Abstract
Antenatal corticosteroids (ACS) are administered where there is risk of preterm birth to promote fetal lung development and improve perinatal survival. However, treatment may be associated with increased risk of developing neurobehavioural disorders. We have recently identified that ACS results in significant changes to DNA methylation patterns in the newborn and juvenile prefrontal cortex (PFC) of exposed guinea pig offspring. Methylation changes at transcription factor binding sites (TFBS) for PLAGL1, TFAP2C, ZNF263, and SP1 were consistently noted at both post-natal stages, suggesting a long-lasting signature of ACS exposure. In this study, we determined if comparable methylation changes are also present in the newborn blood of ACS-exposed guinea pig offspring, as this would determine whether blood methylation patterns may be used as a peripheral biomarker of changes in the brain. Pregnant guinea pigs were treated with saline or betamethasone (1 mg/kg) on gestational days 50/51. gDNA from whole blood of term-born offspring on post-natal day (PND) 1 was used for reduced representation bisulfite sequencing. Overall, 1677 differentially methylated CpG sites (DMCs) were identified in response to ACS. While no specific DMCs identified in the blood overlapped with those previously reported in the PFC of PND1 offspring, significant differential methylation at TFBSs for PLAGL1, TFAP2C, EGR1, ZNF263, and SP1 was persistently observed. Furthermore, re-examination of our previously reported data of DMCs in human neonatal blood following ACS identified the presence of this same TFBS signature in human infants, suggesting the potential for clinical translation of our epigenomic data.
Similar content being viewed by others
Introduction
Antenatal corticosteroids (ACS), including betamethasone or dexamethasone, are administered to pregnant persons to promote fetal lung maturation and improve perinatal survival when there is risk of preterm delivery. Despite established benefits during the perinatal period, ACS exposure influences development in multiple tissue types, leading to long-term effects on cardiometabolic, immune and neurodevelopmental outcomes in offspring [1,2,3].
In humans, ACS exposure has been associated with a heightened stress response in children (6–11 years) [4] and adolescents (14–18) [5]. Effects were greater in female as compared to male children [4]. Altered neurobehavioural outcomes, such as decreased attention, have also been reported in children (8 years) [6] and young adults (14–26 years) [7], who had been exposed to ACS. Furthermore, a recent population-based study identified that ACS exposure increased risk of mental or behavioural disorders at 5 years [8], and that mental and behavioural disorders were more prevalent among ACS exposed children (12.01%) as compared to unexposed children (6.45%) [9]. Exposure to multiple courses, as compared to a single course of ACS, resulted in a reduced head circumference at birth [10] and increased risk of neurodevelopmental and neurosensory deficits at 5 years [11, 12]. Imaging studies have identified smaller and a thinner anterior cingulate cortex in term-born children who received ACS [13]. However, investigations into the molecular mechanisms that underlie these changes in the brain are limited in humans, with studies relying on animal models or molecular insight from peripheral tissue such as blood.
In the juvenile baboon, ACS exposure has been shown to result in reduced learning ability [14], while juvenile guinea pig offspring demonstrated increased locomotor activity following ACS [15]. Notably, behavioural effects were greatest in female offspring, suggesting a higher sensitivity of females to ACS exposure. In juvenile female guinea pig offspring, ACS exposure resulted in significant changes to DNA methylation patterns in the hippocampus, in gene pathways involved in neurodevelopment. In these same animals, significant methylation changes were also identified in the whole blood, suggesting that the whole blood represent a useful surrogate tissue for examining methylation changes in the brain. Indeed, studies have shown that blood methylation status is highly correlated (r = 0.86–0.89) to both post-mortem and surgically resected brain tissue in humans, though correlations were dependent on the brain region examined [16, 17].
More recently, we reported that exposure to a single course of ACS results in significant changes to the DNA methylation pattern in the prefrontal cortex (PFC) of newborn and juvenile female guinea pig offspring. Identified genes were part of networks involved in ‘developmental cellular process’ and ‘neuronal proliferation’ in the newborn PFC, and ‘synaptic regulation’ in the PFC at post-natal day 14 [18]. Furthermore, mRNA levels of key differentially methylated genes identified in the gene networks were found to be altered in the PFC, suggesting a functional correlation of the methylation data. Interestingly, altered DNA methylation in the PFC occurred at multiple transcription factor binding sites, indicating a potential functional relevance of the methylation changes. Specifically, four key binding sites, for transcription factors PLAGL1, TFAP2C, ZNF263, and SP1 were found to be differentially methylated in the newborn and juvenile PFC, presenting a signature consistently observed at multiple developmental stages in response to ACS. It is not known whether this methylation signature associated with ACS exposure is also present in surrogate tissues such as the whole blood.
In the present study, we investigated the differential methylation patterns in the peripheral whole blood of newborn guinea pig offspring to determine whether a consistent signature is left by ACS exposure in the blood as was left in the PFC. Furthermore, we re-examined DNA methylation data from human neonatal blood following ACS exposure, where we had reported 505 differentially methylated sites following exposure to ACS [19]. This previous human study identified gene networks in blood that linked to neuronal proliferation, synaptic development, proteasome activity and intracellular protein trafficking, indicating comparable pathways to those identified in the guinea pig PFC. As such, we sought to examine the differential DNA methylation pattern in the blood of newborn guinea pigs following ACS, and whether the methylation changes in the blood of guinea pigs and humans occur at the same transcription factor binding sites that were previously identified in the PFC [18].
