Abstract
Background
While there is increasing recognition of the morbidity of cardiovascular disease in cancer survivors, including accelerated atherosclerosis following thoracic radiotherapy, patients are frequently under-optimized for cardiovascular risk.
Methods
In this prospective single-arm cohort pilot study, patients were treated with high-dose thoracic radiotherapy and had early consultation with cardio-oncology. Twenty patients were enrolled. The primary endpoint was adherence to cardio-oncology consultation. Secondary endpoints were cardiovascular medication intervention rate and patient-reported intervention perspectives. Clonal hematopoiesis of indeterminate potential, a major cardiovascular risk marker enriched in patients with cancer and induced by radiation exposure, was measured as an exploratory endpoint.
Results
The cohort median age is 71 years. Most patients are female (13/20), have primary lung or esophageal carcinoma (16/20), and 7/20 have pre-existing cardiovascular disease. We show that cardio-oncology consultation adherence is high (19/20) and results in cardiovascular medication optimization changes in most patients (12/19), most commonly to initiate or intensify statin therapy (8/12). 8/12 patients with a primary cardiologist prior to enrollment have medication changes recommended. Most (12/17) participants are glad to learn about their heart health during cancer treatment. Clonal hematopoiesis is detectable prior to treatment in 8/20 patients and three develop new variants after treatment (1/3 de novo).
Conclusions
We observe that early cardio-oncology consultation is feasible, leads to cardiovascular medication optimization in the majority (>60%) of participants, most commonly to initiate or intensify statin therapy. New clonal hematopoiesis variants are detectable early after radiotherapy and the impact on post-treatment cardiovascular risk is worthy of further study.
Plain language summary
Patients with cancer are at high risk for heart disease. Cancer treatments such as chemotherapy, immunotherapy, and radiation therapy can worsen heart disease risk. In this study, we enrolled twenty patients treated with high dose radiation therapy to the chest (near the heart) and had them see a heart doctor who specializes in how cancer treatment affects the heart (called a ‘cardio-oncologist’). Most of these patients (even those who already had a regular heart doctor) still needed heart medicine changes, once the risks of cancer treatment(s) were considered. This study shows that patients with cancer at increased risk of radiation injury to the heart may benefit from optimization of their heart medications to reduce the risk of heart disease.
Similar content being viewed by others
Introduction
There is increasing recognition of the morbidity of cardiovascular disease (CVD) in cancer survivors, including CV toxicity from cancer therapies—such as chemotherapy, molecular targeted agents, radiotherapy (RT), and immunotherapy1. However, patients with cancer are frequently under-optimized for cardiovascular (CV) risk2, including less than half of high CV risk patients on statin therapy3,4. Coronary artery radiation exposure can accelerate the natural history of atherosclerosis5 and has been associated with an increased risk of major cardiac events in patients with lung6,7,8,9, esophageal10,11, and breast12,13 cancers. While RT associated CV toxicity was historically presumed to have a prolonged latency (>5–10 years) to clinical manifestation, recent evidence observes a more compressed timeframe following high dose thoracic RT (such as in lung or esophageal cancer), with 2-year cardiac event rates up to 10–23% and related to baseline CV risk and coronary radiation dose exposure6,10,11,14. Further, statin therapy has been shown to be protective for stroke risk in patients receiving head and neck or thoracic RT15 and has been associated with improved survival in a dose-dependent manner following lung cancer chemoradiotherapy3,16. While consensus cardio-oncology guidelines are now available, they do not incorporate detailed cardiac radiation dose exposure limits in risk assessment or surveillance recommendations, nor reflect the need for more aggressive CV risk mitigation in this patient population17,18,19.
Multiple biomarkers are available in clinical practice that help refine baseline CV risk prediction compared to traditional clinical CV risk factors. Coronary artery calcium (CAC) is a marker of atherosclerotic burden and can aid decision-making regarding initiation or intensification of statin therapy in patients at borderline or intermediate atherosclerotic CVD (ASCVD) risk1. Given that existing primary prevention models do not include cancer treatment(s) or cancer as CV risk factors, they likely underrepresent CV risk in patients with cancer, underscoring the usefulness of CAC and/or other CV risk markers in this setting. Additionally, clonal hematopoiesis of indeterminate potential (CHIP; the clonal expansion of hematopoietic stem cells with somatic mutations20), has recently been identified as a major risk factor for ASCVD, doubling the risk of coronary heart disease independent of traditional CV risk factors21,22,23. Indeed, CHIP is enriched in patients with solid malignancies, and RT is among the strongest risk factors22,24, however there is a paucity of data including CHIP as a CV risk factor in cancer studies.
Collectively, the above knowledge gaps underscore the need to better identify high CV risk patients with cancer who may benefit from early intervention with more aggressive CV risk mitigation strategies. To address these critical gaps in CV risk assessment, we launched a prospective single-arm pilot study measuring the feasibility and impact of early cardio-oncology intervention in patients treated with thoracic RT.
