Abstract
Pyroptosis is a type of programmed cell death characterized by cell swelling and osmotic lysis, resulting in cytomembrane rupture and release of immunostimulatory components, which play a role in several pathological processes. Significant cellular responses to various stimuli involve the formation of inflammasomes, maturation of inflammatory caspases, and caspase-mediated cleavage of gasdermin. The function of pyroptosis in disease is complex but not a simple angelic or demonic role. While inflammatory diseases such as sepsis are associated with uncontrollable pyroptosis, the potent immune response induced by pyroptosis can be exploited as a therapeutic target for anti-tumor therapy. Thus, a comprehensive review of the role of pyroptosis in disease is crucial for further research and clinical translation from bench to bedside. In this review, we summarize the recent advancements in understanding the role of pyroptosis in disease, covering the related development history, molecular mechanisms including canonical, non-canonical, caspase 3/8, and granzyme-mediated pathways, and its regulatory function in health and multiple diseases. Moreover, this review also provides updates on promising therapeutic strategies by applying novel small molecule inhibitors and traditional medicines to regulate pyroptosis. The present dilemmas and future directions in the landscape of pyroptosis are also discussed from a clinical perspective, providing clues for scientists to develop novel drugs targeting pyroptosis.
Similar content being viewed by others
Introduction
Cell death is critical for homeostasis in the human body because it eliminates unwanted cells.1 Among the different cell death types, regulated cell death (RCD) is encoded by genetic information and is accompanied by normal cell senescence.2 The absence of RCD contributes to the initiation and progression of diseases, such as cancer, which is characterized by uncontrolled cell growth and immortality. Apart from immortalization, excessive cell death is also correlated with the development of disease. For example, some neurodegenerative diseases, such as Alzheimer’s disease, are associated with abnormal loss of neurons during the human aging process.3 Therefore, understanding the different RCD modes and methods of reprogramming cell death is vital for disease therapy innovations.
RCD results from complex cellular responses to different stimuli and includes multiple forms, such as apoptosis, necroptosis, pyroptosis, and ferroptosis.4 With the expansion of research on cell death building on previous scientific endeavors. Pyroptosis is regulated by inflammatory caspases, inflammasome formation, and gasdermin aggregation on the membrane, which is induced by pathogen-associated molecular patterns (PAMPs), damage-associated molecular patterns (DAMPs), and pathogen infection. Pyroptosis involves plasma membrane rupture, chromatin condensation, DNA fragmentation, intact nuclei, pore formation, cell swelling, and osmotic lysis. DAMPs such as interleukin (IL)-18, IL-1β, dsDNA, ATP, and high mobility group box 1 (HMGB1) are also released during pyroptosis.5
A holistic understanding of pyroptosis has been achieved after decades of exploration, as shown in Fig. 1. Notably, Kostura et al. and Black et al. first reported a novel enzyme called IL-1β-converting enzyme (ILE).6,7 In 1993, Yuan et al. reported that CED-3 shares similar functions with ILE.8 The ILE-CED3 enzyme family was named caspase in 1996 and is classified into two groups: inflammatory (caspase-1/4/5/11) and apoptotic (caspase-3/6/7, 2/8/9/10).9 The function and signaling pathways related to caspases were explored in subsequent years. However, pyroptosis and apoptosis were not distinguished from one another until later. For example, pyroptosis in infected macrophages was discovered in 1992 but was misclassified as apoptosis.10 Subsequent publications substantiated the pivotal and unique role of caspase-1 in bacterial-induced cell death.11 Several following studies proposed that caspase-1-mediated cell death differs from apoptosis because membrane integrity is destroyed by the inflammatory response.11,12,13 Later on, additional caspases, such as caspase-4/5/11, were found to be involved in what has now been coined “pyroptosis”.14 The term “pyroptosis” was first proposed in 2001 to describe this proinflammatory cell death type. Subsequently, Martinon et al. proposed the “inflammasome” concept in 2002, and further evidence identifying key components emerged in the following years, including NLRP1-CASP1 and NLRP3-ASC-CASP1.15,16 In addition to caspase-1, the function of caspase-11 in pyroptosis was reported in two studies by the Dixit group.17,18 In 2015, Feng et al. identified the effector proteins and showed that caspase-1/11/4/5 can cleave gasdermin D (GSDMD) to release its N-terminal domain.19 Dixit et al. and Han et al. proposed that GSDMD is an effector protein in pyroptosis.20,21 N-terminal GSDMD can aggregate on the membrane, forming holes that alter the intracellular osmotic pressure and lead to cell swelling until membrane fracture and cytokine release (e.g., IL-1β, IL-18).22 Shao et al. found that caspase-3 recognizes and hydrolyzes gasdermin E (GSDME) to induce pyroptosis.23 Other groups have reported a caspase-8-mediated pyroptosis pathway.24,25 These studies verified the hypothesis that the cell death pattern should not be classified by the caspase type but the substrate hydrolyzed by the caspase. Pyroptosis was classified as RCD in 2018 and is characterized by its reliance on the gasdermin protein family.2 In 2020, the mechanism through which granzyme A cleaves gasdermin B (GSDMB) and granzyme B cleaves GSDME to trigger pyroptosis were also discovered.26,27 Additional structural mechanisms and signaling pathways have been reported more recently.28,29
Milestones of research on the discovery and development of pyroptosis. From 1989 to 2001, pyroptosis was observed in cells, and related molecules such as IL-1β-converting enzyme (ILE) were identified. The inflammasome concept was formed and developed in the following decade. The discovery of GSDMD as an effector protein initiated the quick development of pyroptosis research with canonical, non-canonical, caspase-3/8 or granzyme-mediated pathways reported. Increasing pyroptosis studies related to specific diseases have also been performed in recent years
A close correlation between pyroptosis and disease has also been widely reported, and the gasdermin protein family’s functionality varies across cells.30,31 GSDMA is predominantly expressed within skin and epithelial tissues such as the hair follicle, epidermis, and gastric epithelium, which correlate to skin inflammation and alopecia.32,33 As for the GSDMB, it is widely expressed in the gastrointestinal epithelium, liver, and immune cells, which participates in the initiation and development of asthma and inflammatory bowel disease (IBD).34,35 The granzyme A released by CD8+ T and NK cells can activate GSDMB, leading to the pyroptosis.27 Furthermore, cancer cell pyroptosis was frequently activated by distinct molecular mechanisms relying on GSDMC and GSDME. The signal transduction after the binding of TNFα can activate the caspase-8/GSDMC pathway leading to tumor necrosis.36 In GSDME-expressing tumor cells, the granzyme B released from NK and CD8+ T lymphocytes and chimeric antigen receptor T (CAR-T) cells can cleave and activate caspase-3 and GSDME, triggering pyroptosis.37 Chemotherapy drugs also increase the expression of caspase-3 in GSDME cells, transform apoptosis into pyroptosis, and enhance anti-tumor immunity.23 However, higher GSDME expression level is correlated to unsatisfactory prognosis, indicating that GSDME may also possess the function of regulating cell proliferation rather than pyroptosis.38 Finally, GSDMD is the most widely researched protein of the gasdermin family, which can be activated by caspase-1/3/4/5/11, participating in multiple inflammatory diseases because of the immunogenic cellular content release during the pyroptosis.39 In macrophage and dendritic cells, a hyperactive caspase 1/GSDMD pathway correlates to excessive proinflammatory substance release (IL-1, IL-6, and tumor necrosis factor (TNF)-α) and an uncontrollable inflammatory status such as the cytokine release syndrome.40 From the perspective of treatment, small-molecule drugs targeting inflammasomes have increasingly emerged in the latest decade.41 Nanomaterials targeting the pyroptotic pathway have been increasingly developed and used in disease therapy.42,43 Therefore, as clinical scientists, in this review, we aimed to summarize the recent progress in the mechanism, regulation, and therapeutic perspectives of pyroptosis in disease. The current limitations and clinical dilemmas are also presented to inspire readers to propel further fundamental and clinical translational research.
Molecular mechanisms of pyroptosis
Pyroptosis is a novel type of RCD with lytic and pro-inflammatory features. Pyroptotic and apoptotic cells both exhibit chromatin condensation and DNA fragmentation, but pyroptotic cells are distinguished by cell swelling, pore formation, osmotic lysis, and release of proinflammatory contents.44,45,46,47,48,49 Other unique forms of RCD, such as necroptosis and ferroptosis, have also recently emerged.50,51,52,53 Pyroptosis and necroptosis share the outcome of inflammatory stimuli release and immune responses, but the molecular pathways involved are different.54,55,56,57 Although iron can trigger both ferroptosis and iron-induced pyroptosis, ferroptosis is characterized by phospholipid peroxidation as a unique mode of cell death.58,59,60,61 A comparison of key aspects of apoptosis, necroptosis, ferroptosis, and pyroptosis, involving inducing factors, biochemical events, cell morphology, and cell release, is summarized in Table 1.
Characterization of pyroptosis in humans in 2015 revealed that the cleavage of GSDMD is primarily induced by inflammatory caspases, such as caspase-1, -4, -5, and -11.19 This cleavage results in the loss of interaction between the amino-terminal fragment and carboxy-terminal fragment in GSDMD. GSDMD belongs to the gasdermin superfamily, encompassing gasdermin A/B/C/D/E and DFNB59 (Pejvakin, PJVK) in humans and Gsdma1/2/3, Gsdmc1/2/3/4, Gsdmd, Dfna5, and Dfnb59 in mice. GSDME is also known as DFNA5.20,22 Each member comprises two conserved domains: the N-terminal pore-forming domain and the C-terminal repressor domain (PFD and RD), except DFNB59.62,63,64 Generally, the RD interacts with the PFD to maintain gasdermin oligomerization, suppressing its cytotoxic effect.62,64 However, when the PFD is separated from the RD, it assembles and forms perforations in the cell membrane in response to various internal and external stimuli. This process leads to the release of molecules associated with inflammation and pyroptosis.22,65 Therefore, gasdermin is regarded as an executor of pyroptosis. Several pathways that induce GSDMD cleavage have been identified, as shown in Fig. 2.
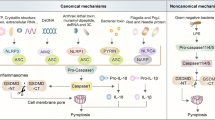
Signaling pathways of pyroptosis. In the canonical pathway of pyroptosis, PAMPs, and DAMPs are stimulated by intracellular signaling molecules. They combine with pro-caspase-1 and the adaptor protein ASC to form inflammasomes, leading to the activation of caspase-1. Cleaved caspase-1 then proceeds to cleave GSDMD and pro-IL-1β/IL-18. N-terminal GSDMD forms non-selective pores in the cell membrane, resulting in water influx, cell lysis, and ultimately cell death. Additionally, IL-1β and IL-18 are released through the pores formed by N-terminal GSDMD. In the non-canonical pathway, LPS activates caspase-4/5/11, triggering pyroptosis by cleaving GSDMD. The cleavage of GSDMD also leads to the efflux of K+, facilitating the assembly of the NLRP3 inflammasome and cleavage of pro-IL-1β and pro-IL-18. In the caspase-8-mediated pathway, the inhibition of TAK1 leads to the activation of caspase-8, which cleaves GSDMD, resulting in pyroptosis. Under hypoxic conditions, PD-L1 translocates to the nucleus and, in conjunction with phosphorylated Stat3, regulates the transcription of GSDMC, leading to the conversion of apoptosis to pyroptosis following TNFα-activated caspase-8. In the granzyme-mediated pathway, CAR-T cells rapidly activate caspase-3 in target cells by releasing GzmB. Subsequently, GSDME is activated, causing extensive pyroptosis. GzmA and GzmB from cytotoxic lymphocytes enter target cells through perforin and induce pyroptosis. GzmA hydrolyzes GSDMB, and GzmB directly activates GSDME. The figure was created by Figdraw
Canonical pathway
Canonical pyroptosis is regulated by the integration of inflammasomes, which induce the cleavage of GSDMD and release of inflammatory factors such as IL-18 and IL-1β.66,67,68,69,70 Inflammasomes are activated and assembled to respond to microbial infections and endogenous danger signals, promoting host immune responses and cellular damage. During this process, released cell contents ultimately recruit innate immune cells to the infection site and modulate adaptive immune cells.71,72,73
The congregation of inflammasomes is initiated when cytosolic pattern recognition receptors (PRRs), known as detectors of inflammasomes, identify PAMPs and DAMPs.30,74,75 The activation of PRRs triggers downstream signaling cascades, leading to the presentation of type I interferons as well as the release of proinflammatory cytokines.71,76,77,78 Upon detection of bacterial or viral signals, PRRs assemble with pro-caspase-1 and adapter apoptosis-associated speck-like proteins containing a caspase activation and recruitment domain (CARD) (ASC) to form inflammasomes.15,79,80,81
Most inflammasomes consist of three segments: (i) leucine-rich repeat-containing proteins (NLRs), (ii) ASC, and (iii) pro-caspase-1.40 NLRs are composed of leucine-rich repeats (LRRs), a NACHT nucleotide-binding domain, and either a pyrin or CARD domain (PYD) at the N-terminus, which categorizes them into NLRP or NLRC subtypes, respectively.82,83,84 NLRC family members possess one or more N-terminal CARDs, as exemplified by NLRC4, whereas NLRP proteins, such as NLRP1 and NLRP3, harbor N-terminal PYDs.78,85,86
Extensive studies have been performed to characterize NLRC4, including ligand detection by NLR family apoptosis inhibitory protein (NAIP), which is the initial step in NLRC4 inflammasome activation.87,88 Additionally, non-NLR proteins, including AIM2 and pyrin, possess inflammasome-forming capacity. ASC, containing PYD and CARD, mediates inflammasome interactions and pro-caspase-1 recruitment.85 Following inflammasome assembly, caspase-1 undergoes autocatalytic cleavage, leading to the generation of active mature enzymes.89 Caspase-1 has a crucial impact on cleaving GSDMD, creating membrane pores, releasing IL-18 and IL-1β, and ultimately generating cell swelling and pyroptosis.90,91 Additionally, caspase-1 drives pro-IL-1β and pro-IL-18 into their mature, active forms.19,65,92
In some cases, inflammasome stimulation leads to cytokine secretion without cell lysis. However, the mechanisms that regulate the phenomenon remain to be further unveiled. Research suggests that the Toll-like receptor adapter SARM may be involved in this process.93
Non-canonical pathway
In non-canonical pyroptosis, human caspase-4/5 (caspase-11 in mice) is activated by the intimate binding of lipopolysaccharides (LPS) to their N-terminal CARDs.94 Upon activation, caspase-4/5/11 cleaves GSDMD, generating an N-terminus that is inserted into the membrane to form pores.95 However, unlike caspase-1, caspase-4/5/11 are unable to cleave pro-IL-1β/IL-18 precursors on their own, and IL-1β/IL-18 release requires engagement of the NLRP3/caspase-1 pathway in specific cellular contexts.96 The processing of GSDMD by caspase-4/5/11 elicits K+ efflux, which leads to NLRP3 inflammasome formation and pyroptotic cell death similar to the classical pathway.19,97,98,99 Unlike the classical pathway, in non-canonical pyroptosis, only cleavage of pro-IL-1β/IL-18 depends on caspase-1, while cleavage of GSDMD is performed by other activated inflammatory caspases. Pannexin-1 is another critical mediator of non-canonical caspase-11-induced pyroptosis.100 LPS exposure activates caspase-11, which cleaves pannexin-1, leading to ATP efflux that triggers P2X7-mediated pyroptosis. Notably, pannexin-1 deficient mice exhibit endotoxin shock resistance, indicating that K+ channels are selective modulators of non-canonical NLRP3 stimulation.
Additionally, the NLRP3 inflammasome regulates GSDME expression in human T cells. The NLRP3 inflammasome significantly impacts the activation of caspase-8, caspase-3, and GSDME cleavage, ultimately releasing the alarm signal IL-1α in a specific subset of T helper cells targeting Candida albicans. This process is triggered by calcium-licensed calpain maturation of pro-IL-1α after T cell receptor stimulation. The mechanism of GSDME pore formation in T cells for cytokine release depends on the NLRP3 inflammasome.101
Caspase-3/8–mediated pathway
Previously thought to be inert to gasdermin activation, caspase-3, an apoptotic caspase, has been found to lead to chemotherapy-induced GSDME fragmentation in cells with abundant GSDME, resulting in the liberation of pyroptosis-inducing N-termini in tumors.23,102,103 This phenomenon has been observed in paclitaxel- and cisplatin-induced lung cancer cells and lobaplatin-induced colon cancer cells.104,105 Additionally, the Yersinia effector YopJ stimulates caspase-8 to cleave GSDMD by hindering TAK1 in mouse macrophages, further expanding our mechanistic understanding of pyroptosis.24,25
Caspase-8, another apoptosis-related caspase, has also been implicated in pyroptosis. In breast cancer cells, PD-L1 redirects TNF-induced death from apoptosis to pyroptosis. Under hypoxic conditions, activated STAT3 enters the nucleus along PD-L1, amplifying GSDMC transcription. TNF-α then promotes caspase-8-mediated cleavage of GSDMC, generating N-terminal fragments that perforate the membrane and elicit pyroptosis. Macrophage TNF-α-driven tumor cell pyroptotic death requires nuclear PD-L1, caspase-8, and GSDMC in vivo. Furthermore, certain chemotherapies induce caspase-8- and GSDMC-dependent pyroptotic death.36 Caspase-8 is often referred to as a molecular switch because it is critical in determining whether a cell’s fate is apoptosis, necroptosis, or pyroptosis.106
Granzyme-mediated pathway
In 2020, Liu et al. demonstrated that chimeric antigen receptor (CAR) T-cells can quickly engage caspase-3 in target cells through granzyme B release, triggering the caspase-3/GSDME pyroptosis pathway and leading to widespread pyroptotic cell death.37 In addition, granzyme B directly fragments GSDME to stimulate pyroptosis, enhance anti-tumor immunity, and restrict tumor expansion.26 Natural killer and cytotoxic T cells have been observed to eliminate GSDMB+ cells via pyroptosis, with cytotoxicity resulting from granzyme A (GzmA)-mediated GSDMB fragmentation at Lys229/Lys244. Notably, non-aspartic gasdermin processing and pore formation by GzmA redefined our previous understanding and expanded the potential for pyroptosis induction beyond caspases.27 Different splicing variants of GSDMB have distinct functions, with the cleaved N-terminal fragments of only certain isoforms causing pyroptosis.107
Biological functions in health
The large GSDM family is representative of the widespread phenomenon of pyroptosis in mammals, suggesting that pyroptosis is a vital mode of RCD.108 Scientists have investigated the role of pyroptosis in biological processes, and evidence suggests that pyroptosis likely plays a role in combating infections by eliminating intracellular pathogen replication sites and supporting downstream immune responses.
Inflammasomes are PRRs that recognize intracellular and extracellular pathogen ligands. Sensors of pyroptosis respond to components of bacteria and viruses, leading to the secretion of IL-18 and IL-1β through gasdermin-forming pores and plasma membrane rupture with the release of DAMPs, including HMGB1, ATP, and others. These cellular contents subsequently trigger several cellular events, including inflammation, proliferation, and differentiation.22,47,109 According to preliminary studies, IL-18 and IL-1β are critical for innate and adaptive immunity. NK and Th1 cells express IL18R on their surface and are highly sensitive to IL-18, which forms a positive loop with interferon-γ.110 Thus, pyroptosis is vital in the crosstalk between innate and adaptive immunity, inducing an immunostimulatory response against pathogens.111 As a crucial contributor to innate immunity, pyroptosis leads to the generation of pore-induced intracellular traps (PITs), i.e., structures that entrap previously intracellular microbes. PITs facilitate intracellular bacterial clearance by containing the pathogen and producing signals that promote recruitment and uptake by neutrophils.80 Besides, emerging research has suggested that pyroptosis exerts an antagonistic effect on endogenous danger signals, such as oxidative stress, in addition to triggering a microbe-mediated immune response.112,113
Aside from lytic cell death, gasdermins, the key executors of pyroptosis, participate in a wide range of physiological processes, including cell differentiation, tissue homeostasis, mitochondrial homeostasis, immune tolerance, and neutrophil extracellular trap (NET) formation.114,115,116 GSDMA3 was reported to play a vital role in normal hair follicle differentiation by participating in the Msx2/Foxn1/acidic hair keratin pathway.117 Li et al. reported that in osteoblasts, GSDMD underwent cleavage to generate non-lytic p20 products, which helped prevent bone loss and maintain bone homeostasis.118 Besides, GSDMD was identified as a key factor in the separation of bacteria from the epithelium in the colon owing to its role in goblet cell-mediated mucus layer formation. This biological function of GSDMD depends on its regulation of cortical F-actin disassembly in the process of mucin secretion.114 According to a recent study, the N-terminal domain of Gsdma3 was associated with Hsp90 and targeted mitochondria, thus regulating mitochondrial oxidative stress.115 In intestinal epithelial cells (IECs), GSDMD was cleaved by caspase-3/7 to produce a 13 kD N-terminal fragment upon exposure to dietary antigens. This fragment translocated to the nucleus and stimulated the expression of CIITA and MHCII molecules, which induced type 1 regulatory T (Tr1) cells and enabled immune tolerance of IECs to dietary antigens.119 Moreover, neutrophil proteases catalyze the proteolytic activation of GSDMD during NETosis, affecting nuclear expansion and protease activation via a feed-forward loop.116
Regulatory function of pyroptosis in diseases
Pyroptosis facilitates the clearance of injured or infected cells, comprising a crucial defense against pathogen infection and DAMP stimulation. Persistent pyroptotic cell death can result in ion gradient dissipation, cellular content release, and exaggerated inflammatory responses, contributing to the pathophysiology of various diseases, including cancer. Two diagrams summarize the regulatory mechanisms of the pyroptotic pathway in cancerous and non-cancerous diseases (Figs. 3 and 4).
