Abstract
Background
Schizophrenia significantly impairs everyday communication, affecting education and employment. Such communication difficulties may arise from deficits in syntax—understanding and generating grammatical structures. Research on syntactic impairments in schizophrenia is underpowered, with inconsistent findings, and it is unclear if deficits are specific to certain patient subgroups, regardless of symptom profiles, age, sex, or illness severity.
Methods
A pre-registered (Open Science Framework: https://doi.org/10.17605/OSF.IO/7FZUC) search using PubMed, Scopus, PsycINFO, and Web of Science databases up to May 1, 2024, for all studies investigating syntax comprehension and production in schizophrenia vs. healthy controls. Excluding studies on those <18 years of age and qualitative research, we extracted Cohen’s d and log coefficient of variation ratio and used Bayesian meta-analysis across 6 domains: 2 in comprehension and 4 in production in patient-control comparisons. Study quality was evaluated using a modified Newcastle–Ottawa Scale, with moderators (age, sex, study quality, language) tested via meta-regression.
Results
We identify 86 relevant articles, of which 45 have sufficient data for meta-analysis (n = 2960 participants, 64.4% English, weighted mean age(sd) = 32.3(5.6)). Bayesian meta-analysis shows strong evidence of syntactic deficits in schizophrenia across all domains (d = 0.65–1.01, overall random-effects d = 0.86, 95% CrI [0.67–1.03]), with syntax comprehension being most affected, with weak publication bias. People with schizophrenia show increased variability in comprehension and production of long and complex utterances (lnCVR = 0.21, 95% CrI [0.07–0.36]), hinting at subgroups with differing performance.
Conclusions
Robust impairments in grammatical comprehension and production in schizophrenia suggest opportunities for targeted interventions focusing on syntax, a rule-based feature amenable to cognitive, educational, and linguistic interventions.
Plain Language Summary
Schizophrenia is a mental condition that alters a person’s thoughts in relation to the world around them. People with schizophrenia often appear to struggle with language use, such as sentence structure and grammar. However, the nature of these language deficits have not yet been fully understood. Here, we performed a comprehensive analysis of published studies up to May 1, 2024, exploring the extent to which schizophrenia affects language comprehension and production. We found that people with schizophrenia have notable challenges with grammar, especially in understanding and creating sentences. As a group, they also have varying degrees of impairment, with some being more affected than others. Overall, these findings could help with improved early detection approaches using language markers and may lead to the development of specific personalized therapies to better support verbal communication for people with schizophrenia.
Similar content being viewed by others
Introduction
The cognitive faculty of language supports interpersonal communication and thinking1, both of which are disrupted in psychotic disorders such as schizophrenia. The thought and communication disorders observed in individuals with schizophrenia appear to stem from a structural disruption in language, i.e., grammatical impairment due to a divergence of syntax from healthy speakers2,3,4,5,6. However, despite the substantial body of work, the existing literature presents a fragmented understanding of the precise nature and extent of syntactic deficits.
Disorganised speech, a diagnostic feature of schizophrenia in DSM-57, is assessed on the basis of incoherence that leads to a failure of effective communication. Syntax production, if impaired, can generate conversational incoherence. Similarly, impaired comprehension of syntax (i.e., who did what to whom?) may contribute to impaired meaning and misinterpretations that typify positive psychotic symptoms such as persecutory delusions, as well as uncooperativeness, and lack of insight. Estimating the relative impairments in syntax production and comprehension is important because these processes rely on distinct cognitive mechanisms, despite sharing the common structural substrate (representation) of language8,9. Production involves generating grammatically correct and contextually appropriate sentences, while comprehension requires decoding and interpreting syntactic structures in real-time. Understanding the nature of the relationship between deficits in syntactic comprehension and production can clarify the level (shared structural vs. distinct cognitive) at which the mechanisms of language disturbances operate in schizophrenia. In the current study, we systematically review the literature published to date on both syntactic production and comprehension in schizophrenia.
Producing and inferring meaning via language is not based on isolated lexical concepts (semantic categories), but involves the interactional basis offered by grammatical constructions. Grammar enables the signifiers and the signified to be put together. Thus, there is a strong case to be made for syntax-level deficits, i.e., an aberration in the way words are composed in an order, to have primacy in the language disorder of schizophrenia10,11,12,13. Several thoughtful reviews in recent times have hinted at the critical importance of syntactic deficits in schizophrenia4,14,15,16,17. Bora and colleagues highlighted a role for impaired syntactic comprehension when analyzing the linguistic correlates of the burden of formal thought disorder18. Nonetheless, to our knowledge, a comprehensive meta-analytic quantification of the overall magnitude of grammatical impairment in both comprehension and production in schizophrenia is still lacking.
Quantifying the degree of grammatical impairment in schizophrenia is critical for two reasons. Firstly, the use of the various linguistic markers in speech to predict clinically important outcomes is an emerging pursuit in the field (e.g., onset of first episode19,20,21, relapses22). Despite the many studies carried out to date, one major obstacle in bringing such predictive analytics to routine clinical use is the lack of empirical guidance on feature selection in these models. As a result, many automatically derived linguistic variables are being tested in clinical prediction models, with minimal overlap among different studies, impeding interpretability and successful external validation (e.g., not a single linguistic feature overlapped across the 18 prediction analysis studies identified in a recent review14). This can be addressed via evidence-based preselection of variables that most proximally relate to the clinical construct of interest i.e., the presence of schizophrenia in our case [see Meehan and colleagues23 for a state-of-the-art review]. Meta-analytic estimation of the effect size of syntax production/comprehension variables will provide evidence for their utility in speech-based predictive analytics.
Secondly, given the relevance of social interaction for functional recovery24, interventions that ameliorate communication deficits in schizophrenia are steadily growing in recent times25,26,27. Yield from these trials can be improved by identifying the most affected syntactic markers as treatment targets and establishing if distinct subgroups with varying degrees of deficits are likely to occur in schizophrenia. In the presence of a high degree of interindividual variability in syntactic deficits, stratified RCTs for communicative remediation are likely to have a better yield. Thus, meta-analytic estimation of the effect size and variability of syntactic deficits will inform forthcoming intervention trials.
Our primary goal of this review is to provide a quantitative synthesis of the degree and interindividual variability of syntactic language deficit across the domains of syntactic comprehension, anomaly/error detection, and various levels of complexity and integrity of syntactic production in schizophrenia. We also aim to investigate the relationship between syntactic production, comprehension, and symptom severity and identify potential research gaps and opportunities in this area of work.
In this meta-analysis, we find robust evidence for grammatical impairments in schizophrenia across all domains examined, with particularly strong effects for syntax comprehension. People with schizophrenia show increased variability for some of the indices of syntax processing, suggesting the existence of potential subgroups with differing degrees of grammatical impairment.
