Abstract
Negative-stranded segmented RNA viruses (NSVs) employ a cap-snatching mechanism for transcription, which makes cap-dependent endonuclease (CEN) an attractive target for drug development. Pathogenic arenaviruses pose a serious threat to humans, yet no approved treatments exist, underscoring the importance of discovering novel compounds targeting arenaviral CENs. Therefore, this study aimed to identify novel CEN inhibitors for arenaviruses and investigate their antiviral mechanisms. A high-throughput screening system based on enzymatic activity of CEN was established for discovering inhibitors of lymphocytic choriomeningitis virus (LCMV). Several hit compounds were screened from a vast natural product library, and then evaluated for both toxicity and inhibition through cellular and animal experiments. One candidate compound was finally identified, and its mechanism of action on CEN was elucidated through simulation analysis and biochemical studies. Moreover, its broad-spectrum effects were investigated among pathogenic arenaviruses as well as representative NSVs. Consequently, salvianolic acid A (SAA) from Salvia miltiorrhiza was identified as a promising compound that effectively inhibited LCMV infection and significantly reduced the viral load via intravenous administration. It was shown to bind to the active pocket of arenaviral CENs while chelating their metal ions through its acid carboxyl group, acting in a substrate-competitive manner. Additionally, SAA exhibited broad-spectrum inhibition of pathogenic arenaviruses as well as representative viruses from the order Bunyavirales. This study identified SAA as a novel CEN inhibitor, particularly for pathogenic arenaviruses, showcasing its promise for antiviral drug development.
Similar content being viewed by others
Introduction
Murine-borne mammarenaviruses, comprising the Old World (OW) and New World (NW) arenaviruses, sporadically cause outbreaks in Africa and South America, respectively, thereby posing significant threats to human health. These viruses disproportionately affect individuals in resource-limited regions with restricted access to medical care [1, 2]. Mammarenaviruses are classified in the family Arenaviridae of the order Bunyavirales [3], among which lymphocytic choriomeningitis virus (LCMV) is globally distributed and can cause severe symptoms, particularly in immuno-compromised individuals and during pregnancies [4]. Other mammarenaviruses include NW arenaviruses Machupo (MACV), Junin virus (JUNV), Guanarito virus (GTOV), Chapare virus (CHAPV), and Sabia virus (SABV), as well as the OW arenaviruses, Lassa virus (LASV), Lujo virus (LUJV), and Dandenong virus (DANV). These are virulent pathogens that cause severe hemorrhagic fever with high mortality and necessitate handling in bio-safety level 4 facilities [5, 6]. However, no approved vaccines or drugs are available for most of the aforementioned viruses, thereby highlighting a definite need for drug development.
Arenaviruses, members of the family Arenaviridae, are classical negative-stranded segmented RNA viruses (NSVs). For example, the LCMV genome consists of two segments: the small segment (S) and the large segment (L) [7]. The S segment encodes the glycoprotein (GP) and nucleoprotein (NP), which facilitates viral entry and is essential for genome assembly, respectively. The L segment encodes the matrix protein (ZP) for virion assembly and a large RNA-dependent RNA polymerase (LP) crucial for genome replication and mRNA transcription [8, 9]. Specifically, the LP includes three known functional domains: the cap-dependent endonuclease domain (CEN), RNA-dependent RNA polymerase (RdRP) domain, and cap-binding domain (CBD). These domains collectively mediate 5’ cap snatching from host RNAs, consequently initiating cap-dependent transcription and viral mRNA synthesis [10]. This mechanism is characteristic of NSVs, including orthomyxoviruses (e.g., influenza virus) and viruses from families such as Phenuividae, Nairoviridae, Hantaviridae, and Peribunyaviridae (for example, severe fever with thrombocytopenia syndrome virus (SFTSV), Heartland virus (HRTV), Guertu virus (GTV), Crimean-Congo hemorrhagic fever virus (CCHFV), Hantaan virus (HTNV), and La Crosse virus (LACV)) [11, 12]. Therefore, CEN is a reliable target for drug development as well as broad-spectrum drug discovery. Currently, baloxavir (BXA) is the only marketed drug targeting the influenza virus CEN. This highlights the urgency for the exploration of more extensive-spectrum cap-dependent endonuclease inhibitors (CENis) to combat against a broader range of NSVs.
In recent years, few drug candidates for arenaviruses have advanced to clinical trials [13], except for ARN-75039 which targets the fusion domain of GP and has completed its phase I trial. Additionally, favipiravir (T-705), an anti-influenza virus drug, has been repurposed and evaluated in a phase II trial. Therapeutic antibodies [14,15,16], CENis, derived from BXA [17] and other strategies [18, 19] were reported to be effective against arenaviruses either in vitro or in vivo. Despite the historical use of ribavirin (RBV) in treating arenavirus infections, conclusive clinical data supporting its efficacy is lacking, and its use is associated with notable potential adverse effects such as thrombocytopenia and anemia [20]. Given these challenges and limitations, there is an urgent need to develop safe and effective antiviral treatments specifically targeting arenaviruses to improve patient outcomes and ensure public health preparedness in the face of potential outbreaks.
In this study, a natural product library comprising 3519 compounds was employed to screen CENis for arenaviruses. This library showcases a diverse range of chemical structures and contains numerous general acids with potential chelation abilities towards the divalent metal ions of CENs. By establishing a high-throughput screening system based on in vitro enzymatic reactions, we unveiled several compounds that effectively inhibit both enzymatic activity and viral infection. Noteworthy among these is the compound salvianolic acid A (SAA), a key pharmacological component of the traditional Chinese medicinal plant Salvia miltiorrhiza, identified as a broad-spectrum antiviral agent against arenaviruses as well as pathogens in the order of Bunyavirales. Subsequent investigations confirmed its capacity to bind to the active pocket of CEN leading to the inhibition of enzymatic function and LCMV infection both in vitro and in vivo. Overall, this study introduces a potential CENi for combating arenaviruses, thus, highlighting the potential of discovering CENis from plant natural products.
Materials and methods
Natural product library and compounds
The natural product library was acquired from MedChemExpress (MCE, Shanghai, China). It comprised 5413 natural products, mainly sugars, phenylpropanoids, quinones, flavonoids, terpenoids, glycosides, steroids, alkaloids, phenols, acids, and aldehydes. A smaller library of 3519 compounds, selected by removing compounds with poor solubility or instability, was used in this study. SAA, chebulinic acid, tannic acid, and punicalagin, with purities exceeding 98.8%, were obtained from MedChemExpress (MCE, Shanghai, China). T-705, RBV, and BXA were obtained from Target Mol Chemicals (Shanghai, China).
Cell lines and viruses
BHK-21, A549, VERO, and VERO E6 cells used in this study were sourced from the American Type Culture Collection (Manassas, VA, USA). Cells were maintained in Dulbecco’s modified Eagle’s medium (DMEM) (Gibco, Grand Island, NY, USA) supplemented with 10% fetal bovine serum (FBS) (Gibco, Grand Island, NY, USA) at 37 °C with 5% CO2.