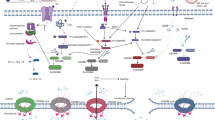
Regulatory mechanisms of pyroptosis in cancer. Over recent years, a series of molecules have been proven to target different sites of the pyroptosis pathway, thereby influencing the occurrence and progression of various cancers, including lung cancer, gastric cancer, breast cancer, HCC, and colorectal cancer. AZU1 Azurocidin 1, Akt protein kinase B, ASAH2 sphingolipid metabolic enzyme ceramidase, CPSF6 cleavage and polyadenylation factor 6, CHMP3 charged multivesicular body protein 3, DRD2 D2 dopamine receptor, IBSP integrin binding sialoprotein, MUC20v2 MUC20 variant 2, MMP mitochondrial membrane potential, P2X7R P2X7 receptor, PDK1 3-phosphoinositide-dependent kinase 1, Sorcin soluble resistance-related calcium-binding protein, TRAF3 tumor necrosis factor receptor-associated factor 3, TCEA3 transcription elongation factor a3, UCP uncoupling protein 1, ZNF Zinc finger protein 148. The figure was created by Figdraw
Regulatory mechanisms of pyroptotic cell death in non-cancer disease. Research on pyroptosis fosters the pathological process comprehension of systemic diseases and the underlying mechanisms involving numerous proteins, RNAs, and other molecules. ALPK1 alpha‐kinase1, CTSB cathepsin B, HRC histidine-rich calcium-binding protein, HNE 4-hydroxynonena, METTL14 methyltransferase-like 14, NETs neutrophil extracellular traps, PI(4;5)P2 phosphatidylinositol-(4;5)-bisphosphate, PI4P phosphatidylinositol-4-monophosphate, S1PR2 sphingosine-1-phosphate receptor 2, Txnrd3 thioredoxin reductase 3, TRIM29 tripartite motif containing 29. The figure was created by Figdraw
Cancer
Pyroptosis plays a dual role in cancer, exhibiting context-dependent pro- or anti-tumor effects during tumorigenesis, because of its significant impact on inflammation and immunity.120,121 Its divergent functions in cancer are influenced by tumor characteristics, genetic background, host immunity status, and the specific pyroptotic effectors involved, emphasizing the complex interplay between pyroptosis, innate immunity, and the tumor microenvironment.122,123,124,125,126 Below, we summarize the involvement of pyroptosis in a range of cancers, covering lung, gastric, breast, hepatocellular carcinoma (HCC), and colorectal cancer.127,128,129,130,131
Lung cancer
Lung cancer is highly prevalent and a major factor contributing to death worldwide.132 Inflammasomes, the key initiators of the pyroptotic pathway, were highly expressed in various types of lung cancer. Several genes have been implicated in the regulation of inflammasome expression, most notably, NLRP3. In lung cancer cells, long non-coding RNA (lncRNA) LINC00969 promotes resistance to gefitinib via epigenetic inhibition of NLRP3. Mechanistically, LINC00969 binds to both METTL3 and EZH2, transcriptionally modifies the level of H3K27me3 in the region of NLRP3 promoter, and post-transcriptionally regulates the m6A level in NLRP3.127 Inhibition of tumor necrosis factor receptor-associated factor 3 (TRAF3) promotes the progression of lung adenocarcinoma (LUAD) cells and weakens the sensitivity of the cells to paclitaxel, partly through activation of caspase-1-dependent pyroptosis.133 In lung and pancreatic cancer models, β5-integrin upregulated the Src-STAT3-ASAH2 signaling axis, thus reducing ROS production to prevent chemotherapy-induced pyroptosis.134 ANGPTL4, a critical regulatory gene for lipid and glucose metabolism, was found to contribute to gefitinib resistance in non-small cell lung cancer (NSCLC) cells by regulating the NLRP3/ASC/caspase 8 pathway.135 Silencing lncRNA-XIST promotes NLRP3-mediated pyroptosis and NSCLC progression via activating the miR-335/SOD2/ROS pathway.136 Shi et al. downregulated miR-556-5p expression to increase cisplatin sensitivity in cisplatin-resistant NSCLC (CR-NSCLC) tissues. Mechanistically, the downregulation of miR-556-5p contributes to the expression of NLRP3, thereby provoking pyroptosis in cisplatin-treated CR-NSCLC cells.137 NLRP3-independent pyroptotic pathways influence the onset and progression of lung cancer. For example, AMIGO2 attenuated the (caspase-8 and caspase-9)/caspase-3 cascade by initiating the PDK1/Akt (T308) signal axis, attenuating GSDME-mediated pyroptosis, which reduces the innate sensitivity of NSCLC cells to cisplatin.138
Gastric cancer
Gastric cancer is among the primary causes of cancer-related mortality worldwide and is often discovered at an advanced stage when surgical intervention is no longer appropriate.139 MUC20 variant 2 (MUC20v2) protects gastric cancer cells from apoptosis and pyroptosis by maintaining mitochondrial calcium levels and membrane potential homeostasis, which enhances cell survival and chemoresistance.128 LncRNA ADAMTS9-AS2 is a tumor suppressor and sensitizes chemoresistant gastric cancer cells to cisplatin by upregulating the miR-223-3p/NLRP3 signaling axis.140 USP50 enhances bile acid-induced NLRP3-mediated pyroptosis of macrophages, releasing HMGB1 and leading to the genesis of gastric tumor cells through the PI3K/AKT and mitogen-activated protein kinase (MAPK)/extracellular signal-regulated kinase (ERK) pathways.141 Cleavage and polyadenylation factor 6 (CPSF6) and Helicobacter pylori also regulate pyroptosis in gastric cancer.142,143
Breast cancer
Breast cancer is one of the most prevalent malignancies worldwide.144 In the tumor microenvironment (TIM) of breast cancer, modulation of macrophages to the M1 phenotype by DRD2, a D2 dopamine receptor, leads to NLRP3 inflammasome assembly and subsequent pyroptosis.145 Azurocidin 1 (AZU1), a heparin‑binding protein, was reported to induce pyroptosis by initiating the pNF‑κB/NLRP3/caspase‑1/GSDMD signaling axis in triple-negative breast cancer (TNBC) in vitro.146 Zinc finger protein 148 (ZNF-148) was found to aggravate the progression of breast cancer through induction of miR-335/SOD2/ROS-mediated pyroptosis.147 Mitochondrial protein UCP1 was reported to inhibit TNBC progression by activating caspase-3-mediated pyroptosis.129 In addition, the PD-L1/PD-1 immune checkpoint has been found to engage in the pyroptosis pathway under specific circumstances when PD-L1 translocates to the nucleus, where it enhances GSDMC transcription. Subsequent treatment with TNF-α results in caspase-8-mediated cleavage of GSDMC, leading to pyroptosis and tumor necrosis in breast cancer.36
Hepatocellular carcinoma
HCC is one of the primary causes of cancer-related death, and its prevalence is anticipated to increase globally.148 A recent study found that soluble resistance-related calcium-binding protein (sorcin) impairs the assembly of the NLRP3 inflammasome, thus inhibiting cell pyroptosis and provoking HCC progression.130 A noteworthy association was found in HCC cells between the expression of GSDMD and NEK7. Moreover, NEK7 knockdown elevated pyroptosis-related markers NLRP3, caspase-1, and GSDMD, reducing HCC stimulation of hepatic stellate cells, hinting at NEK7’s considerable role in both tumor progression and cancer-stromal interactions in HCC.149 Additionally, USP48 stabilized GSDME by removing K48-linked ubiquitination at K120 and K189, thereby promoting pyroptotic death in liver cancer cells.150 Additionally, charged multivesicular body protein 3 (CHMP3) contributed to liver cancer via caspase-1-dependent pyroptotic cell death.151
Colorectal cancer
Owing to environmental degradation and population aging, colorectal cancer ranks third globally in terms of frequency of malignant tumors and has emerged as the world’s fourth most deadly cancer.152 IL-17A has been suspected of inducing mitochondrial dysfunction, intracellular ROS generation, and activation of the NLRP3/caspase-4/GSDMD pyroptotic pathway, consequently upregulating the secretion of inflammatory substances and recruiting infiltrating CD8+ T cells to colorectal tumors.131 The highly selective P2X7R antagonist, A438079, was observed to inhibit pyroptosis via the NLRP3/caspase-1 pathway, although it remains unclear whether this inhibition could ultimately prevent colorectal cancer progression.153 Moreover, a recent study revealed that Nrf2 inhibition increases the sensitivity of CRC cells to oxaliplatin by promoting pyroptosis and ferroptosis.154 USP47-driven deubiquitination and stabilization of transcription elongation factor a3 (TCEA3) suppressed pyroptosis of colorectal cancer cells promoted by chemotherapeutic doxorubicin.155
Neurological disorders
Accumulating evidence points to pyroptosis as a critical factor in Alzheimer’s disease (AD), Parkinson’s disease (PD), ischemic stroke, multiple sclerosis (MS), traumatic brain injury, spinal cord injury, epilepsy, and neuropsychiatric and neurodevelopmental diseases.156,157,158,159,160,161,162 Below, we summarize the regulation of pyroptosis in a range of neurological diseases.
Alzheimer’s disease
AD is the most common neurodegenerative disease in the elderly and is characterized by cognitive decline. The major neuropathological features of AD include amyloid β (Aβ) plaques and neurofibrillary tangles (NFT), along with neuroinflammation and neuronal loss.163 Accumulating evidence indicates that the regulation of pyroptosis plays a role in the pathology of AD.164 Moonen et al. demonstrated differential activation of the pyroptotic pathway in a cell type-dependent manner in AD cases, implicating pyroptosis activation in neuronal death.165 Recent studies have revealed that β-amyloid could promote NLRP3-caspase-1-GSDMD signaling in neurons, leading to nerve injury in AD.166,167 β-amyloid protein aggregation around ASC fibrils could amplify the inflammatory response, leading to pyroptotic cell death.168 Other neuropathogenic proteins, including tau proteins, can induce NLRP3 inflammasome activation.169 In a vicious cycle, ASC released by microglia binds to β-amyloid, enhancing the formation of β-amyloid oligomers and aggregates, which can be attenuated by the anti-ASC antibody.170 Cholesterol overload in neuronal cells provokes pyroptosis via increasing Aβ‑induced oxidative stress in the mitochondria.171 Overall, neuropathogenic proteins can induce inflammasome formation and proinflammatory cytokine release, exacerbating AD pathology.
Parkinson’s disease
PD is a neurodegenerative disorder featuring misfolding of α-synuclein proteins and Lewy body formation in affected neurons.172 These neuropathogenic protein aggregations lead to the loss of dopaminergic neurons in the substantia nigra pars compacta, with symptoms of tremors and bradykinesia.173 The pathophysiology of PD correlates with the neuroinflammation theory and involves pyroptosis.174 A recent study reported that salsolinol induces the expression of pyroptosis-related proteins, such as NLRP3, ASC, and caspase-1, in SH-SY5Y cell lines and mouse models.175 In addition, elevated levels of cleaved caspase-1 and ASC have been identified in substantia nigra samples from patients with PD compared to healthy controls.176 Therefore, growing evidence from cellular, animal, and clinical studies supports a correlation between pyroptosis and PD progression.
Ischemic stroke
Known as a crippling central nervous system disease caused by arterial blockage with high mortality, ischemic stroke imposes a high burden on the economy and society. Increasing evidence suggests that inflammation promoted by inflammasomes and pyroptosis is essential for the pathophysiological process of ischemic stroke and is strongly linked to prognosis. METTL14 (methyltransferase-like 14) was found to activate the NLRP3 inflammasome/pyroptosis axis via KAT3B-STING signaling after oxygen-glucose deprivation/reperfusion (OGD/R), which simulates ischemic stroke in vitro.177 Wei et al. demonstrated that TRIM27 downregulated NLRP3 inflammasome-mediated pyroptosis through Akt/Nrf2/HO-1 signaling, ameliorating ischemic stroke.178 Likewise, TRIM29 (Tripartite Motif Containing 29) was reported to exert a negative regulatory effect on pyroptosis in ischemic stroke.179
Other neurological disorders
Associations have also been found between pyroptosis and some other neurological disorders, including MS, amyotrophic lateral sclerosis, and neuropsychiatric diseases. As a chronic inflammatory demyelination disorder, the development of MS is closely related to pyroptosis. Caspase-1-mediated GSDMD pyroptosis and inflammasome activation have been observed in oligodendrocytes (ODCs) and microglial cells in vitro and patients with MS.180 Activated caspase-3 with GSDMD immunopositivity has also been identified in macrophages/microglia within demyelinating lesions of patients with progressive MS and in experimental autoimmune encephalomyelitis (EAE) models.181 Motor neuron loss in the motor cortex is associated with increased inflammasome-triggered pyroptosis in microglia.182 NLRP3 inflammasome activation has also been observed in TDP43A315T and SOD1 transgenic animals.183,184
Neuropsychiatric diseases, such as anxiety and depression, are characterized by cognitive and mental abnormalities. It has been demonstrated that in addition to hereditary variables, elevated inflammation plays a substantial role in many neuropsychiatric illnesses. Simon et al. reported that the monocytes of patients with major depressive disorder (MDD) exhibited signs of premature aging and inflammation, as well as a tendency toward pyroptotic cell death.185 A recent study illustrated that the Kir6.1/K-ATP channel in astrocytes negatively modulated astrocytic pyroptosis by preventing the assembly and activation of the NLRP3 inflammasome, which plays a vital role in the pathogenesis of depression.186 Furthermore, inorganic arsenic exposure may result in the development of generalized anxiety disorder (GAD) by downregulating the expression of miR-425–3p in the prefrontal cortex, which targets the NF-κB/NLRP3/caspase-1/GSDMD signaling axis and causes the release of IL-1β and IL-18.187
Respiratory diseases
Research indicates that pyroptosis is critical for inflammatory diseases, with increasing evidence linking respiratory diseases to inflammation, bringing the role of pyroptosis in respiratory diseases to the forefront of research. Pyroptosis participates in the genesis of chronic obstructive pulmonary disease (COPD), acute lung injury (ALI), asthma, silicosis, pulmonary hypertension, cystic fibrosis, and pulmonary tuberculosis.188,189,190,191,192,193,194 In this section, we sum up the roles and corresponding mechanisms of pyroptosis in inflammatory respiratory diseases.
Chronic obstructive pulmonary disease (COPD)
COPD is a severe health issue and the third leading cause of inflammatory respiratory-related mortality globally.195 The most significant risk factor for COPD is cigarette smoke, which can draw inflammatory cells to the lung tissues and trigger the induction of a range of cytokines. Pyroptosis is irreversible when cigarette smoke induces tissue inflammation. For example, cigarette smoking can enhance the expression of sphingosine-1-phosphate receptor 2 (S1PR2) in human bronchial epithelial cells, thus triggering the NLRP3/ASC/caspase-1 pathway, leading to airway inflammation and injury.189 Likewise, cigarette smoke extract contributed to the pyroptosis of human bronchial epithelial cells via the ROS/NLRP3/caspase-1 axis.196 Meanwhile, hydrogen sulfide was reported to alleviate pyroptosis and lung injury in a model of smoking-induced COPD by downregulating the TLR4/NF-κB signaling pathway.197 Furthermore, Wang et al. proved that TREM-1 could activate NLRP3-mediated pyroptosis, thereby aggravating the injury and inflammation that COPD caused to lung tissues.198 Additional studies have revealed that factors, including lncRNA GAS5, TRPV4, and WSPM2.5, aggravated COPD by activating pyroptotic cell death.199,200,201 Inhibition of pyroptosis by exosomes from adipose-derived stem cells and the Nrf2/HO-1 signaling axis might alleviate COPD.202,203
Acute lung injury (ALI)
ALI is a lethal disease characterized by cytokine storms, leukocyte infiltration, and diffuse alveolar injury.204 Polymorphonuclear neutrophils (PMNs) are essential to inducing sepsis-related ALI. Specifically, exosomal miR-30d-5p from PMNs facilitated sepsis-related ALI by inducing M1 macrophage polarization and activating NF-κB to prime macrophage pyroptosis.205 A recent study revealed that pyroptosis contributed to ALI caused by cecal ligation and puncture (CLP), and aldehyde dehydrogenase 2 (ALDH2) acted as a buffer by limiting pyroptosis.206 A lipid peroxidation product, 4-hydroxynonenal (HNE), was found to attenuate NLRP3 inflammasome-mediated pyroptosis and downstream IL-1β release, independent of the Nrf2 and NF-κB signaling pathways, contributing to sepsis-related ALI.207 Furthermore, many other substances had a regulatory effect on pyroptosis, thus affecting the development of ALI induced by sepsis and other causes.208,209,210,211,212
Asthma
Asthma is a chronic respiratory inflammatory disease featuring reversible airflow limitation and airway hyperresponsiveness.213 Increasing evidence has shown the critical role of pyroptosis in asthmatic airway inflammation and injury. In an ovalbumin-induced asthmatic mouse model, GSDMD silencing significantly reduced Th17 and Th2 inflammatory responses as well as M2 macrophage polarization, all of which are involved in airway remodeling and inflammation.214 Dectin-1 activation in asthma provoked caspase-11/4-mediated macrophage pyroptosis, thereby stimulating the secretion of chemokines and aggravating airway neutrophil inflammation.215 MUC1 suppressed NLRP3 inflammasome-mediated pyroptosis via blocking the TLR4/ MyD88/NF-κB pathway, subsequently alleviating neutrophil airway inflammation in asthma.216
Silicosis
Attributed to long-term inhalation of crystalline silica (CS) particles, silicosis is a severe lung disease marked by irreversible pulmonary fibrosis.217 Emerging evidence has indicated that CS-induced lung injury was closely associated with the activation of pyroptosis. Meiyue Song et al found that macrophages in silicosis lung tissue underwent GSDMD-induced pyroptosis, mediated by both canonical and non-canonical signaling pathways.218 Another mechanistic study elucidated that silica exposure triggered the dysfunction of the P2X7 ion channel, causing intracellular K+ efflux and the formation of the NLRP3 inflammasome, which cooperated with LPS-primed activities to provoke macrophage pyroptosis and pulmonary inflammation.219 Furthermore, CS particles induced damaged mitochondria to release DAMPs, which initiated downstream NLRP3 inflammasome-mediated pyroptosis to promote pulmonary fibrosis.191
Pulmonary tuberculosis
Caused by Mycobacterium tuberculosis (Mtb), tuberculosis (TB) is a highly contagious illness that continues to be the most common infectious agent-related cause of mortality.220 Accumulating evidence has supported pyroptosis as a critical factor in the development of TB. Mycobacterial EST12 binds to the receptor for activated C kinase 1 (RACK1) in macrophages to assemble a complex that engages the deubiquitinase UCHL5 to facilitate the K48-linked deubiquitination of NLRP3, consequently promoting the NLRP3-caspase-1/11-IL-1β immune pathway.221 PtpB, an Mtb phospholipid phosphatase, was recently reported to suppress the host inflammasome-pyroptosis pathway. Mechanistically, PtpB dephosphorylates phosphatidylinositol-4-monophosphate (PI4P) and phosphatidylinositol-(4,5)-bisphosphate [PI(4,5)P2] in the host cell membrane, thereby interfering with the membrane localization of cleaved GSDMD and preventing macrophage pyroptosis.222 In addition to Mtb, host molecules regulate pyroptosis, driving TB disease progression. According to a recent study, S100A4 provoked the pyroptosis of THP-1 macrophages induced by BCG infection via activation of the NF-κB/NLRP3/caspase-1/GSDMD signaling pathways.194 Moreover, researchers have recently discovered many other factors regulating pyroptosis during pulmonary tuberculosis.223,224,225,226,227,228
Kidney disease
Pyroptosis participates in the initiation and progression of kidney diseases, as most share characteristics such as inflammation and parenchymal cell death. Several studies have shown regulation of pyroptosis in the pathogenesis of kidney diseases, such as acute kidney injury (AKI), chronic kidney disease (CKD), diabetic kidney disease (DKD), lupus nephritis, and kidney allograft transplantation.229,230,231,232,233 This section reviews studies implicating pyroptosis in kidney disease models.
Acute kidney injury
Acute kidney injury (AKI) is a prevalent clinical complication with high worldwide morbidity and mortality rates.234 Increasing evidence suggests that pyroptosis significantly contributes to the pathogenesis of AKI. USF2 upregulated THBS1 to activate the TGF-β/NLRP3 signaling pathway, provoking pyroptosis and further aggravating sepsis-induced AKI.235 According to a recent study, dsDNA-induced AIM2 pyroptosis rapidly removes macrophages, which unexpectedly halts aberrant inflammation during AKI triggered by rhabdomyolysis.236 Baatarjav et al. revealed the pivotal role of GSDME-mediated pyroptosis in AKI development. The cleavage of GSDME by caspase-3 is responsible for forming membrane pores and cell lysis, exacerbating inflammation and renal tubular damage.229 Furthermore, recent investigations have identified numerous proteins, RNAs, and other molecules that implicate pyroptosis as a driver of AKI pathogenesis.237,238,239,240,241
Chronic kidney disease
CKD is an irreversible, progressive illness that massively affects health outcomes.242 Recent research has indicated that pyroptosis contributes to CKD progression. Butyrate plays a renoprotective role, alleviating renal fibrosis in CKD. Mechanistically, STING/NF-κB/p65 pathway downregulation attenuated NLRP3-mediated pyroptosis.243 GSDME was found to contribute to renal tubulointerstitial fibrosis and renal dysfunction induced by ureteral obstruction and 5/6 nephrectomy via pyroptotic cell death.244 In addition, a new study illustrated the collaboration of GSDMD and GSDME in the transition of AKI to CKD.242
Diabetic kidney disease
DKD is the main cause of end-stage renal disease, with a dramatically increased prevalence worldwide over the past decades.245 Recent studies have confirmed that pyroptotic death is essential in the development of DKD. During DKD, caspase-11/4 and GSDMD-mediated pyroptosis are activated, contributing to podocyte loss.246 A recent study demonstrated that alpha‐kinase1 (ALPK1) was activated by hyperglycemia and caused phosphorylation of NF-κB in renal tubular epithelial cells in DKD. Subsequently, the canonical caspase-1-GSDMD pyroptosis pathway is induced, contributing to tubular injury and interstitial inflammation.231 NETs have also been reported to induce glomerular endothelial cell (GEC) pyroptosis, mediated by charge, further inducing the development of DKD.247 Over the past few years, other molecules and substances, such as circRNA, LncRNA, and lysophosphatidic acid have also been reported to target the regulation of pyroptosis in DKD.248,249,250,251,252
Cardiovascular diseases
Accumulating evidence has demonstrated that the regulation of pyroptosis influences the pathogenesis of cardiovascular diseases (CVD). Pyroptosis induces the amplification of inflammatory responses and accelerates the occurrence of cardiovascular diseases such as atherosclerosis, myocardial infarction (MI), arrhythmia, and cardiac hypertrophy.253,254,255
Atherosclerosis
Arteriosclerosis is a chronic inflammatory process featuring lipid deposition, plaque build-up, and endarterium thickening.256 As the primary etiological factor for cardiovascular and cerebrovascular disorders, atherosclerosis has become a significant global cause of death and disability.257 Emerging studies have suggested that pyroptosis is critical for the development of atherosclerosis. The lncRNA NEAT1 positively regulates the expression of NLRP3 at the transcription level, initiating pyroptosis of vascular endothelial cells. In contrast, exercise attenuates the function of NEAT1, impeding atherosclerosis plaque formation.258 Rnd3 was reported to downregulate the TRAF6/NF-κB/NLRP3 signaling pathway via regulation of TRAF ubiquitination, thus impairing endothelial pyroptosis in atherosclerosis.259 During atherosclerosis, STAT3 in macrophages is activated by ox-LDL or inflammatory cytokines and upregulates GSDME transcription, which increases caspase-3 activity and contributes to the transition from apoptosis to pyroptosis.260 Additionally, many other molecules participate in atherosclerosis by regulating the pyroptosis of endothelial cells or macrophages, including uric acid, homocysteine, nicotine, IQGAP1, and hormones.261,262,263,264,265,266
Myocardial infarction
Ischemic heart disease, particularly MI, remains the leading cause of death worldwide.267 Directly restoring blood flow in the ischemic region immediately is the most effective method for rescuing dying cardiomyocytes. Reperfusion, however, is a double-edged sword; while it can save ischemic myocardia, it also risks inflicting further damage, a condition known as myocardial ischemia/reperfusion (MI/R) injury.268 Emerging studies have attempted to uncover the pathophysiological role of pyroptosis in MI and MI/R injury. Uric acid was reported to exacerbate MI/R injury via upregulation of the ROS/ NLRP3 pyroptosis pathway.269 Oxytocin (OT) can exert protective effects against myocardial I/R injury with hyperglycemia via regulation of the AMPK/NLRP3 signaling pathway and pyroptosis.270 GSDMD-induced pyroptosis plays a critical role during MI/R injury, and the caspase-11/GSDMD pathway mediated by oxidative stress may be essential for the process.271 Furthermore, over the past few years, several additional mechanisms have been found to contribute to the pathogenesis of MI, such as circHMGA2, HIF-1α/TUG1/FUS, and lncRNA H19.254,272,273,274,275,276
Diabetic cardiomyopathy
Diabetic cardiomyopathy (DCM) is one of the most detrimental consequences of type 2 diabetes. DCM involves abnormal structural remodeling and aberrant cardiac function.277 Recent studies have widely reported the essential role of pyroptosis regulation in cardiomyopathy, particularly in DCM. Meng et al. discovered that METTL14 attenuates pyroptosis and DCM progression via m6A methylation of lncRNA TINCR by downregulating NLRP3 expression.278 Critically, CD38 deficiency alleviates type 2 diabetes-induced DCM by reducing pyroptosis and apoptosis in vitro and in vivo via activation of the NAD+/Sirt3/FOXO3a signaling pathways.279 CircRNA DICAR was identified as a novel endogenous regulator for DCM, with the potential to protect against cardiomyocyte pyroptosis via DICAR-VCP-Med12 degradation.280 What’s more, many other molecules in the human body exert regulatory effects on pyroptosis involved in DCM disease progression, such as folic acid, ghrelin, miR-21-3p, microRNA-223-3p, and lncRNA MIAT.281,282,283,284,285
Arrhythmia
Among the arrhythmia types, atrial fibrillation has been most frequently reported to be associated with cell pyroptosis. Atrial fibrillation is a prevalent arrhythmia in clinical practice, occurring in 1-2% of people worldwide, and has been linked to an increased risk of heart failure and hospitalization.286 Luo et al. discovered that Akkermansia muciniphila (A.muciniphila) protected rats against cold-related atrial fibrillation. Mechanically, cold exposure decreased the abundance of A. muciniphila, further causing augmented TMAO in plasma and TMAO-mediated atrial pyroptosis, which resulted in atrial structural remodeling and atrial fibrillation.287 According to a new study, the extracellular vesicles from adipose tissue-derived mesenchymal stem cells (AMSCs) carrying LncRNA XIST attenuated myocardial cell pyroptosis in atrial fibrillation via blocking miR-214-3p-mediated Arl2 inhibition.288
Cardiac hypertrophy
Pathological hypertrophy of the myocardium is one of the largest contributors to heart failure and has been associated with pyroptosis in recent studies.289 In a model of doxorubicin (Dox)-induced non-ischemic dilated cardiomyopathy, Dox increased the expression of NOX1 and NOX4 and triggered mitochondrial fission via dynamin-related protein 1 (Drp1) activation, resulting in caspase-1-dependent pyroptosis in cardiomyocytes.290 Moreover, TRPA1 deficiency aggravated dilated cardiomyopathy through augmenting S100A8 expression to induce pyroptosis in M1 macrophages.291 Yue et al also reported that NLRP3/caspase-1-dependent pyroptosis has a pathological role in myocardial hypertrophy.292 In a model of pressure-overload cardiac hypertrophy, NLRP3 deficiency exerted cardioprotective effects via activation of TAK1.293 Another study found that Sema4D contributed to pathological myocardial hypertrophy via the MAPK/NF-κB/NLRP3 pathway.294
Drug-induced cardiac injuries
Antineoplastics, antipsychotics, and other drugs can cause toxicity that adversely influences the heart and may result in cardiomyopathies, known as drug-induced cardiac injuries.295 Increasingly, studies have found an association between drug-induced cardiac injury and pyroptosis. Dox enhanced the expression of Bnip3, which activated caspase-3 to induce GSDME-mediated pyroptosis in cardiomyocytes.296 Additionally, mir-34a-5p selectively attenuated the expression of Sirt3 and then augmented autophagy and mitoROS generation, ultimately exacerbating Dox-induced myocardial cell pyroptosis.297 Antipsychotics competitively bonded to CB1R, which was then internalized and directly interacted with NLRP3 inflammasome through amino acid residues 177–209, mediating stabilization of the inflammasome and activation of cardiomyocyte pyroptosis.298
Digestive diseases
The pyroptotic pathway has emerged as an indispensable component of digestive disorders, including gastric ulcer, ulcerative colitis (UC), acute pancreatitis (AP), and hepatic fibrosis.299,300,301,302 In this section, we summarize the role of pyroptosis in the pathological processes of digestive diseases.