Methods
Animal cohort development
Female Dunkin-Hartley guinea pigs (Charles River) were singly-housed and mated while food and water were available ad libitum on a 12 h light-dark cycle under approval by the Animal Care Committee at the University of Toronto. Pregnant dams were randomly assigned into treatment or control groups. Dams were subcutaneously injected with a clinically relevant single-course (two doses, provided 24 h apart) of betamethasone (1 mg/kg; n = 7) or saline (Veh: equi-volume; n = 7) on gestational days (GD) 50/51. The guinea pig glucocorticoid receptor (GR) has a four-fold lower affinity for synthetic glucocorticoids compared to the human GR [20], as such the dose used in this study (1 mg/kg) is equivalent to that used clinically in humans (12 mg intramuscularly 24 h apart) [21]. Whole blood was collected from PND1 offspring by cardiac puncture under isoflurane anaesthesia. One female offspring per litter was used for DNA methylation analysis, as we have previously shown that female offspring demonstrate greater behavioural and neuroendocrine phenotypes after antenatal corticosteroid treatment compared to males [3].
Sample preparation
Whole blood without EDTA was used to create blood spots (70 µL per spot) and dried at room temperature for 4 h using FTA Blood Stain Cards (Whatman, Sigma). Blood spot cards were stored with silica gel at −80 °C until use. Dried blood spots (DBS) provide a stable medium for DNA that can be easily transported and stored for extended periods without altering DNA methylation [22, 23]. It is not possible to get fresh whole blood from human neonates, however, blood spot cards are routinely collected for newborn screening and the Ontario Birth Study allows additional blood spots (1–5 per neonate) to be collected for research. By producing blood spot cards in the guinea pig cohort, we are assessing the same sample medium in both guinea pigs and humans. We have also previously reported that there are no significant differences in DNA methylation derived from DBS compared to frozen whole blood [23], indicating that dried blood spot cards are a reliable resource for the assessment of blood methylation patterns. To extract gDNA, DBS were cut into small pieces using sterile scissors and processed using the Quick-DNA™ Microprep Plus Kit (Zymo Research), according to the manufacturer’s instructions. gDNA was quantified with Quant-iT Picogreen dsDNA assay (ThermoFisher, Waltham, MA, USA) and quality was assessed using TapeStation (Agilent, Santa Clara, CA, USA) at the Centre for Applied Genomics (Peter Gilgan Centre for Research and Learning, Hospital for Sick Children, Toronto, Canada). Reduced Representation Bisulfite Sequencing (RRBS) libraries were prepared from 100 ng of high-quality dsDNA (DNA integrity number >6) using the Ovation RRBS Methyl-Seq System 1–16 (Tecan Genomics, Redwood City, CA, USA) and EpiTect Fast DNA Bisulfite kit (Qiagen, Germany) following the manufacturer’s protocols. RRBS libraries were sequenced using Illumina’s NextSeq500 platform at the Donnelly Sequencing Centre (University of Toronto, Canada), using single-end reads of 75 bp read lengths as per manufacturer protocol. Samples were processed in coded lots and sequenced in pooled multiplexes of 10, balanced for treatment condition to not introduce group-biased batch effects during sample processing and sequencing.
Bioinformatic analysis
Identification of differentially methylated sites and genes & pathway enrichment
Quality control of sequenced data was performed using Trim Galore (v0.6.4), a wrapper script around CutAdapt [24], to remove low-quality reads (Phred quality scores <30) and accessory sequences from adaptors. A Python script provided by NuGEN (Github: trimRRBSdiversityAdaptCustomers.py) removed reads without an MspI site signature at the 5’ end. Bismark (v0.16.0) [25] and Bowtie2 (v2.3.4.3) [26] were used to align the filtered and trimmed reads to the guinea pig genome (CavPor3.0) and Samtools (v1.9) [27] was used to sort the reads. Differentially methylated CpG sites (DMC) were identified using MethPipe [28, 29], to compare between ACS-treated and untreated samples. methpipe/methcounts was used to obtain methylation levels at individual cytosines, which was used to perform beta-binomial regression analyses in the methpipe/radmeth programme under the regression option. Identified CpG sites were adjusted (bins 1:200:1) using the adjust option in methpipe/radmeth to obtain a false discovery rate (FDR)-corrected list of significant DMCs. All bioinformatic analysis was performed blind until the final step when treatment groups had to be specified for regression analysis.
Analysis was limited to DMCs with greater than 10 reads, showing greater than 5% methylation difference, and FDR < 0.05. DMCs were annotated using CompEpiTools [30] to known genes based on the Ensembl database. DMCs were localized to genomic features (promoter, intragenic–including both introns and exons, or intergenic regions), based on distance from a transcription start site (TSS). Promoter regions were defined as DMCs within 1000 bps up/downstream of a TSS, while DMCs further than 1000 bps were categorized as intergenic regions. Computations were performed on the Niagara supercomputer at the SciNet HPC Consortium [31].
Gene ontology analysis
DMCs were annotated to known genes to understand the functional implications of the altered methylome. Gene ontology (GO) analysis was performed on g:Profiler [32] to determine gene networks, or groups of genes with similar function, that were differentially methylated in response to ACS exposure. An unranked list of genes, independent of the number of CpG sites or the average % methylation difference, was used for analysis. Searches were performed against the guinea pig genomic database using ‘All known genes’ as the statistical domain scope, which is more extensively documented.
