Abstract
Objectives
This study evaluated outcomes in eyes with retinal disorders with moderate to profound vision impairment after Excimer-Laser-Multiple-Light-Redirection (Excimer-MLR), a non-central, superficial and multifocal corneal photoablation for novel refractive neuroadaptive vision restoration.
Methods
Retrospective analysis of seven consecutively treated phakic and pseudophakic eyes followed for 18–24 months after unilateral Excimer-MLR.
Results
Control untreated fellow eyes: No significant change in median best-corrected distance visual acuity (BCDVA) or contrast sensitivity (CS).
Treated eyes: Excimer-MLR temporarily modified corneal radii of curvature, defocused light at the PRL, decreased relative refractive error at multiple non-PRL retinal areas and shifted fixation. Median BCDVA improved significantly (p = 0.018) from baseline at all measured times between 3- and 24-months, with ≥15 ETDRS-letter-gain in 86% at 18–24 months. Preoperative mean and median BCDVA were 20/225 (range 20/100–20/604) and 0.98 logMAR, respectively. Mean BCDVA was 20/58, 20/68, 20/64, 20/60, mean (±SD) ETDRS-letter-gain was 28.9 (±10.6), 25.5 (±13.7), 26.8 (±14.9), 28.4 (±14.1) and median BCDVA was 0.40, 0.38, 0.48, 0.40 at 3, 6, 10–12, and 18–24 months, respectively. Median CS significantly improved from baseline. All treated eyes had preoperative-central scotomas that rapidly disappeared or became smaller and peripheral. Amsler grids were normal in 6/7 eyes at ≥18 months. Mean (±SD) retinal sensitivity average thresholds (RS) increased by 1.53 dB (±1.16) at 6 months. There were no changes in distance- or near-glasses and no Excimer-MLR-related adverse events.
Conclusion
Excimer-MLR, a rapid corneal procedure beneficially synergistic with retinal therapies, safely produced, without customisation, magnification or visual training, significant multiyear-visual improvements and increased retinal sensitivity in eyes with macular degeneration or surgically treated macular holes.
Similar content being viewed by others
Introduction
Central visual loss from macular disorders remains a huge unmet need. Multiple-light-redirection (MLR) therapy was proposed by Serdarevic [1] to harness the visual system’s complex light capture, processing and integration capabilities for vision restoration by redirecting light away from a dysfunctional preferred retinal locus of fixation (PRL) to multiple non-PRL retinal areas within and outside of the fovea. Redirection of enough light away from the small PRL (located either within or outside foveal regions in eyes with central visual loss) to multiple non-PRL areas disrupts the habitually used and overweighted PRL pathway. The resulting unconscious natural increase in visual search, sampling and photoreceptor stimulation of non-PRL areas promotes ongoing spontaneous recruitment of still-functioning photoreceptors and visual pathways in multiple non-PRL areas. The capability of fixational eye movements and the brain to integrate fragmentary input from multiple retinal areas is well-established. Natural integration of additional and better visual signals about previously missing visual information allows the brain to receive enough fragments of objects in the field of view from multiple retinal areas simultaneously to invoke memory representations for sharper, more complete and more accurate perception [2] with ongoing beneficial neuroadaptation [3,4,5] and visual improvement.
Multiple-light-redirection can improve visual function without any intraocular surgery, pre-treatment localisation or customisation or post-treatment magnification or patient training [6]. Excimer-Laser-Multiple-Light-Redirection (Excimer-MLR) is a corneal laser treatment for macular disorders with moderate to profound vision impairment. Excimer-MLR ablates very small, shallow, non-central and multifocal corneal portions to temporarily modify anterior radii of curvature (ROC). Customisation is not required regardless of varying preoperative corneal topography, ROC, refractive error and pupil size in different eyes. Although postoperative widespread ROC modifications vary in eyes with different topographies, Excimer-MLR changes defocus at the PRL and increases relative focusing at multiple retinal areas peripheral to the PRL in all eyes. Moreover, the ROC modifications are temporary, because natural corneal hyperplasia and stromal remodelling fill in Excimer MLR’s very small and shallow ablations. The ROC modifications last just long enough to cause redirection of light in all directions away from the PRL to multiple non-PRL retinal areas to facilitate rewiring and reorganisation of the visual system without any permanent corneal topographic or refractive changes [1, 6]. Therefore, Excimer-MLR’s desired and achieved temporary corneal effects are unlike excimer laser vision correction of refractive errors in normal eyes.
Large visual improvements in pseudophakic worse-seeing eyes with non-exudative age-related macular degeneration (AMD) with geographic atrophy (GA) were reported [6] after a single Excimer-MLR comprising focal photoablation of a very small portion of Bowman’s Layer in each corneal quadrant. Therapeutic corneal photoablation (PTK) is a well-established procedure since its introduction [7] in 1985, and photoablation of Bowman’s Layer has been widely and safely performed for decades. Topographic, raytracing and microperimetric analyses demonstrated that Excimer-MLR performed without any modification of commercially available excimer lasers and software temporarily modified corneal ROC, increased defocus at the PRL, redirected environmental light away from PRL pathways and simultaneously reduced relative refractive error at multiple non-PRL areas in all quadrants [6]. A single Excimer-MLR procedure produced letter-gains of 30 and 59 within 12 days and 25 and 61 at 9 months in operated eyes of two patients, with improved Amsler grids, no distance- or near-glasses-change, no surgical complications and no MLR-related adverse events [6].