Ulcerative colitis
UC is an aggressive, chronic inflammatory bowel disease accompanied by a high risk of bowel cancer.303 Accumulating evidence supports pyroptosis as a critical factor in the development of UC. Inhibition of the C3a/C3aR axis in the early stages of UC induces poor prognosis of UC by promoting cell pyroptosis. However, stimulation with a C3aR inhibitor in the later stages of UC alleviated the symptoms of UC by suppressing pyroptosis.304 Overexpression of Txnrd3 induced intracellular calcium outflow, endoplasmic reticulum stress, and ROS accumulation, ultimately leading to pyroptosis and necrosis of colon cancer cells.305 APOL1 overexpression induces the NLRP3/caspase1/GSDMD pyroptosis pathway and promotes the release of the chemokine CXCL1, further aggravating UC.306 Besides, lncRNA MEG3, miR-141-3p, and SLC6A1 have been found to influence the progression of UC via the regulation of pyroptosis.307,308,309
Acute pancreatitis
AP is an urgent and severe abdominal disease that can have both local and systemic consequences.310 Growing evidence has suggested a close correlation between the inflammasome and its downstream effectors and AP.311 Cathepsin B (CTSB) was reported to induce NLRP3/caspase-1-mediated cell pyroptosis, aggravating AP.312 A recent study uncovered that IL-37 impaired phosphorylated STAT3 and protected against pyroptosis of injured acinar cells in AP.313 Inhibition of TRAF6 was found to attenuate pancreatic injury in hyperlipidemic AP, along with relief of the inflammatory response via pyroptotic cell death.314 High-density lipoprotein (HDL) was reported to relieve oxidative stress and suppress acinar cell pyroptosis, reducing the severity of AP.315 Furthermore, additional factors upregulate cell pyroptosis and promote the progression of AP, such as circHIPK3, endogenous tRNA-derived small RNA, USP25, endoplasmic reticulum stress, and exosomal miR-155-5p.316,317,318,319,320
Liver fibrosis
Liver fibrosis is a chronic liver injury with the potential to progress to cirrhosis and correlates with an elevated risk of HCC over time.321 Accumulating evidence has illustrated that pyroptosis plays a vital role in the development of liver fibrosis. According to a recent study, NLRP3 overactivation resulted in hepatocyte pyroptosis and the release of inflammasome particles into the extracellular space, engendering hepatic stellate cell activation and liver fibrosis.322 STING was found to mediate hepatocyte pyroptosis through epigenetic activation of the IRF3/WDR5/DOT1L transcription activator complex, contributing to liver fibrosis.323 Hepatic histidine-rich calcium-binding protein (HRC) induced paracrine activation of hepatic stem cells (HSCs) by triggering hepatocyte pyroptosis via the NLRP3/caspase-1/HMGB1 signaling axis in liver fibrosis, promoting fibrogenesis.324 In addition, angiotensin II, S100A8, and the METTL3/MALAT1/PTBP1/USP8/TAK1 axis were proven to aggravate liver fibrosis. Concurrently, growth arrest-specific 5 (GAS5) and exosomes produced from bone marrow mesenchymal stem cells exerted an inhibitory effect on liver fibrosis.325,326,327,328,329
Therapeutic strategy regulating pyroptosis
Cancer
It is widely accepted that pyroptotic cell death is implicated in tumor suppression. Generally, activation of the pyroptosis signaling pathway augments anti-tumor immunity, whereas inhibition of the pyroptosis signaling pathway facilitates tumor growth and metastasis. In this section, we review the current literature on molecules that induce or enhance pyroptosis. Notably, some of these molecules have been used in cancer treatment for years, and their mechanisms have previously been solely attributed to the induction of apoptosis.
Therapeutic strategies targeting inflammasome
Inflammasomes are involved in various stages of tumor development and provide tumorigenic and tumor-suppressive functions.5,330,331 Owing to the role of pyroptosis in tumor initiation, targeting inflammasome activation is an encouraging therapeutic policy for cancer treatment.
Molecules that target the NLRP3 inflammasome have received extensive attention. Studies have shown that in tumor cells undergoing Snail-mediated epithelial-mesenchymal transition (EMT), the activity of NLRP3 inflammasomes in tumor-associated macrophages (TAMs) is diminished in response to chemotherapeutic agents. This suppression occurs via the transfer of exosomal miR-21, downregulating PTEN and BRCC3, leading to phosphorylation and lysine-63 ubiquitination of NLRP3. This process effectively prevents the assembly of the NLRP3 inflammasome, resulting in reduced chemotherapy efficacy.332 Therefore, triggering NLRP3-mediated pyroptosis of cells in the tumor microenvironment possesses treatment potential.
Yuan et al. identified cucurbitacin B, derived from muskmelon pedicel, as a natural bioactive compound demonstrating potent anti-tumor effects in lung carcinoma. Cucurbitacin B directly combined with TLR4, triggering the activation of the NLRP3 inflammasome. Subsequently, this activation led to the cleavage of gasdermin D into its N- and C-terminal domains, facilitating the induction of pyroptosis.333 The therapeutic potential of alpine pine flavones (AIF) against HCC has been investigated and demonstrated suppression of proliferation, migration, and invasion in Huh7 and SMMC7721 cell lines, possibly through the excitement of NLRP3 inflammasome assembly, which has also been verified by in vivo findings.334 Metformin, recognized for its glucose-lowering effects, also exhibits antineoplastic effects in HCC by triggering apoptosis and pyroptosis. This activity is mediated by the enhancement of FOXO3 expression by metformin, which subsequently increases NLRP3 transcription and facilitates pyroptosis.335 Another camptothecin anticancer drug, FL118, can also inhibit the progression and metastasis of colorectal cancer by inducing NLRP3-ASC-Caspase-1 mediated pyroptosis.336 A significant correlation was noted between the expression of GSDMD and the presence of NEK7. Silencing NEK7 led to the upregulation of markers associated with pyroptosis, including NLRP3, caspase-1, and GSDMD, thereby diminishing the activation of stellate cells in HCC, suggesting that NEK7 modulates tumor progression and the interaction between cancer and stromal cells in HCC.149 Besides, the administration of IL-17A triggers pyroptosis via the ROS/NLRP3/caspase-4/GSDMD axis, enhancing the release of proinflammatory cytokines and resulting in increased infiltration of CD8+ T cells within tumor tissues.131 Zhao et al. discovered that enhanced expression of circAR-3 escalates cell proliferation and inflammation in prostate cancer (PCa), whereas its suppression exerted contrary effects. This process is facilitated by the acetylation of NLRP3 by KAT2B, furthering the subcellular localization and assembly of the NLRP3 inflammasome complex. This finding suggests that inhibiting NLRP3 acetylation or inflammasome assembly could be a viable strategy for halting the advancement of PCa.337 Yan et al. demonstrated that cisplatin induces the NLRP3/caspase-1/GSDMD pyroptosis pathway through the elevation of long non-coding RNA (lncRNA) maternally expressed gene 3 (MEG3) in TNBC, contributing to its anti-tumor activity. This finding offers a potential new approach for therapeutic intervention in TNBC.338 However, because of the pyroptosis activation effect of cisplatin, there can be severe side effects including acute kidney injury and hearing loss.339,340 To alleviate the side effects induced by cisplatin, animal and cell tests of two potential therapeutic oral anticancer drugs, AZD5438 and dabrafenib, a phase-2 clinical trial protein kinase CDK2 inhibitor and a US Food and Drug Administration-approved drug BRAF inhibitor, respectively, were conducted with the promising result of reduced cell death.341 Therefore, it exemplifies that the side effect of pyroptosis-targeted drugs can be improved when they are combined with other targeted drugs. Conversely, coenzyme Q0, a derivative quinone from Antrodia camphorate, exerted anticancer activity by counteracting NLRP3-mediated inflammation. Coenzyme Q0 suppresses HIF-1α expression and inhibits the NLRP3 inflammasome, as well as ASC/caspase-1 expression, resulting in the downregulation of IL-1β and IL-18 expression in MDA-MB-231 and 468 cells and inhibition of EMT/metastasis of human TNBC and HNSCC cells.342,343 This opposite result implies that in different cancers and different periods of tumor progression, pyroptosis may play different roles, and the treatment strategy should vary accordingly.
Extracts from natural crops have been shown to regulate pyroptosis as well. Radix Sophorae tonkinensis oxymatrine extract possesses anticancer properties. Oxymatrine has anticancer effects against CRC by suppressing LRPPRC, promoting mitophagy, and inhibiting the NLRP3 inflammasome in CRC cell xenografts and liver metastasis models.344 A clinical trial of oxymatrine for the treatment of severe plaque psoriasis found only minor adverse effects, and a clinical trial for CRC is expected.345 Peimine (PM), derived from Fritillaria, was found to mitigate inflammasome activity by reducing endoplasmic reticulum (ER) stress and decreasing the levels of various proteins within the NF-κB and mitogen-activated protein kinase (MAPKs) pathways, limiting the proliferation of breast cancer cells.346
Moreover, applying nanoparticles to regulate NLRP3-mediated pyroptosis is another promising therapeutic strategy. Guo et al. developed novel VB12-tethered nano micelles by enhancing sericin with poly(benzyl-l-glutamate) (PBLG) and encapsulating the near-infrared dye IR780. Under near-infrared light exposure, these specialized nano micelles disrupt ATP synthase function, leading to mitochondrial impairment and subsequent ROS generation. This sequence of events triggered the NLRP3/caspase-1/GSDMD pathway, ultimately resulting in the maturation of dendritic cells.347
Targeting the AIM2 inflammasome also affects cancer development. Curcumin, the principal active compound in turmeric, triggers caspase-1/GSDMD-dependent pyroptosis in leukemia cells by boosting the expression of the IFI16, AIM2, and NLRC4 inflammasomes through the stimulation of ISG3 transcription factor activity.348 Li et al. showed that dihydroartemisinin (DHA) activates the AIM2/caspase-3/GSDME axis, initiating pyroptosis in breast cancer cells.349 Moreover, elevated CCL19 expression markedly suppresses the proliferation of gastric cancer cells and tumor progression, both in vitro and in vivo, by enhancing the CCR7/AIM2 pathway. These findings present an encouraging therapeutic tactic for the treatment of gastric cancer in cases of combination with chemical materials.350 Xu et al. introduced a virus-mimicking particle self-assembled from elongated DNA structures produced via rolling circle amplification (RCA) enveloped in cationic liposomes. This construction initiates the activation of the AIM2 inflammasome, leading to gasdermin D-mediated pyroptosis and enhanced anti-tumor immune responses.351 Furthermore, the development of biodegradable Ca2+ nanomodulators (CaNMs) for pyroptosis-mediated cancer immunotherapy has shown promise, as they cause mitochondrial Ca2+ overload and promote ROS generation, cytochrome C release, and ultimately, the caspase-3/GSDME-dependent pyroptotic pathway.352 Furthermore, in an anaplastic thyroid cancer clinical trial, apatinib upregulates caspase‐1 and melittin activates AIM2 inducing caspase‐3–GSDMD and caspase‐1–GSDME pyroptosis.353
Therapeutic strategies targeting caspases
Caspases are critical effectors of pyroptotic cell death and, therefore, have received much attention as a potentially novel strategy for tumor therapy.104,105,354 Compounds targeting caspase-3 have been favored. For instance, triptolide treatment inhibits the expression of mitochondrial hexokinase and c-Myc II in cancer cells, activating the BAD/BAX caspase-3 cascade and ultimately leading to GSDME-dependent pyroptosis.355 Another small compound, gambogic acid, can also induce caspase-3/GSDME pyroptosis, simultaneously enhancing cancer immunotherapy.356 Miltirone, a natural substance with anticancer activity, simultaneously prompts the proteolytic cleavage of GSDME and caspase-3, thereby inhibiting the viability of HepG2 or Hepa1-6 cells. Mechanistically, miltirone elicits intracellular ROS accumulation, inhibiting the phosphorylation of mitogen-activated and extracellular signal-regulated kinase (MEK) and extracellular regulated protein kinase 1/2 (ERK1/2) and inducing GSDME-dependent pyroptosis. ROS/ERK1/2-BAX-caspase-9-caspase-3-GSDME has been confirmed as the central signaling axis in the regulation of pyroptosis.357 Similarly, tetraarsenic hexoxide can also induce the generation of mitochondrial ROS leading to the caspase-3/GSDME-mediated pyroptosis in cancer cells.358 Nitidine chloride (NC), a benzophenanthridine alkaloid extracted from the Chinese medicinal herb Zanthoxylum nitidum, inhibits the phosphorylation of PI3K and Akt, thus increasing caspase-3/GSDME-mediated pyroptosis in lung cancer cells, indicating that NC is a prospective therapeutic agent for the treatment of lung cancer.359 Curaxin CBL0137, designed to modulate p53 and nuclear factor-κB, has demonstrated the capacity to deactivate the chromatin remodeling complex. This deactivation facilitates chromatin transcription, thereby reducing the transcription of antioxidant genes and promoting oxidative stress, leading to elevated ROS levels. Subsequently, mitochondrial ROS recruit BAX to the mitochondrial membrane, releasing cytochrome c and subsequently activating caspase-3. This cascade prompts caspase-3/GSDME-mediated pyroptosis in ovarian cancer cells.360 Germacrone, a sesquiterpene component obtained from the essential oil of Ezhu, demonstrated anticancer properties by inducing pyroptosis in liver cancer. This effect is mediated through the proteolytic cleavage of caspase 3, accompanied by the cleavage of GSDME, and is notably associated with elevated ROS production.361 Besides, cordyceps militaris extract can also induce caspase-3/PARP/GSDME pyroptosis.362 Although these molecules have not been evaluated in clinical trials, their pyroptosis-targeting effects can harbor great potential for disease treatment.
Xie et al. developed inhaled poly(lactic-co-glycolic acid) (PLGA) porous microspheres loaded with doxorubicin (DOX) and decitabine (DAC), which resulted in elevated expression of cleaved caspase-3 and promotion of cell pyroptosis.363 Additionally, two self-assembling protein nanotoxins, T22-DITOX-H6 and T22-PE24-H6, were designed to target chemokine receptor 4 (CXCR4) in head and neck squamous cell carcinoma (HNSCC) cells and to promote caspase-3/GSDME-mediated pyroptosis.364 Hu et al. reported a delivery strategy using arsenic trioxide nanoparticles (As2O3-NPs) for HCC treatment. In this approach, caspase-3 is activated to cleave GSDME and release its free N-terminal domain, triggering pyroptosis. Compared with free As2O3 and the control, As2O3-NPs showed better inhibition and induced more pronounced pyroptosis, increasing GSDME-N expression in Huh7 cells.365 These studies show that treatment in the form of nanoparticles may provide an ideal effect; however, more evidence and clinical trials are needed before they can be clinically applied because of potential side effects induced by the components and concerns of biocompatibility.
Targeting caspase-1/GSDMD-induced pyroptosis requires further extensive research. Simvastatin, traditionally used to manage hyperlipidemia, has garnered attention for its novel anticancer properties. Recent studies have revealed its ability to generate intracellular ROS in colon cancer cells, subsequently activating caspase-1 and initiating caspase-1-dependent pyroptosis.366 However, the ROS-generating effect of simvastatin was evaluated in 2016, with the results showing nonsignificant differences with the placebo group.367 Therefore, further evidence is required for the treatment outcome of colon cancer in vivo. Likewise, Chen et al. reported a similar phenomenon for secoisolariciresinol diglucoside in colorectal cancer cells and saikosaponin D in lung cancer cells.368,369 Additionally, a combination of drugs may enhance the ability to target caspase-1. For example, combined with ruthenium (II) polypyridyl complex Δ-Ru1, taxol improved caspase-1/GSDMD induced pyroptosis in Taxol-resistant cancer cells.370
Furthermore, other caspases, such as caspase-8 and caspase-9, have been explored for their interactions with pyroptosis in tumor cells, illustrating a diverse range of potential therapeutic approaches.349,371,372,373 Moreover, a mechanism of sorafenib therapy against HCC tumors through inducing macrophage (MΦ) pyroptosis and triggering an NK-cell response was explored. Pyroptosis in MΦ via upregulating caspase-1 activity caused the release of proinflammatory cytokines, enhancing the proliferation and activation of NK cells. Subsequently, tumor cells undergo apoptosis due to NK cell cytotoxicity, degranulation, and release of perforin.374 Notably, a phase III clinical trial of sorafenib in the treatment for HCC produced outcomes of unsatisfactory response and survival benefit, which was less significant than that of tislelizumab. However, the potential of combinations with other components is receiving extensive attention.375,376,377
These developments highlight the potential of targeting caspases and other related pathways as effective strategies for promoting pyroptosis in tumor therapy.
Therapeutic strategies targeting GSDM
Gasdermin is a potential target for anti-tumor therapeutic strategies because it is a critical effector of the pyroptosis axis. Endosomal sorting complexes necessary for transport (ESCRT) III-dependent cell membrane repair have been shown to effectively reduce pyroptosis in tumor cells by repairing and removing GSDM pores. Zhao et al. used a biodegradable, sustained-release calcium chelator based on nanoparticles to design a hydrogel-based delivery system aimed at blocking ESCRT III-dependent membrane repair, with the potential for improved immunotherapy efficacy.378 Another approach involves constructing a recombinant adeno-associated virus (rAAV) system to produce and deliver GSDMNT into tumor cells. Utilizing the Sf9/rBac system, a mammal-specific promoter for packaging rAAV-GSDMDNT, and employing the Cre/lox system to recover and express double-floxed inverted GSDMNT, the system stimulated immune responses by triggering pyroptosis and temporarily opening the blood-brain barrier (BBB).379 These works showed that innovative delivery approaches may improve targeting efficacy and safety.
In addition, the PD-L1/PD-1 immune checkpoint has been found to engage the pyroptosis pathway. Under specific circumstances, PD-L1 translocates to the nucleus, where it enhances GSDMC transcription. Subsequent treatment with TNF-α results in caspase-8-mediated cleavage of GSDMC, leading to pyroptosis and tumor necrosis in breast cancer.36 Furthermore, tumor cell ablation of mixed-lineage leukemia 4 (MLL4) was found to activate the transcriptional reactivation of GSDMD-dependent pyroptosis through enhancer decommissioning and oncolytic parapoxvirus ovis (ORFV) and was shown to pre-stabilize GSDME by mitigating its ubiquitination and subsequently inducing tumor cell pyroptosis.330,380 Additionally, USP48 stabilizes GSDME by removing K48-linked ubiquitination at K120 and K189, thereby promoting pyroptotic death in cancer cells.150
These innovative and diverse approaches demonstrate the potential of targeting GSDM and the pyroptotic pathway in anti-tumor therapeutic strategies, offering promising avenues for further exploration and development, as summarized in Fig. 5 and Table 2.
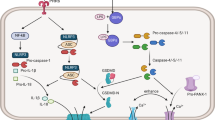
Potential cancer treatment strategies that target pyroptosis. Tumor cells undergoing pyroptosis release immunogenic substances and pro-inflammatory cytokines that promote immune cell activation and recruitment. This may engage in a positive feedback loop that augments tumor-specific immunity and further increases tumor-specific immunity. Thus, recent research has examined the therapeutic viability and potential of manipulating pyroptosis as an anticancer treatment. Key components in the process, such as NLRP3, AIM2, caspase-1, caspase-3, and gasdermins, have been developed into several specific therapeutic agents for malignancies. The figure was created by Figdraw
Neurological disease
Alzheimer’s disease
AD is distinguished by its neuropathological features, such as amyloid-β plaques outside cells, neurofibrillary tangles inside cells, neuroinflammation, and loss of neurons.165 Targeted suppression of inflammasome-dependent pyroptosis alleviates AD-related symptoms. Sodium houttuyfonate (SH) suppressed the expression of NLRP3 and cleavage of GSDMD, ameliorated pyroptosis in hippocampal neurons, and mitigated deficits in spatial learning and memory in mice with AD induced by Aβ1-42.381 L7 (derivative of N-salicyloyl tryptamine) counteracts pyroptosis in BV2 cells triggered by Aβ through the inhibition of the NLRP3-caspase-1 signaling cascade, thereby offering neuroprotection through reduced GSDMD expression.382 1,7-diphenyl-4-hepten-3-one (C1), a natural diarylheptanoid, was found to alleviate AD-like pathology by suppressing pyroptosis via activation of the Nrf2 pathway, thus downregulating the expression of NLRP3, GSDMD, and caspase-1.383 Telmisartan, a widely used anti-hypertensive drug approved by the FDA, can further ameliorate inflammatory effects via the inhibition of the microglial PPARγ/NLRP3 pathway.384 Additionally, artificial silencing of the INPP5D gene could promote NLRP3 production in microglia, providing therapeutic potential for AD treatment.385 The selective blockade of NLRP1, caspase-1, and caspase-6 ameliorates neuroinflammation and cognitive deficits in transgenic mice with AD.386 Chinese traditional medicine acupuncture also exhibits similar treatment efficiency. The Bushen Huoxue acupuncture technique diminishes Aβ generation in the hippocampal tissue of SAMP8 mice, suppresses NLRP1 inflammasome activation-medicated pyroptosis, and ultimately enhances cognitive function in mice with AD.387 Specific blockades of caspase-GSDM-mediated pyroptosis have demonstrated noteworthy neuroprotective effects in animal models of AD. In experiments involving APP/PS1 mice, mafenide (MAF) derivatives restrained GSDMD activation-induced pyroptosis and neuroinflammation by impeding cleavage at the GSDMD-Asp275 site.388 Another study showed that inhibition of inflammasome activation by MCC950, an NLRP3 inhibitor, improved cognitive function in APP/PS1 mice.170
Preserving BBB integrity through suppression of pyroptosis diminishes Aβ aggregation. Research has indicated that inflammatory mediators released from pyroptotic neurons during cerebrovascular disease significantly undermine BBB integrity.389 Traumatic brain injury (TBI) triggers pyroptosis in impaired brain microvascular endothelial cells (BMVECs) via NLRP3 inflammasome activation. The caspase-1 inhibitor AcYVAD-CMK can impede NLRP3 inflammasome activation by preventing GSDMD cleavage and ASC oligomerization, thus preserving the integrity of the BBB.330
Parkinson’s disease
PD symptoms, similar to those of AD, can be alleviated by targeting the suppression of inflammasome-dependent pyroptosis. A small molecular NLRP3 inhibitor, MCC950, inhibited α-synuclein-mediated inflammasome activation, attenuating nigrostriatal dopaminergic degeneration and motor deficits.176 Salidroside (Sal) safeguards dopaminergic neurons by attenuating NLRP3-dependent pyroptosis via (1) indirectly diminishing the synthesis of NLRP3, pro-IL-1β, and pro-IL-18 through the inhibition of the TLR4/MyD88/NF-κB signaling cascade, and (2) directly inhibiting pyroptosis by targeting the TXNIP/NLRP3/caspase-1 signaling pathway.390 Additionally, synchronized upregulation of pyroptosis protein expression and Parkinson’s-like symptoms in 6-hydroxydopamine-induced PD rat models can be inhibited by kaemperfol via the p38MAPK/NF-κB signaling pathway.391 Experimental data from both in vitro and in vivo studies have revealed that the Prussian blue nanozyme (PBzyme) mitigates the activation of microglial NLRP3 inflammasomes and caspase-1 by neutralizing ROS. This action results in reduced cleavage of GSDMD and decreased production of inflammatory mediators, culminating in the suppression of microglial pyroptosis.392 In both in vivo and in vitro PD models, β-hydroxybutyrate (BHB) supplementation suppresses pyroptosis by attenuating the activation of the NLRP3 inflammasome via downregulation of STAT3-mediated signaling.393 Traditional Chinese drugs also exhibited the ability to regulate pyroptosis. Qiji Shujiang granule (QJG) mediates the reduction of pyroptosis by inhibiting the NLRP3/caspase-1 axis, thereby conferring a neuroprotective benefit.394
Targeting the upstream molecules of the pyroptosis pathway is another option. TLR4 has been shown to sense LPS and induce pyroptosis through a signaling cascade.395,396 TLR4 cell-surface expression, LPS sensing, dimerization, and signaling depend on TLR4 binding to MD-2.397 Disulfiram (DSF) was found to modify Cys133 of MD-2 and inhibit TLR4 sensing, suppressing neuroinflammation and dopaminergic neuron loss.398
Stroke
Stroke remains the predominant cause of mortality and persistent impairment globally, with ischemic stroke accounting for approximately 85% of total occurrences.399 Targeting the NLRP3 inflammasome to inhibit pyroptosis has been the dominant strategy for alleviating stroke symptoms in the past few years. Pinocembrin, a natural product, was discovered to inhibit the activation of the TLR4/NF-κB pathway, suppressing the downstream assembly of the NLRP3 inflammasome and ameliorating vascular lesions.400 RRx-001 shows promise as a specific inhibitor of NLRP3, primarily exerting its effects by binding to cysteine 409 of NLRP3 to disrupt the NLRP3-NEK7 interaction, which is crucial for NLRP3 activation. RRx-001, currently undergoing phase III clinical trials, has a reasonably good safety profile and is a promising therapeutic candidate for stroke.401 Meanwhile, inhibition of Janus kinase can ameliorate ischemic stroke injury and neuroinflammation through reducing NLRP3 inflammasome activation via JAK2/STAT3 pathway inhibition.402 More drugs, such as lonidamine, edaravone dexborneol, thiolutin, and β-1, 3-galactosyltransferase-2, may inhibit NLRP3 assembly. However, few of these drug candidates could be translated into clinical research.403,404,405,406
Multiple sclerosis
The NLRP3 inhibitors JC-171, OLT1177, and MCC950 can efficiently ameliorate EAE pathogenesis, improve deficient symptoms, and prevent cognitive deficits in patients with MS.407,408,409 Pyroptosis was inhibited by the caspase-1 inhibitor, VX-765, in an EAE model. VX-765 treatment improved neurobehavioral performance and reduced pyroptosis-related protein expression and axonal injury.180,409
Respiratory diseases
Asthma
Yanghe Pingchuan has been used to treat asthma for many years in China. While the detailed mechanism remains unknown, Yanghe Pingchuan was discovered to inhibit airway smooth muscle cell pyroptosis in asthma by suppressing the TLR4/NF-κB/NLRP3 signaling pathway.410 JT002 treats asthma by inhibiting NLRP3 assembly, thus alleviating airway hyperresponsiveness and neutrophilia.411 Likewise, heme oxygenase-1 (HO-1), an enzyme inducible for degrading heme, was shown to inhibit GSDMD-mediated pyroptosis and release of cytokine TSLP in airway epithelial cells by interacting with the RHD domain of NF-κB p65 and modulating NF-κB-dependent pyroptosis.412 Furthermore, additional NF-κB/NRLP3-mediated asthma treatment candidates include Schisandrin B and Protopine.413,414
Chronic obstructive pulmonary disease
COPD, a prevalent condition that can be prevented and managed, is characterized by persistent respiratory symptoms and restricted airflow resulting from airway and alveolar anomalies. Several pyroptosis inhibitors have been explored to treat COPD, with most targeting the NLRP3 inflammasome. Schisandrin A, a lignan of the diphenyl cyclooctadiene class extracted from Schisandra chinensis fruits, possesses a range of pharmacological activities; it mitigates ROS generation and NLRP3 inflammasome activation through the upregulation of Nrf-2, thus exerting anti-inflammatory effects and diminishing pulmonary damage in mouse models of COPD.415 Tian et al. discovered that (-)-epicatechin, a flavonoid compound, suppresses NLRP3 inflammasome activation by enhancing Nrf2 activity and alleviating lung inflammation triggered by cigarette smoke, which was validated through the reduced secretion of IL-1β and IL-18 in a rat model of COPD.416 Similarly, propofol, an anesthetic agent, increases Nrf2 expression and inhibits NLRP3 expression.417
In addition, physical or support methods can alleviate pyroptosis in COPD. For instance, halotherapy was found to alleviate oxidative stress in the lung tissues of COPD rats, diminish the accumulation of CD4+ and CD8+ T cells in the lungs, and reduce the production of inflammatory factors in the serum by suppressing the TLR4/NF-κB/GSDMD and NLRP3/ASC/caspase-1 pathways.418 The success of halotherapy in alleviating pyroptosis in patients with COPD provides incentives for the more detailed exploration of physical treatments in respiratory diseases.