The LCMV, SFTSV, HRTV, GTV, HTNV, and CCHFV strains were obtained from the National Viral Resource Center (Wuhan, China). LCMV displays cytotropism for BHK-21 and A549 cells and is propagated in BHK-21. In contrast, SFTSV, HRTV, GTV, and CCHFV exhibit cytotropism for VERO cells, are propagated in them, and are cultured in DMEM supplemented with 2% FBS. HTNV shows cytotropism for VERO E6 cells and is propagated with them.
Protein expression purification
The MACV L fragment N-terminal (1–201 aa), LASV L fragment N-terminal (1–169 aa), lymphocytic choriomeningitis virus (LCMV), Junin virus (JUNV), Guanarito virus (GTOV), Chapare virus (CHAPV), Sabia virus (SABV), and LUJV L fragment N-terminal (1–196 aa), DANV L fragment N-terminal (1–171 aa), HTNV L fragment N-terminal (1–180 aa), and LACV L fragment N-terminal (1–185 aa) were inserted into the pET-28a-sumo expression vector. The gene sequence encoding amino acids 1–210 at the N-terminal of the SFTSV L fragment was cloned into the pET-42a vector, while the gene sequence covering amino acids 587–782 at the N-terminal of the CCHFV L fragment was inserted into the pGEX-6P-1 vector. The plasmids were separately introduced into BL21 (DE3) strains (Beyotime, Shanghai, China). LCMV CEN expression was induced with 1 mM isopropyl-β-d-thiogalactoside (IPTG) at 37 °C for 4 h. The expression of other CENs was induced with 0.5 mM IPTG at 16 °C for 20 h. Following centrifugation to harvest the bacterial cells, sonication was employed for cell lysis. Supernatants were obtained after high-speed centrifugation to separate the precipitate, and the soluble proteins were subsequently purified via nickel column or GST column affinity chromatography. The mutant LCMV CEN proteins underwent purification using conditions identical to those of the wild-type.
High-throughput screening by fluorescence resonance energy transfer (FRET)
In a 384-well plate, 1 µM LCMV CEN proteins diluted in reaction buffer (50 mM HEPES, 150 mM KCl, 1 mM MnCl2, pH = 7.8) was co-incubated with 50 µM compounds at 37 °C for 30 min in a 20 µL reaction system. Subsequently, the single-stranded RNA substrate (5’FAM-AGGAAGAUUAAUAAUUUUUUUUUCCU-BHQ13’) at a final concentration of 0.3 µM was introduced, and the fluorescence signals were immediately measured at λex/λem = 485 nm/ 535 nm (Synergy H1, BioTek, Winooski, VT, USA). LCMV CEN inhibition was assessed by measuring changes in fluorescence intensity and calculated using the following formula:
where V is the enzyme reaction rate, t1 is the reaction starting time, t2 is the reaction ending time, F1 is the fluorescence value at t1, and F2 is the fluorescence value at t2.
Cellular antiviral assay
The cells were seeded at a density of 1 × 104 cells per well in 96-well plates overnight. Preceding viral inoculation, cells were incubated with gradient-diluted compounds for 1 h. BHK-21 and A549 cells were infected with LCMV at a multiplicity of infection (MOI) of 0.1, while VERO cells were infected with SFTSV, HRTV, GTV, and CCHFV at an MOI = 0.1, A549 cells were infected with HTNV at MOI = 1. Following 1 h incubation, the supernatants were removed, and cells were replenished with 100 µL of culture medium containing the respective compound concentrations. After 48 h, the supernatants were harvested for viral copy quantification using real time-quantitative PCR (RT-qPCR).
In preparation for RT-qPCR analysis, viral RNA from the culture supernatants was extracted using an automatic nucleic acid extractor with a compatible kit (Vazyme, Nanjing, China) in accordance with the manufacturer’s guidelines. Viral RNA copies were then quantified using a one-step quantification reagent (Vazyme, Nanjing, China), along with specific primers and template plasmids containing the relevant genes, on an ABI platform (QuantStudio 6 Pro, Thermo Fisher, Waltham, MA, USA).
Cytotoxicity assay
Cells were seeded at a density of 1 × 104 cells per well in 96-well plates overnight. The following day, cells were treated with gradient-diluted compounds. Forty-eight hours later, the cultural supernatants were aspirated, and 100 µL diluted Cell Counting Kit-8 reagent (CCK8) (GLPBIO, Montclair, CA, USA) was added to each well. After incubation at 37 °C for 1 h, the absorbance at 450 nm was measured to assess cell viability (Synergy H1, BioTek, Winooski, VT, USA).
Acrylamide-urea gel electrophoresis
The CEN of LCMV, MACV, or LASV was incubated with SAA at concentrations of 50, 17, 5.6, and 0 µM for 30 min. Next, a single-stranded RNA substrate (sequence: 5’-AGGAAGAUUAAUAAUUUUCCU-3’) was introduced, and cleavage of the RNA substrate was assessed via acrylamide-urea gel electrophoresis after 5, 10, and 20 min. The acrylamide-urea gel was formulated with 4.5 mL of 40% polyacrylamide solution, 1 mL of 10× Tris-Borate-Ethylenediaminetetraacetic acid (EDTA) (TBE) buffer, 4.2 g urea, 0.05 mL of 10% ammonium persulphate, and 0.005 mL of coagulant promoter. Diethyl pyrocarbonate (DEPC) water was added to reach a final volume of 10 mL. Samples from different time points were loaded into the gel wells. RNA electrophoresis was then conducted in TBE buffer at 180 V for 0.5 h on ice. Images were captured using a ChemiDoc MP Imaging System (ChemiDoc Touch, Bio-Rad, Hercules, CA, USA).
Surface plasmon resonance (SPR)
The affinity of compounds to CEN proteins was measured using the Biacore 1 K system (Cytiva, Marlborough, MA, USA) at 25 °C. CENs of LCMV, MACV, LASV, SFTSV or mutant LCMV were immobilized on the CM5 chip using an amino coupling kit (Cytiva, Marlborough, MA, USA) with a loading capacity of approximately 15,000 RU. SAA was diluted at concentrations of 400, 200, 100, 50, 25, 12.5, and 6.25 µM, and flowed over the chip at a rate of 30 µL/min for 120 s of association and 90 s of dissociation. Data were analyzed using the Biacore evaluation software (version 1 K) fitted to a 1:1 binding model, and the fitted curves were drawn using GraphPad Prism 9.0.
Enzyme kinetic analysis
The effects of SAA on the kinetics of CENs derived from LCMV, MACV, and LASV were investigated using a multifunctional automatic microplate reader (Synergy H1, BioTek, Winooski, VT, USA). Fluorescence values were measured by fixing the substrate concentration at 1 µM while altering the CEN concentration from 0.2 to 1.4 µM. Alternatively, fluorescence values were measured by fixing the enzyme concentration while altering the substrate concentration. The reaction rates at different enzyme concentrations and at different substrate concentrations were fitted separately. Kinetic equations for the enzymatic reactions were used to determine the interference mode of the compounds on CENs.