Transcription factor motif search
To determine if DMCs were localized within known transcription factor binding sites (TFBS), a motif enrichment search was performed using MEME [33]. Briefly, the dataset of DMCs were expanded by 100 bps (50 bps up/downstream) using BEDTools ‘slop’ [34] and converted into a. bed file to use as input into MEME Suite. Parameters were specified for motif distribution (any number of repetitions) to indicate that any number of non-overlapping motifs may be present per sequence, and as ‘1st order model of sequences’ for the background model to adjust for dimer biases (such as high GC content). Default significance threshold was set at E-value ≤ 0.05. Identified motifs were inputted to TOMTOM [35] to identify transcription factors binding sequences that align to the motifs. Significance was defined at a default threshold of E-value ≤ 10. Targeted searches of known TFBS motifs were also performed with position-weight-matrices (PWM) obtained from JASPAR [36] using FIMO [37] on Meme-Suite.
Human blood methylation data
Differentially methylated sites in the human neonatal blood following ACS exposure were assessed in our previous study [19]. Data were re-examined as part of this study for transcription factor binding site analyses. This was not undertaken in our original publication [19]. The initial dataset was collected from infants born at term and exposed to ACS who were retrospectively identified through the Ontario Birth Study (OBS) conducted at Sinai Health Systems as previously described [19]. Briefly, identified case subjects (n = 14, 8 M, 6 F) were matched to controls based on maternal age, BMI, parity, and fetal sex. Neonatal blood from heel pricks was collected at 24 h post-birth on blood spot cards, as part of routine clinical practice for diagnostic purposes. gDNA from blood spot cards was extracted using the GenSolve DNA COMPLETE Kit (GenTegra, Pleasanton, CA, USA) and prepared and sequenced using Reduced Representation Bisulfite Sequencing (RRBS) as described above. Bioinformatic analyses were performed using the above-mentioned pipeline as previously described [19]. Gene alignment was performed against the human genome (hg38). Identified DMCs were expanded by 100 bps using Bedtools > ‘slop’ and assessed using MEME [33] and TOMTOM [35] to identify transcription factors binding sites that significantly aligned with the identified motif sequences.
Results
Differentially methylated CpG sites in PND1 blood following ACS exposure
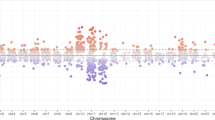
Differential methylation in the PND1 guinea pig blood was examined following ACS exposure. No variation in methylation was observed between offspring. In the PND1 guinea pig blood, 1677 DMCs were identified following ACS exposure (10x coverage, FDR < 0.05, ≥5% methylation difference), of which 956 sites (57%) were hypermethylated and 721 sites (43%) were hypomethylated (Fig. 1A and Supplementary Table 1). Categorizing DMCs to genomic features, 73 sites were localized to the promoter region, while 693 sites were found within intragenic regions (within gene body) and 911 sites were at intergenic regions (Fig. 1B). The DMCs annotated to 171 genes, 108 of which were hypermethylated (63%) and 63 (37%) of which were hypomethylated (Fig. 1C). The top 20 differentially methylated genes are presented in Table 1.
A Scatterplot of individual CpG sites that were significantly differentially methylated in ACS-exposed animals as compared to unexposed controls. 956 sites were hypermethylated, 721 sites were hypomethylated. >10× reads; ≥5% methylation difference; FDR ≤ 0.05 (B) DMCs were localized to various genomic features, based on distance from transcription start sites. Promoter: ≤1000 bp, Intergenic: ≥1000 bp, Intragenic: within gene body, including introns and exons (C) DMCs were annotated to known genes. 171 genes were identified in total, of which 108 genes were hypermethylated and 63 genes were hypomethylated. D The top 20 terms from the gene set enrichment analysis are represented from most to least significant. All differentially methylated genes discovered in the PND1 Blood (171 genes) were used in the analysis.
Of the top 20 genes, nine had known function in immune regulation, representing the largest fraction of genes (Parp12, Padi2, Slco3a1, Igsf21, Stab2, Kcnh2, Il12rb1, Il1rl2, Pbxip1) [38]. Notably, all DMCs annotated to these nine genes were hypermethylated, ranging from 7.18% hypermethylated (Il1rl2) to 41.8% hypermethylated (Padi2). One other DMC was also localized in the promoter region for Kcnh2 (14.66%), while all other DMCs were within the gene body of the annotated genes. Genes involved in regulation of cytoskeletal components, cell adhesion, or cell migration were also frequently observed, comprising seven of the top 20 genes (Adamts17, Igsf21, Bcam, Stab2, Wrap73, Rreb1, Nphp4). Identified DMCs ranged from −36.65% (Wrap73) to 31.48% (Adamts17), all localized to the gene body.
An examination of all 171 genes through gene ontology (GO) analysis identified significant gene interactions and networks, demonstrating enrichment of terms such as ‘DNA binding’, ‘transcription regulation’, ‘response to stimulus’, ‘signalling’, ‘cell migration’, and ‘cell motility’ (Supplementary Table 2). The top 20 terms are represented in Fig. 1D. Secondary GO analysis using only the set of hypomethylated genes (Supplementary Table 3) identified terms related to ‘transcription’ and ‘DNA binding’. Among the set of hypermethylated genes ‘protein binding’, ‘cellular response stimulus’ and ‘regulation of cellular process’ terms were most enriched (Supplementary Table 4).
Comparison with differential methylation profiles in the guinea pig PFC
Comparing the DMCs identified in the newborn guinea pig blood to those that were previously observed in the newborn guinea pig PFC [18], there were no sites that were differentially methylated in both tissue types following ACS exposure. However, seven genes (Igsf21, Stab2, Fzr1, Dnah17, Tshz2, Prex1, Setd1b) were differentially methylated in both blood and PFC of newborn guinea pigs following exposure to ACS (Table 2). The sites at which differential methylation occur were distinct between the two tissue types. Gene set enrichment analysis of these seven genes did not identify any significant gene networks.
