Abstract
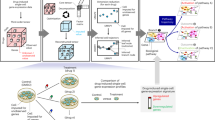
Understanding how drugs affect the immune system has consequences for treating disease and minimizing unwanted side effects. Here we present an integrative computational approach for predicting interactions between drugs and immune cells in a system-wide manner. The approach matches gene sets between transcriptional signatures to determine their similarity. We apply the method to model the interactions between 1,309 drugs and 221 immune cell types and predict 69,995 interactions. The resulting immune-cell pharmacology map is used to predict how five drugs influence four immune cell types in humans and mice. To validate the predictions, we analyzed patient records and examined cell population changes from in vivo experiments. Our method offers a tool for screening thousands of interactions to identify relationships between drugs and the immune system.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to the full article PDF.
USD 39.95
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Kuhn, M., Campillos, M., Letunic, I., Jensen, L.J. & Bork, P. A side effect resource to capture phenotypic effects of drugs. Mol. Syst. Biol. 6, 343 (2010).
Tatonetti, N.P., Ye, P.P., Daneshjou, R. & Altman, R.B. Data-driven prediction of drug effects and interactions. Sci. Transl. Med. 4, 125ra131 (2012).
Beghi, E. & Shorvon, S. Antiepileptic drugs and the immune system. Epilepsia 52 (suppl. 3), 40–44 (2011).
Røge, R., Moller, B.K., Andersen, C.R., Correll, C.U. & Nielsen, J. Immunomodulatory effects of clozapine and their clinical implications: what have we learned so far? Schizophr. Res. 140, 204–213 (2012).
Maloney, D.G. et al. IDEC-C2B8 (Rituximab) anti-CD20 monoclonal antibody therapy in patients with relapsed low-grade non-Hodgkin's lymphoma. Blood 90, 2188–2195 (1997).
Vogelsang, G.B. et al. Thalidomide for the treatment of chronic graft-versus-host disease. N. Engl. J. Med. 326, 1055–1058 (1992).
Fox, R.I. et al. Mechanism of action for leflunomide in rheumatoid arthritis. Clin. Immunol. 93, 198–208 (1999).
Vézina, C., Kudelski, A. & Sehgal, S.N. Rapamycin (AY-22,989), a new antifungal antibiotic. I. Taxonomy of the producing streptomycete and isolation of the active principle. J. Antibiot. (Tokyo) 28, 721–726 (1975).
Romano, A. & Caubet, J.C. Antibiotic allergies in children and adults: from clinical symptoms to skin testing diagnosis. J. Allergy Clin. Immunol. Pract. 2, 3–12 (2014).
Kim, Y.J. et al. Cross-reactivity to acetaminophen and celecoxib according to the type of nonsteroidal anti-inflammatory drug hypersensitivity. Allergy Asthma Immunol. Res. 6, 156–162 (2014).
Sicherer, S.H. & Leung, D.Y. Advances in allergic skin disease, anaphylaxis, and hypersensitivity reactions to foods, drugs, and insects in 2013. J. Allergy Clin. Immunol. 133, 324–334 (2014).
Alvir, J.M., Lieberman, J.A., Safferman, A.Z., Schwimmer, J.L. & Schaaf, J.A. Clozapine-induced agranulocytosis. Incidence and risk factors in the United States. N. Engl. J. Med. 329, 162–167 (1993).
Ward, K.E., Archambault, R. & Mersfelder, T.L. Severe adverse skin reactions to nonsteroidal antiinflammatory drugs: A review of the literature. Am. J. Health Syst. Pharm. 67, 206–213 (2010).
Garratty, G. Drug-induced immune hemolytic anemia. Hematology (Am Soc Hematol Educ Program) 2009, 73–79 (2009).
Cipolletta, D. et al. PPAR-gamma is a major driver of the accumulation and phenotype of adipose tissue Treg cells. Nature 486, 549–553 (2012).
Krönke, J. et al. Lenalidomide causes selective degradation of IKZF1 and IKZF3 in multiple myeloma cells. Science 343, 301–305 (2014).