Molecular docking
The 3D structure of SAA was retrieved in SDF format from the PubChem database, and the SDF file was subsequently converted to PDB format. Protein PDB structures of LCMV CEN (5t2t), MACV CEN (7elc), and LASV CEN (4miw) were obtained from the RCSB PDB. The compound and protein PDB files were converted to PDBQT files using the AutoDock Vina software. Boxes of the CEN active pocket were then established for docking, and conformations with relatively low binding energies were chosen for analysis using the online PLIP tool and mapped using PyMOL.
Molecular dynamics (MD) simulations
MD simulations were conducted using Gromacs software (version 2022.3). The GAFF force field was incorporated into the small molecules via AmberTools22, followed by hydrogenation and calculation of RESP potential using Gaussian16W. Simulations were carried out at a fixed temperature of 300 K and atmospheric pressure (1 bar), applying the Amber99sb-ildn force field, with water (Tip3p water model) as the solvent. The system’s total charge was balanced through the addition of Na+ ions. Initially, the molecular dynamics simulation system underwent energy minimization using the steepest descent method, followed by 100,000 steps of isothermal-isovolumic system equilibrium and isothermal-isobaric system equilibrium, each maintaining a coupling constant of 0.1 ps over a duration of 100 ps. Subsequently, a free molecular dynamics simulation was carried out for 5,000,000 steps with a step size of 2 fs, totaling a duration of 100 ns. Following the simulation, trajectory analysis was performed using the software tool, the root mean square deviation of each amino acid trajectory was calculated.
In vivo efficacy experiments
All animal experimental procedures were performed according to ethical guidelines and were approved by the Animal Care Committee of the Wuhan Institute of Virology (WIVA25202305). Female BALB/c mice aged 6–8 weeks were obtained from GemPharmatech (Nanjing, China) and housed in a pathogen-free facility. The animal challenge experiments were performed in a biosafety Level 2 laboratory. Mice were randomly divided into groups (n = 5), and each group received an intraperitoneal injection of 1 × 105 plaque-forming units (PFUs) of LCMV. Upon challenge, mice in the vehicle group were administered the solvent, and mice in the positive control group were orally administered 300 mg/kg T-705 or 30 mg/kg RBV. In the treatment groups, mice were orally administered SAA, chebulinic acid, tannic acid, and punicalagin at a dose of 20 mg/kg, or severally administered SAA through intragavage (i.g.), intraperitoneal injection (i.p.), and tail vein injection (i.v.) at both 20 mg/kg and 40 mg/kg. The treatments were given once daily for 3 d. At the experimental endpoint, the liver or spleen was dissected for viral RNA extraction using a QIAGEN RNA extraction kit (QIAGEN, Venlo, Netherlands), and viral RNA copies were quantified as described above.
Pharmacokinetic experiment
The pharmacokinetics of SAA were investigated in rats. Six female rats were divided evenly into two groups. SAA was administered via both i.g. and i.v. at a dose of 40 mg/kg, and blood samples were collected at 2, 10, 20, and 30 min and at 1, 2, 4, 6, and 24 h post-administration. Plasma samples (10 µL) were mixed with an internal standard (25 µL), followed by the addition of acetonitrile (300 µL), vortexed for 10 min, and centrifuged at 1700× g for 15 min in a refrigerated high-speed centrifuge at 4 °C. The supernatant (50 µL) was mixed with acetonitrile and water (2:8 ratio) containing 100 µg/mL VC, vortexed for 5 min, and prepared for LC-MS/MS analysis using the TRIPLE QUAD 6500 + AB SCIEX system (Sciex, Framingham, MA, USA). Pharmacokinetic parameters were calculated using the WinNonlin software (Version 8.3) by applying the statistical method of moments.
Statistical analysis
Data were obtained from at least three independent biological replicates, unless otherwise specified. Statistical analyses were conducted using the t-test or one-way ANOVA, with a P-value < 0.05 considered statistically significant. Plot and histograms were generated and visualized using GraphPad Prism 9.0. Statistical significance was indicated as follows: ns for P > 0.05, * for P < 0.05, ** for P < 0.01, and *** for P < 0.001.
Results
SAA was hit from a natural product library for LCMV
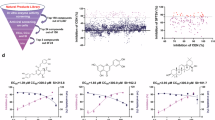
A high-throughput screening system for LCMV CEN was established in a 384-well plate, optimized for enzyme concentration, substrate selection, and reaction duration. A standardized set of conditions (enzyme concentration at 1 µM, substrate concentration at 0.3 µM, and a reaction time of 1 h) in a 20 μL reaction system was validated and utilized for swift screening, following the entire screening process (Fig. 1a). Initially, 150 compounds were selected from the 3519 natural compounds based on a threshold of ≥40% anti-CEN inhibition at 50 μM (Fig. 1b). Subsequently, a dual criterion based on both antiviral inhibition on live LCMV and anti-CEN inhibition was applied to identify hits, resulting in 23 selected compounds (the experiments were independently performed at least three times, Fig. 1c). The 23 hits were tested in BHK-21 cells to evaluate antiviral efficacy and cytotoxicity, calculating 50% effective concentration (EC50) and 50% cytotoxic concentration (CC50) values. Selectivity indices (SI, CC50/EC50) are detailed in Table 1. Notably, four compounds—SAA, chebulinic acid, tannic acid, and punicalagin exhibited promisingly low EC50 values and high CC50 values, with SIs exceeding 30.
a Flowchart of the screening process for cap-dependent endonuclease (CEN) inhibitors from a natural product library, based on both in vitro anti-CEN assays and cellular antiviral assays. b Inhibition of the enzymatic activity of the lymphocytic choriomeningitis virus (LCMV) CEN was detected using our established high-throughput screening system; 150 compounds at 50 µM inhibited LCMV CEN, with over 40% selected for cellular analysis. c The inhibition of viral infection was detected using real-time quantitative PCR; 23 compounds at 50 µM inhibited LCMV infection, with over 40% selected from the initial 150. d Chemical structures of the four hits: salvianolic acid A (SAA), chebulinic acid, tannic acid, and punicalagin. e, f Antiviral effects and cytotoxicity of SAA, chebulinic acid, tannic acid, and punicalagin on LCMV were determined using BHK-21 cells (e) and confirmed using A549 cells (f); EC50s and CC50s, noted along with the curves, were calculated from at least three independent biological replicates.
The chemical structures of the four compounds, depicted in Fig. 1d, were used for further study. SAA exhibited the most favorable EC50 values in antiviral assays conducted on BHK-21 (the experiments were independently performed at least three times, Fig. 1e) and A549 cells (the experiments were independently performed at least three times, Fig. 1f), while tannic acid showed relatively high cytotoxicity. Additionally, immunofluorescence analysis performed on BHK-21 and A549 cells (n = 3, Supplementary Figs. S1a and S1b) illustrated concentration-dependent viral inhibition by the four compounds, with SAA achieving complete inhibition on LCMV infection at a relatively low concentration. Therefore, SAA was selected for further research.
