Abstract
The voltage-gated calcium channel (VGCC) subunit complex is comprised of the α1 subunit, the ion-permeable channel, and three auxiliary subunits: β, α2δ, and γ. β is the most extensively studied auxiliary subunit and is necessary for forward trafficking of the α1 subunit to the plasma membrane. VGCCs mediate voltage-dependent movement of calcium ions into neuronal cytoplasm, including at dendrites, where intracellular calcium spikes initiate signaling cascades that shape the structural plasticity of dendritic spines. Genetic studies strongly implicate calcium signaling dysfunction in the etiology of neurodevelopmental disorders including schizophrenia. Dendritic spine density is significantly decreased in schizophrenia in the primary auditory cortex where it is driven by the loss of small spines, and small spine loss associated with increased peptide levels of ALFDFLK found in the VGCC β subunit β4. Overexpressing the gene that encodes the voltage-gated calcium channel subunit β4, CACNB4, selectively reduced small spine density in vitro. In the current study we extended this observation in an intact mammalian system within a relevant neurodevelopmental context. We overexpressed CACNB4 in early development, assessed spine density and morphology in adult male and female mouse cortex, and characterized β1-4 protein levels and β4 protein-protein interactions. Overexpression reduced small spine density in females. This effect was not dependent on the estrous stage. Instead, it corresponded to sex differences in the murine β4 interactome. The VGCC subunit β1b was significantly enriched in the β4 interactome of male relative to female mice, and thus may have served to mitigate VGCC overexpression-mediated spine loss in male mice.
Similar content being viewed by others
Introduction
β is the most extensively studied auxiliary subunit of voltage-gated calcium channels (VGCCs) [1]. β and the two other auxiliary VGCC subunits α2δ and γ, bind to the α1 VGCC subunit with 1:1:1:1 reversible stoichiometry [2,3,4]. There are four β protein subfamilies, β1-4 [5]. Each β protein subtype is encoded by a separate gene with multiple splice variants. β1b is the only β1 isoform expressed in the brain. Otherwise, β subunits are highly expressed at transcript and protein levels in mouse brains except β2d and β2e [2, 6]. β4a expression is limited to the cerebellum [2].
β4 is the focus of the current study and is encoded by the gene CACNB4, which has five known splice variants [7]. β4 variants are generally highly expressed in the brain, including in rodents, where transcript and protein levels are altered in several brain regions as a function of age [2, 8, 9]. β4 preferentially binds the α1 CaV2.1 subunits [10, 11], 40% of α1 CaV1s, and less frequently to CaV2.2 and CaV2.3 [2, 12, 13]. The neuronal subcellular distribution of β4 is diffuse; β4 is located at the plasma membrane, in intracellular space, axons, dendrites, and in dendritic spines [9]. β4 is also present in synapse preparations for mass spectrometry [14].
β subunits perform similar functions, the most important being to forward traffick the α1 subunit to the plasma membrane [2, 15,16,17,18]. α1 subunits established at the plasma membrane mediate voltage-dependent movement of calcium into the cytoplasm of neurons [19,20,21]. Synaptic depolarizations and back-propagating action potentials trigger VGCC-mediated calcium influx in dendrites [19]. Dendritic calcium spikes initiate signaling cascades that shape the structural and functional plasticity of dendritic spines [19, 22, 23].
Genetic studies strongly implicate calcium signaling dysfunction, including altered VGCC function, in the etiology of neurodevelopmental disorders, including schizophrenia [24, 25]. Dendritic spine density (DSD) is significantly reduced in the primary auditory cortex (A1) in schizophrenia and loss of spines with small volumes drives this reduction [26,27,28,29,30]. Our previous study revealed levels of the tryptic peptide ALFDFLK found in β4 inversely correlated with DSD of small, but not large, spines. CACNB4 overexpression (abbreviated here: β4OE) selectively reduced small spine density in dissociated cortical neuron culture [30]. While β4 expression is not altered in schizophrenia, it may nevertheless represent a modifiable target to enhance spine density [31].
The goal of the current study was to extend our previous in vitro observation to the neurodevelopmental context most relevant to schizophrenia onset (adolescence to early adulthood) and study both sexes in mice, leveraging our previous work. To accomplish this we assessed DSD and spine morphology in the cortex of male and female mice at the adult timepoint of postnatal day 84 (P84) following β4OE. We also characterized β1-4 protein levels and β4 protein-protein interactions using co-immunoprecipitation mass spectrometry (CoIP-MS). β4OE selectively reduced the density of small spines, but this effect was significant only in female mice. β4OE-mediated spine loss did not result from the estrous stage. Instead, the sex-dependent effect of β4OE on DSD corresponded to sex differences in the β4 interactome. The VGCC subunit β1b, the brain β1 isoform, was significantly enriched in the β4 interactome of brain tissue from males and thus may have served to mitigate spine loss in β4OE male mice.
Materials and methods
Experimental animals
All experiments were approved by the IACUC at the University of Pittsburgh in accordance with the guidelines in the USPHS Guide for Care and Use of Laboratory Animals. E16 pregnant C57BL/6J dams were acquired from The Jackson Laboratory and singly housed in standard micro isolator cages on a 12 h light/dark cycle with food and water provided ad libitum.
AAV-exposed C57BL/6J mice
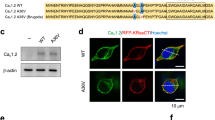
For quantitative microscopy, the adeno-associated virus (AAV) AAV2-hSyn-mCherry (Titer ≥5 × 1012 vg/mL), which confers neuronal expression of mCherry, was obtained from AddGene (#114472 Watertown, MA, USA). AAV2-CaMKII-eGFP-WPRE (Titer = 1.088e13 gc/mL) and AAV2-CaMKII-CACNB4-P2A-eGFP-WPRE (Titer = 4.94e13 gc/mL), both of which express GFP in excitatory cells, were acquired from Penn Vector Core. Two AAV solutions were prepared by combining AAV2-hSyn-mCherry with either control AAV (AAV2-CaMKII-eGFP-WPRE) or AAV-CACNB4 (AAV2-CaMKII-CACNB4-P2A-eGFP-WPRE) 1:1 with dilution 1:10 in 1× PBS to achieve sparse AAV transduction [32, 33]. Control (CN) mice were produced by exposure to 1 μL control AAV injectate. β4OE mice were produced by exposure to 1 μL AAV-CACNB4 injectate. β4OE was verified by comparing tissue samples from three mice (1 AAV-naïve, 1 CN, 1 β4OE) (Fig. 1A) using SDS-PAGE and western blot (see Supplemental Methods).
