Abstract
Inflammation is a probable biological pathway underlying the relationship between diabetes and depression, but data on differences between diabetes types and symptom clusters of depression are scarce. Therefore, this cross-sectional study aimed to compare associations of a multimarker panel of biomarkers of inflammation with depressive symptoms and its symptom clusters between people with type 1 diabetes (T1D) and type 2 diabetes (T2D). This cross-sectional study combined data from five studies including 1260 participants (n = 706 T1D, n = 454 T2D). Depressive symptoms were assessed using the Center for Epidemiological Studies-Depression Scale (CES-D). Serum levels of 92 biomarkers of inflammation were quantified with proximity extension assay technology. After quality control, 76 biomarkers of inflammation remained for statistical analysis. Associations between biomarkers and depressive symptom scores and clusters (cognitive-affective, somatic, anhedonia) were estimated with multivariable linear regression models. Nine biomarkers were positively associated with depressive symptoms in the total sample (CCL11/eotaxin, CCL25, CDCP1, FGF-21, IL-8, IL-10RB, IL-18, MMP-10, TNFRSF9; all p < 0.05) without interaction by diabetes type. Associations differed for eight biomarkers (pinteraction < 0.05). TNFβ was inversely associated with depressive symptoms in T1D, whereas three biomarkers (GDNF, IL-18R1, LIF-R) were positively associated with depressive symptoms in T2D. For the remaining four biomarkers (CD6, CD244, FGF-5, IFNγ) associations were not significant in either subgroup. Biomarker associations were more pronounced with somatic and anhedonia than with cognitive-affective symptoms. These results indicate that different proinflammatory pathways may contribute to depression in T1D and T2D and that there may be a symptom specificity in the link between subclinical inflammation and depression.
Similar content being viewed by others
Introduction
Depression is one of the most frequent mental comorbidities of type 1 diabetes (T1D) and type 2 diabetes (T2D). The lifetime risk of major depression in the general population has been estimated to reach ~16% [1], but for individuals with diabetes it is probably about twofold higher [2,3,4]. This excess risk in people with diabetes is important because depression is associated with less optimal diabetes management, higher risk of diabetes-related complications such as cardiovascular disease, nephropathy and cancer, and premature mortality [5,6,7].
One common mechanism that contributes to the development of diabetes, including its complications and depression as a comorbidity, is subclinical inflammation [8,9,10,11]. Both cross-sectional and prospective studies have demonstrated associations of biomarkers of subclinical inflammation with both subclinical depression and clinical depression, i.e. major depressive disorder (MDD), in the general population [12,13,14,15,16]. Additionally, there is evidence that people with subclinical inflammation may be less responsive to antidepressant treatment [6, 17].
Given the higher risk for depression among people with diabetes, data on the potential relevance of inflammation in this particular population group are still relatively scarce [18, 19]. We previously showed that associations between biomarkers of subclinical inflammation and depressive symptoms may be more pronounced in people with T2D than in those with T1D [20, 21]. This finding could reflect differences in the underlying pathophysiology of both diabetes types, but was based on only six biomarkers so more comprehensive studies with better immunological phenotyping are needed.
Another knowledge gap in understanding the bidirectionality between depression and diabetes is the heterogeneity of depression. Depression is a multi-faceted syndrome that includes a variety of different symptoms ranging from cognitive-affective symptoms (e.g., feeling blue, hopelessness), somatic symptoms (e.g., issues with appetite, sleep) and anhedonia (e.g., lack of interest/pleasure). Notably, it is possible for two individuals with a depression diagnosis to share only one symptom based on the 10th revision of the International Statistical Classification of Diseases and Related Health Problems. Thus, identifying possible pathways between depression and inflammation can be masked by potential differential effects of symptom clusters [22]. It has been suggested that subclinical inflammation may be primarily associated with somatic symptoms [23,24,25], but data are only based on selected biomarkers and few cohorts.
Therefore, this study aimed to characterise the associations between a large panel of biomarkers of inflammation and depressive symptoms in people with diabetes and to test the hypotheses (i) that these associations differ between diabetes types (i.e. stronger in T2D than in T1D) and (ii) that these associations also differ between symptom clusters of depression (i.e. stronger for somatic than for cognitive-affective symptoms).
Methods
Study population
This cross-sectional study combines five study samples that underwent standardised phenotyping at a specialised diabetes clinic (Diabetes Center Mergentheim, Bad Mergentheim, Germany). A detailed description of the study populations is given in Supplementary Table 1. In brief, DIAMOS (Diabetes Motivation Strengthening, NCT01009138; [26]), ECCE HOMO (Evaluation of a Stepped Care Approach to Manage Depression in Diabetes, NCT01812291; [27]) and DDCT (Depression and Diabetes Control Trial, NCT02675257) were randomised controlled trials aiming to reduce depressive symptoms and diabetes distress in people with diabetes. DIA-LINK1 and DIA-LINK2 (Towards a Better Understanding of Diabetes Distress, Depression and Poor Glycaemic Control in T1D/T2D, NCT03811132, NCT04438018; [28]) were prospective observational studies using ecological momentary assessment to analyse associations and mediating links between depressive symptoms, diabetes distress and glycaemic outcomes in people with T1D and T2D, respectively. The present study used data and serum samples from the baseline examinations of these five studies. All baseline examinations were conducted before randomisation in the RCTs.
All studies were approved by the relevant ethics boards, i.e. either by the Ethics Committee of the State Medical Chamber of Baden-Württemberg, Germany (file numbers: DIAMOS, 2009-034-f; ECCE-HOMO, F-2013-011; DDCT, F-2015-056) or the Ethics Committee of the German Psychological Society (DIA-LINK1, NH082018; DIA-LINK2: HermannsNorbert2020-03-05AM) and performed in accordance with the Declaration of Helsinki. All participants provided written informed consent.