Methods
Search strategy and selection criteria
The original protocol was registered on the Open Science Framework registry (May 2024), with an update after the initial search but before undertaking statistical analysis (October 2024)28. This update included missing information on meta-analytic methods and bias assessment framework, adding specifications (grouping of syntactic domains, metaregression variables) and planned deviations (reporting pronoun aberrations separately from the current report, dropping reaction time and parts-of-speech measures to reduce bias from reporting inconsistencies). Any further deviations that occurred after the data analysis (the use of a multivariate approach to meta-analysis) are explicitly reported as such. Institutional review board approval was not required as this study involved analysis of previously published data. This review adheres to the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines29 and recent recommendations to protect against researcher bias in meta-analysis30. We performed a literature search across multiple electronic databases, with PubMed (MEDLINE) and Scopus, Web of Science (Core Collection) as primary sources, followed by non-MEDLINE-indexed studies identified using PsycINFO up to May 1, 2024. Search terms included a combination of keywords and Medical Subject Headings (MeSH) terms related to schizophrenia (schizophrenia OR schizo* OR psychos* OR psychot*), language (language OR verbal OR linguistic OR speech OR communicat* OR thought), syntax (syntax OR syntactic OR gramma*) with the ‘explode’ option for non-MeSH variations of language when appropriate (e.g., language, verbal, linguistic, speech, communicat*, pronoun* with ‘exp’ in PubMed; See Supplementary Note 1). Two reviewers (DE and LP) independently screened titles and abstracts against the inclusion criteria using Rayyan software31 after removing duplicates. Full texts of relevant studies were assessed for eligibility. We then added further studies to the pool by screening the bibliography and hand-searching all citations received by the identified studies via Google Scholar. Imported databases with all studies retrieved via primary search are provided as links in Supplement (Supplementary Data 1) and PRISMA checklist as Supplementary Data 2.
We included English language publications describing studies that (1) enrolled adults (aged 18 or above) diagnosed with schizophrenia spectrum disorders (schizophrenia, schizoaffective, or schizophreniform psychosis) and a control group of healthy adults without known psychiatric disorders (2) assessed speech production and/or comprehension, focusing on grammar and syntax. This includes evaluating either grammatical comprehension (by quantifying a person’s ability to understand complex sentences or detect errors in the syntactic formation) and/or production (by assessing the degree of global [narrative level] or local [clausal/phrasal level] complexity, length and integrity in the utterances or sentences). This grouping of domains of interest was based on Morice and Ingram’s original work32 that separated complexity and integrity in syntax production in schizophrenia, with phrasal/clausal level complexity (coordination) later included by Thomas and colleagues33. This set was further extended as per Lu’s Syntactic Complexity Analyzer approach34 to distinguish production length from other complexity measures.
Only empirical studies with quantitative measures derived in the same manner from both groups were included. Studies focused on subjects <18 years of age35,36,37, case reports/case series38, and those without a healthy control group13,39,40,41,42,43 were excluded. Additionally, studies focused on high-risk subjects without a diagnosed schizophrenia spectrum disorder44, studies reporting verbal outputs that were either restricted (e.g., scripted conversations45) or likely to have been edited after production (e.g., written reports and social media texts46,47,48,49,50), non-naturalistic speech (e.g., word list generation, repetition, monitoring or recall of memorized text51,52,53), analysis restricted to parts-of-speech tagging (with no sentential syntax)54,55,56,57 or providing only second order derivatives (e.g., speech graph metrics58 or factor scores59) without direct indices of syntax production/comprehension were not eligible. One study with a retraction notice was also excluded60. Studies with unconventional criteria for syntactic complexity61,62,63 and those without quantitative measures or plots that allowed effect size estimation were also excluded51,64,65. For a list of articles excluded at the stage of data extraction with the reasons for exclusion and main results, see Supplementary Table 1 embedded in Supplementary Information.
Data extraction
We extracted the available clinical/demographic data (author(s), publication year, country, sample size, mean age, and symptom severity based on standardized scales [e.g., PANSS, SANS/SAPS, BPRS/BPRS-E, with the reported total scores in each patient sample converted to a scale of 0 to 1 via min-max transformation (See Supplementary Note 2)], sex distribution, chlorpromazine equivalent of antipsychotic dose (conversions from other drug equivalents or Defined Daily Doses as per ref. 66), mode [free speech, visual/verbal stimulus such as picture/proverb elaboration, sentence to picture matching] and the language of task administration). When overlapping samples were published in more than one paper, we extracted data from the largest reported sample. The instances where two studies reported data from overlapping participant samples are cited here32,67,68,69,70,71,72,73,74,75. In each case, to avoid duplication and ensure accurate effect size calculations, we extracted data from the largest reported sample. We reached out to selected authors (12.2%) when quantitative measures were unclear for clarifications. For studies where numerical values were not provided33,59,76,77,78,79 we extracted these values from published plots using a visual data extraction tool (plotdigitizer.com). When more than one mean was reported on the same measurement from the same sample (e.g., on/off medications as in ref. 80), we included the average as the summary measure. Some of the studies reported median and range values instead of mean and SD required for Cohen’s d estimation53,77,81. In such instances, we used the five-number summary approach82, available at https://www.math.hkbu.edu.hk/~tongt/papers/median2mean.html.
Quality assessment
The quality of the studies was assessed using a purposively modified Newcastle–Ottawa Scale83, widely used in psychiatry, where rating scale use for exposure assignment is a common practice84. The following indicators were evaluated: case definition, representativeness, selection of control group, comparability of groups, ascertainment of ‘exposure’ (i.e., measurement of syntactic variables of interest), and quality of data reporting. Items in the Newcastle-Ottawa framework are known to have low reliability among raters85 (e.g., demonstrating the timing of measurements) and lack of clarity86 (e.g., emphasis on independent validation of the case status, response proportions, the practice of higher scores for population-based controls, statistical adjustment and blinding which are often unsatisfactory in case-control designs) were replaced these with items specific to psychiatric diagnoses and linguistic variable assessment (see Supplementary Table 2). Furthermore, we defined likely confounders a priori for bias assessment (age, sex, education, and native language being different from the language of assessment). Each study was independently rated by two authors (DE and LP), with disagreements resolved by discussion.
Statistics and reproducibility
Statistical analyses were conducted using the JASP 0.19.0.0 package87. Effect sizes were calculated from available means and standard deviations (Cohen’s d = (M2 − M1)/SDpooled) from each set of analyses. As some studies reported error rates while others reported accuracy rates, all effect sizes were sign-adjusted to read as controls > schizophrenia when producing summary values (See Supplementary Note 3).
We pooled the d values using Bayesian model-averaged (BMA) meta-analysis via metaBMA R package implemented in JASP88, with default priors for heterogeneity (Inverse-Gamma [1, 0.15] and effect size (Cauchy [0, 0.707]). BMA evaluates the likelihood of the data under a combination of models regarding the meta-analytic effect and heterogeneity, reporting model-averaged effects. Evidence in favor of a group difference was categorized as weak (for BF10 1 to <3), moderate (BF10 3 to <10), strong (BF10 10 to <30), very strong (BF10 30 to <100), and extreme (BF10 > 100).
Meta-regression analyses were performed when sufficient evidence for heterogeneity between studies was uncovered in any domain. We included the mean age of People with schizophrenia, proportion of females with schizophrenia, mean chlorpromazine equivalent dose, language of the study assessment (English vs. non-English), and study quality scores as potential moderators.
Robust Bayesian meta-analysis89 was used to assess the sensitivity of the results to the potential presence of publication bias and heterogeneity.
Log Coefficient of Variation Ratio90 (lnCVR: natural log of ratio of the estimated total coefficient of variation between the patient and the control group) was used to quantify the difference in variability after scaling to the mean of each group [lnCVR = 0 indicates equal variability; >0 greater variability, while <0 indicates lower variability in schizophrenia vs. controls].
Given the between-domain heterogeneity, we used a random-effects model to pool the 6 lnCVR measures and the 6 Cohen’s d estimates across the domains and assessed the overall effect.
