_{0}(t)\) and \({h}_{0}\) indices, d, e shows the dynamics of the phases of the neurons \(\theta (t)\), and f, g shows the local curvatures \(D(t)\) for weak \(P/{\omega }_{0}=0.5\) (left) and strong \(P/{{{{{{\rm{\omega }}}}}}}_{0}=2.6\) (right panels) pump amplitudes. Here, we set \(K/{\omega }_{0}=0.008\) and \(0.017\) for weak and strong pump cases, respectively. From Fig. 4d–g, we can see that the regions of desynchronization are not stable for a long time and soon fall back into synchronization. Despite this, these regions tend to reappear out of the synchronized regions. As a result, the local curvature graph shows bubble-like patterns corresponding to neurons falling in and out of synchronization. The category of these chimera states differs from those of the aforementioned ones with constant interactions, where a stationary chimera was observed. As shown in Fig. 4b, c, \({g}_{0}\left(t\right)\) varies irregularly when \({h}_{0} \sim 0\), indicating that the category is a moving-turbulent chimera. Note that the chimera state with a strong pump (right panels) has a characteristic feature and consists of the synchronized firing region and desynchronized non-firing region. Namely, we again found a spontaneous mode shift and coexistence of different spiking dynamics, firing and non-firing, which is analogous to “chimera death”31. This non-firing region corresponds to four stable fixed points resulting from pitchfork bifurcations of v- and w-DOPOs45. As shown in Supplementary Note 5, we confirmed that the numerical simulations reproduce the chimera states discussed above, and we also found transient chimera states and inhomogeneous steady states related to these fixed points in the experiments49. Finally, we should note that antiphase correlation develops in a part of the\(D\ne 0\) regions (see Supplementary Note 6), suggesting that the observed chimera consists of a mixture of a few types of synchronized and desynchronized regions. A similar characteristic chimera state has been reported in refs. 24,50.
Conclusions
We demonstrated that an SNN composed of DOPOs could exhibit various chimera states by arranging interactions on a one-dimensional ring lattice. A constant non-local interaction induces a moving-stationary chimera state, which is multi-headed and meandering. An exponentially decaying interaction induces moving-turbulent chimera states, where the desynchronized regions are only stable for short durations, and a bubble-like creation-and-annihilation pattern can be observed. The current DOPO neuron has two degrees of freedom, \(v\) and \(w\), and these variables have inversion symmetry. This means that the fast-slow multiple dynamics in standard SNNs do not exist in the present system and further that the multiple dynamics are not a prerequisite condition for chimeras. Instead of intrinsic multiple-time-scale dynamics, however, the present system exhibits a spontaneous shift in spiking modes from Class-I to Class-II of Hodgkin’s classification. As a result, high-frequency synchronized and low-frequency desynchronized regions appear. This self-organized change in time scale may stabilize the chimera states. Note that the observed chimeras with a high-frequency synchronized region are analogous to neuronal signals observed in Parkinsonian patients with limb tremor12, while most of the chimeras observed in the previous studies show low-frequency synchronized regions1,2,3,15,16,17,20. In addition, characteristic meandering movement in the observed moving stationary chimera is induced by this spontaneous change of the spiking modes. We found various chimera states by changing the interaction and pump parameters. However, it was difficult to precisely control the pump parameter in the experiments; thus, more systematic studies, e.g., making a chimera phase diagram (see Supplementary Note 8), will be required in the future. We should finally note that the experimental study of chimeras in the SNN is as yet not well established, whereas our system provides an ideal platform for experimental research since it offers a very high degree of control involving spiking modes, time scales, arbitrary interaction geometry, and so on. The controllability of our system allows us to use it to perform computations44,51, and then to investigate the effects of chimeras on the computation in the future. Furthermore, the controllability of the interactions provides us with a platform for researching other interesting synchrony phenomena, such as for observing solitary states52.