In total, data from 1226 participants of these studies were available. After exclusion of people with (i) diabetes types other than type 1 or type 2 (n = 7), (ii) missing covariates for statistical analysis (n = 6), (iii) missing data for biomarkers of subclinical inflammation (mainly due to missing serum samples; n = 50) and (iv) missing data on depressive symptoms (n = 20), the analysis dataset was based on 1160 people, 706 of whom with type 1 diabetes and 454 with type 2 diabetes (Supplementary Fig. 1)
Assessment of depressive symptoms
Depressive symptoms were assessed using the German version of the Center for Epidemiological Studies-Depression Scale (CES-D) [29, 30], a sensitive 20-item instrument to detect depressive symptoms within the previous week and to monitor changes over time [31, 32]. The questionnaires were filled in by the study participants with precise instructions and the opportunity to clarify any questions. Each CES-D item is scored from 0 (“rarely or none of the time”) to 3 (“most or almost all the time”) with a summary score ranging from 0 to 60 and higher scores indicating stronger depressive symptoms. We used the continuous CES-D symptom score (rather than a binary, cut-off-based depression variable) in all analyses to make optimal use of the variation in depressive symptoms. Symptom clusters of depressive symptoms were identified according to the recommendations by Shafer [33], i.e. subscores were calculated for cognitive-affective symptoms (items 3, 6, 9, 10, 14, 17, 18), somatic symptoms (items 1, 2, 5, 7, 11, 13, 20) and anhedonia symptoms (items 4, 8, 12, 16 [reversed scoring]).
Measurement of biomarkers of inflammation
Fasting blood samples were taken between 06:30 a.m. and 08:00 a.m. and serum was stored at -80°C until analysis. Serum levels of biomarkers of inflammation were quantified using the OLINK Target 96 Inflammation assay (Olink, Uppsala, Sweden) as described [34, 35]. This assay is based on proximity extension assay (PEA) technology and designed for the simultaneous measurement of 92 protein biomarkers including cytokines, chemokines, growth factors and factors involved in acute inflammatory and immune responses, angiogenesis, fibrosis and endothelial activation. In this manuscript, “biomarkers of inflammation” refers to all biomarkers from this panel although some of these biomarkers have additional functions in other pathways as well. The PEA provides a relative quantification of protein levels which are given as normalised protein expression (NPX) values. The NPX values are comparable in their distribution to log2-transformed biomarker levels.
Supplementary Table 2 lists all biomarkers with UniProt numbers, gene symbols, intra-assay coefficients of variation (CV) and inter-assay CV. Intra- and inter-assay CV were calculated based on three control serum samples measured in duplicates on each plate (n = 20) as in previous studies [34, 35]. We had a priori defined threshold levels of intra-assay CV > 15%, inter-assay CV > 20% and a proportion of >25% of values below the respective limit of detection for exclusion of biomarkers from further analysis. Six biomarkers fulfilled all three criteria, and additional ten biomarkers were excluded based on the third criterion so that 76 biomarkers of inflammation remained for statistical analysis.
Assessment of covariates
Demographic, anthropometric and clinical data were assessed as described before [26,27,28]. Information on demographic and diabetes-related characteristics comprised age, sex, height and weight (from which BMI was calculated), diabetes type, known diabetes duration, diabetes treatment and co-medication, which was extracted from medical records or interview data. Data about the prevalence or history of diabetes-related complications were based on an entry examination including laboratory analysis and recorded complications in the medical files. History of myocardial infarction, stroke or peripheral arterial occlusive disease was based on previous events or previous revascularization measures. Diabetes-related chronic kidney disease was defined based on a glomerular filtration rate of <60 ml/min/1.73 m² and/or persistent micro-/macroalbuminuria. Diabetic retinopathy was established by an ophthalmologic eye examination or previous laser coagulation treatment. Diabetic neuropathy was based on the neuropathy deficit score.
Statistical analysis
Baseline characteristics and biomarkers of inflammation are presented as mean (SD) and counts (%) for continuous and categorical variables, respectively. Differences between people with T1D and T2D were tested by using Pearson’s chi-squared test (for categorical variables) or the Wilcoxon rank-sum test (for continuous variables). We additionally compared the baseline characteristics by study cohorts. The correlation among biomarkers of inflammation was analysed by calculating Pearson’s correlation coefficients.
Associations between biomarkers of inflammation (independent variables, separate models for standardised serum levels of each biomarker) and CES-D scores or symptom clusters (dependent variables, also standardised) were estimated using multivariable linear regression models. We reported standardised regression coefficients β (per 1 SD of standardised biomarkers level) and p-values from regression models adjusted for different covariates. Model 1 was adjusted for age, sex and study cohort. Model 2 was additionally adjusted for BMI, HbA1c, diabetes duration, total cholesterol, triglycerides, use of lipid-lowering drugs, use of NSAIDs, use of antithrombotic medication and use of antidepressant medication. Model 3 was additionally adjusted for the number of diabetes-related complications. All analyses were further stratified by diabetes type. In addition, differences in the association between biomarkers of inflammation and depressive symptoms were assessed by analysing the interaction between biomarkers of inflammation and diabetes type.
For data visualisation, we plotted the correlation matrix of biomarkers of inflammation and present heat maps to compare biomarkers of inflammation that were significantly associated with CES-D scores and depressive symptom clusters among study participants.
We considered P-values of <0.05 to indicate statistically significant differences or associations. Given the exploratory nature of our study using a panel of multiple biomarkers of inflammation and given the complex correlations between these biomarkers we refrained from adjustment for multiple testing. This approach reduces the risk of type II errors and also the overemphasis on P-values.