We observe in this high cardiovascular risk cohort that early cardio-oncology consultation is feasible, leads to cardiovascular medication optimization in majority (>60%) of participants, and is viewed positively by patients. New clonal hematopoiesis variants are detectable early after radiotherapy and the impact on post-treatment cardiovascular risk is worthy of further study.
Methods
Clinical trial eligibility and cohort characteristics
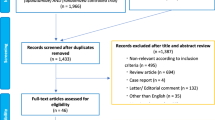
The Cardiac Aggressive Risk Mitigation (CARMA) in thoracic RT trial (NCT05403736) was a prospective single-arm pilot study at Cedars-Sinai Medical Center (Los Angeles, California, U.S.). Eligible patients were adults (age ≥18 years) with Stage I-III or oligometastatic Stage IV cancer, and good performance status (Eastern Cooperative Oncology Group [ECOG] 0-1) who were receiving standard of care RT and were at increased risk for developing cardiac dysfunction as defined by the 2017 American Society of Clinical Oncology (ASCO) clinical practice guideline25, which includes patients treated with high-dose thoracic RT (prescription dose ≥30 Gy) with the heart in the RT field (Fig. 1). Patients with myocardial infarction within 6 months prior to study enrollment or severe and/or active scleroderma or systemic lupus erythematosus were excluded. Patients were identified by research staff who screened the radiation oncology clinic schedules for potentially eligible new patients. For breast cancer specifically, ideal candidates were considered those being treated with regional nodal irradiation. Patients were recruited between October 2022 and January 2023 and data was collected until June 2023. This study is registered on ClinicalTrials.gov (NCT05403736) and was approved by the Cedars-Sinai Medical Center (CSMC) Institutional Review Board and all participants signed informed consent.
Early cardio-oncology intervention
Participants were referred to cardio-oncology (a cardiologist with specialized expertise in the cardiovascular impact of cancer and cancer therapy exposure: A.P.N.) for early consultation (defined as occurring prior to or during RT), including assessment of all potentially cardiotoxic therapies (e.g., RT, chemotherapy, immunotherapy, molecular targeted agents), to develop a personalized CV risk mitigation plan. Given that cardio-oncology referral/consultation is not strictly necessary prior to initiating RT and would not impact the technical aspects of standard RT planning, priority was such that treatment initiation was not delayed. The specific timing of cardio-oncology consultation occurring prior to (vs during) RT was mainly dependent on logistics (e.g., cardiologist clinic availability, patient preference, and/or insurance authorization status), as the protocol was not written or intended to be such that the visit was required prior to RT initiation. In addition, cardio-oncology referral was considered standard of care, given baseline cardiovascular risk factors and/or exposure to cardio-toxic cancer therapies. Patients with a pre-existing primary cardiologist received a one-time cardio-oncology consultation with direct physician-to-physician communication of trial enrollment (i.e., principal investigator and cardio-oncologist notified the existing cardiologist of the clinical trial and plan for one-time second opinion), including notice of planned cardio-oncology consultation and subsequent recommendations. 10-year ASCVD risk calculation was performed according the Pooled Cohort Equation (PCE)26.
Radiotherapy planning
Radiotherapy was typically delivered at CSMC, although two patients had RT delivered at outside institutions, for which their RT simulation CT scans and RT plans were transferred to CSMC for subsequent analyses. Organs at risk (OARs) were contoured manually. The heart and LAD were contoured based on cardiac atlas guidelines27, with an 8-mm brush size for the LAD to encompass cardiac and respiratory motion uncertainties and/or absence of intravenous constrast6,28. Institutional cardiac dose constraints for standard fractionation lung and esophageal cancer included mean heart dose (MHD) < 20 Gy29 and LAD V15 Gy <10%6,30. For breast cancer with regional nodal irradiation, MHD < 2–4 Gy (depending on cancer laterality). At the time of accrual, there were no LAD specific metrics for breast cancer. For 5-fraction SBRT, heart/pericardium constraints were V32 Gy <15 cc, D0.035 cc <38 Gy31 (up to 52.5 Gy)29.”
Coronary artery calcium and clonal hematopoiesis
CAC score was manually measured on non-contrast enhanced RT planning CT scans (non-ECG gated, slice thickness 1.25–2.5 mm) using syngo.via (Siemens Healthineers, Malvern, PA) and expressed as a sum score (Agatston method)32. If the RT planning scan was contrast-enhanced, then either a non-contrast diagnostic chest CT scan or attenuation CT from a positron emission tomography (PET)/CT was used instead.
Clonal hematopoiesis of indeterminate potential (CHIP) was analyzed using the 75-gene multiplex Archer VariantPlex Myeloid panel with additional variant filtering (Z-AM-Pipeline v1.0). The variant allele fraction was expressed as a percentage (%). CHIP was considered positive at a variant allele frequency of 0.025 or higher. Blood samples were collected at baseline and post-RT (at completion of RT and at clinical follow-up 6–14 weeks later). Due to the number of timepoint samples not collected (n = 5 follow-up) or unanalyzable (n = 1 follow-up, n = 1 end of RT), analysis of ‘post-RT’ was considered as a single timepoint—reflecting either the completion of RT or follow-up, whichever came last.
