Abstract
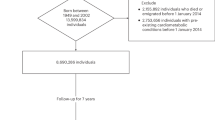
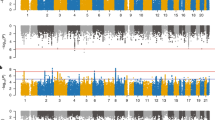
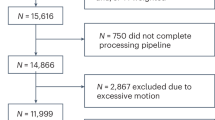
Neurodevelopmental conditions such as attention-deficit/hyperactivity disorder (ADHD) and autism co-occur with cardiometabolic conditions. However, little is known about the mechanisms underlying this co-occurrence. In this nationwide three-generation study using population-based registers in the Netherlands (n = 15 million), we assessed the familial (co-)aggregation of ADHD, autism and cardiometabolic conditions, and estimated their heritabilities and genetic correlations. ADHD, autism and cardiometabolic conditions showed aggregation and co-aggregation within families and between spouses. Estimated heritabilities of ADHD and autism were moderate (both h2 = 0.5), while those of cardiometabolic conditions ranged from low to moderate (h2 = 0.1–0.4). Genetic correlations between neurodevelopmental and cardiometabolic conditions were modest (rg = –0.02–0.20). Together, these results suggest a partly shared familial liability for neurodevelopmental and cardiometabolic conditions, and environmental factors likely play a more important role in the co-occurrence of neurodevelopmental and cardiometabolic conditions than genetics. These new insights can advance research toward specific etiological mechanisms and inform preventive strategies.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 digital issues and online access to articles
$79.00 per year
only $6.58 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to the full article PDF.
USD 39.95
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
Data availability
Under certain conditions, microdata from CBS are accessible for statistical and scientific research. For further information: microdata@cbs.nl.
Data of Lifelines may be obtained from a third party and are not publicly available. Researchers can apply to use the Lifelines data used in this study. More information about how to request Lifelines data and the conditions of use can be found on their website (https://www.lifelines-biobank.com/researchers/working-with-us).
Code availability
Code for the main analysis is publicly available at https://github.com/yiranli-hi/Familial-coaggregation-project (ref. 61).
References
Thapar, A., Cooper, M. & Rutter, M. Neurodevelopmental disorders. Lancet Psychiatry 4, 339–346 (2017).
Dobrosavljevic, M. et al. Attention-deficit/hyperactivity disorder symptoms and subsequent cardiometabolic disorders in adults: investigating underlying mechanisms using a longitudinal twin study. BMC Med. 21, 452 (2023).
Khachadourian, V. et al. Comorbidities in autism spectrum disorder and their etiologies. Transl. Psychiatry 13, 71 (2023).
Garcia-Argibay, M. et al. The association between type 2 diabetes and attention-deficit/hyperactivity disorder: a systematic review, meta-analysis, and population-based sibling study. Neurosci. Biobehav. Rev. 147, 105076 (2023).
Li, L. et al. Attention-deficit/hyperactivity disorder is associated with increased risk of cardiovascular diseases: a systematic review and meta-analysis. JCPP Adv. 3, e12158 (2023).
Dhanasekara, C. S. et al. Association between autism spectrum disorders and cardiometabolic diseases: a systematic review and meta-analysis. JAMA Pediatr. 177, 248–257 (2023).
Landau, Z. & Pinhas-Hamiel, O. Attention deficit/hyperactivity, the metabolic syndrome, and type 2 diabetes. Curr. Diabetes Rep. 19, 46 (2019).
Momen, N. C. et al. Association between mental disorders and subsequent medical conditions. N. Engl. J. Med. 382, 1721–1731 (2020).
Gidziela, A. et al. A meta-analysis of genetic effects associated with neurodevelopmental disorders and co-occurring conditions. Nat. Hum. Behav. 7, 642–656 (2023).
Sandin, S. et al. The familial risk of autism. JAMA 311, 1770–1777 (2014).
Jermendy, G. et al. Effect of genetic and environmental influences on cardiometabolic risk factors: a twin study. Cardiovasc. Diabetol. 10, 96 (2011).