All figures: n.s. p > 0.05, *p < =0.05, **p < =0.01, ***p < =0.001. A Western blot confirming β4 overexpression. (Left) Mean optical density (a.u.) of β4 was significantly increased in β4OE compared to AAV Naïve (t = 3.818, DF = 2, p = 0.0311) and separately to CN (t = 3.016, DF = 2, p = 0.0473). Error bars = SEM. Unpaired one-tailed t-tests (α = 0.05). (Right) western blot showing β4 bands at ~51–55 kD, as well as a band at ~82 kD in β4OE tissue, which is the predicted molecular weight of a β4-GFP fusion protein (GFP molecular weight = 27 kD). The mean optical density of β4-GFP fusion protein band was not included in the assessment of overall β4 overexpression. B β4OE significantly reduced mean DSD at the mouse level (F = 9.249, DF = 3, p = 0.01). Data points are mean DSD values from individual mice. Error bars = SD. ANOVA (α = 0.05). C DSD was significantly reduced in β4OE, compared to control (CN) mice at the neuron level (t-value = 3.171, DF = 13, p = 0.0074) (n = 42 from CN males, n = 28 from β4OE males, n = 30 from CN females and n = 70 from β4OE females). D β4OE significantly reduced DSD in neurons from female mice (t-value = 3.655, DF = 7, p = 0.008). E In contrast, β4OE did not significantly impact DSD in neurons from male mice (t-value = 1.757, DF = 5, p = 0.1392). For C–E data points are DSD from individual neurons; Error bars = SD; LMM with random effect of mouse (α = 0.05).
AAV solutions were coded “A” or “B”. Twenty-four P0-P2 C57BL/6J mouse pups were exposed to injectate A (50%) or B using the BReVI method [34, 35]. AAV was injected intracranially 1 mm rostral to the left earbud and 1 mm lateral from the midline. Mice were weaned at P21, provided environmental enrichment (hut and exercise wheel), and housed with same-sex littermates until estrous stage assessment (see Supplemental Methods) and sacrifice on P84.
AAV-naïve C57BL/6J mice
Four male P28, four female P28, four male P84, and four female P84 mice were used for SDS-PAGE and western blot. Four additional male P84 and four additional female P84 C57BL/6J mice were used for CoIP-MS.
Quantitative microscopy
Perfusion and tissue processing
See Supplemental Fig. 1 for a detailed summary of mouse and cell numbers, and quantitative microscopy schema. Mice were deeply anesthetized with 150 mg/kg Pentobarbital and transcardially perfused with 1xPBS and 4% PFA. Brains were extracted, post-fixed, and moved to 18% sucrose. Sixty micrometer-thick coronal sections were cryostat cut into 12-well plates filled with cryoprotectant.
Immunohistochemistry
The region of interest (ROI) was the sensory cortex: primary and secondary auditory (A1, A2) and visual (V1, V2) cortices and temporal association cortex (TeA) [36, 37]. Four tissue sections per mouse corresponding to plates 55, 57, 59, and 61 in The Mouse Brain In Stereotaxic Coordinates [37] were washed, incubated in 1% NaBH4, incubated in blocking solution and then in primary antibody solution with 1:2000 guinea pig anti-NeuN (Millipore #ABN90 Danvers, MA, USA) and 1:1000 rabbit anti-RFP (Rockland Immunochemicals, Inc #600-401-376 Pottstown, PA, USA). Sections were subsequently incubated in secondary antibody solution with 1:500 goat anti-guinea pig 405 (Abcam #Ab175678 Branford, CT, USA) and 1:500 goat anti-rabbit Alexa Fluor 568 (Thermo Fisher Scientific #A11036 Waltham, MA, USA) and mounted on TruBond380 micro slide glass (Matsunami, Osaka, Japan) using ProLong Gold antifade mountant (Invitrogen, Waltham, MA, USA).
Imaging and analysis
See Supplemental Methods for complete details for imaging, processing, and spine volume assessment. Briefly, images were captured using a spinning disk confocal microscope and Slidebook 6 (3i-Intelligent Imaging Innovations, Denver, CO, USA) software. 2-D images of each tissue section were acquired at 1.25× and the mouse atlas [37] and examination of NeuN and mCherry fluorescence were used to verify layer 5 (L5) and region (Supplemental Fig. 2A) [38,39,40]. mCherry+ L5 pyramidal cells with somal GFP fluorescence, i.e. GFP + mCherry + cells (n = 170), were captured in 3-D image stacks at 60× (Supplemental Fig. 2B, C). mCherry+ L5 pyramidal cells lacking GFP in sections from female β4OE mice (β4OE-cells) (n = 86) were captured in 3-D image stacks and served as within mouse internal control.
3-D image stacks were smoothed. mCherry+ basal dendritic segments > 50 μm in length, located >10 μm away from the cell body and >3 μm from a dendrite branch point were cropped into individual image stacks. The mean (SD) distance from the soma was 15.85 (4.48) μm, indicating dendritic segments were on proximal branches. Segment lengths were measured and spine counting and categorization were performed. DSD for each neuron was calculated using:
Two neurons per mouse were further assessed to determine if the significant main effect of the group on mean DSD was driven by a significant difference in the density of spines of a particular volume. Each spine object (n = 1543) was masked (Supplemental Fig. 2D), volume (μm3) calculated and organized into ten size bins based on volume i.e. <0.1 μm3, 0.1–0.2 μm3, and so on [30]. Dendritic protrusions were classified into types: short stubby, long stubby, short mushroom, long mushroom, thin, branched, or atypical spine or filopodia (Supplemental Fig. 2E) [36, 41, 42].
Protein assays
SDS-PAGE and western blot
β protein levels in male and female C57BL/6J wildtype mouse A1 were assessed at two developmental time points relevant to the risk period for schizophrenia onset [36, 43, 44]: P28, which is associated with murine adolescence/estrous cycle commencement and P84, corresponding to adulthood. See Supplemental Methods for complete details. Briefly, mice were anesthetized and brains extracted. Bilateral A1 tissue was manually cut from cryostat sections and combined with extraction buffer. Twenty microgram protein samples were loaded into 4–20% SDS-PAGE gradient gels and separated. β-tubulin (50 kD) was the loading control. Samples were transferred to polyvinylidene fluoride membranes and incubated in a blocking solution. Membranes were incubated in primary antibody solutions containing: either 1:100 mouse anti-CaVβ1 (Neuromab #73-052 Davis, CA, USA), 1:800 rabbit anti-CaVβ3 (Alomone Labs #AAC-008 Jerusalem, Israel) or 1:1000 mouse anti-CaVβ4 (Neuromab #75-054) and 1:600,000 rabbit anti-β-tubulin (Abcam #ab6046). Prior to scanning membranes were incubated in Li-Cor Biosciences (Lincoln, NE, USA) IRDye secondary antibody solutions containing: either 1:10,000 goat anti-mouse 800 nm (#926-32210) and 1:10,000 goat anti-rabbit 680 nm (#926-68071) or 1:10,000 goat anti-rabbit 800 nm (#926-32211) and 1:10,000 goat anti-mouse 680 nm (#926-68070).