Statistical analysis
The primary endpoint was adherence to cardio-oncology consultation. We tested the null hypothesis that the proportion of adherent patients is no more than 70% using an exact Binomial test at 5% nominal significance level with 3.5% as actual significance level. Thus, if the number of adherent patients was greater or equal to 18 patients, then we reject the null hypothesis and declare the study as feasible. Secondary endpoints included the rate of CV medication intervention by cardio-oncology and patient-reported intervention perspectives by survey. Data was summarized using descriptive statistics, including n (%), median (interquartile rage [IQR]), or mean (±standard deviation) where indicated. No participants were excluded from analysis. Analyses were performed using StataSE (version 17.0; StataCorp LLC) statistical software.
Reporting summary
Further information on research design is available in the Nature Portfolio Reporting Summary linked to this article.
Results
Cohort characteristics
Twenty patients were enrolled. The median age was 71 years (interquartile range [IQR], 63–77). Most were female (13/20), white (17/20), or Latin(x) (4/20). Hyperlipidemia (10/20), hypertension (9/20), tobacco use (9/20 = former, 1/20 = current), and pre-existing ASCVD (7/20) were common (Table 1). Cancers were primary lung/mediastinal (12/20), esophageal (4/20), breast (2/20), or secondary lung metastases (2/20, Table 2). Among the 13 patients without ASCVD, the mean ASCVD risk score was 14.8% (±12.7%) and six had CAC > 0 (1–99 [n = 2]; 100–399 [n = 3]; ≥400 [n = 1]) on pre-radiotherapy CT scans. Additionally, of the 5 patients with low 10-year ASCVD risk score (<7.5%), CAC, LDL, presence of CHIP mutation, and/or LAD dose exposure, identified two (2/5) patients who may benefit from initiating or intensifying statin therapy (Table 3). Several (7/20) received induction systemic therapy (molecularly targeted agent [4/7]; docetaxel, carboplatin, trastuzumab, pertuzumab [2/7]). Most (12/20) received concurrent chemoradiotherapy (carboplatin or cisplatin with pemetrexed [7/12]; carboplatin/paclitaxel [4/12]), while 5/20 received adjuvant immunotherapy. The mean whole heart and LAD radiation dose was 7.0 Gy (±4.8 Gy) and 3.8 Gy (±3.9 Gy), respectively.
Cardio-Oncology Intervention
Cardio-oncology consultation was completed before or during RT in 19/20 patients, and the intervention was therefore deemed feasible. In one patient, the principal investigator placed the cardio-oncology referral on hold during a protracted hospitalization for possible paraneoplastic syndrome. Ultimately a diagnosis of intracranial metastatic disease was reached after the completion of RT and the cardio-oncology referral was deemed no longer clinically warranted by the principal investigator. Cardio-oncology consultation resulted in CV medication optimization changes in most patients (12/19). The most common recommendations were statin-related (either to start statin [2/8] or increase statin intensity [6/8]). Other changes were to start/stop/change dosage of anti-hypertensives (4/12), start low-dose (81 mg) aspirin (1/12) or start sacubitril/valsartan (1/12).
Of the 12 patients with a primary cardiologist prior to enrollment, 8/12 had medication changes recommended (start [n = 1] or increase [n = 4] statin, start aspirin [n = 1], or anti-hypertensive change [n = 4]). There was no significant difference in the rate of CV medication change recommendation in those with or without a primary cardiologist prior to enrollment (66.7% [8/12] vs 57.1% [4/7]; p > 0.99) nor among those with a primary cardiologist with most recent cardiology visit >18 months/unknown duration vs <6 months (75.0% [3/4] vs 71.4% [5/7]; p > 0.99). Based on consensus guidelines advising baseline transthoracic echocardiography (TTE) be performed for patients undergoing thoracic RT if the treatment will expose the heart and there is pre-existing cardiac disease33, 6/20 patients warranted baseline TTE evaluation (n = 2 completed same day, n = 4 completed within 3 weeks). Additionally, five patients were recommended additional cardiovascular testing (n = 2 coronary CT angiogram [CCTA], n = 3 stress echocardiogram). Of these five patients, n = 3 had stress echocardiogram performed 22 days, 12 days, and 76 days following consultation, respectively. Of the 2 patients recommended CCTA, one declined due to feeling overwhelmed with appointments, and the other instead had an emergent left heart catheterization performed (see cardiac event details in the paragraph below).
Cardiac events
At a median follow up of 14 weeks (IQR 11–15), one patient experienced a non-fatal major cardiac event (non-ST elevation myocardial infarction [NSTEMI] and acute decompensated heart failure during chemoradiotherapy). Specifically, this was a 67-year-old man with known ASCVD and prior coronary artery bypass graft with esophageal squamous cell carcinoma who experienced shortness of breath in the setting of severe anemia (hemoglobin 5.6 g/dL) cycle 1 day 2 of carboplatin/paclitaxel plus radiotherapy, then developed acute hypoxemia respiratory failure, acute heart failure with reduced ejection fraction (ejection fraction 40% with right ventricle systolic dysfunction) and NSTEMI; underwent emergent left heart catheterization six days following presentation (no coronary intervention warranted).