Acute lung injury
Several molecules have been found to improve ALI symptoms by regulating pyroptosis, most of which target the upstream molecules of the NLRP3 inflammasome. For example, buformin, a hypoglycemic agent, can drive phosphorylation of AMPK, inhibit downstream NLRP3 inflammasome production, and accelerate autophagy, which, in turn, promotes NLRP3 inflammasome degradation, inducing a therapeutic effect after ALI.190 Emodin, an anthraquinone compound derived from rhubarb, and Polygonum cuspidatum treat AP-associated ALI by regulating macrophages and neutrophils via the targeting of NLRP3 production. Emodin mitigates the pyroptotic process of alveolar macrophages by decreasing the level of inflammatory cytokines and lactate dehydrogenase via NLRP3 inhibition and cold-inducible RNA-binding protein-activated NLRP3/IL-1β/CXCL1 signaling to dampen neutrophil infiltration.419,420 Furthermore, dehydroandrographolide, sourced from the traditional Chinese herb Andrographis paniculata, mitigates NLRP3-mediated pyroptosis in acute lung injury models by causing ROS-induced mitochondrial damage, which is achieved through the suppression of the Akt/Nrf2 signaling pathway, mediated by PDPK1 ubiquitination.421 Similarly, chicoric acid effectively inhibited pyroptosis by inducing mitochondrial damage via ROS generation. This effect is mediated by the activation of the Akt/Nrf2 pathway via PDPK1 ubiquitination.422 Other potential drugs regulate pyroptosis by targeting distinct pathways. Tangeretin mitigates ALI induced by sepsis by suppressing ROS-mediated activation of the NLRP3 inflammasome by modulating the PLK1/AMPK/DRP1 signaling axis.423 EuHD1 hinders the formation and activation of the NLRP3 inflammasome by suppressing ROS production and ASC oligomerization.424 Ren et al. revealed that the anti-pyroptotic effect of ergolide is conferred through direct targeting of the NACHT domain of NLRP3.425 Finally, britannin is an effective and natural NLRP3 inhibitor.426 The diversity of upstream molecules of NLRP3 offers various choices, but the interactions between these molecules and which one possesses the dominant role remain to be determined.
Other targets of pyroptosis have the potential to treat ALI. Zhang et al. found that Xuebijing, a traditional Chinese medicine recognized for its potent anti-inflammatory properties, inhibited gasdermin-E-mediated pyroptosis of lung cells by suppressing TNF-α production.427 Moreover, targeting pyroptosis has therapeutic implications for other respiratory diseases. For example, targeting caspase-1 by tetracycline blocks pyroptotic cell death in macrophages exposed to silica particles during silicosis.428 Colchicine inhibits NLRP3 inflammasome and inflammation to ameliorate COVID-19 pneumonia which are verified in clinical trials.429
In summary, targeting the NLRP3 inflammasome is a viable strategy for inhibiting pyroptosis and treating respiratory diseases, whereas other targets, such as caspase-1, also exhibit therapeutic potential.
Kidney diseases
Acute kidney injury
Similarly, the NLRP3 inflammasome serves as a potential target for AKI treatment. The carboxy-terminus of Hsc70-interacting protein (CHIP), a U-box E3 ligase, is known to modulate oxidative stress by degrading its target proteins and has been found to engage with NLRP3, ubiquitinating it to facilitate its degradation via the proteasome, thereby suppressing pyroptosis mediated by the NLRP3/ASC inflammasome.430 The Klotho protein, which is beneficial in AKI, including for anti-senescence, anti-oxidation, anti-inflammation, and anti-fibrosis, inhibited the NLRP3 inflammasome by promoting cell autophagy, improving AKI.431 miR-30c-5p can suppress the expression of NLRP3, ASC, and caspase-1 by directly interacting and inhibiting TXNIP.432 Additionally, circ DENND4C, secreted by urine-derived stem cells in the form of exosomes, interacts with the miR 138-5p/FOXO3a axis, inhibiting the NLRP3 inflammasome.433 In addition to the NLRP3 inflammasome, caspase-1 is another candidate target for pyroptosis inhibition. Carnosine, a dipeptide known for its antioxidant and anti-inflammatory effects, reduces damage in kidney tubular epithelial cells by targeting caspase-1 and suppressing caspase-1/GSDMD-driven pyroptosis.434 Although these targets are embedded upstream of the pyroptosis pathway, GSDMD, an executor of pyroptosis, exhibits therapeutic potential. Recent studies have highlighted that dual-specificity phosphatase 2 (DUSP2) is a pivotal modulator of cell death and inflammation in several diseases. Functioning as a nuclear phosphatase, DUSP2 deactivates STAT1, a transcriptional suppressor of GSDMD, thereby limiting GSDMD-mediated pyroptosis in renal tubular epithelial cells.435
Diabetic kidney disease
Three PubMed-indexed studies have targeted pyroptosis for DKD treatment, and all targeted NLRP3-mediated inflammation. Loganin mitigates pyroptosis in HK-2 cells triggered by high glucose levels by suppressing ROS generation and NLRP3 inflammasome activation, resolving renal pathologies in DKD mice, similar to ManNAc in podocytes.436,437 Likewise, the anti-pyroptotic effect of the Tangshen formula acts via the TXNIP-NLRP3-GSDMD axis.438
Lupus nephritis
Honokiol, derived from the bark of Magnolia officinalis, is a versatile lignan with diverse pharmacological effects, including anti-inflammatory, antioxidant, and anti-tumor properties, with minimal side effects.439 Ma et al. unveiled that honokiol can suppress the renal activation of the NLRP3 inflammasome in macrophages, inhibiting the release of IL-33 and IL-1β and avoiding renal tubular epithelial cell death during lupus nephritis.440 Additionally, quercetin, a bioactive compound naturally occurring in various plants, has been identified for its role in improving pyroptosis mediated by inflammasomes and GSDMD. This effect is primarily attributed to the regulation of the IL-33/ST2 pathway and potentially involves the IL-33/TLR4 pathway in renal tubular epithelial cells.441
Cardiovascular diseases
Atherosclerosis
Pyroptosis has been a treatment target for atherosclerosis, as exemplified by the candidate molecule salvianolic acid A in diabetic atherosclerosis.442 Typically, apigenin, lncRNA H19, organogermanium compound 3-(trihydroxygermyl) propanoic acid (THGP), and Z-LLSD-FMK or Z-YVAD-FMK improve atherosclerosis by targeting NF-κB, caspase-1, caspase-1, and GSDMD, respectively.443,444,445,446 However, some molecules regulate pyroptosis through distinct targets. Melatonin, a neuroendocrine hormone produced in the pineal gland and other organs, has been shown to suppress macrophage pyroptosis in atherosclerosis by downregulating the SIRT3/FOXO3α/ROS pathway, consequently diminishing caspase-1 dependent pyroptosis.262 Estrogen was reported to recognize estrogen receptor α and promote autophagy of endothelial cells, thereby downregulating the expression of NLRP3, cleaved caspase 1, and GSDMD.265 Likewise, Liu et al. revealed that blocking the p62/Nrf2/ARE signaling pathway using chloroquine through autophagy impairment can promote pyroptosis in macrophages, providing a novel therapeutic target for atherosclerosis treatment.447 Thus, the MALAT1/miR-30c-5p/Cx43 axis is a potential target for atherosclerosis therapy. Yang et al. revealed that the expression of MALAT1 and Cx43 was upregulated, while miR-30c-5p was downregulated in rat aortic endothelial cells during pyroptosis following atherosclerosis. Furthermore, MALAT1/miR-30c-5p/Cx43 comprises a signal cascade that regulates pyroptosis.448
Myocardial infarction
In recent years, numerous drugs and molecules have been explored to treat myocardial injury. Downstream regulation of NLRP3 expression has been the prominent target, by focusing on various upstream molecules. For example, geniposide promotes AMPK phosphorylation, Tanshinone IIA and Qighen granule suppress the TLR4/NF-κB p65 signaling pathway, hydrogen gas inhalation reduces the production of ROS, and chlorogenic acid depresses lncRNA Neat1 expression, ultimately impacting cardiomyocyte pyroptosis.449,450,451,452,453 In clinical trials, coenzyme Q10 is verified to suppress the recruitment of pro-inflammatory CCR2+ macrophages by attenuating the activation of the NLRP3/ IL-1β pathway, ameliorating MI.454 The AIM2 inflammasome also exhibits therapeutic potential in MI. Epigallocatechin-3-gallate, a bioactive polyphenol isolated from green tea, protects cardiomyocytes from pyroptosis via the MEG3/TAF15/AIM2 axis, thereby inhibiting AIM2 expression.455 Moreover, other targets in the canonical pathway, such as GSDMD, have gained attention in MI. Danhong, a traditional Chinese medicine, injection directly blocks GSDMD-N oligomerization and pore formation.101 MicroRNA-182-5p, carried by MSC-derived exosomes, also directly targets GSDMD and suppresses pyroptosis.456 In addition to the direct effects of upstream molecules on the NLRP3 inflammasome, additional targets have been of interest. Magnetic stimulation therapy also contributes to the treatment of MI. Lu et al. found that magnetic vagus nerve stimulation inhibited cardiomyocyte pyroptosis by activating M2AChR to suppress oxoglutarate dehydrogenase-like expression.457 In summary, various targets are involved in the regulation of pyroptosis in MI treatment.
Diabetic cardiomyopathy
Pyroptosis-inhibitory compounds targeting upstream molecules of NLRP3 have been discovered in the context of DCM. For example, the bone morphogenetic protein-7 cascade represses the expression of Nek7, GBP5, and TLR4, eventually inhibiting NLRP3.458 Metformin can activate the AMPK/mTOR pathway, thereby improving cell autophagy and disrupting NLRP3-mediated pyroptosis.459 Similarly, berberine suppresses NLRP3 by disturbing mTOR/mtROS.460 Fufang Zhenzhu Tiaozhi, a Chinese herbal medicine, and pomegranate peel extract exhibit cardioprotective potential through NLRP3 inhibition.461,462 GSDMD is also a target for DCM treatment. Lu et al. discovered that the protective role of irisin and mitochondrial ubiquitin ligase (MITOL/MARCH 5) in DCM was partially offset by the activation of cGAS/STING signaling, inhibiting GSDMD-mediated pyroptosis.463 Targeting Nrf2 has a pivotal role in inhibiting pyroptosis. For instance, curcumin can reduce the accumulation of superoxide in the myocardium through AKT/Nrf2/ARE pathway activation and inhibit pyroptosis, promoting nuclear translocation of Nrf2, increasing expression of antioxidant factors in cells and inhibiting the progression of cell pyroptosis.464,465 Similarly, puerarin and quercetin can inhibit pyroptosis by regulating P2X7 receptor expression. However, these two compounds exhibit opposite effects on the expression of the P2X7 receptor, leaving the mechanism of pyroptosis inhibition to be elucidated.465,466
Digestive diseases
Inflammatory bowel disease
Xu et al. proposed a novel approach using self-adaptive pyroptosis-responsive liposomes to treat autoimmune inflammatory diseases, including inflammatory bowel disease. Following pyroptosis, the activated GSDME-N bound to the cardiolipin on the liposome surface, forming pores releasing encapsulated dimethyl fumarate and inhibiting the caspase 3/GSDME pathway.467 A PLGA-microsphere-carried circGMCL1 was designed to protects against Crohn’s colitis through suppressing NLRP3 inflammasome-dependent pyroptosis via regulation of miR-124-3p/ANXA7-induced autophagy.468 Human umbilical cord mesenchymal stem cell-derived exosomes were found to protect against colitis via the regulation of macrophage pyroptosis in a miR-378a-5p-denpendent manner.469 Furthermore, β-sitosterol, Tou Nong powder, necrosulfonamide, Munronoid I, and ginsenoside Rg3 have been associated with inflammatory bowel disease treatment.470,471,472,473,474
Acute pancreatitis
The natural compound wedelolactone has been reported to impede AP progression and associated lung injury through the canonical caspase-1-mediated pyroptotic pathway and caspase-11-mediated non-canonical pyroptotic pathway.475 Li et al. demonstrated that hair follicle-derived mesenchymal stem cell-derived small extracellular vesicles alleviated AP by suppressing inflammation and pyroptosis in pancreatic acinar cells. Furthermore, compared to the effect of small extracellular vesicles administered intraperitoneally, the therapeutic effect of small extracellular vesicles following intravenous injection seems to be enhanced.476 Qingjie Huagong decoction, a formula consisting of seven traditional medicines, has been reported to exert its anti-AP effects by regulating the circHipk3/miR-193a-5p/ NLRP3 pathway.477 Other therapeutic strategies have also emerged to target pyroptosis in the pathology of AP, including baicalein, disulfiram, sinapic acid, and salidrosidet.478,479,480,481
Liver fibrosis
Forsythiaside A (FA), a natural bioactive ingredient derived from traditional Chinese medicine, was loaded into a CD44-targeting exosome nanocarrier and mitigated liver fibrosis. The anti-liver fibrosis mechanism could be attributed to the suppression of NLRP3-mediated pyroptosis.482 Stem cells from human exfoliated deciduous teeth attenuated liver cirrhosis by suppressing the GSDMD-mediated pyroptosis pathway via decreasing ROS in hepatocytes.483 Nicotinic acid (NA), a vitamin used to treat dyslipidemia, was found to inhibit the NF/κB/NLRP3 signaling axis, thereby preventing pyroptosis and liver fibrosis during non-alcoholic steatohepatitis progression.484 Furthermore, researchers have identified various pioneering compounds that target pyroptosis for treating liver fibrosis, such as JT001, trilobatin, ursolic acid, and auranofin.485,486,487,488 Additionally, the clinical efficacy of some drugs was tested. A phase I clinical trial verified that emricasan inhibits excessive caspase activation and lowers ALT in patients with non‐alcoholic fatty liver disease, which may ameliorate the progression from fatty liver to liver fibrosis.489 These molecules and targets’ potential for non-cancer disease treatment are summarized and visualized in Fig. 6.
Potential targeted therapeutic strategies for pyroptosis in non-cancer diseases. Investigations into pyroptosis enhance our understanding of the pathological mechanisms underlying various diseases. Key elements involved in pyroptosis, including NLRP3, caspase-1, gasdermin D, and upstream molecules such as TLR4/NF-κB axis, are crucial for human health and have led to the development of numerous targeted therapies for several inflammatory conditions. The figure was created by Figdraw
Conclusion and perspective
Pyroptosis is a distinct form of cell death characterized by inflammasome formation, caspase-mediated gasdermin cleavage, and inflammatory cytokine release. As an RCD form, the ubiquity of pyroptosis in cells indicates its great promise and remarkable drug market potential of pyroptosis-targeted drugs. For example, in cancer cells, pyroptosis can induce regional inflammation and convert “cold tumors” to “hot tumors,” triggering enhanced antitumor immunity and sensitizing checkpoint blockade immunotherapy.490 Pyroptosis-targeted therapy can assist in antitumor immunotherapy by inducing tumor cell pyroptosis, similar to chemotherapy but with fewer side effects. Pyroptosis-targeted drugs are promising therapeutic methods for tumors in addition to classical chemo-, radio, and immunotherapies. In addition, the intraepithelial mast cell-driven gasdermin C-mediated type 2 immunity has also been identified recently, which can be exploited as a potential drug target for allergic diseases.491 Besides, studies focusing on pyroptosis have made significant progress in recent decades at multiple levels, including elucidation of signaling mechanisms, disease regulation, and molecular structures. NLRP3/caspase-1/GSDMD and caspase-3/GSDME are two main druggable pathways for pyroptosis-related diseases. Previous research widely reported that the therapeutic effect of some classical drugs and traditional Chinese medicines is partly mediated through pyroptosis pathways. For instance, the IL-1R inhibitor, Anakinra, can relieve liver inflammation by targeting NLRP3 inflammasome.492 Atorvastatin inhibits the expression of NLRP3 to prohibit the progression of atherosclerosis. As for targeting caspase, numerous natural substances such as dendrobine can inhibit the expression or maturation of caspase-1 to achieve therapeutic outcome.493 Most chemotherapy drugs such as doxorubicin (Dox) and cisplatin induce cancer pyroptosis by activating caspase-3. Furthermore, disulfiram, a drug for alcoholism treatment, can modify the Cys191 amino acid of GSDMD to inhibit its oligomerization, which is promising for inflammatory disease therapy.396 Therefore, large amounts of approved drugs possess the potential to regulate pyroptosis for therapy. Although drug innovation targeting pyroptosis pathways is hard because of the interlaced signal pathway and complicated molecular structures such as NLRP3 inflammasome, increasing small molecule drugs have been reported recently. For example, while UK5099 can selectively inhibit NLRP3 inflammasome, necrosulfonamide, a chemical inhibitor, can selectively bind to GSDMD inhibiting its oligomerization to serve as a potential drug for inflammatory diseases.494,495 Molecular structures identified by cryo-electron microscopy or artificial intelligence such as Alphafold 3 provide increasing structures for scientists to identify novel drug targets by molecular docking, experiment verification, and further clinical trials.496 However, several challenges remain regarding the research and development of pyroptosis drugs with potential therapeutic strategies.
Disease heterogeneity presents a formidable challenge for pyroptosis drugs. For example, tumor heterogeneity exists not only between but also within different malignancies. Variations in the expression and function of pyroptosis-related molecules among different tumors may affect the responsiveness of tumors to pyroptosis-inducing therapies. Crosstalk between pyroptosis and other signaling pathways also complicates the cytoplasmic microenvironment. Given the heterogeneous intracellular and tumor microenvironments, pyroptosis-based therapies are challenging. Identifying a suitable patient population for pyroptosis therapy is crucial for future clinical trials. Inspired by targeted therapy in patients with cancer based on molecular classification, data-driven patient selection is promising for improving the therapeutic efficacy of pyroptosis-based therapies. Integrating omics and clinical information may also help predict the therapeutic outcomes of pyroptosis drugs in patients. For example, the expression level of GSDME in cancer cells may predict the therapeutic response of GSDME-targeted therapy. Molecular classification for selecting suitable patients to receive pyroptosis-targeted therapy is the guarantee of a favorable outcome.
Second, effective and selective drugs that can safely induce or inhibit pyroptosis remain far from satisfactory. Research on pyroptosis-based drugs remains at the laboratory stage, with limited candidates entering the preclinical and clinical trial phases. These clinical trials are summarized in Table 3 among which butyrate and inulin, zinc supplementation, and dapansutrile ameliorate type 2 diabetes, Bechet’s disease, and gout by regulating pyroptosis, respectively.497,498,499 However, the safety concerns associated with the induction or inhibition of pyroptosis are paramount. Extensive inhibition of pyroptosis inevitably increases the probability of pathogen invasion which may bring unforeseen risks for patients. Off-target effects also deserve wide attention. For example, previous research demonstrated that GSDME activation is reliant on caspase-3 and that the expression level of GSDME is closely linked to the type of cell death induced by chemotherapeutic agents. Cells with high GSDME expression underwent pyroptosis in response to chemotherapy, whereas cells with low or absent GSDME expression experienced apoptosis. However, researchers also noted that GSDME expression is typically low in most tumor cell lines because of methylation of the GSDME gene promoter, while GSDME is broadly overexpressed in normal cell lines.23,103 Therefore, chemotherapy might also trigger caspase-3-mediated pyroptosis in normal cells with high GSDME expression, potentially contributing to the toxicity and side effects observed during chemotherapy. For instance, Zheng et al. demonstrated that the activation of the Bnip3-caspase-3-GSDME pathway following Dox treatment initiated GSDME-mediated pyroptosis, contributing to Dox-induced cardiotoxicity in vivo.296 Dox treatment results in the hyperactivation of the NLRP3 inflammasome and pyroptotic cell death in cardiomyocytes, which is a key mechanism underlying dilated cardiomyopathy in Dox-treated heart tissues. The absence of either NLRP3 or caspase-1 protects mice from Dox-induced dilated cardiomyopathy.290 The specificity of pyroptosis-targeted agents has emerged as a critical issue, emphasizing the need to minimize unintended consequences in healthy cells. Selecting the appropriate drug target, integrating nano-delivery and protein-degradation technologies, and combining other existing therapy strategies are viable pathways to improving therapeutic efficiency while mitigating the risk of toxicity. The identity of molecules’ structure helps to design and deliver drugs. The structure and related mechanism of NLRP1, NLRP3, GSDMB, and GSDMD have been analyzed by cryo-electron microscopy.29,500,501,502 Specific cell-targeted adeno-associated virus systems also possess the potential to precisely regulate pyroptosis. Furthermore, as immune cells can distinguish cancer cells from normal cells, pyroptosis-mediated immune mobilization by engineered immune cells such as CAR-T can selectively alter the tumor microenvironment, which can greatly reduce side effects.
Finally, robust preclinical and clinical trials are warranted to determine the clinical applications of pyroptosis-based drugs. In addition to classical cell lines and primary cells from patients, organoids are novel approaches to evaluate drug effectiveness and safety before entering clinical trials. Organoids derived from adult and pluripotent stem cells serve as crucial preclinical models for cancer research and therapy development.503 For instance, Zhou et al. found that NLRP3 inflammasome facilitates silica-induced injury to lung epithelial cells and abnormal regeneration in lung stem/progenitor cell-derived organotypic models.504 In addition to in vitro studies, multi-species in vivo animal model verification is the cornerstone of further clinical trials. For example, MCC950, an NLRP3 inhibitor, is discovered to function in neurodegenerative diseases and myocardial infarction (MI) in mice models.505 Meanwhile, NLRP3-inflammasome inhibition reduces infarct size and preserves cardiac function in a pig MI model.506 Finally, unintended consequences, long-term effects, and a balance between therapeutic benefits and potential risks are issues that need to be addressed in further clinical trials. Therefore, a complete verification chain of in vitro-in vivo-clinical trials is the basis for the clinical translation of pyroptosis-targeted drugs.
In conclusion, scientific progress has pushed us into an exciting era in the field of pyroptosis research. Addressing the previously mentioned challenges is imperative to realizing the full potential of pyroptosis in clinical practice. We expect that the successful translation of pyroptosis-targeted therapy into clinical treatment will soon be a reality.
References
Hotchkiss, R. S., Strasser, A., McDunn, J. E. & Swanson, P. E. Cell death. N. Engl. J. Med. 361, 1570–1583 (2009).
Galluzzi, L. et al. Molecular mechanisms of cell death: recommendations of the Nomenclature Committee on Cell Death 2018. Cell Death Differ. 25, 486–541 (2018).
Querfurth, H. W. & LaFerla, F. M. Alzheimer’s disease. N. Engl. J. Med. 362, 329–344 (2010).
Green, D. R. The Coming Decade of Cell Death Research: Five Riddles. Cell 177, 1094–1107 (2019).
Wei, X. et al. Role of pyroptosis in inflammation and cancer. Cell Mol. Immunol. 19, 971–992 (2022).
Black, R. A., Kronheim, S. R. & Sleath, P. R. Activation of interleukin-1 beta by a co-induced protease. FEBS Lett. 247, 386–390 (1989).
Kostura, M. J. et al. Identification of a monocyte specific pre-interleukin 1 beta convertase activity. Proc. Natl Acad. Sci. USA 86, 5227–5231 (1989).
Yuan, J. et al. The C. elegans cell death gene ced-3 encodes a protein similar to mammalian interleukin-1 beta-converting enzyme. Cell 75, 641–652 (1993).