All analyses were conducted in R software (version: 4.2.2, R Core Team, R Foundation for Statistical Computing, Vienna, Austria).
Results
Study population
The characteristics of the total study sample and stratified by diabetes type are shown in Table 1. People with T2D were older, more often male, had a higher BMI, higher HbA1c, shorter time since diabetes diagnosis, lower cholesterol and higher triglyceride levels than people with T1D. Additionally, people with T2D more frequently used lipid-lowering, antithrombotic and antidepressant drugs and had a higher number of comorbidities than people with T1D. Supplementary Table 3 gives an overview of the characteristics of the study participants stratified by cohort. Overall, 20.6% of all study participants were first-generation migrants which is similar to the nationwide percentage of 19.2% [36].
Out of 76 biomarkers of inflammation, serum levels differed between diabetes types for 44 biomarkers (10 higher in T1D, 34 higher in T2D, all P < 0.05; Supplementary Table 4). Most biomarkers showed positive pairwise correlations with weak or moderate effect sizes (Supplementary Fig. 2). Among the clinical characteristics and covariates, triglyceride levels, age, BMI, diabetes-related comorbidities and diabetes type were most frequently associated with biomarkers of inflammation (Supplementary Fig. 3). Directions and effect sizes of correlations between biomarkers of inflammation and clinical characteristics are summarised in Supplementary Fig. 4.
Association between biomarkers of inflammation and depressive symptoms (CES-D score)
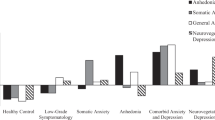
Associations between all biomarkers of inflammation and depressive symptoms in the total study sample and stratified by diabetes type are listed in Supplementary Tables 5-7 for models 1-3, respectively. Results for the biomarkers with significant associations with depressive symptoms in model 3 (fully adjusted) are listed in Table 2 and visualised in Fig. 1. Nine biomarkers of inflammation (CDCP1, FGF-21, IL-8, IL-18, eotaxin, MMP-10, TNFRSF9, CCL25, IL-10RB; see Supplementary Table 2 for full biomarker names) were positively associated with depressive symptoms without significant interaction by diabetes type. Thus, higher levels of these biomarkers of inflammation were associated with greater depressive symptoms (CES-D sum score). In stratified analyses, these associations were also significant for eotaxin, MMP-10 and TNFRSF9 in people with T1D and for CCL25 and IL-10RB in people with T2D.
*Only results with P < 0.05 (in the total sample or at least one of the subgroups) or pinteraction < 0.05 (CD244, CD6, FGF-5, IFNγ) are shown. β coefficients indicate standardised changes in CES-D scores by per 1-SD increase in biomarkers of inflammation. Regression models were adjusted for age, sex, study, body mass index, HbA1c, diabetes duration, total cholesterol, triglycerides, use of lipid-lowering drugs, non-steroidal anti-inflammatory drugs, antithrombotic medication, antidepressant medication, and number of diabetes-related comorbidities (model 3). T1D, type 1 diabetes; T2D, type 2 diabetes; Total, all people with type 1 or 2 diabetes.
For another eight biomarkers (TNFβ, GDNF, IL-18R1, LIF-R, CD244, CD6, FGF-5, IFNγ), a significant interaction by diabetes type was observed. TNFβ was significantly inversely associated with depressive symptoms in people with T1D only, whereas GDNF, IL18R1 and LIF-R were significantly positively associated with deprerssive symptoms in people with T2D.
Associations between biomarkers of inflammation and symptom clusters of depression
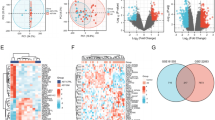
Figure 2A–D summarises all associations of biomarkers of inflammation with the overall depressive symptom (CES-D total) score and the three depressive symptom clusters for the total study sample as well as stratified by diabetes type. For better readability, the four panels are additionally given as separate Supplementary Figs. 5–8. Fully adjusted results for the symptom clusters are also listed in Supplementary Tables 8–10.
a CES-D score. b Cognitive-affective symptoms. c Somatic symptoms. d Anhedoniasymptoms. *P < 0.05 for associations between biomarkers of inflammation and CES-D or symptom clusters. β coefficients indicate standardised changes in CES-D scores or its symptom clusters per 1-SD increase in biomarkers of inflammation. Regression models were adjusted for age, sex, study, body mass index, HbA1c, diabetes duration, total cholesterol, triglycerides, use of lipid-lowering drugs, non-steroidal anti-inflammatory drugs, antithrombotic medication, antidepressant medication, and number of diabetes-related comorbidities (model 3). Abbreviations:T1D, type 1 diabetes; T2D, type 2 diabetes; Total, all people with type 1 or 2 diabetes.
Out of the nine biomarkers with positive associations with depressive symptoms in the total sample, four (CCL25, eotaxin, FGF-21, TNFRSF9) were also associated with cognitive-affective symptoms, six (CCL25, FGF-21, eotaxin, IL-18, MMP-10, TNFRSF9) with somatic symptoms and two (IL-8, MMP-10) with anhedonia. In addition, CXCL5 was positively associated with cognitive-affective symptoms in the total study sample.
In the analysis stratified by diabetes type, two biomarkers were positively associated with cognitive-affective symptoms in T2D (CCL25, GDNF). For somatic symptoms, five positive associations (CDCP1, FGF-5, MMP-10, TNFRSF9, TRAIL) and one negative association (TNFβ) were observed in people with T1D, while four biomarkers (FGF-19, FGF-21, GDNF, LIF-R) showed positive associations in people with T2D. For anhedonia, two biomarkers (eotaxin, MMP-10) had positive and three biomarkers (NT-3, TNFβ, TWEAK) had inverse associations in people with T1D, whereas in people with T2D positive associations were found for ten biomarkers (CD5, CD8A, CXCL6, GDNF, IL-10RB, IL-18R1, IL-8, LIF-R, MIP1α, SIRT2).