The 6 domains of interest are outcomes that are likely to be correlated with each other, though the participant-level correlations were seldom reported in the individual studies. Based on the assumption that individual-level correlation will lead to population (study) level correlation91, we employed multivariate meta-analysis with a missing at random assumption to analyze all correlated outcomes jointly. This enabled increased efficiency of the meta-analysis, allowing us to synthesize across domain-specific effect sizes using a random-effects approach91 to estimate the overall meta-effect of grammatical impairment using BMA. We used the mvmeta R package92. Due to the large amount of missing values in the aggregated data (only n = 18 had measurements for more than two domain indicators), all the data were subjected to multiple imputation by chained equations using the mice93 R package. We then estimated the within-study correlation using the metavcov R package94. All effect sizes were transformed into Hedge’s g for this analysis. A random-effect model was used because of the significant heterogeneity between individual studies. Borrowing of strength (BoS)95 was calculated to compare the results of multivariate meta-analyses to separate univariate methods. Note that this was not a pre-registered analysis and should only be considered as a supplemental analysis, given the amount of missing data.
Reporting summary
Further information on research design is available in the Nature Portfolio Reporting Summary linked to this article.
Results
Study selection
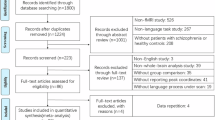
A total of 820 studies were identified through the initial database search. After removing duplicates, 509 unique studies remained. Following title and abstract screening, and adding hand-searched references, 86 articles were retrieved as relevant, of which 45 studies met the inclusion criteria for numerical synthesis for the meta-analysis11,32,33,53,67,70,74,76,77,78,80,81,96,97,98,99,100,101,102,103,104,105,106,107,108,109,110,111,112,113,114,115,116,117,118,119,120,121,122,123,124,125,126 (see Fig. 1).
Study characteristics
The final list included studies published between 1982 and 2024, with summary data from a total of n = 1679 people with schizophrenia and n = 1281 controls available from 79 comparisons across 6 domains of interest. The weighted mean age across studies was 32.31 (SD = 5.6) years, with no difference in distribution among people with schizophrenia and control cohorts (paired t = 0.85, p = 0.4). Only 29.2% of participants were women, with 5 studies recruiting only men80,110,117,127,128; only 8 studies had >40% women. The studies predominantly included individuals diagnosed with established schizophrenia spectrum disorders (n = 1292), with first-episode samples forming 33.59% of the total sample (n = 564). A great majority of studies (64.4%) recruited English-speaking participants. Some studies reported separate contrasts based on the presence of Formal Thought Disorder (FTD/no-FTD104,105,129) or stage of illness (FEP/established schizophrenia11,67,103). Quality scores are presented in Supplementary Table 3. See Supplementary Table 4 for a description of included studies.
There is no single accepted index to measure grammatical impairment in mental health conditions. As a result, we found a notable variation in the method used to quantify the variables of interest, and in some cases, more than one variable for the same domain was reported. As a general principle, we chose the measures with the closest theoretical alignment to the 6 domains of interest for this meta-analysis. Within each domain, we chose tasks and variables that were most commonly used across studies. Other study-specific decisions in variable choices are discussed in the Supplementary Note 3.
Information availability
While mean age (93.3% of studies), language of testing (100%), and sex distribution (93.3%) were available for most studies, an estimate of antipsychotic dose exposure (48.9%) and overall symptom severity (40%) were less often reported. Most studies only provided the overall proportion of antipsychotic use and domain-specific symptom scores (generally positive symptoms: See Supplementary Tables 5 and 6 embedded in Supplementary Information). As a result, we included age, assessment language, sex, and the study quality scores in the meta-regression analyses, but only reported moderator/effect-size bivariate correlations for antipsychotic dose and total symptom severity index.
Meta-analytical results
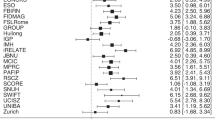
The results of Bayesian Meta-Analysis for each group of studies are shown in Table 1 along with the data on between-studies heterogeneity, log coefficient of variation ratios, and publication bias. BMA showed extreme evidence for reduced syntactic comprehension, error detection, production length, phrasal complexity, production integrity, and global complexity in people with schizophrenia (all BF10 > 100; Fig. 2). Random effects analysis across the 6 domain-specific effects indicated extreme evidence (BF10 = 3173; estimated d = 0.87) for an overall grammatical impairment in schizophrenia. See the Supplementary Results 1 for multivariate meta-analysis of correlated outcomes.
a Forest plots for Bayesian Model-Averaged estimates of group differences in syntactic comprehension (n = 16 studies). b Error detection (n = 6). c Production length (n = 17). d Phrasal complexity (n = 16). e Production integrity (n = 11). f Global complexity (n = 13). Estimated Cohen’s d values (with 95% credible intervals) reflect patient-control differences. FTD Formal Thought Disorder, nFTD no-FTD, FEP First Episode Psychosis, SCZ Established schizophrenia. Circles represent the effect of individual studies, with their size weighted by sample size; diamond lozenges represent pooled effects.
Between-study heterogeneity (tau) was strong for global complexity, production length, and integrity. Of these domains, the meta-regression analysis revealed age as a significant moderator for global complexity while study quality was the most significant known source of heterogeneity for production integrity (Table 1; Fig. 3). The moderator/effect-size bivariate correlations were not significant for antipsychotic dose (r31 = 0.27, p = 0.14) or total symptom severity index (r33 = 0.06, p = 0.74) across all domains. While the number of studies on clinically detectable FTD was insufficient for a meta-regression, visual inspection of the forest plots revealed that all FTD contrasts had above-average Cohen’s d values for syntactic comprehension and phrasal complexity but not for production integrity.
Meta-analysis of within-group variations indicated higher inter-individual variability in people with schizophrenia for syntactic comprehension, phrasal complexity, and production length (lnCVR = 0.13–0.41; medium to large variation effect130) but not for other measures (Fig. 4). Random effects analysis across the 6 domain-specific variation estimates indicated moderate evidence (BF10 = 5.27; estimated lnCVR = 0.21) for excess variability among people with schizophrenia compared to healthy controls.
a Forest plots for Bayesian Model-Averaged estimates of patient-control differences in variability (log coefficient of variation ratio, lnCVR) for syntactic comprehension (n = 16). b Error detection (n = 6). c Production length (n = 17). d Phrasal complexity (n = 16). e Production integrity (n = 11). f Global complexity (n = 13). Positive lnCVR values (95% credible intervals) indicate greater variability in people with schizophrenia. FTD Formal Thought Disorder, nFTD no-FTD, FEP First Episode Psychosis, SCZ Established schizophrenia. Circles represent the effect of individual studies, with their size weighted by sample size; diamond lozenges represent pooled effects.
Using Robust BMA, we found no or weak evidence for publication bias in all of the individual meta-analyses, with moderate to extreme evidence for group differences retained for domain-specific impairments in syntax (Table 1).
The unregistered multivariate meta-analysis improved the precision of estimates (Fig. 5), again indicating error detection and syntactic comprehension to be the most affected domains, followed by all 4 production domains. Correlations were not robust, but hinted that an impairment in error detection (comprehension) may co-occur with reduced production integrity and lower global complexity (production). But these results were affected by the randomness of imputation due to the large amount of missing data. As shown by Borrowing of Strength analysis, all precision estimates were boosted (median of 75%) by the adjustment for correlations among the domains.
Top panel: Left: Imputed study-level correlations (heatmap) indicated that an impairment in error detection (comprehension) may co-occur with reduced production integrity and lower global complexity (production), but the strength of the imputed relationships was unstable due to a large volume of missing data. Right: Borrowing of Strength analysis indicating the gain in domain-specific estimates with the unregistered multi-variate approach. Bottom panel: Forest plot of random effects multivariate analysis across domains. Circles represent the effect of individual studies, with their size weighted by sample size; diamond lozenges represent pooled effects.