Methods
Oscillation description of dynamics of neurons
Equations (1) and (2) in the main text can be rewritten by using \({v}_{i}+i{w}_{i}=\sqrt{{R}_{i}}{{\exp }}i{\theta }_{i}\) as follows:
where \({\omega }_{0}=\sqrt{-{J}_{{vw}}{J}_{{wv}}}\) is the basic spiking frequency of a neuron in the case of pump amplitude \({P}_{i}=-1+{p}_{i}=0\), \({\epsilon }_{ij}={R}_{j}/{R}_{i}\) and \(\phi ={{{\tan }}}^{-1}\frac{\beta }{\alpha }\) with parallel and cross-type coupling strengths \(\alpha\) and \(\beta\). Note that the basic frequency \({\omega }_{0}\) determines the physical time scale, and in the experiments, it was on the order of 1–10 KHz. Thus, the time scale of the spike signals in our system is about a sub-microsecond. Equation (3) shows an analogy to the Kuramoto model (and also Stuart Landau model) with exception of the second term on the right-hand side. However, the connection between (3) and (4) induced by the second term causes the characteristic behavior of chimera states discussed in the main text. In the present study, we chose\(\phi =\frac{{{{{{\rm{\pi }}}}}}}{2}\) (\(\alpha =1\) and \(\beta =0\)) because the Kuramoto model of the ring structure lattice shows chimera states at around \(\phi \sim \frac{{{{{{\rm{\pi }}}}}}}{2}\)1,3\(.\)
Bifurcation models and change in spiking frequencies without interneuron couplings
Without any interneuron couplings (\(\alpha = 0\) and \(\beta =0\)), the change in spiking frequencies is described by the following equation44
In the limit \(P/{\omega }_{0}\to {0}_{+}\), the spiking frequency changes discontinuously from zero to \({\omega }_{0}\), which characterizes the Class-II spiking mode. This Class-II mode results from the supercritical Andronov–Hopf bifurcation44, corresponding to a monostable resonator53. This can be easily understood by rewriting Eq. (4) without a coupling term by using \({r}_{i}={\sqrt{R}}_{i}\), which leads to the normal form of the supercritical Andronov-Hopf bifurcation: \(\frac{{{dr}}_{i}}{{dt}}=P{r}_{i}-{r}_{i}^{3}\). On the other hand, at \(P=\sqrt{8}{{{{{{\rm{\omega }}}}}}}_{0}\), the spiking frequency vanishes continuously, which is a characteristic of the Class-I spiking mode. This mode originates from the saddle node in the limit-cycle bifurcation44, corresponding to a monostable integrator53. The normal form of this bifurcation can be derived as follows from the expansion of Eq. (3) around, e.g., \(4{{{{{\rm{\theta }}}}}}\sim \frac{{{{{{\rm{\pi }}}}}}}{2}\), where a saddle point appears: \(\frac{d\theta }{{dt}}={\omega }_{0}-\frac{P}{\sqrt{8}}+\sqrt{8}P{\theta }^{2}\) with \(0 < \frac{{{{{{\rm{P}}}}}}}{{{{{{{\rm{\omega }}}}}}}_{0}} < \sqrt{8}\). Note that we used an averaged value of \(R\sim \frac{2P}{\pi }{\int }_{-\pi }^{\pi }\frac{d\theta }{3+{{\cos }}4\theta }=\sqrt{2}P\). Crossover between these two classes occurs between \(P/{{{{{{\rm{\omega }}}}}}}_{0}\) = 0 and \(\sqrt{8}\). The bifurcation physics of the DOPO neurons has been detailed in the Supplementary Materials of ref. 44.
Self-organized spiking-mode shifts
As described in the prior paper of Inagaki et al.44, the order (and hence the synchronization) of the parallel coupled neurons with \(\phi =0\) results in a renormalization of the effective pump power. This can be understood from Eq. (3) by considering, for simplicity, a global coupling \({J}_{{ij}}=K\), where the term \(2{R}_{i}K{\sum }_{{{{{{\rm{j}}}}}}}{\epsilon }_{{ij}}{{\cos }}({\theta }_{i}-{\theta }_{j})\) can be rewritten as \(2{R}_{i}{rNK}\) by using the order parameter \(r=|{\sum }_{j}{{\exp }}(-i{\theta }_{j})|/N\) with an assumption of \({\epsilon }_{{ij}}\sim 1\), and then the term \(2{P}_{i}{R}_{{{{{{\rm{i}}}}}}}\) is renormalized as \(2\left({P}_{i}+{rNK}\right){R}_{i}\). Note that synchronized neurons have similar amplitudes, and thus, the assumption \({\epsilon }_{{ij}}\sim 1\) is reasonable. This renormalization results in an increase in the effective pump \({P}_{{{{{\rm{eff}}}}}}=P+{rNK}\) and indirectly decreases the frequency of the oscillators, as described by Eq. (5) in the Methods (see also ref. 44). In the cross-coupling case, which is used in this experiment, a similar effect occurs. In this case, the effective pump power stays constant, and instead, the effective basic frequency \({{{{{{\rm{\omega }}}}}}}_{0}\) increases in proportion to the order of the neurons. Namely, when \(\phi =\frac{{{{{{\rm{\pi }}}}}}}{2}\), the order parameter can be found in Eq. (4), and the renormalization of \({\omega }_{0}+{rNK}\) can be found from this equation. As a result, we can see that the spiking frequency increases in proportion to the synchronization of the neurons. The renormalization proportional to the order parameter \(r\) indicates that this increase/decrease in spiking frequency is spontaneously introduced in a self-organized manner. Supplementary Note 2 provides experimental evidence of the above discussion.