The purpose of the present study was to evaluate multiple functional outcomes in treated and control untreated fellow eyes at multiple time periods up to 24 months after Excimer-MLR in another larger series of consecutively treated phakic and pseudophakic eyes with various macular disorders and moderate to profound vision impairment.
Methods
This was a retrospective, consecutive case series of a cohort of patients with macular disorders and central visual loss treated unilaterally (with the fellow untreated eye serving as a control) with Excimer-MLR performed by one surgeon and followed for 18–24 months. The study was conducted with approval of the University of Illinois College of Medicine Investigational Review Board and adhered to the Declaration of Helsinki. Informed consent was obtained from all patients.
Pre-existing medical records of the patients were reviewed for patient demographics, clinical features from ophthalmic exams, treatments and treatment outcomes. Reviewed patient characteristics included retinal diagnoses, retinal medical and surgical treatments prior to MLR, age and sex. Clinical features analysed included best-corrected distance and near visual acuities (BCDVA, BCNVA) measured with the examiner masked as to the treated eye and blinded to prior measurements using the Early Treatment Diabetic Retinopathy Study (ETDRS) charts from at least 3 months prior to MLR treatment, within 1 day prior to treatment, within 12 days after treatment and at 3 months, 6 months, 10–12 months and 18–24 months after MLR. Gonzalez Markowitz (GM) Potential Visual Acuity (PVA) multiple E-optotype-reversed-polarity- test charts (Precision Vision, Woodstock, IL) had been used prior to the Excimer-MLR procedure to predict the potential optimal visual acuity obtainable by integrating multiple retinal areas, including areas peripheral to the PRL, in candidate eyes. Screening with GM PVA testing had been done to recommend and perform unilateral Excimer-MLR only in eyes with PVA results showing improved visual acuity compared to ETDRS-measured BCDVA, which is a measurement of visual acuity only at the PRL. The screening PVA of each treated eye was compared to actual ETDRS-measured BCDVA achieved from a few days up to 12 months postoperatively to analyse the usefulness of PVA as a screening test for Excimer-MLR. Additional clinical data analysed included contrast sensitivity (CS) measured (right eye measured first by examiner masked as to eye treated and blinded to results of previous measurements) by Pelli-Robson charts at 1 week-3 months, 6–12 months and 18–24 months after MLR. Other clinical data analysed before and after Excimer-MLR included fundus exams, subjective manifest refractions, central visual fields on Amsler Grids, Random Dot stereopsis, anterior corneal radii of curvature maps, corneal z-elevation maps and retinal spot diagrams of retinal ray tracings measured by the iTrace analyser (Tracey Technologies, Houston, TX) and optical coherence tomography (OCT) (Carl Zeiss Meditec, Jena, Germany). Mesopic microperimetry exams performed with the Macular Integrity Assessment (MAIA) microperimeter, software version 1.7.0, (Centervue, Fremont, CA) were analysed for PRL locations and retinal sensitivities. The MAIA microperimeter measured threshold retinal sensitivity as the minimum light intensity perceived by the eye when white light emitting diode spots stimulated an area of the retina. The average threshold retinal sensitivity measured using the standard 4.2 strategy was the average of minimum light intensities perceived at 37 points of the retina within the 10° diameter area centred on the PRL, with the decibel value range calculated among the minimum and the maximum intensity level of the projected stimuli. The stimuli size was Goldmann III, the background luminance was 4 apostilb (asb) and the maximum luminance was 1000 asb, with a 36 decibels (dB) dynamic range. The decibel value range was calculated among the minimum and the maximum intensity level of the projected stimuli.
The unilateral Excimer-MLR procedure, which has been fully described previously [6], was performed by an experienced LASIK/PRK surgeon (EQY) in the worse-seeing eye of all patients. The rapid procedure [6] used standard Star S4 excimer laser myopic LASIK software 5.32 (Johnson and Johnson Vision, Santa Ana, CA) to produce shallow gradated ablations up to about 20μm deep of only four ~1.5-mm-wide paracentral areas spaced 45 degrees apart after mechanical removal of the overlying epithelium. The photoablation pattern was chosen [6] to modify corneal ROC to redirect environmental light impinging on the retina and change focusing at the PRL and numerous retinal locations peripheral to the PRL in all quadrants. The locations and depths of ablation were chosen to remove, without any photoablation over the central optical zone, the smallest portions of Bowman’s Layer, the most rigid part of the cornea, to most effectively and safely achieve widespread ROC modifications within and outside of the ablated area to facilitate natural visual neuroadaptation. The gradated ablations were located at 1:30, 4:30, 7:30 and 10:30 clock hours of the cornea and were centred at 3 mm from the eye’s PRL, which had been located by having the patient look at the laser’s fixation light while the surgeon centred on the Purkinje images.