Walker, R.G. et al. High throughput screens yield small molecule inhibitors of Leishmania CRK3:CYC6 cyclin-dependent kinase. PLoS Negl. Trop. Dis. 5, e1033 (2011).
Emert-Sedlak, L.A. et al. Effector kinase coupling enables high-throughput screens for direct HIV-1 Nef antagonists with antiretroviral activity. Chem. Biol. 20, 82–91 (2013).
Bellucci, R. et al. Tyrosine kinase pathways modulate tumor susceptibility to natural killer cells. J. Clin. Invest. 122, 2369–2383 (2012).
Sirota, M. et al. Discovery and preclinical validation of drug indications using compendia of public gene expression data. Sci. Transl. Med. 3, 96ra77 (2011).
Dudley, J.T. et al. Computational repositioning of the anticonvulsant topiramate for inflammatory bowel disease. Sci. Transl. Med. 3, 96ra76 (2011).
Jahchan, N.S. et al. A drug repositioning approach identifies tricyclic antidepressants as inhibitors of small cell lung cancer and other neuroendocrine tumors. Cancer Discov. 3, 1364–1377 (2013).
Lamb, J. et al. The Connectivity Map: using gene-expression signatures to connect small molecules, genes, and disease. Science 313, 1929–1935 (2006).
Heng, T.S., Painter, M.W. & Consortium, I.G. The Immunological Genome Project: networks of gene expression in immune cells. Nat. Immunol. 9, 1091–1094 (2008).
Jojic, V. et al. Identification of transcriptional regulators in the mouse immune system. Nat. Immunol. 14, 633–643 (2013).
Benoist, C., Lanier, L., Merad, M., Mathis, D. & Immunological Genome, P. Consortium biology in immunology: the perspective from the Immunological Genome Project. Nat. Rev. Immunol. 12, 734–740 (2012).
Miller, J.C. et al. Deciphering the transcriptional network of the dendritic cell lineage. Nat. Immunol. 13, 888–899 (2012).
Iorio, F. et al. Discovery of drug mode of action and drug repositioning from transcriptional responses. Proc. Natl. Acad. Sci. USA 107, 14621–14626 (2010).
Iorio, F., Tagliaferri, R. & di Bernardo, D. Identifying network of drug mode of action by gene expression profiling. J. Comput. Biol. 16, 241–251 (2009).
Zhang, X. & Young, H.A. PPAR and immune system–what do we know? Int. Immunopharmacol. 2, 1029–1044 (2002).
Lewis, J.D. et al. Rosiglitazone for active ulcerative colitis: a randomized placebo-controlled trial. Gastroenterology 134, 688–695 (2008).
Rouleau, M., Patel, A., Hendzel, M.J., Kaufmann, S.H. & Poirier, G.G. PARP inhibition: PARP1 and beyond. Nat. Rev. Cancer 10, 293–301 (2010).
Jagtap, P. & Szabo, C. Poly(ADP-ribose) polymerase and the therapeutic effects of its inhibitors. Nat. Rev. Drug Discov. 4, 421–440 (2005).
Maugeri-Saccá, M., Bartucci, M. & De Maria, R. DNA damage repair pathways in cancer stem cells. Mol. Cancer Ther. 11, 1627–1636 (2012).
Johannessen, T.C., Bjerkvig, R. & Tysnes, B.B. DNA repair and cancer stem-like cells–potential partners in glioma drug resistance? Cancer Treat. Rev. 34, 558–567 (2008).
Suzuki, R. & Shimodaira, H. Pvclust: an R package for assessing the uncertainty in hierarchical clustering. Bioinformatics 22, 1540–1542 (2006).
Fagius, J., Osterman, P.O., Siden, A. & Wiholm, B.E. Guillain-Barre syndrome following zimeldine treatment. J. Neurol. Neurosurg. Psychiatry 48, 65–69 (1985).
Goh, K.-I. et al. The human disease network. Proc. Natl. Acad. Sci. USA 104, 8685–8690 (2007).
Kolar, D. et al. Treatment of adults with attention-deficit/hyperactivity disorder. Neuropsychiatr. Dis. Treat. 4, 389–403 (2008).