Data availability
The data that support the plots within this paper and other findings of this study are available from the corresponding authors upon reasonable request.
Code availability
The modeling is described in the Methods and Supplementary Information and the code is available from the corresponding authors upon reasonable request.
References
Kuramoto, Y. & Battogtokh, D. Coexistence of coherence and incoherence in nonlocally coupled phase oscillators. Nonlinear Phenom. Complex Syst. 5, 380–385 (2002).
Abrams, D. M. & Strogatz, S. H. Chimera states for coupled oscillators. Phys. Rev. Lett. 93, 174102 (2004).
Panaggio, M. J. & Abrams, D. M. Chimera states: coexistence of coherence and incoherence in networks of coupled oscillators. Nonlinearity 28, R67 (2015).
Motter, A. E., Myers, S. A., Anghel, M. & Nishikawa, T. Spontaneous synchrony in power-grid networks. Nat. Phys. 9, 191–197 (2013).
Xu, Hong-Ya, Wang, Guang-Lei, Huang, L. & Lai, Ying-Cheng Chaos in dirac electron optics: emergence of a relativistic quantum chimera. Phys. Rev. Lett. 120, 124101 (2018).
Sakurai, A., Bastidas, V. M., Munro, W. J. & Nemoto, K. Chimera time-crystalline order in quantum spin networks. Phys. Rev. Lett. 126, 120606 (2021).
Gonzalez-Avella, J. C., Cosenza, M. G. & Miguel, M. S. Localized coherence in two interacting populations of social agents. Physica A 399, 24–30 (2014).
Lugo, H., González-Avella, J. C. & Miguel, M. S. Chimera and anticoordination states in learning dynamics. Front. Appl. Math. Stat. 5, 16 (2019).
Laing, C. R. & Chow, C. C. Stationary bumps in networks of spiking neurons. Neural Comput. 13, 1473 (2001).
Cherry, E. M. & Fenton, F. H. Visualization of spiral and scroll waves in simulated and experimental cardiac tissue. New J. Phys. 10, 125016 (2008).
Rattenborg, N. C., Amlaner, C. J. & Lima, S. L. Behavioral, neurophysiological and evolutionary perspectives on unihemispheric sleep. Neurosci. Biobehav. Rev. 24, 817–842 (2000).
Levy, R., Hutchison, W. D., Lozano, A. M. & Dostrovsky, J. O. High-frequency synchronization of neuronal activity in the subthalamic nucleus of Parkinsonian patients with limb tremor. J. Neurosci. 20, 7766–7775 (2000).
Ayala, G. F., Dichter, M., Gumnit, R. J., Matsumoto, H. & Spencer, W. A. Genesis of epileptic interictal spikes. New knowledge of cortical feedback systems suggests a neurophysiological explanation of brief paroxysms. Brain Res. 52, 1–17 (1973).
Tognoli, E. & Kelso, J. S. The metastable brain. Neuron 81, 35–48 (2014).
Majhi, S., Bera, B. K., Ghosh, D. & Perc, M. Chimera states in neuronal networks: a review. Phys. Life Rev. 28, 100–121 (2019).
Wang, Z. & Liu, Z. A brief review of chimera state in empirical brain networks. Front. Physiol. 11, 724 (2020).
Omelchenko, I., Omel’chenko, O. E., Hövel, P. & Schöll, E. When nonlocal coupling between oscillators becomes stronger: patched synchrony or multichimera states. Phys. Rev. Lett. 110, 224101 (2013).