Statistical analyses were performed using Intellectus Statistics (2019, Intellectus Statistics, Online computer software). Statistical significance of paired outcomes of pre- and post-logMAR BCDVA, -logMAR BCNVA and -log CS were assessed by two-tailed Wilcoxon signed rank tests, which compared the median of two paired groups. A p-value less than 0.05 was considered statistically significant.
Results
Seven patients (4 men, 3 women) who had been consecutively treated with unilateral Excimer-MLR satisfied the inclusion criteria. The mean patient age was 77 years (±11.5). Retinal pathologies confirmed by fundus examination and OCT and causing moderate to profound vision impairment included non-neovascular AMD with GA (three eyes), neovascular AMD with GA but without persistent intraretinal or subretinal fluid (two eyes), and surgically repaired-macular holes (surgery two years prior to Excimer-MLR in one eye and five years prior to Excimer-MLR in another eye with myopic macular degeneration) (see Table 1). None of the Excimer-MLR-treated eyes received any other retinal medical or surgical treatments from at least 6 months prior to Excimer-MLR through the entire post-operative follow-up period. All treated eyes had stable refractions and visual acuities for at least 3 months prihor to Excimer-MLR.
Table 1 lists patient characteristics, including age, sex, retinal macular pathologies, prior retinal medical and surgical treatments, lens status, screening test GM PVA and pre-operative MRSE, BCDVA, BCNVA and CS. Table 1 also lists Excimer-MLR outcomes, including postoperative BCDVA, BCNVA and CS of treated eyes with p-values.
Refractive multiple-light-redirection from the PRL
Excimer-MLR created four very small, shallow and paracentral depressions of the anterior corneal surface overlying the photoablated portions of Bowman’s Layer (Fig. 1A) and modified ROC in all corneal quadrants (Fig. 1B). Retinal spot diagrams showing on-axis ray tracings confirmed that Excimer-MLR caused light in the field of view to be directed away from the PRL (Fig. 1C) with temporary defocusing at the PRL. After Excimer-MLR, rapid natural corneal epithelial migration and hyperplasia, together with subsequent stromal remodelling, filled in the depressions leading to progressive reversion to preoperative corneal topography and optical aberrations with ongoing multiyear visual improvement (Fig. 1A–C).
A iTrace analyser maps of differences in corneal anterior elevation: pre-MLR vs 5 days after Excimer-MLR (left) in eye 6OD showing remaining four paracentral depressions in ablated regions centred at 1:30, 4:30, 7:30 and 10:30 clock hours; pre-MLR vs 6 months after MLR (right) demonstrating progressive reversion to preoperative corneal topographic elevation; B iTrace analyser maps of differences of local anterior corneal radii of curvature (ROC): pre-MLR vs 5 days after MLR (left) in eye 6OD showing ROC changes in all four corneal quadrants; pre-MLR vs 1month after MLR (right) demonstrating progressive reversion to preoperative corneal topographic ROC; C retinal ray-tracings centred on the PRL using the iTrace analyser before MLR (left) in eye 6OD, at 5 days after MLR (middle) demonstrating that environmental light within the field of view was directed away from the PRL by MLR and at 1 year after MLR (right) showing return to pre-MLR defocus and other optical aberration levels with ongoing visual improvement. Total lower order aberrations and defocus were 0.544 µ and 0.376 µ, 0.787 µ and 0.675 µ, and 0.541 µ and 0.407 µ preoperatively, at 5 days postoperatively and at 1 year postoperatively, respectively; D MAIA microperimetry before and after Excimer-MLR of eyes 6OD (left top and bottom) with a macular hole surgically two years prior to MLR, 4OS (middle top and bottom) left) with a macular hole surgically repaired five years prior to MLR with residual atrophy and myopic macular degeneration and eye 7OS (right top and bottom) with non-exudative AMD and geographic atrophy demonstrating shifting by the visual system (without any patient visual training) of the locations of fixation points (blue smallest dots) inferotemporally in eye 6OD, superiorly, superotemporally and superonasally in eye 4OS and superiorly, superotemporally and inferiorly in eye 7OS and the locations of the PRL (blue larger dot, which is the centroid of fixation points);
Microperimetry PRL locations and retinal sensitivity
Excimer-MLR’s effect of directing light away from the PRL caused the locations of fixation points on microperimetry to spontaneously shift (Figs. 1D and 2) in all treated eyes. The angle direction and amount of shifting of the PRL on the first postoperative microperimetry exam were different in all seven eyes; the amount ranged between 0.2° (about 0.06 mm) and 3.3° (about 1 mm), with a mean(±SD) of 1.186°(±0.96°). The eyes with smaller areas of retinal pathology around the pre-MLR PRL, such as the two eyes with repaired macular holes, displayed smaller shifts (0.2° and 0.8°) than eyes with AMD with more extensive pathology around the pre-MLR PRL (1.6° and 3.3°). Microperimetry was a worse indicator of the potential for visual improvement after Excimer-MLR than GM PVA testing. For example, during preoperative measurement of retinal sensitivity within the 10° surrounding the PRL (Fig. 2), eye 2OS did not respond at any of the 37 measured retinal points to the brightest stimulus produced by the MAIA microperimeter and projected onto the retina. Despite preoperative microperimetry suggesting no visual potential (<0 dB sensitivity) within the 10° surrounding the PRL in eye 2OS (Fig. 2), PVA testing predicted BCDVA-improvement from 20/604 to 20/200 and actual achieved postoperative BVCVA was 20/240, 20/152 and 20/160 at 6, 12 and 18 months after Excimer-MLR. Retinal sensitivity average thresholds (RS) increased by 10 dB by 18 months after Excimer-MLR in eye 2OS, despite demonstrating the smallest increase, 0.1 dB, of all treated eyes at 6 months. The range of RS-increases was 0.1 dB to 3.1 dB and the mean(±SD) was 1.53 dB (±1.16) at 6 months, at which time, apart from eye 2OS, retinal sensitivity increased less in eyes with macular holes than in eyes with AMD with GA. Microperimetry was performed continuously though 18 months only in eye 2OS.