Fisahn, A. et al. Muscarinic induction of hippocampal gamma oscillations requires coupling of the M1 receptor to two mixed cation currents. Neuron 33, 615–624 (2002).
Summers, C. et al. Neutrophil kinetics in health and disease. Trends Immunol. 31, 318–324 (2010).
Fortunati, E., Kazemier, K.M., Grutters, J.C., Koenderman, L. & Van den Bosch, v.J. Human neutrophils switch to an activated phenotype after homing to the lung irrespective of inflammatory disease. Clin. Exp. Immunol. 155, 559–566 (2009).
Beyrau, M., Bodkin, J.V. & Nourshargh, S. Neutrophil heterogeneity in health and disease: a revitalized avenue in inflammation and immunity. Open Biol. 2, 120134 (2012).
Cherny, R.A. et al. Treatment with a copper-zinc chelator markedly and rapidly inhibits beta-amyloid accumulation in Alzheimer's disease transgenic mice. Neuron 30, 665–676 (2001).
Grossi, C. et al. Clioquinol decreases amyloid-beta burden and reduces working memory impairment in a transgenic mouse model of Alzheimer's disease. J. Alzheimers Dis. 17, 423–440 (2009).
Qiu, P. et al. Extracting a cellular hierarchy from high-dimensional cytometry data with SPADE. Nat. Biotechnol. 29, 886–891 (2011).
Devi, S. et al. Neutrophil mobilization via plerixafor-mediated CXCR4 inhibition arises from lung demargination and blockade of neutrophil homing to the bone marrow. J. Exp. Med. 210, 2321–2336 (2013).
Robertson, A.L. et al. A zebrafish compound screen reveals modulation of neutrophil reverse migration as an anti-inflammatory mechanism. Sci. Transl. Med. 6, 225ra229 (2014).
Seok, J. et al. Genomic responses in mouse models poorly mimic human inflammatory diseases. Proc. Natl. Acad. Sci. USA 110, 3507–3512 (2013).
Lin, S. et al. Comparison of the transcriptional landscapes between human and mouse tissues. Proc. Natl. Acad. Sci. USA 111, 17224–17229 (2014).
Cheng, Y. et al. Principles of regulatory information conservation between mouse and human. Nature 515, 371–375 (2014).
Shay, T. et al. Conservation and divergence in the transcriptional programs of the human and mouse immune systems. Proc. Natl. Acad. Sci. USA 110, 2946–2951 (2013).
Novershtern, N. et al. Densely interconnected transcriptional circuits control cell states in human hematopoiesis. Cell 144, 296–309 (2011).
Stepan, A.F. et al. Structural alert/reactive metabolite concept as applied in medicinal chemistry to mitigate the risk of idiosyncratic drug toxicity: a perspective based on the critical examination of trends in the top 200 drugs marketed in the United States. Chem. Res. Toxicol. 24, 1345–1410 (2011).
Evans, D.C. & Baillie, T.A. Minimizing the potential for metabolic activation as an integral part of drug design. Curr. Opin. Drug Discov. Devel. 8, 44–50 (2005).
Subramanian, A. et al. Gene set enrichment analysis: a knowledge-based approach for interpreting genome-wide expression profiles. Proc. Natl. Acad. Sci. USA 102, 15545–15550 (2005).
Nam, D. De-correlating expression in gene-set analysis. Bioinformatics 26, i511–i516 (2010).
Wu, D. & Smyth, G.K. Camera: a competitive gene set test accounting for inter-gene correlation. Nucleic Acids Res. 40, e133 (2012).
Yaari, G., Bolen, C.R., Thakar, J. & Kleinstein, S.H. Quantitative set analysis for gene expression: a method to quantify gene set differential expression including gene-gene correlations. Nucleic Acids Res. 41, e170 (2013).
Rahmatallah, Y., Emmert-Streib, F. & Glazko, G. Gene Sets Net Correlations Analysis (GSNCA): a multivariate differential coexpression test for gene sets. Bioinformatics 30, 360–368 (2014).
Painter, M.W. et al. Transcriptomes of the B and T lineages compared by multiplatform microarray profiling. J. Immunol. 186, 3047–3057 (2011).