Discussion
To our knowledge, this is the first meta-analysis on the association between schizophrenia and the use of grammar/syntax. BMA reveals extreme evidence in support of a global grammatical impairment across the domains of interest in schizophrenia, with the most robust effects being noted for comprehension of complex syntax and detection of errors, followed by production length and integrity. The evidence favoring illness-related differences was moderately strong for global and phrasal complexity, even after taking between-studies heterogeneity and publication bias into account. This implies that people with schizophrenia understand simpler sentences better, ignore syntactical errors, and speak in less sophisticated, shorter sentences that may not have a complete syntactic structure. Within the patient group, variability in grammar production/comprehension was higher than that of the healthy control group; this may occur in the presence of subgroups with varying degrees of impairment in schizophrenia. Taken together, a broad spectrum of grammatical impairment appears to be a key feature of schizophrenia.
Given the relatively modest sample sizes in individual studies (median patient n = 32), our meta-analytic synthesis offers a more robust and representative effect size of grammatical impairment in people with schizophrenia. Nevertheless, one limitation is our reliance on summary measures reported by authors instead of individual participant data. As 40% of case-control contrasts came from studies completed 20 years ago, we assessed (a priori) the likelihood of data availability to be low. Notable variation in study quality was noted, with representativeness across sexes and assessment languages being poor. Our synthesis is also limited by the diversity of variables used to define the domain-specific divergence; this likely accounts for the high heterogeneity observed in certain domains. Individual studies seldom reported the subject-level correlations among the various domains (especially between production and comprehension divergence), precluding our ability to test one of our pre-registered aims (but see the unregistered multivariate meta-analysis).
We also record notable variations in clinical sampling, with some studies focusing exclusively on those with FTD110. We found insufficient data to estimate the effect of FTD across all domains and excluded studies that only compared FTD and non-FTD patient groups131. But our results indicate that grammatical impairment occurs irrespective of the presence of FTD. It is important to note that at an individual level, the degree of grammatical impairment is likely to be much higher among people with schizophrenia as it is influenced by comorbid developmental disorders and poor proficiency in a non-native language, both of which led to participant exclusion in the studies we identified. Furthermore, people with schizophrenia with more severe linguistic deficits often lack the capacity to provide written informed consent; given the fluctuating nature of clinical symptoms (including thought disorder), it is likely that cross-sectional assessments reported in primary studies fail to capture the most symptomatic phases of the illness, wherein syntactic deficits may be more prominent. Thus, the effect size reported here should be considered as a conservative estimate of the real-world complexities of grammatical impairment in schizophrenia.
One of the strengths of our review is the depth of our literature search - covering 50 years of work. In contrast to Ehlen and colleagues4 who recently “identified no studies evaluating syntax production in individuals with schizophrenia”, our search strategy located n = 29 studies on syntax production. Furthermore, our robust BMA analytical approach accounts for the uncertainty in heterogeneity and publication bias estimates and offers a comprehensive meta-analytic quantification of the overall magnitude of grammatical impairment in schizophrenia. The robust medium-to-large deficit in syntax production makes a strong case for including speech-based predictive analytics for early detection of schizophrenia, reinforcing prior37,132 and ongoing studies in this regard133. We offer specific recommendations for future studies of grammatical impairment in schizophrenia that can refine the effect size estimates reported in the current meta-analysis (see Box 1).
Several domains of language function, such as pronoun use134, semantic coherence55, and fluency135, are affected in schizophrenia. Compared to the other reported impairments, deficits in syntax, being a rule-based feature of language, are potentially remediable across the lifespan. Syntactic improvement may also affect other levels of linguistic processing (see Box 2). Therapeutic gain has been shown in aphasic disorders with structured rehabilitation/education approaches (e.g., mapping therapy, syntax stimulation136,137) or via targeted cognitive training (e.g., working memory138). In schizophrenia, studies investigating the causal relationship between syntactic deficits and other linguistic domains are needed, along with those investigating the neural basis of these deficits. By demonstrating evidence for a small-to-medium-sized increase in inter-individual variability in syntactic deficit (especially for phrasal complexity and syntactic comprehension), our synthesis encourages pre-trial selection of patients for communicative remediation. In particular, for syntactic comprehension, the combination of a large effect-size deficit, low between-studies heterogeneity, and the possibility of finding highly impaired subgroups indicates its suitability as an outcome measure for linguistic intervention trials.
The neural and social interactional basis of the observed syntactic deficits warrants attention in future studies. Emerging arguments against the presence of specific neural substrates for syntax/combinatorial processing in human language139,140, indicate that the syntactic aberrations in schizophrenia may reflect deficits at multiple levels of language processing, especially semantic cognition; this remains to be empirically studied . Our observation of a generalized syntactic deficit across patient samples argues against focusing exclusively on those with clinically detectable FTD in mechanistic studies of linguistic divergence in psychosis (see refs. 67,141,142 for a similar argument).
Our estimate of overall syntactic impairment (d = 0.87) can be considered as a large effect by convention, but smaller than the generalized cognitive impairment reported in schizophrenia (d = 1.2143) and comparable to mechanistic observations relevant to its pathophysiology (e.g., presynaptic dopamine excess in neuroimaging studies d = 0.79144). Studies included in our meta-analysis either excluded participants with notably low IQ or matched IQ between groups; thus, we cannot attribute the observed syntactic divergence to a generalized cognitive impairment. Unlike the constrained neuropsychological tests used to assess cognitive deficits, syntactic deficits (especially in production) reported here has been observed on the basis of narratives/conversations that occur in more natural contexts. Thus, grammatical impairments, often carried by patients without much self-awareness, are likely to have intrusive effects on one’s everyday social functions.
In conclusion, our meta-analysis substantiates the long-suspected role of grammatical aberrations in schizophrenia. The question of whether these deficits occur independently of lexico-semantic abnormalities or are part of a broader linguistic impairment remains unresolved. Nonetheless, the findings underscore the need for targeted interventions to address these linguistic differences. More general implications include the importance of adjusting verbal exchanges in therapeutic and other settings (e.g., inpatient units, educational, vocational and legal institutions) for schizophrenia.
Data availability
All data that support the findings are provided as supplementary information. The source data for Fig. 1 can be found in Supplementary Data 1 (Imported Databases). The source data for Fig. 2 and Fig. 4 are available in Supplementary Table 4 (Description of included studies) and Supplementary Table 6 (Medications, linguistic variables, and task details); for Fig. 3, the source data is presented in Supplementary Table 3 (Quality scores) and Supplementary Table 6 (Medications, linguistic variables and task details). The source data for Table 1 (summary statistics) is provided in Supplementary Tables 4 and 5 (Key variables and moderators), and list of papers provided as links in Supplementary Data 1. Supplementary Table 1 lists excluded studies and the reasons for the exclusion. Supplementary Table 2 describes the modified Newcastle–Ottawa Scale used for quality assessment. Supplementary Table 6 includes the information on medications, linguistic variables and task details from the included studies. Supplementary Note 1 provides all search terms on PubMed. Supplementary Note 2 explains how illness severity index was calculated across studies. Supplementary Note 3 explains how domain specific variables were chosen. Any further data requests can be made to the corresponding author.
References
Carruthers, P. The cognitive functions of language. Behav. Brain Sci. 25, 657–674 (2002).
Chaika, E. A unified explanation for the diverse structural deviations reported for adult schizophrenics with disrupted speech. J. Commun. Disord. 15, 167–189 (1982).
Covington, M. A. et al. Schizophrenia and the structure of language: the linguist’s view. Schizophr. Res. 77, 85–98 (2005).
Ehlen, F., Montag, C., Leopold, K. & Heinz, A. Linguistic findings in persons with schizophrenia—a review of the current literature. Front. Psychol. 14, https://www.frontiersin.org/articles/10.3389/fpsyg.2023.1287706 (2023).
Hinzen, W. & Rosselló, J. The linguistics of schizophrenia: thought disturbance as language pathology across positive symptoms. Front. Psychol. 6, 971 (2015).