Differential methylation at transcription factor binding sites in PND1 blood
A key observation in the guinea pig PFC following ACS exposure was the differential methylation identified at the binding sites for transcription factors PLAGL1, TFAP2C, ZNF263, and SP1 [18]. The TFBS were differentially methylated at both PND1 and PND14, indicating that this methylation signature may be an important mediator of ACS exposure on long-term neurodevelopmental sequelae. As such, TFBS analysis was performed among the DMCs identified in the guinea pig blood derived on PND1 following ACS exposure, to determine whether the same TFBS signature is evident in peripheral tissue. A similar signature of altered methylation in the TFBS in blood and PFC would suggest that the blood could provide a surrogate biomarker reflecting TFBS methylation changes in the brain following ACS.
Using MEME Suite v5.5.3 [33], we performed motif enrichment analysis to identify fixed-length patterns of sequences which are 6–50 bp long, that are repeatedly observed amongst the provided input (significance threshold E-value ≤ 0.05). TOMTOM [35] was subsequently used to compare the identified sequences against a database of known transcription factor binding motifs to highlight the top matches.
In the blood of guinea pigs on PND1, significant changes in methylation were identified at motifs that correspond to the binding sites for transcription factors PLAGL1, TFAP2C, EGR1, SP1, and ZNF263 following ACS exposure (Fig. 2 and Table 3). Binding sequences for PLAGL1 were found within 454 sites (27% of all differentially methylated sites; Supplementary Table 5), annotating to 80 genes enriched in ‘cellular response to stimulus’, ‘signal transduction’, ‘response to chemical’, and ‘cell communication’. TFAP2C binding sites were identified at 356 DMCs (22%; Supplementary Table 6), which annotated to 71 genes involved in ‘cell differentiation’, ‘cell proliferation’, ‘transcription regulation’, and ‘cell motility’, while EGR1 binding sites were identified among 313 sites (19%), in 63 genes that were involved in ‘transcription’, ‘cellular biosynthetic process’, ‘signalling’, ‘response to stimulus’, and ‘cell communication’ (Supplementary Table 7). SP1 binding sites were identified at 84 sites (5%), which annotated to 55 genes enriched in ‘regulation of cell population and proliferation’, ‘cell differentiation’, ‘cell migration’, ‘cell motility’, and ‘signal transduction’ (Supplementary Table 8). ZNF263 binding sites were identified among 213 DMCs (13%) annotating to 50 genes enriched for ‘transcription regulation activity’, ‘protein kinase binding’, ‘calcium-dependent protein serine/threonine kinase activity’, ‘cell motility’, ‘cell migration’, and ‘cellular response to stimulus’ (Supplementary Table 9).
Binding motifs for PLAGL1, TFAP2C, EGR1, ZNF263, and SP1 were identified to be significantly differentially methylated in the newborn guinea pig blood following ACS exposure. Significant motif sequences (E-value ≤ 0.05) that were identified surrounding the DMCs identified in the PND1 guinea pig blood following ACS are indicated in the bottom row. Transcription factor binding sites that significantly aligned (E-value ≤ 10) to the identified motifs are indicated in the top row. Each nucleotide is represented in a different colour, and the size of each letter represents the probability of the nucleotide at the position.
Methylation at transcription factor binding site in human blood
We previously reported extensive changes in DNA methylation, where 505 differentially methylated sites were identified in newborn human blood following ACS treatment [19]. No variation in methylation values for observed between offspring. Initial comparison of DNA methylation in guinea pig and human blood following ACS revealed no overlap of altered methylation at specific genes between the species. However, following our finding that key transcription factor binding sites are differentially methylated in both the guinea pig blood and PFC following ACS treatment, we re-examined our human neonatal blood methylation data to perform transcription factor binding site enrichment analysis. Similar to the guinea pig, significant differential methylation was identified at binding sites for PLAGL1, TFAP2C, EGR1, ZNF263 and SP1 following ACS (Fig. 3 and Table 3). Binding sites for PLAGL1 were identified among 104 DMCs (20%) annotating to 26 genes (Supplementary Table 10) involved in regulating ‘gene transcription’, while binding sites for TFAP2C were annotated to 49 DMCs (10%) and 17 genes (Supplementary Table 11). EGR1 binding sites was observed within 136 DMCs (27%) and 26 genes following ACS (Supplementary Table 12), and ZNF263 binding sites were identified at 84 DMCs (17%) which annotated to 26 genes (Supplementary Table 13). None of the annotated genes for TFAP2C, EGR1, nor ZNF263 were significantly enriched in any known gene pathways. SP1 binding sites were found within 82 DMCs (16%), annotating to 23 genes (Supplementary Table 14) that were enriched in ‘RNA polymerase II-specific DNA-binding transcription factor binding’, ‘NuRD complex’, ‘positive regulation of proteolysis’, ‘myeloid cell differentiation’, and ‘membrane-bounded organelle’. This suggests that though individual genes may not be overlapping targets of differential DNA methylation following ACS treatment between guinea pigs and humans, there are five core transcription factors and their downstream pathways which are affected in common in both species in response to ACS exposure.
Binding motifs for PLAGL1, TFAP2C, EGR1, ZNF263, and SP1 were identified to be significantly differentially methylated in human neonatal blood following ACS exposure. Significant motif sequences (E-value ≤ 0.05) represented on the bottom row, were identified among the full dataset of DMCs identified in the human blood following ACS. Transcription factor binding sequences that aligned significantly (E-value ≤ 10) to the identified motifs are represented in the row above. Each nucleotide is represented in a separate colour, and the size of each letter represents the probability of the nucleotide at the position.
