Abstract
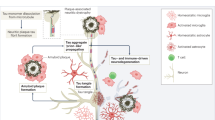
The reconceptualization of Alzheimer’s disease (AD) as a clinical and biological construct has facilitated the development of biomarker-guided, pathway-based targeted therapies, many of which have reached late-stage development with the near-term potential to enter global clinical practice. These medical advances mark an unprecedented paradigm shift and requires an optimized global framework for clinical care pathways for AD. In this Perspective, we describe the blueprint for transitioning from the current, clinical symptom-focused and inherently late-stage diagnosis and management of AD to the next-generation pathway that incorporates biomarker-guided and digitally facilitated decision-making algorithms for risk stratification, early detection, timely diagnosis, and preventative or therapeutic interventions. We address critical and high-priority challenges, propose evidence-based strategic solutions, and emphasize that the perspectives of affected individuals and care partners need to be considered and integrated.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$32.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 digital issues and online access to articles
$119.00 per year
only $9.92 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to the full article PDF.
USD 39.95
Prices may be subject to local taxes which are calculated during checkout

Similar content being viewed by others
References
Alzheimer’s Association. 2018 Alzheimer’s disease facts and figures. Alzheimers Dement. 14, 367–429 (2018).
GBD 2019 Dementia Forecasting Collaborators Estimation of the global prevalence of dementia in 2019 and forecasted prevalence in 2050: an analysis for the Global Burden of Disease Study 2019. Lancet Public Health 7, e105–e125 (2022).
Scheltens, P. et al. Alzheimer’s disease. Lancet 397, 1577–1590 (2021).
Dubois, B. et al. Clinical diagnosis of Alzheimer’s disease: recommendations of the International Working Group. Lancet Neurol. 20, 484–496 (2021).
Jack, C. R. Jr. et al. NIA-AA Research Framework: toward a biological definition of Alzheimer’s disease. Alzheimers Dement. 14, 535–562 (2018).
Aisen, P. S., Vellas, B. & Hampel, H. Moving towards early clinical trials for amyloid-targeted therapy in Alzheimer’s disease. Nat. Rev. Drug Discov. 12, 324 (2013).
Hampel, H. et al. The amyloid-β pathway in Alzheimer’s disease. Mol. Psychiatry 26, 5481–5503 (2021).
Cummings, J. The National Institute on Aging-Alzheimer’s Association Framework on Alzheimer’s disease: application to clinical trials. Alzheimers Dement. 15, 172–178 (2019).
Hampel, H. et al. Developing the ATX(N) classification for use across the Alzheimer disease continuum. Nat. Rev. Neurology 17, 580–589 (2021).
US Food and Drug Administration. FDA’s decision to approve new treatment for Alzheimer’s Disease (2021).
Cummings, J., Lee, G., Zhong, K., Fonseca, J. & Taghva, K. Alzheimer’s disease drug development pipeline: 2021. Alzheimer’s Dement. 7, e12179 (2021).
Biogen. Aduhelm (aducanumab-avwa) [package insert]. US Food and Drug Administration. https://www.accessdata.fda.gov/drugsatfda_docs/label/2021/761178s000lbl.pdf (2021).
Alzheimer’s Association. 2021 Alzheimer’s disease facts and figures. Alzheimers Dement. 17, 327–406 (2021).
Majoka, M. A. & Schimming, C. Effect of social determinants of health on cognition and risk of Alzheimer disease and related dementias. Clin. Ther. 43, 922–929 (2021).
Ferri, C. P. & Jacob, K. S. Dementia in low-income and middle-income countries: different realities mandate tailored solutions. PLoS Med. 14, e1002271 (2017).
Hampel, H. & Lista, S. Dementia: the rising global tide of cognitive impairment. Nat. Rev. Neurol. 12, 131–132 (2016).
Ngandu, T. et al. A 2 year multidomain intervention of diet, exercise, cognitive training, and vascular risk monitoring versus control to prevent cognitive decline in at-risk elderly people (FINGER): a randomised controlled trial. Lancet 385, 2255–2263 (2015).
Kivipelto, M. et al. World-Wide FINGERS Network: a global approach to risk reduction and prevention of dementia. Alzheimers Dement. 16, 1078–1094 (2020).
Alzheimer’s Association. 2019 Alzheimer’s disease facts and figures. Alzheimers Dement. 15, 321–387 (2019).
Dubois, B., Padovani, A., Scheltens, P., Rossi, A. & Dell’Agnello, G. Timely diagnosis for Alzheimer’s disease: a literature review on benefits and challenges. J. Alzheimers Dis. 49, 617–631 (2016).
Eichler, T. et al. Rates of formal diagnosis in people screened positive for dementia in primary care: results of the DelpHi-Trial. J. Alzheimers Dis. 42, 451–458 (2014).
Lang, L. et al. Prevalence and determinants of undetected dementia in the community: a systematic literature review and a meta-analysis. BMJ Open 7, e011146 (2017).
Toepper, M. Dissociating normal aging from Alzheimer’s disease: a view from cognitive neuroscience. J. Alzheimers Dis. 57, 331–352 (2017).