Discussion
This study identified novel biomarkers of inflammation that are positively associated with depressive symptoms in people with diabetes. Our data demonstrate that associations between biomarkers of inflammation and depressive symptoms partly differ between T1D and T2D. Additionally, the analysis of symptom clusters supports the hypothesis that positive associations between biomarkers of inflammation are particularly pronounced for somatic symptoms in the total study sample and with anhedonia symptoms in people with T2D.
Association between biomarkers of inflammation and depressive symptoms (CES-D score) in the total study sample
This study demonstrates positive associations of CDCP-1, FGF-21, IL-8, IL-18, eotaxin/CCL11, MMP-10, TNFRSF9, CCL25 and IL-10RB with depressive symptoms in people with diabetes without interaction with diabetes type. We are not aware of any previous study that assessed the aforementioned biomarkers in people with diabetes except IL-18 as discussed below [20], which underlines the novelty of our findings.
When comparing our results with studies that did not focus on people with diabetes, our data are in line with positive associations of the chemokine IL-8/CXCL8 and the proinflammatory cytokine IL-18 with depression that were found in previous meta-analyses based on study cohorts with a wide range of participant characteristics such as age, BMI and burden of comorbidities [13, 37]. However, no significant association was found for eotaxin/CCL11 [37].
Two small studies on FGF-21 and MDD reported conflicting results. One study found higher FGF-21 levels in people with chronic, early-onset MDD than in healthy people [38], which is in line with our data. Another study reported lower FGF-21 levels in people with MDD compared to healthy people but no associations between FGF-21 and symptom score [39]. There is evidence from mouse models that endogenous FGF-21 counteracts depression-like behaviour [40] so that higher FGF-21 levels might reflect a compensatory response but these data are difficult to extrapolate to depression in humans.
One cross-sectional study in women with and without postpartum depressive symptoms used the same multimarker panel as our analysis [41]. However, there was no overlap in biomarkers showing significant associations with depressive symptoms, which may be attributable to considerable differences in the aetiology of postpartum depression and the development of depression in adulthood that is not related to pregnancy.
Taken together, IL-8/CXCL8 and IL-18 appear related with depressive symptoms irrespectively of diabetes status. Whether the novel associations of the other biomarkers discussed here are specific to people with diabetes is unclear because of the lack of comparable data from other cohorts. Thus, our findings should be corroborated in both cohorts based on people with diabetes and in cohorts from the general population.
Differences in associations between biomarkers of inflammation and depressive symptoms between T1D and T2D
Given the differences in the aetiology of T1D and T2D in particular regarding the role of inflammation and the immune system, analyses of potential differences between diabetes types are relevant for all comorbidities of diabetes. This study extends the current knowledge about potential differences between diabetes types [20] and provides novel evidence that associations between biomarkers and inflammation and depressive symptoms partly differ between T1D and T2D. Associations for eight biomarkers showed a significant interaction by diabetes type, although only four of them were also significantly associated with depressive symptoms in one of the subgroups.
Higher levels of TNFβ, also known as lymphotoxin-α or TNFSF1, were associated with less pronounced depressive symptoms in people with T1D. In contrast, higher levels of GDNF, IL-18R1 and LIF-R were associated with more pronounced depressive symptoms in people with T2D. Of these four biomarkers, only GDNF has been investigated in the context of depression before. GDNF is a potent neurotrophic factor that promotes the growth and survival of various types of neurons and protects them from oxidative stress. It has been hypothesised that GDNF has anti-depressant properties [42] so that the positive association between GDNF levels and depressive symptoms in T2D in our study could reflect a counterregulation. However, reports on associations with MDD in other studies were conflicting. No differences in GDNF levels between people without and with MDD were found in a recent meta-analysis but the reasons for the large heterogeneity between the studies are not known [43]. Our finding for IL-18R1 supports the aforementioned association of IL-18 with depression and highlights the potential relevance of inflammasome activation and IL-18/IL-18R1 signalling.
This analysis focused on the comparison between T1D and T2D, whereas future studies addressing the heterogeneity of diabetes and depression should also take into account the novel five subtypes as suggested by Ahlqvist et al. [44]. These identified diabetes subtypes differ in circulating biomarkers of inflammation [35] and quality of health [45] but data for depression in the subtypes have not been published yet.
Associations between biomarkers of inflammation and symptom clusters of depression
Not only diabetes but also depression is a heterogeneous disease with diverse clinical manifestations [46, 47], thus studies focusing on symptom clusters of depression could provide additional insights into the neurobiology of the different facets of depression. In our analysis of biomarkers of inflammation and symptoms clusters, the largest number of significant associations in the total sample were found for somatic symptoms, whereas the stratified analysis showed most associations with anhedonia in people with T2D.
We are not aware of comparable analyses in people with diabetes so that our data extend the current literature on inflammation in the context of the heterogeneity of both diabetes and depression. Previous studies on symptom clusters of depression focused on high-sensitivity C-reactive protein (hsCRP) and few additional biomarkers [46, 47], whereas studies with more comprehensive immunological phenotyping are lacking. In these studies, hsCRP showed the strongest associations with somatic symptoms of depression [23, 25, 46,47,48]. This observation is supported by multiple lines of evidence linking inflammation with coordinated behavioural changes (termed sickness behaviour) which are related to physical illness and infection [8]. Associations were also found for anhedonia and were weakest with cognitive-affective symptoms [23, 46,47,48]. This is in line with mechanistic and translational studies that demonstrated the involvement of inflammatory stimuli for the development of anhedonia [49]. Our data are consistent with these aforementioned studies in that we found more evidence for associations of inflammation with somatic symptoms and anhedonia than with cognitive-affective symptoms.
