Abstract
Residential stem cells sense extrinsic and intrinsic signals to proliferate accordingly to maintain homeostasis. However, how differentiated cells control stem cell proliferation still remains elusive. Here, we find that Auxilin (Aux) maintains enterocyte (EC) integrity to prevent unlimited intestinal stem cell (ISC) proliferation. Depleting aux in ECs leads to excessive ISC proliferation and intestinal homeostasis disruption. Ectopic cytokine production from dying aux-depleted ECs activates JAK/STAT signaling and promotes ISC proliferation. Mechanistically, Aux facilitates anterograde ER-to-Golgi apparatus (GA) vesicle transport by associating with COPII coatomer. Further, the presentation of cell adhesion molecules (CAMs) by ER-to-GA transport is required for intestinal homeostasis. Together, these data demonstrate that Aux maintains EC integrity by mediating ER-to-GA trafficking of CAMs to restrain excessive ISC proliferation. Thus our study uncovers the underlying mechanism of how differentiated cells control stem cell proliferation through inter-cell communication during tissue homeostasis and pathogenesis.

Similar content being viewed by others
Introduction
Adult fitness is closely determined by intestinal physiology as intestine is the organ to digest and absorb nutrients. Adult stem cells must constantly respond to the needs of residential tissue to adopt a proper proliferation rate to maintain tissue homeostasis. Communications between differentiated cells and residential stem cells are critical for homeostasis maintenance. Stem cell proliferation and progeny differentiation must be tightly balanced to prevent unrestricted stem cell proliferation or stem cell depletion which eventually results in various diseases such as cancer [1,2,3]. Therefore, understanding inter-cell communication networks between differentiated cells and stem cells will yield insights into the mechanisms of tissue homeostasis control and related human diseases.
The Drosophila posterior midgut is an equivalent of the mammalian small intestine and an attractive system to investigate stem cell regulation and homeostasis control. Without crypt-villus structure, the Drosophila midgut epithelium is a monolayer of epithelial cells composed of ISCs and their intermediate/terminally differentiated progeny [4,5,6,7]. ISCs are interspersed along the basement membrane of the adult Drosophila midgut [8, 9]. Under physiological conditions, ISC often undergoes asymmetric self-renewal division to produce either an enteroblast (EB) which is directly differentiated into absorptive enterocyte (EC) by high levels of Notch signaling or an enteroendocrine progenitor (EEP) which divides once to produce two EEs [8,9,10,11,12]. In response to differentiation and subsequent loss of neighboring ISCs (or vice versa), a significant proportion of ISCs undergoes symmetric division to replace lost ISCs [13, 14]. ISC proliferation under stressed conditions is regulated by multiple signaling pathways such as EGFR and JAK/STAT [7, 15]. The damaged ECs are the major source of stress-induced ligands [16,17,18,19,20]. However, how the fitness of ECs is maintained to communicate with ISCs for intestinal homeostasis and regeneration remains poorly understood.
Cells constantly exchange information and materials with their outside environment and/or neighbors through endocytosis and exocytosis. Once Clathrin-coated endocytic vesicles (CCVs) pinch off from the plasma membrane (PM). The DnaJ-domain chaperone Auxilin (Aux) facilitates the disassembly of the Clathrin lattice of CCVs and participates in other steps of the CCV cycle [21,22,23]. Two Aux isoforms, the brain-specific Aux 1 and the ubiquitous Aux 2 (or GAK, cyclin G-associated kinase), exist in vertebrates, while only one Aux ortholog is found in Drosophila which is more structurally similar to GAK [22, 23]. Aux has been found to function in the signal-sending cells as an integral regulator of Notch signaling in several Notch-dependent processes in Drosophila [24,25,26]. Our previous work showed that Aux in ISCs restrains EGFR signaling by promoting EGFR clearance from the PM through endocytosis [27]. SH3Ps were reported to recruit Aux to endosomes recently [28]. Further, Aux is a putative Parkinson disease factor and involved in neurodegeneration in glia [29].
Many eukaryotic proteins are processed in the ER for proper folding, modifications and assembly before entering into the secretory pathway to their destinations [30]. Anterograde ER-to-GA trafficking starts from the ER where proteins are packed into COPII-coated vesicles which bud off at ER exit sites (ERES), COPII vesicles are then delivered to the GA where these proteins are further processed and transported to their final destinations [31, 32]. Sec23/Sec24 coatomer is recruited to the cytoplasmic side of the ERES to form the inner shell of COPII, followed by binding of the outer layer coat proteins Sec13 and Sec31 [31, 32]. Conditions like protein overload in the ER often cause ER stress (ERS) and ERUPR to restore cellular protein homeostasis. Three sub-pathways of ERUPR, mediated by IRE1/Xbp1, ATF6/Hsc3, and PERK/eIF2α, are executed [33]. Previous report revealed that Aux facilitates membrane traffic in the early secretory pathway in budding yeast [34]. However, it remains elusive whether and how Aux functions in mature ECs to non-cell autonomously control ISC proliferation and intestinal homeostasis.
In this study, we provide evidence that Aux in ECs non-cell autonomously restricts ISC proliferation under normal conditions. Importantly, Aux associates with COPII coatomer to facilitate anterograde ER-to-GA transport of cell adhesion molecules (CAMs) to maintain EC integrity. Thus, our data uncover the underlying mechanism of how differentiated cells control stem cell proliferation through inter-cell communication.
Results
Aux in ECs is required for intestinal homeostasis
We previously demonstrated that Aux is intrinsically required for ISC proliferation and tissue homeostasis [27]. Using Aux-specific antibody, we found that Aux is expressed in all intestinal cell types (Supplementary Fig. S1) [27]. To determine whether Aux in ECs plays any roles for intestinal homeostasis, we specifically depleted aux in ECs using several effective auxRNAi lines by the EC-specific driver Myo1AGal4 (Myo1Ats) [27]. Interestingly, we found that the life span of Myo1Ats>auxRNAi flies was significantly shortened in comparison to control animals, indicating that Aux plays important role in ECs. Upon closer examinations, we found that the size of Myo1Ats>auxRNAi intestine and the intestinal cell density were significantly increased compared to those of control, suggesting that Aux in ECs is essential for fitness and intestinal homeostasis (Fig. 1A).
