Abstract
Background
Excessive daytime napping has been associated with neurodegeneration in older adults, but prior research has focused on nap duration and frequency. Emerging frameworks emphasize the multidimensionality of sleep, but it remains unknown whether other dimensions of napping (e.g., timing, variability) are linked to neurodegeneration. To address this gap, we investigated the associations of daytime nap timing and intraindividual variability of nap duration with incident Alzheimer’s dementia and Alzheimer’s disease pathology.
Methods
We analyzed data from 936 older adults (age range: 56–99; 77% female) in the Rush Memory and Aging Project to examine incident Alzheimer’s dementia and from 320 deceased participants (age range at death: 71–105; 70% female) to examine Alzheimer’s pathology. The proportions of morning (9–11am) and early afternoon naps (1–3 pm) and the intraindividual variability of nap duration were assessed using actigraphy. Participants completed neurological assessments at baseline and annually for up to 17 years. In deceased participants, amyloid β and neurofibrillary tangles were examined.
Results
Here we show that more morning naps are linked to a higher risk of Alzheimer’s dementia, whereas more early afternoon naps are linked to reduced amyloid β levels. Higher intraindividual variability of nap duration is shown to be associated with increased amyloid β and neurofibrillary tangles.
Conclusions
Our findings suggest that specific timing patterns and irregularities in daytime napping are linked to Alzheimer’s disease risk and pathology. Multi-dimensional assessments of nap behaviors may aid in risk stratification for neurocognitive impairment and offer a potential target for interventions aimed at promoting healthy cognitive aging.
Plain Language Summary
Research suggests that too much daytime napping is associated with adverse brain health in older adults. However, how the timing and regularity of daytime naps link to cognitive health remain unclear. We followed over 1000 older adults for up to 17 years and used wrist-worn watches to measure their nap patterns. We found that more frequent morning naps were linked to a higher risk of developing Alzheimer’s dementia. Additionally, more naps in the early afternoon and more consistent nap patterns were linked to lower levels of Alzheimer’s pathology. These findings suggest that when and how people nap during the day may help inform the risk of developing dementia.
Similar content being viewed by others
Introduction
Daytime napping is common among older adults, with a prevalence rate ranging between 20% and 60%1. Growing evidence suggests that excessive daytime naps may be associated with poorer neurocognitive health. For example, in a sample of 2751 community-dwelling older men, longer nap duration, as measured by actigraphy, was associated with greater cognitive decline and a higher risk for cognitive impairment during a 12-year follow-up period2. In addition, our recent study employed objective measurement of daytime naps and found that prolonged nap durations and increased nap frequency were associated with an elevated risk of Alzheimer’s dementia3. However, these existing studies mainly focus on the duration and frequency of naps. In the realm of sleep health, there is a growing body of evidence supporting a multi-dimensional assessment framework to better comprehend the complexities of this vital process4. The rationale behind adopting such a multi-dimensional approach is rooted in the understanding that sleep health encompasses more than just the quantity of sleep or the presence/absence of sleep disorders4. Additional dimensions, including timing and (ir)regularity, provide valuable insights for a more holistic understanding.
Previous studies have offered important implications regarding the timing and (ir)regularity of daytime naps. For example, naps in the morning may be due to fatigue upon awakening caused by nonrestorative sleep and inflammation5. Naps taken in the early afternoon is considered closely aligned with the circadian rhythms, coinciding with the post-lunch dips in alertness and body temperature that also occur at nocturnal bedtime6. Additionally, afternoon naps tend to be rich in slow-wave sleep7, which has been associated with the clearance of amyloid beta (Aβ) plaques and slower cognitive decline8. These prior observations suggest that the timing of naps may play a role in modulating Alzheimer’s disease (AD) pathologies and progression. In terms of (ir)regularity, the intraindividual variability (IIV) in sleep has been associated with a broad spectrum of negative health consequences, including cognition9. It remains unclear whether IIV in napping behaviors exhibits similar adverse associations with neurocognitive health.