In vivo antiviral effect of SAA on LCMV
The in vivo efficacy of SAA and three other compounds was evaluated in BALB/c mouse model. Viral copies in the targeted organs were detected using RT-qPCR 3 days post-infection (dpi) after diverse drug treatments (Fig. 2a). Mice challenged with LCMV were treated with 20 mg/kg of SAA, chebulinic acid, tannic acid, punicalagin, or 300 mg/kg of T-705 [21], via i.g. The body weight of the mice in the vehicle group decreased for 3 days, while this decrease was halted by T-705 and SAA (Fig. 2b). In addition, T-705 and SAA significantly reduced viral copies in the liver, unlike the other three treatments, which had no effect (Fig. 2c).
a Schematic diagram for in vivo efficacy evaluation with BALB/c mouse model. Mice of 6–8 weeks (n = 5) were randomly divided into vehicle, positive drug control, and experimental groups. In detail, the mice either daily received 20 mg/kg SAA, chebulinic acid, tannic acid, and punicalagin via intragavage (i.g.), or daily received 20 or 40 mg/kg SAA via i.g., intraperitoneal injection (i.p.) or tail vein injection (i.v.) upon challenge of 1 × 105 plaque-forming units of LCMV via i.p. Mice received 300 mg/kg Favipiravir (T-705, a nucleic acid analog drug with broad-spectrum antiviral activity to target viral RNA-dependent RNA polymerase) or 30 mg/kg of Ribavirin (RBV) daily via i.g., as controls. Viral copies in spleens or livers were taken for analysis at 3 days post-infection (dpi). b Body weight changes in mice after treatment with 20 mg/kg of SAA, chebulinic acid, tannic acid, or punicalagin via i.g. upon challenge. 300 mg/kg T-705 administered via i.g. was used as a positive control. c, d Viral copies in livers and spleens were determined at 3 dpi using RT-qPCR through the standard curve method, after the treatments of 20 or 40 mg/kg SAA via i.g., i.p., or i.v., or the treatment of 300 mg/kg T-705 or 30 mg/kg RBV via i.g. Data were represented as mean ± standard deviation from five animals. e Metabolic kinetic data of SAA in rats (n = 3) were determined through both i.g. and i.v. administration routes. The table presents the half-life (t1/2), maximum concentration time (Tmax), maximum blood concentration (Cmax), area under curve (AUC), and bioavailability (F) of SAA across various administration methods. Data were represented as mean ± standard deviation from three animals.
Further experiments were conducted to investigate the influence of the administration route on the efficacy of SAA. Specifically, mice were subjected to i.g., i.p., and i.v. of either 20 or 40 mg/kg of SAA once a day, and viral copies from the liver and spleen were taken for analysis at 3 dpi (Fig. 2a). It was found that the positive control, T-705, significantly decreased viral loads in both the liver and spleen by approximately two logs, while RBV [17] only mildly reduced viral loads in the liver, which is consistent with previous reports [21, 22]. For SAA, i.v. administration reduced viral loads in the liver or spleen by 1–1.5 logs, while i.g. administration showed mild reductions, and i.p. administration was ineffective (Fig. 2d). Pharmacokinetic analysis in rats revealed that the half-life of SAA following i.v. administration was 2.34 h, which exceeded that observed with i.g. administration. This discrepancy suggests that the in vivo effectiveness of SAA is impacted by its bioavailability across diverse administration routes, thereby implying the suboptimal oral bioavailability of SAA (Fig. 2e). Collectively, these data demonstrate the definite in vivo efficacy of SAA on LCMV and highlight the necessity for an optimized administration route for SAA.
SAA inhibited LCMV CEN in a substrate-competing manner
To elucidate the underlying mechanism, a series of biochemical assays were performed on LCMV CEN. It was observed that, in the presence of equal concentrations of single-stranded RNA, SAA markedly impeded the cleavage of LCMV CEN on the RNA substrate in a dose-dependent and time-dependent manner (n = 3, Fig. 3a and Supplementary Fig. S2a). FRET based enzymatic assay revealed that SAA inhibited LCMV CEN with an IC50 at the micromolar level (n = 3, Fig. 3b). Moreover, the mechanism of action of SAA on CEN was investigated through a series of traditional enzyme kinetic experiments with varying enzyme concentrations at a fixed substrate level, and substrate concentrations at a fixed enzyme level. Enzyme kinetic experiments collectively demonstrated that the interaction between SAA and LCMV CEN was reversible and involved substrate-competition (n = 3, Fig. 3c).
a The inhibitory effect of SAA on the cleavage of LCMV CEN on ssRNA substrate was examined at 5, 10, and 20 min via acrylamide-urea gel electrophoresis. Gray analysis of the corresponding strips was calculated for the inhibition rate, shown in the right panel. The divalent metal ion chelator, EDTA, was used as a positive control. The representative image was derived from three replicate experiments. b Dose-dependent inhibitory effect of SAA on LCMV CEN activity was detected using fluorescence resonance energy transfer (FRET), and an IC50 was calculated based on the fluorescent signal value. Baloxavir (BXA) was used as a control. Data were from three independent experiments. c Enzymatic reaction rate was determined by adding different concentrations of LCMV CEN (left) to the reaction system while maintaining a constant substrate concentration, or adding different concentrations of substrate (right) to the reaction system in the presence of 1 µM of LCMV CEN. SAA was used as 5, 10, and 20 µM. Data were from three independent experiments. d Affinity of SAA to LCMV CEN was measured using surface plasmon resonance (SPR); KD was calculated and is shown in the fitted curves. Data were from once representative experiment. e Interaction between SAA and LCMV CEN was analyzed by docking. SAA is shown in green, the blue sticks indicate amino acids interacting with SAA, and the Mn2+ is shown in purple. f Molecular dynamics simulations of SAA with LCMV CEN at 100 ns.
Subsequent surface plasmon resonance (SPR) experiments demonstrated that SAA bound to LCMV CEN with micromolar-level affinity, thereby indicating rapid association and dissociation kinetics (Fig. 3d). Moreover, molecular docking analysis suggested that SAA interacted with key residues, such as Lys43, Ser46, Ile86, Asp88, Glu101, Cys102, Lys114, and Lys121, situated within the active site of LCMV CEN. Additionally, the docking analysis suggested that SAA chelates Mn2+ ions in the active center, with a predicted binding energy of -7.9 kcal/mol (Fig. 3e). For further validation of the molecular docking outcomes of SAA and LCMV CEN, MD simulations indicated that SAA maintained stability upon binding to LCMV CEN for a duration of 10 ns (Fig. 3f). These findings confirm that SAA exerts antiviral effects by targeting LCMV CEN, competitively binding to the enzymatic active site and chelating divalent metal ions.