Knijnenburg, T., Wessels, L., Reinders, M. & Shmulevich, I. Fewer permutations, more accurate P-values. Bioinformatics 25, i161 (2009).
Benjamini, Y. & Hochberg, Y. Controlling the false discovery rate–a practical and powerful approach to multiple testing. J. R. Stat. Soc., B 57, 289–300 (1995).
Morgan, A.A., Khatri, P., Jones, R.H., Sarwal, M.M. & Butte, A.J. Comparison of multiplex meta analysis techniques for understanding the acute rejection of solid organ transplants. BMC Bioinformatics 11 (suppl. 9), S6 (2010).
Shannon, P. et al. Cytoscape: a software environment for integrated models of biomolecular interaction networks. Genome Res. 13, 2498–2504 (2003).
Linderman, M.D. et al. CytoSPADE: high-performance analysis and visualization of high-dimensional cytometry data. Bioinformatics 28, 2400–2401 (2012).
Choi, B.Y. et al. Copper/zinc chelation by clioquinol reduces spinal cord white matter damage and behavioral deficits in a murine MOG-induced multiple sclerosis model. Neurobiol. Dis. 54, 382–391 (2013).
Acknowledgements
We thank G. Hoffman and B. Readhead for useful conversations about the computational methods and suggestions on the manuscript, L. Li for assistance with the electronic medical records from Mount Sinai, K. Oguntuyo for assistance with the qPCR reactions, and A. Rahman for sample preparation and acquisition on the CyTOF. This work was supported by the US National Institutes of Health grants R01AI104848, R33CA182377 and R01AI113221 (to B.D.B.), U54CA189201 and R01DK098242, and Jonathan E. Gray TCI Young Scientist Cancer Research Award (to J.T.D.).
Author information
Authors and Affiliations
Contributions
B.A.K. and J.T.D. conceived of and designed the study. B.A.K. implemented the computational methods and performed the data analysis. A.W., J.A., M.M. and B.D.B. designed the experiments. A.W. conducted all in vivo assays. M.R.B. analyzed electronic medical records from Columbia University Medical Center. B.A.K. and J.T.D. wrote the manuscript with review and feedback from A.W., B.D.B. and N.P.T.
Corresponding authors
Ethics declarations
Competing interests
B.A.K. has received consulting fees from Merck. J.T.D. owns equity in NuMedii Inc. and has received consulting fees or honoraria from Janssen Pharmaceuticals, GlaxoSmithKline, AstraZeneca and LAM Therapeutics.
Integrated supplementary information
Supplementary Figure 1 Cell type and tissue location counts in the Immunological Genome data set.
Barplots indicate the sums of the table rows and columns. Abbreviated cell type labels represent: Neut = neutrophil, Mono = monocyte, NKT = natural killer T cell, NK = natural killer cell, Mac = macrophage, T4 = CD4+ T cell, T8 = CD8+ T cell, Tgd = gamma-delta T cell, DC = dendritic cell. Abbreviated tissue labels represent: BM = Bone marrow, scLN = Subcutaneous lymph nodes, msLN = mesenteric lymph nodes, sdLN = skin-draining lymph nodes, SI = Small intestine, FLN = Follicular lymph nodes, IE = Intraepithelial, mdLN = mediastinal lymph nodes, PP = Peyer’s patch, AA = abdominal adipose, LN = Lymph nodes, MsLN = Medullary sinus lymph nodes, PLN = Pancreatic lymph nodes, SF = synovial fluid, ssLN = Subcapsular lymph nodes.
Supplementary Figure 2 Clustered heatmap of cell state change similarity.
Hierarchical clustering applied to the matrix of Jaccard distances calculated between gene sets from the extremes—top/bottom 5% fold-change—of each cell state change expression profile. The full heatmap shows complete linkage clustering for all pairwise combinations of the 304 cell state changes. Colored bars on the rows and columns represent the categories of state changes and the cell types respectively.
Supplementary Figure 3 Jaccard distance between immune cell state change gene sets.