Kuperberg, G. R. Language in schizophrenia Part 1: an Introduction. Lang. Linguist Compass 4, 576–589 (2010).
Tandon, R. et al. Definition and description of schizophrenia in the DSM-5. Schizophrenia Res. 150, 3–10 (2013).
Lukic, S. et al. Common and distinct neural substrates of sentence production and comprehension. NeuroImage 224, 117374 (2021).
Segaert, K., Menenti, L., Weber, K., Petersson, K. M. & Hagoort, P. Shared syntax in language production and language comprehension—an fMRI study. Cereb. Cortex 22, 1662–1670 (2012).
Crow, T. J. The nuclear symptoms of schizophrenia reveal the four quadrant structure of language and its deictic frame. J. Neurolinguist. 23, 1–9 (2010).
DeLisi, L. E. Speech disorder in schizophrenia: review of the literature and exploration of its relation to the uniquely human capacity for language. Schizophr. Bull. 27, 481–496 (2001).
Hinzen, W. & Palaniyappan, L. The ‘L-factor’: language as a transdiagnostic dimension in psychopathology. Prog. Neuro Psychopharmacol. Biol. Psychiatry 131, 110952 (2024).
Pylyshyn, Z. W. Clinical correlates of some syntactic features of patients’ speech. J. Nerv. Ment. Dis. 150, 307 (1970).
Deneault, A., Dumais, A., Désilets, M. & Hudon, A. Natural language processing and schizophrenia: a scoping review of uses and challenges. J. Personalized Med. 14, 744 (2024).
Elvevåg, B. et al. An examination of the language construct in NIMH’s research domain criteria: time for reconceptualization! Am. J. Med. Genet. Part B Neuropsychiatr. Genet. 171, 904–919 (2016).
Kircher, T., Bröhl, H., Meier, F. & Engelen, J. Formal thought disorders: from phenomenology to neurobiology. Lancet Psychiatry 5, 515–526 (2018).
Low, D. M., Bentley, K. H. & Ghosh, S. S. Automated assessment of psychiatric disorders using speech: a systematic review. Laryngoscope Investig. Otolaryngol. 5, 96–116 (2020).
Bora, E., Yalincetin, B., Akdede, B. B. & Alptekin, K. Neurocognitive and linguistic correlates of positive and negative formal thought disorder: a meta-analysis. Schizophrenia Res. 209, 2–11 (2019).
Corcoran, C. M. & Cecchi, G. A. Using language processing and speech analysis for the identification of psychosis and other disorders. Biol. Psychiatry Cogn. Neurosci. Neuroimaging 5, 770–779 (2020).
Corona Hernández, H. et al. Natural language processing markers for psychosis and other psychiatric disorders: emerging themes and research agenda from a cross-linguistic workshop. Schizophr. Bull. 49, S86–S92 (2023).
de Boer, J. N., Brederoo, S. G., Voppel, A. E. & Sommer, I. E. C. Anomalies in language as a biomarker for schizophrenia. Curr. Opin. Psychiatry 33, 212–218 (2020).
Zaher, F. et al. Speech markers to predict and prevent recurrent episodes of psychosis: a narrative overview and emerging opportunities. Schizophrenia Res. 266, 205–215 (2024).
Meehan, A. J. et al. Clinical prediction models in psychiatry: a systematic review of two decades of progress and challenges. Mol. Psychiatry 27, 2700–2708 (2022).
Adamczyk, P. et al. Do better communication skills promote sheltered employment in schizophrenia? Schizophrenia Res. 176, 331–339 (2016).
Bambini, V. et al. It is time to address language disorders in schizophrenia: a RCT on the efficacy of a novel training targeting the pragmatics of communication (PragmaCom). J. Commun. Disord. 97, 106196 (2022).
Jimeno, N. Language and communication rehabilitation in patients with schizophrenia: a narrative review. Heliyon 10, e24897 (2024).
Bosco, F. M., Gabbatore, I., Gastaldo, L. & Sacco, K. Communicative-pragmatic treatment in schizophrenia: a pilot study. Front. Psychol. 7, https://doi.org/10.3389/fpsyg.2016.00166 (2016).
Elleuch, D. & Palaniyappan, L. Grammar and psychosis: a systematic review of language production and comprehension studies. https://doi.org/10.17605/OSF.IO/7FZUC (2024).
Page, M. J. et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ 372, n71 (2021).
Baldwin, J. R., Pingault, J.-B., Schoeler, T., Sallis, H. M. & Munafò, M. R. Protecting against researcher bias in secondary data analysis: challenges and potential solutions. Eur. J. Epidemiol. 37, 1 (2022).
Ouzzani, M., Hammady, H., Fedorowicz, Z. & Elmagarmid, A. Rayyan—a web and mobile app for systematic reviews. Syst. Rev. 5, https://doi.org/10.1186/s13643-016-0384-4 (2016).
Morice, R. D. & Ingram, J. C. L. Language analysis in schizophrenia: diagnostic implications. Aust. N. Z. J. Psychiatry 16, 11–21 (1982).
Thomas, P., King, K. & Fraser, W. I. Positive and negative symptoms of schizophrenia and linguistic performance. Acta Psychiatr. Scand. 76, 144–151 (1987).
Lu, X. Automatic analysis of syntactic complexity in second language writing. Int. J. Corpus Linguist. 15, 474–496 (2010).
Silberg, J. L. The development of pronoun usage in the psychotic child. J. Autism Dev. Disord. 8, 413–425 (1978).
Solomon, M. et al. From lumping to splitting and back again: atypical social and language development in individuals with clinical-high-risk for psychosis, first episode schizophrenia, and autism spectrum disorders. Schizophr. Res. 131, 146–151 (2011).
Corcoran, C. M. et al. Prediction of psychosis across protocols and risk cohorts using automated language analysis. World Psychiatry 17, 67–75 (2018).
Noël-Jorand, M. C., Reinert, M., Giudicelli, S. & Dassa, D. A new approach to discourse analysis in psychiatry, applied to a schizophrenic patient’s speech. Schizophr. Res. 25, 183–198 (1997).
Barch, D. M. & Berenbaum, H. The effect of language production manipulations on negative thought disorder and discourse coherence disturbances in schizophrenia. Psychiatry Res. 71, 115–127 (1997).
Barch, D. M. & Berenbaum, H. Language generation in schizophrenia and mania: the relationships among verbosity, syntactic complexity, and pausing. J. Psycholinguist. Res. 26, 401–412 (1997).
Lelekov, T., Franck, N., Dominey, P. F. & Georgieff, N. Cognitive sequence processing and syntactic comprehension in schizophrenia. Neuroreport 11, 2145–2149 (2000).
Lott, P. R., Guggenbühl, S., Schneeberger, A., Pulver, A. E. & Stassen, H. H. Linguistic analysis of the speech output of schizophrenic, bipolar, and depressive patients. PSP 35, 220–227 (2002).
Jeong, L. et al. Exploring the use of natural language processing for objective assessment of disorganized speech in schizophrenia. Psychiatr. Res. Clin. Pract. 5, 84–92 (2023).
Haas, S. S. et al. Linking language features to clinical symptoms and multimodal imaging in individuals at clinical high risk for psychosis. Eur. Psychiatry 63, e72 (2020).
Dwyer, K., David, A. S., McCarthy, R., McKenna, P. & Peters, E. Linguistic alignment and theory of mind impairments in schizophrenia patients’ dialogic interactions. Psychological Med. 50, 2194–2202 (2020).
Thomas, P., Leudar, I., Newby, D. & Johnston, M. Syntactic processing and written language output in first onset psychosis. J. Commun. Disord. 26, 209–230 (1993).