Huo, S., Tian, C., Kang, L. & Liu, Z. Chimera states of neuron networks with adaptive coupling. Nonlinear Dyn. 96, 75–86 (2019).
Semenova, N., Zakharova, A., Anishchenko, V. & Schöll, E. Coherence-resonance chimeras in a network of excitable elements. Phys. Rev. Lett. 117, 014102 (2016).
Bera, B. K., Ghosh, D. & Lakshmanan, M. Chimera states in bursting neurons. Phys. Rev. E 93, 012205 (2016).
Santos, M. S. et al. Chimera-like states in a neuronal network model of the cat brain. Chaos Solitons Fract. 101, 86–91 (2017).
Mitchell, H. M., Sheridan, P., Matthew, J. & Danforth, M. Chimera states and seizures in a mouse neuronal model. Int. J. Bifurc. Chaos 30, 2050256 (2020).
Hizanidis, J., Kanas, V. G., Bezerianos, A. & Bountis, T. Chimera states in networks of nonlocally coupled Hindmarsh–Rose neuron models. Int. J. Bifurc. Chaos 24, 1450030 (2014).
Calim, A., Torres, J. J., Ozer, M. & Uzuntarla, M. Chimera states in hybrid coupled neuron populations. Neural Netw. 126, 108–117 (2020).
Bansal, K. et al. Cognitive chimera states in human brain networks. Sci. Adv. 5, eaau8535 (2019).
Glaze, T. A., Lewis, S. & Bahar, S. Chimera states in a Hodgkin-Huxley model of thermally sensitive neurons. Chaos 26, 083119 (2016).
Schmidt, A., Kasimatis, T., Hizanidis, J., Provata, A. & Hövel, P. Chimera patterns in two-dimensional networks of coupled neurons. Phys. Rev. E 95, 032224 (2017).
Laing, C. R. Chimeras in networks with purely local coupling. Phys. Rev. E 92, 050904 (2015).
Hizanidis, J., Lazarides, N. & Tsironis, G. P. Robust chimera states in SQUID metamaterials with local interactions. Phys. Rev. E 94, 032219 (2016).
Yeldesbay, A., Pikovsky, A. & Rosenblum, M. Chimeralike states in an ensemble of globally coupled oscillators. Phys. Rev. Lett. 112, 144103 (2014).
Zakharova, A., Kapeller, M. & Schöll, E. Chimera death: symmetry breaking in dynamical networks. Phys. Rev. Lett. 112, 154101 (2014).
Kemeth, F. P., Haugland, S. W., Schmidt, L., Kevrekidis, I. G. & Krischer, K. A classification scheme for chimera states. Chaos 26, 094815 (2016).
Gambuzza, L. V. et al. Experimental investigation of chimera states with quiescent and synchronous domains in coupled electronic oscillators. Phys. Rev. E 90, 032905 (2014).
Martens, E. A., Thutupalli, S., Fourrire, A. & Hallatschek, O. Chimera states in mechanical oscillator networks. Proc. Natl. Acad. Sci. USA 110, 10563–10567 (2013).
Tinsley, M. R., Nkomo, S. & Showalter, K. Chimera and phase-cluster states in populations of coupled chemical oscillators. Nat. Phys. 8, 662–665 (2012).
Schmidt, L., Schönleber, K., Krischer, K. & García-Morales, V. Coexistence of synchrony and incoherence in oscillatory media under nonlinear global coupling. Chaos 24, 013102 (2014).
Larger, L., Penkovsky, B. & Maistrenko, Y. Laser chimeras as a paradigm for multistable patterns in complex systems. Nat. Commun. 6, 7752 (2015).
Shena, J., Hizanidis, J., Kovanis, V. & Tsironis, G. P. Turbulent chimeras in large semiconductor laser arrays. Sci. Rep. 7, 42116 (2017).
Clerc, M. G., Coulibaly, S., Ferré, M. A. & Tlidi, M. Two-dimensional optical chimera states in an array of coupled waveguide resonators. Chaos 30, 043107 (2020).
Böhm, F., Zakharova, A., Schöll, E. & Lüdge, K. Amplitude-phase coupling drives chimera states in globally coupled laser networks. Phys. Rev. E 91, 040901 (2015).