Clonal hematopoiesis of indeterminate potential
Clonal hematopoiesis of indeterminate potential (CHIP) was detectable prior to RT in 8/20 patients. By post-RT follow-up, 9/20 had detectable CHIP, including 2/19 with pre-existing DNMT3A (deoxyribonucleic acid methyltransferase 3A) clones who developed additional variants (PPM1D [protein phosphatase Mn2+/Mg2+-dependent 1D], TP53 [tumor protein 53], NF1 [neurofibromatosis type 1]), and 1/19 with no pre-existing CHIP developed a TET2 (Tet methylcytosine dioxygenase 2) variant (Table 4).
Intervention perspectives survey
By study conclusion, among 17 responding participants, most agreed or strongly agreed that they learned new information about their heart health (12/17), while 1/17 was neutral, 2/17 disagreed, and 1/17 strongly disagreed. Most agreed or strongly agree that they were glad to know new information about their heart health (12/17), though 4/17 were unsure or neutral and 1/17 disagreed. Most agreed or strongly agreed that this information did not make them feel more anxious (13/17), though 2/17 were unsure or neutral and 2/17 disagreed. Most agreed or strongly agreed it was not overwhelming to focus on their heart health during cancer treatment (14/17), though 1/17 was unsure one each disagreed or strongly disagreed.
Discussion
To our best knowledge, this is the first study reporting adherence and outcomes of early cardio-oncology intervention and patient reported perspectives of cardio-oncology intervention. We observed in this high CV risk cohort that early cardio-oncology consultation was feasible, led to CV medication optimization in majority (>60%) of participants—including two-thirds of those with a primary cardiologist, and was viewed positively by patients. The most common intervention was initiating/intensifying statin therapy, for which this recommendation in several participants was based on CAC and/or consideration of potentially cardiotoxic RT/chemotherapy/systemic therapy treatment(s), underscoring the importance of cardio-oncology assessment of the totality of CV risks. Even among low ASCVD risk patients, 40% (2/5) harbored CAC with LDL > 130 mg/dL (including one of two patients with detectable CHIP) for which statins were started/increased (Table 2), highlighting the need for comprehensive CV risk assessment. While there may be concern amongst oncologists that emphasizing cardiovascular health during cancer treatment may add excess fear and worry to patients, most reported that they were glad to know this information and it was not overwhelming or anxiety-inducing.
Our study demonstrated how readily available CV risk markers, such as LAD radiation dose exposure and/or the presence of CAC on RT planning CTs, can capture additional at-risk patients for CV events. Evidence suggests that cardiac substructure radiation dose exposure, particularly to the left coronary arteries, is a better predictor of MACE compared to whole heart dose (i.e., mean heart dose) and warrants inclusion into the global CV risk assessment of patients receiving cardiotoxic cancer therapies6,10,11,12,13. Indeed, LAD and other coronary radiation dose limits based this data have recently been implemented into U.S. National Comprehensive Cancer Network guidelines for Hodgkin lymphoma30. Further, baseline CAC on RT planning CT scans has been associated with increased risk of major cardiac events in patients with breast34,35 and lung cancer9,36,37,38, yet CAC assessment prior to initiation of cancer treatment is not routinely performed. It is notable that approximately 50% of patients without known ASCVD had CAC > 0 in this cohort, which is consistent with data supporting shared risk factors and epidemiology for CVD and cancer. Results from a large retrospective multicenter cohort of nearly 9,000 adults aged 40–75 years without known CVD and with ASCVD risk of 5–7.5% showed that approximately 35% had CAC = 0, 38% with CAC 1–99, and 27% with CAC ≥ 10039. In a study of U.S. adults aged 30–45 years, prevalence of CAC > 0 was 26% among White males, 16% among Black males, 10% among White females, and 7% among Black females40. This has not been systematically explored in the lowest risk cohorts (i.e., ASCVD risk <5%), but several studies have shown that rates of CAC > 0 can vary based on certain risk-enhancing factors and thus CAC assessment can be considered when these are present as per the 2018 American Heart Association (AHA) guidelines26. Similarly, the Canadian Cardiovascular Society (CCS) reports that CAC screening can be considered for low-risk individuals >40 years of age with a family history of premature ASCVD and genetic risk-enhancing factors (i.e., familial hypercholesterolemia or elevated lipoprotein[a], as these patients may have more than a 40% rate of CAC > 041. Together with the data demonstrating the pooled cohort equation for ASCVD risk in the U.S. does not perform well in patients with cancer and may in fact underestimate CV risk in this setting42, incorporation of novel CV risk markers in patient with cancer, and particularly those exposed to cardiotoxic cancer therapies, is urgently needed.