Jo, Y. T., Lee, J., Park, J., Lee, J. & Joo, Y. Linguistic anomalies observed in the Sentence Completion Test in patients with schizophrenia. Cognit. Neuropsychiatry 28, https://doi.org/10.1080/13546805.2023.2209313 (2023).
Hoffman, R. E., Hogben, G. L., Smith, H. & Calhoun, W. F. Message disruptions during syntactic processing in schizophrenia. J. Commun. Disord. 18, 183–202 (1985).
Ellsworth, R. B. The regression of schizophrenic language. J. Consult Psychol. 15, 387–391 (1951).
Gupta, T., Hespos, S. J., Horton, W. S. & Mittal, V. A. Automated analysis of written narratives reveals abnormalities in referential cohesion in youth at ultra high risk for psychosis. Schizophr. Res. 192, 82–88 (2018).
Ruchsow, M., Trippel, N., Groen, G., Spitzer, M. & Kiefer, M. Semantic and syntactic processes during sentence comprehension in patients with schizophrenia: evidence from event-related potentials. Schizophr. Res. 64, 147–156 (2003).
Kuperberg, G. R., McGuire, P. K. & David, A. S. Sensitivity to linguistic anomalies in spoken sentences: a case study approach to understanding thought disorder in schizophrenia. Psychol. Med. 30, 345–357 (2000).
Dwyer, K., David, A., McCarthy, R., McKenna, P. & Peters, E. Higher-order semantic processing in formal thought disorder in schizophrenia. Psychiatry Res. 216, 168–176 (2014).
Tan, E. J., Meyer, D., Neill, E. & Rossell, S. L. Investigating the diagnostic utility of speech patterns in schizophrenia and their symptom associations. Schizophr. Res. 238, 91–98 (2021).
He, R. et al. Navigating the semantic space: Unraveling the structure of meaning in psychosis using different computational language models. Psychiatry Res. 333, 115752 (2024).
Takashima, A., Ohta, K., Matsushima, E. & Toru, M. The event-related potentials elicited by content and function words during the reading of sentences by patients with schizophrenia. Psychiatry Clin. Neurosci. 55, 611–618 (2001).
Rossell, S. L. & Batty, R. A. Elucidating semantic disorganisation from a word comprehension task: do patients with schizophrenia and bipolar disorder show differential processing of nouns, verbs and adjectives? Schizophr. Res. 102, 63–68 (2008).
Ciampelli, S. et al. Syntactic network analysis in schizophrenia-spectrum disorders. Schizophrenia Bull. 49, S172–S182 (2023).
Voleti, R. et al. Language analytics for assessment of mental health status and functional competency. Schizophrenia Bull. 49, S183–S195 (2023).
Retraction Notice. A study of cohesive patterns and dynamic choices utilized by two schizophrenic patients in dialog, pre- and post medication. Lang. Speech. 40, 331–351 (2003).
Alqahtani, A., Kayi, E. S., Hamidian, S., Compton, M. & Diab, M. A quantitative and qualitative analysis of schizophrenia language. In Proceedings of the 13th international workshop on health text mining and information analysis (LOUHI) (eds, Lavelli, A. et al.) 173–183 (Association for Computational Linguistics, 2022).
Zhang, H. et al. Linguistic markers of psychosis in Mandarin Chinese: relations to theory of mind. Psychiatry Res. 325, 115253 (2023).
Wiltschko, M. Is grammar affected in Schizophrenia? Psychiatry Res. 339, 116061 (2024).
DeLisi, L. E. et al. Anomalous cerebral asymmetry and language processing in schizophrenia. Schizophr. Bull. 23, 255–271 (1997).
Thomas, P., King, K., Fraser, W. I. & Kendell, R. E. Linguistic performance in schizophrenia: a comparison of acute and chronic patients. Br. J. Psychiatry 156, 204–210 (1990).
Leucht, S., Samara, M., Heres, S. & Davis, J. M. Dose equivalents for antipsychotic drugs: the DDD method. Schizophr. Bull. 42, S90–S94 (2016).
Dalal, T. C. et al. Speech based natural language profile before, during and after the onset of psychosis: a cluster analysis. Acta Psychiatrica Scand. https://doi.org/10.1111/acps.13685 (2024).
Silva, A. M. et al. Syntactic complexity of spoken language in the diagnosis of schizophrenia: a probabilistic Bayes network model. Schizophr. Res. 259, 88–96 (2023).
de Boer, J. N., Voppel, A. E., Brederoo, S. G., Wijnen, F. N. K. & Sommer, I. E. C. Language disturbances in schizophrenia: the relation with antipsychotic medication. npj Schizophrenia 6, 1–9 (2020).
de Boer, J. N. et al. Language in schizophrenia: relation with diagnosis, symptomatology and white matter tracts. npj Schizophrenia 6, 1–10 (2020).
Obrębska, M. Frequency analysis of singular first-person pronouns and verbs in the utterances of schizophrenia patients and healthy controls: a research report. Ling. Posnan. 55, 87–98 (2013).
Obrębska, M. & Kleka, P. Lexical indicators of anxiety in schizophrenia. Anxiety Stress Coping 36, 382–397 (2023).
Morice, R. D. & Ingram, J. C. L. Language complexity and age of onset of schizophrenia. Psychiatry Res. 9, 233–242 (1983).
Thomas, P. et al. Speech and language in first onset psychosis differences between people with schizophrenia, mania, and controls. BR J. Psychiatry 168, 337–343 (1996).
Thomas, P. et al. Syntactic complexity and negative symptoms in first onset schizophrenia. Cogn. Neuropsychiatry 1, 191–200 (1996).
Lee, C. W. et al. P600 alteration of syntactic language processing in patients with bipolar mania: comparison to schizophrenic patients and healthy subjects. J. Affect. Disord. 201, 101–111 (2016).
Morgan, S. E. et al. Natural Language Processing markers in first episode psychosis and people at clinical high-risk. Transl. Psychiatry 11, 630 (2021).
Stephane, M., Pellizzer, G., Fletcher, C. R. & McClannahan, K. Empirical evaluation of language disorder in schizophrenia. J. Psychiatry Neurosci. 32, 250–258 (2007).
Ziv, I. et al. Morphological characteristics of spoken language in schizophrenia patients - an exploratory study. Scand. J. Psychol. 63, 91–99 (2022).
Condray, R., van Kammen, D. P., Steinhauer, S. R., Kasparek, A. & Yao, J. K. Language comprehension in schizophrenia: Trait or state indicator? Biol. Psychiatry 38, 287–296 (1995).
Li, R. et al. Deciphering language disturbances in schizophrenia: a study using fine-tuned language models. Schizophrenia Res. 271, 120–128 (2024).
Shi, J. et al. Optimally estimating the sample standard deviation from the five-number summary. Res Synth. Methods 11, 641–654 (2020).
Wells, G. et al. The Newcastle-Ottawa Scale (NOS) for assessing the quality of nonrandomised studies in meta-analyses. https://www.ohri.ca/programs/clinical_epidemiology/oxford.asp 2018.
Luchini, C., Stubbs, B., Solmi, M. & Veronese, N. Assessing the quality of studies in meta-analyses: advantages and limitations of the Newcastle Ottawa Scale. World J. Meta Anal. 5, 80–84 (2017).
Hartling, L. et al. Testing the Newcastle Ottawa Scale showed low reliability between individual reviewers. J. Clin. Epidemiol. 66, 982–993 (2013).
Stang, A. Critical evaluation of the Newcastle-Ottawa scale for the assessment of the quality of nonrandomized studies in meta-analyses. Eur. J. Epidemiol. 25, 603–605 (2010).