SAA exerted a broad-spectrum effect on pathogens in the order Bunyavirales including arenaviruses
Thereafter, we explored the broad-spectrum effects of SAA on representative NSVs in Arenaviridae, Phenuiviridae, Nairoviridae, Hantaviridae, and Peribunyaviridae in the order Bunyavirales (Fig. 4a). Initially, we analyzed the primary and secondary structures of CENs from LCMV, NW arenavirus MACV, and OW arenavirus LASV, revealing classical features of the PD-D/ExK nuclease superfamily and over 70% sequence similarity (Fig. 4b). Further research revealed that SAA notably inhibited the cleavage of single-stranded RNA substrates by MACV CEN and LASV CEN within 20 min (n = 3, Fig. 4c). The IC50 values of SAA on MACV CEN and LASV CEN were 6.9 µM and 6.5 µM, respectively (n = 3, Fig. 4d), which was comparable to LCMV CEN. Moreover, SAA competitively inhibited MACV CEN and LASV CEN in a substrate-competing manner (n = 3, Fig. 4e) and bound to MACV CEN and LASV CEN with KD of 85.4 μM and 46.0 μM, respectively (Fig. 4f). Subsequent results revealed comparable inhibitory effects of SAA on the CEN of JUNV, GTOV, CHAPV, SABV, LUJV, and DANV within the family Arenaviridae, with its potency exceeding that of BXA (n = 3, Fig. 4g and Supplementary Fig. S2b). These findings indicated that SAA possesses broad-spectrum anti-CEN effects against the pathogenic arenaviruses.
a Phylogenetic tree of the pathogens from family Phenuiviridae, Nairoviridae, Arenaviridae, Hantaviridae, and Peribunyaviridae in the order of Bunyavirales were made based on L protein sequences, pathogens mentioned in this study were marked. b Alignment of the primary amino acid sequences and secondary spatial structures of LCMV, MACV, and LASV CENs, and analysis on enzyme active pocket. Blue triangles indicate the active sites of LCMV CEN. Red triangles indicate mutational sites of LCMV CEN. Amino acid sites of PD-D/E(X)K are marked by red pentagrams. c The inhibitory effect of SAA on the cleavage of MACV and LASV CENs on ssRNA substrate was examined at a number of different time points via acrylamide-urea gel electrophoresis. Gray analysis of the corresponding strips was calculated into the inhibition rate, shown in the right panel. The divalent metal ion chelator, EDTA, was used as a positive control. The representative image was derived from three replicate experiments. d IC50 of SAA on MACV and LASV CENs were determined using FRET. BXA was used as a control. e SAA inhibited MACV and LASV CENs in a substrate-competing manner. f Affinity of SAA to MACV and LASV CENs was detected using SPR. Data were from once representative experiment. g In vitro inhibitory activity of SAA against CHAPV CEN, DANV CEN, GTOV CEN, JUNV CEN, LUJV CEN and SABV CEN in Arenaviridae. BXA was used as a control. Data from enzymatic assays were obtained from at least three independent experiments.
Additionally, we investigated the antiviral activity of SAA against NSVs beyond Arenaviridae. The effects of SAA on Phenuiviridae were determined using cellular antiviral assays, revealing significant antiviral activity of SAA against SFTSV (n = 3, Fig. 5a and b). Subsequent enzymatic assays revealed that SAA effectively inhibited SFTSV CEN, with an IC50 of 7.8 µM (n = 3, Fig. 5c). Moreover, the docking analysis predicted that it bound to the active pocket of SFTSV CEN with an affinity of -7.1 kcal/mol (Fig. 5d), and SPR experiment indicated a robust affinity between SAA and SFTSV CEN, with a KD = 33.5 μM (Fig. 5e). MD simulations further validated the outcomes of SAA docking with SFTSV CEN, demonstrating the complex reaching equilibrium after 60 ns (Fig. 5f). These results collectively indicate that SAA exhibits antiviral activity by targeting SFTSV CEN.
a Dose-dependent antiviral effect of SAA on SFTSV. T-705 was used as a positive control. b The antiviral effect of SAA on SFTSV in VERO cells was detected using indirect immunofluorescence analysis; representative images are shown. Green fluorescence represents the NP protein of the virus and blue fluorescence represents the nucleus of the VERO cells. T-705 was used as a positive control. c IC50 of SAA on SFTSV CEN was detected using FRET. The CEN inhibitor BXA was used as a control. d Interaction between SAA and SFTSV CEN was analyzed by docking. SAA is shown in green, blue sticks indicate amino acids interacting with SAA, and the Mn2+ is shown in purple. e SPR assay to determine the affinity between SAA and SFTSV CEN. Data were from once representative experiment. f Molecular dynamics simulations of SAA and SFTSV CEN at 100 ns. g Determination of antiviral activity of SAA against GTV and HRTV by antiviral experiments. T-705 was used as a positive control. h Inhibitory activity of SAA on HTNV CEN, LACV CEN and CCHFV CEN in vitro. BXA was used as a control. i Antiviral activity of SAA against HTNV and CCHFV was determined using RT-qPCR. Data from enzymatic and cellular assays were obtained from at least three independent experiments.
Subsequently, GTV and HRTV, both belonging to the same family as SFTSV, were selected to confirm the antiviral activity. Our results also showed that SAA displayed antiviral activity against GTV and HRTV (n = 3, Fig. 5g). Besides, we chose CEN of CCHFV from Nairoviridae, HTNV from Hantaviridae, and LACV from Peribunyaviridae, to further validate the broad-spectrum effects of SAA. The inhibitory and antiviral activities of SAA against the CEN of NSVs across various families were also confirmed (n = 3, Fig. 5h and i). From the IC50s (n = 3, Supplementary Fig. S2c), we found that SAA exhibited comparative inhibitory activity on CENs from Arenaviridae, Phenuiviridae, Hantaviridae, and Peribunyaviridae, while demonstrating suboptimal inhibitory activity on CEN from Nairoviridae, indicating a limited selectivity of SAA in vitro. In conclusion, these findings illustrated the broad-spectrum inhibition and potential antiviral activity of SAA against NSVs in the order of Bunyavirales.
Binding mechanism of SAA on LCMV cap-dependent endonuclease
We conducted a detailed analysis to validate the binding sites and interaction patterns of SAA, enhancing the understanding of how it influences LCMV CEN. From the target view, we utilized docking analysis to predict the binding sites on CEN across the six most favorable conformations, followed by quantifying the frequency of amino acid interactions with SAA. As a result, we identified 17 amino acids that are likely to interact with SAA. Among these, residues Lys43, Arg47, Ile86, Asp88, Glu101, Lys114, and Lys121 were observed three or more times and were subsequently subjected to affinity analysis (Figs. 4b and 6a). Significantly, these amino acids represent key sites within the active center of CEN. Specifically, Lys43, Asp88, Glu101, Lys114, and Lys121 were conserved across the CENs of LCMV, MACV, and LASV. Furthermore, Asp88, Glu101, Lys114, and Lys121 were conserved across all arenaviruses [23]. Through a series of SPR assays, we observed that mutations at Glu101 and Lys114 significantly reduced the affinity of SAA for CEN, whereas mutations at the remaining five sites had a less pronounced impact (Fig. 6b). These findings confirm that SAA definitely interacts with CEN, thus, suggesting a potential mechanism for its broad-spectrum effects.
a Analysis of amino acids in LCMV CEN active pocket bond by SAA in docking under diverse conformations; amino acids with binding frequencies equal to or greater than three were taken for mutation analysis. b Affinity of SAA to the LCMV wildtype and mutant CENs. Data were from once representative experiment. c Comparison of the structural formula of SAA and methyl salvionolate A (MSA). d Alignment on the binding conformation of SAA and MSA with LCMV CEN. SAA is shown in green, MSA is shown in blue, and Mn2+ is shown in purple. e Inhibition of MSA on LCMV CEN (left) and LCMV infection (right). SAA was used as a control. Data from enzymatic and cellular assays were obtained from at least three independent experiments.
