Abstract
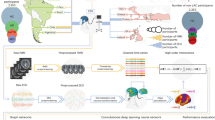
Brain clocks track the deviations between predicted brain age and chronological age (brain age gaps, BAGs). These BAGs can be used to measure accelerated aging, monitoring deviations from the healthy brain trajectories associated with brain diseases and different cumulative burdens. However, the underlying biophysical mechanisms associated with BAGs in aging and dementia remain unclear. Here we combine source space connectivity (via electroencephalography) with generative brain modeling in healthy controls from the global south and north, alongside patients with Alzheimer’s disease and behavioral variant frontotemporal dementia (bvFTD) (N = 1,399). BAGs in aging were influenced by geography (south > north), income (low > high), sex (female > male) and education (low > high), with larger BAGs in patients, especially females, with Alzheimer’s disease. Biophysical modeling revealed BAGs related to hyperexcitability and structural disintegration in aging, while hypoexcitability and severe disintegration were linked to dementia. Our work sheds light on the biophysical mechanisms of accelerated aging and dementia in diverse populations.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 digital issues and online access to articles
$79.00 per year
only $6.58 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to the full article PDF.
USD 39.95
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
Data availability
Raw data are available upon request from the corresponding authors. Functional and structural connectivity matrices, demographics and the data required to reproduce the results of this work are available at https://github.com/carlosmig/EEG-Dementias, with a link to Zenodo at https://doi.org/10.5281/zenodo.16420033 ref. 139.
Code availability
Analyses were conducted in MATLAB R2022a and Python (v.3.12.3). Machine learning and modeling used scikit-learn (v.1.4.2) and Numba (v.0.59.1). Statistical analysis used numpy (v.1.26.4), scipy (v.1.15.2), pandas (v.2.2.2) and statsmodels (v.0.14.2). Image processing used scikit-image (v.0.23.2). Visualization used matplotlib (v.3.8.4). Brain network analyses were performed using Brain Connectivity Toolbox (BCT, v.0.6.0) and NetworkX (v.3.2.1). EEG preprocessing used EEGLAB (v.2022.1). The codes for simulations are available at https://github.com/carlosmig/EEG-Dementias, and mirrored to a Zenodo repository at https://doi.org/10.5281/zenodo.16420033 ref. 139. The Brain Connectivity Toolbox for Python (https://github.com/fiuneuro/brainconn)133 was used for graph analysis and the BrainNet Viewer toolbox140 for visualization.
References
Moguilner, S. et al. Brain clocks capture diversity and disparities in aging and dementia across geographically diverse populations. Nat. Med. 30, 3646–3657 (2024).
Tian, Y. E. et al. Heterogeneous aging across multiple organ systems and prediction of chronic disease and mortality. Nat. Med. 29, 1221–1231 (2023).
Jones, D. T., Lee, J. & Topol, E. J. Digitising brain age. Lancet 400, 988–988 (2022).
Cole, J. H. & Franke, K. Predicting age using neuroimaging: innovative brain ageing biomarkers. Trends Neurosci. 40, 681–690 (2017).
Bashyam, V. M. et al. MRI signatures of brain age and disease over the lifespan based on a deep brain network and 14,468 individuals worldwide. Brain 143, 2312–2324 (2020).
Hou, Y. et al. Ageing as a risk factor for neurodegenerative disease. Nat. Rev. Neurol. 15, 565–581 (2019).
Legaz, A. et al. Structural inequality linked to brain volume and network dynamics in aging and dementia across the Americas. Nat. Aging 5, 259–274 (2024).
Scheltens, P. et al. Alzheimer’s disease. Lancet 388, 505–517 (2016).
Tseng, W.-Y. I., Hsu, Y.-C. & Kao, T.-W. Brain age difference at baseline predicts clinical dementia rating change in approximately two years. J. Alzheimers Dis. 86, 613–627 (2022).