JASP Team. JASP (Version 0.19.0). https://jasp-stats.org/ (2024).
Berkhout, S. W., Haaf, J. M., Gronau, Q. F., Heck, D. W. & Wagenmakers, E.-J. A tutorial on Bayesian model-averaged meta-analysis in JASP. Behav. Res. 56, 1260–1282 (2024).
Bartoš, F., Maier, M., Quintana, D. S. & Wagenmakers, E.-J. Adjusting for publication bias in JASP and R: selection models, PET-PEESE, and robust Bayesian meta-analysis. Adv. Methods Pract. Psychological Sci. 5, 25152459221109259 (2022).
Senior, A. M., Viechtbauer, W. & Nakagawa, S. Revisiting and expanding the meta-analysis of variation: the log coefficient of variation ratio. Res Synth. Methods 11, 553–567 (2020).
Riley, R. D. et al. Multivariate and network meta-analysis of multiple outcomes and multiple treatments: rationale, concepts, and examples. BMJ 358, j3932 (2017).
Gasparrini, A., Armstrong, B. & Kenward, M. G. Multivariate meta-analysis for non-linear and other multi-parameter associations. Stat. Med. 31, 3821–3839 (2012).
van Buuren, S. & Groothuis-Oudshoorn, K. mice: multivariate imputation by chained equations in R. J. Stat. Softw. 45, 1–67 (2011).
Lu, M. Computing within-study covariances, data visualization, and missing data solutions for multivariate meta-analysis with metavcov. Front. Psychol. 14, https://doi.org/10.3389/fpsyg.2023.1185012 (2023).
Hattle, M. et al. Multivariate meta-analysis of multiple outcomes: characteristics and predictors of borrowing of strength from Cochrane reviews. Syst. Rev. 11, 149 (2022).
Anand, A., Wales, R. J., Jackson, H. J. & Copolov, D. L. Linguistic impairment in early psychosis. J. Nerv. Ment. Dis. 182, 488–493 (1994).
Arslan, B. et al. Computational analysis of linguistic features in speech samples of first-episode bipolar disorder and psychosis. J. Affect Disord. 363, 340–347 (2024).
Bagner, D. M., Melinder, M. R. D. & Barch, D. M. Language comprehension and working memory language comprehension and working memory deficits in patients with schizophrenia. Schizophr. Res. 60, 299–309 (2003).
Barattieri di San Pietro, C., Barbieri, E., Marelli, M., de Girolamo, G. & Luzzatti, C. Processing argument structure and syntactic complexity in people with schizophrenia spectrum disorders. J. Commun. Disord. 96, 106182 (2022).
Barrera, A., McKENNA, P. J. & Berrios, G. E. Formal thought disorder in schizophrenia: an executive or a semantic deficit? Psychological Med. 35, 121–132 (2005).
Buck, B. & Penn, D. L. Lexical characteristics of emotional narratives in schizophrenia: Relationships with symptoms, functioning, and social cognition. J. Nerv. Ment. Dis. 203, 702–708 (2015).
Çabuk, T. et al. Natural language processing for defining linguistic features in schizophrenia: a sample from Turkish speakers. Schizophr. Res. 266, 183–189 (2024).
Chaves, M. F., Rodrigues, C., Ribeiro, S., Mota, N. B. & Copelli, M. Grammatical impairment in schizophrenia: an exploratory study of the pronominal and sentential domains. PLOS One 18, e0291446 (2023).
Çokal, D. et al. The language profile of formal thought disorder. npj Schizophrenia 4, 1–8 (2018).
Çokal, D., Zimmerer, V., Varley, R., Watson, S. & Hinzen, W. Comprehension of embedded clauses in schizophrenia with and without formal thought disorder. J. Nerv. Ment. Dis. 207, 384–392 (2019).
Delvecchio, G. et al. Altered syntactic abilities in first episode patients: An inner phenomenon characterizing psychosis. Eur. Psychiatry 61, 119–126 (2019).
Fraser, W. I., King, K. M., Thomas, P. & Kendell, R. E. The diagnosis of schizophrenia by language analysis. Br. J. Psychiatry 148, 275–278 (1986).
Gargano, G. et al. Language production impairments in patients with a first episode of psychosis. PLOS One 17, e0272873 (2022).
King, K., Fraser, W. I., Thomas, P. & Kendell, R. E. Re-examination of the language of psychotic subjects. Br. J. Psychiatry 156, 211–215 (1990).
Kircher, T. T. J., Oh, T. M., Brammer, M. J. & McGuire, P. K. Neural correlates of syntax production in schizophrenia. Br. J. Psychiatry 186, 209–214 (2005).
Kuperberg, G. R., Kreher, D. A., Goff, D., McGuire, P. K. & David, A. S. Building up linguistic context in schizophrenia: evidence from self-paced reading. Neuropsychology 20, 442–452 (2006).
Kuperberg, G. R., Sitnikova, T., Goff, D. & Holcomb, P. J. Making sense of sentences in schizophrenia: electrophysiological evidence for abnormal interactions between semantic and syntactic processing. J. Abnorm. Psychol. 115, 251–265 (2006).
Liang, L. et al. Widespread cortical thinning, excessive glutamate and impaired linguistic functioning in schizophrenia: a cluster analytic approach. Front. Hum. Neurosci. 16, 954898 (2022).
Morice, R. & McNicol, D. The comprehension and production of complex syntax in schizophrenia. Cortex 21, 567–580 (1985).
Moro, A. et al. Detecting syntactic and semantic anomalies in schizophrenia. Neuropsychologia 79, 147–157 (2015).
Özcan, A. et al. The production of simple sentence structures in schizophrenia. Int. J. Arts Sci. 9, 159–164 (2016).
Panikratova, Y. et al. Executive regulation of speech production in schizophrenia: a pilot neuropsychological study. Neurosci. Behav. Physi 51, 415–422 (2021).
Perlini, C. et al. Linguistic production and comprehension deficits in schizophrenia and bipolar disorder. Eur. Psychiatry 25, 1083 (2010).
Sanders, L. M., Adams, J., Tager-Flusberg, H., Shenton, M. E. & Coleman, M. A comparison of clinical and linguistic indices of deviance in the verbal discourse of schizophrenics. Appl. Psycholinguist. 16, 325–338 (1995).
Schneider, K. et al. Syntactic complexity and diversity of spontaneous speech production in schizophrenia spectrum and major depressive disorders. Schizophr 9, 1–10 (2023).
Sevilla, G. et al. Deficits in nominal reference identify thought disordered speech in a narrative production task. PLOS ONE 13, e0201545 (2018).
Shedlack, K. et al. Language processing and memory in ill and well siblings from multiplex families affected with schizophrenia. Schizophr. Res. 25, 43–52 (1997).
Stirling, J., Hellewell, J., Blakey, A. & Deakin, W. Thought disorder in schizophrenia is associated with both executive dysfunction and circumscribed impairments in semantic function. Psychol. Med. 36, 475–484 (2006).
Tan, E. J., Yelland, G. W. & Rossell, S. L. Characterising receptive language processing in schizophrenia using word and sentence tasks. Cogn. Neuropsychiatry 21, 14–31 (2016).
Tang, S. X. et al. Natural language processing methods are sensitive to sub-clinical linguistic differences in schizophrenia spectrum disorders. npj Schizophrenia 7, 1–8 (2021).
Tavano, A. et al. Specific linguistic and pragmatic deficits in Italian patients with schizophrenia. Schizophrenia Res. 102, 53–62 (2008).
Condray, R., Steinhauer, S. R., van Kammen, D. P. & Kasparek, A. The language system in schizophrenia: effects of capacity and linguistic structure. Schizophrenia Bull. 28, 475–490 (2002).