Meijsen, J. et al. Quantifying the relative importance of genetics and environment on the comorbidity between mental and cardiometabolic disorders using 17 million Scandinavians. Nat. Commun. 15, 5064 (2024).
Karhunen, V. et al. The link between attention deficit hyperactivity disorder (ADHD) symptoms and obesity-related traits: genetic and prenatal explanations. Transl. Psychiatry 11, 455 (2021).
Baranova, A., Chandhoke, V., Cao, H. B. & Zhang, F. Q. Shared genetics and bidirectional causal relationships between type 2 diabetes and attention-deficit/hyperactivity disorder. Gen. Psychiatry 36, e100996c (2023).
Zhang, J. et al. Mediating pathways between attention deficit hyperactivity disorder and type 2 diabetes mellitus: evidence from a two-step and multivariable Mendelian randomization study. Epidemiol. Psychiatr. Sci. 33, e54 (2024).
Garcia-Argibay, M. et al. The role of ADHD genetic risk in mid-to-late life somatic health conditions. Transl. Psychiatry 12, 166 (2022).
Du Rietz, E. et al. The contribution of attention-deficit/hyperactivity disorder polygenic load to metabolic and cardiovascular health outcomes: a large-scale population and sibling study. Transl. Psychiatry 14, 470 (2024).
Du Rietz, E. et al. Mapping phenotypic and aetiological associations between ADHD and physical conditions in adulthood in Sweden: a genetically informed register study. Lancet Psychiatry 8, 774–783 (2021).
Schendel, D. et al. 3-generation family histories of mental, neurologic, cardiometabolic, birth defect, asthma, allergy, and autoimmune conditions associated with autism: an open-source catalog of findings. Autism Res. 17, 2144–2155 (2024).
Triatin, R. D. et al. Familial co-aggregation and shared genetics of cardiometabolic disorders and traits: data from the multi-generational Lifelines Cohort Study. Cardiovasc. Diabetol. 22, 282 (2023).
Athanasiadis, G. et al. A comprehensive map of genetic relationships among diagnostic categories based on 48.6 million relative pairs from the Danish genealogy. Proc. Natl Acad. Sci. USA 119, e2118688119 (2022).
Vos, M. et al. Familial co-aggregation and shared familiality among neurodevelopmental problems and with aggressive behavior, depression, anxiety, and substance use. Psychol. Med. 54, 4820–4832 (2024).
Peyrot, W. J., Robinson, M. R., Penninx, B. W. & Wray, N. R. Exploring boundaries for the genetic consequences of assortative mating for psychiatric traits. JAMA Psychiatry 73, 1189–1195 (2016).
Nordsletten, A. E. et al. Patterns of nonrandom mating within and across 11 major psychiatric disorders. JAMA Psychiatry 73, 354–361 (2016).
Nakaya, N. et al. Spousal similarities in cardiometabolic risk factors: a cross-sectional comparison between Dutch and Japanese data from two large biobank studies. Atherosclerosis 334, 85–92 (2021).
Nielsen, J., Hulman, A. & Witte, D. R. Spousal cardiometabolic risk factors and incidence of type 2 diabetes: a prospective analysis from the English Longitudinal Study of Ageing. Diabetologia 61, 1572–1580 (2018).
Tick, B., Bolton, P., Happé, F., Rutter, M. & Rijsdijk, F. Heritability of autism spectrum disorders: a meta-analysis of twin studies. J. Child Psychol. Psychiatry 57, 585–595 (2016).
Faraone, S. V. & Larsson, H. Genetics of attention deficit hyperactivity disorder. Mol. Psychiatry 24, 562–575 (2019).
Polderman, T. J. et al. Meta-analysis of the heritability of human traits based on fifty years of twin studies. Nat. Genet. 47, 702–709 (2015).
Rosen, N. E., Lord, C. & Volkmar, F. R. The diagnosis of autism: from Kanner to DSM-III to DSM-5 and beyond. J. Autism Dev. Disord. 51, 4253–4270 (2021).