Given the emerging role of CHIP as a potent CV risk marker and its enrichment in patients with cancer and RT as a power risk factor, we further characterized the prevalence of CHIP at baseline and after completion of RT. Consistent with others24, we observed a high baseline prevalence of CHIP (8/20, 40%) as well as the development of new variants in 3/20 patients following completion of RT. This is consistent with the observation that radiation exposure is one of the strongest risk factors for CHIP, causing genotoxic stress that preferentially selects for mutations in DNA damage response genes43, such as TP53, PPM1D, Ataxia-telangiectasia-mutated (ATM), and Checkpoint kinases 1,2 (CHK1, CHK2)44. Specifically, experimental studies have demonstrated that the powerful selective pressure from the genotoxic stress of RT results in robust expansion of clones harboring mutations in DNA damage response genes that confer resistance to these stressors45,46,47,48. Moreover, specific CHIP variants, including RT-induced clones, may better predict for CV risk, metabolic perturbations, and/or response to anti-inflammatory CVD therapies49,50,51. Together, these observations highlight the need for further investigation of the utility of CHIP monitoring in patients with cancer, particularly those treated with RT or exposed to other cardiotoxic cancer therapies.
Statins are the cornerstone for ASCVD risk mitigation due to their pleiotropic lipid-lowering, anti-inflammatory, and anti-fibrotic properties. A recent study suggests statin dose intensity was independently associated with improved survival following definitive chemoradiotherapy for NSCLC16 A similar study in patients with locally advanced NSCLC observed that high heart radiation dose was associated with mortality in statin naïve patients, but not in patients on statin therapy, suggesting a potential cardioprotective effect in these high risk patients3. Notably, there was no difference in cardiac event rates in the above studies, which may be explained in part by the observation that patients with lung cancer are typically under optimized for CV risk and that patients on statin therapy in retrospective cohorts often represent the highest CV risk strata. This is consistent with the current findings that even among patients on statin therapy and/or with a baseline cardiologist, initiation and/or intensification of statin therapy was frequently warranted. Further, ASCVD risk estimation based solely on traditional risk models, such as the PCE, may under capture at risk populations that would benefit from primary prevention statin use. As such, there is a need for improved CV risk estimation models in patients with cancer treated with cardiotoxic cancer therapies (such as thoracic RT), that incorporate the diagnosis of cancer, cardiotoxic cancer treatment(s), and enhanced biomarkers, such as CAC, CHIP, and potentially others. The ongoing CARTIER study (NCT03711110) is a much awaited randomized, open-label clinical trial studying the impact of intensive CV monitoring and treatment vs usual care in patients ≥65 years old with colon, breast, or hematologic cancers. Together, these studies and the current study highlight the existing knowledge gaps in identifying patients with cancer who may benefit most from earlier and/or intensified CV risk intervention strategies.
Importantly, we recognize that cardio-oncology is not a widely available resource geographically and that cardiovascular optimization and guidelines-based care—including utilization of cardio-oncology consensus statements and guidelines, can be performed and delivered by primary care physicians and cardiologists, as well as specialized cardio-oncologists. It will be important for future national and international guidelines to aid in more clearly defining risk criteria that, if met, should warrant cardiology or cardio-oncology referral based on risk factors and treatment exposures. In the current trial, we utilized the specialized expertise of cardio-oncology to reliably measure the optimization gap that exists in these high cardiovascular risk patients. In future studies it will be important to leverage shared communication between oncology, primary care physicians, and cardiology/cardio-oncology to find sustainable solutions to cardiovascular risk optimization on a larger scale. Moreover, the oncology community may benefit from lessons learned in the arena of perioperative cardiovascular risk assessment52. As patients embark on potentially cardiotoxic cancer therapies, we should standardly seek to identify unstable or undiagnosed cardiac conditions, estimate the risk of major adverse cardiac events (MACE) based on patient- and exposure-specific factors, and to determine whether additional tests or coronary revascularization is warranted.
There are several limitations to this study. Eligibility criteria were permissive for many cancer types, though our cohort was largely comprised of patients with lung and esophageal cancers. A heterogenous cohort was considered reasonable to accrue given the pilot nature of the intervention and that the need for CV optimization in the setting of cardiac radiation exposure (+/- baseline CV risk factors) is not histology-specific, but rather CV risk-specific. There was a wide variance of baseline CV risk factors, though overall this was a high CV risk population with 35% having known ASCVD. The inclusion of patients both with and without an established primary cardiologist introduced heterogeneity in potential for intervention, though the observation that these patients were intervened upon at high frequency was notable, and worthy of further study. Follow up duration was short, given the pilot nature of the trial, though one serious event was observed during the trial period. While most participants had positive feedback about the trial at study completion, two participants reported feeling overwhelmed and more anxious during the intervention, which may reflect a need for greater psychosocial support during cancer therapy and interventions such as these. Lastly, the sample size was small, and it was not powered to detect a difference in MACE vs historical controls nor randomized to detect an incremental benefit of aggressive cardiovascular optimization by cardio-oncology versus primary care or general cardiology. Rather, this study may serve as early, hypothesis generating data to support larger, randomized studies of enhanced cardiovascular risk stratification and early cardiovascular optimization. Despite its small size, our study demonstrates a clear cardiovascular optimization gap and role for enhanced cardiovascular risk assessment to inform guidelines-based care. Future studies analyzing the cost-effectiveness of early cardio-oncology intervention and enhanced risk assessment are warranted.