Sets based on the extremes—top/bottom 5% fold-change—of each cell state change expression profile. Each bar height reflects the average distance within a cell type and the error bars are the s.e.m. Gray horizontal line shows the average distance between all gene sets. Group differences assessed by ANOVA.
Supplementary Figure 4 Schematic depiction of our method to test for significant interactions and identify robust connections.
(a) Diagram of ranked list comparison to calculate the immunemod score for each pair of drug (j) and cell state change (k) at a particular gene set size (l). (b) Gene set size variation procedure to evaluate the robustness of each predicted interaction. (c) Schematic of the permutation testing to assess the significance of an immunemod score for a given drug (j), cell state change (k), and gene set size (l).
Supplementary Figure 5 Quantile-Quantile plot for gene expression models.
P-value distribution of independent (naïve) and correlated permutation models for the IP map.
Supplementary Figure 6 Number of predicted interactions between drugs and immune cells across all categories of immunological cell state changes.
(a) Overall, (b) Subset, (c) Tissue, (d) Genetic, (e) Time, and (f) Perturbation.
Supplementary Figure 7 Drug class enrichments among the stable drug clusters.
Enrichment value for each of the 13 level 1 ATC categories: H = Systemic hormonal preparations, excl. sex hormones and insulins, V = Various, B = Blood and blood forming organs, P = Anti-parasitic products, M = Musculo-skeletal system, L = Anti-neoplastic and immunomodulating agents, G = Genito urinary system and sex hormones, R = Respiratory system, A = Alimentary tract and metabolism, D = Dermatologicals, J = Anti-infectives for systemic use, S = Sensory organs, N = Nervous system, and C = Cardiovascular system. Asterisks indicate significant over- or under-enrichment (P < 0.01 and E > 1 or E < 1 respectively). Significance assessed by hypergeometric distribution.
Supplementary Figure 8 Circular layout of the predicted connections between drugs and cell subsets found in different tissues.
All connections statistically significant P < 0.05 with FDR < 5%, see methods. Line widths correspond to the number of interactions. A drug-tissue connection represents a drug that is predicted to influence a particular immune cell in the specific tissue as compared to the same cell in a contrasting tissue. The diagram has been organized by sorting the tissues counter-clockwise (drugs clockwise) in order of decreasing number of connections. Each single letter for of the drug half corresponds to a level 1 label of the ATC system. H = Systemic hormonal preparations, excl. sex hormones and insulins, V = Various, B = Blood and blood forming organs, P = Anti-parasitic products, M = Musculo-skeletal system, L = Anti-neoplastic and immunomodulating agents, G = Genito urinary system and sex hormones, R = Respiratory system, A = Alimentary tract and metabolism, D = Dermatologicals, J = Anti-infectives for systemic use, S = Sensory organs, N = Nervous system, and C = Cardiovascular system. Codes for each tissue label are as follows: PC = peritoneal cavity, br = brain, AA = abdominal adipose, pan = pancreas, SI = small intestine, SF = synovial fluid, LN = lymph nodes, PLN = pancreatic lymph nodes, mdLN = mediastinal lymph nodes, FLN = follicular lymph nodes, thy = thymus, sdLN = skin-draining lymph nodes, PP = Peyer’s patch, scLN = subcutaneous lymph nodes, msLN = mesenteric lymph nodes, and BM = bone marrow.
Supplementary Figure 9 Distribution and enrichment of drug hubs and islands.
(a) Histogram of drugs binned according to the number of unique cell types they are predicted to interact with (top right shows drug hubs with connections to 11 or more cell types). ATC level 1 categories enriched in drug (b) islands and (c) hubs. Significance assessed by fold-enrichment analysis and hypergeometric distribution.
Supplementary Figure 10 Drug hubs and islands.
Examples of drug (a) hubs or (b) islands associated with immune cell types.
Supplementary Figure 11 Two-dimensional colored heatmap of the T cell subnetwork pulled from the IP map.