Co-immunoprecipitation
Anti-CaVβ4 (Neuromab #75-054) was used to immunoprecipitate β4 from synaptosome preparations previously [14] and by us for β4 immunoprecipitation. Anti-CaVβ4 immunoprecipitated β4 from cortical homogenate in a co-immunoprecipitation western blot using 1:1000 goat anti-CACNB4 (Everest Biotech Ltd #EB06591 Oxfordshire, UK) and 1:10,000 Li-Cor donkey anti-goat 800 secondary antibody (#926-32214) for detection (Supplemental Fig. 3A). Tissue samples were prepared from mice (Supplemental Fig. 3B–E) as previously described [45]. Briefly, mice were sacrificed and brains were extracted. The bilateral cerebral cortex and underlying structures were separated from the olfactory bulb and tissue posterior to the cerebral cortex-midbrain junction. Tissue samples were homogenized in lysis buffer [14] and brain lysate was incubated on ice. The supernatant was RNAse treated for 30 min. Fifty microliter input samples were generated. Antibody-coupled beads were prepared by incubating 3 mg Dynabeads (Thermo Fisher Scientific #10018D) in 30 μg mouse anti-CaVβ4. The supernatant was incubated with pre-washed Dynabeads beads not coupled to antibody (one “CN” per mouse) or antibody-coupled beads (one “β4-IP” sample per mouse). Finally, protein in β4-IP and CN samples (n = 16) was eluted from beads.
Liquid chromatography mass spectrometry (LC-MS/MS)
One microliter (1667fmol) 13C615N4-l-Arginine13C615N2-l-Lysine Stable Isotope Labeled (SIL) β4 protein standard (Origene #PH310440 Rockville, MD, USA) was added to each sample (Supplemental Fig. 3C). Samples were digested with trypsin using high-yield S-Trap™ Micro Spin columns (Protifi #C02-micro-80 Farmingdale, NY, USA) following manufacturer protocol and desalted using 0.6 μL C18 resin ZipTips (Millipore Sigma #ZTC18S096). A pooled instrument control (PIC) was made by combining 1 μL from each sample (Supplemental Fig. 3D). 2 μL (~1 μg) from each sample plus three 2 μL aliquots of the PIC were resolved on an EASY C18 column (1.7 µm, 2.1 × 50 cm) at 300 nL/min with an UltiMate™ 3000 RSLCnano HPLC system over a 90 min gradient and analyzed on an Orbitrap Eclipse™ Tribrid™ MS (Thermo Fisher Scientific) operated in MS/MS after a final sample reordering (Supplemental Fig. 3E). Peptide/protein identification, quantification and initial peptide peak area normalization were performed in Skyline Software [46].
Calculations and statistics
Quantitative microscopy
For quantitative microscopy statistical tests were performed at: 1) mouse level (n = 16) and 2) neuron level (n = 170) in IBM SPSS Statistics. For mice, ANOVA (α = 0.05) was used to test the main effects of group, sex, and group-by-sex interaction on mean DSD. Separately, for neurons, the potential effects of the mean distance of the dendritic segment from soma, and the mean number of GFP + mCherry + cells in the sensory cortex and in individual regions were evaluated. None were significantly associated with DSD and thus not included in the final statistical model for neurons. For neurons, a linear mixed effects model fitted with restricted maximum likelihood (LMM REML) and random effect of the mouse was run to test the main effects of group, sex, and group-by-sex interaction on DSD. ANOVA (α = 0.05) was used to test the significance of the impact of β4OE on the density of spines based on volume, with the main effects of group, sex, and group-by-sex interaction on DSD of each individual size bin. ANOVA (α = 0.05) was used to detect significant differences in mean densities among dendritic protrusion types; the main effects of group, sex, and group-by-sex interaction were again tested with dendritic protrusion types. LMM REMLs with random effect of the mouse were also used to evaluate densities based on estrous stage, condition (β4OE + v. internal control), volume, and protrusion types (Supplemental Tables 1–3).
Western blot
For the western blot, ANOVA (α = 0.05) was used to detect group differences in mean optical density. Mean optical density was calculated by averaging β-tubulin normalized optical density of β protein technical replicates for each mouse and then creating group means. For each β protein assessment, blot significantly impacted the primary outcome measure: mean β/mean β-tubulin, therefore, blot was included as a covariate in the statistical analysis for each β protein assessed.
CoIP-MS
For CoIP-MS, β4 interactome data was analyzed as previously described by our group [45]. Peptide peak areas were exported from Skyline Software. β4 peptide levels were normalized to SIL β4 peptide levels to control for differences in immunoprecipitation efficiency. Peptide peak areas were divided by normalized β4 peptide levels to generate normalized peak area ratios. Mean overall (includes samples from male and female mice) CN-IP peak area and mean overall β4-IP peak area ratios were then calculated. Next, log2 fold change values were calculated using the overall means to detect shifts in distributions of β4-IP enriched protein levels, relative to CN. Peptides with Coefficients of Variation (CV) < 0.3 were compared using paired Student’s t-tests of log2-transformed peak ratios. Significantly altered peptides were defined as those with p < 0.05, p value greater than CV (calculated using false discovery rate at 0.05) and log2 fold change > 1 (peptides enriched in males) or log2 fold change < −1 (peptides enriched in females).
Results
β4OE reduced DSD of L5 pyramidal cells in the sensory cortex
At the mouse level, β4OE significantly reduced mean DSD on basal branches of L5 pyramidal cells in the sensory cortex (F = 9.249, DF = 3, p = 0.01) (Fig. 1B). At the neuron level, LMM REML fitting with random effect of mouse revealed β4OE significantly reduced DSD (t-value = 3.171, DF = 13, p = 0.0074)(Fig. 1C). Male- and female- specific LMMs indicated DSD in males was not significantly associated with β4OE (t-value = 1.757, DF = 5, p = 0.1392), and in female mice, β4OE significantly reduced DSD (t-value = 3.655, DF = 5, p = 0.0081) (Fig. 1E, D), respectively.
β4OE-mediated DSD reduction in female mice is independent of the estrous stage
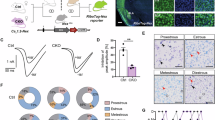
Metestrus/diestrus was the only stage with neurons from both CN and β4OE mice (Fig. 2A). DSD was significantly reduced in female β4OE mice in metestrus/diestrus alone (F = 6.190, DF = 1, p = 0.017)(Fig. 2B). Within mouse assessments of three randomly selected β4OE female mice (Ms10-87, Ms5-10 and Ms16-169) revealed β4OE+ significantly decreased neuron DSD compared to β4OE− internal control (F = 103.646, DF = 1, p < 0.001) regardless of estrous stage. There was a significant interaction between the estrous stage and condition (β4OE+ vs β4OE−) (F = 8.105, DF = 1, p = 0.006), however, the main effect of the estrous stage was not significant (F = 0.985, DF = 1, p = 0.324). DSD of β4OE+ neurons was significantly lower in each mouse: Ms10-87 was in estrus (F = 67.688, DF = 1, p < 0.001) and Ms5-10 (F = 9.502, DF = 1, p = 0.004) and Ms16-169 (F = 126.518, DF = 1, p < 0.001) were in metestrus/diestrus (Fig. 2C). β4OE− DSD across the three β4OE mice described above did not significantly differ based on estrous stage (F = 2.108, DF = 1, p = 0.154) (Fig. 2D).