Dubois, B. et al. Advancing research diagnostic criteria for Alzheimer’s disease: the IWG-2 criteria. Lancet Neurol. 13, 614–629 (2014).
Mandelblatt, J. S. et al. Cognitive effects of cancer and its treatments at the intersection of aging: what do we know; what do we need to know? Semin. Oncol. 40, 709–725 (2013).
Spudich, S. & Nath, A. Nervous system consequences of COVID-19. Science 375, 267–269 (2022).
Porsteinsson, A. P., Isaacson, R. S., Knox, S., Sabbagh, M. N. & Rubino, I. Diagnosis of early Alzheimer’s disease: clinical practice in 2021. J. Prev. Alzheimers Dis. 8, 371–386 (2021).
Ng, K. P. et al. The influence of language and culture on cognitive assessment tools in the diagnosis of early cognitive impairment and dementia. Expert Rev. Neurother. 18, 859–869 (2018).
Sabbagh, M. N. et al. Early detection of mild cognitive impairment in primary care. J. Prev. Alzheimers Dis. 7, 165–170 (2020).
Rhodius-Meester, H. F. M. et al. cCOG: a web-based cognitive test tool for detecting neurodegenerative disorders. Alzheimer’s Dement. 12, e12083 (2020).
Mattke, S., Cho, S. K., Bittner, T., Hlávka, J. & Hanson, M. Blood-based biomarkers for Alzheimer’s pathology and the diagnostic process for a disease-modifying treatment: projecting the impact on the cost and wait times. Alzheimer’s Dement. 12, e12081 (2020).
Hampel, H. et al. Blood-based biomarkers for Alzheimer disease: mapping the road to the clinic. Nat. Rev. Neurol. 14, 639–652 (2018).
Teunissen, C. E. et al. Blood-based biomarkers for Alzheimer’s disease: towards clinical implementation. Lancet Neurol. 21, 66–77 (2022).
Zetterberg, H. & Bendlin, B. B. Biomarkers for Alzheimer’s disease—preparing for a new era of disease-modifying therapies. Mol. Psychiatry 26, 296–308 (2021).
Gauthier, S., Rosa-Neto, P., Morais, J. & Webster, C. World Alzheimer Report 2021: journey through the diagnosis of dementia. Alzheimer’s Disease International https://www.alzint.org/resource/world-alzheimer-report-2021/ (2021).
Robinson, L., Tang, E. & Taylor, J. P. Dementia: timely diagnosis and early intervention. BMJ 350, h3029 (2015).
Nelson, P. T. et al. Limbic-predominant age-related TDP-43 encephalopathy (LATE): consensus working group report. Brain 142, 1503–1527 (2019).
Graus, F. et al. A clinical approach to diagnosis of autoimmune encephalitis. Lancet Neurol. 15, 391–404 (2016).
Scialo, C. et al. TDP-43 real-time quaking induced conversion reaction optimization and detection of seeding activity in CSF of amyotrophic lateral sclerosis and frontotemporal dementia patients. Brain Commun. 2, fcaa142 (2020).
Mank, A. et al. Identifying relevant outcomes in the progression of Alzheimer’s disease; what do patients and care partners want to know about prognosis? Alzheimer’s Dement. 7, e12189 (2021).
Hane, F. T. et al. Recent progress in Alzheimer’s disease research, part 3: diagnosis and treatment. J. Alzheimers Dis. 57, 645–665 (2017).
Kourtis, L.C., Regele, O.B., Wright, J.M. & Jones, G.B. Digital biomarkers for Alzheimer’s disease: the mobile/wearable devices opportunity. NPJ Digit. Med. 2, 9 (2019).
Ritchie, C. W. et al. The Edinburgh Consensus: preparing for the advent of disease-modifying therapies for Alzheimer’s disease. Alzheimer’s Res. Ther. 9, 85 (2017).
Keshavan, A. et al. Population-based blood screening for preclinical Alzheimer’s disease in a British birth cohort at age 70. Brain 144, 434–449 (2021).
Frozza, R. L., Lourenco, M. V. & De Felice, F. G. Challenges for Alzheimer’s disease therapy: insights from novel mechanisms beyond memory defects. Front Neurosci. 12, 37 (2018).
Khan, T. K. An algorithm for preclinical diagnosis of Alzheimer’s disease. Front. Neurosci. 12, 275 (2018).
Kivipelto, M., Mangialasche, F. & Ngandu, T. Lifestyle interventions to prevent cognitive impairment, dementia and Alzheimer disease. Nat. Rev. Neurol. 14, 653–666 (2018).
Prorok, J. C., Horgan, S. & Seitz, D. P. Health care experiences of people with dementia and their caregivers: a meta-ethnographic analysis of qualitative studies. CMAJ 185, E669–E680 (2013).
Frank, C. & Forbes, R. F. A patient’s experience in dementia care: using the ‘lived experience’ to improve care. Can. Fam. Physician 63, 22–26 (2017).
Kowe, A. et al. Stakeholder involvement in dementia research: a qualitative approach with healthy senior citizens and providers of dementia care in Germany. Health Soc. Care Community 30, 908–917 (2020).