In conclusion, we observed that early cardio-oncology intervention in high CV risk patients treated with thoracic RT is feasible, resulted in CV medication optimization changes in most patients, and was viewed positively by patients. As oncologic outcomes continue to improve, it will become increasingly important to improve CV assessment and mitigation approaches, including integration with novel CV risk markers (e.g., CHIP), additive effects of cardiotoxic therapies, and optimal surveillance schedules.
Data availability
Individual deidentified participant data (including data dictionaries) supporting the manuscript findings will be shared beginning three months after publication and without end date with qualified investigators whose proposed use of the data has been approved by an institutional review board and following execution of a data use agreement per Cedars-Sinai Medical Center institutional policy. The study protocol will additionally be shared.
References
Velusamy, R., Nolan, M., Murphy, A., Thavendiranathan, P. & Marwick, T. H. Screening for Coronary Artery Disease in Cancer Survivors: JACC: CardioOncology State-of-the-Art Review. JACC CardioOncol. 5, 22–38 (2023).
Al-Kindi, S. G. & Oliveira, G. H. Prevalence of Preexisting Cardiovascular Disease in Patients With Different Types of Cancer: The Unmet Need for Onco-Cardiology. Mayo Clin. Proc. 91, 81–83 (2016).
Atkins, K. M. et al. Statin Use, Heart Radiation Dose, and Survival in Locally Advanced Lung Cancer. Pr. Radiat. Oncol. https://doi.org/10.1016/j.prro.2020.12.006 (2021).
Pope, A. et al. Detection of subclinical atherosclerosis from PET-CT in patients with breast cancer. J. Cardiovasc. Comput. Tomogr. 16, 189–190 (2022).
Stewart, F. A. et al. Ionizing radiation accelerates the development of atherosclerotic lesions in ApoE-/- mice and predisposes to an inflammatory plaque phenotype prone to hemorrhage. Am. J. Pathol. 168, 649–658 (2006).
Atkins, K. M. et al. Association of Left Anterior Descending Coronary Artery Radiation Dose With Major Adverse Cardiac Events and Mortality in Patients With Non–Small Cell Lung Cancer. JAMA Oncol. 7, 206–219 (2021).
Tjong, M. C. et al. Major adverse cardiac event risk prediction model incorporating baseline Cardiac disease, Hypertension, and Logarithmic Left anterior descending coronary artery radiation dose in lung cancer (CHyLL). Radiother. Oncol. 169, 105–113 (2022).
Tjong, M. C. et al. External validation of Cardiac disease, Hypertension, and Logarithmic Left anterior descending coronary artery radiation dose (CHyLL) for predicting major adverse cardiac events after lung cancer radiotherapy. Clin. Transl. Radiat. Oncol. 42, 100660 (2023).
No, H. J. et al. Predicting Adverse Cardiac Events After Radiotherapy for Locally Advanced Non–Small Cell Lung Cancer. JACC: CardioOncol. https://doi.org/10.1016/j.jaccao.2023.08.007 (2023).
Cai, G. et al. Cardiac Substructures Dosimetric Predictors for Cardiac Toxicity After Definitive Radiotherapy in Esophageal Cancer. Int J. Radiat. Oncol. Biol. Phys. 115, 366–381 (2023).
Wang, X. et al. The Impact of Radiation Dose to Heart Substructures on Major Coronary Events and Patient Survival after Chemoradiation Therapy for Esophageal Cancer. Cancers 14, 1304 (2022).
Zureick, A. H. et al. Dose to the Left Anterior Descending Artery Correlates With Cardiac Events After Irradiation for Breast Cancer. Int. J. Radiat. Oncol. Biol. Phys. 114, 130–139 (2022).
Wennstig, A. K. et al. The relationship between radiation doses to coronary arteries and location of coronary stenosis requiring intervention in breast cancer survivors. Radiat. Oncol. 14, 40 (2019).
Atkins, K. M., Rawal, B. & Mak, R. H. Reply: Two Statistical Methodological Issues Worth of Attention for Survival Data Using Multivariable Models. J. Am. Coll. Cardiol. 74, 1739 (2019).
Boulet, J. et al. Statin Use and Risk of Vascular Events Among Cancer Patients After Radiotherapy to the Thorax, Head, and Neck. J. Am. Heart Assoc. 8, e005996 (2019).
Walls, G. M. et al. Association between statin therapy dose intensity and radiation cardiotoxicity in non-small cell lung cancer: Results from the NI-HEART study. Radiother. Oncol. 186, 109762 (2023).
Herrmann, J. et al. Defining cardiovascular toxicities of cancer therapies: an International Cardio-Oncology Society (IC-OS) consensus statement. Eur. Heart J. 43, 280–299 (2022).