A Compared to control intestines, the number of esg-lacZ+ cells (red, white arrowheads) is dramatically increased in Myo1Ats>auxRNAi intestines at 29 °C for 7 days. The white lines indicate the diameter of intestines with indicated genotypes. Quantification of esg-lacZ+ cell No/images in different genotypes indicated. Mean ± SD is shown. ****p < 0.0001. Please note that Myo1Ats>auxRNAi intestines are highly deformed preventing accurate cell number quantification (the same as follows). B Compared to control intestines, the number of ISCs (by Dl-lacZ, red, white arrowheads) is dramatically increased in Myo1Ats>auxRNAi intestines at 29 °C for 7 days. Quantification of Dl-lacZ+ cell No/images in different genotypes indicated. Mean ± SD is shown. ****p < 0.0001. C Compared to control intestines, the number of EBs (by Gbe + Su(H)-lacZ, red, white arrowheads) is dramatically increased in Myo1Ats>auxRNAi intestines at 29 °C for 7 days. Quantification of Gbe + Su(H)-lacZ+ cell No/images in different genotypes indicated. Mean ± SD is shown. ****p < 0.0001. D Compared to control intestines, the number of mitotic cells (white arrowheads) is significantly increased in Myo1Ats>auxRNAi intestines at 29 °C for 7 days. Quantification of the number of pH3+ cells/images in different genotypes indicated. Mean ± SD is shown. ****p < 0.0001. Scale bars, 20 μm.
Next, we characterized the identities of these increased intestinal cells in Myo1Ats>auxRNAi intestines. Compared to in control intestines where progenitors are frequently observed as 1–3 cells/cluster, the number of progenitors in Myo1Ats>auxRNAi intestines was dramatically increased which formed large clusters with varied cell sizes, indicative of excessive progenitor accumulation and intestinal homeostasis disruption (Fig. 1A and Supplementary Fig. S2). We found that simultaneous expression of UAS-aux-flag-GFP completely restored midgut homeostasis determined by esg-lacZ, confirming the specificity of aux depletion (Fig. 1A and Supplementary Fig. S2). When ISC was examined (by Dl-lacZ), the number of Dl-lacZ+ cells in Myo1Ats>auxRNAi intestines was also significantly increased compared to those in control flies (Fig. 1B). Further, these Dl-lacZ+ cells were heterogeneous in size, indicative of mixed identity (Fig. 1B). Furthermore, the number and percentage of EBs (by Gbe + Su(H)-lacZ) were dramatically increased in Myo1Ats>auxRNAi intestines compared to those in control flies (Fig. 1C). Moreover, the number of dividing ISCs was significantly increased in aux-depleted intestines compared to those in control flies (Fig. 1D). Together, these data demonstrate that Aux in ECs non-cell autonomously restricts ISC proliferation to maintain intestinal homeostasis under physiological conditions.
Ectopic cytokine expression promotes ISC proliferation
We then explored the mechanism(s) by which Aux non-cell autonomously inhibits ISC proliferation. Our previous study showed that Aux actively clears EGFR from ISC PM to quench EGFR signaling [27]. However, co-depletion of Egfr could not suppress the observed defects (Supplementary Fig. S3) [27], suggesting that Aux may not function in EGFR internalization and EGFR signaling is unlikely responsible for the defects observed in Myo1Ats>auxRNAi intestines.
Previous studies showed that JAK/STAT signaling activated by cytokines from ECs plays essential role in intestinal homeostasis when ECs are defective in certain signaling pathways or assaulted by environmental challenges [19, 35,36,37,38]. We examined JAK/STAT signal activation in Myo1Ats>auxRNAi intestines. Compared with control [19, 39], the number of 10XSTAT-GFP+ cells and the fluorescence intensity of 10XSTAT-GFP were significantly increased in Myo1Ats>auxRNAi intestines, indicative of ectopic JAK/STAT signaling (Fig. 2A). The ectopic JAK/STAT signaling was independently verified with a pSTAT antibody (Supplementary Fig. S4) [40]. Consistently, the expression of upd-lacZ and upd3-lacZ in ECs was significantly increased in Myo1Ats>auxRNAi intestines compared to those of control intestines (Fig. 2B, C). The ectopic JAK/STAT signaling in these intestines was further verified by transcript analysis of cytokines and socs36E, the downstream target of JSK/STAT signaling (Fig. 2D). These data show that ectopic JAK/STAT signaling activated by increased EC-derived cytokines promotes ISC proliferation and disrupts intestinal homeostasis in the absence of Aux. Supporting this notion, complete removal of upd3 effectively suppressed the defects observed in Myo1Ats>auxRNAi intestines (Fig. 2E). Further, Tofacitinib administration, the JAK inhibitor, also greatly suppressed the defects observed in Myo1Ats>auxRNAi intestines (Supplementary Fig. S5). Altogether, these data show that ectopic JAK/STAT signaling is mainly responsible for the defects observed in Myo1Ats> auxRNAi intestines.
A Compared to control intestines, the number of 10XSTAT-GFP cells and the fluorescence intensity of 10XSTAT-GFP (green, white arrowheads) are significantly increased in Myo1Ats>auxRNAi intestines at 29 °C for 7 days. 10XSTAT-GFP channel is showed separately in black white. Quantification of the fluorescence intensity of 10XSTAT-GFP in control and Myo1Ats>auxRNAi intestines. Mean ± SD is shown. ****p < 0.0001. B, C Compared to control ECs in which cytokines (by upd-lacZ and upd3-lacZ) are barely detectable, cytokine expression (red, white arrowheads) is significantly increased in aux-defective ECs at 29 °C for 7 days. lacZ channel is showed separately in black white. Quantification of the fluorescence intensity of Upds-lacZ in control and Myo1Ats>auxRNAi ECs. Mean ± SD is shown. ****p < 0.0001. D The transcripts of cytokines and socs36E are significantly increased in Myo1Ats>auxRNAi intestines. Mean ± SD is shown. ****p < 0.0001. E The dramatic increase of Dl+ cells (red, white arrowheads) in Myo1Ats>auxRNAi intestines is significantly suppressed by further removal of upd3. Quantification of the number of Dl+ cells in intestines with indicated genotypes. Mean ± SD is shown. ****p < 0.0001. Scale bars: 20 μm.
aux-defective ECs undergo apoptosis due to ER stress
What is the function of Aux in ECs? The homeostatic defects observed in Myo1Ats> auxRNAi intestines phenocopy those midguts damaged/stressed by chemical agents and challenged by enteric bacterial infection [18, 38, 41, 42]. Thus, we speculated that Aux in ECs may protect ECs from death induced by internal or environmental stresses. Indeed, we observed increased number of apoptotic ECs upon aux depletion, indicating that aux-defective ECs are unhealthy and undergo apoptosis (Fig. 3A). Consistently, simultaneous expression of anti-apoptotic p35 almost completely suppressed the defects observed in Myo1Ats>auxRNAi intestines (Fig. 3B).