Data organized by drug (rows) and T cell categories (columns). Predicted interactions between drug categories (ATC level 1 categories: H = Systemic hormonal preparations, excl. sex hormones and insulins, B = Blood and blood forming organs, V = Various, L = Anti-neoplastic and immunomodulating agents, P = Anti-parasitic products, M = Musculo-skeletal system, G = Genito urinary system and sex hormones, R = Respiratory system, S = Sensory organs, D = Dermatologicals, A = Alimentary tract and metabolism, N = Nervous system, J = Anti-infectives for systemic use, and C = Cardiovascular system) and (left) T cell subsets “Cell Types” or (right) identical T cell subset found in different tissues. All connections are statistically significant (P < 0.05 and FDR < 5%, see methods). Each colored cell in the heatmap represents the fraction of drugs from a particular ATC level 1 category (0–0.7, max = 1.0) predicted to influence the specific Cell Type or Tissue location. Colored circles represent the number of drugs in each category where the size is proportional to the number of unique drugs (16–144).
Supplementary Figure 12 Drugs predicted to influence T cell subset changes.
T4 = CD4+ cells, T8 = CD8+ cells, FP3 = FoxP3, mem = memory subset. Drugs ranked in descending order by immunemod score.
Supplementary Figure 13 Influence of guanfacine on regulatory T cells.
(a) Graph showing connections for a single drug, Guanfacine, predicted to influence regulatory T cells based on the immunemod score (shown as numbers next to each node). (b) Percentage of regulatory T cells (CD4+FoxP3+CD62L+) isolated from mouse spleens following treatment with the anti-hypertensive drug guanfacine. Each circle is a separate mouse from five separate experiments—grouped by color—for a total of 14 or 15 mice in the Ctrl and Guanfacine groups respectively. Dashed lines connect the mean frequency values between groups in each experiment. Significance assessed by meta analysis (see below).
Morgan, A.A., Khatri, P., Jones, R.H., Sarwal, M.M. & Butte, A.J. Comparison of multiplex meta analysis techniques for understanding the acute rejection of solid organ transplants. BMC Bioinformatics 11 Suppl 9, S6 (2010).
Supplementary Figure 14 Flow cytometry plots.
Representative biaxial flow cytometry plots showing the gating strategy for identifying Ly6G+CD11b+ cells from blood, bone marrow and peritoneal cavity of treated and untreated mice. Data are representative of three to four experiments with 8 mice in each condition.
Supplementary Figure 15 SPADE trees of CyTOF data collected during a single experiment.
Cells isolated from spleens of mice treated with drug (clioquinol) and vehicle control (PEG400). Trees show the lymphocyte lineage of CD45+ cells. Key for trees shown to orient toward CD4+ and CD8+ cells. Arrows highlight CD45+CD3+CD4–CD8+Thy1.2+TCRb+CD62LhiCD44lo population increase after clioquinol treatment. Horizontal color scales represent median intensity (blue = low, red = high) for marker indicated.
Supplementary information
Supplementary Text and Figures
Supplementary Figures 1–15 (PDF 15357 kb)
Rights and permissions
About this article
Cite this article
Kidd, B., Wroblewska, A., Boland, M. et al. Mapping the effects of drugs on the immune system. Nat Biotechnol 34, 47–54 (2016). https://doi.org/10.1038/nbt.3367
Received:
Accepted:
Published:
Issue date:
DOI: https://doi.org/10.1038/nbt.3367
This article is cited by
-
Pharmaceutical therapeutics for articular regeneration and restoration: state-of-the-art technology for screening small molecular drugs
Cellular and Molecular Life Sciences (2021)
-
Synergy from gene expression and network mining (SynGeNet) method predicts synergistic drug combinations for diverse melanoma genomic subtypes
npj Systems Biology and Applications (2019)
-
RETRACTED ARTICLE: Computational Drug Repositioning for Gastric Cancer using Reversal Gene Expression Profiles
Scientific Reports (2019)
-
Genome-Wide Expression Profiles Drive Discovery of Novel Compounds that Reduce Binge Drinking in Mice
Neuropsychopharmacology (2018)
-
Clinical pharmacodynamic/exposure characterisation of the multikinase inhibitor ilorasertib (ABT-348) in a phase 1 dose-escalation trial
British Journal of Cancer (2018)