Kunneman, M. et al. Patients’ and caregivers’ views on conversations and shared decision making in diagnostic testing for Alzheimer’s disease: the ABIDE project. Alzheimer’s Dement. 3, 314–322 (2017).
Rostamzadeh, A. et al. Health literacy in individuals at risk for Alzheimer’s dementia: a systematic review. J. Prev. Alzheimers Dis. 7, 47–55 (2020).
Fruijtier, A. D. et al. What patients want to know, and what we actually tell them: the ABIDE project. Alzheimer’s Dement. 6, e12113 (2020).
Fruijtier, A. D. et al. ABIDE Delphi study: topics to discuss in diagnostic consultations in memory clinics. Alzheimer’s Res. Ther. 11, 77 (2019).
DiBenedetti, D. B. et al. Assessing what matters most to patients with or at risk for Alzheimer’s and care partners: a qualitative study evaluating symptoms, impacts, and outcomes. Alzheimers Res. Ther. 12, 90 (2020).
Tochel, C. et al. What outcomes are important to patients with mild cognitive impairment or Alzheimer’s disease, their caregivers, and health-care professionals? a systematic review. Alzheimers Dement. 11, 231–247 (2019).
Amyvid (florbetapir F18 injection) [highlights of prescribing information]. US Food and Drug Administration. https://www.accessdata.fda.gov/drugsatfda_docs/label/2012/202008s000lbl.pdf (2012).
Vizamyl (flutemetamol F18 injection) [highlights of prescribing information]. US Food and Drug Administration. https://www.accessdata.fda.gov/drugsatfda_docs/label/2013/203137s000lbl.pdf (2013).
Neuraceq (florbetaben F18 injection) [highlights of prescribing information]. US Food and Drug Administration. https://www.accessdata.fda.gov/drugsatfda_docs/label/2014/204677s000lbl.pdf (2014).
Johnson, K. A. et al. Appropriate use criteria for amyloid PET: a report of the Amyloid Imaging Task Force, the Society of Nuclear Medicine and Molecular Imaging, and the Alzheimer’s Association. Alzheimers Dement. 9, e1–e16 (2013).
Johnson, K. A. et al. Update on appropriate use criteria for amyloid PET imaging: dementia experts, mild cognitive impairment, and education. Amyloid Imaging Task Force of the Alzheimer’s Association and Society for Nuclear Medicine and Molecular Imaging. Alzheimers Dement. 9, e106–e109 (2013).
Altomare, D. et al. Quantitative appraisal of the Amyloid Imaging Taskforce appropriate use criteria for amyloid-PET. Alzheimers Dement. 14, 1088–1098 (2018).
Rabinovici, G. D. et al. Association of amyloid positron emission tomography with subsequent change in clinical management among medicare beneficiaries with mild cognitive impairment or dementia. JAMA 321, 1286–1294 (2019).
Tousi, B. & Sabbagh, M. N. Editorial: a time of transition of Alzheimer’s disease in the advent of anti-amyloid monoclonal antibodies. Neurol. Ther. 10, 409–413 (2021).
Zetterberg, H. & Blennow, K. Moving fluid biomarkers for Alzheimer’s disease from research tools to routine clinical diagnostics. Mol. Neurodegener. 16, 10 (2021).
Shaw, L. M. et al. Appropriate use criteria for lumbar puncture and cerebrospinal fluid testing in the diagnosis of Alzheimer’s disease. Alzheimers Dement. 14, 1505–1521 (2018).
Hampel, H. et al. State-of-the-art of lumbar puncture and its place in the journey of patients with Alzheimer’s disease. Alzheimers Dement. 18, 159–177 (2021).
Hansson, O. et al. Pre-analytical protocol for measuring Alzheimer’s disease biomarkers in fresh CSF. Alzheimers Dement 12, e12137 (2020).
Vanderstichele, H. et al. Standardization of preanalytical aspects of cerebrospinal fluid biomarker testing for Alzheimer’s disease diagnosis: a consensus paper from the Alzheimer’s Biomarkers Standardization Initiative. Alzheimers Dement. 8, 65–73 (2012).
Tauvid (flortaucipir F 18 injection) [highlights of prescribing information]. US Food and Drug Administration. https://www.accessdata.fda.gov/drugsatfda_docs/label/2020/212123s000lbl.pdf (2020).
Moscoso, A. et al. Longitudinal associations of blood phosphorylated Tau181 and neurofilament light chain with neurodegeneration in Alzheimer disease. JAMA Neurol. 78, 396–406 (2021).
Chen, S. D. et al. Longitudinal plasma phosphorylated tau 181 tracks disease progression in Alzheimer’s disease. Transl. Psychiatry 11, 356 (2021).
Moscoso, A. et al. Time course of phosphorylated-tau181 in blood across the Alzheimer’s disease spectrum. Brain 144, 325–339 (2021).
Janelidze, S. et al. Plasma beta-amyloid in Alzheimer’s disease and vascular disease. Sci. Rep. 6, 26801 (2016).
Schindler, S. E. et al. High-precision plasma beta-amyloid 42/40 predicts current and future brain amyloidosis. Neurology 93, e1647–e1659 (2019).