A Estrous stage breakdown of DSD of neurons from female CN and β4OE mice. Spine density was significantly different based on estrous stage (F = 6.450, DF = 2, p = 0.002). Importantly, note both genotypes are represented in metestrus/diestrus, not proestrus, estrus. Data points are from individual neurons. Error bars = SD. ANOVA (α = 0.05). B β4OE significantly reduced DSD in female mice in metestrus/diestrus (F = 6.190, DF = 1, p = 0.017). Data points are from individual neurons. Error bars = SD. ANOVA (α = 0.05). C Within mouse internal control comparison. DSD of β4OE+ neurons was significantly lower than DSD of β4OE− internal control neurons in three β4OE mice overall (F = 103.646, DF = 1, p < 0.001). There was a significant interaction between the estrous stage and condition (β4OE+ vs β4OE−) (F = 8.105, DF = 1, p = 0.006), however the main effect of the estrous stage was not significant (F = 0.985, DF = 1, p = 0.324). Mice were in different estrous stages (L-R): Ms10-87 was in Estrus (F = 67.688, DF = 1, p < 0.001), and Ms5-10 (F = 9.502, DF = 1, p = 0.004) and Ms16-169 (F = 126.518, DF = 1, p < 0.001) were in metestrus/diestrus on day of sacrifice. Data points from individual neurons. Error bars = SD. ANOVA (α = 0.05). D DSD of β4OE− internal control neurons in the three β4OE mice (in C) did not significantly differ based on estrous stage (F = 2.108, DF = 1, p = 0.154). Data points from individual neurons. Error bars = SD. ANOVA (α = 0.05).
β4OE selectively reduced the density of spines with small volumes in female mice
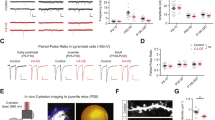
Because β4OE-mediated DSD reductions were significant only in females, subsequent spine analyses focused on female mice. Mean DSD of small <0.1 μm3 spines was significantly decreased in neurons from female β4OE mice (F = 5.276, DF = 1, p = 0.035). The mean DSD of spine objects >0.1 μm3 did not significantly differ based on group (Fig. 3A). β4OE significantly decreased the mean DSD of small spines with volume 0.1 μm3–0.2 μm3 (F = 6.748, DF = 1, p = 0.032) in female β4OE mice in metestrus/diestrus alone (Fig. 3B). The effect of reduced small spine objects was not observed in male mice (Supplemental Fig. 4A).
A Mean DSD of small <0.1 μm3 objects was significantly reduced in neurons from female β4OE mice, relative to CN (F = 5.276, DF = 1, p = 0.035). The mean DSD of larger objects with volumes > 0.1 μm3 did not significantly differ based on genotype in female mice. Error bars = SEM. ANOVA (α = 0.05). B Focusing exclusively on female mice in metestrus/diestrus, β4OE significantly decreased the mean DSD of small objects of 0.1 μm3–0.2 μm3 volume (F = 6.748, DF = 1, p = 0.032). Error bars = SEM. ANOVA (α = 0.05). C β4OE significantly reduced mean density of short stubby (F = 33.939, DF = 1, p < 0.001), long stubby (F = 7.043, DF = 1, p = 0.009), short mushroom (F = 4.342, DF = 1, p = 0.040) and long mushroom (F = 30.354, DF = 1, p < 0.001) spines in female mice (all estrous stages represented) using ANOVA (α = 0.05). Collectively, spines in these four categories made up 95.19% of all protrusions observed in female mice. Error bars = SEM. D Focusing exclusively on female mice in metestrus/diestrus, β4OE significantly decreased mean density of short mushroom spines (F = 20.160, DF = 1, p < 0.001). Error bars = SEM.
β4OE decreased the density of dendritic protrusions in female mice
β4OE significantly decreased the mean density of: short stubby, long stubby, short mushroom, and long mushroom spines (Fig. 3C). These categories comprised the majority (95.19%) of all protrusions observed in females at P84. For metestrus/diestrus, β4OE significantly decreased the mean density of short mushroom spines (F = 20.160, DF = 1, p < 0.001) (Fig. 3D). Alterations to spine morphologic categories of males were subtle (Supplemental Fig. 4B).
β subunit protein levels varied based on age and sex in C57BL/6J mice
β4 levels in A1 were significantly lower at P84, compared to P28, in male and female mice (F = 9.635, DF = 3, p = 0.013) (Fig. 4A), with no significant sex nor age-by-sex interaction. β1 levels were also significantly lower at P84 (F = 21.499, DF = 3, p = 0.001) with a significant main effect of sex (F = 6.944, DF = 3, p = 0.027) and age by sex interaction (F = 6.835, DF = 3, p = 0.028). β1 levels was significantly lower in females at P28 (F = 12.138, DF = 1, p = 0.040) but not P84 (F = 0.016, DF = 1, p = 0.906) (Fig. 4B). Neither age nor sex significantly impacted levels of β3 (Fig. 4C).
A (Left) Mean optical density (a.u.) of β4 was significantly lower in P84, relative to P28 male and female mice (F = 9.635, DF = 3, p = 0.013), with no significant sex or age-by-sex interactions. Error bars = SEM. ANOVA (α = 0.05). (Right) Representative blot showing β4 bands at ~51–55 kD. B (Left) Mean optical density (a.u.) of β1 was significantly lower in P84, relative to P28 male and female mice (F = 21.499, DF = 3, p = 0.001). There was also a main effect of sex (F = 6.944, DF = 3, p = 0.027) and a significant age-by-sex interaction (F = 6.835, DF = 3, p = 0.028). Main effect of sex was significant at P28 (F = 12.138, DF = 1, p = 0.040) but not P84 (F = 0.016, DF = 1, p = 0.906). Error bars = SEM. ANOVA (α = 0.05). (Right) Representative blot showing β1 bands at ~50–80 kD. C (Left) Neither age nor sex significantly impacted the mean optical density (a.u.) of β3 and there was not a significant sex by age interaction. Error bars = SEM. ANOVA (α = 0.05). (Right) Representative blot showing β3 bands at ~55 kD (molecular weight of the predominant β3 isoform).
β1b significantly enriched in the β4 interactome of male C57BL/6J mice
We identified expected peptides/proteins consistent with previous reports2 in the 13023 peptides that comprised the β4 interactome in P84 mouse brain homogenate in the current study including the VGCC subunits CaV2.1, CaV2.2, CaV2.3, and α2δ1 (Supplemental Table 4). Among these peptides (Fig. 5A), eighteen were significantly enriched in males and twenty-four in female mice (Fig. 5B). Of particular interest was the peptide TMATAALAASPAPVSNLQGPYLASGDQPLDR, which was significantly enriched in the β4 interactome of male relative to female P84 mice (log2 fold change = 3.9357, p = 0.0201). This peptide is contained in the auxiliary VGCC subunit β1. The amino acid sequence of this peptide was aligned to the amino acid sequences of each of the four β1 isoforms and revealed this peptide is exclusively found in the only β1 isoform that is expressed in the brain, β1b.
A Volcano plot showing 13023 β4-IP enriched peptides that make up the β4 interactome. Enriched peptides with p < 0.05 are represented either in green (enriched in females) or red (enriched in males). Gray vertical lines (L–R) located at log2 fold change = −1, 1. B Table with the 18 significantly enriched peptides in males (with log2 fold change > 1) and 24 significantly enriched peptides in females (with log2 fold change < −1) revealed using paired Student’s t-tests of log2-transformed peak ratios. TMATAALAASPAPVSNLQGPYLASGDQPLDR (bolded, italicized) is significantly enriched in the β4 interactome of male relative to female P84 wildtype mice and is contained in the b isoform of the β1 subunit of voltage-gated calcium channels.