Therefore, in this work, we seek to utilize the multi-dimensional sleep health framework4 to examine the relationships of timing and IIV of daytime napping with AD in older adults. We examine whether these napping characteristics at baseline are associated with the risk of Alzheimer’s dementia during the follow-up. Additionally, we investigate whether these napping characteristics before death are associated with AD pathology. We hypothesize that more naps in the morning and increased IIV in daytime napping are associated with a higher risk for Alzheimer’s dementia and greater AD pathological burdens, independent of mean nap duration or frequency. We also hypothesize that napping in the early afternoon is associated with better neurocognitive outcomes.
Methods
Participants
We analyzed data from the Rush Memory and Aging Project (MAP), which is a clinical-pathologic cohort study launched in 1997. Participants were recruited from retirement communities, senior and subsidized housing, and via church groups in northeastern Illinois (USA) and were followed annually10. The study baseline was defined as the participants’ initial assessment in the Rush MAP. In 2005, wrist actigraphy (Actical, Phillips Respironics; Bend, OR, USA) was incorporated into the study protocol as an annual assessment. The analytic baseline for the current study was defined as the initial actigraphy assessment. The data utilized in this study, including wrist actigraphy, dementia diagnosis, and AD pathologies, were gathered up until December 2022, marking the cutoff point for our dataset.
Figure 1 shows the flow of participants for different sets of analyses. For the analyses on incident Alzheimer’s dementia, we excluded participants diagnosed with Alzheimer’s dementia at baseline, those without follow-up neurocognitive assessments, and those with mean nap durations <15 min or >4 h. A final sample of 936 participants were included in the incident Alzheimer’s dementia analyses (mean age=81.34; range: 56.14-98.90; 77% female).
Incident Alzheimer’s dementia analyses included participants free of Alzheimer’s dementia at baseline, with follow-up neurocognitive assessments, and moderate nap durations (15 min to 4 h/day). AD pathology analyses included participants who died within three years of their last actigraphy assessment and had moderate nap durations. The two sub-samples (n = 936 and n = 320) overlap partially and are not entirely distinct; n = 320 is not a subset of n = 936.
For the analyses on AD pathology, we focused on participants who had died and undergone brain autopsy. From this group, we excluded individuals who died more than three years after their last actigraphy assessment and those with mean nap durations <15 min or >4 h. A final sample of 320 participants were included in the AD pathology analyses (mean age at death = 90.14; range: 71.15–104.59; 70% female).
The Rush MAP was approved by an institutional review board of the Rush University Medical Center. All participants signed informed consent, a repository consent for data sharing, and Anatomical Gift Act for brain donation. Access to MAP resources was granted through an approved application (#2708) with the Rush Alzheimer’s Disease Center via the Rush Alzheimer’s Disease Center Research Resource Sharing Hub. The current study involves secondary analyses of previously collected MAP data. The protocol was reviewed by the Mass General Brigham Institutional Review Board (MGB IRB) and determined to be non-human subjects research.
Assessment of daytime napping
Participants wore the Actical device on their non-dominant wrist continuously for up to 14 days (Mean = 10, standard deviation [SD] = 1). The Actical device recorded three-dimensional acceleration in 15-second epochs with a 32 Hz sampling rate, which were then integrated into one-dimensional activity counts. The previously established and validated Cole-Kripke algorithm was employed for sleep detection11,12. To be consistent with this algorithm, we first re-sampled the activity count signal based on a 1-min epoch length. To eliminate periods where Actical devices were potentially off the wrist, we excluded the data segments with zero activity counts consecutively for ≥2 h3.
Daytime napping was defined as daytime sleep episodes between 9am and 7pm3. If two nap segments were separated by ≤3 min, they were merged as one. If two nap segments were more than 3 min apart, they were counted as two nap episodes. Driven by our hypotheses, we calculated the percentage of naps for each time window—morning (9am–11am; am%) and early afternoon (1pm–3pm; pm%)—by dividing the duration of naps within each window by the total duration of daytime naps (9am-7pm). Naps occurring outside these time windows (11am–1pm and 3pm–7pm) were not analyzed, as there were no specific hypotheses related to these periods. This approach helped minimize exploratory analyses and reduce the risk of type I errors. To quantify intraindividual variability in naps, we calculated each participant’s daily nap duration and then computed the standard deviation of these durations across all days9.