Discussion
ACS exposure is associated with increased risk of developing neurodevelopmental disorders in humans, yet mechanistic studies have been limited due to the inaccessibility of brain tissue for examination. Studies have relied on animal models for brain-specific molecular investigation. Previously, we have reported genome-wide DNA methylation changes in multiple brain regions (prefrontal cortex, hippocampus) at various stages of development (newborn, juvenile) following ACS exposure in guinea pigs [18, 39, 40]. However, whether animal-derived mechanistic data can be translated to humans remained unclear.
We recently identified a conserved methylation signature in the PFC of newborn and juvenile guinea pigs where the TFBS of PLAGL1, TFAP2C, ZNF263 and SP1 were significantly differentially methylated in response to ACS exposure [18]. In the current study, we identified that the same ACS-induced TFBS signature is also present in the newborn blood of both guinea pigs and humans. Across our two studies, we have identified an epigenetic signature that is not only consistently observed in the brain and blood of guinea pigs but also in human blood, indicating potential for clinical translatability.
Exposure to ACS results in genome-wide DNA methylation differences in the peripheral blood of PND1 guinea pig offspring. Affected pathways included RNA transcription regulation or cellular response to stimulus, including terms such as ‘DNA binding’, ‘regulation of transcription by RNA polymerase II’, ‘response to stimulus’, ‘signalling’, ‘cell migration’, ‘cell motility’, and ‘endothelial adhesion’. These terms may represent pathways involved in immune regulation, as might be expected for glucocorticoid-mediated effects on leukocyte function [41]. Comparison of the methylation differences found in the blood to those previously found in the PFC indicated no common DMCs. This was unexpected as previous studies have identified significant correlations in DNA methylation between the hippocampus and peripheral blood of PND14 guinea pig offspring following multiple courses of ACS [40]. This may be due to key differences in the experimental designs, as the previous study administered multiple courses of ACS, with treatment administered across a wider phase of fetal development [40]. The present study also examined the newborn (PND1) blood, unlike the previous study which focused on PND14 offspring [40]. While no specific DMCs were shared between the PFC and blood, differential methylation at TFBS for ZNF263, EGR1 and SP1 was observed in both studies, indicating a glucocorticoid-specific biomarker that is present, independent of the treatment dose, offspring age, and brain area.
PLAGL1 is a transcription factor which is normally imprinted and only paternally expressed, though it shows biallelic expression in the blood [42]. PLAGL1 has been shown to regulate anti-proliferative effects, especially in inducing differentiation-associated cell cycle arrest pathways [43], by activating cell cycle and apoptosis pathways through transcriptional activation of p53 [44]. In blood, PLAGL1 has also been shown to downregulate cytokine production through SOCS3 (suppressor of cytokine signalling) activation [45, 46]. In the PND1 guinea pig blood, SOCS3 was hypermethylated in its promoter region at a putative binding site for TFAP2C, thus presenting a possible genomic network by which ACS treatment targets inflammatory suppression [47, 48].
TFAP2C, which encodes for the AP2-gamma protein, is a member of the AP2 family of transcription factors that is known for its ability to inhibit cell migration/invasion [49, 50] while promoting stemness [51] and a state of naïve pluripotency [52]. These downstream functions of TFAP2C align well with known effects of glucocorticoids to impede leukocyte migration and extravasation [53], and direct T-cell migration away from sites of inflammation [54, 55]. In our gene set enrichment analysis, target genes of TFAP2C were enriched in terms such as ‘cell differentiation’, ‘cell motility’, ‘cell migration’, and ‘plasma-membrane bound cell projection’, consistent with known effects of glucocorticoids and TFAP2C [49, 50].
While little is known concerning the effects of glucocorticoid exposure on PLAGL1 and TFAP2C activity, relationships between glucocorticoids and the transcription factors EGR1, ZNF263, and SP1 have been previously reported. EGR1, also known as NGFI-A, is a zinc finger transcription factor that is a downstream target of glucocorticoid signalling [56] known to regulate cellular proliferation, differentiation, and apoptosis [57]. We have previously reported an increase in EGR1 expression following ACS exposure in the hippocampus 24 h after treatment [58], while others have reported hypomethylation at the binding sites for EGR1, ZNF263, and SP1 in the blood of 5-year-old children who also showed high cortisol concentrations in their hair [59], indicating a strong association between these transcription factor binding sites and exposure to endogenous and synthetic glucocorticoids.
In this project, we have demonstrated significant differential methylation at key transcription factor binding sites in peripheral tissue following ACS exposure. Importantly, the same methylation signature was identified in multiple tissue types (PFC vs blood), at multiple developmental timepoints (PND1 vs PND14 PFCs), and in multiple species (guinea pigs vs humans). That the signature was also evident in association with high levels of endogenous glucocorticoids in 5-year-old children highlights the potential strength of this signature as a biomarker for fetal exposure to high levels of exogenous and endogenous glucocorticoids in early life (fetal and neonatal). The latter occurs in cases of high fetal, neonatal or maternal stress. However, we acknowledge that the data reported here were analysed using bioinformatic approaches and may not directly reflect the activity levels of the transcription factor [60]. DNA-protein interactions are also dependent on various additional environmental triggers [61]. As such, future studies to examine such DNA-protein interactions will be required to delineate which of the gene pathways we identified are differentially regulated at a functional level. Furthermore, studies to perform comprehensive DNA methylation and transcriptomic association analyses will support the functional relevance of the differential methylation at TFBS and downstream molecular changes. Nonetheless, our identification of the same signatures in human neonatal blood is highly valuable, emphasizing the potential for the clinical translation of molecular epigenetic data. We are currently assessing neurocognitive outcomes in the OBS cohort as children reach 5-years of age. In future studies, we will be positioned to relate DNA methylation changes to functional outcomes. Further prospective longitudinal studies with larger sample sizes will be important in establishing whether the methylation signature identified in this study can be used for predictive or diagnostic purposes. The identification of biomarkers at early timepoints is critical as the brain is most malleable in these early stages of development. Recognizing epigenetic differences at this early stage provides a deeper understanding of the molecular pathways during a sensitive window of development. Supported by future studies, these data can enable the advancement of individualized intervention strategies, designed to identify infants and children most at risk of developing poor phenotypes following prenatal adversities and provide therapeutic solutions to reshape the molecular landscape and ameliorate developmental outcomes.