A Compared to control intestines in which apoptosis (by GC3Ai, green) is barely detectable, the number of GC3Ai+ cells (white arrowheads) is significantly increased in Myo1Ats>auxRNAi intestines at 29 °C for 3 days. Quantification of GC3Ai+ cell No/image in control and Myo1Ats>auxRNAi intestines. Mean ± SD is shown. ****p < 0.0001. B Simultaneous expression of p35 almost completely rescued the defects observed in Myo1Ats>auxRNAi intestines at 29 °C for 7 days (by esg-lacZ, red, white arrowheads). Quantification of esg-lacZ+ cell No/images in different genotypes indicated. Mean ± SD is shown. ****p < 0.0001. C Compared to ECs in control intestines in which ER stress (by Hsc3, red, white arrowheads) is barely detectable, Hsc3 is dramatically increased in Myo1Ats>auxRNAi intestines at 29 °C for 3 days. Please note that ER stress is constantly occurred in some EEs (yellow arrowheads) in control and Myo1Ats>auxRNAi intestines. Hsc3 channel is showed separately in black white. Quantification of the fluorescence intensity of Hsc3 in ECs of control and Myo1Ats>auxRNAi intestines. Mean ± SD is shown. ****p < 0.0001. D Compared to control intestines in which Xbp1-GFP (white arrowhead) is barely detectable, Xbp1-GFP is significantly increased in Myo1Ats>auxRNAi intestines at 29 °C for 3 days. Xbp1-GFP channel is showed separately in black white. Quantification of the fluorescence intensity of Xbp1-GFP in control and Myo1Ats>auxRNAi intestines. Mean ± SD is shown. ****p < 0.0001. Scale bars, 5 μm (C, 20 μm).
We then investigated the cause of EC death. Interestingly, compared to control intestines, we found that extensive ER stress was observed in aux-defective ECs (by Hsc3) [43,44,45,46], indicating that Aux may function in the ER or ER-to-GA transport (Fig. 3C). Further, the IRE1/Xbp1 branch of ERS (by Xbp1-GFP) was also significantly increased in aux-defective ECs (Fig. 3D) [43, 44]. Collectively, these data show that Aux may function in the ER or ER-to-GA transport to maintain EC stability, thereby maintaining intestinal homeostasis.
Aux facilitates in ER-to-GA vesicle trafficking
Next, we examined the status of the ER and the GA in the absence of Aux. Compared to control, the fluorescence intensity of the ER (by KDEL-GFP) was significantly increased in aux-defective ECs, suggesting that the ER in ECs is affected in the absence of Aux (Fig. 4A). Meanwhile, no obvious defects in the morphology of the GA were observed in aux-defective ECs (by ManII-GFP and Grasp65-RFP) (Supplementary Fig. S6). These data indicate that Aux may function in the ER and/or ER-to-GA transport.
A Compared to control intestines, the fluorescence intensity of KDEL-GFP (green, white arrowheads) is significantly increased in Myo1Ats>auxRNAi intestines at 29 °C for 7 days. KDEL-GFP channel is showed separately in black white. Quantification of the fluorescence intensity of KDEL-GFP in control and Myo1Ats>auxRNAi intestines. Mean ± SD is shown. ****p < 0.0001. B Compared to control intestines, the fluorescence intensity of Ergic53-GFP (green, white arrowheads) is significantly increased in Myo1Ats>auxRNAi intestines at 29 °C for 7 days. Ergic53-GFP channel is showed separately in black white. Quantification of the fluorescence intensity of Ergic53-GFP in control and Myo1Ats>auxRNAi intestines. Mean ± SD is shown. ****p < 0.0001. C Compared to control intestines, the number of Sec13-RFP+ puncta (red, white arrowheads) is significantly increased in Myo1Ats>auxRNAi intestines at 29 °C for 7 days. Sec13-RFP channel is showed separately in black white. Quantification of the size of Sec13-RFP+ puncta and the number of Sec13-RFP+ puncta/EC in control and Myo1Ats>auxRNAi intestines. Mean ± SD is shown. ****p < 0.0001. D Compared to control intestines, the number of Sec31-RFP+ puncta (red, white arrowheads) is significantly increased in Myo1Ats>auxRNAi intestines at 29 °C for 7 days. Sec31-RFP channel is showed separately in black white. Quantification of the size of Sec31-RFP+ puncta and the number of Sec31-RFP+ puncta/EC in control and Myo1Ats>auxRNAi intestines. Mean ± SD is shown. ****p < 0.0001. Scale bars, 5 μm (D, 10 μm).
ER-to-GA trafficking is bridged by a morphologically defined ER-Golgi intermediate compartment (ERGIC) [47]. Compared to control, the morphology and the fluorescence intensity of the ERGIC compartment (by Ergic53-GFP) were significantly increased in Myo1Ats>auxRNAi intestines, indicating that Aux may participate in ER-to-GA transport in ECs (Fig. 4B). Proteins secreted from the ER are packed into COPII-coated vesicles which bud off at ERES [31, 32]. Interestingly, we found that the number and the size of COPII-coated vesicles (by Sec13-RFP) were significantly increased in Myo1Ats> auxRNAi intestines, indicative of defective COPII-mediated transport (Fig. 4C). This conclusion was further verified with Sec31-RFP (Fig. 4D). Altogether, these data indicate that Aux participates in anterograde ER-to-GA vesicle trafficking to maintain EC integrity.