Hart, J. D., Bansal, K., Murphy, T. E. & Roy, R. Experimental observation of chimera and cluster states in a minimal globally coupled network. Chaos 26, 094801 (2016).
Shena, J., Hizanidis, J., Hövel, P. & Tsironis, G. P. Multiclustered chimeras in large semiconductor laser arrays with nonlocal interactions. Phys. Rev. E 96, 032215 (2017).
Hodgkin, A. L. The local electric changes associated with repetitive action in a nonmedullated axon. J. Physiol. 107, 165–181 (1948).
Inagaki, T. et al. Collective and synchronous dynamics of photonic spiking neurons. Nat. Commun. 12, 2325 (2021).
Wang, Z., Marandi, A., Wen, K., Byer, R. L. & Yamamoto, Y. Coherent Ising machine based on degenerate optical parametric oscillators. Phys. Rev. A 88, 063853 (2013).
Marandi, A., Wang, Z., Takata, K., Byer, R. L. & Yamamoto, Y. Network of time-multiplexed optical parametric oscillators as a coherent Ising machine. Nat. Photon. 8, 937–942 (2014).
Inagaki, T. et al. A coherent Ising machine for 2000-node optimization problems. Science 354, 603–606 (2016).
Umeki, T., Tadanaga, O., Takada, A. & Asobe, M. Phase sensitive degenerate parametric amplification using directly-bonded PPLN ridge waveguides. Opt. Express 19, 6326–6332 (2011).
Bar-Eli, K. On the stability of coupled chemical oscillators. Physica D 14, 242–252 (1985).
Omelchenko, I., Maistrenko, Y., Hövel, P. & Schöll, E. Loss of coherence in dynamical networks: spatial chaos and chimera states. Phys. Rev. Lett. 106, 234102 (2011).
Chalkiadakis, D. & Hizanidis, J. Dynamical properties of neuromorphic Josephson junctions. Phys. Rev. E 106, 044206 (2022).
Maistrenko, Y., Penkovsky, B. & Rosenblum, M. Solitary state at the edge of synchrony in ensembles with attractive and repulsive interactions. Phys. Rev. E 89, 060901 (2014).
Izhikevich, E. M. Dynamical Systems in Neuroscience. (MIT Press, Cambridge, MA, 2007).
Acknowledgements
This research was partially supported by the Impulsing Paradigm Change through Disruptive Technologies (ImPACT) Program of the Council of Science, Technology, and Innovation (Cabinet Office, Government of Japan). T.L. is partially supported by JSPS KAKENHI Grant Number JP22K03545. K.A. is partially supported by the Japan Agency for Medical Research and Development (AMED) under Grant Number JP22dm0307009, the Japan Science and Technology Agency Moonshot R&D Grant Number JPMJMS2021, and JSPS KAKENHI Grant Number JP20H05921. The authors thank Hiroyuki Tamura for his support during this research.
Author information
Authors and Affiliations
Contributions
K.I., T.M., and T. Inagaki proposed the project. T.M. and T. Inagaki performed the experiments. K.I., T.M., and Y.Y. performed the data analysis and numerical simulations. T.L. and K.A. supported the theoretical analysis of computational neuroscience. T. Inagaki, K.I., T. Ikuta, T.H., and H.T. contributed to building the DOPO network system. K.E., T.U., and R.K. contributed to building the PPLN modules. T.M., K.I., T. Inagaki, Y.Y., T.L., K.A., and H.T. wrote the paper with input from all authors.
Corresponding authors
Ethics declarations
Competing interests
K.I., H.T., T.H., T. Inagaki, and T. Ikuta are inventors on patent JP6996457 awarded in December 2021 to NTT that covers a scheme of coupled optical oscillators for SNNs. T.U. and K.E. are inventors on patent JP5856083 awarded in February 2016 to NTT that covers phase-sensitive amplifiers based on periodically poled lithium niobate waveguides. The remaining authors declare no competing interests.
Peer review
Peer review information
Communications Physics thanks Bogdan Penkovsky and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Makinwa, T., Inaba, K., Inagaki, T. et al. Experimental observation of chimera states in spiking neural networks based on degenerate optical parametric oscillators. Commun Phys 6, 121 (2023). https://doi.org/10.1038/s42005-023-01240-x
Received:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s42005-023-01240-x