In our study, we excluded participants who napped for <15 min or > 4 h per day. Minimal napping of <15 min raises questions about whether the behavior reflects a meaningful nap habit at a specific time (e.g., morning). Similarly, extensive napping > 4 h may indicate a predominant daytime sleep pattern with frequent awakenings and fragmented sleep episodes throughout the day. In both instances, identifying a clear nap pattern or a preference for specific nap timing becomes challenging.
Assessment of AD outcomes
Clinical assessments were conducted annually and clinical diagnosis of cognitive status was rendered based on participants’ performance on the cognitive tests, clinical judgment of neuropsychologists, and diagnostic classification by clinicians13. Diagnoses of Alzheimer’s dementia was based on the criteria recommended by the National Institute of Neurological and Communicative Disorders and Stroke and the Alzheimer’s Disease and Related Disorders Association (NINCDS/ADRDA)14.
Postmortem brain autopsy was performed by staff who were masked to participants’ clinical data. Researchers labeled Aβ-immunoreactive plaques with an N terminus directed monoclonal antibody (10D5, Elan Pharmaceuticals, Dublin, Ireland; 1:1000). Aβ protein was identified using molecularly-specific immunohistochemistry and were quantified through image analysis with the value presented as the percent area of cortex occupied by Aβ15. In addition, researchers labeled paired helical filament tau tangles with an antibody specific for phosphorylated tau (AT8, Innogenetics, San Ramon, California, USA; 1:1000). Neuronal neurofibrillary tangles were identified through molecularly specific immunohistochemistry, and their density was determined by stereology15. The mean Aβ and neurofibrillary tangles of eight brain regions (hippocampus, entorhinal cortex, midfrontal cortex, inferior temporal cortex, angular gyrus, calcarine cortex, anterior cingulate cortex, and superior frontal cortex) were calculated to represent the average Aβ burden and tangle density, respectively. The average pathological burdens across these eight brain regions were used to align with established methods and prior publications from the Rush MAP15. Regional dissociations are not possible due to the high inter-correlations among the regions.
Assessment of covariates
Demographic covariates included participants’ age, sex, race/ethnicity (White vs. non-White), and education (number of years of regular school). Body mass index (BMI) was calculated as weight in kilograms divided by height in meters squared. Alcohol consumption was assessed at the study baseline and dichotomized as consuming less than one drink/glass per month or consuming one drink/glass per month or more. Smoking status was similarly assessed at the study baseline and dichotomized as never or former/current smoker. Physical activity was calculated as the sum of hours per week that the participant engaged in 5 categories of activities: walking for exercise, gardening or yard work, calisthenics or general exercise, bicycle riding (including stationary bikes), and swimming or water exercises.
Sleep-related covariates included nighttime sleep duration, sleep fragmentation, and wake after sleep onset. Nighttime sleep duration was the total hours scored as sleep between 9 pm and 7am on actigraphy, determined by the Cole-Kripke algorithm11. Sleep fragmentation was the probability of having an arousal (e.g., a non-zero activity count) after a long (~5 min) period of sleep16. Wake after sleep onset was the total minutes of wakefulness between the first and last nighttime sleep epochs. Rest-activity rhythm variables included interdaily stability and intradaily variability derived from actigraphy data using non-parametric analyses17. Interdaily stability measures the robustness of daily activity rhythm across days, and intradaily variability measures the fragmentation of daily activity rhythms within a day.
Baseline comorbidities and medications included depressive symptoms measured by the Center for Epidemiologic Studies-Depression Scale18, self-reported thyroid disease (Yes/No), vascular disease burden score (0–4; included claudication, stroke, heart conditions, and congestive heart failure), vascular disease risk factors (0–3; included hypertension, diabetes, and smoking history), medications for anxiety and insomnia, analgesics, anticonvulsants, and beta blockers.
To assess APOE genotype, DNA was extracted from peripheral blood mononuclear cells or brain tissue. Genotyping was conducted by Agencourt Bioscience Corporation (Beverly, MA, USA) using high-throughput sequencing of codon 112 (position 3937) and codon 158 (position 4075) of exon 4 of the APOE gene on chromosome 19. Participants were classified as APOE ε4 carriers if they had at least one ε4 allele.