Nakamura, A. et al. High performance plasma amyloid-beta biomarkers for Alzheimer’s disease. Nature 554, 249–254 (2018).
West, T. et al. A blood-based diagnostic test incorporating plasma Abeta42/40 ratio, ApoE proteotype, and age accurately identifies brain amyloid status: findings from a multi cohort validity analysis. Mol. Neurodegener. 16, 30 (2021).
Barthelemy, N.R., Horie, K., Sato, C. & Bateman, R.J. Blood plasma phosphorylated-tau isoforms track CNS change in Alzheimer’s disease. J. Exp. Med. 217, e20200861 (2020).
Janelidze, S. et al. Plasma p-tau181 in Alzheimer’s disease: relationship to other biomarkers, differential diagnosis, neuropathology and longitudinal progression to Alzheimer’s dementia. Nat. Med. 26, 379–386 (2020).
Karikari, T. K. et al. Blood phosphorylated tau 181 as a biomarker for Alzheimer’s disease: a diagnostic performance and prediction modelling study using data from four prospective cohorts. Lancet Neurol. 19, 422–433 (2020).
Mielke, M. M. et al. Plasma phospho-tau181 increases with Alzheimer’s disease clinical severity and is associated with tau- and amyloid-positron emission tomography. Alzheimers Dement. 14, 989–997 (2018).
Palmqvist, S. et al. Discriminative accuracy of plasma phospho-tau217 for Alzheimer disease vs other neurodegenerative disorders. JAMA 324, 772–781 (2020).
Thijssen, E. H. et al. Diagnostic value of plasma phosphorylated tau181 in Alzheimer’s disease and frontotemporal lobar degeneration. Nat. Med. 26, 387–397 (2020).
Ashton, N. J. et al. Plasma p-tau231: a new biomarker for incipient Alzheimer’s disease pathology. Acta Neuropathol. 141, 709–724 (2021).
Ashton, N. J. et al. The validation status of blood biomarkers of amyloid and phospho-tau assessed with the 5-phase development framework for AD biomarkers. Eur. J. Nucl. Med. Mol. Imaging 48, 2140–2156 (2021).
Langa, K. M., Foster, N. L. & Larson, E. B. Mixed dementia: emerging concepts and therapeutic implications. JAMA 292, 2901–2908 (2004).
McAleese, K. E. et al. TDP-43 pathology in Alzheimer’s disease, dementia with Lewy bodies and ageing. Brain Pathol. 27, 472–479 (2017).
Visser, L. N. C. et al. Clinicians’ communication with patients receiving a MCI diagnosis: the ABIDE project. PLoS ONE 15, e0227282 (2020).
van Maurik, I. S. et al. Development and usability of ADappt: web-based tool to support clinicians, patients and caregivers in the diagnosis of mild cognitive impairment and Alzheimer disease. JMIR Form. Res. 3, e13417 (2019).
Chiotis, K. et al. Clinical validity of increased cortical uptake of amyloid ligands on PET as a biomarker for Alzheimer’s disease in the context of a structured 5-phase development framework. Neurobiol. Aging 52, 214–227 (2017).
Sabbagh, M. N. et al. Early detection of mild cognitive impairment in an at-home setting. J. Prev. Alzheimers Dis. 7, 171–178 (2020).
Stroud, C., Onnela, J. P. & Manji, H. Harnessing digital technology to predict, diagnose, monitor, and develop treatments for brain disorders. NPJ Digit. Med. 2, 44 (2019).
Au, R., Ritchie, M., Hardy, S., Ang, T. F. A. & Lin, H. Aging well: using precision to drive down costs and increase health quality. Adv. Geriatr. Med. Res. 1, e190003 (2019).
Seelye, A. et al. Embedded online questionnaire measures are sensitive to identifying mild cognitive impairment. Alzheimer Dis. Assoc. Disord. 30, 152–159 (2016).
Kirste, T. et al. Detecting the effect of Alzheimer’s disease on everyday motion behavior. J. Alzheimers Dis. 38, 121–132 (2014).
Lüdtke, S., Hermann, W., Kirste, T., Beneš, H. & Teipel, S. An algorithm for actigraphy-based sleep/wake scoring: comparison with polysomnography. Clin. Neurophysiol. 132, 137–145 (2021).
Sabbagh, M. N. et al. Rationale for early diagnosis of mild cognitive impairment supported by emerging digital technologies. J. Prev. Alzheimers Dis. 7, 158–164 (2020).
Chen, R. et al. Developing measures of cognitive impairment in the real world from consumer-grade multimodal sensor streams. in Proceedings of the 25th ACM SIGKDD International Conference on Knowledge Discovery & Data Mining 2145–2155 (2019).
Beattie, Z. et al. The Collaborative Aging Research Using Technology Initiative: an open, sharable, technology-agnostic platform for the research community. Digit. Biomark. 4, 100–118 (2020).
Wu, C. Y. et al. Unobtrusive sensing technology detects ecologically valid spatiotemporal patterns of daily routines distinctive to persons with mild cognitive impairment. J. Gerontol. A Biol. Sci. Med. Sci. glab293 (2021).