Bonvicini, C., Faraone, S. V. & Scassellati, C. Common and specific genes and peripheral biomarkers in children and adults with attention-deficit/hyperactivity disorder. World J. Biol. Psychiatry 19, 80–100 (2018).
Poveda, A. et al. The heritable basis of gene-environment interactions in cardiometabolic traits. Diabetologia 60, 442–452 (2017).
Tandon, P. S. et al. Physical activity, screen time, and sleep in children with ADHD. J. Phys. Act. Health 16, 416–422 (2019).
Kodak, T. & Piazza, C. C. Assessment and behavioral treatment of feeding and sleeping disorders in children with autism spectrum disorders. Child Adolesc. Psychiatr. Clin. N. Am. 17, 887–905 (2008).
Elkins, I. J. et al. Increased risk of smoking in female adolescents who had childhood ADHD. Am. J. Psychiatry 175, 63–70 (2018).
Charlier, L. et al. Effects of physical exercise on cardiometabolic health in individuals with autism spectrum disorder: a systematic review. Healthcare 13, 439 (2025).
Shahane, V., Kilyk, A. & Srinivasan, S. M. Effects of physical activity and exercise-based interventions in young adults with autism spectrum disorder: a systematic review. Autism 28, 276–300 (2024).
Wang, S. et al. Effectiveness of physical activity interventions for core symptoms of autism spectrum disorder: a systematic review and meta-analysis. Autism Res. 16, 1811–1824 (2023).
Corona, R. et al. Integrating tobacco prevention skills into an evidence-based intervention for adolescents with ADHD: results from a pilot efficacy randomized controlled trial. J. Abnorm. Child Psychol. 48, 1439–1453 (2020).
Kendler, K. S., Maes, H. H., Sundquist, K., Ohisson, H. & Sundquist, J. Genetic and family and community environmental effects on drug abuse in adolescence: a Swedish national twin and sibling study. Am. J. Psychiatry 171, 209–217 (2014).
Kendler, K. S., Ohlsson, H., Mezuk, B., Sundquist, K. & Sundquist, J. Exposure to peer deviance during childhood and risk for drug abuse: a Swedish national co-relative control study. Psychol. Med. 45, 855–864 (2015).
van Rheenen, W., Peyrot, W. J., Schork, A. J., Lee, S. H. & Wray, N. R. Genetic correlations of polygenic disease traits: from theory to practice. Nat. Rev. Genet. 20, 567–581 (2019).
Ormhoj, S. S., Pottegard, A., Gasse, C. & Rasmussen, L. Use of attention-deficit/hyperactivity disorder medication among older adults in Denmark. Br. J. Clin. Pharmacol. 84, 1505–1513 (2018).
Statistics Netherlands. CBS https://www.cbs.nl (2025).
Swiers, R. & Kleijwegt, R. Individual product determination in the new Dutch DBC system: how to make the system transparent for its users. BMC Health Serv. Res. 11, A17 (2011).
Sijtsma, A. et al. Cohort profile update: Lifelines, a three-generation cohort study and biobank. Int. J. Epidemiol. 51, E295–E302 (2021).
Bell, C. C. DSM-IV: diagnostic and statistical manual of mental disorders. JAMA 272, 828–829 (1994).
Risch, N. Linkage strategies for genetically complex traits. I. Multilocus models. Am. J. Hum. Genet. 46, 222 (1990).
Muller, C. J. & MacLehose, R. F. Estimating predicted probabilities from logistic regression: different methods correspond to different target populations. Int. J. Epidemiol. 43, 962–970 (2014).
Dickerman, B. A. & Hernan, M. A. Counterfactual prediction is not only for causal inference. Eur. J. Epidemiol. 35, 615–617 (2020).
Zeileis, A., Köll, S. & Graham, N. Various versatile variances: an object-oriented implementation of clustered covariances in R. J. Stat. Softw. 95, 1–36 (2020).