Of note, biomarkers associated with somatic symptoms overlapped largely with biomarkers also associated with the CES-D score overall. In addition, FGF-5, TRAIL and FGF-19 showed positive associations with somatic symptoms in the stratified analysis. So far, only higher levels of the proinflammatory cytokine TRAIL have been implicated with MDD in a small study [50].
In contrast, associations with anhedonia were mainly seen for T2D in the stratified analysis. In addition to biomarkers also associated with the CES-D score in the total study sample, there were positive associations of CD5, CD8A, CXCL6, MIP-1α/CCL3 and SIRT2 with anhedonia. CD5 and CD8A are expressed on T cells which have been implicated in the pathogenesis of depression [51]. CXCL6 and MIP1α/CCL3 add to the growing list of chemokines associated with depression, and a previous meta-analysis supports our finding regarding MIP1α/CCL3 and anhedonia [37]. SIRT2 (sirtuin 2), an NAD-dependent deacetylase, has been linked to various neuroinflammatory diseases but the underlying mechanisms are still poorly understood [52]. On the one hand, SIRT2 has anti-inflammatory properties [52]. On the other hand, SIRT2 inhibitors ameliorate depression-like behaviour in mouse models [52, 53], and SIRT2 expression is also downregulated by anti-depressants [54]. Therefore, the positive association between SIRT2 levels and anhedonia from our study appears biologically plausible.
Unfortunately, there were other aspects of the heterogeneity of depression that we could not address in our study. We did not have data on the temporal relationship between diabetes diagnosis and depressive symptomology. Additionally, we relied on the CES-D score but did not have clinical interviews, data on remission status or, for those with high CES-D scores, information on first or recurrent depression. The proportion of people on antidepressant medication was too small for subgroup analyses but the use of this medication was adjusted for as confounder in our regression models.
Clinical relevance and implications for precision medicine
Our study identified multiple biomarkers of inflammation that are associated with depressive symptoms in people with diabetes. These include proinflammatory cytokines and their receptors (IL-18, IL-18R1, LIF-R), chemokines (IL-8/CXCL8, eotaxin/CCL11, CCL25, CXCL6, MIP-1α/CCL3), biomarkers involved in the cross-talk between innate and adaptive immunity (TNFRSF9, CD5, CD8A) and lastly biomarkers that might be upregulated in depression as part of a counterregulatory response (FGF-21, GDNF). Since these biomarkers were measured in serum, it is not possible to directly link our findings to specific cell types that might be responsible for their expression and release into the circulation. However, our findings suggest the implication of cell types from both innate and adaptive immunity and point towards a role of cell-cell communication and migration in the inflammation-related processes that are associated with higher depressive symptoms. This is supported by a recent meta-analysis showing that blood counts of multiple subsets of immune cells are altered in people with depression [55].
In order to translate our findings to improved therapy, a more comprehensive phenotyping and the search for distinct upstream regulators that could represent actionable targets would be of interest. Several meta-analyses provided evidence that drugs targeting cytokines such as IL-6 or IL-12/IL-23 reduced depressive symptoms [56]. This suggests that anti-inflammatory drugs could be useful as adjunctive therapy in people with depression. However, most of these data were based on people with depression and inflammatory diseases such as rheumatoid arthritis or systemic lupus erythematosus who are characterised by a more pronounced immune activation than people with depression but without such diseases. Therefore, the potential of anti-inflammatory approaches in the treatment of depression as primary disease remains unclear. Of note, our findings support the hypothesis that different inflammatory mechanisms may be relevant in T1D and T2D and also for specific symptoms of depression. However, the identification of inflammation-related subtypes is still an unmet clinical need in both diabetology and psychiatry but might have considerable potential for patient stratification and implementation of precision medicine [6].
Given the fact that people with subclinical inflammation are also characterised by higher risk of cardiovascular and neurodegenerative diseases, cancer and frailty [9], it remains to be seen to what extent immunomodulatory aproaches might have broader benefits, in particular in the prevention of multimorbidity.
Strengths and limitations
Strengths of our study include the relatively large sample size, the extensive biomarker measurement, the comprehensive adjustment for confounders and the analyses focusing on diabetes type and on symptom clusters of depression. This study also has limitations. First, the cross-sectional study does not allow conclusions on temporal and causal relationships between inflammation and depressive symptoms. The lack of temporal ordering also precluded us from performing mediation analysis [57] which would be of interest to better understand the bidirectional relationship of diabetes and depression. Second, the study sample consisted of mainly middle-aged people with moderate to high levels of depressive symptoms up to mild/moderate MDD (while people with severe MDD were excluded from the studies for ethical reasons), so our findings might not be generalisable to other age groups or to people with severe forms of MDD. In addition, the study was performed in a tertiary care setting which is characterised by worse glycaemic control compared to all people with diabetes, which might lead to some selection bias. Unfortunately, we did not have data on ethnicity but with respect to the percentage of first-generation migrants our study sample was representative for Germany. Third, the high prevalence of T1D in our study means that our results for the total study sample cannot be directly compared to population-based studies on diabetes which are highly dominated by T2D. Fourth, given the exploratory nature of our study and the complex correlations between biomarkers of inflammation we did not adjust for multiple testing so our results need replication in other studies. Lastly, the biological interpretation of some of the findings is unclear because some of the biomarkers discussed above represent circulating forms of primarily cytoplasmatic or transmembrane proteins, and it is not known how their circulating concentrations in blood are regulated.