Prado, P. et al. The BrainLat project, a multimodal neuroimaging dataset of neurodegeneration from underrepresented backgrounds. Sci. Data 10, 889 (2023).
Baez, S., Alladi, S. & Ibanez, A. Global South research is critical for understanding brain health, ageing and dementia. Clin. Transl. Med. 13, e1486 (2023).
Greene, A. S. et al. Brain–phenotype models fail for individuals who defy sample stereotypes. Nature 609, 109–118 (2022).
Marek, S. et al. Reproducible brain-wide association studies require thousands of individuals. Nature 603, 654–660 (2022).
Lewandowska, P. et al. Association between real-time strategy video game learning outcomes and pre-training brain white matter structure: preliminary study. Sci. Rep. 12, 20741 (2022).
Ranasinghe, K. G. et al. Altered excitatory and inhibitory neuronal subpopulation parameters are distinctly associated with tau and amyloid in Alzheimer’s disease. eLife 11, e77850 (2022).
Ibanez, A., Kringelbach, M. L. & Deco, G. A synergetic turn in cognitive neuroscience of brain diseases. Trends Cogn. Sci. https://doi.org/10.1016/j.tics.2023.12.006 (2024).
Santamaria-Garcia, H. et al. Factors associated with healthy aging in Latin American populations. Nat. Med. 29, 2248–2258 (2023).
Parra, M. A. et al. Dementia in Latin America. Neurology 90, 222–231 (2018).
McGlinchey, E. et al. Biomarkers of neurodegeneration across the Global South. Lancet Healthy Longev 5, 100616 (2024).
Lotze, M. et al. Income is associated with hippocampal/amygdala and education with cingulate cortex grey matter volume. Sci. Rep. 10, 18786 (2020).
De Looze, C. et al. Examining the impact of socioeconomic position across the life course on cognitive function and brain structure in healthy aging. J. Gerontol. A 78, 890–901 (2023).
Yaple, Z. A. & Yu, R. Functional and structural brain correlates of socioeconomic status. Cerebral Cortex 30, 181–196 (2020).
Wang, A. Y. et al. Socioeconomic status and risks of cognitive impairment and dementia: a systematic review and meta-analysis of 39 prospective studies. J. Prevent. Alzheimers Dis 10, 83–94 (2023).
Migeot, J., Calivar, M., Granchetti, H., Ibáñez, A. & Fittipaldi, S. Socioeconomic status impacts cognitive and socioemotional processes in healthy ageing. Sci. Rep. 12, 6048 (2022).
Hatzenbuehler, M. L., McLaughlin, K. A., Weissman, D. G. & Cikara, M. A research agenda for understanding how social inequality is linked to brain structure and function. Nat. Hum. Behav. 8, 20–31 (2024).
Ibáñez, A., Legaz, A. & Ruiz-Adame, M. Addressing the gaps between socioeconomic disparities and biological models of dementia. Brain 146, 3561–3564 (2023).
Parra-Rodriguez, M. A. et al. The EuroLaD‐EEG consortium: towards a global EEG platform for dementia, for seeking to reduce the regional impact of dementia. Alzheimers Dement. 18, e059944 (2022).
Ribeiro, F., Teixeira-Santos, A. C., Caramelli, P. & Leist, A. K. Prevalence of dementia in Latin America and Caribbean countries: systematic review and meta-analyses exploring age, sex, rurality and education as possible determinants. Ageing Res. Rev. 81, 101703 (2022).
Vega, I. E., Cabrera, L. Y., Wygant, C. M., Velez-Ortiz, D. & Counts, S. E. Alzheimer’s disease in the Latino community: intersection of genetics and social determinants of health. J. Alzheimers Dis. 58, 979–992 (2017).
Gatica, M. et al. High-order functional redundancy in ageing explained via alterations in the connectome in a whole-brain model. PLoS Comput. Biol. 18, e1010431 (2022).