Discussion
We employed sparse labeling to assess dendritic spines in our tissue. This necessitated expanding the ROI from A1, the region specified in our scientific premise, to regions in the broader sensory cortex. We and others have shown DSD does not significantly differ based on region in the cortex of mice [41, 47,48,49,50]. A limitation of sparse labeling in our study is this led to an uneven number of mice per group. We mitigated this by assessing DSD at both mouse and neuron levels, focusing on neurons since group sizes with n = cell were better balanced.
Several influential studies report circulating hormone levels across the estrous cycle shape DSD in rat hippocampus [51] with elevated levels of 17β-estradiol (E2) during proestrus associated with higher DSD [52,53,54]. Results from studies performed in mouse cortex indicate no change in DSD due to estrous stage: L5 DSD across estrous in somatosensory cortex was unchanged [51], as was L5 DSD in frontal cortex in hormone-treated vs ovariectomized female mice [55]. Nevertheless, we explored the possibility that β4OE-mediated spine loss could be driven by the estrous stage in the mouse cortex in our study. Vaginal cytology was performed on sacrifice day, P84, to control for DSD fluctuations based on age [36, 44]. This resulted in the estrous stage not being equally represented across groups. Thus we focused on comparing DSD in mice in metestrus/diestrus, the only estrous stage represented in both groups. DSD was significantly reduced in female β4OE relative to CN mice in this estrous stage, replicating the overall pattern in females. Importantly, the DSD of β4OE+ neurons was significantly decreased compared to β4OE− (internal control) DSD in 3 β4OE mice. Further, internal control neuron DSD did not significantly differ based on the estrous stage overall in β4OE mice. Our data collectively indicate that β4OE-mediated DSD reduction in female mice was not an artifact of the estrous stage. Moreover, our internal control data suggest that among pyramidal cells β4OE exerts a cell-autonomous effect on DSD.
The primary roles of VGCC β subunits are to forward traffick the VGCC α1 subunit toward the plasma membrane and regulate the biophysical properties of the channel [15,16,17,18]. Given that VGCC currents substantially increase when β subunits are coexpressed with α1 subunits, compared to α1 expression alone [2, 3] and β oligomerization selectively increases VGCC current density [56,57,58], it seems likely that β4OE may have increased calcium current density through VGCCs in the current study. Postsynaptic calcium spikes are compulsory for initiating signaling cascades that shape synaptic plasticity induction and actin cytoskeleton remodeling [19, 22, 23]. If β4OE mediates calcium entry into spines at levels that exceed the regulatory capacity of these spines, synaptotoxicity and subsequent spine loss may result. Synaptotoxicity, a feature of early pathogenesis, is well described in Alzheimer’s disease where Aβ oligomer-induced synaptotoxicity results in a reduced density of excitatory synapses [59,60,61,62]. A number of underlying molecular mechanisms involving elevated cytosolic calcium have been suggested including calcium dyshomeostasis at synaptic sites [63], increased CaV1-mediated calcium entry [64,65,66,67], decreased cytosolic calcium efflux via plasma membrane calcium ATPase and/or Na+/Ca2+ exchanger inhibition [68,69,70,71], and perforated cell membranes [72, 73]. Similarly, emerging research suggests that synptotoxicity and subsequent dendritic spine loss in Parkinson’s disease may result from significantly increased CaV1.3-mediated calcium influx following dopamine depletion [74, 75]. Although synaptotoxicity is typically discussed in the neurodegeneration literature, the data generated in our studies may provide evidence that a similar molecular mechanism could be involved in β4OE-mediated spine loss and/or DSD in schizophrenia more broadly [30].
Dendritic spine numbers undergo changes over the course of neurodevelopment. During normal neurodevelopment, spine numbers increase during the perinatal period and childhood, followed by a decrease starting at the transition from childhood into adolescence and early adulthood. In schizophrenia, this developmental trajectory is altered resulting in significantly lower spine density and spine numbers in adulthood [26,27,28, 76,77,78,79]. Our study found that cortical β4 protein levels are higher in adolescence compared to adulthood, mirroring the age-dependent changes in β4 levels reported in the hippocampus and cerebellum [9]. Increased β4 levels in wildtype mice during this period may contribute to normal synaptic remodeling leading to the normative changes in spine densities over development. In β4OE mice, which have consistently elevated β4 levels since birth, the further increase in β4 levels during adolescence may thus culminate in an exaggerated gain-of-function which results in the observed spine loss in adulthood.
Could there be a potential explanation for why spines of small volumes are particularly susceptible to β4OE? In the current study, small spines (<0.3 μm3) made up 68.96% of all spines observed in P84 mice. The actin cytoskeleton of small spines, many of which are transient and/or lacking a mature postsynaptic density, are particularly vulnerable to the destabilizing effects of altered calcium transients whether through activity-dependent synaptic plasticity or pathological mechanisms [80,81,82]. Recent modeling studies shed light on these observations, finding spine head volume to be inversely proportional to intracellular calcium content, due to calcium efflux and buffering source variables in spines with large surface area. Modeled spine volume was inversely proportional to peak calcium and area under the curve of calcium ions regardless of spine shape [83]. If β4OE does indeed increase calcium transients into the spines of β4OE mice, the smallest spines would be the most susceptible to high calcium concentrations with the potential to induce synaptotoxicity and spine loss.
We observed significant small spine loss only in female β4OE mice, leading us to evaluate whether the β4 interactome differed between males and females. We found enrichment of β1b, (the brain β1 isoform) in the β4 interactome of male mice, suggesting that β1 might mitigate the effects of β4OE in males. Although the presence of β1 in the β4 interactome does not specifically indicate that the two proteins are directly interacting and it is currently unknown if β1 and β4 oligomerize, we cannot rule out this possibility. β subunits readily heterooligomerize including β1 and β3 [56]. β2d–β3 oligomers alter the biophysical properties of CaV1 shifting the peak I–V curve by 20 mV in the depolarized direction compared to monomer expression alone [56]. A potential implication of β1 and β4 oligomerization is to similarly shift the peak I–V curve of VGCCs to depolarized voltages, which would be expected to decrease VGCC-mediated inward flow of calcium, an outcome that could mitigate synaptotoxicity and spine loss.