Lyon, A. R. et al. 2022 ESC Guidelines on cardio-oncology developed in collaboration with the European Hematology Association (EHA), the European Society for Therapeutic Radiology and Oncology (ESTRO) and the International Cardio-Oncology Society (IC-OS) Developed by the task force on cardio-oncology of the European Society of Cardiology (ESC). Eur. Heart J. 43, 4229–4361 (2022).
Mitchell, J. D. et al. Cardiovascular Manifestations From Therapeutic Radiation: A Multidisciplinary Expert Consensus Statement From the International Cardio-Oncology Society. JACC CardioOncol. 3, 360–380 (2021).
Calvillo-Argüelles, O. et al. Cardiovascular Disease Among Patients With AML and CHIP-Related Mutations. JACC CardioOncol 4, 38–49 (2022).
Fuster, J. J. et al. Clonal hematopoiesis associated with TET2 deficiency accelerates atherosclerosis development in mice. Science 355, 842–847 (2017).
Jaiswal, S. et al. Clonal Hematopoiesis and Risk of Atherosclerotic Cardiovascular Disease. N. Engl. J. Med. 377, 111–121 (2017).
Libby, P. et al. Clonal Hematopoiesis: Crossroads of Aging, Cardiovascular Disease, and Cancer: JACC Review Topic of the Week. J. Am. Coll. Cardiol. 74, 567–577 (2019).
Coombs, C. C. et al. Therapy-Related Clonal Hematopoiesis in Patients with Non-hematologic Cancers Is Common and Associated with Adverse Clinical Outcomes. Cell Stem Cell 21, 374–382.e4 (2017).
Armenian, S. H. et al. Prevention and Monitoring of Cardiac Dysfunction in Survivors of Adult Cancers: American Society of Clinical Oncology Clinical Practice Guideline. J. Clin. Oncol. 35, 893–911 (2017).
Grundy, S. M. et al. 2018 AHA/ACC/AACVPR/AAPA/ABC/ACPM/ADA/AGS/APhA/ASPC/NLA/PCNA guideline on the management of blood cholesterol: A report of the American college of cardiology/American heart association task force on clinical practice guidelines. Circulation, 139, https://doi.org/10.1161/cir.0000000000000625 (2019).
Feng, M. et al. Development and validation of a heart atlas to study cardiac exposure to radiation following treatment for breast cancer. Int J. Radiat. Oncol. Biol. Phys. 79, 10–18 (2011).
Atkins, K. M. et al. Mean Heart Dose Is an Inadequate Surrogate for Left Anterior Descending Coronary Artery Dose and the Risk of Major Adverse Cardiac Events in Lung Cancer Radiation Therapy. Int J. Radiat. Oncol. Biol. Phys. 110, 1473–1479 (2021).
Non-Small Cell Lung Cancer Version: 3.2023. National Comprehensive Cancer Network (NCCN) Guidelines. Available at https://www.nccn.org/professionals/physician_gls/pdf/nscl.pdf (2023). Accessed May 15, 2023.
NCCN Clinical Practice Guidelines in Oncology. NCCN Guidelines Version 3.2024 Hodgkin Lymphoma. National Comprehensive Cancer Network (NCCN) Guidelines. Available at https://www.nccn.org/professionals/physician_gls/pdf/hodgkins.pdf (2024). Accessed July 12, 2024.
Timmerman, R. A story of hypofractionation and the table on the wall. Int. J. Radiat. Oncol. Biol. Phys. 112, 4–21 (2022).
Agatston, A. S. et al. Quantification of coronary artery calcium using ultrafast computed tomography. J. Am. Coll. Cardiol. 15, 827–832 (1990).
Lyon, A. R. et al. Baseline cardiovascular risk assessment in cancer patients scheduled to receive cardiotoxic cancer therapies: a position statement and new risk assessment tools from the Cardio-Oncology Study Group of the Heart Failure Association of the European Society of Cardiology in collaboration with the International Cardio-Oncology Society. Eur. J. Heart Fail 22, 1945–1960 (2020).
Roos, C. T. G. et al. Is the coronary artery calcium score associated with acute coronary events in breast cancer patients treated with radiotherapy? Radiother. Oncol. 126, 170–176 (2018).
Gal, R. et al. Others. Identification of risk of cardiovascular disease by automatic quantification of coronary artery calcifications on radiotherapy planning CT scans in patients with breast cancer. JAMA Oncol. 7, 1024–1032 (2021).
Atkins, K. M. et al. Elevated Coronary Artery Calcium Quantified by a Validated Deep Learning Model From Lung Cancer Radiotherapy Planning Scans Predicts Mortality. JCO Clin. Cancer Inf. 6, e2100095 (2022).
Wang, K. et al. Coronary Artery Calcifications and Cardiac Risk After Radiation Therapy for Stage III Lung Cancer. Int. J. Radiat. Oncol. Biol. Phys. 112, 188–196 (2022).