Alnemri, E. S. et al. Human ICE/CED-3 protease nomenclature. Cell 87, 171 (1996).
Zychlinsky, A., Prevost, M. C. & Sansonetti, P. J. Shigella flexneri induces apoptosis in infected macrophages. Nature 358, 167–169 (1992).
Hersh, D. et al. The Salmonella invasin SipB induces macrophage apoptosis by binding to caspase-1. Proc. Natl Acad. Sci. USA 96, 2396–2401 (1999).
Brennan, M. A. & Cookson, B. T. Salmonella induces macrophage death by caspase-1-dependent necrosis. Mol. Microbiol. 38, 31–40 (2000).
Boise, L. H. & Collins, C. M. Salmonella-induced cell death: apoptosis, necrosis or programmed cell death? Trends Microbiol. 9, 64–67 (2001).
Man, S. M. & Kanneganti, T. D. Converging roles of caspases in inflammasome activation, cell death and innate immunity. Nat. Rev. Immunol. 16, 7–21 (2016).
Martinon, F., Burns, K. & Tschopp, J. The inflammasome: a molecular platform triggering activation of inflammatory caspases and processing of proIL-beta. Mol. Cell 10, 417–426 (2002).
Agostini, L. et al. NALP3 forms an IL-1beta-processing inflammasome with increased activity in Muckle-Wells autoinflammatory disorder. Immunity 20, 319–325 (2004).
Kayagaki, N. et al. Non-canonical inflammasome activation targets caspase-11. Nature 479, 117–121 (2011).
Kayagaki, N. et al. Noncanonical inflammasome activation by intracellular LPS independent of TLR4. Science 341, 1246–1249 (2013).
Shi, J. et al. Cleavage of GSDMD by inflammatory caspases determines pyroptotic cell death. Nature 526, 660–665 (2015).
He, W. T. et al. Gasdermin D is an executor of pyroptosis and required for interleukin-1β secretion. Cell Res 25, 1285–1298 (2015).
Kayagaki, N. et al. Caspase-11 cleaves gasdermin D for non-canonical inflammasome signalling. Nature 526, 666–671 (2015).
Ding, J. et al. Pore-forming activity and structural autoinhibition of the gasdermin family. Nature 535, 111–116 (2016).
Wang, Y. et al. Chemotherapy drugs induce pyroptosis through caspase-3 cleavage of a gasdermin. Nature 547, 99–103 (2017).
Orning, P. et al. Pathogen blockade of TAK1 triggers caspase-8-dependent cleavage of gasdermin D and cell death. Science 362, 1064–1069 (2018).
Sarhan, J. et al. Caspase-8 induces cleavage of gasdermin D to elicit pyroptosis during Yersinia infection. Proc. Natl Acad. Sci. USA 115, E10888–e10897 (2018).
Zhang, Z. et al. Gasdermin E suppresses tumour growth by activating anti-tumour immunity. Nature 579, 415–420 (2020).
Zhou, Z. et al. Granzyme A from cytotoxic lymphocytes cleaves GSDMB to trigger pyroptosis in target cells. Science 368, eaaz7548 (2020).
Deng, W. et al. Streptococcal pyrogenic exotoxin B cleaves GSDMA and triggers pyroptosis. Nature 602, 496–502 (2022).
Zhong, X. et al. Structural mechanisms for regulation of GSDMB pore-forming activity. Nature 616, 598–605 (2023).
Strowig, T., Henao-Mejia, J., Elinav, E. & Flavell, R. Inflammasomes in health and disease. Nature 481, 278–286 (2012).
Zhu, C. et al. The gasdermin family: emerging therapeutic targets in diseases. Signal Transduct. Target Ther. 9, 87 (2024).
Li, J. et al. Gsdma3 is required for hair follicle differentiation in mice. Biochem Biophys. Res Commun. 403, 18–23 (2010).
Saeki, N. et al. GASDERMIN, suppressed frequently in gastric cancer, is a target of LMO1 in TGF-beta-dependent apoptotic signalling. Oncogene 26, 6488–6498 (2007).
Saeki, N. et al. Distinctive expression and function of four GSDM family genes (GSDMA-D) in normal and malignant upper gastrointestinal epithelium. Genes Chromosomes Cancer 48, 261–271 (2009).
Chen, Q. et al. GSDMB promotes non-canonical pyroptosis by enhancing caspase-4 activity. J. Mol. Cell Biol. 11, 496–508 (2019).
Hou, J. et al. PD-L1-mediated gasdermin C expression switches apoptosis to pyroptosis in cancer cells and facilitates tumour necrosis. Nat. Cell Biol. 22, 1264–1275 (2020).
Liu, Y. et al. Gasdermin E-mediated target cell pyroptosis by CAR T cells triggers cytokine release syndrome. Sci. Immunol. 5, eaax7969 (2020).
Hergueta-Redondo, M. et al. Gasdermin B expression predicts poor clinical outcome in HER2-positive breast cancer. Oncotarget 7, 56295–56308 (2016).
Orning, P., Lien, E. & Fitzgerald, K. A. Gasdermins and their role in immunity and inflammation. J. Exp. Med. 216, 2453–2465 (2019).
Yu, P. et al. Pyroptosis: mechanisms and diseases. Signal Transduct. Target Ther. 6, 128 (2021).
Vande Walle, L. & Lamkanfi, M. Drugging the NLRP3 inflammasome: from signalling mechanisms to therapeutic targets. Nat. Rev. Drug Discov. 23, 43–66 (2024).
Liu, J. et al. Engineering materials for pyroptosis induction in cancer treatment. Bioact. Mater. 33, 30–45 (2024).
Wang, M. & Fu, Q. Nanomaterials for Disease Treatment by Modulating the Pyroptosis Pathway. Adv. Health. Mater. 13, e2301266 (2024).
Liu, X. et al. Induction of apoptotic program in cell-free extracts: requirement for dATP and cytochrome c. Cell 86, 147–157 (1996).
Fraser, A. & Evan, G. A license to kill. Cell 85, 781–784 (1996).
Bertheloot, D., Latz, E. & Franklin, B. S. Necroptosis, pyroptosis and apoptosis: an intricate game of cell death. Cell Mol. Immunol. 18, 1106–1121 (2021).
Chen, X. et al. Pyroptosis is driven by non-selective gasdermin-D pore and its morphology is different from MLKL channel-mediated necroptosis. Cell Res. 26, 1007–1020 (2016).
Xu, X., Lai, Y. & Hua, Z. C. Apoptosis and apoptotic body: disease message and therapeutic target potentials. Biosci. Rep. 39, BSR20180992 (2019).
Li, J. & Yuan, J. Caspases in apoptosis and beyond. Oncogene 27, 6194–6206 (2008).
Mompeán, M. et al. The Structure of the Necrosome RIPK1-RIPK3 Core, a Human Hetero-Amyloid Signaling Complex. Cell 173, 1244–1253.e1210 (2018).
Sun, L. et al. Mixed lineage kinase domain-like protein mediates necrosis signaling downstream of RIP3 kinase. Cell 148, 213–227 (2012).
Stockwell, B. R. et al. Ferroptosis: A Regulated Cell Death Nexus Linking Metabolism. Redox Biol., Dis. Cell. 171, 273–285 (2017).
Dixon, S. J. et al. Ferroptosis: an iron-dependent form of nonapoptotic cell death. Cell 149, 1060–1072 (2012).
Degterev, A. et al. Chemical inhibitor of nonapoptotic cell death with therapeutic potential for ischemic brain injury. Nat. Chem. Biol. 1, 112–119 (2005).
Temkin, V. et al. Inhibition of ADP/ATP exchange in receptor-interacting protein-mediated necrosis. Mol. Cell Biol. 26, 2215–2225 (2006).
Kaiser, W. J. et al. Toll-like receptor 3-mediated necrosis via TRIF, RIP3, and MLKL. J. Biol. Chem. 288, 31268–31279 (2013).
Frank, D. & Vince, J. E. Pyroptosis versus necroptosis: similarities, differences, and crosstalk. Cell Death Differ. 26, 99–114 (2019).
Sun, Y. et al. The emerging role of ferroptosis in inflammation. Biomed. Pharmacother. 127, 110108 (2020).
Friedmann Angeli, J. P., Krysko, D. V. & Conrad, M. Ferroptosis at the crossroads of cancer-acquired drug resistance and immune evasion. Nat. Rev. Cancer 19, 405–414 (2019).
Chen, X. et al. Ferroptosis: machinery and regulation. Autophagy 17, 2054–2081 (2021).
Tang, D. et al. The molecular machinery of regulated cell death. Cell Res. 29, 347–364 (2019).
Kuang, S. et al. Structure insight of GSDMD reveals the basis of GSDMD autoinhibition in cell pyroptosis. Proc. Natl Acad. Sci. USA 114, 10642–10647 (2017).
Liu, Z. et al. Crystal Structures of the Full-Length Murine and Human Gasdermin D Reveal Mechanisms of Autoinhibition, Lipid Binding, and Oligomerization. Immunity 51, 43–49.e44 (2019).
Rogers, C. et al. Gasdermin pores permeabilize mitochondria to augment caspase-3 activation during apoptosis and inflammasome activation. Nat. Commun. 10, 1689 (2019).
Liu, X. et al. Inflammasome-activated gasdermin D causes pyroptosis by forming membrane pores. Nature 535, 153–158 (2016).
Cookson, B. T. & Brennan, M. A. Pro-inflammatory programmed cell death. Trends Microbiol. 9, 113–114 (2001).
He, Y. & Amer, A. O. Microbial modulation of host apoptosis and pyroptosis. Front Cell Infect. Microbiol. 4, 83 (2014).
Jackson, D. N. & Theiss, A. L. Gut bacteria signaling to mitochondria in intestinal inflammation and cancer. Gut Microbes 11, 285–304 (2020).
Li, P. et al. Mice deficient in IL-1 beta-converting enzyme are defective in production of mature IL-1 beta and resistant to endotoxic shock. Cell 80, 401–411 (1995).
Nunes, T. & de Souza, H. S. Inflammasome in intestinal inflammation and cancer. Mediators Inflamm. 2013, 654963 (2013).
He, Y., Hara, H. & Núñez, G. Mechanism and Regulation of NLRP3 Inflammasome Activation. Trends Biochem Sci. 41, 1012–1021 (2016).
Sha, W. et al. Human NLRP3 inflammasome senses multiple types of bacterial RNAs. Proc. Natl Acad. Sci. USA 111, 16059–16064 (2014).
Dowling, J. K. & O’Neill, L. A. J. Biochemical regulation of the inflammasome. Crit. Rev. Biochem. Mol. Biol. 47, 424–443 (2012).
Liston, A. & Masters, S. L. Homeostasis-altering molecular processes as mechanisms of inflammasome activation. Nat. Rev. Immunol. 17, 208–214 (2017).
Wang, Y. Y., Liu, X. L. & Zhao, R. Induction of Pyroptosis and Its Implications in Cancer Management. Front Oncol. 9, 971 (2019).
Broz, P. et al. Caspase-11 increases susceptibility to Salmonella infection in the absence of caspase-1. Nature 490, 288–291 (2012).
Chen, G. Y. & Nuñez, G. Sterile inflammation: sensing and reacting to damage. Nat. Rev. Immunol. 10, 826–837 (2010).
Lamkanfi, M. & Dixit, V. M. Mechanisms and functions of inflammasomes. Cell 157, 1013–1022 (2014).
Fernandes-Alnemri, T. et al. AIM2 activates the inflammasome and cell death in response to cytoplasmic DNA. Nature 458, 509–513 (2009).
Jorgensen, I., Zhang, Y., Krantz, B. A. & Miao, E. A. Pyroptosis triggers pore-induced intracellular traps (PITs) that capture bacteria and lead to their clearance by efferocytosis. J. Exp. Med. 213, 2113–2128 (2016).
Martinon, F. et al. Gout-associated uric acid crystals activate the NALP3 inflammasome. Nature 440, 237–241 (2006).
Barton, G. M. & Medzhitov, R. Toll-like receptor signaling pathways. Science 300, 1524–1525 (2003).
Lamkanfi, M. Emerging inflammasome effector mechanisms. Nat. Rev. Immunol. 11, 213–220 (2011).
Zitvogel, L., Kepp, O., Galluzzi, L. & Kroemer, G. Inflammasomes in carcinogenesis and anticancer immune responses. Nat. Immunol. 13, 343–351 (2012).
Broz, P. & Dixit, V. M. Inflammasomes: mechanism of assembly, regulation and signalling. Nat. Rev. Immunol. 16, 407–420 (2016).
Lamkanfi, M. & Dixit, V. M. Inflammasomes and their roles in health and disease. Annu Rev. Cell Dev. Biol. 28, 137–161 (2012).
Duncan, J. A. & Canna, S. W. The NLRC4 Inflammasome. Immunol. Rev. 281, 115–123 (2018).
Zhao, Y. et al. The NLRC4 inflammasome receptors for bacterial flagellin and type III secretion apparatus. Nature 477, 596–600 (2011).
Sollberger, G. et al. Caspase-1: the inflammasome and beyond. Innate Immun. 20, 115–125 (2014).
Liu, S. et al. MLKL forms disulfide bond-dependent amyloid-like polymers to induce necroptosis. Proc. Natl Acad. Sci. USA 114, E7450–e7459 (2017).
Sborgi, L. et al. GSDMD membrane pore formation constitutes the mechanism of pyroptotic cell death. Embo j. 35, 1766–1778 (2016).
Thornberry, N. A. et al. A novel heterodimeric cysteine protease is required for interleukin-1 beta processing in monocytes. Nature 356, 768–774 (1992).
Carty, M. et al. Cell Survival and Cytokine Release after Inflammasome Activation Is Regulated by the Toll-IL-1R Protein SARM. Immunity 50, 1412–1424.e1416 (2019).
Shi, J. et al. Inflammatory caspases are innate immune receptors for intracellular LPS. Nature 514, 187–192 (2014).
Chu, L. H. et al. The oxidized phospholipid oxPAPC protects from septic shock by targeting the non-canonical inflammasome in macrophages. Nat. Commun. 9, 996 (2018).
Shi, J., Gao, W. & Shao, F. Pyroptosis: Gasdermin-Mediated Programmed Necrotic Cell Death. Trends Biochem Sci. 42, 245–254 (2017).
Baker, P. J. et al. NLRP3 inflammasome activation downstream of cytoplasmic LPS recognition by both caspase-4 and caspase-5. Eur. J. Immunol. 45, 2918–2926 (2015).
Rühl, S. & Broz, P. Caspase-11 activates a canonical NLRP3 inflammasome by promoting K(+) efflux. Eur. J. Immunol. 45, 2927–2936 (2015).
Schmid-Burgk, J. L. et al. Caspase-4 mediates non-canonical activation of the NLRP3 inflammasome in human myeloid cells. Eur. J. Immunol. 45, 2911–2917 (2015).
Yang, D. et al. Caspase-11 Requires the Pannexin-1 Channel and the Purinergic P2X7 Pore to Mediate Pyroptosis and Endotoxic Shock. Immunity 43, 923–932 (2015).
Chao, Y. Y. et al. Human T(H)17 cells engage gasdermin E pores to release IL-1α on NLRP3 inflammasome activation. Nat. Immunol. 24, 295–308 (2023).
Hu, L. et al. Chemotherapy-induced pyroptosis is mediated by BAK/BAX-caspase-3-GSDME pathway and inhibited by 2-bromopalmitate. Cell Death Dis. 11, 281 (2020).
Rogers, C. et al. Cleavage of DFNA5 by caspase-3 during apoptosis mediates progression to secondary necrotic/pyroptotic cell death. Nat. Commun. 8, 14128 (2017).
Yu, J. et al. Cleavage of GSDME by caspase-3 determines lobaplatin-induced pyroptosis in colon cancer cells. Cell Death Dis. 10, 193 (2019).
Zhang, C. C. et al. Chemotherapeutic paclitaxel and cisplatin differentially induce pyroptosis in A549 lung cancer cells via caspase-3/GSDME activation. Apoptosis 24, 312–325 (2019).
Fritsch, M. et al. Caspase-8 is the molecular switch for apoptosis, necroptosis and pyroptosis. Nature 575, 683–687 (2019).
Kong, Q. et al. Alternative splicing of GSDMB modulates killer lymphocyte-triggered pyroptosis. Sci. Immunol. 8, eadg3196 (2023).
Zou, J. et al. The Versatile Gasdermin Family: Their Function and Roles in Diseases. Front Immunol. 12, 751533 (2021).
Volchuk, A. et al. Indirect regulation of HMGB1 release by gasdermin D. Nat. Commun. 11, 4561 (2020).
Mantovani, A., Dinarello, C. A., Molgora, M. & Garlanda, C. Interleukin-1 and Related Cytokines in the Regulation of Inflammation and Immunity. Immunity 50, 778–795 (2019).
Dinarello, C. A. Overview of the IL-1 family in innate inflammation and acquired immunity. Immunol. Rev. 281, 8–27 (2018).
Robinson, N. et al. Programmed necrotic cell death of macrophages: Focus on pyroptosis, necroptosis, and parthanatos. Redox Biol. 26, 101239 (2019).
Zheng, D. et al. ROS-triggered endothelial cell death mechanisms: Focus on pyroptosis, parthanatos, and ferroptosis. Front Immunol. 13, 1039241 (2022).
Zhang, J. et al. Epithelial Gasdermin D shapes the host-microbial interface by driving mucus layer formation. Sci. Immunol. 7, eabk2092 (2022).
Lin, P. H., Lin, H. Y., Kuo, C. C. & Yang, L. T. N-terminal functional domain of Gasdermin A3 regulates mitochondrial homeostasis via mitochondrial targeting. J. Biomed. Sci. 22, 44 (2015).
Sollberger, G. et al. Gasdermin D plays a vital role in the generation of neutrophil extracellular traps. Sci. Immunol. 3, eaar6689 (2018).
Tanaka, S. et al. A new Gsdma3 mutation affecting anagen phase of first hair cycle. Biochem. Biophys. Res. Commun. 359, 902–907 (2007).
Li, M. et al. Gasdermin D maintains bone mass by rewiring the endo-lysosomal pathway of osteoclastic bone resorption. Dev. Cell 57, 2365–2380.e2368 (2022).
He, K. et al. Gasdermin D licenses MHCII induction to maintain food tolerance in small intestine. Cell 186, 3033–3048.e3020 (2023).
Ahechu, P. et al. NLRP3 Inflammasome: A Possible Link Between Obesity-Associated Low-Grade Chronic Inflammation and Colorectal Cancer Development. Front Immunol. 9, 2918 (2018).
Galluzzi, L. et al. Immunogenic cell death in cancer and infectious disease. Nat. Rev. Immunol. 17, 97–111 (2017).
Parker, K. H., Horn, L. A. & Ostrand-Rosenberg, S. High-mobility group box protein 1 promotes the survival of myeloid-derived suppressor cells by inducing autophagy. J. Leukoc. Biol. 100, 463–470 (2016).
Ben-Sasson, S. Z. et al. IL-1 enhances expansion, effector function, tissue localization, and memory response of antigen-specific CD8 T cells. J. Exp. Med. 210, 491–502 (2013).
Jin, S. et al. Roles of HMGB1 in regulating myeloid-derived suppressor cells in the tumor microenvironment. Biomark. Res. 8, 21 (2020).
Ostrand-Rosenberg, S., Beury, D. W., Parker, K. H. & Horn, L. A. Survival of the fittest: how myeloid-derived suppressor cells survive in the inhospitable tumor microenvironment. Cancer Immunol. Immunother. 69, 215–221 (2020).
Jain, A., Song, R., Wakeland, E. K. & Pasare, C. T cell-intrinsic IL-1R signaling licenses effector cytokine production by memory CD4 T cells. Nat. Commun. 9, 3185 (2018).
Dai, J. et al. LncRNA LINC00969 promotes acquired gefitinib resistance by epigenetically suppressing of NLRP3 at transcriptional and posttranscriptional levels to inhibit pyroptosis in lung cancer. Cell Death Dis. 14, 312 (2023).
Fu, L. et al. Intracellular MUC20 variant 2 maintains mitochondrial calcium homeostasis and enhances drug resistance in gastric cancer. Gastric Cancer 25, 542–557 (2022).
Xia, J. et al. Mitochondrial Protein UCP1 Inhibits the Malignant Behaviors of Triple-negative Breast Cancer through Activation of Mitophagy and Pyroptosis. Int J. Biol. Sci. 18, 2949–2961 (2022).
Li, Z. et al. Sorcin regulate pyroptosis by interacting with NLRP3 inflammasomes to facilitate the progression of hepatocellular carcinoma. Cell Death Dis. 14, 678 (2023).
Feng, W.-Q. et al. IL-17A-mediated mitochondrial dysfunction induces pyroptosis in colorectal cancer cells and promotes CD8 + T-cell tumour infiltration. J. Transl. Med. 21, 335 (2023).
Thai, A. A. et al. Lung cancer. Lancet 398, 535–554 (2021).
Wang, W. et al. Effects of TRAF3 on the proliferation and migration of lung adenocarcinoma depend partly on pyroptosis. BMC Cancer 23, 942 (2023).
Su, L. et al. Targeting Src reactivates pyroptosis to reverse chemoresistance in lung and pancreatic cancer models. Sci. Transl. Med. 15, eabl7895 (2023).
Fang, Y. et al. ANGPTL4 Regulates Lung Adenocarcinoma Pyroptosis and Apoptosis via NLRP3\ASC\Caspase 8 Signaling Pathway to Promote Resistance to Gefitinib. J. Oncol. 2022, 3623570 (2022).
Liu, J. et al. Downregulation of LncRNA-XIST inhibited development of non-small cell lung cancer by activating miR-335/SOD2/ROS signal pathway mediated pyroptotic cell death. Aging (Albany NY) 11, 7830–7846 (2019).
Shi, F., Zhang, L., Liu, X. & Wang, Y. Knock-down of microRNA miR-556-5p increases cisplatin-sensitivity in non-small cell lung cancer (NSCLC) via activating NLR family pyrin domain containing 3 (NLRP3)-mediated pyroptotic cell death. Bioengineered 12, 6332–6342 (2021).
Chen, L. K. et al. AMIGO2 attenuates innate cisplatin sensitivity by suppression of GSDME-conferred pyroptosis in non-small cell lung cancer. J. Cell Mol. Med. 27, 2412–2423 (2023).
Smyth, E. C. et al. Gastric cancer. Lancet 396, 635–648 (2020).
Ren, N. et al. LncRNA ADAMTS9-AS2 inhibits gastric cancer (GC) development and sensitizes chemoresistant GC cells to cisplatin by regulating miR-223-3p/NLRP3 axis. Aging (Albany NY) 12, 11025–11041 (2020).
Zhao, C. et al. USP50 regulates NLRP3 inflammasome activation in duodenogastric reflux-induced gastric tumorigenesis. Front Immunol. 15, 1326137 (2024).
Pachathundikandi, S. K., Blaser, N., Bruns, H. & Backert, S. Helicobacter pylori Avoids the Critical Activation of NLRP3 Inflammasome-Mediated Production of Oncogenic Mature IL-1β in Human Immune Cells. Cancers (Basel) 12, 803 (2020).
Wang, X. J. et al. RNA-binding protein CPSF6 regulates IBSP to affect pyroptosis in gastric cancer. World J. Gastrointest. Oncol. 15, 1531–1543 (2023).
Loibl, S. et al. Breast cancer. Lancet 397, 1750–1769 (2021).
Tan, Y. et al. Tumor suppressor DRD2 facilitates M1 macrophages and restricts NF-κB signaling to trigger pyroptosis in breast cancer. Theranostics 11, 5214–5231 (2021).
Lei, S. et al. Azurocidin 1 inhibits the aberrant proliferation of triple‑negative breast cancer through the regulation of pyroptosis. Oncol. Rep. 50, 188 (2023).
Wang, Y. et al. Targeting the ZNF-148/miR-335/SOD2 signaling cascade triggers oxidative stress-mediated pyroptosis and suppresses breast cancer progression. Cancer Med. 12, 21308–21320 (2023).
Vogel, A. et al. Hepatocellular carcinoma. Lancet 400, 1345–1362 (2022).
Yan, Z. et al. Inhibition of NEK7 Suppressed Hepatocellular Carcinoma Progression by Mediating Cancer Cell Pyroptosis. Front Oncol. 12, 812655 (2022).
Ren, Y. et al. USP48 Stabilizes Gasdermin E to Promote Pyroptosis in Cancer. Cancer Res. 83, 1074–1093 (2023).
Zheng, Y. et al. CHMP3 promotes the progression of hepatocellular carcinoma by inhibiting caspase‑1‑dependent pyroptosis. Int J. Oncol. 64, 8 (2024).
Dekker, E. et al. Colorectal cancer. Lancet 394, 1467–1480 (2019).
Zhang, Y., Li, F., Wang, L. & Lou, Y. A438079 affects colorectal cancer cell proliferation, migration, apoptosis, and pyroptosis by inhibiting the P2X7 receptor. Biochem Biophys. Res. Commun. 558, 147–153 (2021).
Huang, Y. et al. Nrf2 inhibition increases sensitivity to chemotherapy of colorectal cancer by promoting ferroptosis and pyroptosis. Sci. Rep. 13, 14359 (2023).
Hou, X. et al. USP47-Mediated Deubiquitination and Stabilization of TCEA3 Attenuates Pyroptosis and Apoptosis of Colorectal Cancer Cells Induced by Chemotherapeutic Doxorubicin. Front Pharm. 12, 713322 (2021).