Deco, G. & Kringelbach, M. L. Great expectations: using whole-brain computational connectomics for understanding neuropsychiatric disorders. Neuron 84, 892–905 (2014).
Lynn, C. W. & Bassett, D. S. The physics of brain network structure, function and control. Nat. Rev. Phys. 1, 318–332 (2019).
Coronel-Oliveros, C., Gießing, C., Medel, V., Cofré, R. & Orio, P. Whole-brain modeling explains the context-dependent effects of cholinergic neuromodulation. NeuroImage 265, 119782 (2023).
Gatica, M. et al. High-order interdependencies in the aging brain. Brain Connect. 11, 734–744 (2021).
Prado, P. et al. Dementia ConnEEGtome: towards multicentric harmonization of EEG connectivity in neurodegeneration. Int. J. Psychophysiol. 172, 24–38 (2022).
Alber, M. et al. Integrating machine learning and multiscale modeling—perspectives, challenges and opportunities in the biological, biomedical and behavioral sciences. npj Digit. Med. 2, 115 (2019).
Al Zoubi, O. et al. Predicting age from brain EEG signals—a machine learning approach. Front. Aging Neurosci 10, 184 (2018).
Khayretdinova, M. et al. Predicting age from resting-state scalp EEG signals with deep convolutional neural networks on TD-brain dataset. Front. Aging Neurosci 14, 1019869 (2022).
Signorino, M., Pucci, E., Belardinelli, N., Nolfe, G. & Angeleri, F. EEG spectral analysis in vascular and Alzheimer dementia. Electroencephalogr. Clin. Neurophysiol. 94, 313–325 (1995).
Besthorn, C. et al. Discrimination of Alzheimer’s disease and normal aging by EEG data. Electroencephalogr. Clin. Neurophysiol. 103, 241–248 (1997).
Stefanovski, L. et al. Linking molecular pathways and large-scale computational modeling to assess candidate disease mechanisms and pharmacodynamics in Alzheimer’s disease. Front. Comput. Neurosci 13, 54 (2019).
Coronel‐Oliveros, C. et al. Viscous dynamics associated with hypoexcitation and structural disintegration in neurodegeneration via generative whole‐brain modeling. Alzheimers Dement. https://doi.org/10.1002/alz.13788 (2024).
Maestú, F., de Haan, W., Busche, M. A. & DeFelipe, J. Neuronal excitation/inhibition imbalance: core element of a translational perspective on Alzheimer pathophysiology. Ageing Res. Rev. 69, 101372 (2021).
Lopatina, O. L. et al. Excitation/inhibition imbalance and impaired neurogenesis in neurodevelopmental and neurodegenerative disorders. Rev. Neurosci. 30, 807–820 (2019).
Martínez‐Cañada, P., Perez‐Valero, E., Minguillon, J., Pelayo, F., López Gordo, M. A. & Morillas, C. Combining aperiodic 1/f slopes and brain simulation: an EEG/MEG proxy marker of excitation/inhibition imbalance in Alzheimer's disease. Alzheimers Dement. 15, e12477 (2023).
van den Heuvel, M. P. & Sporns, O. Network hubs in the human brain. Trends Cogn. Sci. 17, 683–696 (2013).
van den Heuvel, M. P. & Sporns, O. Rich-club organization of the human connectome. J. Neurosci. 31, 15775–15786 (2011).
Coronel-Oliveros, C., Castro, S., Cofré, R. & Orio, P. Structural features of the human connectome that facilitate the switching of brain dynamics via noradrenergic neuromodulation. Front. Comput. Neurosci. https://doi.org/10.3389/fncom.2021.687075 (2021).
Deco, G., Van Hartevelt, T. J., Fernandes, H. M., Stevner, A. & Kringelbach, M. L. The most relevant human brain regions for functional connectivity: evidence for a dynamical workspace of binding nodes from whole-brain computational modelling. NeuroImage 146, 197–210 (2017).