Statistics and reproducibility
Pearson’s correlations or independent samples t-tests were performed to examine whether baseline napping characteristics were associated with age or sex.
Cox proportional hazards models were performed to test the associations of percentage of naps in the morning (hereafter am%), percentage of naps in the early afternoon (hereafter pm%), and intraindividual variability in nap duration across days (hereafter IIV) at baseline with incident Alzheimer’s dementia. We performed an initial model (i.e., Model A) for each of the three variables with adjustments of demographics, BMI, smoking, alcohol consumption, mean nap duration, and mean nap frequency. We performed additional models by adding the following covariates to Model A: baseline nighttime sleep duration, sleep fragmentation index, wake after sleep onset, interdaily stability, and intradaily variability (Model B); baseline comorbidities and medication use (Model C); APOE ε4 carrier status (Model D). In Model E, we adjusted for all covariates mentioned above. The overall follow-up of survivors is about 95%. Thus, there are very few people who withdrew and only a few lost to follow-up. Participants were right censored if deceased, withdrawn, or lost to follow-up before developing Alzheimer’s dementia. Time was treated as a continuous variable in integer years since baseline actigraphy assessment, and Efron’s approximation was used to handle tied events in the Cox proportional hazards models. Additionally, to reduce the effect of reverse causality, we conducted sensitivity analyses, excluding participants who developed Alzheimer’s dementia within two years of actigraphy assessment.
Linear regression models were performed to regress the Aβ and neurofibrillary tangles against am%, pm%, and IIV of napping, separately, using nap data obtained from the last actigraphy assessment (i.e., proximate to death). The models were adjusted for all covariates aforementioned, as well as the time lag between the last actigraphy assessment and death.
All analyses were conducted in JMP Pro 16. Square-root transformation (to correct for right skewness) and standardization have been applied to nap duration, am%, pm%, IIV, Aβ, and neurofibrillary tangles. Listwise deletion was used for handling missing data. All tests were two-tailed, with p < 0.05 considered statistically significant.
Reporting summary
Further information on research design is available in the Nature Portfolio Reporting Summary linked to this article.
Results
Figure 1 shows the flow of participants for different sets of analyses. Table 1 shows the participants’ characteristics for the incident Alzheimer’s dementia analyses. The 936 participants were on average 81.3 years old (SD = 7.2) at actigraphy baseline, 77% female, and 93% White. Table 2 shows the participants’ characteristics for AD pathology analyses. The 320 participants were on average 90.1 years old (SD = 6.2) at death, 70% female, and 98% White.
We first examined the associations between age, sex, and nap characteristics at baseline. As shown in Fig. 2, older age was associated with greater daily nap duration (Pearson’s r = 0.14, p < 0.0001), daily nap frequency (r = 0.17, p < 0.0001), and pm% (r = 0.19, p < 0.0001), but not with am% (r = −0.03, p = 0.431) or IIV (r = −0.05, p = 0.113). No significant sex difference was observed in daily nap duration (t = 0.13, p = 0.893), frequency (t = 0.42, p = 0.677), nap timing (am%: t = 0.90, p = 0.369; pm%: t = 0.36, p = 0.719), or IIV (t = 0.15, p = 0.877).
The relationship between a age and nap duration, b sex and nap duration, c age and nap frequency, d sex and nap frequency, e age and proportion of morning naps, f sex and proportion of morning naps, g age and proportion of early afternoon naps, h sex and proportion of early afternoon naps, i age and intraindividual variability in nap duration, and j sex and intraindividual variability in nap duration in n = 936 participants (n = 721 female; n = 215 male). The red solid lines and shaded areas represent lines of best fit and the corresponding 95% confidence intervals. Black bars represent means and standard deviation.