Stancu, I.-C. et al. Aggregated Tau activates NLRP3–ASC inflammasome exacerbating exogenously seeded and non-exogenously seeded Tau pathology in vivo. Acta Neuropathol. 137, 599–617 (2019).
Li, S. et al. Gasdermin D in peripheral myeloid cells drives neuroinflammation in experimental autoimmune encephalomyelitis. J. Exp. Med. 216, 2562–2581 (2019).
Zhou, Y., Gu, Y. & Liu, J. BRD4 suppression alleviates cerebral ischemia-induced brain injury by blocking glial activation via the inhibition of inflammatory response and pyroptosis. Biochem Biophys. Res. Commun. 519, 481–488 (2019).
Xu, S. et al. CD73 alleviates GSDMD-mediated microglia pyroptosis in spinal cord injury through PI3K/AKT/Foxo1 signaling. Clin. Transl. Med. 11, e269 (2021).
Xu, X.-X. et al. Neuronal nitric oxide synthase/reactive oxygen species pathway is involved in apoptosis and pyroptosis in epilepsy. Neural Regen. Res. 18, 1277–1285 (2023).
Zhan, T. et al. Implication of lncRNA MSTRG.81401 in Hippocampal Pyroptosis Induced by P2X7 Receptor in Type 2 Diabetic Rats with Neuropathic Pain Combined with Depression. Int J. Mol. Sci. 25, 1186 (2024).
Lammert, C. R. et al. AIM2 inflammasome surveillance of DNA damage shapes neurodevelopment. Nature 580, 647–652 (2020).
Trejo-Lopez, J. A., Yachnis, A. T. & Prokop, S. Neuropathology of Alzheimer’s Disease. Neurotherapeutics 19, 173–185 (2022).
Oladapo, A., Jackson, T., Menolascino, J. & Periyasamy, P. Role of pyroptosis in the pathogenesis of various neurological diseases. Brain Behav. Immun. 117, 428–446 (2024).
Moonen, S. et al. Pyroptosis in Alzheimer’s disease: cell type-specific activation in microglia, astrocytes and neurons. Acta Neuropathol. 145, 175–195 (2023).
Han, C. et al. New mechanism of nerve injury in Alzheimer’s disease: β-amyloid-induced neuronal pyroptosis. J. Cell Mol. Med. 24, 8078–8090 (2020).
Liu, Y. et al. Beta-amyloid activates NLRP3 inflammasome via TLR4 in mouse microglia. Neurosci. Lett. 736, 135279 (2020).
Friker, L. L. et al. β-Amyloid Clustering around ASC Fibrils Boosts Its Toxicity in Microglia. Cell Rep. 30, 3743–3754.e3746 (2020).
Stancu, I. C. et al. Aggregated Tau activates NLRP3-ASC inflammasome exacerbating exogenously seeded and non-exogenously seeded Tau pathology in vivo. Acta Neuropathol. 137, 599–617 (2019).
Venegas, C. et al. Microglia-derived ASC specks cross-seed amyloid-β in Alzheimer’s disease. Nature 552, 355–361 (2017).
de Dios, C. et al. Inflammasome activation under high cholesterol load triggers a protective microglial phenotype while promoting neuronal pyroptosis. Transl. Neurodegener. 12, 10 (2023).
Nussbaum, R. L. & Ellis, C. E. Alzheimer’s disease and Parkinson’s disease. N. Engl. J. Med 348, 1356–1364 (2003).
Nutt, J. G. & Wooten, G. F. Clinical practice. Diagnosis and initial management of Parkinson’s disease. N. Engl. J. Med. 353, 1021–1027 (2005).
De Virgilio, A. et al. Parkinson’s disease: Autoimmunity and neuroinflammation. Autoimmun. Rev. 15, 1005–1011 (2016).
Tong, X. et al. Targeting cell death pathways for cancer therapy: recent developments in necroptosis, pyroptosis, ferroptosis, and cuproptosis research. J. Hematol. Oncol. 15, 174 (2022).
Gordon, R. et al. Inflammasome inhibition prevents α-synuclein pathology and dopaminergic neurodegeneration in mice. Sci. Transl. Med. 10, eaah4066 (2018).
Li, Y. et al. METTL14 regulates microglia/macrophage polarization and NLRP3 inflammasome activation after ischemic stroke by the KAT3B-STING axis. Neurobiol. Dis. 185, 106253 (2023).
Wei, X. et al. TRIM27 ameliorates ischemic stroke by regulating NLRP3 inflammasome-mediated pyroptosis via the Akt/Nrf2/HO-1 signaling. Exp. Neurol. 371, 114599 (2024).
Deng, Y. et al. TRIM29 (Tripartite Motif Containing 29) Alleviates NLRC4 (NLR Family CARD Domain Containing Protein 4) Inflammasome Related Cerebral Injury via Promoting Proteasomal Degradation of NLRC4 in Ischemic Stroke. Stroke 54, 1377–1389 (2023).
McKenzie, B. A. et al. Caspase-1 inhibition prevents glial inflammasome activation and pyroptosis in models of multiple sclerosis. Proc. Natl Acad. Sci. USA 115, E6065–e6074 (2018).
McKenzie, B. A. et al. Activation of the executioner caspases-3 and -7 promotes microglial pyroptosis in models of multiple sclerosis. J. Neuroinflammation 17, 253 (2020).
Van Schoor, E. et al. Increased pyroptosis activation in white matter microglia is associated with neuronal loss in ALS motor cortex. Acta Neuropathol. 144, 393–411 (2022).
Johann, S. et al. NLRP3 inflammasome is expressed by astrocytes in the SOD1 mouse model of ALS and in human sporadic ALS patients. Glia 63, 2260–2273 (2015).
Gugliandolo, A., Giacoppo, S., Bramanti, P. & Mazzon, E. NLRP3 Inflammasome Activation in a Transgenic Amyotrophic Lateral Sclerosis Model. Inflammation 41, 93–103 (2018).
Simon, M. S. et al. Monocyte mitochondrial dysfunction, inflammaging, and inflammatory pyroptosis in major depression. Prog. Neuro-Psychopharmacol. Biol. Psychiatry 111, 110391 (2021).
Li, F. et al. Kir6.1/K-ATP channel in astrocytes is an essential negative modulator of astrocytic pyroptosis in mouse model of depression. Theranostics 12, 6611–6625 (2022).
Lei, W.-X. et al. The role and mechanism of miR-425–3p regulating neuronal pyroptosis -mediated inorganic arsenic-induced generalized anxiety disorder. Ecotoxicol. Environ. Saf. 269, 115781 (2024).
Wang, L.-Q. et al. Perfluoroalkyl substance pollutants activate the innate immune system through the AIM2 inflammasome. Nat. Commun. 12, 2915 (2021).
Xu, H. et al. S1PR2 is Important for Cigarette Smoke-induced Pyroptosis in Human Bronchial Epithelial Cells. Arch. Med. Res. 54, 277–286 (2023).
Liu, B. et al. Buformin alleviates sepsis-induced acute lung injury via inhibiting NLRP3-mediated pyroptosis through an AMPK-dependent pathway. Clin. Sci. (Lond.) 136, 273–289 (2022).
Wei, Y. et al. Crystalline silica-induced macrophage pyroptosis interacting with mitophagy contributes to pulmonary fibrosis via modulating mitochondria homeostasis. J. Hazard Mater. 454, 131562 (2023).
Jiang, Y. et al. Hypoxia activates GPR146 which participates in pulmonary vascular remodeling by promoting pyroptosis of pulmonary artery endothelial cells. Eur. J. Pharmacol. 941, 175502 (2023).
Gabillard-Lefort, C. et al. Trikafta Rescues CFTR and Lowers Monocyte P2X7R-induced Inflammasome Activation in Cystic Fibrosis. Am. J. Respir. Crit. Care Med. 205, 783–794 (2022).
Li, M. et al. S100A4 Promotes BCG-Induced Pyroptosis of Macrophages by Activating the NF-κB/NLRP3 Inflammasome Signaling Pathway. Int J. Mol. Sci. 24, 12709 (2023).
Halpin, D. M. G. et al. Global Initiative for the Diagnosis, Management, and Prevention of Chronic Obstructive Lung Disease. The 2020 GOLD Science Committee Report on COVID-19 and Chronic Obstructive Pulmonary Disease. Am. J. Respir. Crit. Care Med. 203, 24–36 (2021).
Zhang, M. Y. et al. Cigarette smoke extract induces pyroptosis in human bronchial epithelial cells through the ROS/NLRP3/caspase-1 pathway. Life Sci. 269, 119090 (2021).
Wang, L. et al. Hydrogen sulfide attenuates cigarette smoke‑induced pyroptosis through the TLR4/NF‑κB signaling pathway. Int J. Mol. Med. 49, 56 (2022).
Wang, L. et al. TREM-1 aggravates chronic obstructive pulmonary disease development via activation NLRP3 inflammasome-mediated pyroptosis. Inflamm. Res. 70, 971–980 (2021).
Mo, R., Li, J., Chen, Y. & Ding, Y. lncRNA GAS5 promotes pyroptosis in COPD by functioning as a ceRNA to regulate the miR‑223‑3p/NLRP3 axis. Mol. Med. Rep. 26, 219 (2022).
Rao, Y. et al. Transient Receptor Potential Cation Channel Subfamily V Member 4 Mediates Pyroptosis in Chronic Obstructive Pulmonary Disease. Front Physiol. 12, 783891 (2021).
Fu, X. et al. Wood smoke particulate matter (WSPM2.5) induces pyroptosis through both Caspase-1/IL-1β/IL-18 and ATP/P2Y-dependent mechanisms in human bronchial epithelial cells. Chemosphere 307, 135726 (2022).
Zhu, Z. et al. Exosomes derived from adipose-derived stem cells alleviate cigarette smoke-induced lung inflammation and injury by inhibiting alveolar macrophages pyroptosis. Respir. Res. 23, 5 (2022).
Zhang, Y., Wang, J., Wang, Y. & Lei, K. Nrf2/HO-1 signaling activation alleviates cigarette smoke-induced inflammation in chronic obstructive pulmonary disease by suppressing NLRP3-mediated pyroptosis. J. Cardiothorac. Surg. 19, 58 (2024).
Ackermann, M. et al. Pulmonary Vascular Endothelialitis, Thrombosis, and Angiogenesis in Covid-19. N. Engl. J. Med. 383, 120–128 (2020).
Jiao, Y. et al. Exosomal miR-30d-5p of neutrophils induces M1 macrophage polarization and primes macrophage pyroptosis in sepsis-related acute lung injury. Crit. Care 25, 356 (2021).
Cao, Z. et al. Crosstalk of pyroptosis, ferroptosis, and mitochondrial aldehyde dehydrogenase 2-related mechanisms in sepsis-induced lung injury in a mouse model. Bioengineered 13, 4810–4820 (2022).
Hsu, C. G. et al. The lipid peroxidation product 4-hydroxynonenal inhibits NLRP3 inflammasome activation and macrophage pyroptosis. Cell Death Differ. 29, 1790–1803 (2022).
Pu, Z., Wang, W., Xie, H. & Wang, W. Apolipoprotein C3 (ApoC3) facilitates NLRP3 mediated pyroptosis of macrophages through mitochondrial damage by accelerating of the interaction between SCIMP and SYK pathway in acute lung injury. Int Immunopharmacol. 128, 111537 (2024).
Zhang, T. et al. Exosome from BMMSC Attenuates Cardiopulmonary Bypass-Induced Acute Lung Injury Via YAP/β-Catenin Pathway: Downregulation of Pyroptosis. Stem Cells 40, 1122–1133 (2022).
Xie, W. M. et al. FTO Deficiency Alleviates LPS-induced Acute Lung Injury by TXNIP/NLRP3-mediated Alveolar Epithelial Cell Pyroptosis. Am. J. Respir. Cell Mol. Biol. 70, 351–363 (2024).
Huang, W. et al. GBP2 upregulated in LPS-stimulated macrophages-derived exosomes accelerates septic lung injury by activating epithelial cell NLRP3 signaling. Int Immunopharmacol. 124, 111017 (2023).
Li, N. et al. HDAC3 promotes macrophage pyroptosis via regulating histone deacetylation in acute lung injury. iScience 26, 107158 (2023).
Lommatzsch, M. et al. A(2)BCD: a concise guide for asthma management. Lancet Respir. Med. 11, 573–576 (2023).
Wu, J. et al. Gasdermin D silencing alleviates airway inflammation and remodeling in an ovalbumin-induced asthmatic mouse model. Cell Death Dis. 15, 400 (2024).
Cai, R. et al. Dectin-1 aggravates neutrophil inflammation through caspase-11/4-mediated macrophage pyroptosis in asthma. Respir. Res 25, 119 (2024).
Liu, L. et al. MUC1 attenuates neutrophilic airway inflammation in asthma by reducing NLRP3 inflammasome-mediated pyroptosis through the inhibition of the TLR4/MyD88/NF-κB pathway. Respir. Res 24, 255 (2023).
Leung, C. C., Yu, I. T. & Chen, W. Silicosis. Lancet 379, 2008–2018 (2012).
Song, M. et al. Inhibition of gasdermin D-dependent pyroptosis attenuates the progression of silica-induced pulmonary inflammation and fibrosis. Acta Pharm. Sin. B 12, 1213–1224 (2022).
Zhang, L., Tian, J., Ma, L. & Duan, S. Mechanistic insights into severe pulmonary inflammation caused by silica stimulation: The role of macrophage pyroptosis. Ecotoxicol. Environ. Saf. 258, 114975 (2023).
Bagcchi, S. Dismal global tuberculosis situation due to COVID-19. Lancet Infect. Dis. 21, 1636 (2021).
Zhao, P. et al. Programming cell pyroptosis with biomimetic nanoparticles for solid tumor immunotherapy. Biomaterials 254, 120142 (2020).
Chai, Q. et al. A bacterial phospholipid phosphatase inhibits host pyroptosis by hijacking ubiquitin. Science 378, eabq0132 (2022).
Ma, B. et al. GSK2656157, a PERK Inhibitor, Alleviates Pyroptosis of Macrophages Induced by Mycobacterium Bacillus Calmette-Guerin Infection. Int J. Mol. Sci. 24, 16239 (2023).
Yao, Q. et al. Lnc-EST12, which is negatively regulated by mycobacterial EST12, suppresses antimycobacterial innate immunity through its interaction with FUBP3. Cell Mol. Immunol. 19, 883–897 (2022).
Rastogi, S. et al. Mycobacterium tuberculosis inhibits the NLRP3 inflammasome activation via its phosphokinase PknF. PLoS Pathog. 17, e1009712 (2021).
Qian, J. et al. Mycobacterium tuberculosis PE_PGRS19 Induces Pyroptosis through a Non-Classical Caspase-11/GSDMD Pathway in Macrophages. Microorganisms 10, 2473 (2022).
Escobar-Chavarría, O. et al. Necrotic Cell Death and Inflammasome NLRP3 Activity in Mycobacterium bovis-Infected Bovine Macrophages. Cells 12, 2079 (2023).
Nie, X. et al. Endoplasmic Reticulum Stress Mediated NLRP3 Inflammasome Activation and Pyroptosis in THP-1 Macrophages Infected with Bacillus Calmette-Guérin. Int J. Mol. Sci. 24, 11692 (2023).
Xia, W. et al. Gasdermin E deficiency attenuates acute kidney injury by inhibiting pyroptosis and inflammation. Cell Death Dis. 12, 139 (2021).
Li, Y. et al. GSDME-mediated pyroptosis promotes inflammation and fibrosis in obstructive nephropathy. Cell Death Differ. 28, 2333–2350 (2021).
Cui, X. et al. Alpha-kinase1 promotes tubular injury and interstitial inflammation in diabetic nephropathy by canonical pyroptosis pathway. Biol. Res. 56, 5 (2023).
Zou, H. et al. C/EBPβ isoform-specific regulation of podocyte pyroptosis in lupus nephritis-induced renal injury. J. Pathol. 261, 269–285 (2023).
Su, X. et al. NLRP3 inflammasome: A potential therapeutic target to minimize renal ischemia/reperfusion injury during transplantation. Transpl. Immunol. 75, 101718 (2022).
Lameire, N. H. et al. Acute kidney injury: an increasing global concern. Lancet 382, 170–179 (2013).
Sun, J. et al. USF2 knockdown downregulates THBS1 to inhibit the TGF-β signaling pathway and reduce pyroptosis in sepsis-induced acute kidney injury. Pharm. Res. 176, 105962 (2022).
Baatarjav, C. et al. dsDNA-induced AIM2 pyroptosis halts aberrant inflammation during rhabdomyolysis-induced acute kidney injury. Cell Death Differ. 29, 2487–2502 (2022).
Ling, H. et al. LncRNA GAS5 inhibits miR-579-3p to activate SIRT1/PGC-1α/Nrf2 signaling pathway to reduce cell pyroptosis in sepsis-associated renal injury. Am. J. Physiol. Cell Physiol. 321, C117–c133 (2021).
Ni, J. et al. Hydrogen sulfide reduces pyroptosis and alleviates ischemia-reperfusion-induced acute kidney injury by inhibiting NLRP3 inflammasome. Life Sci. 284, 119466 (2021).
Zhang, Y., Zhang, Y., Yang, A. & Xia, F. Downregulation of IRF2 Alleviates Sepsis-Related Acute Kidney Injury in vitro and in vivo. Drug Des. Devel Ther. 15, 5123–5132 (2021).
Li, N. et al. Myoglobin promotes macrophage polarization to M1 type and pyroptosis via the RIG-I/Caspase1/GSDMD signaling pathway in CS-AKI. Cell Death Discov. 8, 90 (2022).
Cao, Y. et al. STING contributes to lipopolysaccharide-induced tubular cell inflammation and pyroptosis by activating endoplasmic reticulum stress in acute kidney injury. Cell Death Dis. 15, 217 (2024).
Chen, Z. et al. GSDMD and GSDME synergy in the transition of acute kidney injury to chronic kidney disease. Nephrol. Dialysis Transplant. 39, 1344–1359 (2024).
Tian, X. et al. Butyrate alleviates renal fibrosis in CKD by regulating NLRP3-mediated pyroptosis via the STING/NF-κB/p65 pathway. Int Immunopharmacol. 124, 111010 (2023).
Wu, M. et al. Gasdermin E Deletion Attenuates Ureteral Obstruction- and 5/6 Nephrectomy-Induced Renal Fibrosis and Kidney Dysfunction. Front Cell Dev. Biol. 9, 754134 (2021).
Thomas, M. C., Cooper, M. E. & Zimmet, P. Changing epidemiology of type 2 diabetes mellitus and associated chronic kidney disease. Nat. Rev. Nephrol. 12, 73–81 (2016).
Cheng, Q. et al. Caspase-11/4 and gasdermin D-mediated pyroptosis contributes to podocyte injury in mouse diabetic nephropathy. Acta Pharm. Sin. 42, 954–963 (2021).
Zheng, F. et al. Neutrophil Extracellular Traps Induce Glomerular Endothelial Cell Dysfunction and Pyroptosis in Diabetic Kidney Disease. Diabetes 71, 2739–2750 (2022).
Li, Y., Yu, W., Xiong, H. & Yuan, F. Circ_0000181 regulates miR-667-5p/NLRC4 axis to promote pyroptosis progression in diabetic nephropathy. Sci. Rep. 12, 11994 (2022).
Shoeib, H. M. et al. Interplay between long non-coding RNA MALAT1 and pyroptosis in diabetic nephropathy patients. Gene 851, 146978 (2023).
Qin, Y. et al. N6-methyladenosine methylation regulator RBM15 promotes the progression of diabetic nephropathy by regulating cell proliferation, inflammation, oxidative stress, and pyroptosis through activating the AGE-RAGE pathway. Environ. Toxicol. 38, 2772–2782 (2023).
Lan, J. et al. WTAP-mediated N(6)-methyladenosine modification of NLRP3 mRNA in kidney injury of diabetic nephropathy. Cell Mol. Biol. Lett. 27, 51 (2022).
Kim, D., Ban, K. Y., Lee, G. H. & Jun, H. S. Lysophosphatidic Acid Induces Podocyte Pyroptosis in Diabetic Nephropathy by an Increase of Egr1 Expression via Downregulation of EzH2. Int J. Mol. Sci. 24, 9968 (2023).
Liang, Y. et al. Linc00657 promoted pyroptosis in THP-1-derived macrophages and exacerbated atherosclerosis via the miR-106b-5p/TXNIP/NLRP3 axis. Int. J. Biol. Macromol. 253, 126953 (2023).
Wang, Y. W. et al. HIF-1α-regulated lncRNA-TUG1 promotes mitochondrial dysfunction and pyroptosis by directly binding to FUS in myocardial infarction. Cell Death Discov. 8, 178 (2022).
You, J. et al. GSDMD-mediated pyroptosis promotes cardiac remodeling in pressure overload. Clin. Exp. Hypertens. 45, 2189138 (2023).
Blaschke, F. et al. Liver X Receptor Agonists Suppress Vascular Smooth Muscle Cell Proliferation and Inhibit Neointima Formation in Balloon-Injured Rat Carotid Arteries. Circ. Res. 95, e110–e123 (2004).
Herrington, W. et al. Epidemiology of Atherosclerosis and the Potential to Reduce the Global Burden of Atherothrombotic Disease. Circ. Res. 118, 535–546 (2016).
Yang, Q. et al. Exercise Mitigates Endothelial Pyroptosis and Atherosclerosis by Downregulating NEAT1 Through N6-Methyladenosine Modifications. Arterioscler Thromb. Vasc. Biol. 43, 910–926 (2023).
Zhang, Y. et al. Rnd3 suppresses endothelial cell pyroptosis in atherosclerosis through regulation of ubiquitination of TRAF6. Clin. Transl. Med. 13, e1406 (2023).
Wei, Y. et al. GSDME-mediated pyroptosis promotes the progression and associated inflammation of atherosclerosis. Nat. Commun. 14, 929 (2023).
An, C. et al. IQGAP1 promotes mitochondrial damage and activation of the mtDNA sensor cGAS-STING pathway to induce endothelial cell pyroptosis leading to atherosclerosis. Int Immunopharmacol. 123, 110795 (2023).
Cong, L. et al. Melatonin alleviates pyroptosis by regulating the SIRT3/FOXO3α/ROS axis and interacting with apoptosis in Atherosclerosis progression. Biol. Res 56, 62 (2023).
He, B. et al. Hyperuricemia promotes the progression of atherosclerosis by activating endothelial cell pyroptosis via the ROS/NLRP3 pathway. J. Cell Physiol. 238, 1808–1822 (2023).
Hou, L. et al. Nicotine induces macrophage pyroptosis via LINC01272/miR-515/KLF6 axis. Ecotoxicol. Environ. Saf. 263, 115265 (2023).
Meng, Q. et al. Estrogen prevent atherosclerosis by attenuating endothelial cell pyroptosis via activation of estrogen receptor α-mediated autophagy. J. Adv. Res. 28, 149–164 (2021).
Zhang, S. et al. Homocysteine promotes atherosclerosis through macrophage pyroptosis via endoplasmic reticulum stress and calcium disorder. Mol. Med. 29, 73 (2023).
O’Gara, P. T. et al. 2013 ACCF/AHA guideline for the management of ST-elevation myocardial infarction: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. Circulation 127, e362–e425 (2013).
Vander Heide, R. S. & Steenbergen, C. Cardioprotection and myocardial reperfusion: pitfalls to clinical application. Circ. Res. 113, 464–477 (2013).
Shen, S. et al. Uric acid aggravates myocardial ischemia-reperfusion injury via ROS/NLRP3 pyroptosis pathway. Biomed. Pharmacother. 133, 110990 (2021).
Yao, M. et al. Oxytocin ameliorates high glucose- and ischemia/reperfusion-induced myocardial injury by suppressing pyroptosis via AMPK signaling pathway. Biomed. Pharmacother. 153, 113498 (2022).
Shi, H. et al. GSDMD-Mediated Cardiomyocyte Pyroptosis Promotes Myocardial I/R Injury. Circ. Res. 129, 383–396 (2021).
Feng, P. et al. Effect and mechanism of circHMGA2 on ferroptosis and pyroptosis in myocardial ischemia-reperfusion model CircHMGA2 exacerbates MI/R injury. Heliyon 9, e17849 (2023).
Piamsiri, C. et al. GSDMD-mediated pyroptosis dominantly promotes left ventricular remodeling and dysfunction in post-myocardial infarction: a comparison across modes of programmed cell death and mitochondrial involvement. J. Transl. Med. 21, 16 (2023).
Han, Y., Dong, B., Chen, M. & Yao, C. LncRNA H19 suppresses pyroptosis of cardiomyocytes to attenuate myocardial infarction in a PBX3/CYP1B1-dependent manner. Mol. Cell Biochem. 476, 1387–1400 (2021).
Ma, M. et al. Mitofilin Mitigates Myocardial Damage in Acute Myocardial Infarction by Regulating Pyroptosis of Cardiomyocytes. Front Cardiovasc Med 9, 823591 (2022).
Shi, M. et al. TRIM16 exerts protective function on myocardial ischemia/reperfusion injury through reducing pyroptosis and inflammation via NLRP3 signaling. Biochem Biophys. Res. Commun. 632, 122–128 (2022).
Tan, Y. et al. Mechanisms of diabetic cardiomyopathy and potential therapeutic strategies: preclinical and clinical evidence. Nat. Rev. Cardiol. 17, 585–607 (2020).
Meng, L. et al. METTL14 suppresses pyroptosis and diabetic cardiomyopathy by downregulating TINCR lncRNA. Cell Death Dis. 13, 38 (2022).