Dai, Z. et al. Identifying and mapping connectivity patterns of brain network hubs in Alzheimer’s disease. Cerebral Cortex 25, 3723–3742 (2015).
Cohen, J. R. & D’Esposito, M. The segregation and integration of distinct brain networks and their relationship to cognition. J. Neurosci. 36, 12083–12094 (2016).
Lord, L.-D., Stevner, A. B., Deco, G. & Kringelbach, M. L. Understanding principles of integration and segregation using whole-brain computational connectomics: implications for neuropsychiatric disorders. Phil. Trans. R. Soc. A 375, 20160283 (2017).
Moguilner, S. et al. Biophysical models applied to dementia patients reveal links between geographical origin, gender, disease duration and loss of neural inhibition. Alzheimers Res. Ther. 16, 79 (2024).
Amato, L. G. et al. Personalized modeling of Alzheimer’s disease progression estimates neurodegeneration severity from EEG recordings. Alzheimers Dement. 16, e12526 (2024).
Snyder, H. M. et al. Sex biology contributions to vulnerability to Alzheimer’s disease: a think tank convened by the Women’s Alzheimer’s Research Initiative. Alzheimers Dement. 12, 1186–1196 (2016).
de Boer, S. C. M. et al. Differences in sex distribution between genetic and sporadic frontotemporal dementia. J. Alzheimers Dis. 84, 1153–1161 (2021).
Fisher, D. W., Bennett, D. A. & Dong, H. Sexual dimorphism in predisposition to Alzheimer’s disease. Neurobiol. Aging 70, 308–324 (2018).
Ganaie, M. A., Tanveer, M. & Beheshti, I. Brain age prediction with improved least squares twin SVR. IEEE J. Biomed. Health Inform. 27, 1661–1669 (2023).
Birba, A. et al. Allostatic-interoceptive overload in frontotemporal dementia. Biol. Psychiatry 92, 54–67 (2022).
Migeot, J. A., Duran-Aniotz, C. A., Signorelli, C. M., Piguet, O. & Ibáñez, A. A predictive coding framework of allostatic-interoceptive overload in frontotemporal dementia. Trends Neurosci. 45, 838–853 (2022).
Abdel-Naseer, M. Epidemiology of dementia in developing countries. J. Neurol. Sci. 405, 72–73 (2019).
Ibañez, A. & Manes, F. Contextual social cognition and the behavioral variant of frontotemporal dementia. Neurology 78, 1354–1362 (2012).
Pandics, T. et al. Exposome and unhealthy aging: environmental drivers from air pollution to occupational exposures. GeroScience 45, 3381–3408 (2023).
Finch, C. E. & Kulminski, A. M. The Alzheimer’s disease exposome. Alzheimers Dement. 15, 1123–1132 (2019).
Risk factors related to population diversity and disparity determine healthy aging. Nat. Med. 29, 2183–2184 (2023).
Griffa, A. & Van den Heuvel, M. P. Rich-club neurocircuitry: function, evolution and vulnerability. Dialogues Clin. Neurosci. 20, 121–132 (2018).
Chenot, Q., Lepron, E., De Boissezon, X. & Scannella, S. Functional connectivity within the fronto-parietal network predicts complex task performance: a fNIRS study. Front. Neuroergonomics 2, 718176 (2021).
Ptak, R. The frontoparietal attention network of the human brain. Neuroscientist 18, 502–515 (2012).
Küchenhoff, S. et al. Visual processing speed is linked to functional connectivity between right frontoparietal and visual networks. Eur. J. Neurosci. 53, 3362–3377 (2021).
Kim, Y. H. et al. Real-time strategy video game experience and visual perceptual learning. J. Neurosci. 35, 10485–10492 (2015).
Campbell, K. L., Grady, C. L., Ng, C. & Hasher, L. Age differences in the frontoparietal cognitive control network: implications for distractibility. Neuropsychologia 50, 2212–2223 (2012).