Piau, A. et al. Intrinsic capacitiy monitoring by digital biomarkers in integrated care for older people (ICOPE). J. Frailty Aging 10, 132–138 (2021).
Takeda, C., Guyonnet, S., Sumi, Y., Vellas, B. & Araujo de Carvalho, I. Integrated care for older people and the implementation in the INSPIRE Care Cohort. J. Prev. Alzheimers Dis. 7, 70–74 (2020).
Tavassoli, N. et al. Implementation of the WHO integrated care for older people (ICOPE) programme in clinical practice: a prospective study. Lancet Healthy Longev. 3, 394–404 (2022).
van Gils, A. M. et al. Assessing the views of professionals, patients and care partners concerning the use of computer tools in memory clinics: International Survey Study. JMIR Form. Res. 5, e31053 (2021).
McAlearney, A. S. et al. High Touch and High Tech (HT2) proposal: transforming patient engagement throughout the continuum of care by engaging patients with portal technology at the bedside. JMIR Res. Protoc. 5, e221 (2016).
Hampel, H. & Vergallo, A. The Sars-CoV-2 pandemic and the brave new digital world of environmental enrichment to prevent brain aging and cognitive decline. J. Prev. Alzheimers Dis. 7, 294–298 (2020).
Liu, J. L., Hlavka, J. P., Hillestad, R. & Mattke, S. Assessing the Preparedness of the US Health Care System Infrastructure for an Alzheimer’s Treatment (RAND Corporation, 2017).
Hlavka, J. P., Mattke, S. & Liu, J. L. Assessing the preparedness of the health care system infrastructure in six European countries for an Alzheimer’s treatment. Rand Health Q. 8, 2 (2019).
Mattke, S., Hlavka, J. P., Yoong, J., Wang, M. & Goto, R. Assessing the preparedness of the Japanese health care system infrastructure for an Alzheimer’s treatment. (USC Dornsife: Center for Economic and Social Research, 2019).
Jun, H., Cho, S. K., Yoong, J. & Mattke, S. Assessing the preparedness of the Korean health care system infrastructure for an Alzheimer’s treatment. (USC Dornsife: Center for Economic and Social Research, 2020).
Mattke, S. & Hanson, M. Expected wait times for access to a disease-modifying Alzheimer’s treatment in the United States. Alzheimers Dement. 18, 1071–1074 (2021).
Mattke, S., Ullrich, A. & Wang, M. Implications of Alzheimer’s treatment for organization and payment of medical practices in the EU-5 countries. (USC Dornsife: Center for Economic and Social Research, 2020).
Mattke, S. & Wang, M. Implications of Alzheimer’s treatment for organization and payment of medical practices in the United States. (USC Dornsife: Center for Economic and Social Research, 2020).
Prince, M., Comas-Herrera, A, Knapp, M, Guerchet, M. & Karagiannidou, M. World Alzheimer Report 2016: improving healthcare for people living with dementia: coverage, quality and costs now and in the future. (Alzheimer’s Disease International, 2016).
Sperling, R. A. et al. Amyloid-related imaging abnormalities (ARIA) in amyloid modifying therapeutic trials: recommendations from the Alzheimer’s Association Research Roundtable Workgroup. Alzheimers Dement. 7, 367–385 (2011).
Liu, J. L., et al. Assessing the preparedness of the Canadian health care system infrastructure for an Alzheimer’s treatment (RAND Corporation, 2019).
Au, R. K., Kolachalama V. B., Paschalidis, I. C. H. Redefining and validating digital biomarkers as fluid, dynamic multi-dimensional digital signal patterns. Front. Digit. Health 3, 751629 (2022).
Lloyd-Jones, D. M. et al. Use of risk assessment tools to guide decision-making in the primary prevention of atherosclerotic cardiovascular disease: a special report from the American Heart Association and American College of Cardiology. Circulation 139, e1162–e1177 (2019).
Rossini, P. M. et al. The Italian INTERCEPTOR Project: from the early identification of patients eligible for prescription of antidementia drugs to a nationwide organizational model for early Alzheimer’s disease diagnosis. J. Alzheimers Dis. 72, 373–388 (2019).
National Institute on Aging. Telehealth: improving dementia care. https://www.nia.nih.gov/news/telehealth-improving-dementia-care (2020).
Chin, A. L., Negash, S. & Hamilton, R. Diversity and disparity in dementia: the impact of ethnoracial differences in Alzheimer disease. Alzheimer Dis. Assoc. Disord. 25, 187–195 (2011).
US Department of Health and Human Services. Racial and ethnic disparities in Alzheimer’s Disease: a literature review. https://aspe.hhs.gov/sites/default/files/migrated_legacy_files//138596/RacEthDis.pdf (2014).
Vega, I. E., Cabrera, L. Y., Wygant, C. M., Velez-Ortiz, D. & Counts, S. E. Alzheimer’s disease in the Latino community: intersection of genetics and social determinants of health. J. Alzheimers Dis. 58, 979–992 (2017).
McKindra, L. Dementia researchers issue urgent call for more diversity in clinical trial participation. (University of Kansas Medical Center, 2021).