Incident Alzheimer’s dementia
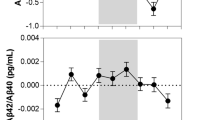
In this set of analysis, 936 participants were included, and out of them, 269 (28.74%) developed Alzheimer’s dementia within on average 6.0 years (SD = 3.7; range: 0.9–17.0) after analytic baseline. Table 1 presents the characteristics of these participants, Table 3 presents the summary results, and Supplementary Tables 1–3 present the full results. Cox proportional hazards models showed that a greater am% was associated with a higher risk for Alzheimer’s dementia, after adjusting for demographic characteristics, BMI, smoking, alcohol consumption, physical activity, and mean nap duration and frequency. Specifically, as shown in Fig. 3a, for each 1 SD increase in am%, the risk of developing Alzheimer’s dementia increased by 15% (hazard ratio [HR] = 1.15, 95% confidence interval [CI]: 1.01–1.30, p = 0.035). This effect was equivalent to the effect of being 1.3 years older, and the results were consistent after additionally adjusting for sleep and rest-activity rhythms (Model B: HR = 1.15, 95%CI: 1.01–1.32, p = 0.040) or comorbidities and medications (Model C: HR = 1.14, 95%CI: 1.002–1.30, p = 0.047). Nevertheless, this association became marginal or non-significant when APOE e4 carrier status was adjusted (Model D: HR = 1.11, 95%CI: 0.97–1.26, p = 0.124) or when all covariates were accounted for (Model E: HR = 1.10, 95%CI: 0.95–1.27, p = 0.192). The pm% or IIV was not associated with incident Alzheimer’s dementia (see Supplementary Tables 2–3 for full results).
a Relationship between the proportion of morning naps and incidents of Alzheimer’s dementia. The lines represent individual participants at 10th and 90th percentiles based on their proportions of naps in the morning. b Relationship between the proportion of early afternoon naps and amyloid β. c Relationship between intraindividual variability in nap duration and amyloid β. d Relationship between intraindividual variability in nap duration and neurofibrillary tangles. The red lines and shaded areas represent lines of best fit and the corresponding 95% confidence intervals.
Sensitivity analyses results are presented in Supplementary Tables 4–6. After excluding participants who developed Alzheimer’s dementia within 2 years after baseline actigraphy assessment, greater am% showed a trend of association with greater risk for Alzheimer’s dementia incidents in Models A and C (Model A: HR = 1.12, 95%CI: 0.98-1.28, p = 0.092; Model C: HR = 1.12, 95%CI: 0.98-1.29, p = 0.093). The pm% or IIV was not associated with incident Alzheimer’s dementia (see Supplementary Tables 5–6 for full results).
AD pathologies
In this set of analyses, n = 320 participants were included (Fig. 1). They died on average 1.32 years (SD = 0.83; range: 0.0-3.0) after their last actigraphy assessment. Table 2 presents the characteristics of these participants, Table 3 presents the summary results, and Supplementary Tables 7–8 present the full results. Linear regression models showed that a 1-SD increase in am% was associated with a non-significant trend of 0.15 SD increase in Aβ level (β = 0.15, SE = 0.09, p = 0.085; see Supplementary Table 7 for full results). Interestingly, as shown in Fig. 3b, a 1 SD increase in pm% was associated with a 0.19 SD decrease in Aβ (β = −0.19, SE = 0.09, p = 0.034). Neither the proportion of morning naps nor early-afternoon naps was associated with neurofibrillary tangles (Table 3; see Supplementary Table 8 for full results). As shown in Fig. 3c, d, greater IIV was associated with greater AD pathologies at death. Specifically, a 1 SD increase in the IIV was associated with a 0.23 SD (SE = 0.11, p = 0.033; Table 3) increase in Aβ and a 0.21 SD (SE = 0.08, p = 0.006) increase in neurofibrillary tangles.
Discussion
We investigated the relationships between the timing and variability of daytime nap with AD-related outcomes in older adults. We found that aging was associated with more naps in the early afternoon. Besides, more naps in the morning were associated with a higher risk of Alzheimer’s dementia, whereas early afternoon naps were associated with a decreased Aβ level. Additionally, we found that the variability in daytime naps was associated with increased levels of Aβ and neurofibrillary tangles.
Daytime napping, particularly in the morning, may be an indicator or consequence of disruptions in sleep and/or circadian function, thereby connecting to neurodegeneration. Prior studies have revealed multiple mechanisms for the links between sleep and circadian disruptions with neurodegenerative processes. For instance, sleep disruptions and circadian dysfunction may decrease glymphatic flow19, leading to increased Aβ deposition20 and inflammation21. Conversely, neurodegeneration may interfere with brain regions responsible for the clock regulation or regulating sleep-wake, thus compromising sleep at different times of the day22. Supporting the notion that daytime napping may be linked to nighttime sleep disturbances, previous studies have found that individuals experiencing longer wake after sleep onset at night (i.e., indicative of poor sleep quality) initiated daytime naps earlier during the day23.