Our findings extend previous in vitro findings of β4OE-mediated loss of small dendritic spines to a developed mammalian system relevant to the highest risk period for schizophrenia onset. They further reveal nuances including sex differences: (1) β4OE-mediated spine loss was significant in female but not male mice and (2) the VGCC subunit β1 was significantly enriched in the β4 interactome of a male relative to female C57BL/6J mice. The current studies help inform our understanding of mechanisms leading to structural spine plasticity generally, emphasizing the need to study both males and females [84]. Including complementary knockdown experiments would assist in decreasing the possibility that β4OE represents a dominant negative phenotype rather than a gain-of-function phenotype. We attempted to measure the impact of knocking down β4 in primary neuron cortical culture on DSD to help guide β4OE-mediated spine loss interpretations. However, the introduction of β4 shRNA induced impairments of neuronal and dendritic health which precluded DSD measurements, perhaps reflecting additional functions of β4. Indeed β4 has been shown to target the nuclear compartment, unique among β VGCC subunits [85, 86]. Our spine findings do not fully recapitulate the spine phenotype in A1 in postmortem schizophrenia, in which small spine loss extends to both sexes [29, 30], but this divergence is not surprising. Schizophrenia is a polygenic disorder with hundreds of genetic variants and environmental exposures associated with risk [25, 87, 88]. Postmortem human studies demonstrating small spine loss include individuals who likely develop this loss via different combinations of molecular mechanisms. Although our spine findings may not translate to the schizophrenia population as a whole, we cannot rule out the possibility that sex differences in DSD are present in pairs of schizophrenia subjects with shared genetic liability, for example, in individuals that harbor variants in VGCCs that fundamentally alter postsynaptic calcium signaling, a future direction for fruitful inquiry.
Data availability
Data are made available from the corresponding author upon request.
References
Zamponi, GW. Voltage-gated calcium channels. New York: Springer; 2005.
Buraei GW, Yang J. The β subunit of voltage-gated Ca 2+ channels. Physiol Rev. 2010;90:1461–506.
Buraei Z, Yang J. Structure and function of the β subunit of voltage-gated Ca2+ channels. Biochim Biophys Acta Biomembr. 2013;1828:1530–40.
Dolphin AC. Voltage‐gated calcium channels and their auxiliary subunits: physiology and pathophysiology and pharmacology. J Physiol. 2016;594:5369–90.
Castellano A, Perez-Reyes E. Molecular diversity of Ca2+ channel β subunits. Biochem Soc Trans. 1994;22:483–8.
Schlick B, Flucher B, Obermair G. Voltage-activated calcium channel expression profiles in mouse brain and cultured hippocampal neurons. Neuroscience. 2010;167:786–98.
Etemad S, Obermair GJ, Bindreither D, Benedetti A, Stanika R, Di Biase V, et al. Differential neuronal targeting of a new and two known calcium channel β4 subunit splice variants correlates with their regulation of gene expression. J Neurosci. 2014;34:1446–61.
Ludwig A, Flockerzi V, Hofmann F. Regional expression and cellular localization of the α1 and β subunit of high voltage-activated calcium channels in rat brain. J Neurosci. 1997;17:1339–49.
Ferrándiz‐Huertas C, Gil‐Mínguez M, Luján R. Regional expression and subcellular localization of the voltage‐gated calcium channel β subunits in the developing mouse brain. J Neurochem. 2012;122:1095–107.
Tanaka O, Sakagami H, Kondo H. Localization of mRNAs of voltage-dependent Ca2+-channels: four subtypes of α1-and β-subunits in developing and mature rat brain. Mol Brain Res. 1995;30:1–16.
Wittemann S, Mark MD, Rettig J, Herlitze S. Synaptic localization and presynaptic function of calcium channel β4-subunits in cultured hippocampal neurons. J Biol Chem. 2000;275:37807–14.
Scott VE, De Waard M, Liu H, Gurnett CA, Venzke DP, Lennon VA, et al. Beta subunit heterogeneity in N-type Ca2+ channels. J Biol Chem. 1996;271:3207–12.
McEnery MW, Vance CL, Begg CM, Lee WL, Choi Y, Dubel SJ. Differential expression and association of calcium channel subunits in development and disease. J Bioenerg Biomembr. 1998;30:409–18.
Klemmer P, Smit AB, Li KW. Proteomics analysis of immuno-precipitated synaptic protein complexes. J Proteom. 2009;72:82–90.
Gonzalez-Gutierrez G, Miranda-Laferte E, Naranjo D, Hidalgo P, Neely A. Mutations of nonconserved residues within the calcium channel α1-interaction domain inhibit β-subunit potentiation. J Gen Physiol. 2008;132:383–95.
Maltez JM, Nunziato DA, Kim J, Pitt GS. Essential Ca v β modulatory properties are AID-independent. Nat Struct Mol Biol. 2005;12:372–7.
Josephson IR, Varadi G. The beta subunit increases Ca2+ currents and gating charge movements of human cardiac L-type Ca2+ channels. Biophys J. 1996;70:1285–93.
Jones LP, Wei S-K, Yue DT. Mechanism of auxiliary subunit modulation of neuronal α1E calcium channels. J Gen Physiol. 1998;112:125–43.
Higley MJ, Sabatini BL. Calcium signaling in dendritic spines. Cold Spring Harb Perspect Biol. 2012;4:a005686.
Sabatini BL, Svoboda K. Analysis of calcium channels in single spines using optical fluctuation analysis. Nature. 2000;408:589–93.
Yasuda R, Sabatini BL, Svoboda K. Plasticity of calcium channels in dendritic spines. Nat Neurosci. 2003;6:948–55.
Yuste R, Majewska A, Holthoff K. From form to function: calcium compartmentalization in dendritic spines. Nat Neurosci. 2000;3:653–9.
Kasai H, Matsuzaki M, Noguchi J, Yasumatsu N, Nakahara H. Structure–stability–function relationships of dendritic spines. Trends Neurosci. 2003;26:360–8.
Ripke S, O'Dushlaine C, Chambert K, Moran JL, Kähler AK, Akterin S, et al. Genome-wide association analysis identifies 13 new risk loci for schizophrenia. Nat Genet. 2013;45:1150–9.
Stephan R, Benjamin MN, Aiden C, James TRW, Kai-How F, et al. Biological insights from 108 schizophrenia-associated genetic loci. Nature. 2014;511:421–7.
Sweet RA, Henteleff RA, Zhang W, Sampson AR, Lewis DA. Reduced dendritic spine density in auditory cortex of subjects with schizophrenia. Neuropsychopharmacology. 2009;34:374–89.
Moyer CE, Shelton MA, Sweet RA. Dendritic spine alterations in schizophrenia. Neurosci Lett. 2015;601:46–53.
Shelton MA, Newman JT, Gu H, Sampson AR, Fish KN, MacDonald ML, et al. Loss of microtubule-associated protein 2 immunoreactivity linked to dendritic spine loss in schizophrenia. Biol Psychiatry. 2015;78:374–85.
McKinney BC, MacDonald ML, Newman JT, Shelton MA, DeGiosio RA, Kelly RM, et al. Density of small dendritic spines and microtubule-associated-protein-2 immunoreactivity in the primary auditory cortex of subjects with schizophrenia. Neuropsychopharmacology. 2019;44:1055–61.
MacDonald ML, Alhassan J, Newman JT, Richard M, Gu H, Kelly RM, et al. Selective loss of smaller spines in schizophrenia. Am J Psychiatry. 2017;174:586–94.
Gandal MJ, Zhang P, Hadjimichael E, Walker RL, Chen C, Liu S, et al. Transcriptome-wide isoform-level dysregulation in ASD, schizophrenia, and bipolar disorder. Science. 2018;362:eaat8127.