Wang, L. F. et al. CD38 Deficiency Alleviates Diabetic Cardiomyopathy by Coordinately Inhibiting Pyroptosis and Apoptosis. Int J. Mol. Sci. 24, 16008 (2023).
Yuan, Q. et al. CircRNA DICAR as a novel endogenous regulator for diabetic cardiomyopathy and diabetic pyroptosis of cardiomyocytes. Signal Transduct. Target Ther. 8, 99 (2023).
Hong, L. et al. Folic Acid Alleviates High Glucose and Fat-Induced Pyroptosis via Inhibition of the Hippo Signal Pathway on H9C2 Cells. Front Mol. Biosci. 8, 698698 (2021).
Shi, P. et al. MiR-21-3p triggers cardiac fibroblasts pyroptosis in diabetic cardiac fibrosis via inhibiting androgen receptor. Exp. Cell Res. 399, 112464 (2021).
Xiao, W. et al. Long non-coding RNA MIAT is involved in the regulation of pyroptosis in diabetic cardiomyopathy via targeting miR-214-3p. iScience 24, 103518 (2021).
Zhao, S. et al. MicroRNA-223-3p promotes pyroptosis of cardiomyocyte and release of inflammasome factors via downregulating the expression level of SPI1 (PU.1). Toxicology 476, 153252 (2022).
Wang, F. et al. Ghrelin inhibits myocardial pyroptosis in diabetic cardiomyopathy by regulating ERS and NLRP3 inflammasome crosstalk through the PI3K/AKT pathway. J. Drug Target 32, 148–158 (2024).
Al-Khatib, S. M. Atrial Fibrillation. Ann. Intern Med 176, Itc97–itc112 (2023).
Luo, Y. et al. Akkermansia muciniphila prevents cold-related atrial fibrillation in rats by modulation of TMAO induced cardiac pyroptosis. EBioMedicine 82, 104087 (2022).
Yan, B. et al. LncRNA XIST shuttled by adipose tissue-derived mesenchymal stem cell-derived extracellular vesicles suppresses myocardial pyroptosis in atrial fibrillation by disrupting miR-214-3p-mediated Arl2 inhibition. Lab Invest 101, 1427–1438 (2021).
Nakamura, M. & Sadoshima, J. Mechanisms of physiological and pathological cardiac hypertrophy. Nat. Rev. Cardiol. 15, 387–407 (2018).
Zeng, C. et al. NLRP3 inflammasome-mediated pyroptosis contributes to the pathogenesis of non-ischemic dilated cardiomyopathy. Redox Biol. 34, 101523 (2020).
Wang, M. et al. TRPA1 deficiency aggravates dilated cardiomyopathy by promoting S100A8 expression to induce M1 macrophage polarization in rats. Faseb j. 37, e22982 (2023).
Yue, R. et al. NLRP3-mediated pyroptosis aggravates pressure overload-induced cardiac hypertrophy, fibrosis, and dysfunction in mice: cardioprotective role of irisin. Cell Death Discov. 7, 50 (2021).
Li, X. et al. TAK1 Activation by NLRP3 Deficiency Confers Cardioprotection Against Pressure Overload-Induced Cardiomyocyte Pyroptosis and Hypertrophy. JACC Basic Transl. Sci. 8, 1555–1573 (2023).
Wu, B. et al. Inhibition of Sema4D attenuates pressure overload-induced pathological myocardial hypertrophy via the MAPK/NF-κB/NLRP3 pathways. Biochim Biophys. Acta Mol. Basis Dis. 1870, 166944 (2024).
Ewer, M. S. & Ewer, S. M. Cardiotoxicity of anticancer treatments. Nat. Rev. Cardiol. 12, 547–558 (2015).
Zheng, X. et al. Bnip3 mediates doxorubicin-induced cardiomyocyte pyroptosis via caspase-3/GSDME. Life Sci. 242, 117186 (2020).
Zhong, Z. et al. Inhibiting mir-34a-5p regulates doxorubicin-induced autophagy disorder and alleviates myocardial pyroptosis by targeting Sirt3-AMPK pathway. Biomed. Pharmacother. 168, 115654 (2023).
Li, L. et al. CB1R-stabilized NLRP3 inflammasome drives antipsychotics cardiotoxicity. Signal Transduct. Target Ther. 7, 190 (2022).
Selim, H. M. et al. Fucoidan mitigates gastric ulcer injury through managing inflammation, oxidative stress, and NLRP3-mediated pyroptosis. Int Immunopharmacol. 120, 110335 (2023).
Sun, S. et al. Lactic Acid-Producing Probiotic Saccharomyces cerevisiae Attenuates Ulcerative Colitis via Suppressing Macrophage Pyroptosis and Modulating Gut Microbiota. Front Immunol. 12, 777665 (2021).
Al Mamun, A. et al. Pyroptosis in acute pancreatitis and its therapeutic regulation. Apoptosis 27, 465–481 (2022).
Zhao, Q. et al. Parabacteroides distasonis ameliorates hepatic fibrosis potentially via modulating intestinal bile acid metabolism and hepatocyte pyroptosis in male mice. Nat. Commun. 14, 1829 (2023).
Ungaro, R. et al. Ulcerative colitis. Lancet 389, 1756–1770 (2017).
Zhang, X. et al. Inhibition of C3a/C3aR Axis in Diverse Stages of Ulcerative Colitis Affected the Prognosis of UC by Modulating the Pyroptosis and Expression of Caspase-11. Inflammation 43, 2128–2136 (2020).
Liu, Q. et al. Thioredoxin reductase 3 suppression promotes colitis and carcinogenesis via activating pyroptosis and necrosis. Cell Mol. Life Sci. 79, 106 (2022).
Zhu, F. et al. APOL1 Induces Pyroptosis of Fibroblasts Through NLRP3/Caspase-1/GSDMD Signaling Pathway in Ulcerative Colitis. J. Inflamm. Res. 16, 6385–6396 (2023).
Wang, Y. et al. Long Non-coding RNA MEG3 Alleviated Ulcerative Colitis Through Upregulating miR-98-5p-Sponged IL-10. Inflammation 44, 1049–1059 (2021).
Yan, R., Liang, X. & Hu, J. miR-141-3p alleviates ulcerative colitis by targeting SUGT1 to inhibit colonic epithelial cell pyroptosis. Autoimmunity 56, 2220988 (2023).
Gu, Q. et al. SLC6A14 promotes ulcerative colitis progression by facilitating NLRP3 inflammasome-mediated pyroptosis. World J. Gastroenterol. 30, 252–267 (2024).
Tenner, S., Baillie, J., DeWitt, J. & Vege, S. S. American College of Gastroenterology Guideline: Management of Acute Pancreatitis. Am. J. Gastroenterol. 108, 1400–1415 (2013).
Gao, L. et al. Acinar cell NLRP3 inflammasome and gasdermin D (GSDMD) activation mediates pyroptosis and systemic inflammation in acute pancreatitis. Br. J. Pharm. 178, 3533–3552 (2021).
Wang, J. et al. Cathepsin B aggravates acute pancreatitis by activating the NLRP3 inflammasome and promoting the caspase-1-induced pyroptosis. Int Immunopharmacol. 94, 107496 (2021).
Ma, N. et al. Interleukin-37 protects against acinar cell pyroptosis in acute pancreatitis. JCI Insight 7, e161244 (2022).
Wei, B. et al. Inhibition of TRAF6 improves hyperlipidemic acute pancreatitis by alleviating pyroptosis in vitro and in vivo rat models. Biol. Direct 18, 23 (2023).
Lu, Y. et al. HDL inhibits pancreatic acinar cell NLRP3 inflammasome activation and protect against acinar cell pyroptosis in acute pancreatitis. Int Immunopharmacol. 125, 110950 (2023).
Wang, J. et al. CircHIPK3 Promotes Pyroptosis in Acinar Cells Through Regulation of the miR-193a-5p/GSDMD Axis. Front Med. (Lausanne) 7, 88 (2020).
Sun, B. et al. Endogenous tRNA-derived small RNA (tRF3-Thr-AGT) inhibits ZBP1/NLRP3 pathway-mediated cell pyroptosis to attenuate acute pancreatitis (AP). J. Cell Mol. Med. 25, 10441–10453 (2021).
Lv, H., Liu, X. & Zhou, H. USP25 UPREGULATION BOOSTS GSDMD -MEDIATED PYROPTOSIS OF ACINAR CELLS IN ACUTE PANCREATITIS. Shock 58, 408–416 (2022).
Shao, Y. et al. Circulating exosomal miR-155-5p contributes to severe acute pancreatitis-associated intestinal barrier injury by targeting SOCS1 to activate NLRP3 inflammasome-mediated pyroptosis. Faseb j. 37, e23003 (2023).
Yan, C. et al. Endoplasmic reticulum stress promotes caspase-1-dependent acinar cell pyroptosis through the PERK pathway to aggravate acute pancreatitis. Int Immunopharmacol. 120, 110293 (2023).
Hernandez-Gea, V. & Friedman, S. L. Pathogenesis of liver fibrosis. Annu Rev. Pathol. 6, 425–456 (2011).
Gaul, S. et al. Hepatocyte pyroptosis and release of inflammasome particles induce stellate cell activation and liver fibrosis. J. Hepatol. 74, 156–167 (2021).
Xiao, Y. et al. STING mediates hepatocyte pyroptosis in liver fibrosis by Epigenetically activating the NLRP3 inflammasome. Redox Biol. 62, 102691 (2023).
Wu, J. et al. Hepatic HRC induces hepatocyte pyroptosis and HSCs activation via NLRP3/caspase-1 pathway. J. Mol. Med. (Berl.) 100, 1787–1799 (2022).
Xie, Z. Y., Xu, Y. X. & Yao, L. Angiotensin II can trigger HSC-LX2 pyroptosis through both classical and non-classical pathways. Life Sci. 307, 120878 (2022).
Zhang, Y. et al. Exosomes Derived from BMMSCs Mitigate the Hepatic Fibrosis via Anti-Pyroptosis Pathway in a Cirrhosis Model. Cells 11, 4004 (2022).
Lang, Z. et al. GAS5-inhibited hepatocyte pyroptosis contributes to hepatic stellate cell inactivation via microRNA-684 and AHR. iScience 26, 107326 (2023).
Shu, B. et al. The METTL3/MALAT1/PTBP1/USP8/TAK1 axis promotes pyroptosis and M1 polarization of macrophages and contributes to liver fibrosis. Cell Death Discov. 7, 368 (2021).
Liu, Y. et al. S100A8-Mediated NLRP3 Inflammasome-Dependent Pyroptosis in Macrophages Facilitates Liver Fibrosis Progression. Cells 11, 3579 (2022).
Rao, Z. et al. Pyroptosis in inflammatory diseases and cancer. Theranostics 12, 4310–4329 (2022).
Sharma, B. R. & Kanneganti, T. D. NLRP3 inflammasome in cancer and metabolic diseases. Nat. Immunol. 22, 550–559 (2021).
Cheng, H. Y. et al. Snail-regulated exosomal microRNA-21 suppresses NLRP3 inflammasome activity to enhance cisplatin resistance. J. Immunother. Cancer 10, e004832 (2022).
Yuan, R. et al. Cucurbitacin B inhibits non-small cell lung cancer in vivo and in vitro by triggering TLR4/NLRP3/GSDMD-dependent pyroptosis. Pharm. Res 170, 105748 (2021).
Zhang, Y. et al. Alpinumisoflavone suppresses hepatocellular carcinoma cell growth and metastasis via NLRP3 inflammasome-mediated pyroptosis. Pharm. Rep. 72, 1370–1382 (2020).
Shen, Z. et al. Metformin inhibits hepatocellular carcinoma development by inducing apoptosis and pyroptosis through regulating FOXO3. Aging (Albany NY) 13, 22120–22133 (2021).
Tang, Z. et al. Pyroptosis is involved in the inhibitory effect of FL118 on growth and metastasis in colorectal cancer. Life Sci. 257, 118065 (2020).
Zhao, A. N. et al. Disturbing NLRP3 acetylation and inflammasome assembly inhibits androgen receptor-promoted inflammatory responses and prostate cancer progression. Faseb j. 36, e22602 (2022).
Yan, H. et al. Cisplatin Induces Pyroptosis via Activation of MEG3/NLRP3/caspase-1/GSDMD Pathway in Triple-Negative Breast Cancer. Int J. Biol. Sci. 17, 2606–2621 (2021).
McSweeney, K. R. et al. Mechanisms of Cisplatin-Induced Acute Kidney Injury: Pathological Mechanisms, Pharmacological Interventions, and Genetic Mitigations. Cancers 13, 1572 (2021).
Waissbluth, S., Maass, J. C., Sanchez, H. A. & Martínez, A. D. Supporting Cells and Their Potential Roles in Cisplatin-Induced Ototoxicity. Front Neurosci. 16, 867034 (2022).
Pushpan, C. K. et al. Repurposing AZD5438 and Dabrafenib for Cisplatin-Induced AKI. J. Am. Soc. Nephrol. 35, 22–40 (2024).
Yang, H. L. et al. Coenzyme Q(0) defeats NLRP3-mediated inflammation, EMT/metastasis, and Warburg effects by inhibiting HIF-1α expression in human triple-negative breast cancer cells. Arch. Toxicol. 97, 1047–1068 (2023).
Yang, H. L. et al. Coenzyme Q(0) inhibited the NLRP3 inflammasome, metastasis/EMT, and Warburg effect by suppressing hypoxia-induced HIF-1α expression in HNSCC cells. Int J. Biol. Sci. 20, 2790–2813 (2024).
Liang, L. et al. Oxymatrine suppresses colorectal cancer progression by inhibiting NLRP3 inflammasome activation through mitophagy induction in vitro and in vivo. Phytother. Res 37, 3342–3362 (2023).
Zhou, H. et al. Efficacy of oxymatrine for treatment and relapse suppression of severe plaque psoriasis: results from a single-blinded randomized controlled clinical trial. Br. J. Dermatol 176, 1446–1455 (2017).
Sun, J., Li, J., Kong, X. & Guo, Q. Peimine Inhibits MCF-7 Breast Cancer Cell Growth by Modulating Inflammasome Activation: Critical Roles of MAPK and NF-κB Signaling. Anticancer Agents Med Chem. 23, 317–327 (2023).
Guo, W. et al. VB12-Sericin-PBLG-IR780 Nanomicelles for Programming Cell Pyroptosis via Photothermal (PTT)/Photodynamic (PDT) Effect-Induced Mitochondrial DNA (mitoDNA) Oxidative Damage. ACS Appl Mater. Interfaces 14, 17008–17021 (2022).
Zhou, Y. et al. Curcumin activates NLRC4, AIM2, and IFI16 inflammasomes and induces pyroptosis by up-regulated ISG3 transcript factor in acute myeloid leukemia cell lines. Cancer Biol. Ther. 23, 328–335 (2022).
Li, Y. et al. Dihydroartemisinin induces pyroptosis by promoting the AIM2/caspase-3/DFNA5 axis in breast cancer cells. Chem. Biol. Interact. 340, 109434 (2021).
Zhou, R. et al. CCL19 suppresses gastric cancer cell proliferation, migration, and invasion through the CCL19/CCR7/AIM2 pathway. Hum. Cell 33, 1120–1132 (2020).
Xu, X. et al. Virus-Like Particle-Induced cGAS-STING Activation and AIM2 Inflammasome-Mediated Pyroptosis for Robust Cancer Immunotherapy. Angew. Chem. Int Ed. Engl. 62, e202303010 (2023).
Zheng, P. et al. Biodegradable Ca(2+) Nanomodulators Activate Pyroptosis through Mitochondrial Ca(2+) Overload for Cancer Immunotherapy. Angew. Chem. Int Ed. Engl. 61, e202204904 (2022).
Zhao, Q. et al. The central role of a two-way positive feedback pathway in molecular targeted therapies-mediated pyroptosis in anaplastic thyroid cancer. Clin. Transl. Med. 12, e727 (2022).
Zheng, Z. et al. Metformin activates AMPK/SIRT1/NF-κB pathway and induces mitochondrial dysfunction to drive caspase3/GSDME-mediated cancer cell pyroptosis. Cell Cycle 19, 1089–1104 (2020).
Cai, J. et al. Natural product triptolide induces GSDME-mediated pyroptosis in head and neck cancer through suppressing mitochondrial hexokinase-ΙΙ. J. Exp. Clin. Cancer Res. 40, 190 (2021).
Xu, H. et al. Gambogic Acid Induces Pyroptosis of Colorectal Cancer Cells through the GSDME-Dependent Pathway and Elicits an Antitumor Immune Response. Cancers 14, 5505 (2022).
Zhang, X. et al. Miltirone induces cell death in hepatocellular carcinoma cell through GSDME-dependent pyroptosis. Acta Pharm. Sin. B 10, 1397–1413 (2020).
An, H. et al. Tetraarsenic hexoxide enhances generation of mitochondrial ROS to promote pyroptosis by inducing the activation of caspase-3/GSDME in triple-negative breast cancer cells. Cell Death Dis. 12, 159 (2021).
Yu, F. et al. Nitidine chloride induces caspase 3/GSDME-dependent pyroptosis by inhibting PI3K/Akt pathway in lung cancer. Chin. Med 17, 115 (2022).
Yang, C. et al. CBL0137 activates ROS/BAX signaling to promote caspase-3/GSDME-dependent pyroptosis in ovarian cancer cells. Biomed. Pharmacother. 161, 114529 (2023).
Sun, X. et al. Germacrone induces caspase-3/GSDME activation and enhances ROS production, causing HepG2 pyroptosis. Exp. Ther. Med. 24, 456 (2022).
Hu, Z. et al. Cordyceps militaris extract induces apoptosis and pyroptosis via caspase-3/PARP/GSDME pathways in A549 cell line. Food Sci. Nutr. 10, 21–38 (2022).
Xie, B. et al. Combination of DNA demethylation and chemotherapy to trigger cell pyroptosis for inhalation treatment of lung cancer. Nanoscale 13, 18608–18615 (2021).
Rioja-Blanco, E. et al. CXCR4-targeted nanotoxins induce GSDME-dependent pyroptosis in head and neck squamous cell carcinoma. J. Exp. Clin. Cancer Res. 41, 49 (2022).
Hu, J. et al. Local delivery of arsenic trioxide nanoparticles for hepatocellular carcinoma treatment. Signal Transduct. Target Ther. 4, 28 (2019).
Xie, W. et al. Simvastatin induces pyroptosis via ROS/caspase-1/GSDMD pathway in colon cancer. Cell Commun. Signal 21, 329 (2023).
Rasmussen, S. T. et al. Simvastatin and oxidative stress in humans: A randomized, double-blinded, placebo-controlled clinical trial. Redox Biol. 9, 32–38 (2016).
Chen, T. et al. Secoisolariciresinol diglucoside induces pyroptosis by activating caspase-1 to cleave GSDMD in colorectal cancer cells. Drug Dev. Res. 83, 1152–1166 (2022).
Chen, M. et al. Saikosaponin-D induces the pyroptosis of lung cancer by increasing ROS and activating the NF-κB/NLRP3/caspase-1/GSDMD pathway. J. Biochem Mol. Toxicol. 37, e23444 (2023).
Chen, D. et al. Combination of ruthenium (II) polypyridyl complex Δ-Ru1 and Taxol enhances the anti-cancer effect on Taxol-resistant cancer cells through Caspase-1/GSDMD-mediated pyroptosis. J. Inorg. Biochem. 230, 111749 (2022).
Li, L. et al. Photodynamic therapy induces human esophageal carcinoma cell pyroptosis by targeting the PKM2/caspase-8/caspase-3/GSDME axis. Cancer Lett. 520, 143–159 (2021).
Li, R. Y. et al. Cisplatin-induced pyroptosis is mediated via the CAPN1/CAPN2-BAK/BAX-caspase-9-caspase-3-GSDME axis in esophageal cancer. Chem. Biol. Interact. 361, 109967 (2022).
Liu, Z. et al. Apoptin induces pyroptosis of colorectal cancer cells via the GSDME-dependent pathway. Int J. Biol. Sci. 18, 717–730 (2022).
Hage, C. et al. Sorafenib Induces Pyroptosis in Macrophages and Triggers Natural Killer Cell-Mediated Cytotoxicity Against Hepatocellular Carcinoma. Hepatology 70, 1280–1297 (2019).
Yoon, S. M. et al. Efficacy and Safety of Transarterial Chemoembolization Plus External Beam Radiotherapy vs Sorafenib in Hepatocellular Carcinoma With Macroscopic Vascular Invasion: A Randomized Clinical Trial. JAMA Oncol. 4, 661–669 (2018).
Qin, S. et al. Tislelizumab vs Sorafenib as First-Line Treatment for Unresectable Hepatocellular Carcinoma: A Phase 3 Randomized Clinical Trial. JAMA Oncol. 9, 1651–1659 (2023).
Yau, T. et al. Efficacy and Safety of Nivolumab Plus Ipilimumab in Patients With Advanced Hepatocellular Carcinoma Previously Treated With Sorafenib: The CheckMate 040 Randomized Clinical Trial. JAMA Oncol. 6, e204564 (2020).
Li, Z. et al. Enhancing Gasdermin-induced tumor pyroptosis through preventing ESCRT-dependent cell membrane repair augments antitumor immune response. Nat. Commun. 13, 6321 (2022).
Lu, Y. et al. Strategies to package recombinant Adeno-Associated Virus expressing the N-terminal gasdermin domain for tumor treatment. Nat. Commun. 12, 7155 (2021).
Lin, J. et al. Oncolytic Parapoxvirus induces Gasdermin E-mediated pyroptosis and activates antitumor immunity. Nat. Commun. 14, 224 (2023).
Zhao, Y., Tian, Y. & Feng, T. Sodium Houttuyfonate Ameliorates β-amyloid(1-42)-Induced Memory Impairment and Neuroinflammation through Inhibiting the NLRP3/GSDMD Pathway in Alzheimer’s Disease. Mediators Inflamm. 2021, 8817698 (2021).
Bai, Y. et al. N-salicyloyl tryptamine derivatives as potential therapeutic agents for Alzheimer’s disease with neuroprotective effects. Bioorg. Chem. 115, 105255 (2021).
Shi, Y.-S. et al. 1,7-diphenyl-4-hepten-3-one mitigates Alzheimer’s-like pathology by inhibiting pyroptosis via activating the Nrf2 pathway. Naunyn-Schmiedeberg’s. Arch. Pharmacol. 397, 3065–3075 (2024).
Fu, X. X. et al. Telmisartan Alleviates Alzheimer’s Disease-Related Neuropathologies and Cognitive Impairments. J. Alzheimers Dis. 94, 919–933 (2023).
Chou, V. et al. INPP5D regulates inflammasome activation in human microglia. Nat. Commun. 14, 7552 (2023).
Flores, J., Noël, A., Fillion, M. L. & LeBlanc, A. C. Therapeutic potential of Nlrp1 inflammasome, Caspase-1, or Caspase-6 against Alzheimer disease cognitive impairment. Cell Death Differ. 29, 657–669 (2022).
Zhang, T. et al. Bushen Huoxue Acupuncture Inhibits NLRP1 Inflammasome-Mediated Neuronal Pyroptosis in SAMP8 Mouse Model of Alzheimer’s Disease. Neuropsychiatr. Dis. Treat. 17, 339–346 (2021).
Esmaeili-Mahani, S. et al. Apelin-13 prevents hippocampal synaptic plasticity impairment in Parkinsonism rats. J. Chem. Neuroanat. 111, 101884 (2021).
Ye, X. et al. Caspase-1: A Promising Target for Preserving Blood-Brain Barrier Integrity in Acute Stroke. Front Mol. Neurosci. 15, 856372 (2022).
Zhang, X. et al. Salidroside ameliorates Parkinson’s disease by inhibiting NLRP3-dependent pyroptosis. Aging (Albany NY) 12, 9405–9426 (2020).
Cai, M. et al. Kaemperfol alleviates pyroptosis and microglia-mediated neuroinflammation in Parkinson’s disease via inhibiting p38MAPK/NF-κB signaling pathway. Neurochem Int 152, 105221 (2022).
Ma, X. et al. Prussian Blue Nanozyme as a Pyroptosis Inhibitor Alleviates Neurodegeneration. Adv. Mater. 34, e2106723 (2022).
Jiang, Z. et al. β-Hydroxybutyrate alleviates pyroptosis in MPP(+)/MPTP-induced Parkinson’s disease models via inhibiting STAT3/NLRP3/GSDMD pathway. Int Immunopharmacol. 113, 109451 (2022).
Huan, P. et al. Qiji Shujiang granules alleviates dopaminergic neuronal injury of parkinson’s disease by inhibiting NLRP3/Caspase-1 pathway mediated pyroptosis. Phytomedicine 120, 155019 (2023).
Poltorak, A. et al. Defective LPS signaling in C3H/HeJ and C57BL/10ScCr mice: mutations in Tlr4 gene. Science 282, 2085–2088 (1998).
Hu, J. J. et al. FDA-approved disulfiram inhibits pyroptosis by blocking gasdermin D pore formation. Nat. Immunol. 21, 736–745 (2020).
Saitoh, S. Chaperones and transport proteins regulate TLR4 trafficking and activation. Immunobiology 214, 594–600 (2009).
Bai, Y. et al. Disulfiram blocks inflammatory TLR4 signaling by targeting MD-2. Proc. Natl Acad. Sci. USA 120, e2306399120 (2023).
Zhao, L. R. & Willing, A. Enhancing endogenous capacity to repair a stroke-damaged brain: An evolving field for stroke research. Prog. Neurobiol. 163-164, 5–26 (2018).
Kong, L. L. et al. Pinocembrin attenuates hemorrhagic transformation after delayed t-PA treatment in thromboembolic stroke rats by regulating endogenous metabolites. Acta Pharm. Sin. 42, 1223–1234 (2021).