Code availability
All codes used in this study are posted at https://github.com/bkim-lab/DNAm/.
Data availability
The original guinea pig datasets can be found on GSE275411. Datasets generated from the Ontario Birth Study (OBS) cohort are available from the OBS executive committee on reasonable request and in accordance with OBS guidelines.
References
Dean F, Yu C, Lingas RI, Matthews SG. Prenatal glucocorticoid modifies hypothalamo-pituitary-adrenal regulation in prepubertal guinea pigs. Neuroendocrinology. 2001;73:194–202. https://doi.org/10.1159/000054636
Sparrow S, Manning JR, Cartier J, Anblagan D, Bastin ME, Piyasena C, et al. Epigenomic profiling of preterm infants reveals DNA methylation differences at sites associated with neural function. Transl Psychiatry. 2016;6:e716 https://doi.org/10.1038/tp.2015.210
Moisiadis VG, Constantinof A, Kostaki A, Szyf M, Matthews SG. Prenatal glucocorticoid exposure modifies endocrine function and behaviour for 3 generations following maternal and paternal transmission. Sci Rep. 2017;7:11814.
Alexander N, Rosenlöcher F, Stalder T, Linke J, Distler W, Morgner J, et al. Impact of antenatal synthetic glucocorticoid exposure on endocrine stress reactivity in term-born children. J Clin Endocrinol Metab. 2012;97:3538–44. https://doi.org/10.1210/jc.2012-1970
Ilg L, Kirschbaum C, Li SC, Rosenlöcher F, Miller R, Alexander N. Persistent effects of antenatal synthetic glucocorticoids on endocrine stress reactivity from childhood to adolescence. J Clin Endocrinol Metab. 2019;104:827–94. https://doi.org/10.1210/jc.2018-01566
Khalife N, Glover V, Taanila A, Ebeling H, Järvelin MR, Rodriguez A. Prenatal glucocorticoid treatment and later mental health in children and adolescents. PLoS ONE. 2013;8:e81394.
Stålnacke J, Diaz Heijtz R, Norberg H, Norman M, Smedler AC, Forssberg H. Cognitive outcome in adolescents and young adults after repeat courses of antenatal corticosteroids. J Pediatr. 2013;163:441–6. https://doi.org/10.1016/j.jpeds.2013.01.030
Räikkönen K, Gissler M, Tapiainen T, Kajantie E. Associations between maternal antenatal corticosteroid treatment and psychological developmental and neurosensory disorders in children. JAMA Netw Open. 2022;5:E2228518.
Räikkönen K, Gissler M, Kajantie E. Associations between maternal antenatal corticosteroid treatment and mental and behavioral disorders in children. JAMA J Am Med Assoc 2020;323:1924–33. https://doi.org/10.1001/jama.2020.3937
Murphy KE, Willan AR, Hannah ME, Ohlsson A, Kelly EN, Matthews SG, et al. Effect of antenatal corticosteroids on fetal growth and gestational age at birth. Obstet Gynecol. 2012;119:917–23.
Asztalos EV, Murphy KE, Willan AR, Matthews SG, Ohlsson A, Saigal S, et al. Multiple courses of antenatal corticosteroids for preterm Birth study outcomes in children at 5 years of age (MACS-5). JAMA Pediatr. 2013;167:1102–10.
Asztalos E, Willan A, Murphy K, Matthews S, Ohlsson A, Saigal S, et al. Association between gestational age at birth, antenatal corticosteroids, and outcomes at 5 years: multiple courses of antenatal corticosteroids for preterm birth study at 5 years of age (MACS-5). BMC Pregnancy Childbirth 2014;14::272.
Davis EP, Sandman CA, Buss C, Wing DA, Head K. Fetal glucocorticoid exposure is associated with preadolescent brain development. Biol Psychiatry. 2013;74:647–55.
Rodriguez JS, Zrcher NR, Keenan KE, Bartlett TQ, Nathanielsz PW, Nijland MJ. Prenatal betamethasone exposure has sex specific effects in reversal learning and attention in juvenile baboons. Am J Obstet Gynecol. 2011;204:545.e1–10. https://doi.org/10.1016/j.ajog.2011.01.063
Owen D, Matthews SG. Repeated maternal glucocorticoid treatment affects activity and hippocampal NMDA receptor expression in juvenile guinea pigs. J Physiol. 2007;578:249–57.
Edgar RD, Jones MJ, Meaney MJ, Turecki G, Kobor MS. BECon: a tool for interpreting DNA methylation findings from blood in the context of brain. Transl Psychiatry. 2017;7:e1187–10.