Xia, H., He, Q. & Chen, A. Understanding cognitive control in aging: a brain network perspective. Front. Aging Neurosci 14, 1038756 (2022).
Musa, G. et al. Alzheimer’s disease or behavioral variant frontotemporal dementia? Review of key points toward an accurate clinical and neuropsychological diagnosis. J. Alzheimers Dis. 73, 833–848 (2020).
Boroshok, A. L. et al. Individual differences in frontoparietal plasticity in humans. npj Sci. Learn. 7, 14 (2022).
Berry, K. P. & Nedivi, E. Experience-dependent structural plasticity in the visual system. Annu. Rev. Vis. Sci. 2, 17–35 (2016).
Nikolaidis, A., Voss, M. W., Lee, H., Vo, L. T. K. & Kramer, A. F. Parietal plasticity after training with a complex video game is associated with individual differences in improvements in an untrained working memory task. Front. Hum. Neurosci 8, 169 (2014).
Schmidt, S. et al. Experience-dependent structural plasticity in the adult brain: how the learning brain grows. NeuroImage 225, 117502 (2021).
Kowalczyk, N. et al. Real‐time strategy video game experience and structural connectivity–a diffusion tensor imaging study. Hum. Brain Mapp. 39, 3742–3758 (2018).
Toepper, M. Dissociating normal aging from Alzheimer’s disease: a view from cognitive neuroscience. J. Alzheimers Dis. 57, 331–352 (2017).
Illán‐Gala, I. et al. Sex differences in the behavioral variant of frontotemporal dementia: a new window to executive and behavioral reserve. Alzheimers Dement. 17, 1329–1341 (2021).
Moore, K. M. et al. Age at symptom onset and death and disease duration in genetic frontotemporal dementia: an international retrospective cohort study. Lancet Neurol. 19, 145–156 (2020).
Perry, D. C. et al. Clinicopathological correlations in behavioural variant frontotemporal dementia. Brain 140, 3329–3345 (2017).
Pini, L. et al. Brain atrophy in Alzheimer’s disease and aging. Ageing Res. Rev. 30, 25–48 (2016).
Gili, T. et al. Regional brain atrophy and functional disconnection across Alzheimer’s disease evolution. J. Neurol. Neurosurg. Psychiatry 82, 58–66 (2011).
La Joie, R. et al. Region-specific hierarchy between atrophy, hypometabolism and β-amyloid (Aβ) load in Alzheimer’s disease dementia. J. Neurosci. 32, 16265–16273 (2012).
Klupp, E. et al. Prefrontal hypometabolism in Alzheimer disease is related to longitudinal amyloid accumulation in remote brain regions. J. Nucl. Med. 56, 399–404 (2015).
Förster, S. et al. Regional expansion of hypometabolism in Alzheimer’s disease follows amyloid deposition with temporal delay. Biol. Psychiatry 71, 792–797 (2012).
Herman, J. P., Nawreen, N., Smail, M. A. & Cotella, E. M. Brain mechanisms of HPA axis regulation: neurocircuitry and feedback in context Richard Kvetnansky lecture. Stress 23, 617–632 (2020).
Dedovic, K., Duchesne, A., Andrews, J., Engert, V. & Pruessner, J. C. The brain and the stress axis: the neural correlates of cortisol regulation in response to stress. NeuroImage 47, 864–871 (2009).
Kelberman, M. A. et al. Consequences of hyperphosphorylated tau in the locus coeruleus on behavior and cognition in a rat model of Alzheimer’s disease. J. Alzheimers Dis. 86, 1037–1059 (2022).
Kelberman, M. A. et al. Age-dependent dysregulation of locus coeruleus firing in a transgenic rat model of Alzheimer’s disease. Neurobiol. Aging 125, 98–108 (2023).