A Before MLR, MAIA showing the retinal sensitivity map, <0dB sensitivity at all points on the 10° grid, 100% reduced thresholds and average threshold of 0dB, and the Amsler grid demonstrating a large positive scotoma (lower right); B At 6 months after treatment, MAIA showing the retinal sensitivity map, all sensitivities on the 10° grid, 100% reduced thresholds and average threshold of 0.1dB, and the normal Amsler grid (lower right); C At 12 months after treatment, MAIA showing the retinal sensitivity map, all sensitivities on the 10° grid, 100% reduced thresholds and average threshold of 1.6dB, and the normal Amsler grid (lower right); D At 18 months after treatment, MAIA showing the retinal sensitivity map, all sensitivities on the 10° grid, 97.3% reduced thresholds and average threshold of 10.0dB, and the normal Amsler grid (lower right).
Amsler grid testing
All eyes had large positive central scotomas on pre-operative Amsler grid testing (Fig. 3). Within 30 min to 12 days after MLR, 50% of eyes developed normal Amsler grids and, in the remainder, the scotomas became much smaller and moved peripherally. At 6 months and 18 months after MLR, 5 of 7 eyes (71.4%) and 6 of 7 eyes (85.7%), respectively, had normal Amsler grids with no scotomas.
Visual acuity and contrast sensitivity
Treated eyes
Visual acuity and contrast sensitivity results with statistically significant p-values of treated eyes are listed in Table 1. Postoperative BCDVA and BCNVA were achieved with no postoperative change in refractive correction for any patient.
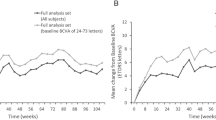
Before Excimer-MLR, mean BCDVA was 20/224 (range 20/100–20/604) in treated eyes. After Excimer-MLR, all patients preferred to gaze directly at ETDRS charts during visual acuity testing, including patients who needed to gaze eccentrically before treatment. Mean BCDVA of treated eyes was 20/96, 20/58, 20/68. 20/65 and 20/60 at 1–12 days, 3 months, 6 months, 10–12 months and 18–24 months after treatment, respectively. Mean (±SD) ETDRS-letter-gains were 18.9 (±10.7), 28.9 (±10.6), 25.5 (±13.7), 26.8 (±14.9) and 28.4 (±14.1), at 1–12 days, 3 months, 6 months, 10–12 months and 18–24 months, respectively. All treated eyes demonstrated gains ≥5 letters and 86% of treated eyes demonstrated gains ≥15 letters in BCDVA at 18–24 months. No treated eyes lost any letters from baseline at any time during follow-up. BCDVA better than or equal to the screening GM PVA was achieved in 43% of treated eyes within 1–12 days, 86% within 3 months and 100% within 12 months (actual achieved BCDVA range 20/25–20/152 at 10–12 months). Median logMAR BCDVA of treated eyes was 0.98 before MLR and 0.43, 0.40, 0.38, 0.48 and 0.40 at 1–12 days, 3, 6, 10–12 and 18–24 months postoperatively, respectively. Median logMAR BCDVA of treated eyes increased significantly (p = 0.018 at 3–24 months, p = 0.043 at 1–12 days) compared to baseline at all post-op exams.
Mean preoperative BCNVA was 20/148 in treated eyes. Mean BCNVA was 20/78, 20/43, 20/56, 20/54 and 20/55 at 1–12 days, 3 months, 6 months, 10–12 months and 18–24 months after treatment, respectively. Median logMAR BCNVA of treated eyes was 0.78 before MLR and 0.50, 0.30, 0.38, 0.46 and 0.40 at 1–12 days, 3, 6, 10–12 and 18–24 months postoperatively, respectively. Median logMAR BCNVA of treated eyes increased significantly (p = 0.018 at 3–12 months, p = 0.028 at 18–24 months, p = 0.043 at 1–12 days) compared to baseline at all post-op exams.