Oehlert, G. W. A note on the delta method. Am. Stat. 46, 27–29 (1992).
Arel-Bundock, V., Greifer, N. & Heiss, A. How to interpret statistical models using marginaleffects for R and Python. J. Stat. Softw. 111, 1–32 (2024).
Falconer, D. S. The inheritance of liability to certain diseases, estimated from the incidence among relatives. Ann. Hum. Genet. 29, 51–76 (1965).
Reich, T., James, J. & Morris, C. The use of multiple thresholds in determining the mode of transmission of semi-continuous traits. Ann. Hum. Genet. 36, 163–184 (1972).
Wray, N. R. & Gottesman, I. I. Using summary data from the Danish national registers to estimate heritabilities for schizophrenia, bipolar disorder, and major depressive disorder. Front. Genet. 3, 118 (2012).
Baselmans, B. M. L., Yengo, L., van Rheenen, W. & Wray, N. R. Risk in relatives, heritability, SNP-based heritability, and genetic correlations in psychiatric disorders: a review. Biol. Psychiatry 89, 11–19 (2021).
Kemper, K. E. et al. Phenotypic covariance across the entire spectrum of relatedness for 86 billion pairs of individuals. Nat. Commun. 12, 1050 (2021).
Noyan, F. & Şimşek, G. G. Tetrachoric correlation as a measure of default correlation. Procedia Soc. Behav. Sci. 62, 1230–1234 (2012).
Digby, P. G. N. Approximating the tetrachoric correlation-coefficient. Biometrics 39, 753–757 (1983).
Li, Y. & Zhou, Y. Familial-coaggregation-project. GitHub https://github.com/yiranli-hi/Familial-coaggregation-project (2025).
Acknowledgements
This research was supported by the European Union Horizon 2020 Research and Innovation Program (grant 956381 (C.A.H.)). This research reflects only the authors’ view, and the European Commission is not responsible for any use that may be made of the information it contains. This research used the data from the Lifelines cohort study. The Lifelines cohort study is supported by the Netherlands Organization of Scientific Research NWO (grant 175.010.2007.006), the Economic Structure Enhancing Fund (FES) of the Dutch government, the Dutch Ministry of Economic Affairs, the Ministry of Education, Culture and Science, the Dutch Ministry of Health, Welfare and Sports, the Northern Netherlands Collaboration of Provinces (SNN), the Province of Groningen, the University Medical Center Groningen, the University of Groningen, Dutch Kidney Foundation and Dutch Diabetes Research Foundation. Y.L. and Y.Z. acknowledge the support of a joint scholarship from the China Scholarship Council and the University of Groningen. M.B. acknowledges the support by NIMH grant R01MH125902. N.R.W. acknowledges the Australian National Health and Medical Research Council (1173790 and 1113400) and the Michael Davys Trust. The funders had no role in study design, data collection and analysis, decision to publish or preparation of the paper.
Author information
Authors and Affiliations
Contributions
Y.L. and Y.Z. conducted the data analyses. The study was conceptualized under the supervision of M.V., C.A.H. and H.S. Analysis code for the methods was originally developed by N.R.W. and M.B. and subsequently adapted by Y.L. and Y.Z. N.R.W. and M.B. provided additional methodological advice. O.S. performed the validation analyses using the Lifelines cohort. Y.L. and Y.Z. drafted the original paper. All authors reviewed the paper and contributed to its editing and revision.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Mental Health thanks Joeri Meijsen and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Supplementary Information
Supplementary Figs. 1–7 and Supplementary Methods.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Li, Y., Zhou, Y., Vos, M. et al. Familial co-aggregation and shared heritability between neurodevelopmental problems and cardiometabolic conditions. Nat. Mental Health 3, 1545–1554 (2025). https://doi.org/10.1038/s44220-025-00535-y
Received:
Accepted:
Published:
Version of record:
Issue date:
DOI: https://doi.org/10.1038/s44220-025-00535-y