Corriveau-Lecavalier, N., Mellah, S., Clément, F. & Belleville, S. Evidence of parietal hyperactivation in individuals with mild cognitive impairment who progressed to dementia: a longitudinal fMRI study. NeuroImage Clin. 24, 101958 (2019).
Arbabyazd, L. et al. Virtual connectomic datasets in Alzheimer’s disease and aging using whole-brain network dynamics modelling. eNeuro https://doi.org/10.1523/ENEURO.0475-20.2021 (2021).
Sanz Perl, Y. et al. Model-based whole-brain perturbational landscape of neurodegenerative diseases. eLife 12, e83970 (2023).
Ipiña, I. P. et al. Modeling regional changes in dynamic stability during sleep and wakefulness. NeuroImage 215, 116833 (2020).
Sanz Perl, Y. et al. Perturbations in dynamical models of whole-brain activity dissociate between the level and stability of consciousness. PLoS Comput. Biol. 17, e1009139 (2021).
Woodard, J. L. & Sugarman, M. A. in Behavioral Neurobiology of Aging 113–136 (Springer, 2011).
Fujita, S. et al. Characterization of brain volume changes in aging individuals with normal cognition using serial magnetic resonance imaging. JAMA Netw. Open 6, e2318153 (2023).
Ibañez, A. et al. Predicting and characterizing neurodegenerative subtypes with multimodal neurocognitive signatures of social and cognitive processes. J. Alzheimers Dis. 83, 227–248 (2021).
Walhovd, K. B. et al. Education and income show heterogeneous relationships to lifespan brain and cognitive differences across European and US cohorts. Cerebral Cortex 32, 839–854 (2022).
Hunt, J. F. V. et al. Association of neighborhood-level disadvantage with cerebral and hippocampal volume. JAMA Neurol. 77, 451–460 (2020).
Hernandez, H. et al. Brain health in diverse settings: how age, demographics and cognition shape brain function. NeuroImage 295, 120636 (2024).
Marek, S. & Laumann, T. O. Replicability and generalizability in population psychiatric neuroimaging. Neuropsychopharmacology 50, 52–57 (2024).
Ibanez, A. et al. The multi-partner consortium to expand dementia research in Latin America (ReDLat): driving multicentric research and implementation science. Front. Neurol 12, 631722 (2021).
McKhann, G. M. et al. The diagnosis of dementia due to Alzheimer’s disease: recommendations from the National Institute on Aging‐Alzheimer’s Association workgroups on diagnostic guidelines for Alzheimer’s disease. Alzheimers Dement. 7, 263–269 (2011).
Dubois, B. et al. Research criteria for the diagnosis of Alzheimer’s disease: revising the NINCDS–ADRDA criteria. Lancet Neurol. 6, 734–746 (2007).
Rascovsky, K. et al. Sensitivity of revised diagnostic criteria for the behavioural variant of frontotemporal dementia. Brain 134, 2456–2477 (2011).
Hu, S., Lai, Y., Valdes-Sosa, P. A., Bringas-Vega, M. L. & Yao, D. How do reference montage and electrodes setup affect the measured scalp EEG potentials? J. Neural Eng. 15, 026013 (2018).
Delorme, A. & Makeig, S. EEGLAB: an open source toolbox for analysis of single-trial EEG dynamics including independent component analysis. J. Neurosci. Methods 134, 9–21 (2004).
Pion-Tonachini, L., Kreutz-Delgado, K. & Makeig, S. ICLabel: an automated electroencephalographic independent component classifier, dataset and website. NeuroImage 198, 181–197 (2019).
Zhao, L. et al. Quantitative signal quality assessment for large-scale continuous scalp electroencephalography from a big data perspective. Physiol. Meas. 44, 035009–035009 (2023).
Grech, R. et al. Review on solving the inverse problem in EEG source analysis. J. NeuroEng. Rehabil. 5, 25 (2008).