Early afternoon napping was associated with decreased Aβ deposition. Past studies have found self-report afternoon or midday naps to be associated with better cognitive performance24. Afternoon naps tend to be efficient and rich in slow-wave sleep7. During sleep, especially slow-wave sleep, the interstitial space is enlarged, which promotes the exchange of cerebrospinal fluid and interstitial fluid and increases the rate of Aβ clearance19. Therefore, the potential of having more slow-wave sleep during early afternoon hours may benefit Aβ clearance, explaining our observed decrease in Aβ in association with more prevalent early afternoon naps.
Increased variability in napping is linked to greater levels of Aβ and neurofibrillary tangles. This is partially in keeping with previous research that showed a connection between IIV in napping and health outcomes, such as chronic diseseases25. Similarly, such variability in naps may reflect disruptions in sleep and circadian rhythm regulation, which are bidirectionally associated with neurodegeneration as discussed above22. Interestingly, we did not find any associations between the variability in daytime naps with incident Alzheimer’s dementia. It is conceivable that increased variability in napping patterns across days may be a consequence of the pathological processes associated with AD. However, clinical manifestations of these pathologies can vary due to multiple resilience factors26, which may compromise the direct relationship between nap variability and Alzheimer’s dementia. Future studies should leverage in vivo assessments of AD pathology along with assessments of sleep and cognitive functions to further clarify these possibilities.
While our analyses provide correlational and prospective evidence, additional efforts are needed to elucidate the causal directions of these associations. Some studies have attempted to explore the causal relationships by employing Mendelian randomization, providing preliminary evidence linking habitual daytime napping with Alzheimer’s disease27 and total brain volume28. Mendelian randomization provides valuable initial insights by leveraging genetic variations as natural experiments to infer causality. However, such causal findings must be confirmed through randomized controlled trials (RCT) in future studies. High-quality RCTs are essential to translate these findings into interventions for Alzheimer’s dementia prevention.
The current study has notable strengths, including the use of longitudinal data, the inclusion of both clinical diagnosis and pathology data, and the objective measurement of daytime naps. However, it is important to acknowledge certain limitations. Firstly, while actigraphy for sleep scoring has been validated and is extensively used in field studies, we recognize that polysomnography remains the gold standard for sleep assessment. Secondly, more granular approaches should be developed to clearly separate naps from the primary sleep window, as well as to distinguish individual nap episodes, potentially incorporating sleep diaries or logs and chronotype. Our use of a constant time window for napping detection may have mislabeled certain primary sleep episodes as naps, particularly in the morning hours. With advances in nap assessment, future research could investigate the relationship between other aspects of napping behaviors, such as IIV in nap timing, and cognitive aging to provide a more comprehensive understanding of napping’s role in neurodegenerative diseases. Thirdly, the predominantly White sample in this study precluded an exploration of racial/ethnic or cultural differences. Afternoon napping is a common practice in some cultures (e.g., Chinese and Latino culture)1, and therefore, future study should also investigate whether the associations between afternoon napping and neurocognitive outcomes are consistent across different cultures.
It is also worth noting that the public health implications of our findings are crucial. On the one hand, monitoring nap behaviors, especially excessive morning naps or irregular napping behaviors, should be considered in the ongoing endeavors for early risk stratifications for cognitive impairment or dementia. If causal links can be established, these napping behaviors may also aid in the search of intervenable targets for preventing or slowing down the neurodegenerative processes. On the other hand, sleep disturbances, including excessive daytime naps, can notably impact the quality of life of individuals with AD and their caregivers. Such studies will contribute to furthering the understanding of factors contributing to extended daytime napping in AD, offering insights for healthcare professionals in addressing sleep-related issues to improve overall well-being.
Data availability
The data are available under restricted access from the Rush Alzheimer’s Disease Center (RADC) following the data and resource sharing policy. Access of data can be obtained by submitting requests through the RADC platform https://www.radc.rush.edu/requests.htm. The source data for Fig. 2 is in Supplementary Data 1. The source data for Fig. 3 is in Supplementary Data 2.