From the compound perspective, we examined the functional group responsible for chelating the divalent metal ions within CENs leading to deactivation of the metalloenzyme. Utilizing a derivative of SAA known as methyl salvionolate A (MSA), where the carboxyl group had been esterified by methanol thereby eliminating its acidity and chelating ability, we substantiated the role of the lone exposed carboxyl group of SAA, which was predicted to interact with the Mn2+ ion in LCMV CEN (Fig. 6c). Consequently, MSA displayed a weaker affinity (-6.9 kcal/mol) for LCMV CEN than that for SAA (−7.9 kcal/mol) (Fig. 6d). Moreover, MSA entirely abrogated its inhibitory impact on LCMV CEN and its antiviral efficacy against LCMV (n = 3, Fig. 6e). These findings collectively elucidated the action mechanism of SAA on CENs, thus, indicating the prospect of SAA as a promising compound for drug development.
Discussion
Cap-snatching mediated by viral endonuclease is a distinctive mechanism utilized by NSVs. This mechanism plays a critical role in viral transcription, thus, rendering CEN a critical target for drug discovery and development [11]. Until now, only BXA has been approved for the clinical treatment of influenza virus infection due to its excellent efficacy and pharmacokinetics [24]. However, BXA has been shown to induce mutations in CEN leading to drug resistance [25]. In addition, BXA has been demonstrated to inhibit SFTSV and HRTV with EC50s of 0.26 µM and 0.25 µM, respectively, and inhibit LCMV and JUNV with an EC50 > 1 µM [26]. Thus, there is a pressing need of CENis that are effective against NSVs beyond influenza virus.
Recent studies have highlighted three main routes for discovering CENis: high-throughput screening of metal-chelating agent compound libraries [17], de novo synthesis based on rational design [27], and screening of natural products that contain diverse general acids [28]. Historically, natural products and their structural analogs have been pivotal in therapies for cancer and infectious diseases [29]. Salvianolic acid C derived from Salvia miltiorrhizafrom has been previously proved to inhibit severe acute respiratory syndrome coronavirus 2 by blocking membrane fusion [30]. Our previous study has also screened a mini natural product library containing 71 compounds, and found that flavonoids such as tanshione I from Salvia miltiorrhiza exhibits broad-spectrum antiviral effects on NSVs including SFTSV, influenza A virus, and LCMV, which was associated with CEN inhibition [28]. In addition, our recent work has also revealed Licoflavone C as an alternative inhibitor of SFTSV, thereby offering insights into targeting CEN with flavonoids in drug discovery [31]. Here, we reported a polyphenol component of Salvia miltiorrhiza, called SAA, inhibiting CENs from viruses in Arenaviridae, Phenuiviridae, Hantaviridae, Peribunyaviridae, and Nairoviridae, through screening a large natural compound library containing 3519 compounds. In comparison to the previous study, SAA discovered in this study exhibits a broader antiviral spectrum and higher efficacy against NSV CENs in comparison to tanshinone I and Licoflavone C. Moreover, the mechanism of SAA was shown to bind with conserved sites in the CEN active pocket while chelating metal ion via the carboxyl group, thus, demonstrating explicit in vitro and in vivo effects. In view of the above, natural products from Salvia miltiorrhiza are important resources for antiviral discovery, and medicinal ingredients such as flavonoids and polyphenols are worth in-depth study.
To the best of our knowledge, this study represents the first report of SAA as a novel CENi to inhibit NSVs, although previous studies have reported its multifaceted roles. Specifically, SAA alleviates oxidative stress-induced osteoporosis by acting as an antioxidant and modulating bone metabolic pathways [32]. Moreover, in the context of acute cerebral ischemia-reperfusion injury, SAA migrates neuroinflammation by inhibiting the microglial TLR2/4 pathway [33]. Additionally, SAA has been shown to alleviate heart failure with preserved ejection fraction (HFpEF) through the modulation of TLR/Myd88/TRAF/NF-κB and p38MAPK/CREB signaling pathways [34]. Thus, SAA has been documented to exert anti-inflammatory and antioxidant effects. Studies have indicated that viral infection triggers a cascade of pro-inflammatory factors by activating the Toll-like receptor (TLR) and RIG-I-like receptor (RLR) pathways, and that mitochondrial dysfunction leads to burst of reactive oxygen species (ROS) [35, 36]; therefore, it is plausible to hypothesize that SAA might play a role in viral infections through its anti-inflammatory and antioxidant properties. Thus, the contribution of off-target effects due to the anti-inflammatory actions of SAA cannot be entirely excluded in this study. In addition to the aforementioned anti-inflammatory effects, SAA may exert a direct inactivation effect on enveloped RNA/DNA viruses by interfering with the phospholipid bilayer, thereby representing an inhibition at the viral entry stage by disrupting the virion integrity. This mechanism is widely found in natural products [37,38,39], and may also constitute a part of its common anti-viral mechanism. Thus, an off-target effect resulting from envelope glycoprotein interference may also contribute to the antiviral performance of SAA against arenavirus in this study.
Moreover, previous studies have also demonstrated that SAA exerts antiviral activity by targeting envelope glycoproteins, for example, binding specifically to glycoprotein B of HSV and the receptor-binding domain (RBD) of SARS-CoV-2 [40, 41] to block viral entry. Thus, SAA may interact with different viral proteins to exhibit unique mechanisms while combating various viruses. In this study, SAA exhibited a high affinity for the arenavirus CEN (KD = 5.1 µM) demonstrating an antiviral effect by inhibiting viral replication. Consequently, we reported a unique mechanism of its antiviral activity, which may enhance the understanding of SAA pharmacology.
Although the off-target effects of natural products are often considered drawbacks compared to targeted synthetic compounds, these effects may offer unexpected benefits in combating complex viral infections because they allow for targeting multiple viral replication stages simultaneously. Despite this, data on the toxicity of SAA in dogs has indicated that SAA lacks mutagenic properties but exhibits hepatotoxicity and nephrotoxicity; however, these effects have been shown to be transient and reversible [42]. Hence, further research on developing SAA as an antiviral targeted at CEN must fully consider its potential primary and side effects, as well as the mechanism of action derived from its multi-target properties.