All treated eyes achieved gains ≥0.15 logCS after Excimer-MLR, with 71% (5/7) demonstrating gains ≥0.30 logCS at 18–24 months. Mean logCS in treated eyes was 0.71 preoperatively, and 1.22, 1.20 and 1.18 at 1 week-3 months, 6–12 months and 18–24 months post-operatively, respectively. Median logCS in treated eyes was 0.90 preoperatively, and 1.20, 1.05 and 1.20 at 1 week-3 months, 6–12 months and 18–24 months post-operatively, respectively. Median Pelli-Robson log CS significantly improved from baseline in treated eyes after Excimer-MLR during all follow up-time periods.
Untreated fellow control eyes
Median logMAR BCDVA of control untreated fellow eyes did not change significantly (p = 0.225 to p = 0.917) compared to baseline at any time. Median logMAR BCDVA of control untreated eyes was 0.38 before treatment of the contralateral eye and 0.40, 0.18, 0.20, 0.40 and 0.38 at 1–12 days, 3, 6, 10–12 and 18–24 months. Mean(±SD) ETDRS-letter-changes in control untreated fellow eyes were 0.7 (±3.9), 6.9 (±9.9), 5.1 (±12.7), 0 (±8.6) and 0.9 (±11.7), at 1–12 days, 3 months, 6 months, 10–12 months and 18–24 months, respectively. At 1–12 days after MLR, three control untreated fellow eyes had no change from baseline, three had a loss of 1 or 2 letters, and one untreated eye with AMD and GA had a gain of 10 letters. The gain in that untreated eye 1OS increased to 16 and 19 letters at 3 and 6 months, respectively, with microperimetry showing that Excimer-MLR shifted the locations of fixation points in treated eye 1OD and caused a corresponding oculomotor fixation response in the fellow untreated eye. Control untreated fellow eye 5OD with neovascular AMD and GA also had a letter gain by 3 months after corresponding bilateral PRL relocation. In both control fellow eyes in which visual acuities improved, the treated eye preoperatively had BCDVA only a little worse than that of the untreated eye and became the better-seeing eye controlling binocular viewing fixation between several days to 3 months postoperatively. Control untreated fellow eyes of the other patients with AMD lost ≥2 letters from baseline from 6 to 24 months. The maximum loss from disease progression in the control untreated better-seeing fellow eyes was 16 letters at 24 months. Median logMAR BCNVA and median logCS of control untreated fellow eyes did not change significantly compared to baseline at any time.
Stereopsis
Stereopsis was measured before and after Excimer-MLR only in the patient with poor vision in eye 6OD at two years after macular hole surgery. Stereopsis was 160 sec-arc before Excimer-MLR and improved to 32 sec-arc at 3 and 6 months and 50 sec-arc at 12 and 30 months after Excimer-MLR.
Safety
There was no Excimer-MLR-related pain, glare, halos, diplopia, decreased vision or other adverse event or surgical complication.
One patient with progressive GA (eye 7OS) requested a repeat Excimer-MLR at 13 months after Excimer-MLR because of a new negative scotoma. At one day after repeat Excimer-MLR, the Amsler grid returned to normal with disappearance of the negative scotoma.
Discussion
Excimer-MLR produced statistically significant improvements in BCDVA, BCNVA and CS from a few days through 18–24 months, with functionally meaningful disappearance of scotomas and gains ≥15 letters maintained in 86% of treated eyes at 18–24 months. This study confirmed the shorter-term results achieved in our previous smaller case series of patients with AMD with GA [6] and demonstrated efficacy for additional macular disorders. Our results also were consistent with results achieved in single and multicentre studies in adequately screened eyes after unilateral or bilateral modification of corneal ROC by a non-commercially available custom corneal photovitrification (non-ablative) laser [8,9,10]. The multicentre custom laser study [9] reported letter-gains for mean binocular BCNVA/BCDVA of 22/15, 25/19, 25/18 at 1, 6, and 12 months, respectively, and improved quality of life (NEI VFQ-25) after bilateral treatment for dry and wet AMD. In the single centre custom laser study [10], dry AMD eyes with screening-PVA improvement ≥15 letters relative to preoperative BCDVA achieved mean(± SD) letter gains of 21.0 (±12.1) and 15.2 (±13.6) letters at 1 and 3 months, respectively.
Median BCDVA, BCNVA and CS did not significantly change compared to baseline in untreated control fellow eyes at any time in this study. Although some small losses in untreated fellow eyes could be attributed to testing variability [11], the maximum 16-letter loss in an untreated eye at 24 months was consistent with previous studies of the natural course of bilateral dry AMD with GA showing a mean loss of 12.4 letters by 24 months [12]. Interestingly, this study revealed that vision of untreated slightly better-seeing fellow eyes can improve after unilateral Excimer-MLR if postoperative visual gain in the treated eye causes the untreated eye to become the worse-seeing eye. In eyes with AMD, the better-seeing eye has been reported to drive oculomotor fixational control when viewing binocularly [13].