Aux associates with COPII-coated vesicles to maintain intestinal homeostasis
We then determined how Aux participates in ER-to-GA transport. Extensive co-localization of transiently expressed Aux-GFP and Sec31-RFP+ puncta was observed in ECs (Fig. 5A). Further, transiently expressed Aux-GFP also extensively co-localized with Sec13-RFP+ puncta (Fig. 5B). These data suggest that Aux may associate with COPII-coated vesicles in ECs. We then performed co-IP experiments to examine whether Aux physically associates with COPII coatomer. Indeed, the co-IP results showed that transiently expressed Aux-GFP associates with Sec13-RFP in vivo (Fig. 5C and Supplementary Fig. S7). We further examined whether COPII-mediated vesicle transport is required for intestinal homeostasis. Consistently, depleting either Sec31 or Sec13 in ECs led to significant increase of progenitors (by esg-lacZ) and intestinal homeostasis disruption (Fig. 5D). Mimicking those of aux knockdown, extensive ER stress/ERUPR was observed in Sec31- and Sec13-depleted ECs (Fig. 5E). Collectively, these data suggest that Aux associates with COPII vesicles to maintain EC integrity and intestinal homeostasis.
A Co-localization of Sec31-RFP+ puncta (red) and Aux-GFP (green) in ECs (white arrowheads). Sec31-RFP and Aux-GFP channels are showed separately in black white. B Co-localization of Sec13-RFP+ puncta (red) and Aux-GFP (green) in ECs (white arrowheads). Sec13-RFP and Aux-GFP channels are showed separately in black white. C co-IP results of transiently expressed Aux-GFP by transiently expressed Sec13-RFP. NIP negative immunoprecipitation control. D Compared to control intestines, the number of progenitors (white arrowheads) is dramatically increased in Myo1Ats>sec31RNAi and Myo1Ats>sec13RNAi intestines at 29 °C for 7 days. Quantification of esg-lacZ+ cell No/images in different genotypes indicated. Mean ± SD is shown. ****p < 0.0001. E Compared to ECs in control intestines in which Hsc3 (red, white arrowheads) is barely detectable, Hsc3 is dramatically increased in Myo1Ats>Sec31RNAi and Myo1Ats>Sec13RNAi intestines at 29 °C for 3 days. Please note that ERS is constantly occurred in some EEs (yellow arrowheads) in control and RNAi knockdown intestines. Hsc3 channel is showed separately in black white. Quantification of the fluorescence intensity of Hsc3 in ECs of intestines with indicated genotypes. Mean ± SD is shown. ****p < 0.0001. Scale bars, 5 μm (D, E, 20 μm).
Aux mediates the presentation of cell adhesion molecules (CAMs) on the PM
Next, we investigated the cargos transported by Aux-mediated ER-to-GA trafficking in ECs. CAMs, such as E-Cadherin (E-Cad) and integrin, are presented on the PM or the extracellular matrix (ECM) by COPII-coated vesicles for cell adhesion, tissue integrity, signaling, and tumorigenesis [48,49,50,51]. We first examined the subcellular localization of E-Cad in ECs. E-Cad (Shotgun in fly, Shg) outlines the contour of different intestinal cell types in control intestines (by Shg-GFP) (Fig. 6A). However, the presence of Shg-GFP on EC PM was almost completely abolished in aux-depleted ECs, indicating that Aux is required for the presentation of E-Cad on the PM (Fig. 6A). This conclusion was further confirmed by E-Cad antibody and an independent Shg-mTomato reporter line (Supplementary Fig. S8A, B). We then examined the subcellular localization of the intracellular partner of E-Cad, beta-Catenin (β-Cat, Arm in fly), in ECs. Similar as E-Cad, Arm also localizes to the PM of all intestinal cell types in control intestines, whilst Arm on EC PM was diminished in the absence of Aux (Fig. 6B). Further, compared to control, the localization of the alpha subunit of integrin (αPS1, by Mew-GFP) on the PM of aux-depleted ECs was significantly reduced (Fig. 6C and Supplementary Fig. S8C). The great reduction of integrin on EC PM was independently confirmed by examination of another alpha subunit of integrin (αPS2, by If-GFP) (Fig. 6D and Supplementary Fig. S8D). Together, these data show that Aux facilitates CAM presentation on the PM/surface through ER-to-GA vesicle trafficking in ECs.
A Compared to control intestines, Shg-GFP levels (green, white arrowheads) on EC PM are diminished in Myo1Ats>auxRNAi intestines at 29 °C for 2 days. Please note that Shg-GFP levels on the PM of progenitors and EEs (yellow arrowheads) are unaffected in these intestines. Shg-GFP channel is showed separately in black white. Quantification of the fluorescence intensity of Shg-GFP in ECs in control and Myo1Ats>auxRNAi intestines. Mean ± SD is shown. ****p < 0.0001. B Compared to control intestines, the protein levels of Arm (red, white arrowheads) on EC PM are diminished in Myo1Ats>auxRNAi intestines at 29 °C for 2 days. Please note that Arm levels on the PM of progenitors and EEs (yellow arrowheads) are unaffected in these intestines. Arm channel is showed separately in black white. Quantification of the fluorescence intensity of Arm in ECs in control and Myo1Ats>auxRNAi intestines. Mean ± SD is shown. ****p < 0.0001. C Compared to control intestines, Mew-GFP levels (green, white arrowheads) on EC PM are significantly decreased in Myo1Ats>auxRNAi intestines at 29 °C for 2 days. Mew-GFP channel is showed separately in black white. Quantification of the fluorescence intensity of Mew-GFP in ECs in control and Myo1Ats>auxRNAi intestines. Mean ± SD is shown. ****p < 0.0001. D Compared to control intestines, If-GFP levels (green, white arrowheads) in ECs are significantly decreased in Myo1Ats>auxRNAi intestines at 29 °C for 2 days. If-GFP channel is showed separately in black white. Quantification of the fluorescence intensity of If-GFP in ECs in control and Myo1Ats>auxRNAi intestines. Mean ± SD is shown. ****p < 0.0001. Scale bars, 10 μm.