Notably, our pharmacokinetic investigation of SAA in rats unveiled a markedly low oral bioavailability (F) utilization of SAA (F = 1%), aligning with previous research on the in vivo metabolic pathways of SAA. Thus, an effective concentration might not be achieved in the liver or spleen through administration via i.g. [43]. This low bioavailability can be attributed to poor membrane permeability in the gastrointestinal tract and predominant fecal metabolism [44]. Furthermore, existing clinical studies on SAA have demonstrated its favorable tolerability in humans within a single dose range of 10–300 mg [43]. Hence, SAA holds promise for development as an antiviral drug targeting NSVs CEN. Further research and consideration are needed to address the challenges related to enhancing its oral bioavailability and antiviral activity. A potential optimization strategy involves adding large side-chain groups to SAA to enhance binding affinity and activity at the CEN active site. The other is to reduce the hydroxyl groups of SAA to retard metabolism, thus, improving its in vivo bioavailability and safety. Although modifications based on natural products can be challenging, increasing plant- or microbe-based fermentation techniques may help [45].
In this study, we performed in-depth research on the mechanism of action of SAA on LCMV CEN by both biochemical and computational analysis, and identified key amino acid sites in the active pocket of LCMV CEN engaging with SAA binding, where a naked SAA carboxyl group might chelate with the divalent ions in it. Interestingly, we found that mutations at positions Glu101 and Lys114 noticeably weakened the affinity of SAA to LCMV CEN. Importantly, these two sites are not only conserved among CENs of LCMV, MACV, and LASV, but also conserved among CENs of all arenaviruses [23]. Additionally, the broad-spectrum effects were validated by enzymatic or antiviral experiments on representative NSVs from different families in the order Bunyavirales. Besides, SAA demonstrated superior inhibitory activity against NSV CENs compared to BXA. Notably, we found that SAA shares a similar ternary ring structure with BXA and its analogs. These broadly target arenaviral CENs when the hydroxyl group at position 24 of SAA forms a ring with the carbon atom at position 32 on the benzene ring [17]. This potentiates the applicability of SAA and implies a possibility of discovering broad-spectrum antivirals against CEN based on natural products, although this should be subjected to further modification. Moreover, the variations in IC50s and EC50s between different viruses’ prompt concerns about the potential off-target effects of SAA, which should be considered and possibly addressed by structure-activity optimization.
This study examined a wide range of natural products and identified SAA as a promising compound. SAA demonstrated its in vitro and in vivo efficacy, broad-spectrum antiviral activity, and detailed mechanism of action on CEN. These findings underscore the potential of SAA against pathogenic arenaviruses, and highlight the feasibility of targeting CENs for drug discovery against bunyaviruses.
References
Charrel RN, Lamballerie XD. Arenaviruses other than Lassa virus. Antivir Res. 2003;57:89–100.
Luo XL, Lu S, Qin C, Shi M, Lu XB, Wang L, et al. Emergence of an ancient and pathogenic mammarenavirus. Emerg Microbes Infect. 2023;12:e2192816.
Current ICTV Taxonomy Release [database on the internet]. [Place unknown]: International Committee on Taxonomy of Viruses (ICTV). c2025 [cited 2024 Jan 22]. Available from: https://ictv.global/taxonomy
Kinori M, Schwartzstein H, Zeid JL, Kurup SP, Mets MB. Congenital lymphocytic choriomeningitis virus—an underdiagnosed fetal teratogen. J Am Assoc Pediatr Ophthalmol Strabismus. 2018;22:79–81.e1.
McLay L, Liang Y, Ly H. Comparative analysis of disease pathogenesis and molecular mechanisms of New World and Old World arenavirus infections. J Gen Virol. 2014;95:1–15.
Shao JJ, Liang YY, Ly H. Human hemorrhagic Fever causing arenaviruses: molecular mechanisms contributing to virus virulence and disease pathogenesis. Pathog Basel Switz. 2015;4:283–306.
Riviere Y, Ahmed R, Southern PJ, Buchmeier MJ, Dutko FJ, Oldstone MB. The S RNA segment of lymphocytic choriomeningitis virus codes for the nucleoprotein and glycoproteins 1 and 2. J Virol. 1985;53:966–8.
Kranzusch PJ, Schenk AD, Rahmeh AA, Radoshitzky SR, Bavari S, Walz T, et al. Assembly of a functional Machupo virus polymerase complex. Proc Natl Acad Sci USA. 2010;107:20069–74.
Morin B, Coutard B, Lelke M, Ferron F, Kerber R, Jamal S, et al. The N-terminal domain of the arenavirus L protein is an RNA endonuclease essential in mRNA transcription. PLoS Pathog. 2010;6:e1001038.
Te Velthuis AJW, Grimes JM, Fodor E. Structural insights into RNA polymerases of negative-sense RNA viruses. Nat Rev Microbiol. 2021;19:303–18.
Olschewski S, Cusack S, Rosenthal M. The cap-snatching mechanism of Bunyaviruses. Trends Microbiol. 2020;28:293–303.
Holm T, Kopicki J-D, Busch C, Olschewski S, Rosenthal M, Uetrecht C, et al. Biochemical and structural studies reveal differences and commonalities among cap-snatching endonucleases from segmented negative-strand RNA viruses. J Biol Chem. 2018;293:19686–98.
Home | ClinicalTrials.gov. no date
Cross RW, Fenton KA, Woolsey C, Prasad AN, Borisevich V, Agans KN, et al. Monoclonal antibody therapy protects nonhuman primates against mucosal exposure to Lassa virus. Cell Rep. Med. 2024;5:101392.
Pan XY, Wu Y, Wang W, Zhang LK, Xiao GF. Development of horse neutralizing immunoglobulin and immunoglobulin fragments against Junín virus. Antivir Res. 2020;174:104666.
Pan XY, Wu Y, Wang W, Zhang LK, Xiao GF. Novel neutralizing monoclonal antibodies against Junin virus. Antivir Res. 2018;156:21–28.
Toba S, Sato A, Kawai M, Taoda Y, Unoh Y, Kusakabe S, et al. Identification of cap-dependent endonuclease inhibitors with broad-spectrum activity against bunyaviruses. Proc Natl Acad Sci USA. 2022;119:e2206104119.
Zhang GS, Cao JY, Cai Y, Liu Y, Li YL, Wang PL, et al. Structure-activity relationship optimization for lassa virus fusion inhibitors targeting the transmembrane domain of GP2. Protein Cell. 2019;10:137–42.
Lan XH, Zhang YL, Jia XY, Dong SQ, Liu Y, Zhang MM, et al. Screening and identification of Lassa virus endonuclease-targeting inhibitors from a fragment-based drug discovery library. Antivir Res. 2022;197:105230.
Gowen BB, Naik S, Westover JB, Brown ER, Gantla VR, Fetsko A, et al. Potent inhibition of arenavirus infection by a novel fusion inhibitor. Antivir Res. 2021;193:105125.
Hickerson BT, Westover JB, Jung K-H, Komeno T, Furuta Y, Gowen BB. Effective treatment of experimental lymphocytic choriomeningitis virus infection: consideration of favipiravir for use with infected organ transplant recipients. J Infect Dis. 2018;218:522–7.
Zadeh VR, Afowowe TO, Abe H, Urata S, Yasuda J. Potential and action mechanism of favipiravir as an antiviral against Junin virus. PLoS Pathog. 2022;18:e1010689.