Braun PR, Han S, Hing B, Nagahama Y, Gaul LN, Heinzman JT, et al. Genome-wide DNA methylation comparison between live human brain and peripheral tissues within individuals. Transl Psychiatry. 2019;9:47.
Kim B, Kostaki A, Matthews SG. Conserved DNA methylation signatures in the prefrontal cortex of newborn and juvenile guinea pigs following antenatal corticosteroid exposure. BioRxiv. 2024:2024.03.26.586671.
Kim B, Sasaki A, Murphy K, Matthews SG. DNA methylation signatures in human neonatal blood following maternal antenatal corticosteroid treatment. Transl Psychiatry. 2022;12:132. https://doi.org/10.1038/s41398-022-01902-4.
Reul JMHM, Van Den Bosch FR, De Kloet ER. Relative occupation of type-I and type-II corticosteroid receptors in rat brain following stress and dexamethasone treatment: functional implications. J Endocrinol. 1987;115:459–67.
Simhan HN. Practice bulletin no. 171: management of preterm labor. Obstet Gynecol. 2016;128:e155–64.
Walker RM, MacGillivray L, McCafferty S, Wrobel N, Murphy L, Kerr SM, et al. Assessment of dried blood spots for DNA methylation profiling [version 1; peer review: 2 approved]. Wellcome Open Res. 2019;4:44.
Sasaki A, Kim B, Murphy KE, Matthews SG. Impact of ex vivo sample handling on DNA methylation profiles in human cord blood and neonatal dried blood spots. Front Genet. 2020;11:224.
Martin M. Cutadapt removes adapter sequences from high-throughput sequencing reads. EMBnet J. 2011;17:10–12.
Krueger F, Andrews SR. Bismark: a flexible aligner and methylation caller for Bisulfite-Seq applications. Bioinformatics. 2011;27:1571–2.
Langmead B, Salzberg SL. Fast gapped-read alignment with Bowtie 2. Nat Methods Nat Publ Group. 2012;9:357–9.
Li H, Handsaker B, Wysoker A, Fennell T, Ruan J, Homer N, et al. The sequence alignment/map format and SAMtools. Bioinformatics. 2009;25:2078–9.
Dolzhenko E, Smith AD. Using beta-binomial regression for high-precision differential methylation analysis in multifactor whole-genome bisulfite sequencing experiments. BMC Bioinforma. 2014;15:215.
Song Q, Decato B, Hong EE, Zhou M, Fang F, Qu J, et al. A reference methylome database and analysis pipeline to facilitate integrative and comparative epigenomics. PLoS ONE. 2013;8:e81148.
Kishore K, de Pretis S, Lister R, Morelli MJ, Bianchi V, Amati B, et al. methylPipe and compEpiTools: a suite of R packages for the integrative analysis of epigenomics data. BMC Bioinforma. 2015;16:1–11.
Loken C, Gruner D, Groer L, Peltier R, Bunn N, Craig M, et al. SciNet: lessons learned from building a power-efficient top-20 system and data centre. J Phys Conf Ser. 2010;256:012026.
Raudvere U, Kolberg L, Kuzmin I, Arak T, Adler P, Peterson H, et al. g:Profiler: a web server for functional enrichment analysis and conversions of gene lists (2019 update). Nucleic Acids Res. 2019;47:W191.
Bailey T, Elkan C. Fitting a mixture model by expectation maximization to discover motifs in biopolymers. Proc Int Conf Intell Syst Mol Biol 1994;2:28–36.
Quinlan AR, Hall IM. BEDTools: a flexible suite of utilities for comparing genomic features. Bioinformatics. 2010;26:841–2.
Gupta S, Stamatoyannopoulos JA, Bailey TL, Noble WS. Quantifying similarity between motifs. Genome Biol. 2007;8:R24.
Castro-Mondragon JA, Riudavets-Puig R, Rauluseviciute I, Berhanu Lemma R, Turchi L, Blanc-Mathieu R, et al. JASPAR 2022: the 9th release of the open-access database of transcription factor binding profiles. Nucleic Acids Res. 2022;50:D165–73.
Grant CE, Bailey TL, Noble WS. FIMO: scanning for occurrences of a given motif. Bioinformatics. 2011;27:1017.
Stelzer G, Rosen N, Plaschkes I, Zimmerman S, Twik M, Fishilevich S, et al. The genecards suite: from gene data mining to disease genome sequence analyses. Curr Protoc Bioinforma. 2016;54:1.30.1–1.30.33.
Constantinof A, Boureau L, Moisiadis VG, Kostaki A, Szyf M, Matthews SG. Prenatal glucocorticoid exposure results in changes in gene transcription and DNA methylation in the female juvenile guinea pig hippocampus across three generations. Sci Rep. 2019;9:18211.
Sasaki A, Eng ME, Lee AH, Kostaki A, Matthews SG. DNA methylome signatures of prenatal exposure to synthetic glucocorticoids in hippocampus and peripheral whole blood of female guinea pigs in early life. Transl Psychiatry. 2021;11:63.
Flaster H, Bernhagen J, Calandra T, Bucala R. The macrophage migration inhibitory factor-glucocorticoid dyad: regulation of inflammation and immunity. Mol Endocrinol. 2007;21:1267–80.
Smith CEL, Alexandraki A, Cordery SF, Parmar R, Bonthron DT, Valleley EMA. A tissue-specific promoter derived from a SINE retrotransposon drives biallelic expression of PLAGL1 in human lymphocytes. PLoS ONE. 2017;12:e0185678.
Du J, Jing J, Yuan Y, Feng J, Han X, Chen S, et al. Arid1a-Plagl1-Hh signaling is indispensable for differentiation-associated cell cycle arrest of tooth root progenitors. Cell Rep. 2021;35:108964.