CAMs are required in ECs for intestinal homeostasis
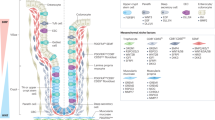
To functionally determine whether the diminished presence of CAMs on EC PM/cell surface is responsible for the defects associated with aux depletion, we examined the consequences of depleting these CAMs in ECs. Phenocopying Myo1Ats>auxRNAi intestines, E-Cad (Shg) knockdown in ECs resulted in dramatic accumulation of progenitors, demonstrating that E-Cadherin is essential for intestinal homeostasis (Fig. 7A). Further, depletion of the alpha subunit of integrin in ECs also led to drastic increase of progenitors and intestinal homeostasis disruption (Fig. 7A). The essential requirement of integrin in midgut homeostasis was further confirmed by depletion of another subunit of integrin, αPS2/If (Fig. 7A). Altogether, our study demonstrates that Aux facilitates ER-to-GA trafficking of CAMs by COPII coatomers to maintain EC integrity, stabilized ECs in turn restrict ISC proliferation, thereby maintaining intestinal homeostasis under physiological conditions (Fig. 7B). When ER-to-GA transport is affected in Aux-depleted ECs which undergo apoptosis due to extensive ER stress and integrity loss. These dying ECs ectopically express cytokines which activates JAK/STAT signaling in ISCs, resulting in excessive ISC proliferation and intestinal homeostasis disruption (Fig. 7B).
A Compared to control, the number of progenitors (by esg-lacZ, red, white arrowheads) is dramatically increased when shg, mew, and if are depleted in ECs at 29 °C for 7 days, while no obvious changes are observed upon arm knockdown. Quantification of esg-lacZ+ cell No/images in different genotypes indicated. Mean ± SD is shown. ****p < 0.0001. B Model of Aux in ECs facilitating COPII-mediated ER-to-GA trafficking of CAMs to control intestinal homeostasis through cell-cell communication. Under physiological conditions, Aux facilitates COPII-mediated transport of CAMs to stabilize EC integrity, thereby maintaining intestinal homeostasis. Whilst in aux-depleted ECs, the COPII-mediated transport of CAMs is blocked. aux-depleted ECs undergo apoptosis due to extended ER stress and secrete cytokines to activate JAK/STAT signaling in ISCs, eventually leading to intestinal homeostasis disruption and pathogenesis. Scale bars, 20 μm.
Discussion
Adult stem cells constantly perceive signals from the niche and terminally differentiated cells to precisely control their proliferation rate to maintain tissue homeostasis. Here we demonstrate that, instead of participating in CCV trafficking, Aux in ECs functions in COPII-mediated ER-to-GA transport of CAMs to maintain EC integrity, thereby restraining excessive ISC proliferation. Our data show that impeding vesicle transport of CAMs in ECs has severe consequences: apoptosis due to extensive ERS, failure in the establishment of cell junctions and cell/tissue integrity disruption, and excessive production of cytokines by dying ECs which drives unlimited ISC proliferation and intestinal homeostasis disruption. Together, our results suggest communication between ECs and ISCs is essential to constrain ISC proliferation and maintain midgut homeostasis.
Aux in ECs facilitates COPII-mediated ER-to-GA vesicle trafficking
CCVs mediate traffic away from the cell surface (endocytosis) and between the trans-Golgi and endosomes [52]. Aux is well established as a co-factor for CCV disassembly [21, 23]. Previous studies show that Aux is required for Notch activation in multiple Notch-dependent processes by facilitating ligand internalization [24,25,26]. Our recent work showed that Aux in ISCs actively internalizes EGFR from the PM to restrict ISCs from excessive proliferation, thereby maintaining intestinal homeostasis [27]. Here our data indicate that Aux in ECs functions in the secretory pathway although we cannot fully exclude the possibility that Aux may also participate in the CCV-mediated vesicle trafficking in ECs. Nevertheless, EGFR signaling is unlikely responsible for the defects observed upon Aux depletion in ECs (Supplementary Fig. S3). Our data demonstrate that Aux in ECs mediates COPII transport based on the observations that: 1) Aux-depleted ECs are extensively stressed, 2) COPII vesicles are greatly accumulated in aux-defective intestines, 3) Aux associates with COPII coatomer, and 4) the status of GA is largely unaffected. These results are consistent with previous report that Aux binds to COPII and COPI coat subunits in budding yeast [34]. As the morphology of the GA is largely normal in the absence of Aux, Aux may play minor role in COPI-mediated retrograde vesicle transport in ECs. This is the first study showing that Aux facilitates COPII-mediated vesicle trafficking in higher organisms, demonstrating that the function of Aux in the early secretory pathway is evolutionarily conserved. The observation that Aux participates in different vesicle trafficking pathways in different intestinal cell types indicates that Aux functions in a cell-context dependent manner for intestinal homeostasis control.
Administration of tunicamycin (TM), a canonical ER inhibitor, could cause ERUPR and induce ERS [53, 54]. As ECs are the major component of midgut and the first barrier facing the gut lumen, ECs are the first victims of TM administration. Thus TM administration changed ER morphology due to ERS and disrupted intestinal homeostasis [45]. In support of the pivotal role of ER-to-GA transport, almost identical defects are observed in aux-depleted ECs as those of TM-treated animals [45]. Although many previous reports show that ECs are the major source of stress-induced ligands and the drivers of intestinal homeostasis loss in response to various stresses [16,17,18,19, 36, 55, 56], only the consequences of EC damage are described. However, the detailed insults imposed to ECs by these stresses are not illustrated. Here we show that Aux facilitates anterograde ER-to-GA transport of CAMs in ECs. Compromising this Aux-mediated process leads to ERS and cell adhesion disruption, resulting in EC death and subsequent secretion of cytokines which eventually leads to intestinal homeostasis loss. Thus, our data demonstrate that Aux in ECs is crucial to non-cell autonomously control ISC proliferation and maintain intestinal homeostasis.