Conclusions
In summary, this study found multiple associations between biomarkers of inflammation and depressive symptoms in people with diabetes and provides evidence that these associations and relevant biomarkers differ depending on diabetes type and symptom clusters of depression. Further elucidation of the specificity for subtypes of diabetes and/or depression could be promising for future patient stratification and the development of more targeted treatment options for depression in diabetes.
Data availability
The data are subject to national data protection laws. Therefore, data cannot be made freely available in a public repository. However, data can be requested through an individual project agreement with FIDAM and DDZ.
References
Kupfer DJ, Frank E, Phillips ML. Major depressive disorder: new clinical, neurobiological, and treatment perspectives. Lancet. 2012;379:1045–55.
Pouwer F. Depression: a common and burdensome complication of diabetes that warrants the continued attention of clinicians, researchers and healthcare policy makers. Diabetologia. 2017;60:30–34.
Lindekilde N, Rutters F, Erik Henriksen J, Lasgaard M, Schram MT, Rubin KH, et al. Psychiatric disorders as risk factors for type 2 diabetes: an umbrella review of systematic reviews with and without meta-analyses. Diabetes Res Clin Pract. 2021;176:108855.
Kremers SHM, Wild SH, Elders PJM, Beulens JWJ, Campbell DJT, Pouwer F, et al. The role of mental disorders in precision medicine for diabetes: a narrative review. Diabetologia. 2022;65:1895–906.
Nouwen A, Adriaanse MC, van Dam K, Iversen MM, Viechtbauer W, Peyrot M, et al. Longitudinal associations between depression and diabetes complications: a systematic review and meta-analysis. Diabet Med. 2019;36:1562–72.
Drevets WC, Wittenberg GM, Bullmore ET, Manji HK. Immune targets for therapeutic development in depression: towards precision medicine. Nat Rev Drug Discov. 2022;21:224–44.
Prigge R, Wild SH, Jackson CA. Depression, diabetes, comorbid depression and diabetes and risk of all-cause and cause-specific mortality: a prospective cohort study. Diabetologia. 2022;65:1450–60.
Miller AH, Raison CL. The role of inflammation in depression: from evolutionary imperative to modern treatment target. Nat Rev Immunol. 2016;16:22–34.
Furman D, Campisi J, Verdin E, Carrera-Bastos P, Targ S, Franceschi C, et al. Chronic inflammation in the etiology of disease across the life span. Nat Med. 2019;25:1822–32.
Beurel E, Toups M, Nemeroff CB. The bidirectional relationship of depression and inflammation: double trouble. Neuron. 2020;107:234–56.
Rohm TV, Meier DT, Olefsky JM, Donath MY. Inflammation in obesity, diabetes, and related disorders. Immunity. 2022;55:31–55.
Köhler CA, Freitas TH, Maes M, de Andrade NQ, Liu CS, Fernandes BS, et al. Peripheral cytokine and chemokine alterations in depression: a meta-analysis of 82 studies. Acta Psychiatr Scand. 2017;135:373–87.
Osimo EF, Pillinger T, Rodriguez IM, Khandaker GM, Pariante CM, Howes OD. Inflammatory markers in depression: a meta-analysis of mean differences and variability in 5,166 patients and 5,083 controls. Brain Behav Immun. 2020;87:901–9.
Mac Giollabhui N, Ng TH, Ellman LM, Alloy LB. The longitudinal associations of inflammatory biomarkers and depression revisited: systematic review, meta-analysis, and meta-regression. Mol Psychiatry. 2021;26:3302–14.
Hayashino Y, Mashitani T, Tsujii S, Ishii H. Elevated levels of hs-CRP are associated with high prevalence of depression in japanese patients with type 2 diabetes: the Diabetes Distress and Care Registry at Tenri (DDCRT 6). Diabetes Care. 2014;37:2459–65.
Moulton CD, Pickup JC, Rokakis AS, Amiel SA, Ismail K, Stahl D. The prospective association between inflammation and depressive symptoms in type 2 diabetes stratified by sex. Diabetes Care. 2019;42:1865–72.
Strawbridge R, Arnone D, Danese A, Papadopoulos A, Herane Vives A, Cleare AJ. Inflammation and clinical response to treatment in depression: a meta-analysis. Eur Neuropsychopharmacol. 2015;25:1532–43.
Herder C, Hermanns N. Subclinical inflammation and depressive symptoms in patients with type 1 and type 2 diabetes. Semin Immunopathol. 2019;41:477–89.
Nguyen MM, Perlman G, Kim N, Wu CY, Daher V, Zhou A, et al. Depression in type 2 diabetes: a systematic review and meta-analysis of blood inflammatory markers. Psychoneuroendocrinology. 2021;134:105448.
Herder C, Schmitt A, Budden F, Reimer A, Kulzer B, Roden M, et al. Association between pro- and anti-inflammatory cytokines and depressive symptoms in patients with diabetes-potential differences by diabetes type and depression scores. Transl Psychiatry. 2018;7:1.
Herder C, Schmitt A, Budden F, Reimer A, Kulzer B, Roden M, et al. Longitudinal associations between biomarkers of inflammation and changes in depressive symptoms in patients with type 1 and type 2 diabetes. Psychoneuroendocrinology. 2018;91:216–25.
de Jonge P. Depression deconstruction lessons from psychosomatic research. J Psychosom Res. 2011;71:59–60.
Jokela M, Virtanen M, Batty GD, Kivimäki M. Inflammation and specific symptoms of depression. JAMA Psychiatry. 2016;73:87–8.