Vega-Benedetti AF, Saucedo CN, Zavattari P, Vanni R, Royo F, Llavero F, et al. PLAGL1 gene function during hepatoma cells proliferation. Oncotarget. 2018;9:32775.
Kuo CL, Liu ST, Chang YL, Wu CC, Huang SM. Zac1 regulates IL-11 expression in osteoarthritis. Oncotarget. 2018;9:32478–95.
Schmidt-Edelkraut U, Hoffmann A, Daniel G, Spengler D. Zac1 regulates astroglial differentiation of neural stem cells through Socs3. Stem Cells. 2013;31:1621–32.
Kemp MW, Newnham JP, Challis JG, Jobe AH, Stock SJ. The clinical use of corticosteroids in pregnancy. Hum Reprod Update. 2016;22:240–59.
Zeng Y, Ge G, Lei C, Zhang M. Beyond fetal immunity: a systematic review and meta-analysis of the association between antenatal corticosteroids and retinopathy of prematurity. Front Pharm. 2022;13:75974.
Chang TH, Tsai MF, Gow CH, Wu SG, Liu YN, Chang YL, et al. Upregulation of microRNA-137 expression by Slug promotes tumor invasion and metastasis of non-small cell lung cancer cells through suppression of TFAP2C. Cancer Lett. 2017;402:190–202.
Wu VT, Kiriazov B, Koch KE, Gu VW, Beck AC, Borcherding N, et al. A TFAP2C gene signature is predictive of outcome in HER2-positive breast cancer. Mol Cancer Res. 2020;18:46–56.
Wang X, Sun D, Tai J, Chen S, Yu M, Ren D, et al. TFAP2C promotes stemness and chemotherapeutic resistance in colorectal cancer via inactivating hippo signaling pathway. J Exp Clin Cancer Res. 2018;37:1–16.
Pastor WA, Liu W, Chen D, Ho J, Kim R, Hunt TJ, et al. TFAP2C regulates transcription in human naive pluripotency by opening enhancers. Nat Cell Biol. 2018;20:553–64.
Ronchetti S, Ricci E, Migliorati G, Gentili M, Riccardi C. Molecular sciences how glucocorticoids affect the neutrophil life. Int J Mol Sci. 2018;19:4090.
Müller N, Fischer HJ, Tischner D, van den Brandt J, Reichardt HM. Glucocorticoids induce effector T cell depolarization via ERM proteins, thereby impeding migration and APC conjugation. J Immunol. 2013;190:4360–70.
Reichardt SD, Amouret A, Muzzi C, Vettorazzi S, Tuckermann JP, Lühder F, et al. The role of glucocorticoids in inflammatory diseases. Cells. 2021;10:2921.
Carvalho C, L’Hôte V, Courbeyrette R, Kratassiouk G, Pinna G, Cintrat JC, et al. Glucocorticoids delay RAF-induced senescence promoted by EGR1. J Cell Sci. 2019;132:jcs230748.
Wang B, Guo H, Yu H, Chen Y, Xu H, Zhao G. The role of the transcription factor EGR1 in cancer. Front Oncol. 2021;11:64254.
Andrews MH, Kostaki A, Setiawan E, McCabe L, Owen D, Banjanin S, et al. Developmental regulation of the 5-HT7 serotonin receptor and transcription factor NGFI-A in the fetal guinea-pig limbic system: influence of GCs. J Physiol. 2004;555:659.
Nätt D, Johansson I, Faresjö T, Ludvigsson J, Thorsell A. High cortisol in 5-year-old children causes loss of DNA methylation in SINE retrotransposons: a possible role for ZNF263 in stress-related diseases. Clin Epigenetics. 2015;7:91.
Lin LH, Lee HC, Li WH, Chen BS. A systematic approach to detecting transcription factors in response to environmental stresses. BMC Bioinforma. 2007;8:1–13.
Ni P, Wilson D, Su Z. A map of cis-regulatory modules and constituent transcription factor binding sites in 80% of the mouse genome. BMC Genom. 2022;23:714.
Acknowledgements
This research was funded by the Canadian Institutes of Health Research (FDN-148368) and by a Canada Research Chair (Tier 1, CRC-2019-00386) awarded to Stephen G. Matthews. Animal work was performed by Alisa Kostaki. Bioinformatic computations were performed on the Niagara supercomputer at the SciNet HPC Consortium, funded by the Canadian Foundation for Innovation; the Government of Ontario; Ontario Research Fund—Research Excellence; and the University of Toronto.
Author information
Authors and Affiliations
Contributions
BK performed the experiment and data analysis and wrote the manuscript; AK produced the guinea pig cohorts; SM contributed to data analysis and reviewed the manuscript; SGM conceptualized and supervised the project and reviewed the manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
All animal experiments detailed in this study were approved by the Animal Care Committee at the University of Toronto (Protocol #12058) and is in accordance with the Canadian Council on Animal Care. This complies with the ARRIVE guidelines [62]. Human subjects were retrospectively identified through the Ontario Birth Study (OBS) [63] at Sinai Health Systems (REB:17-0210-E, Toronto, Canada). Informed consent was provided by all subjects upon OBS enrolment.
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Kim, B., Kostaki, A., McClymont, S. et al. Identification of a DNA methylation signature in whole blood of newborn guinea pigs and human neonates following antenatal betamethasone exposure. Transl Psychiatry 14, 465 (2024). https://doi.org/10.1038/s41398-024-03175-5
Received:
Revised:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s41398-024-03175-5