Gholizadeh S, Tharmalingam S, MacAldaz ME, Hampson DR. Transduction of the central nervous system after intracerebroventricular injection of adeno-associated viral vectors in neonatal and juvenile mice. Hum Gene Ther Methods. 2013;24:205–13.
Stoica L, Ahmed SS, Gao G, Esteves MS. AAV-mediated gene transfer to the mouse CNS. Curr Protoc Microbiol. 2013;Chapter 14:Unit 14D.15.
Cheetham CEJ, Grier BD, Belluscio L. Bulk regional viral injection in neonatal mice enables structural and functional interrogation of defined neuronal populations throughout targeted brain areas. Front Neural Circuits. 2015;9:72.
Phifer CB, Terry LM. Use of hypothermia for general anesthesia in preweaning rodents. J Physiol Behav. 1986;38:887–90.
Parker EM, Kindja NL, Cheetham CEJ, Sweet RA. Sex differences in dendritic spine density and morphology in auditory and visual cortices in adolescence and adulthood. Sci Rep. 2020;10:1–11.
Franklin KBJ, Paxinos G. The mouse brain in stereotaxic coordinates. Orlando: Gulf Professional Publishing; 2004.
Bopp R, Holler-Rickauer S, Martin KAC, Schuhknecht GFP. An ultrastructural study of the thalamic input to layer 4 of primary motor and primary somatosensory cortex in the mouse. J Neurosci. 2017;37:2435–48.
Zhang W, Peterson M, Beyer B, Frankel WN, Zhang Z. Loss of MeCP2 from forebrain excitatory neurons leads to cortical hyperexcitation and seizures. J Neurosci. 2014;34:2754–63.
Li H-S, Wang D, Shen Q, Schonemann MD, Gorski JA, Jones KR, et al. Inactivation of numb and numblike in embryonic dorsal forebrain impairs neurogenesis and disrupts cortical morphogenesis. Neuron. 2003;40:1105–18.
Arellano JI, Benavides-Piccione R, DeFelipe J, Yuste R. Ultrastructure of dendritic spines: correlation between synaptic and spine morphologies. Front Neurosci. 2007;1:131–43.
Risher WC, Ustunkaya T, Alvarado JS, Eroglu C. Rapid Golgi analysis method for efficient and unbiased classification of dendritic spines. PLoS One. 2014;9:107591.
Caligioni CS. Assessing reproductive status/stages in mice. Curr Protoc Neurosci. 2009;48:Appendix-4I.
Moyer CE, Erickson SL, Fish KN, Thiels E, Penzes P, Sweet RA. Developmental trajectories of auditory cortex synaptic structures and gap-prepulse inhibition of acoustic startle between early adolescence and young adulthood in mice. Cereb Cortex. 2015;26:2115–26.
Grubisha MJ et al. MAP2 is hyperphosphorylated in schizophrenia and alters its function. bioRxiv:683912 [Preprint]. 2019.
MacLean B, Tomazela DM, Shulman N, Chambers M, Finney GL, Frewen B, et al. Skyline: an open source document editor for creating and analyzing targeted proteomics experiments. Bioinformatics. 2010;26:966–8.
Harris KD, Shepherd GMG. The neocortical circuit: themes and variations. Nat Neurosci. 2015;18:170–81.
Benavides-Piccione R, Ballesteros-Yáñez I, DeFelipe J, Yuste R. Cortical area and species differences in dendritic spine morphology. J Neurocytol. 2002;31:337–46.
Hsu A, Luebke JI, Medalla M. Comparative ultrastructural features of excitatory synapses in the visual and frontal cortices of the adult mouse and monkey. J Comp Neurol. 2017;525:2175–91.
Luebke JI. Pyramidal neurons are not generalizable building blocks of cortical networks. Front Neuroanat. 2017;11:11.
Alexander BH, Barnes HM, Trimmer E, Davidson AM, Ogola BO, Lindsey SH, Mostany R. Stable density and dynamics of dendritic spines of cortical neurons across the estrous cycle while expressing differential levels of sensory-evoked plasticity. Front Mol Neurosci. 2018;11:83.
Woolley CS, Gould E, Frankfurt M, McEwen BS. Naturally occurring fluctuation in dendritic spine density on adult hippocampal pyramidal neurons. J Neurosci. 1990;10:4035–9.
Woolley CS, McEwen BS. Estradiol mediates fluctuation in hippocampal synapse density during the estrous cycle in the adult rat. J Neurosci. 1992;12:2549–54.
Kato A, Hojo Y, Higo S, Komatsuzaki Y, Murakami G, Yoshino H, et al. Female hippocampal estrogens have a significant correlation with cyclic fluctuation of hippocampal spines. Front Neural Circuits. 2013;7:149.
Boivin JR, Piekarski DJ, Thomas AW, Wilbrecht L. Adolescent pruning and stabilization of dendritic spines on cortical layer 5 pyramidal neurons do not depend on gonadal hormones. Dev Cogn Neurosci. 2018;30:100–7.
Lao QZ, Kobrinsky E, Liu Z, Soldatov NM. Oligomerization of Cavβ subunits is an essential correlate of Ca2+ channel activity. FASEB J. 2010;24:5013–23.
Hullin R, Matthes J, von Vietinghoff S, Bodi I, Rubio M, D'Souza K, et al. Increased expression of the auxiliary β2-subunit of ventricular L-type Ca2+ channels leads to single-channel activity characteristic of heart failure. PLoS One. 2007;2:e292.
Chen X, Zhang X, Kubo H, Harris DM, Mills GD, Moyer J, et al. Ca2+ influx–induced sarcoplasmic reticulum Ca2+ overload causes mitochondrial-dependent apoptosis in ventricular myocytes. Circ. Res. 2005;97:1009–17.
Rush T, Martinez-Hernandez J, Dollmeyer M, Frandemiche ML, Borel E, Boisseau S, et al. Synaptotoxicity in Alzheimer’s disease involved a dysregulation of actin cytoskeleton dynamics through cofilin 1 phosphorylation. J Neurosci. 2018;38:10349–61.
Lacor PN, Buniel MC, Chang L, Fernandez SJ, Gong Y, Viola KL, et al. Synaptic targeting by Alzheimer’s-related amyloid β oligomers. J Neurosci. 2004;24:10191–200.
Lacor PN, Buniel MC, Furlow PW, Clemente AS, Velasco PT, Wood M, et al. Aβ oligomer-induced aberrations in synapse composition, shape, and density provide a molecular basis for loss of connectivity in Alzheimer’s disease. J Neurosci. 2007;27:796–807.
Kervern M, Angeli A, Nicole O, Léveillé F, Parent B, Villette V, et al. Selective impairment of some forms of synaptic plasticity by oligomeric amyloid-β peptide in the mouse hippocampus: implication of extrasynaptic NMDA receptors. J Alzheimer’s Dis. 2012;32:183–96.
Green KN. Calcium in the initiation, progression and as an effector of Alzheimer’s disease pathology. J Cell Mol Med. 2009;13:2787–99.