CAMs, EC integrity, and pathogenesis
Proper association or anchorage between cell and its neighboring cells/environment by CAMs is critical for cellular maintenance and function. Direct cell-to-cell adhesion such as adherens junctions, septate junctions, and integrin-mediated adhesion has been implicated as a strategy for stem cell anchorage to the niche cells or differentiated cells to their neighbors/ECM [48,49,50, 57,58,59,60]. The requirement of integrin in ISCs seems to be discrepant from different reports, ranging from ISC maintenance to asymmetric division [61,62,63,64]. Nevertheless, these reports indicate that integrin-mediated adhesion of ISC to ECM is required for ISC behavior. Further, the duration of adhesion between ISC and its immediate progeny determines the differentiation speed and cell fate decision of its progeny [65,66,67,68,69,70]. Here we show that Aux-mediated transport of CAMs is critical for EC integrity and intestinal homeostasis. We can speculate that aux-defective ECs may also be extruded from the intestinal epithelium because of adhesion loss and undergo anoikis. Thus, the observed death of aux-defective ECs may be a combined consequence of ER stress and integrity loss and we could not differentiate which is the primary cause of EC death. Nevertheless, the strategy that Aux-defective ECs adopts can help to explain what happens to ECs facing other stress conditions in intestines. Our unpublished data showed that when the intestines encountered acute insults such as oxidative stress, the levels of Aux were diminished, the number and size of COPII vesicles (by Sec13-GFP) were significantly increased, and the insulted ECs underwent apoptosis and secreted cytokines to promote ISC proliferation, thereby speeding up the regeneration process. However, during the regeneration process after withdraw of oxidative reagents, the levels of Aux and ER-to-GA transport were greatly restored, indicating that upon acute oxidative stress, insulted ECs are no longer be maintained due to diminished Aux, releasing cytokines to promote ISC proliferation for regeneration, while during recovery, the levels of Aux and ER-to-GA transport are restored to maintain the integrity of newly generated ECs to prevent ISCs from excessive proliferation, acting as a safe-guardian for proper regeneration. Thus, during regeneration process upon acute oxidative stress, Aux likely acts as a sensor for regeneration initiation and as a brake for regeneration exit. Without this brake, ISCs will undergo unlimited proliferation, leading to tumorigenesis eventually.
Importantly, CAMs such as Transmembrane and immunoglobulin domain-containing protein 1 (TMIGD1) and E-Cad have been suggested to be directly participated in Crohn’s disease and gastrointestinal tumorigenesis, respectively [71,72,73]. Aux-defective ECs loss their contact with the basal membrane and their neighbors, undergoing apoptosis and releasing ISC-promoting cytokines to drive tumorigenesis non-cell autonomously. It will be worsened if ISCs are also defective in Aux which will undergo excessive proliferation due to ectopic EGFR signaling [27], as differentiated aux-defective progeny will in turn undergo apoptosis and release cytokines to further promote ISC proliferation and aggravate tumorigenesis. Therefore, based on the conservation of Aux-COPII-CAMs/Aux-EGFR, the structure and function of ECs, and mechanisms controlling stem cell proliferation/differentiation, we would expect that compromising Aux-COPII-CAM and Aux-EGFR cascades could be substantial contributing factors to age-onset diseases and tumorigenesis in mammal intestines. Thus, Aux-COPII-CAM and Aux-EGFR will be promising biomarkers for colorectal cancer diagnosis, while targeting JAK/STAT and EGFR signaling will be an effective treatment of colorectal cancer. It will be interesting to examine these in future studies.
Materials and methods
Flies were maintained on standard cornmeal media at 25 °C. Crosses were raised at 18 °C in humidity controlled incubators or as otherwise noted. In all experiments, only the female posterior midgut was analyzed. Information for alleles and transgenes used in this study can be found either in FlyBase or as noted. Please refer to Supplementary Information for detailed experimental procedures.
Data availability
Drosophila lines and other reagents generated in this study will be available upon request.
References
Morrison SJ, Spradling AC. Stem cells and niches: mechanisms that promote stem cell maintenance throughout life. Cell. 2008;132:598–611.
Radtke F, Clevers H. Self-renewal and cancer of the gut: two sides of a coin. Science. 2005;307:1904–9.
Lin H. Cell biology of stem cells: an enigma of asymmetry and self-renewal. J Cell Biol. 2008;180:257–60.
Casali A, Batlle E. Intestinal stem cells in mammals and Drosophila. Cell Stem Cell. 2009;4:124–7.
Stainier DYR. No organ left behind: tales of gut development and evolution. Science. 2005;307:1902–4.
Edgar BA. Intestinal stem cells: no longer immortal but ever so clever. EMBO J. 2012;31:2441–3.
Colombani J, Andersen DS. The Drosophila gut: a gatekeeper and coordinator of organism fitness and physiology. WIREs Dev Biol. 2020;9:e378.
Ohlstein B, Spradling A. The adult Drosophila posterior midgut is maintained by pluripotent stem cells. Nature. 2006;439:470–4.
Micchelli CA, Perrimon N. Evidence that stem cells reside in the adult Drosophila midgut epithelium. Nature. 2006;439:475–9.
Ohlstein B, Spradling A. Multipotent Drosophila intestinal stem cells specify daughter cell fates by differential notch signaling. Science. 2007;315:988–92.
Biteau B, Jasper H. Slit/Robo signaling regulates cell fate decisions in the intestinal stem cell lineage of Drosophila. Cell Rep. 2014;7:1867–75.
Chen J, Xu N, Wang C, Huang P, Huang H, Jin Z, et al. Transient Scute activation via a self-stimulatory loop directs enteroendocrine cell pair specification from self-renewing intestinal stem cells. Nat Cell Biol. 2018;20:152–61.
de Navascués J, Perdigoto CN, Bian Y, Schneider MH, Bardin AJ, Martínez-Arias A, et al. Drosophila midgut homeostasis involves neutral competition between symmetrically dividing intestinal stem cells. EMBO J. 2012;31:2473–85.
O’Brien LucyE, Soliman SarahS, Li X, Bilder D. Altered modes of stem cell division drive adaptive intestinal growth. Cell. 2011;147:603–14.
Guo Z, Lucchetta E, Rafel N, Ohlstein B. Maintenance of the adult Drosophila intestine: all roads lead to homeostasis. Curr Opin Genet Dev. 2016;40:81–86.
Biteau B, Jasper H. EGF signaling regulates the proliferation of intestinal stem cells in Drosophila. Development. 2011;138:1045–55.
Guo Z, Driver I, Ohlstein B. Injury-induced BMP signaling negatively regulates Drosophila midgut homeostasis. J Cell Biol. 2013;201:945–61.
Jiang H, Grenley MO, Bravo M-J, Blumhagen RZ, Edgar BA. EGFR/Ras/MAPK signaling mediates adult midgut epithelial homeostasis and regeneration in Drosophila. Cell Stem Cell. 2011;8:84–95.