Koutroumpakis, E. et al. Coronary artery calcium score on standard of care oncologic CT scans for the prediction of adverse cardiovascular events in patients with non-small cell lung cancer treated with concurrent chemoradiotherapy. Front. Cardiovasc. Med. 9, 1071701 (2022).
Uddin, S. M. I. et al. Coronary Artery Calcium Scoring for Adults at Borderline 10-Year ASCVD Risk: The CAC Consortium. J. Am. Coll. Cardiol. 78, 537–538 (2021).
Javaid, A. et al. Distribution of Coronary Artery Calcium by Age, Sex, and Race Among Patients 30-45 Years Old. J. Am. Coll. Cardiol. 79, 1873–1886 (2022).
Dudum, R. et al. Coronary artery calcium scoring in low risk patients with family history of coronary heart disease: Validation of the SCCT guideline approach in the coronary artery calcium consortium. J. Cardiovasc. Comput. Tomogr. 13, 21–25 (2019).
Polter, E. J. et al. Performance of the pooled cohort equations in cancer survivors: the Atherosclerosis Risk in Communities study. J. Cancer Surviv 18, 124–134 (2024).
Bolton, K. L. et al. Cancer therapy shapes the fitness landscape of clonal hematopoiesis. Nat. Genet. 52, 1219–1226 (2020).
Jackson, S. P. & Bartek, J. The DNA-damage response in human biology and disease. Nature 461, 1071–1078 (2009).
Bondar, T. & Medzhitov, R. p53-mediated hematopoietic stem and progenitor cell competition. Cell Stem Cell 6, 309–322 (2010).
Hsu, J. I. et al. PPM1D Mutations Drive Clonal Hematopoiesis in Response to Cytotoxic Chemotherapy. Cell Stem Cell 23, 700–713.e6 (2018).
Khan, M. A. et al. Association of left atrial volume index and all-cause mortality in patients referred for routine cardiovascular magnetic resonance: a multicenter study. J. Cardiovasc. Magn. Reson. 21, 4 (2019).
Yura, Y., Cochran, J. D. & Walsh, K. Therapy-Related Clonal Hematopoiesis: A New Link Between Cancer and Cardiovascular Disease. Heart Fail. Clin. 18, 349–359 (2022).
Svensson, E. C. et al. TET2-Driven Clonal Hematopoiesis and Response to Canakinumab: An Exploratory Analysis of the CANTOS Randomized Clinical Trial. JAMA Cardiol. 7, 521–528 (2022).
Yura, Y. et al. The Cancer Therapy-Related Clonal Hematopoiesis Driver Gene Ppm1d Promotes Inflammation and Non-Ischemic Heart Failure in Mice. Circ. Res. 129, 684–698 (2021).
Fuster, J. J. et al. TET2-Loss-of-Function-Driven Clonal Hematopoiesis Exacerbates Experimental Insulin Resistance in Aging and Obesity. Cell Rep. 33, 108326 (2020).
Raslau, D. et al. Preoperative Cardiac Risk Assessment. Mayo Clin. Proc. 95, 1064–1079 (2020).
Acknowledgements
K.M.A. reports funding from the Garber Award for Cancer Research.
Author information
Authors and Affiliations
Contributions
K.M.A., A.P.N., and R.H.M. conceptualized the research idea. K.M.A. and A.P.N. were responsible for over-seeing the project, clinical trial, data collection, data analysis. F.P., J.O.G. assisted with clinical trial management and data collection. S.C.Z., J.O.G., C.E., K.D.S., F.P., W.Z. were responsible for data collection and analysis. K.M.A., A.P.M., R.H.M., S.C.Z. devised the data analysis plan and developed the initial manuscript. E.V., C.E., M.K., B.H., A.M. assisted with management and coordination of the project and helped review the manuscript. All authors performed a critical review and approval of the final manuscript for interpretation of the data, intellectual input, and to confirm accuracy.
Corresponding author
Ethics declarations
Competing interests
K.A. reports honoraria from OncLive. M.K. reports receiving personal fees from Theragenics, Alessa, GTMedical, and Springer, outside the submitted work. E.V. reports consulting with Bayer, Eli Lilly, AstraZeneca, Janssen, Pfizer, Sanofi, Thermo Fisher, Illumina, Caris, Tempus, PierianDx; employment by LungLifeAI. R.M. reports consulting with AstraZeneca, ViewRay, Novartis, Sio Capital Mgmt, Varian Medical Systems; advisory board with ViewRay, AstraZeneca; grant funding from AstraZeneca, ViewRay. The remaining authors have nothing to disclose.
Peer review
Peer review information
Communications Medicine thanks Michael Ewer and the other, anonymous, reviewer(s) for their contribution to the peer review of this work. Peer reviewer reports are available.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Zhang, S.C., Gasho, J.O., Eno, C. et al. Early cardio-oncology intervention in thoracic radiotherapy: prospective single-arm pilot study. Commun Med 5, 43 (2025). https://doi.org/10.1038/s43856-025-00761-6
Received:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s43856-025-00761-6