Vogel, A. P. et al. Verbal fluency, semantics, context and symptom complexes in schizophrenia. J. Psycholinguist. Res. 38, 459–473 (2009).
Çokal, D. et al. Referential noun phrases distribute differently in Turkish speakers with schizophrenia. Schizophr. Res. 259, 104–110 (2023).
Howes, O. D. & Chapman, G. E. Understanding variability: the role of meta-analysis of variance. Psychological Med. 54, 1–4 (2024).
Rodriguez-Ferrera, S., McCarthy, R. A. & McKenna, P. J. Language in schizophrenia and its relationship to formal thought disorder. Psychological Med. 31, 197–205 (2001).
Bedi, G. et al. Automated analysis of free speech predicts psychosis onset in high-risk youths. npj Schizophrenia 1, 1–7 (2015).
Bayer, J. M. M. et al. The SPEAK study rationale and design: a linguistic corpus-based approach to understanding thought disorder. Schizophr. Res. 259, 80–87 (2023).
Elleuch, D., Chen, Y., Luo, Q. & Palaniyappan, L. Speaking of yourself: A meta-analysis of 80 years of research on pronoun use in schizophrenia. Schizophr. Res. 279, 22–30 (2025).
Mackinley, M. et al. More than words: Speech production in first-episode psychosis predicts later social and vocational functioning. Front. Psychiatry 14, 1144281 (2023).
Poirier, S.-È., Fossard, M. & Monetta, L. The efficacy of treatments for sentence production deficits in aphasia: a systematic review. Aphasiology 37, 122–142 (2023).
Rochon, E., Laird, L., Bose, A. & Scofield, J. Mapping therapy for sentence production impairments in nonfluent aphasia. Neuropsychol. Rehabil. 15, 1–36 (2005).
Roque-Gutierrez, E. & Ibbotson, P. Working memory training improves children’s syntactic ability but not vice versa: a randomized control trial. J. Exp. Child Psychol. 227, 105593 (2023).
Fedorenko, E., Blank, I. A., Siegelman, M. & Mineroff, Z. Lack of selectivity for syntax relative to word meanings throughout the language network. Cognition 203, 104348 (2020).
Mollica, F. et al. Composition is the core driver of the language-selective network. Neurobiol. Lang. 1, 104–134 (2020).
Bambini, V. et al. Deconstructing heterogeneity in schizophrenia through language: a semi-automated linguistic analysis and data-driven clustering approach. Schizophr 8, 1–12 (2022).
Oomen, P. P. et al. Characterizing speech heterogeneity in schizophrenia-spectrum disorders. J. Psychopathol. Clin. Sci. 131, 172–181 (2022).
Schaefer, J., Giangrande, E., Weinberger, D. R. & Dickinson, D. The global cognitive impairment in schizophrenia: Consistent over decades and around the world. Schizophrenia Res. 150, 42 (2013).
Howes, O. D. et al. The nature of dopamine dysfunction in schizophrenia and what this means for treatment. Arch. Gen. Psychiatry 69, 776–786 (2012).
McKenna, P. J. & Oh, T. M. The dyssemantic hypothesis of thought disorder. In: Schizophrenic speech: making sense of bathroots and ponds that fall in doorways, 146–171 (Cambridge University Press, 2005).
Spitzer, M. A cognitive neuroscience view of schizophrenic thought disorder. Schizophr. Bull. 23, 29–50 (1997).
Baskak, B., Ozel, E. T., Atbasoglu, E. C. & Baskak, S. C. Peculiar word use as a possible trait marker in schizophrenia. Schizophrenia Res. 103, 311–317 (2008).
Juhasz, B. J., Chambers, D., Shesler, L. W., Haber, A. & Kurtz, M. M. Evaluating lexical characteristics of verbal fluency output in schizophrenia. Psychiatry Res. 200, 177–183 (2012).
Bambini, V. et al. The communicative impairment as a core feature of schizophrenia: frequency of pragmatic deficit, cognitive substrates, and relation with quality of life. Compr. Psychiatry 71, 106–120 (2016).
Docherty N. M. On identifying the processes underlying schizophrenic speech disorder. Schizophr. Bull. https://doi.org/10.1093/schbul/sbr048 (2011).
Parola, A., Gabbatore, I., Berardinelli, L., Salvini, R. & Bosco, F. M. Multimodal assessment of communicative-pragmatic features in schizophrenia: a machine learning approach. NPJ Schizophr. 7, 28 (2021).
Rochester, S. & Martin, J. R. Crazy talk (Springer, 1979).
Bazin, N., Perruchet, P., Hardy-Bayle, M. C. & Feline, A. Context-dependent information processing in patients with schizophrenia. Schizophrenia Res. 45, 93–101 (2000).
Iter, D., Yoon, J. & Jurafsky, D. Automatic detection of incoherent speech for diagnosing schizophrenia. In: Proceedings of the Fifth Workshop on Computational Linguistics and Clinical Psychology: From Keyboard to Clinic, 136–146 (Association for Computational Linguistics, 2018).
Bilgrami, Z. R. et al. Construct validity for computational linguistic metrics in individuals at clinical risk for psychosis: associations with clinical ratings. Schizophr. Res. 245, 90–96 (2022).
Acknowledgements
L.P.’s research is supported by the Canada First Research Excellence Fund, awarded to the Healthy Brains, Healthy Lives initiative at McGill University (through New Investigator Supplement) and Monique H. Bourgeois Chair in Developmental Disorders. He receives a salary award from the Fonds de recherche du Québec-Santé (#366934). This work is supported by the FRQS Partenariat Innovation-Québec-Janssen (PIQ-J) initiative (#338282); Canadian Institutes of Health Research (CIHR) - Strategy for Patient-Oriented Research Priority Announcement (SPOR;#PJK192157) and Project Grant (#PJT195903); Wellcome Trust Discretionary Grant (#226168/Z/22/Z) and Mental Health Award for the DIALOG consortium (#314138/Z/24/Z). Q.L.’s research is supported by grants from the National Key Research and Development Program of China (No. 2023YFE0109700), the National Natural Science Foundation of China (No.s 32441107 and 82272079), and the Program of Shanghai Academic Research Leader (No. 23XD1423400).
Author information
Authors and Affiliations
Contributions
L.P. conceptualized the study; D.E. and L.P. designed, searched, and extracted the data; L.P., Y.C., and Q.L. undertook the meta-analysis. L.P. and D.E. interpreted the findings and drafted the manuscript; L.P. provided the resources and supervised D.E.’s work. All authors revised it critically for important intellectual content.
Corresponding author
Ethics declarations
Competing interests
L.P. reports personal fees from Janssen Canada, Otsuka Canada, SPMM Course Limited, UK, Canadian Psychiatric Association; book royalties from Oxford University Press; investigator-initiated educational grants from Sunovion, Janssen Canada, Otsuka Canada outside the submitted work. All other authors (DE, QL, YC) report no competing interests.
Peer review
Peer review information
Communications Medicine thanks Alexander Michael Rapp and Masahiro Banno for their contribution to the peer review of this work. [A peer review file is available].
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Elleuch, D., Chen, Y., Luo, Q. et al. Relationship between grammar and schizophrenia: a systematic review and meta-analysis. Commun Med 5, 235 (2025). https://doi.org/10.1038/s43856-025-00944-1
Received:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s43856-025-00944-1
This article is cited by
-
Automated Flagging of Cognitive Biases in the Spoken Language of People with Hallucination Experiences
Journal of Technology in Behavioral Science (2025)
-
Collecting language, speech acoustics, and facial expression to predict psychosis and other clinical outcomes: strategies from the AMP® SCZ initiative
Schizophrenia (2025)