Chen, Y. et al. RRx-001 ameliorates inflammatory diseases by acting as a potent covalent NLRP3 inhibitor. Cell Mol. Immunol. 18, 1425–1436 (2021).
Zhu, H. et al. Janus Kinase Inhibition Ameliorates Ischemic Stroke Injury and Neuroinflammation Through Reducing NLRP3 Inflammasome Activation via JAK2/STAT3 Pathway Inhibition. Front Immunol. 12, 714943 (2021).
Chen, C. et al. Directly targeting ASC by lonidamine alleviates inflammasome-driven diseases. J. Neuroinflammation 19, 315 (2022).
Hu, R. et al. Edaravone dexborneol provides neuroprotective benefits by suppressing NLRP3 inflammasome-induced microglial pyroptosis in experimental ischemic stroke. Int Immunopharmacol. 113, 109315 (2022).
Jia, Y., Xue, K., Luo, Y. & Liu, C. Thiolutin attenuates ischemic stroke injury via inhibition of NLRP3 inflammasome: an in vitro and in vivo study. Exp. Brain Res. 241, 839–849 (2023).
Guo, X. et al. Intranasal administration of β-1, 3-galactosyltransferase 2 confers neuroprotection against ischemic stroke by likely inhibiting oxidative stress and NLRP3 inflammasome activation. Faseb j. 36, e22542 (2022).
Guo, C. et al. Development and Characterization of a Hydroxyl-Sulfonamide Analogue, 5-Chloro-N-[2-(4-hydroxysulfamoyl-phenyl)-ethyl]-2-methoxy-benzamide, as a Novel NLRP3 Inflammasome Inhibitor for Potential Treatment of Multiple Sclerosis. ACS Chem. Neurosci. 8, 2194–2201 (2017).
Hou, B. et al. Inhibition of the NLRP3-inflammasome prevents cognitive deficits in experimental autoimmune encephalomyelitis mice via the alteration of astrocyte phenotype. Cell Death Dis. 11, 377 (2020).
Sánchez-Fernández, A., Skouras, D. B., Dinarello, C. A. & López-Vales, R. OLT1177 (Dapansutrile), a Selective NLRP3 Inflammasome Inhibitor, Ameliorates Experimental Autoimmune Encephalomyelitis Pathogenesis. Front Immunol. 10, 2578 (2019).
Pan, L. et al. Yanghe Pingchuan Granules Alleviate Airway Inflammation in Bronchial Asthma and Inhibit Pyroptosis by Blocking the TLR4/NF-κB/NRLP3 Signaling Pathway. Mediators Inflamm. 2022, 6561048 (2022).
Ambrus-Aikelin, G. et al. JT002, a small molecule inhibitor of the NLRP3 inflammasome for the treatment of autoinflammatory disorders. Sci. Rep. 13, 13524 (2023).
Lv, J. et al. Heme oxygenase-1 alleviates allergic airway inflammation by suppressing NF-κB-mediated pyroptosis of bronchial epithelial cells. Faseb j. 38, e23472 (2024).
Yang, J. et al. Protopine ameliorates OVA-induced asthma through modulatingTLR4/MyD88/NF-κB pathway and NLRP3 inflammasome-mediated pyroptosis. Phytomedicine 126, 155410 (2024).
Chen, X. et al. Schisandrin B Attenuates Airway Inflammation and Airway Remodeling in Asthma by Inhibiting NLRP3 Inflammasome Activation and Reducing Pyroptosis. Inflammation 44, 2217–2231 (2021).
Zeng, J. et al. Schisandrin A regulates the Nrf2 signaling pathway and inhibits NLRP3 inflammasome activation to interfere with pyroptosis in a mouse model of COPD. Eur. J. Med. Res. 28, 217 (2023).
Tian, X. et al. -)-Epicatechin ameliorates cigarette smoke-induced lung inflammation via inhibiting ROS/NLRP3 inflammasome pathway in rats with COPD. Toxicol Appl Pharmacol. 429, 115674 (2021). .
Xu, L. et al. Propofol modulates Nrf2/NLRP3 signaling to ameliorate cigarette smoke-induced damage in human bronchial epithelial cells. Tissue Cell 88, 102341 (2024).
Zhang, C. et al. Halotherapy relieves chronic obstructive pulmonary disease by alleviating NLRP3 inflammasome-mediated pyroptosis. Ann. Transl. Med. 10, 1279 (2022).
Wu, X. et al. Emodin Ameliorates Acute Pancreatitis-Associated Lung Injury Through Inhibiting the Alveolar Macrophages Pyroptosis. Front Pharm. 13, 873053 (2022).
Xu, Q. et al. Emodin Alleviates Severe Acute Pancreatitis-Associated Acute Lung Injury by Inhibiting the Cold-Inducible RNA-Binding Protein (CIRP)-Mediated Activation of the NLRP3/IL-1β/CXCL1 Signaling. Front Pharm. 12, 655372 (2021).
Pu, Z. et al. The effects and mechanisms of the anti-COVID-19 traditional Chinese medicine, Dehydroandrographolide from Andrographis paniculata (Burm.f.) Wall, on acute lung injury by the inhibition of NLRP3-mediated pyroptosis. Phytomedicine 114, 154753 (2023).
Zhang, W. et al. Chicoric Acid Presented NLRP3-Mediated Pyroptosis through Mitochondrial Damage by PDPK1 Ubiquitination in an Acute Lung Injury Model. Am. J. Chin. Med. 51, 1431–1457 (2023).
Liu, Y. et al. Tangeretin attenuates acute lung injury in septic mice by inhibiting ROS-mediated NLRP3 inflammasome activation via regulating PLK1/AMPK/DRP1 signaling axis. Inflamm. Res 73, 47–63 (2024).
Qiu, H. et al. EuHD1 protects against inflammatory injury driven by NLRP3 inflammasome. Int Immunopharmacol. 115, 109712 (2023).
Ren, M. et al. Ergolide covalently binds NLRP3 and inhibits NLRP3 inflammasome-mediated pyroptosis. Int Immunopharmacol. 120, 110292 (2023).
Shao, J. J. et al. Britannin as a novel NLRP3 inhibitor, suppresses inflammasome activation in macrophages and alleviates NLRP3-related diseases in mice. Acta Pharm. Sin. 45, 803–814 (2024).
Zhang, C. et al. Xuebijing alleviates LPS-induced acute lung injury by downregulating pro-inflammatory cytokine production and inhibiting gasdermin-E-mediated pyroptosis of alveolar epithelial cells. Chin. J. Nat. Med. 21, 576–588 (2023).
Peukert, K. et al. Tetracycline ameliorates silica-induced pulmonary inflammation and fibrosis via inhibition of caspase-1. Respir. Res 23, 21 (2022).
Amaral, N. B. et al. Colchicine reduces the activation of NLRP3 inflammasome in COVID-19 patients. Inflamm. Res. 72, 895–899 (2023).
Zhang, H. et al. CHIP protects against septic acute kidney injury by inhibiting NLRP3-mediated pyroptosis. iScience 26, 107762 (2023).
Zhu, X. et al. αKlotho protein has therapeutic activity in contrast-induced acute kidney injury by limiting NLRP3 inflammasome-mediated pyroptosis and promoting autophagy. Pharm. Res. 167, 105531 (2021).
Li, X. et al. miR-30c-5p Alleviated Pyroptosis During Sepsis-Induced Acute Kidney Injury via Targeting TXNIP. Inflammation 44, 217–228 (2021).
Yang, B. et al. Circ DENND4C inhibits pyroptosis and alleviates ischemia-reperfusion acute kidney injury by exosomes secreted from human urine-derived stem cells. Chem. Biol. Interact. 391, 110922 (2024).
Luo, X. et al. Carnosine alleviates cisplatin-induced acute kidney injury by targeting Caspase-1 regulated pyroptosis. Biomed. Pharmacother. 167, 115563 (2023).
Xiong, J. et al. DUSP2-mediated inhibition of tubular epithelial cell pyroptosis confers nephroprotection in acute kidney injury. Theranostics 12, 5069–5085 (2022).
Kong, X. et al. Loganin reduces diabetic kidney injury by inhibiting the activation of NLRP3 inflammasome-mediated pyroptosis. Chem. Biol. Interact. 382, 110640 (2023).
Gao, Y., Ma, Y., Xie, D. & Jiang, H. ManNAc protects against podocyte pyroptosis via inhibiting mitochondrial damage and ROS/NLRP3 signaling pathway in diabetic kidney injury model. Int Immunopharmacol. 107, 108711 (2022).
Li, N. et al. Tangshen Formula Attenuates Diabetic Kidney Injury by Imparting Anti-pyroptotic Effects via the TXNIP-NLRP3-GSDMD Axis. Front Pharm. 11, 623489 (2020).
Rauf, A. et al. Honokiol: A review of its pharmacological potential and therapeutic insights. Phytomedicine 90, 153647 (2021).
Ma, Q. et al. Honokiol suppresses the aberrant interactions between renal resident macrophages and tubular epithelial cells in lupus nephritis through the NLRP3/IL-33/ST2 axis. Cell Death Dis. 14, 174 (2023).
Wei, T. et al. Rational design, synthesis, and pharmacological characterisation of dicarbonyl curcuminoid analogues with improved stability against lung cancer via ROS and ER stress mediated cell apoptosis and pyroptosis. J. Enzym. Inhib. Med. Chem. 37, 2357–2369 (2022).
Zhu, J. et al. Salvianolic acid A regulates pyroptosis of endothelial cells via directly targeting PKM2 and ameliorates diabetic atherosclerosis. Front Pharm. 13, 1009229 (2022).
Weng, X. et al. Apigenin inhibits macrophage pyroptosis through regulation of oxidative stress and the NF-κB pathway and ameliorates atherosclerosis. Phytother. Res. 37, 5300–5314 (2023).
Liu, S. et al. LncRNA H19 Mitigates Oxidized Low-Density Lipoprotein Induced Pyroptosis via Caspase-1 in Raw 264.7 Cells. Inflammation 44, 2407–2418 (2021).
Zhang, B. L. et al. Inhibition of GSDMD activation by Z-LLSD-FMK or Z-YVAD-FMK reduces vascular inflammation and atherosclerotic lesion development in ApoE(-/-) mice. Front Pharm. 14, 1184588 (2023).
Azumi, J. et al. The Organogermanium Compound 3-(Trihydroxygermyl) Propanoic Acid (THGP) Suppresses Inflammasome Activation Via Complexation with ATP. Int J. Mol. Sci. 23, 13364 (2022).
Liu, J. et al. Autophagy blockage promotes the pyroptosis of ox-LDL-treated macrophages by modulating the p62/Nrf2/ARE axis. J. Physiol. Biochem. 77, 419–429 (2021).
Yang, Z. J. et al. Knockdown of the long non‑coding RNA MALAT1 ameliorates TNF‑α‑mediated endothelial cell pyroptosis via the miR‑30c‑5p/Cx43 axis. Mol. Med. Rep. 27, 90 (2023).
Li, H. et al. Geniposide suppresses NLRP3 inflammasome-mediated pyroptosis via the AMPK signaling pathway to mitigate myocardial ischemia/reperfusion injury. Chin. Med. 17, 73 (2022).
Chai, R. et al. Tanshinone IIA inhibits cardiomyocyte pyroptosis through TLR4/NF-κB p65 pathway after acute myocardial infarction. Front Cell Dev. Biol. 11, 1252942 (2023).
Dong, H. Q. et al. Liproxstatin‑1 induces cell cycle arrest, apoptosis, and caspase‑3/GSDME‑dependent secondary pyroptosis in K562 cells. Int J. Oncol. 61, 119 (2022).
Nie, C. et al. Hydrogen gas inhalation ameliorates cardiac remodelling and fibrosis by regulating NLRP3 inflammasome in myocardial infarction rats. J. Cell Mol. Med. 25, 8997–9010 (2021).
Chai, X. et al. Chlorogenic acid protects against myocardial ischemia-reperfusion injury in mice by inhibiting Lnc Neat1/NLRP3 inflammasome-mediated pyroptosis. Sci. Rep. 13, 17803 (2023).
Pan, W. et al. Coenzyme Q10 mitigates macrophage mediated inflammation in heart following myocardial infarction via the NLRP3/IL1β pathway. BMC Cardiovasc Disord. 24, 76 (2024).
Qin, C. et al. Epigallocatechin gallate prevents cardiomyocytes from pyroptosis through lncRNA MEG3/TAF15/AIM2 axis in myocardial infarction. Chin. Med. 18, 160 (2023).
Yue, R. et al. Mesenchymal stem cell-derived exosomal microRNA-182-5p alleviates myocardial ischemia/reperfusion injury by targeting GSDMD in mice. Cell Death Discov. 8, 202 (2022).
Lu, Y. et al. Magnetic vagus nerve stimulation alleviates myocardial ischemia-reperfusion injury by the inhibition of pyroptosis through the M(2)AChR/OGDHL/ROS axis in rats. J. Nanobiotechnology 21, 421 (2023).
Elmadbouh, I. & Singla, D. K. BMP-7 Attenuates Inflammation-Induced Pyroptosis and Improves Cardiac Repair in Diabetic Cardiomyopathy. Cells 10, 2640 (2021).
Yang, F. et al. Metformin Inhibits the NLRP3 Inflammasome via AMPK/mTOR-dependent Effects in Diabetic Cardiomyopathy. Int J. Biol. Sci. 15, 1010–1019 (2019).
Zhong, C. et al. Berberine inhibits NLRP3 inflammasome activation by regulating mTOR/mtROS axis to alleviate diabetic cardiomyopathy. Eur. J. Pharm. 964, 176253 (2024).
Abo-Saif, M. A. et al. Pomegranate peel extract protects against the development of diabetic cardiomyopathy in rats by inhibiting pyroptosis and downregulating LncRNA-MALAT1. Front Pharm. 14, 1166653 (2023).
Yan, M. et al. The Chinese herbal medicine Fufang Zhenzhu Tiaozhi protects against diabetic cardiomyopathy by alleviating cardiac lipotoxicity-induced oxidative stress and NLRP3-dependent inflammasome activation. Biomed. Pharmacother. 148, 112709 (2022).
Lu, L. et al. Irisin improves diabetic cardiomyopathy-induced cardiac remodeling by regulating GSDMD-mediated pyroptosis through MITOL/STING signaling. Biomed. Pharmacother. 171, 116007 (2024).
Wei, Z. et al. Curcumin Improves Diabetic Cardiomyopathy by Inhibiting Pyroptosis through AKT/Nrf2/ARE Pathway. Mediators Inflamm. 2023, 3906043 (2023).
Wei, Z. et al. Quercetin Inhibits Pyroptosis in Diabetic Cardiomyopathy through the Nrf2 Pathway. J. Diabetes Res. 2022, 9723632 (2022).
Sun, S. et al. Puerarin Inhibits NLRP3-Caspase-1-GSDMD-Mediated Pyroptosis via P2X7 Receptor in Cardiomyocytes and Macrophages. Int J. Mol. Sci. 24, 13169 (2023).
Xu, K. et al. Self-adaptive pyroptosis-responsive nanoliposomes block pyroptosis in autoimmune inflammatory diseases. Bioact. Mater. 36, 272–286 (2024).
Zhao, J. et al. PLGA-microspheres-carried circGMCL1 protects against Crohn’s colitis through alleviating NLRP3 inflammasome-induced pyroptosis by promoting autophagy. Cell Death Dis. 13, 782 (2022).
Cai, X. et al. hucMSC-derived exosomes attenuate colitis by regulating macrophage pyroptosis via the miR-378a-5p/NLRP3 axis. Stem Cell Res. Ther. 12, 416 (2021).
Zhang, D. et al. β-sitosterol alleviates dextran sulfate sodium-induced experimental colitis via inhibition of NLRP3/Caspase-1/GSDMD-mediated pyroptosis. Front Pharm. 14, 1218477 (2023).
Ye, Z. et al. Tou Nong powder obstructs ulcerative colitis through the regulation of NF-κB/NLRP3/Caspase-1/GSDMD inflammasome pyroptotic pathway. J. Ethnopharmacol. 317, 116846 (2023).
Yang, W. et al. Necrosulfonamide ameliorates intestinal inflammation via inhibiting GSDMD-medicated pyroptosis and MLKL-mediated necroptosis. Biochem. Pharm. 206, 115338 (2022).
Ma, X. et al. Munronoid I Ameliorates DSS-Induced Mouse Colitis by Inhibiting NLRP3 Inflammasome Activation and Pyroptosis Via Modulation of NLRP3. Front Immunol. 13, 853194 (2022).
Liu, D. et al. Ginsenoside Rg3 Ameliorates DSS-Induced Colitis by Inhibiting NLRP3 Inflammasome Activation and Regulating Microbial Homeostasis. J. Agric Food Chem. 71, 3472–3483 (2023).
Fan, R. et al. Wedelolactone alleviates acute pancreatitis and associated lung injury via GPX4 mediated suppression of pyroptosis and ferroptosis. Free Radic. Biol Med. 173, 29–40 (2021).
Li, S. et al. Hair follicle-MSC-derived small extracellular vesicles as a novel remedy for acute pancreatitis. J. Control Release 352, 1104–1115 (2022).
Feng, M. et al. Qingjie Huagong decoction inhibits pancreatic acinar cell pyroptosis by regulating circHipk3/miR-193a-5p/NLRP3 pathway. Phytomedicine 126, 155265 (2024).
Wang, X. et al. Baicalein alleviates pyroptosis and inflammation in hyperlipidemic pancreatitis by inhibiting NLRP3/Caspase-1 pathway through the miR-192-5p/TXNIP axis. Int Immunopharmacol. 101, 108315 (2021).
Wu, J. et al. Treatment of Severe Acute Pancreatitis and Related Lung Injury by Targeting Gasdermin D-Mediated Pyroptosis. Front Cell Dev. Biol. 9, 780142 (2021).
Huang, Z. W. et al. Sinapic Acid Alleviates Acute Pancreatitis in Association with Attenuation of Inflammation, Pyroptosis, and the AMPK/NF-κB Signaling Pathway. Am. J. Chin. Med. 50, 2185–2197 (2022).
Wang, X. et al. Salidroside ameliorates severe acute pancreatitis-induced cell injury and pyroptosis by inactivating Akt/NF-κB and caspase-3/GSDME pathways. Heliyon 9, e13225 (2023).
Gong, L. et al. CD44-Targeting Drug Delivery System of Exosomes Loading Forsythiaside A Combats Liver Fibrosis via Regulating NLRP3-Mediated Pyroptosis. Adv. Health. Mater. 12, e2202228 (2023).
Chen, P. et al. Stem Cells From Human Exfoliated Deciduous Teeth Alleviate Liver Cirrhosis via Inhibition of Gasdermin D-Executed Hepatocyte Pyroptosis. Front Immunol. 13, 860225 (2022).
Cardoso-Lezama, I. et al. Nicotinic acid attenuates experimental non-alcoholic steatohepatitis by inhibiting the NLRP3 inflammasome/pyroptosis pathway. Biochem Pharm. 216, 115762 (2023).
Kim, H. Y. et al. Auranofin prevents liver fibrosis by system Xc-mediated inhibition of NLRP3 inflammasome. Commun. Biol. 4, 824 (2021).
Povero, D. et al. Pharmacology of a Potent and Novel Inhibitor of the NOD-Like Receptor Pyrin Domain-Containing Protein 3 (NLRP3) Inflammasome that Attenuates Development of Nonalcoholic Steatohepatitis and Liver Fibrosis. J. Pharm. Exp. Ther. 386, 242–258 (2023).
Zhang, Z. T. et al. Trilobatin alleviates non-alcoholic fatty liver disease in high-fat diet plus streptozotocin-induced diabetic mice by suppressing NLRP3 inflammasome activation. Eur. J. Pharm. 933, 175291 (2022).
Wan, Y. et al. Ursolic acid alleviates Kupffer cells pyroptosis in liver fibrosis by the NOX2/NLRP3 inflammasome signaling pathway. Int Immunopharmacol. 113, 109321 (2022).
Shiffman, M. et al. Randomised clinical trial: emricasan versus placebo significantly decreases ALT and caspase 3/7 activation in subjects with non-alcoholic fatty liver disease. Aliment Pharm. Ther. 49, 64–73 (2019).
Wang, Q. et al. A bioorthogonal system reveals antitumour immune function of pyroptosis. Nature 579, 421–426 (2020).
Yang, L. et al. Intraepithelial mast cells drive gasdermin C-mediated type 2 immunity. Immunity 57, 1056–1070.e1055 (2024).
Wree, A. et al. NLRP3 inflammasome activation results in hepatocyte pyroptosis, liver inflammation, and fibrosis in mice. Hepatology 59, 898–910 (2014).
Wu, L. M. et al. Atorvastatin inhibits pyroptosis through the lncRNA NEXN-AS1/NEXN pathway in human vascular endothelial cells. Atherosclerosis 293, 26–34 (2020).
Ran, L. et al. UK5099 Inhibits the NLRP3 Inflammasome Independently of its Long-Established Target Mitochondrial Pyruvate Carrier. Adv Sci (Weinh), 11, e2307224 (2024).
Rathkey, J. K. et al. Chemical disruption of the pyroptotic pore-forming protein gasdermin D inhibits inflammatory cell death and sepsis. Sci. Immunol. 3, eaat2738 (2018).
Li, Y. et al. Cleavage-independent activation of ancient eukaryotic gasdermins and structural mechanisms. Science 384, adm9190 (2024).
Roshanravan, N. et al. Effects of oral butyrate and inulin supplementation on inflammation-induced pyroptosis pathway in type 2 diabetes: A randomized, double-blind, placebo-controlled trial. Cytokine 131, 155101 (2020).
Faghfouri, A. H. et al. Regulation of NLRP3 inflammasome by zinc supplementation in Behçet’s disease patients: A double-blind, randomized placebo-controlled clinical trial. Int Immunopharmacol. 109, 108825 (2022).
Klück, V. et al. Dapansutrile, an oral selective NLRP3 inflammasome inhibitor, for treatment of gout flares: an open-label, dose-adaptive, proof-of-concept, phase 2a trial. Lancet Rheumatol. 2, e270–e280 (2020).
Zhang, Z. et al. Structural basis for thioredoxin-mediated suppression of NLRP1 inflammasome. Nature 622, 188–194 (2023).
Hochheiser, I. V. et al. Structure of the NLRP3 decamer bound to the cytokine release inhibitor CRID3. Nature 604, 184–189 (2022).
Xia, S. et al. Gasdermin D pore structure reveals preferential release of mature interleukin-1. Nature 593, 607–611 (2021).
Yan, H. H. N., Chan, A. S., Lai, F. P. & Leung, S. Y. Organoid cultures for cancer modeling. Cell Stem Cell 30, 917–937 (2023).
Zhou, H. et al. NLRP3 Inflammasome Mediates Silica-induced Lung Epithelial Injury and Aberrant Regeneration in Lung Stem/Progenitor Cell-derived Organotypic Models. Int J. Biol. Sci. 19, 1875–1893 (2023).
Gao, R. et al. The selective NLRP3-inflammasome inhibitor MCC950 reduces myocardial fibrosis and improves cardiac remodeling in a mouse model of myocardial infarction. Int Immunopharmacol. 74, 105575 (2019).
van Hout, G. P. et al. The selective NLRP3-inflammasome inhibitor MCC950 reduces infarct size and preserves cardiac function in a pig model of myocardial infarction. Eur. Heart J. 38, 828–836 (2017).
Funding
This work was supported by grants from the National Natural Science Foundation of China (Grant No. 82173201,82272758 and U23A20456), the Key Research and Development Program of Hunan Province (Grant No.2022SK2026, 2023DK2001, and 2024DK2007, China), the Scientific Research Program of FuRong Laboratory (No.2023SK2094, China), the Natural Science Foundation of Hunan Province (Grant No.2022JJ70020, 2023JJ60120 and 2024JJ3036, China) and Hunan Cancer Hospital Climb Plan (Grant No. 2023NSFC-B005 and ZX2021003, China).
Author information
Authors and Affiliations
Contributions
P.C, H.W., and B.X. designed the review; Y.L., R.P., and Y.O. searched for the literature and wrote the manuscript; Y.L, R.P., and Y.O. drew the figures; W.G., T.X., L.T., and H.Y. assisted in editing and revising the manuscript; P.C., H.W., and B.X. acquired the funding; All authors have read and approved the article.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Liu, Y., Pan, R., Ouyang, Y. et al. Pyroptosis in health and disease: mechanisms, regulation and clinical perspective. Sig Transduct Target Ther 9, 245 (2024). https://doi.org/10.1038/s41392-024-01958-2
Received:
Revised:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s41392-024-01958-2
This article is cited by
-
Healthy young human plasma-derived exosomes enhance neural stem cell therapy by suppressing pyroptosis via TXNIP/NLRP3 after intracerebral hemorrhage
Journal of Nanobiotechnology (2026)
-
Microbiota-derived IPA mitigates post-stroke neuroinflammation by inhibiting TREM2-dependent pyroptosis
Journal of Neuroinflammation (2026)
-
Ferroptosis in cancer toward molecular insights and clinical translation in pancreatic cancer
Molecular Cancer (2026)
-
Selenium-doped carbon dots nanozymes hitchhiking tailored liposomes block neuronal pyroptosis through GPX4/ROS/NLRP3/GSDMD axis to attenuate ischemic stroke
Journal of Nanobiotechnology (2026)
-
Activation of NF-κB signaling pathway in GSDME-low esophageal squamous cell carcinoma cells enhances radioresistance
Journal of Translational Medicine (2026)