White J, Kivimäki M, Jokela M, Batty GD. Association of inflammation with specific symptoms of depression in a general population of older people: the english longitudinal study of ageing. Brain Behav Immun. 2017;61:27–30.
Moriarity DP, Slavich GM, Alloy LB, Olino TM. Hierarchical inflammatory phenotypes of depression: a novel approach across five independent samples and 27,730 adults. Biol Psychiatry. 2023;93:253–9.
Hermanns N, Schmitt A, Gahr A, Herder C, Nowotny B, Roden M, et al. The effect of a diabetes-specific cognitive behavioral treatment program (DIAMOS) for patients with diabetes and subclinical depression: results of a randomized controlled trial. Diabetes Care. 2015;38:551–60.
Schmitt A, Kulzer B, Reimer A, Herder C, Roden M, Haak T, et al. Evaluation of a stepped care approach to manage depression and diabetes distress in patients with type 1 diabetes and type 2 diabetes: results of a randomized controlled trial (ECCE HOMO Study). Psychother Psychosom. 2022;91:107–22.
Ehrmann D, Schmitt A, Priesterroth L, Kulzer B, Haak T, Hermanns N. Time with diabetes distress and glycemia-specific distress: new patient-reported outcome measures for the psychosocial Burden of diabetes using ecological momentary assessment in an observational study. Diabetes Care. 2022;45:1522–31.
Hautzinger M. Die CES-D Skala: ein depressionsmeßinstrument für untersuchungen in der Allgemeinbevölkerung [The CES-D scale: a depression-rating scale for research in the general population]. Diagnostica. 1988;34:167–73.
Hautzinger M, Bailer M, Hofmeister D, Keller F. ADS: Allgemeine depressionsskala (2., überarbeitete und neu normierte auflage) [ADS: General depression inventory (2nd revised, newly standardised edition] (in German). Hogrefe: Göttingen, Germany, 2012.
Radloff LS. The CES-D scale. A self-report depression scale for research in the general population. Appl Psychol Measure. 1977;1:385–401.
Vilagut G, Forero CG, Barbaglia G, Alonso J. Screening for depression in the general population with the center for epidemiologic studies depression (CES-D): a systematic review with meta-analysis. PLoS One. 2016;11:e0155431.
Shafer AB. Meta-analysis of the factor structures of four depression questionnaires: Beck, CES-D, Hamilton, and Zung. J Clin Psychol. 2006;62:123–46.
Herder C, Kannenberg JM, Carstensen-Kirberg M, Strom A, Bönhof GJ, Rathmann W, et al. A systemic inflammatory signature reflecting cross talk between innate and adaptive immunity is associated with incident polyneuropathy: KORA F4/FF4 study. Diabetes. 2018;67:2434–42.
Herder C, Maalmi H, Strassburger K, Zaharia OP, Ratter JM, Karusheva Y, et al. Differences in biomarkers of inflammation between novel subgroups of recent-onset diabetes. Diabetes. 2021;70:1198–208.
Bevölkerung mit Migrationshintergrund https://www.bpb.de/kurz-knapp/zahlen-und-fakten/soziale-situation-in-deutschland/61646/bevoelkerung-mit-migrationshintergrund/#:~:text=Jahren%20einen%20Migrationshintergrund.-,Fakten,7%20Prozent%20an%20der%20Gesamtbev%C3%B6lkerung. Accessed July 18, 2024.
Leighton SP, Nerurkar L, Krishnadas R, Johnman C, Graham GJ, Cavanagh J. Chemokines in depression in health and in inflammatory illness: a systematic review and meta-analysis. Mol Psychiatry. 2018;23:48–58.
Mason BL, Minhajuddin A, Czysz AH, Jha MK, Gadad BS, Mayes TL, et al. Fibroblast growth factor 21 (FGF21) is increased in MDD and interacts with body mass index (BMI) to affect depression trajectory. Transl Psychiatry. 2022;12:16.
Tang M, Cheng S, Wang L, Tang H, Liu T, Zhao T, et al. Decreased FGF19 and FGF21: possible underlying common pathogenic mechanism of metabolic and cognitive dysregulation in depression. Front Neurosci. 2023;17:1165443.
Usui N, Yoshida M, Takayanagi Y, Nasanbuyan N, Inutsuka A, Kurosu H, et al. Roles of fibroblast growth factor 21 in the control of depression-like behaviours after social defeat stress in male rodents. J Neuroendocrinol. 2021;33:e13026.
Bränn E, Fransson E, White RA, Papadopoulos FC, Edvinsson Å, Kamali-Moghaddam M, et al. Inflammatory markers in women with postpartum depressive symptoms. J Neurosci Res. 2020;98:1309–1321.
Wang H, Yang Y, Pei G, Wang Z, Chen N. Neurotrophic basis to the pathogenesis of depression and phytotherapy. Front Pharmacol. 2023;14:1182666.
Shi Y, Luan D, Song R, Zhang Z. Value of peripheral neurotrophin levels for the diagnosis of depression and response to treatment: a systematic review and meta-analysis. Eur Neuropsychopharmacol. 2020;41:40–51.
Ahlqvist E, Storm P, Käräjämäki A, Martinell M, Dorkhan M, Carlsson A, et al. Novel subgroups of adult-onset diabetes and their association with outcomes: a data-driven cluster analysis of six variables. Lancet Diabetes Endocrinol. 2018;6:361–369.
Werkman NCC, García-Sáez G, Nielen JTH, Tapia-Galisteo J, Somolinos-Simón FJ, Hernando ME. et al. Disease severity-based subgrouping of type 2 diabetes does not parallel differences in quality of life: the Maastricht Study. Diabetologia. 2024;67:695–702.