As it is obvious that not all eyes with macular disorders and moderate to profound visual impairment could benefit from Excimer-MLR, it was very encouraging that the potential for postoperative visual improvement was predicted by rapid and easy screening with inexpensive handheld GM PVA charts that provided estimation of visual acuity obtainable by integrating multiple retinal areas, including areas peripheral to the PRL. All treated eyes in this study achieved BCDVA better than or equal to PVA within 12 months. Multiple E-optotype-PVA acuity testing was previously described [14] as a measure of the optimal visual acuity of which an eye with AMD [14] is capable and a useful tool for assessing visual rehabilitation. That study [14] showed that visual acuity measured with multiple optotypes did not improve in normal eyes compared to single optotype- or ETDRS-testing, but there was marked improvement in some eyes with AMD. Pinhole and potential acuity meter tests, which test visual acuity at the PRL independently of refractive errors, cannot measure potential non-PRL visual acuity. This study showed that microperimetry, which is expensive and very laborious for both patients and examiners, was less useful than GM PVA testing for screening eyes preoperatively for potential visual gain after Excimer-MLR.
Remarkable survival of cone photoreceptors with highly variable density even in regions of atrophy has been documented using adaptive optics [15]. Excimer-MLR harnessed functional photoreceptors in multiple areas. Excimer-MLR enabled the visual system to spontaneously shift fixational movements to multiple retinal areas with healthier cells, which, for pathologies like macular holes, produced smaller shifts compared with pathologies like very extensive macular degeneration, which produced larger shifts. By changing corneal ROC in all quadrants, Excimer-MLR appeared to allow the visual system to spontaneously minimise shifts to the closest areas with functional photoreceptors.
Visual rehabilitation therapies with prisms [16] or biofeedback fixation training [17, 18] to relocate and confine fixation to a single and very small microperimetry-chosen retinal location without any defocusing at the PRL have shown variable efficacy, require ongoing reinforcement [16,17,18]. and may inhibit neuronal reorganisation processes [4]. Intraocular lenses providing magnification [19] of a small area including the PRL require intraocular surgery, make inadequate use of healthier peripheral retina or binocularity, preclude bilateral wide field-views for safe mobility and often require lengthy visual training for adaptation to abnormal monocular viewing. Our study demonstrated that Excimer-MLR could produce large improvements in vision and binocularity for at least 18–24 months after a single treatment by disrupting the defective PRL pathway and facilitating natural search, attention and integration of better visual information from multiple retinal areas. Near-peripheral retinal areas are known to have varying refractive errors relative to the refractive error at the PRL in phakic and pseudophakic eyes [20]. Excimer-MLR’s increased relative focusing of visual input at multiple retinal areas up to several degrees around the preoperative PRL [6] further facilitated spontaneous attention to non-PRL areas and integration of signals providing improved perception. Visual psychophysical studies [21] showed that the brain, using a remapping system with fine spatial selectivity for object identification, unconsciously integrates even object features that are not in proximity on the retina because of eye movements. Corneal modifications after Excimer-MLR in our study caused light impinging on the retina to be separated into different foci with immediate and ongoing natural neuroadaptation. Increased gamma-aminobutyric acid (GABA) and GABAergic inhibition in the lingual gyrus, such as following implantation of multifocal intraocular lenses [5], may have been factors contributing to neuroadaptation after Excimer-MLR. Neuroimaging studies suggested that GABA suppresses irrelevant sensory information and increases specificity of neural representations in the visual cortex for improved cognition [22]. Increased GABA has been shown to enhance perceptual performance by changing the gain of the neural response and reducing variability and the effective noise in the visual system [23].
Our study demonstrated that Excimer-MLR further improves multiple parameters of visual function after treatment with current retinal therapies for macular disorders. Anatomical success of macular hole surgery is very high, but functional success with logMAR BCDVA improvement ≥0.3 is only about 43% for large holes [24]. Excimer-MLR beneficially added to the success of macular hole surgery by producing additional logMAR BCDVA improvements of 1.20 and 0.52, with improved contrast sensitivity, central fields and retinal sensitivity, in the two eyes that had undergone surgical repair two and five years earlier, respectively. Anti-vascular endothelial growth factor (anti-VEGF) treatment of neovascular AMD inhibits growth of neovascular lesions and resolves exudation to improve and maintain vision, but, despite resolution of retinal oedema, macular atrophy or subretinal fibrosis causes poor residual acuity or a further decline in vision in many eyes [25]. Excimer-MLR produced additional logMAR BCDVA improvements (0.60 and 0.52 at 3 months; 0.64 and 0.42 at 18–24 months), with improved contrast sensitivity, central fields and retinal sensitivity, in the two eyes with neovascular AMD and GA without persistent intraretinal or subretinal fluid and without any anti-VEGF treatment from at least 6 months prior to Excimer-MLR through the entire post-operative follow-up period of our study. Intravitreal complement inhibitor therapies (IVCI) successfully slow anatomical progression of geographic atrophy, but treating physicians have highlighted that lack of visual benefits has limited their acceptance [26, 27]. Excimer-MLR produced clinically meaningful improvements in visual function, with no eyes losing BCDVA compared to baseline at 18–24 months, in all five eyes with geographic atrophy, with or without concomitant neovascular AMD. None of the eyes with GA in our study received IVCI before Excimer-MLR. However, vision improvement from Excimer-MLR performed before or after IVCI has the potential to improve acceptance and compliance with IVCI. Excimer-MLR performed two days apart in each eye at about one week after bilateral IVCI in a patient with GA and very poor vision (BCDVA OU: 20/800; logMAR 1.60) gained 35 and 30 ETDRS letters with disappearance of central negative scotomas at 4 and 6 days after Excimer- MLR in the first- and second-treated eyes, respectively (unpublished data, Serdarevic and Yavitz).