Saez-Ayala M, Yekwa EL, Carcelli M, Canard B, Alvarez K, Ferron F. Crystal structures of Lymphocytic choriomeningitis virus endonuclease domain complexed with diketo-acid ligands. IUCrJ. 2018;5:223–35.
Abraham GM, Morton JB, Saravolatz LD. Baloxavir: a novel antiviral agent in the treatment of influenza. Clin Infect Dis Publ Infect Dis Soc Am. 2020;71:1790–4.
Hickerson BT, Petrovskaya SN, Dickensheets H, Donnelly RP, Ince WL, Ilyushina NA. Impact of baloxavir resistance-associated substitutions on influenza virus growth and drug susceptibility. J Virol. 2023;97:e0015423.
Wang WJ, Shin WJ, Zhang BJ, Choi Y, Yoo JS, Zimmerman MI, et al. The cap-snatching SFTSV endonuclease domain is an antiviral target. Cell Rep. 2020;30:153–163.e5.
Credille CV, Dick BL, Morrison CN, Stokes RW, Adamek RN, Wu NC, et al. Structure–activity relationships in metal-binding pharmacophores for influenza endonuclease. J Med Chem. 2018;61:10206–17.
He XX, Yang F, Wu Y, Lu J, Gao X, Zhu XR, et al. Identification of tanshinone I as cap-dependent endonuclease inhibitor with broad-spectrum antiviral effect. J Virol. 2023;97:e00796–23.
Atanasov AG, Zotchev SB, Dirsch VM. International natural product sciences taskforce, supuran CT. Natural products in drug discovery: advances and opportunities. Nat Rev Drug Discov. 2021;20:200–16.
Yang C, Pan XY, Xu XF, Cheng C, Huang Y, Li L, et al. Salvianolic acid C potently inhibits SARS-CoV-2 infection by blocking the formation of six-helix bundle core of spike protein. Signal Transduct Target Ther. 2020;5:220.
Gao X, He XX, Zhu XR, Wu Y, Lu J, Chen XL, et al. Identification of Licoflavone C as a cap-dependent endonuclease inhibitor against severe fever with thrombocytopenia syndrome virus. Acta Pharmacol Sin. 2025;46:2482–95.
Khan A, Sabella H, Mandlem VKK, Deba F. Salvianolic acid-A alleviates oxidative stress-induced osteoporosis. Life Sci. 2025;375:123727.
Ling Y, Jin L, Ma QX, Huang Y, Yang QQ, Chen ML, et al. Salvianolic acid A alleviated inflammatory response mediated by microglia through inhibiting the activation of TLR2/4 in acute cerebral ischemia-reperfusion. Phytomedicine. 2021;87:153569.
Dawuti A, Sun SC, Wang RR, Gong DF, Liu RQ, Kong DW, et al. Salvianolic acid A alleviates heart failure with preserved ejection fraction via regulating TLR/Myd88/TRAF/NF-κB and p38MAPK/CREB signaling pathways. Biomed Pharmacother. 2023;168:115837.
Huang SZ, Cheng AC, Wang MS, Yin ZQ, Huang J, Jia RY. Viruses utilize ubiquitination systems to escape TLR/RLR-mediated innate immunity. Front Immunol. 2022;13:1065211.
To EE, Erlich JR, Liong F, Luong R, Liong S, Esaq F, et al. Mitochondrial reactive oxygen species contribute to pathological inflammation during influenza A virus infection in mice. Antioxid Redox Signal. 2020;32:929–42.
Lu SS, Pan XY, Chen DW, Xie X, Wu Y, Shang WJ, et al. Broad-spectrum antivirals of protoporphyrins inhibit the entry of highly pathogenic emerging viruses. Bioorg Chem. 2021;107:104619.
Chen DW, Lu SS, Yang G, Pan XY, Fan S, Xie X, et al. The seafood Musculus senhousei shows anti-influenza A virus activity by targeting virion envelope lipids. Biochem Pharmacol. 2020;177:113982.
Chen ZL, Li DL, Wang TL, Li YQ, Qin PP, Zhu HS, et al. Salvianolic acid A inhibits pseudorabies virus infection by directly inactivating the virus particle. Phytomedicine. 2024;134:156015.
Hu SL, Wang J, Zhang YJ, Bai HY, Wang C, Wang N, et al. Three salvianolic acids inhibit 2019‐nCoV spike pseudovirus viropexis by binding to both its RBD and receptor ACE2. J Med Virol. 2021;93:3143–51.
Yan H, Li HH, Chen XY, Wang J, Yang JY, Xu ZQ, et al. Salvianolic acid A acts as a herpes simplex virus dual inhibitor by blocking glycoprotein B-mediated adsorption and membrane fusion. Phytomedicine. 2025;143:156910.
Yang MY, Song ZY, Gan HL, Zheng MH, Liu Q, Meng XT, et al. Non-clinical safety evaluation of salvianolic acid A: acute, 4-week intravenous toxicities and genotoxicity evaluations. BMC Pharm Toxicol. 2022;23:83.
Chen JL, Ruan ZR, Lou HG, Yang DD, Shao R, Xu YC, et al. First-in-human study to investigate the safety and pharmacokinetics of salvianolic acid A and pharmacokinetic simulation using a physiologically based pharmacokinetic model. Front Pharmacol. 2022;13:907208.
Sun JL, Song JK, Zhang W, Jing FB, Xu W, Leng P, et al. Some pharmacokinetic parameters of salvianolic acid A following single-dose oral administration to rats. Pharm Biol. 2018;56:399–406.
Li L, Wang L, Fan WX, Jiang Y, Zhang C, Li JH, et al. The application of fermentation technology in traditional chinese medicine: a review. Am J Chin Med. 2020;48:899–921.
Acknowledgements
We are particularly grateful to the Institutional Center for Shared Technologies and Facilities of Wuhan Institute of Virology, CAS, for their technical support. We also thank the National Virus Resource Center, Wuhan Institute of Virology, CAS, for providing important research materials. We acknowledge the National Natural Science Foundation of China (Grant No. 82130101), the Youth Innovation Promotion Association of CAS (Grant No. 2021333), and the Natural Science Foundation of Wuhan (Grant No. 2024040701010067) for financial support.
Author information
Authors and Affiliations
Contributions
XG designed and performed all of the biochemical, cellular, and animal experiments, and drafted the manuscript; YW constructed the plasmids; XXH and GLL expressed and purified the recombinant proteins; HXY, JL, XRZ, XLC, and CSZ participated in data collection; HYL and ZFZ assisted with data analysis; CY, SS, FD, WX, and SWL coordinated the project; GFX revised the manuscript and contributed to the interpretation of the results; and XYP drafted, revised, and approved the manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Gao, X., Wu, Y., He, Xx. et al. Salvianolic acid A from Salvia miltiorrhiza identified as a cap-dependent endonuclease inhibitor for pathogenic arenaviruses. Acta Pharmacol Sin (2025). https://doi.org/10.1038/s41401-025-01654-z
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41401-025-01654-z