Tzourio-Mazoyer, N. et al. Automated anatomical labeling of activations in SPM using a macroscopic anatomical parcellation of the MNI MRI single-subject brain. NeuroImage 15, 273–289 (2002).
Rommel, C., Paillard, J., Moreau, T. & Gramfort, A. Data augmentation for learning predictive models on EEG: a systematic comparison. J. Neural Eng. 19, 066020 (2022).
He, C., Liu, J., Zhu, Y. & Du, W. Data augmentation for deep neural networks model in EEG classification task: a review. Front. Hum. Neurosci 15, 765525 (2021).
Smith, S. M., Vidaurre, D., Alfaro-Almagro, F., Nichols, T. E. & Miller, K. L. Estimation of brain age delta from brain imaging. NeuroImage 200, 528–539 (2019).
Chen, B. et al. Contrasting inequality in human exposure to greenspace between cities of Global North and Global South. Nat. Commun. 13, 4636 (2022).
Gonzalez-Gomez, R. et al. Qualitative and quantitative educational disparities and brain signatures in healthy aging and dementia across global settings. eClinicalMedicine 82, 103187 (2025).
Filmer, D., Rogers, H., Angrist, N. & Sabarwal, S. Learning-adjusted years of schooling (LAYS): defining a new macro measure of education. Econ. Educ. Rev. 77, 101971 (2020).
David, O. & Friston, K. J. A neural mass model for MEG/EEG. NeuroImage 20, 1743–1755 (2003).
Otero, M., Lea-Carnall, C., Prado, P., Escobar, M.-J. & El-Deredy, W. Modelling neural entrainment and its persistence: influence of frequency of stimulation and phase at the stimulus offset. Biomed. Phys. Eng. Express https://doi.org/10.1088/2057-1976/ac605a (2022).
Jansen, B. H. & Rit, V. G. Electroencephalogram and visual evoked potential generation in a mathematical model of coupled cortical columns. Biol. Cybern. 73, 357–366 (1995).
Gilbert, C. D., Hirsch, J. A. & Wiesel, T. N. Lateral interactions in visual cortex. Cold Spring Harb. Symp. Quant. Biol. 55, 663–677 (1990).
McGuire, B. A., Gilbert, C. D., Rivlin, P. K. & Wiesel, T. N. Targets of horizontal connections in macaque primary visual cortex. J. Comp. Neurol. 305, 370–392 (1991).
Abeysuriya, R. G. et al. A biophysical model of dynamic balancing of excitation and inhibition in fast oscillatory large-scale networks. PLoS Comput. Biol. 14, e1006007 (2018).
Gütig, R., Aharonov, R., Rotter, S. & Sompolinsky, H. Learning input correlations through nonlinear temporally asymmetric Hebbian plasticity. J. Neurosci. 23, 3697–3714 (2003).
Ito, T. et al. Task-evoked activity quenches neural correlations and variability across cortical areas. PLoS Comput. Biol. 16, e1007983 (2020).
Wang, Z., Bovik, A. C., Sheikh, H. R. & Simoncelli, E. P. Image quality assessment: from error visibility to structural similarity. IEEE Trans. Image Process. 13, 600–612 (2004).
Medel, V., Irani, M., Crossley, N., Ossandón, T. & Boncompte, G. Complexity and 1/f slope jointly reflect brain states. Sci. Rep. 13, 21700 (2023).
Daianu, M. et al. Disrupted rich club network in behavioral variant frontotemporal dementia and early-onset Alzheimer’s disease. Hum. Brain Mapp. 37, 868–883 (2016).
de Haan, W., Mott, K., van Straaten, E. C. W., Scheltens, P. & Stam, C. J. Activity dependent degeneration explains hub vulnerability in Alzheimer’s disease. PLoS Comput. Biol. 8, e1002582 (2012).
Kizilirmak, J. M., Soch, J., Richter, A. & Schott, B. H. Age-related differences in fMRI subsequent memory effects are directly linked to local grey matter volume differences. Neurobiol. Aging 134, 160–164 (2024).