Despite the limitations of a retrospective single centre study design with a small number of treated eyes, the strengths of our study were large statistically significant gains for multiple functional endpoints in consecutively treated eyes measured with masking and blinding at multiple times up to 18–24 months, with the fellow eye serving as a control and no eyes lost to follow-up. Moreover, the amount of improvement was much larger than could be attributed to testing variability [11] or repeat test learning. Additionally, our study demonstrated that Excimer-MLR could be successfully repeated in a patient with progressive foveal GA. Prospective multicentre clinical trials of bilateral Excimer-MLR treatments, studies with longer than 2 year-follow up, studies over a longer time period of multiple repeat Excimer-MLR procedures in eyes with very progressive retinal macular disease and further studies of retinal sensitivity and immediate and ongoing neuroadaptation after Excimer-MLR would address other limitations of our study and add to the understanding of the benefits of Excimer-MLR for central visual loss from retinal disorders.
In summary, Excimer-MLR produced large improvements in visual acuity and contrast sensitivity, which were statistically significant within days, improved over months and lasted through the 18–24 month-exams, together with improved central fields and increased retinal sensitivity after a single, rapid, noncentral, superficial and multifocal corneal laser treatment, except in one patient who desired a second treatment at 13 months. Visual improvement after Excimer-MLR was predicted by preoperative screening-GM PVA tests. Very importantly, Excimer-MLR’s safety profile was excellent with no treatment complications or Excimer-MLR-related adverse effects. It also was safely and effectively repeatable for progressive GA. In eyes with macular disorders and moderate to profound vision impairment, Excimer-MLR was beneficially synergistic with prior retinal therapies, such as macular hole surgery and anti-VEGF intravitreal injections. Excimer-MLR could add to the clinical utility of and compliance with anti-complement, anti-angiogenic and anti-inflammatory therapies, as well as future neuroregenerative and genetic therapies for many macular disorders.
Summary
What was known before
-
Excimer-Laser-Multiple-Light-Redirection (Excimer-MLR) is a non-central, superficial and multifocal corneal photoablation for novel refractive neuroadaptive vision restoration.
-
Excimer-MLR temporarily modified corneal radii of curvature, defocused light at the PRL, decreased relative refractive error at multiple non-PRL retinal areas, shifted fixation and produced large improvements in visual acuity and Amsler grids followed up to 9 months in two eyes with age-related macular degeneration with geographic atrophy.
What this study adds
-
Excimer-MLR increased retinal sensitivities and produced statistically significant improvements in best-corrected distance and near visual acuities and contrast sensitivity from a few days through 18–24 months with functionally meaningful disappearance of scotomas and gains ≥15 ETDRS letters maintained in 86% of treated eyes at 18–24 months and no Excimer-MLR-related adverse events in seven consecutively treated eyes with various macular disorders with moderate to profound vision impairment.
Data availability
The datasets generated during the current study are available from the corresponding author on reasonable request. This manuscript includes unlabelled use of a commercially available excimer laser.
References
Serdarevic ON, inventor; Aperture in Motion, LLC, assignee. Devices and Methods for novel retinal irradiance distribution to improve and restore vision without producing corneal vitrification. US Patent No. 10,835,417B2. November 17, 2020, US Patent No. 11,766,354B2. September 26, 2023 and US Patent No. 11,974,945B2. May 7, 2024.
Perez DC, Cook SM, Peterson MA. Prior experience alters the appearance of blurry object borders. Sci Rep. 2020;10:5821.
Sabbah N, Sanda N, Authié CN, Mohand-Saïd S, Sahel JA, Habas C, et al. Reorganization of early visual cortex functional connectivity following selective peripheral and central visual loss. Sci Rep. 2017;7:43223.
d’Almeida OC, Sampaio JM, Ferreira S, Silva ED, Castelo-Branco M. Long term adult visual plasticity after the developmental critical period in genetically determined peripheral visual loss. Heliyon. 2025;11:41970.
Zhang L, Lin D, Wang Y, Chen W, Xiao W, Xiang Y, et al. Comparison of visual neuroadaptations after multifocal and monofocal intraocular lens implantation. Front Neurosci. 2021;15:648863.
Serdarevic ON, Yavitz EQ. Visual improvements after excimer laser multiple light redirection in eyes with central visual loss from macular degeneration and geographic atrophy: first report of cases. J Refract Surg Case Rep. 2024;4:e29–35.
Serdarevic O, Darrell RW, Krueger RR, Trokel SL. Excimer laser therapy for experimental Candida keratitis. Am J Ophthalmol. 1985;99:534–38.