Li Z, Zhang Y, Han L, Shi L, Lin X. Trachea-derived Dpp controls adult midgut homeostasis in Drosophila. Dev Cell. 2013;24:133–43.
Patel PH, Dutta D, Edgar BA. Niche appropriation by Drosophila intestinal stem cell tumours. Nat Cell Biol. 2015;17:1182–92.
Lemmon SK. Clathrin uncoating: auxilin comes to life. Curr Biol. 2001;11:R49–52.
Ungewickell E, Ungewickell H, Holstein SE, Lindner R, Prasad K, Barouch W, et al. Role of auxilin in uncoating clathrin-coated vesicles. Nature. 1995;378:632–5.
Eisenberg E, Greene LE. Multiple roles of auxilin and hsc70 in clathrin-mediated endocytosis. Traffic. 2007;8:640–6.
Eun SH, Banks SM, Fischer JA. Auxilin is essential for Delta signaling. Development. 2008;135:1089–95.
Hagedorn EJ, Bayraktar JL, Kandachar VR, Bai T, Englert DM, Chang HC. Drosophila melanogaster auxilin regulates the internalization of Delta to control activity of the Notch signaling pathway. J Cell Biol. 2006;173:443–52.
Kandachar V, Bai T, Chang HC. The clathrin-binding motif and the J-domain of Drosophila Auxilin are essential for facilitating Notch ligand endocytosis. BMC Dev Biol. 2008;8:50.
Zhao H, Ren X, Kong R, Shi L, Li Z, Wang R, et al. Auxilin regulates intestinal stem cell proliferation through EGFR. Stem Cell Rep. 2022;17:1120–37.
Adamowski M, Randuch M, Matijević I, Narasimhan M, Friml J. SH3Ps recruit auxilin-like vesicle uncoating factors for clathrin-mediated endocytosis. Cell Rep. 2024;43:7.
Zhang S, Wang L, Yi S, Tsai YT, Cheng YH, Lin YT, et al. Drosophila aux orchestrates the phosphorylation-dependent assembly of the lysosomal V-ATPase in glia and contributes to SNCA/α-synuclein degradation. Autophagy. 2025;21:1039–58.
Guerriero CJ, Brodsky JL. The delicate balance between secreted protein folding and endoplasmic reticulum-associated degradation in human physiology. Physiol Rev. 2012;92:537–76.
Lee MCS, Miller EA, Goldberg J, Orci L, Schekman R. Bi-directional protein transport between the ER and Golgi. Annu Rev Cell Dev Biol. 2004;20:87–123.
Barlowe C, Orci L, Yeung T, Hosobuchi M, Hamamoto S, Salama N, et al. COPII: a membrane coat formed by Sec proteins that drive vesicle budding from the endoplasmic reticulum. Cell. 1994;77:895–907.
Frakes AE, Dillin A. The UPRER: sensor and coordinator of organismal homeostasis. Mol Cell. 2017;66:761–71.
Ding J, Segarra VA, Chen S, Cai H, Lemmon SK, Ferro-Novick S. Auxilin facilitates membrane traffic in the early secretory pathway. Mol Biol Cell. 2016;27:127–36.
Staley BK, Irvine KD. Warts and Yorkie mediate intestinal regeneration by influencing stem cell proliferation. Curr Biol. 2010;20:1580–7.
Ren F, Wang B, Yue T, Yun EY, Ip YT, Jiang J. Hippo signaling regulates Drosophila intestine stem cell proliferation through multiple pathways. Proc Natl Acad Sci USA. 2010;107:21064–9.
Jiang H, Patel PH, Kohlmaier A, Grenley MO, McEwen DG, Edgar BA. Cytokine/Jak/Stat signaling mediates regeneration and homeostasis in the Drosophila midgut. Cell. 2009;137:1343–55.
Amcheslavsky A, Jiang J, Ip YT. Tissue damage-induced intestinal stem cell division in Drosophila. Cell Stem Cell. 2009;4:49–61.
Bach EA, Ekas LA, Ayala-Camargo A, Flaherty MS, Lee H, Perrimon N, et al. GFP reporters detect the activation of the Drosophila JAK/STAT pathway in vivo. Gene Expr Patterns. 2007;7:323–31.
Kong R, Li J, Liu F, Ma Y, Zhao H, Zhao H, et al. A feed forward loop between JAK/STAT downstream target p115 and STAT in germline stem cell maintenance in Drosophila adult testis. Stem Cell Rep. 2023;18:1940–53.
Jiang H, Edgar BA. EGFR signaling regulates the proliferation of Drosophila adult midgut progenitors. Development. 2009;136:483–93.
Buchon N, Broderick NA, Poidevin M, Pradervand S, Lemaitre B. Drosophila intestinal response to bacterial infection: activation of host defense and stem cell proliferation. Cell Host Microbe. 2009;5:200–11.
Hetz C, Papa FR. The unfolded protein response and cell fate control. Mol Cell. 2018;69:169–81.
Yoo YS, Han HG, Jeon YJ. Unfolded protein response of the endoplasmic reticulum in tumor progression and immunogenicity. Oxid Med Cell Longev. 2017;2017:2969271.
Shi L, Ma H, Wang J, Ma M, Zhao H, Li Z, et al. An EMC-Hpo-Yki axis maintains intestinal homeostasis under physiological and pathological conditions. Development. 2023;150:dev201958.
Liu F, Zhao H, Kong R, Shi L, Li Z, Ma R. Derlin-1 and TER94/VCP/p97 are required for intestinal homeostasis. J Genet Genom. 2022;49:195–207.
Watanabe S, Kise Y, Yonezawa K, Inoue M, Shimizu N, Nureki O, et al. Structure of full-length ERGIC-53 in complex with MCFD2 for cargo transport. Nat Commun. 2024;15:024–46747.
Hynes RO. Integrins: bidirectional, allosteric signaling machines. Cell. 2002;110:673–87.
Verpoort B, de Wit J. Cell adhesion molecule signaling at the synapse: beyond the scaffold. Cold Spring Harb Perspect Biol. 2024;16:a041501.
Cavallaro U, Dejana E. Adhesion molecule signalling: not always a sticky business. Nat Rev Mol Cell Biol. 2011;12:189–97.