Rubinov, M. & Sporns, O. Complex network measures of brain connectivity: uses and interpretations. NeuroImage 52, 1059–1069 (2010).
Lancichinetti, A. & Fortunato, S. Consensus clustering in complex networks. Sci. Rep. 2, 336 (2012).
Newman, M. E. J. Modularity and community structure in networks. Proc. Natl Acad. Sci. USA 103, 8577–8582 (2006).
Guimerà, R. & Nunes Amaral, L. A. Functional cartography of complex metabolic networks. Nature 433, 895–900 (2005).
Hofmann, M. A. Searching for effects in big data: why p-values are not advised and what to use instead. In Proc. Winter Simulation Conference (eds Yilmaz, L. et al.) 725–736 (IEEE, 2015).
Benjamini, Y. & Hochberg, Y. Controlling the false discovery rate: a practical and powerful approach to multiple testing. J. R. Stat. Soc. B 57, 289–300 (1995).
carlosmig/EEG-Dementias: EEG modeling in aging and dementia. Zenodo https://doi.org/10.5281/zenodo.16420033 (2025).
Xia, M., Wang, J. & He, Y. BrainNet Viewer: a network visualization tool for human brain connectomics. PLoS ONE 8, e68910 (2013).
Acknowledgements
This work was supported by Latin American Brain Health Institute (BrainLat) award BL-SRGP2020-02 to M.A.P. and A.I. A.I. is supported by grants from ReDLat (National Institutes of Health and the Fogarty International Center (FIC), the National Institutes of Aging (R01 AG057234, R01 AG075775, AG021051, R01 AG083799, CARDS-NIH 75N95022C00031), Alzheimer’s Association (SG-20-725707), Rainwater Charitable Foundation, The Bluefield project to cure FTD and the Global Brain Health Institute), ANID/FONDECYT Regular (1210195, 1210176 and 1220995) and ANID/FONDAP/15150012. A.M.G. is partially supported by the National Institute On Aging of the National Institutes of Health (R01 AG075775, R01 AG083799, 2P01AG019724), ANID (FONDECYT Regular 1210176, 1210195) and DICYT-USACH (032351G_DAS). The contents of this publication are solely the author’s responsibility and do not represent the official views of these institutions.
Author information
Authors and Affiliations
Contributions
Conceptualization was provided by A.I. and C.C.-O. Supervision was provided by A.I. Methodology and analyses were performed by C.C.-O., H.H. and J. Cruzat. Data were collated by R.G.-G., J. Cruzat and P.P. The original draft was written by C.C.-O. and A.I. Review and editing of the paper were carried out by C.C.-O., S.M., H.H., J. Cruzat, S.B., V.M., J. Cuadros, H.S.-G., P.A.V.-S., F.L., J.F.O.-G., A.G.-H., J.B.-S., R.A.G.-M., T.A., E.Y., R.A., A.L., S.F., G.G.Y., J.E., C.B., S.L., R.W., A.F., D.H., G.D.C., M.S.-A., R.G.-G., E.H., D.A., K.K., N.R., R.C., R.H., D.Y., B.G., G.D., P.P., M.A.P., P.O., E.T., B.L. and A.I.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Mental Health thanks Carina Fernandes and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Supplementary Information
Supplementary methods, figures, tables and references.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Coronel-Oliveros, C., Moguilner, S., Hernandez, H. et al. Diversity-sensitive brain clocks linked to biophysical mechanisms in aging and dementia. Nat. Mental Health 3, 1214–1229 (2025). https://doi.org/10.1038/s44220-025-00502-7
Received:
Accepted:
Published:
Version of record:
Issue date:
DOI: https://doi.org/10.1038/s44220-025-00502-7
This article is cited by
-
Computational whole-body-exposome models for global precision brain health
Nature Communications (2025)