Wilkins, C. H., Schindler, S. E. & Morris, J. C. Addressing health disparities among minority populations: why clinical trial recruitment is not enough. JAMA Neurol. 77, 1063–1064 (2020).
Anderson, J. G., Flatt, J. D., Jabson Tree, J. M., Gross, A. L. & Rose, K. M. Characteristics of sexual and gender minority caregivers of people with dementia. J. Aging Health 33, 838–851 (2021).
Ferretti, M. T. et al. Optimal Alzheimer’s disease detection and diagnosis under the sex and gender lens: a crucial step towards precision neurology. in World Alzheimer Report 2021: Journey through the diagnosis of dementia. Alzheimer’s Disease International, 238–241 (2021).
Ferretti, M. T. et al. Sex differences in Alzheimer disease—the gateway to precision medicine. Nat. Rev. Neurol. 14, 457–469 (2018).
Fredriksen-Goldsen, K. I., Jen, S., Bryan, A. E. B. & Goldsen, J. Cognitive impairment, Alzheimer’s disease and other dementias in the lives of lesbian, gay, bisexual and transgender (LGBT) older adults and their caregivers: needs and competencies. J. Appl Gerontol. 37, 545–569 (2018).
Galvin, J. E. et al. Early stages of Alzheimer’s disease: evolving the care team for optimal patient management. Front. Neurol. 11, 592302 (2020).
Hampel, H., Lista, S. & Khachaturian, Z. S. Development of biomarkers to chart all Alzheimer’s disease stages: the royal road to cutting the therapeutic Gordian Knot. Alzheimers Dement. 8, 312–336 (2012).
Hampel, H. et al. PRECISION MEDICINE—the golden gate for detection, treatment and prevention of Alzheimer’s disease. J. Prev. Alzheimers Dis. 3, 243–259 (2016).
Hampel, H. et al. A precision medicine framework using artificial intelligence for the identification and confirmation of genomic biomarkers of response to an Alzheimer’s disease therapy: analysis of the blarcamesine (ANAVEX2-73) phase 2a clinical study. Alzheimer’s Dement. 6, e12013 (2020).
Hampel, H., Vergallo, A., Perry, G., Lista, S. & Alzheimer Precision Medicine Inititaive. The Alzheimer Precision Medicine Initiative. J. Alzheimers Dis. 68, 1–24 (2019).
Altomare, D. et al. Brain Health Services: organization, structure, and challenges for implementation. a user manual for Brain Health Services—part 1 of 6. Alzheimers Res. Ther. 13, 168 (2021).
Cummings, J. The role of biomarkers in Alzheimer’s disease drug development. Adv. Exp. Med. Biol. 1118, 29–61 (2019).
McKhann, G. et al. Clinical diagnosis of Alzheimer’s disease: report of the NINCDS-ADRDA Work Group under the auspices of Department of Health and Human Services Task Force on Alzheimer’s Disease. Neurology 34, 939–944 (1984).
Beach, T. G., Monsell, S. E., Phillips, L. E. & Kukull, W. Accuracy of the clinical diagnosis of Alzheimer disease at National Institute on Aging Alzheimer Disease Centers, 2005–2010. J. Neuropathol. Exp. Neurol. 71, 266–273 (2012).
Kovacs, G. G. et al. Non-Alzheimer neurodegenerative pathologies and their combinations are more frequent than commonly believed in the elderly brain: a community-based autopsy series. Acta Neuropathol. 126, 365–384 (2013).
Graff-Radford, J. et al. New insights into atypical Alzheimer’s disease in the era of biomarkers. Lancet Neurol. 20, 222–234 (2021).
Dubois, B. et al. Research criteria for the diagnosis of Alzheimer’s disease: revising the NINCDS-ADRDA criteria. Lancet Neurol. 6, 734–746 (2007).
Dubois, B. et al. Revising the definition of Alzheimer’s disease: a new lexicon. Lancet Neurol. 9, 1118–1127 (2010).
Dubois, B. et al. Preclinical Alzheimer’s disease: definition, natural history, and diagnostic criteria. Alzheimers Dement. 12, 292–323 (2016).
Albert, M. S. et al. The diagnosis of mild cognitive impairment due to Alzheimer’s disease: recommendations from the National Institute on Aging-Alzheimer’s Association workgroups on diagnostic guidelines for Alzheimer’s disease. Alzheimers Dement. 7, 270–279 (2011).
McKhann, G. M. et al. The diagnosis of dementia due to Alzheimer’s disease: recommendations from the National Institute on Aging-Alzheimer’s Association workgroups on diagnostic guidelines for Alzheimer’s disease. Alzheimers Dement. 7, 263–269 (2011).
Sperling, R. A. et al. Toward defining the preclinical stages of Alzheimer’s disease: recommendations from the National Institute on Aging-Alzheimer’s Association workgroups on diagnostic guidelines for Alzheimer’s disease. Alzheimers Dement. 7, 280–292 (2011).
Jack, C. R. et al. A/T/N: an unbiased descriptive classification scheme for Alzheimer disease biomarkers. Neurology 87, 539–547 (2016).