Majd M, Saunders EFH, Engeland CG. Inflammation and the dimensions of depression: a review. Front Neuroendocrinol. 2020;56:100800.
Křenek P, Hořínková J, Bartečků E. Peripheral inflammatory markers in subtypes and core features of depression: a systematized review. Psychopathology. 2023;56:403–416.
Franklyn SI, Stewart J, Beaurepaire C, Thaw E, McQuaid RJ. Developing symptom clusters: linking inflammatory biomarkers to depressive symptom profiles. Transl Psychiatry. 2022;12:133.
Bekhbat M, Treadway MT, Felger JC. Inflammation as a pathophysiologic pathway to anhedonia: mechanisms and therapeutic implications. Curr Top Behav Neurosci. 2022;58:397–419.
Klaus F, Guetter K, Schlegel R, Spiller TR, Seifritz E, Cathomas F. et al. Common and disorder-specific upregulation of the inflammatory markers TRAIL and CCL20 in depression and schizophrenia. Sci Rep. 2021;11:19204.
Beurel E, Medina-Rodriguez EM, Jope RS. Targeting the adaptive immune system in depression: focus on T helper 17 cells. Pharmacol Rev. 2022;74:373–386.
Fan Z, Bin L. Will sirtuin 2 be a promising target for neuroinflammatory disorders? Front Cell Neurosci. 2022;16:915587.
Muñoz-Cobo I, Belloch FB, Díaz-Perdigón T, Puerta E, Tordera RM. SIRT2 inhibition reverses anhedonia in the VGLUT1+/- depression model. Behav Brain Res. 2017;335:128–131.
Erburu M, Muñoz-Cobo I, Domínguez-Andrés J, Beltran E, Suzuki T, Mai A, et al. Chronic stress and antidepressant induced changes in Hdac5 and Sirt2 affect synaptic plasticity. Eur Neuropsychopharmacol. 2015;25:2036–48.
Sørensen NV, Frandsen BH, Orlovska-Waast S, Buus TB, Ødum N, Christensen RH, et al. Immune cell composition in unipolar depression: a comprehensive systematic review and meta-analysis. Mol Psychiatry. 2023;28:391–401.
Wittenberg GM, Stylianou A, Zhang Y, Sun Y, Gupta A, Jagannatha PS, et al. Effects of immunomodulatory drugs on depressive symptoms: a mega-analysis of randomized, placebo-controlled clinical trials in inflammatory disorders. Mol Psychiatry. 2020;25:1275–1285.
Schuler MS, Coffman DL, Stuart EA, Nguyen TG, Vegetabile B, McCaffrey DF. Practical challenges in mediation analysis: a guide for applied researchers. Health Serv Outcomes Res Method. 2024. https://doi.org/10.1007/s10742-024-00327-4.
Acknowledgements
We appreciate the contribution of all study participants, and we thank the laboratory and research staff of the Diabetes Center Mergentheim (DZM) for their excellent work.
Funding
The project was supported by a grant from the German Research Association (DFG) to CH (HE 4510/9-1), DE (EH 538/3-1) and NH (HE 3053/4-1). The German Diabetes Center (DDZ) is funded by the German Federal Ministry of Health (Berlin, Germany) and the Ministry of Culture and Science of the state North Rhine-Westphalia (Düsseldorf, Germany) and receives additional funding from the German Federal Ministry of Education and Research (BMBF) through the German Center for Diabetes Research (DZD e.V.). The conduct of the studies by the Research Institute of the Diabetes Academy Bad Mergentheim was supported by the Federal Ministry of Education and Research (DIAMOS, FKZ 01GI089; ECCE HOMO, FKZ 01GI1105) and the German Center for Diabetes Research (DZD e.V.; DDCT, FKZ 82DZD001101, DIA_LINK, FKZ 82DZD11A02). The funders had no role in study design, data collection, data analysis, data interpretation or writing of the report. Open Access funding enabled and organized by Projekt DEAL.
Author information
Authors and Affiliations
Contributions
CH, DE and NH acquired funding. CH, AS, DE and NH designed the study. AS collected the study data. CH, AS, BK, DE and NH contributed to the data collection. CH, AZ, DE and NH drafted the analysis plan. AZ, MCS and DE performed the statistical analyses. CH, AZ, AS, MCS, BK, MR, DE and NH contributed to data interpretation. CH wrote the manuscript. All authors reviewed and edited the manuscript and approved its submission. CH and NH are the guarantors of this work and, as such, had full access to all the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis.
Corresponding author
Ethics declarations
Competing interests
MR received lecture fees or served on advisory boards for AstraZeneca, Boehringer-Ingelheim, Echosens, Eli Lilly, Madrigal, MSD Sharp & Dohme, Novo Nordisk and Target RWE and performed investigator-initiated research with support from Boehringer-Ingelheim, Novo Nordisk and Nutricia/Danone to the German Diabetes Center (DDZ). NH reports advisory board member fees from Abbott Diabetes Care, Roche Diabetes Care and Insulet as well as honoraria for lectures from Berlin Chemie AG, Becton Dickenson, Sanofi Germany, Roche Diabetes Care and Dexcom Germany. DE reports advisory board member fees from Dexcom Germany and Roche Diabetes Care as well as honoraria for lectures from Berlin Chemie AG, Dexcom Germany, Sanofi-Aventis Germany and Roche Diabetes Care. All other authors declare that there are no relationships or activities that might bias, or be perceived to bias, their work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Herder, C., Zhu, A., Schmitt, A. et al. Associations between biomarkers of inflammation and depressive symptoms—potential differences between diabetes types and symptom clusters of depression. Transl Psychiatry 15, 9 (2025). https://doi.org/10.1038/s41398-024-03209-y
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41398-024-03209-y