Serdarevic ON, Berry MJ, Heller DF, inventors. Corneal vitrification, methods and devices to produce corneal vitrification and methods of use thereof. US Patent No. 9526656B2. 2016.
Serdarevic O, Tasindi E, Dekaris I, Berry M. Vision improvement in dry and wet age-related macular degeneration (AMD) patients after treatment with new corneal CPV procedure for light redirections onto the retina. Acta Ophthalmol. 2017;95:S259.
Stein RM, Markowitz SN, Berry II MJ, Berry MJ. Corneal laser procedure for vision improvement in patients with late stage dry age-related macular degeneration - a retrospective observational cohort study. F1000Research. 2020;9:1500.
van der Zee C, Muijzer MB, Claessens JLJ, Wisse RPL. Determining the variability associated with visual acuity and refractive error measurements: a systematic review. Ophthalmology. 2025;132:1020–32.
Chakravarthy U, Bailey CC, Johnston RL, McKibbin M, Khan RS, Mahmood S, et al. Characterizing disease burden and progression of geographic atrophy secondary to age-related macular degeneration. Ophthalmology. 2018;125:842–49.
Tarita-Nistor L, Brent MH, Steinbach MJ, González EG. Fixation stability during binocular viewing in patients with age-related macular degeneration. Invest Ophthalmol Vis Sci. 2011;52:1887–93.
González EG, Tarita-Nistor L, Markowitz SN, Steinbach MJ. Computer-based test to measure optimal visual acuity in age-related macular degeneration. Invest Ophthalmol Vis Sci. 2007;48:4838–45.
Elsner AE, Papay JA, Johnston KD, Sawides L, de Castro A, King BJ, et al. Cones in ageing and harsh environments: the neural economy hypothesis. Ophthalmic Physiol Opt. 2020;40:88–116.
Smith HJ, Dickinson CM, Cacho I, Reeves BC, Harper RA. A randomized controlled trial to determine the effectiveness of prism spectacles for patients with age-related macular degeneration. Arch Ophthalmol. 2005;123:1042–50.
Morales MU, Saker S, Wilde C, Rubinstein M, Limoli P, Amoaku WM. Biofeedback fixation training method for improving eccentric vision in patients with loss of foveal function secondary to different maculopathies. Int Ophthalmol. 2020;40:305–12.
Rubin GS, Crossland MD, Dunbar HMP, Brown GM, Petriti B, Roche H, et al. Eccentric viewing training for age-related macular disease: results of a randomized controlled trial (the EFFECT Study). Ophthalmol Sci. 2023;4:100422.
Borkenstein AF, Borkenstein EM, Augustin AJ. Implantable vision-enhancing devices and postoperative rehabilitation in advanced age-related macular degeneration. Eye. 2023;37:597–606.
Jaeken B, Mirabet S, Marín JM, Artal P. Comparison of the optical image quality in the periphery of phakic and pseudophakic eyes. Invest Ophthalmol Vis Sci. 2013;54:3594–9.
Drissi-Daoudi L, Ögmen H, Herzog MH, Cicchini GM. Object identity determines trans-saccadic integration. J Vis. 2020;20:33.
Sandberg K, Blicher JU, Dong MY, Rees G, Near J, Kanai R. Occipital GABA correlates with cognitive failures in daily life. Neuroimage. 2014;87:55–60.
Hammett ST, Cook E, Hassan O, Hughes CA, Rooslien H, Tizkar R, et al. GABA, noise and gain in human visual cortex. Neurosci Lett. 2020;736:135294.
Kalur A, Muste J, Singh RP. A review of surgical techniques for the treatment of large idiopathic macular holes. Ophthalmic Surg Lasers Imaging Retin. 2022;53:52–61.
Berni A, Coletto A, Li J, Shen M, Bandello F, Reibaldi M, et al. Macular atrophy in neovascular age-related macular degeneration: a systematic review and meta-analysis. Ophthalmol Retin. 2025;9:625–44.
Del Priore LV. To treat or not to treat geographic atrophy—that is the question. Ophthalmol Retin. 2024;8:207–9.
Dinah C, Enoch J, Ghulakhszian A, Sekhon M, Salvatore S, DeSalvo G et al. Patient-reported importance of functional benefit in geographic atrophy. JAMA Ophthalmol. 2025;143:916–24.
Author information
Authors and Affiliations
Contributions
Olivia N Serdarevic: Conceptualisation (equal); methodology (equal); formal analysis (equal); writing-original draft; writing-review and editing (equal). Edward Q. Yavitz: Conceptualisation (equal); methodology (equal); data curation; formal analysis (equal); writing-review and editing (equal).
Corresponding author
Ethics declarations
Competing interests
ONS and EQY are limited partners of Aperture in Motion, LLC (AIM) assigned patents on MLR (US 10835417, US 11766354 and US 11974945).
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Serdarevic, O.N., Yavitz, E.Q. Outcomes up to two years after Excimer-Laser-Multiple-Light-Redirection (Excimer-MLR) corneal treatment for retinal disorders with central visual loss. Eye Open 2, 3 (2026). https://doi.org/10.1038/s44440-025-00010-8
Received:
Revised:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s44440-025-00010-8