References
Zhang, Z., Xiao, X., Ma, W. & Li, J. Napping in older adults: a review of current literature. Curr. Sleep Med. Rep. 6, 129–135 (2020).
Leng, Y., Redline, S., Stone, K. L., Ancoli-Israel, S. & Yaffe, K. Objective napping, cognitive decline, and risk of cognitive impairment in older men. Alzheimers Dementia 15, 1039–1047 (2019).
Li, P. et al. Daytime napping and Alzheimer’s dementia: A potential bidirectional relationship. Alzheimers Dement. 19, 158–168 (2023).
Buysse, D. J. Sleep health: Can we define it? Does it matter? Sleep 37, 9–17 (2014).
Olszowka, M. et al. Excessive daytime sleepiness, morning tiredness, and prognostic biomarkers in patients with chronic coronary syndrome. Int. J. Cardiol. 394, 131395 (2024).
Haghayegh, S. et al. The circadian rhythm of thermoregulation modulates both the sleep/wake cycle and 24 h pattern of arterial blood pressure. Compr. Physiol. 11, 2645–2658 (2021).
Milner, C. E. & Cote, K. A. Benefits of napping in healthy adults: impact of nap length, time of day, age, and experience with napping. J. Sleep Res. 18, 272–281 (2009).
Lee, Y. F., Gerashchenko, D., Timofeev, I., Bacskai, B. J. & Kastanenka, K. V. Slow wave sleep is a promising intervention target for Alzheimer’s disease. Front Neurosci. 14, 705 (2020).
Bei, B., Wiley, J. F., Trinder, J. & Manber, R. Beyond the mean: a systematic review on the correlates of daily intraindividual variability of sleep/wake patterns. Sleep Med. Rev. 28, 108–124 (2016).
Bennett, D. A. et al. Overview and findings from the rush Memory and Aging Project. Curr. Alzheimer Res. 9, 646–663 (2012).
Cole, R. J., Kripke, D. F., Gruen, W., Mullaney, D. J. & Gillin, J. C. Automatic sleep/wake identification from wrist activity. Sleep 15, 461–469 (1992).
Gao, C. et al. Actigraphy-based sleep detection: Validation with polysomnography and comparison of performance for nighttime and daytime sleep during simulated shift work. Nat. Sci. Sleep 14, 1801–1816 (2022).
Bennett, D. A. et al. Decision rules guiding the clinical diagnosis of Alzheimer’s disease in two community-based cohort studies compared to standard practice in a clinic-based cohort study. Neuroepidemiology 27, 169–176 (2006).
McKhann, G. et al. Clinical diagnosis of Alzheimer’s disease: Report of the NINCDS‐ADRDA Work Group under the auspices of Department of Health and Human Services Task Force on Alzheimer’s Disease. Neurology 34, 939–939 (1984).
Wilson, R. S., Arnold, S. E., Schneider, J. A., Tang, Y. & Bennett, D. A. The relationship between cerebral Alzheimer’s disease pathology and odour identification in old age. J. Neurol. Neurosurg. Psychiatry 78, 30–35 (2007).
Lim, A. S. P. et al. Quantification of the fragmentation of rest-activity patterns in elderly individuals using a state transition analysis. Sleep 34, 1569–1581 (2011).
Gao, C. et al. Approaches for assessing circadian rest-activity patterns using actigraphy in cohort and population-based studies. Curr. Sleep Med. Rep. 9, 247–256 (2023).
Radloff, L. S. The CES-D Scale: A self-report depression scale for research in the general population. Appl. Psychol. Measur. 1, 385–401 (1977).
Xie, L. et al. Sleep drives metabolite clearance from the adult brain. Science 342, 373–377 (2013).
Kang, J.-E. et al. Amyloid-beta dynamics are regulated by orexin and the sleep-wake cycle. Science 326, 1005–1007 (2009).
Poroyko, V. A. et al. Chronic sleep disruption alters gut microbiota, induces systemic and adipose tissue inflammation and insulin resistance in mice. Sci Rep 6, 35405 (2016).