Viotti C. ER to Golgi-dependent protein secretion: the conventional pathway. Methods Mol Biol. 2016:1459:3–29.
Robinson MS. Forty years of clathrin-coated vesicles. Traffic. 2015;16:1210–38.
Stengel ST, Fazio A, Lipinski S, Jahn MT, Aden K, Ito G, et al. Activating transcription factor 6 mediates inflammatory signals in intestinal epithelial cells upon endoplasmic reticulum stress. Gastroenterology. 2020;159:1357–74.e1310.
Yang Z, Wang C, Zhang X, Li J, Zhang Z, Tan Z, et al. Stem cells from human exfoliated deciduous teeth attenuate trigeminal neuralgia in rats by inhibiting endoplasmic reticulum stress. Korean J Pain. 2022;35:383–90.
Shaw RL, Kohlmaier A, Polesello C, Veelken C, Edgar BA, Tapon N. The Hippo pathway regulates intestinal stem cell proliferation during Drosophila adult midgut regeneration. Development. 2010;137:4147–58.
Karpowicz P, Perez J, Perrimon N. The Hippo tumor suppressor pathway regulates intestinal stem cell regeneration. Development. 2010;137:4135–45.
Chen S, Lewallen M, Xie T. Adhesion in the stem cell niche: biological roles and regulation. Development. 2013;140:255–65.
Utech M, Brüwer M, Nusrat A. Tight junctions and cell-cell interactions. Methods Mol Biol. 2006;341:185–95.
Chen J, St Johnston D. Epithelial cell polarity during Drosophila midgut development. Front Cell Dev Biol. 2022;10:886773.
Chen J, Sayadian AC, Lowe N, Lovegrove HE, St Johnston D. An alternative mode of epithelial polarity in the Drosophila midgut. PLoS Biol. 2018;16;e3000041.
Goulas S, Conder R, Knoblich JA. The Par complex and integrins direct asymmetric cell division in adult intestinal stem cells. Cell Stem Cell. 2012;11:529–40.
Lin G, Zhang X, Ren J, Pang Z, Wang C, Xu N, et al. Integrin signaling is required for maintenance and proliferation of intestinal stem cells in Drosophila. Dev Biol. 2013;377:177–87.
You J, Zhang Y, Li Z, Lou Z, Jin L, Lin X. Drosophila perlecan regulates intestinal stem cell activity via cell-matrix attachment. Stem Cell Rep. 2014;2:761–9.
Okumura T, Takeda K, Taniguchi K, Adachi-Yamada T. βν integrin inhibits chronic and high level activation of JNK to repress senescence phenotypes in Drosophila adult midgut. PLoS ONE. 2014;9:e89387.
Guisoni N, Martinez-Corral R, Garcia-Ojalvo J, de Navascués J. Diversity of fate outcomes in cell pairs under lateral inhibition. Development. 2017;144:1177–86.
Sallé J, Gervais L, Boumard B, Stefanutti M, Siudeja K, Bardin AJ. Intrinsic regulation of enteroendocrine fate by Numb. EMBO J. 2017;36:1928–45.
Choi NH, Lucchetta E, Ohlstein B. Nonautonomous regulation of Drosophila midgut stem cell proliferation by the insulin-signaling pathway. Proc Natl Acad Sci USA. 2011;108:18702–7.
Zhai Z, Boquete JP, Lemaitre B. A genetic framework controlling the differentiation of intestinal stem cells during regeneration in Drosophila. PLoS Genet. 2017;13:e1006854.
Maeda K, Takemura M, Umemori M, Adachi-Yamada T. E-cadherin prolongs the moment for interaction between intestinal stem cell and its progenitor cell to ensure Notch signaling in adult Drosophila midgut. Genes Cells. 2008;13:1219–27.
Falo-Sanjuan J, Bray SJ. Membrane architecture and adherens junctions contribute to strong Notch pathway activation. Development. 2021;148:dev199831.
Libusova L, Stemmler MP, Hierholzer A, Schwarz H, Kemler R. N-cadherin can structurally substitute for E-cadherin during intestinal development but leads to polyp formation. Development. 2010;137:2297–305.
Zhou L, Zhu L, Wu X, Hu S, Zhang S, Ning M, et al. Decreased TMIGD1 aggravates colitis and intestinal barrier dysfunction via the BANF1-NF-κB pathway in Crohn’s disease. BMC Med. 2023;21:287.
Wheelock MJ, Shintani Y, Maeda M, Fukumoto Y, Johnson KR. Cadherin switching. J Cell Sci. 2008;121:727–35.
Acknowledgements
We are grateful to Jose Pastor, Norbert Perrimon, Sarah Bray, Steven Hou, Gyeong Hun Baeg, Henry Sun, Wei Zhou, Lei Liu, Zongzhao Zhai, and Yu Cai for generous gifts of reagents, the Bloomington Stock Center, VDRC, Tokyo Stock Center, NIG-FLY Center, TRiP at Harvard Medical School (NIH/NIGMS R01-GM084947), Tsinghua Fly Center (THFC) for fly stocks, and DSHB for antibodies. We apologize to the colleagues whose work could not be cited due to the huge amount of publications and requirement of the journal.
Funding
This work is supported by grants from the National Natural Science Foundation of China (Nos. 32370841, 92054109, 31972893, and 31471384 to ZHL) and Beijing Municipal Commission of Education (Nos. KZ20231002827 and KZ201910028040 to ZHL).
Author information
Authors and Affiliations
Contributions
Conceptualization, ZHL; investigation, RQW, ZRL, JW, RYK, HZ, XJR, DJZ, and XYTL; formal analysis, RQW, ZRL, JW, and ZHL; validation, RQW, ZRL, RYK, and ZHL; writing—original draft preparation, ZHL; writing—review and editing, RQW, ZRL, and ZHL; supervision, ZHL; project administration, ZHL; funding acquisition, ZHL. All authors have read and agreed to the published version of the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Edited by Professor Mauro Piacentini
Supplementary information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Wang, R., Li, Z., Wei, J. et al. Auxilin in enterocytes controls intestinal homeostasis through inter-cell communication. Cell Death Dis 16, 626 (2025). https://doi.org/10.1038/s41419-025-07954-w
Received:
Revised:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s41419-025-07954-w