Min D, Guo F, Zhu S, Xu X, Mao X, Cao Y, et al. The alterations of Ca2+/calmodulin/CaMKII/CaV1. 2 signaling in experimental models of Alzheimer’s disease and vascular dementia. Neurosci Lett. 2013;538:60–65.
Kim S, Rhim H. Effects of amyloid-β peptides on voltage-gated L-type Cav1.2 and Cav1.3 Ca2+ channels. Mol cells. 2011;32:289–94.
Ueda K, Shinohara S, Yagami T, Asakura K, Kawasaki K. Amyloid β protein potentiates Ca2+ influx through L-type voltage-sensitive Ca2+ channels: a possible involvement of free radicals. J Neurochem. 1997;68:265–71.
Anekonda TS, et al. L-type voltage-gated calcium channel blockade with isradipine as a therapeutic strategy for Alzheimer’s disease. Neurobiol Dis 2011;41:62–70.
Mata AM. Functional interplay between plasma membrane Ca2+-ATPase, amyloid β-peptide and tau. Neurosci Lett. 2018;663:55–59.
Kim H-S, Lee J-H, Suh Y-H. C-terminal fragment of Alzheimer’s amyloid precursor protein inhibits sodium/calcium exchanger activity in SK–N–SH cell. Neuroreport. 1999;10:113–6.
Colvin RA, Davis N, Wu A, Murphy CA, Levengood J. Studies of the mechanism underlying increased Na+/Ca2+ exchange activity in Alzheimer’s disease brain. Brain Res. 1994;665:192–200.
Moriguchi S, Kita S, Fukaya M, Osanai M, Inagaki R, Sasaki Y, et al. Reduced expression of Na+/Ca2+ exchangers is associated with cognitive deficits seen in Alzheimer’s disease model mice. Neuropharmacology. 2018;131:291–303.
Sepulveda FJ, Parodi J, Peoples RW, Opazo C, Aguayo LG. Synaptotoxicity of Alzheimer beta amyloid can be explained by its membrane perforating property. PLoS One. 2010;5:e11820.
Pacheco CR, Morales CN, Ramírez AE, Muñoz FJ, Gallegos SS, Caviedes PA, et al. Extracellular α-synuclein alters synaptic transmission in brain neurons by perforating the neuronal plasma membrane. J Neurochem. 2015;132:731–41.
Day M, Wang Z, Ding J, An X, Ingham CA, Shering AF, et al. Selective elimination of glutamatergic synapses on striatopallidal neurons in Parkinson disease models. Nat Neurosci. 2006;9:251–9.
Oertner TG, Matus A. Calcium regulation of actin dynamics in dendritic spines. Cell Calcium. 2005;37:477–82.
Parker EM, Sweet RA. Stereological assessments of neuronal pathology in auditory cortex in schizophrenia. Front Neuroanat. 2018;11:131.
Ziermans TB, Schothorst PF, Sprong M, van Engeland H. Transition and remission in adolescents at ultra-high risk for psychosis. Schizophr Res. 2011;126:58–64.
Penzes P, Cahill ME, Jones KA, VanLeeuwen J-E, Woolfrey KM. Dendritic spine pathology in neuropsychiatric disorders. Nat Neurosci. 2011;14:285–93.
Martínez-Cerdeño V. Dendrite and spine modifications in autism and related neurodevelopmental disorders in patients and animal models. Dev Neurobiol. 2017;77:393–404.
Murmu RP, Li W, Holtmaat A, Li J-Y. Dendritic spine instability leads to progressive neocortical spine loss in a mouse model of Huntington’s disease. J Neurosci. 2013;33:12997–3009.
Holtmaat A, Svoboda K. Experience-dependent structural synaptic plasticity in the mammalian brain. Nat Rev Neurosci. 2009;10:647.
Holtmaat AJ, Trachtenberg JT, Wilbrecht L, Shepherd GM, Zhang X, Knott GW, et al. Transient and persistent dendritic spines in the neocortex in vivo. Neuron. 2005;45:279–91.
Bell M, Bartol T, Sejnowski T, Rangamani P. Dendritic spine geometry and spine apparatus organization govern the spatiotemporal dynamics of calcium. J Gen Physiol. 2019;151:1017–34.
Beery AK, Zucker I. Sex bias in neuroscience and biomedical research. Neurosci Biobehav Rev. 2011;35:565–72.
Subramanyam P, Obermair GJ, Baumgartner S, Gebhart M, Striessnig J, Kaufmann WA, et al. Activity and calcium regulate nuclear targeting of the calcium channel beta4b subunit in nerve and muscle cells. Channels. 2009;3:343–55.
Obermair GJ, Szabo Z, Bourinet E, Flucher BE. Differential targeting of the L-type Ca2+ channel α1C (CaV1. 2) to synaptic and extrasynaptic compartments in hippocampal neurons. Eur J Neurosci. 2004;19:2109–22.
Purcell SM, Moran JL, Fromer M, Ruderfer D, Solovieff N, Roussos P, et al. A polygenic burden of rare disruptive mutations in schizophrenia. Nature. 2014;506:185–90.
Freedman R, Leonard S, Olincy A, Kaufmann CA, Malaspina D, Cloninger CR, et al. Evidence for the multigenic inheritance of schizophrenia. Am J Med Genet 2001;105:794–800.
Acknowledgements
The authors acknowledge support from the National Institute of Mental Health Ruth Kirschstein award 5F31MH117834-02 (Parker), grant no. T32-MH018870 (Kellendonk), grant no. 5R01MH071533-09 (Sweet) and 1R01MH118497-01A1 (MacDonald).
Author information
Authors and Affiliations
Contributions
EMP designed the experiments and wrote up the results with close guidance from RAS and MLM (mass spectrometry experiments only). EMP used MLM’s lab space to complete the mass spec experiments and MLM graciously prioritized EMP’s mass spec samples and personally ran the samples over a weekend so that EMP could complete this set of experiments just prior to the COVID-19 shutdown. EMP generated all the mice used in these studies after completing training with CEJC, who also helped fine-tune the experimental design for the dendritic spine experiments. NLK spent countless hours providing image analysis, spine counting, and classification support. RAD independently performed experiments requested by reviewers and contributed heavily to discussions about data interpretation. RBS trained EMP on LC-MS sample preparation methods and completed sample preparation in tandem with EMP. EMP performed co-immunoprecipitation and western blot experiments following critical training on western blot from JMK, who also taught EMP how to analyze mass spec data following initial guidance from MLM. WF and BC were brought on to provide statistical consultation on the dendritic spine data with WF building and interpreting LMM statistical models using the dendritic spine data.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Parker, E.M., Kindja, N.L., DeGiosio, R.A. et al. Impacts of CACNB4 overexpression on dendritic spine density in both sexes and relevance to schizophrenia. Transl Psychiatry 14, 484 (2024). https://doi.org/10.1038/s41398-024-03181-7
Received:
Revised:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s41398-024-03181-7
This article is cited by
-
CACNB4 attenuates cardiac dysfunction by regulating calcium and ATP levels via interaction with RyR2
European Journal of Medical Research (2025)
-
KChIP3 fosters neuroinflammation and synaptic dysfunction in the 5XFAD mouse model of Alzheimer’s disease
Journal of Neuroinflammation (2025)