Food & Drug Administration. Early Alzheimer’s Disease: Developing Drugs for Treatment: Guidance for Industry. https://www.fda.gov/media/110903/download (2018).
Acknowledgements
R.A.’s grant support includes National Institutes of Health (NIH) grants (AG062109, AG068753, AG072654 and AG063635). Additional support was provided by the American Heart Association (20SFRN35360180 and 20SFRN35490098), the Alzheimer’s Drug Discovery Foundation (201902-2017835) and Gates Ventures. W.M.v.d.F received support from the Research of Alzheimer center Amsterdam, which is part of the neurodegeneration research program of Amsterdam Neuroscience. The chair of W.v.f.F is supported by the Pasman stichting. W.F. is a recipient of ABOARD, which is a public–private partnership receiving funding from ZonMW (73305095007) and Health~Holland, Topsector Life Sciences & Health (PPP allowance, LSHM20106). More than 30 partners participate in ABOARD. H.W. receives research grant from the National Brain Project funded by the Ministry of Science and Technology, China (2021ZD0201805). C.C. is supported by the National Medical Research Council of Singapore (MOH-000707-00, NMRC/OFLCG/2019, NMRC/CIRG/1485/2018 and NMRC/CSA-SI/0007/2016). The Gérontopôle (chair B.V.) has received research grant support from the European Commission as well as industries including Biogen, Green Valley Pharmaceuticals, Novo Nordisk, Pfizer, Pierre-Fabre, Roche, Lily and Eisai. J.C. is supported by NIGMS grant P20GM109025, NINDS grant U01NS093334, NIA grants R01AG053798, P20AG068053 and R35AG71476 and the Alzheimer’s Disease Drug Discovery Foundation. A.S. receives support from multiple NIH grants (P30 AG010133, P30 AG072976, R01 AG019771, R01 AG057739, U01 AG024904, R01 LM013463, R01 AG068193, T32 AG071444, U01 AG068057 and U01 AG072177). The authors thank D. Henley for his contribution to the critical revision of the Perspective. Medical writing support was provided by L. O’Brien of CMC AFFINITY, McCann Health Medical Communications and was funded by Eisai.
Author information
Authors and Affiliations
Contributions
H.H., A.V., S.D.S. and P.G. developed the initial concept and theoretical framework for this Perspective. All authors contributed to researching the literature and data, discussing the content, and writing, reviewing and/or editing of the Perspective.
Corresponding author
Ethics declarations
Competing interests
H.H. is an employee of Eisai and serves as senior associate editor for the Journal Alzheimer’s & Dementia and has not received any fees or honoraria since May 2019. H.H. is inventor of 11 patents and has received no royalties for: In Vitro Multiparameter Determination Method for The Diagnosis and Early Diagnosis of Neurodegenerative Disorders patent no. 8916388; In Vitro Procedure for Diagnosis and Early Diagnosis of Neurodegenerative Diseases patent no. 8298784; Neurodegenerative Markers for Psychiatric Conditions publication no. 20120196300; In Vitro Multiparameter Determination Method for The Diagnosis and Early Diagnosis of Neurodegenerative Disorders publication no. 20100062463; In Vitro Method for The Diagnosis and Early Diagnosis of Neurodegenerative Disorders publication no. 20100035286; In Vitro Procedure for Diagnosis and Early Diagnosis of Neurodegenerative Diseases publication no. 20090263822; In Vitro Method for The Diagnosis of Neurodegenerative Diseases patent no. 7547553; CSF Diagnostic in Vitro Method for Diagnosis of Dementias and Neuroinflammatory Diseases publication no. 20080206797; In Vitro Method for The Diagnosis of Neurodegenerative Diseases publication no. 20080199966; Neurodegenerative Markers for Psychiatric Conditions publication no. 20080131921; Method for diagnosis of dementias and neuroinflammatory diseases based on an increased level of procalcitonin in cerebrospinal fluid: US patent no. 10921330. R.A. is a scientific advisor to Signant Health and consultant to Biogen. S.M. serves on the board of directors of Senscio Systems and the scientific advisory board of AiCure Technologies, and Boston Millennia Partners, and has received consulting fees from AARP, Biogen, Biotronik, Bristol-Myers Squibb, C2N, Eisai and Roche. Research programs of W.M.v.d.F. have been funded by ZonMW, NWO, EU-FP7, EU-JPND, Alzheimer Nederland, CardioVascular Onderzoek Nederland, Health~Holland, Topsector Life Sciences & Health, stichting Dioraphte, Gieskes-Strijbis fonds, stichting Equilibrio, Pasman stichting, stichting Alzheimer & Neuropsychiatrie Foundation, Biogen MA, Boehringer Ingelheim, Life-MI, AVID, Roche BV, Fujifilm and Combinostics. W.F. holds the Pasman chair. W.F. is a recipient of ABOARD, which is a public–private partnership receiving funding from ZonMW (73305095007) and Health~Holland, Topsector Life Sciences & Health (PPP allowance, LSHM20106). W.F. has performed contract research for Biogen