Musiek, E. S. & Holtzman, D. M. Mechanisms linking circadian clocks, sleep, and neurodegeneration. Science 354, 1004–1008 (2016).
Cross, N. et al. Napping in older people ‘at risk’ of dementia: relationships with depression, cognition, medical burden and sleep quality. J Sleep Res 24, 494–502 (2015).
Cai, H. et al. Relationship between afternoon napping and cognitive function in the ageing Chinese population. Gen. Psychiatr. 34, e100361 (2021).
Dautovich, N. D. et al. Day-to-day variability in nap duration predicts medical morbidity in older adults. Health Psychol. 31, 671–676 (2012).
Li, P. et al. Delineating cognitive resilience using fractal regulation: Cross-sectional and longitudinal evidence from the Rush Memory and Aging Project. Alzheimers Dement. 20, 3203–3210 (2024).
Ran, S., Lin, X. & Liu, B. A Mendelian randomization study of Alzheimer’s disease and daytime napping. Alzheimers Dementia 20, 741–742 (2024).
Paz, V., Dashti, H. S. & Garfield, V. Is there an association between daytime napping, cognitive function, and brain volume? A Mendelian randomization study in the UK Biobank. Sleep Health 9, 786–793 (2023).
Acknowledgements
This study is supported by the Alzheimer’s Association Research Fellowship to Promote Diversity (AARFD-22-928372) and the BrightFocus Foundation (A2020886S). CG is additionally supported by the American Academy of Sleep Medicine Foundation (290-FP-22). PL is additionally supported by the Funds to Sustain Research Excellence from the Brigham Research Institute and a start-up fund from the Department of Anesthesia, Critical Care and Pain Medicine at Massachusetts General Hospital. KH is additionally supported by the National Institute on Aging (RF1AG064312; R01AG083799) and a start-up fund from the Department of Anesthesia, Critical Care and Pain Medicine at Massachusetts General Hospital. LG is additionally supported by the Alzheimer’s Association (AACSF-23-1148490). AI is supported by grants from ReDLat [National Institutes of Health and the Fogarty International Center (FIC), National Institutes of Aging (R01 AG057234, R01 AG075775, R01 AG21051, R01 AG083799, CARDS-NIH), Alzheimer’s Association (SG-20-725707), Rainwater Charitable Foundation – The Bluefield project to cure FTD, and Global Brain Health Institute)], ANID/FONDECYT Regular (1210195, 1210176 and 1220995); and ANID/FONDAP/15150012. The Rush Memory and Aging Project is supported by the National Institutes of Health (R01AG056352 and R01AG017917).
Author information
Authors and Affiliations
Contributions
P.L., K.H., C.G., and L.G. had the idea for the study and contributed to study design. C.G. drafted the manuscript. C.G., X.Z., P.L., and R.C. contributed to signal processing and statistical analysis. D.A.B., J.A.S., and A.S.B. contributed to the design of the Rush Memory and Aging Project and obtained data. P.L., K.H., C.G., L.Y., A.S.B., D.A.B., Y.L., A.I., and L.G. revised the manuscript for important intellectual content. All authors reviewed the manuscript and approved the final version to be submitted.
Corresponding authors
Ethics declarations
Competing interests
The authors declare the following competing interests: PL has received a monetary gift to support research from iFutureLab. P.L. serves on the iFutureLab-HEKA Scientific Advisory Board as the Chair of Cardiac Dynamics and Honorary Life-Time Co-Founder and has received consulting fees. P.L. has also received honorarium for lecturing from China Pharmaceutical University. K.H. serves on the iFutureLab-HEKA Scientific Advisory Board as the Chair of Medical Biodynamics and Honorary Life-Time Co-Founder and has received consulting fees. The interests of P.L. and K.H. were reviewed and managed by Mass General Brigham following their conflict of interest policies. These interests are not related to the current work. The other authors have indicated no financial conflicts of interest.
Peer review
Peer review information
Communications Medicine thanks the anonymous reviewers for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Gao, C., Zheng, X., Cai, R. et al. Timing and intraindividual variability of daytime napping and Alzheimer’s disease in older adults. Commun Med 5, 219 (2025). https://doi.org/10.1038/s43856-025-00936-1
Received:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s43856-025-00936-1