MA and Boehringer Ingelheim. W.F. has been an invited speaker at Boehringer Ingelheim, Biogen MA, Danone, Eisai, WebMD Neurology (Medscape) and Springer Healthcare. W.F. is consultant to Oxford Health Policy Forum CIC, Roche and Biogen MA. W.F. participated in advisory boards of Biogen MA and Roche. All funding is paid to the institution of W.F. W.F. was associate editor of Alzheimer, Research & Therapy in 2020/2021. W.F. is associate editor at Brain. P.A. reports research agreements with Janssen, Lilly and Eisai, grants from NIA, the Alzheimer’s Association and FNIH and consulting fees from Biogen, Roche, Merck, Abbvie, Immunobrain Checkpoint, Rainbow Medical and Shionogi. L.A. has provided consultation to Eli Lilly, Biogen, Eisai, GE Healthcare and Two Labs. L.G.A. receives research support from NIA U01 AG057195, NIA R01 AG057739, NIA P30 AG010133, Alzheimer Association LEADS GENETICS 19-639372, Roche Diagnostics RD005665, AVID Pharmaceuticals and Life Molecular Imaging. L.G.A. received honoraria for participating in independent data safety monitoring boards and providing educational CME lectures and programs. L.G.A. has stock in Cassava Sciences and Semiring. C.C. receives research grants from the National Medical Research Council of Singapore. C.C. also receives research support from Moleac, Roche, Eisai and Lundbeck; and has participated in advisory boards for Cerecin and Eisai in the past 3 years. A.I. receives research grant from AMED (Japanese Agency for Medical Research), JSPS (Japan Society for Promotion of Science), Eisai, Daiichi Sankyo, Shionogi, Chugai-Roche and Kyowa Kirin. A.I. also receives consultant fees from Eisai, Biogen and Janssen Pharmaceuticals. A.I. also receives lecture fees from Eisai, Daiichi Sankyo, Otsuka, Ono Pharmaceutical and Fujirebio. A.S. received support from Avid Radiopharmaceuticals, a subsidiary of Eli Lilly (in kind contribution of PET tracer precursor), Bayer Oncology (Scientific Advisory Board), Eisai (Scientific Advisory Board), Siemens Medical Solutions USA (Dementia Advisory Board) and Springer Nature Publishing (Editorial Office Support as Editor-in-Chief, Brain Imaging and Behavior). S.T. has served on the advisory boards of Roche, Biogen, Eisai and Grifols within the last 3 years. B.V. has served as consultant/advisor to Eisai, Biogen, Lilly, Longeveron, Novo Nordisk, TauRx P and Roche in the past 3 years. A.V. declares no competing interests related to the present paper and the contribution of A.V. to this paper reflects entirely and only A.V.’s own academic expertise on the matter. A.V. was an employee of Eisai (November 2019–June 2021). A.V. did not receive any fees or honoraria since November 2019. Before November 2019, A.V. received lecture honoraria from Roche, MagQu and Servier. H.W. has provided consultation to Eisai, Lundbeck, Roche and Signant Health pharmaceutical and assessment companies. H.W. owns the copyright of the individualized management system of neuropsychiatric symptoms (NPSIMS) and the smartphone-based application of brief cognitive screening kit (shairenzhi). J.C. provided consultation to AB Science, Acadia, Alkahest, AlphaCognition, ALZPathFinder, Annovis, AriBio, Artery, Avanir, Biogen, Biosplice, Cassava, Cerevel, Clinilabs, Cortexyme, Diadem, EIP Pharma, Eisai, GatehouseBio, GemVax, Genentech, Green Valley, Grifols, Janssen, Karuna, Lexeo, Lilly, Lundbeck, LSP, Merck, NervGen, Novo Nordisk, Oligomerix, Ono, Otsuka, PharmacotrophiX, PRODEO, Prothena, ReMYND, Renew, Resverlogix, Roche, Signant Health, Suven, Unlearn AI, Vaxxinity, VigilNeuro pharmaceutical, assessment and investment companies. M.C., S.D.S., P.G. and R.K. are employees of Eisai.
Peer review
Peer review information
Nature Aging thanks Niklas Mattsson-Carlgren and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Hampel, H., Au, R., Mattke, S. et al. Designing the next-generation clinical care pathway for Alzheimer’s disease. Nat Aging 2, 692–703 (2022). https://doi.org/10.1038/s43587-022-00269-x
Received:
Accepted:
Published:
Version of record:
Issue date:
DOI: https://doi.org/10.1038/s43587-022-00269-x
This article is cited by
-
A scoping review of remote and unsupervised digital cognitive assessments in preclinical Alzheimer’s disease
npj Digital Medicine (2025)
-
The therapeutic potential of circular RNAs
Nature Reviews Genetics (2025)
-
Optimizing mobile cognitive assessment reduces administration time while maintaining screening accuracy in older adults
Scientific Reports (2025)
-
Subcortical tau deposition and plasma glial fibrillary acidic protein as predictors of cognitive decline in mild cognitive impairment and Alzheimer’s disease
European Journal of Nuclear Medicine and Molecular Imaging (2025)
-
Use of a digital tool to support the diagnostic process in memory clinics–a usability study
Alzheimer's Research & Therapy (2024)