Abstract
The impact of chirality on immune response has attracted great interest in cancer vaccine research recently. However, the study of chiral synthetic polypeptide hydrogels as cancer vaccines as well as of the impact of biomaterials themselves for antitumor immunotherapy has rarely been reported. Here, we show the key role of residue chirality of polypeptide hydrogels in antitumor immunity and local immune microenvironment regulation. Compared to poly(γ-ethyl-L-glutamate)-based hydrogels (L-Gel), poly(γ-ethyl-D-glutamate)-based hydrogels (D-Gel) induces enhanced level of immune cell infiltration. However, D-Gel causes higher levels of suppressive markers on antigen-presenting cells and even induces stronger T cell exhaustion than L-Gel. Finally, D-Gel establishes a local chronic inflammatory and immunosuppressive microenvironment and shows insufficient anti-tumor effects. Conversely, the milder host immune responses induced by L-Gel leads to more effective tumor inhibition. This study provides insights on the role of residue chirality in the regulation of local immune microenvironment and affecting antitumor immune response.
Similar content being viewed by others
Introduction
Cancer is a major threat to human health worldwide1. In recent years, cancer immunotherapy has received significant progress attributed to the fact that the host immune responses can display the advantages of specificity and high efficiency. Current mainstream approaches for cancer immunotherapy include cancer vaccines2, immune-checkpoint blockade3,4, and adoptive immune cell therapy5. Among them, cancer vaccines can exert excellent anti-tumor effects by inducing specific immune responses following the presentation of multiple tumor antigens and generate a long-lasting protection to prevent tumor recurrence6. Pathogen-associated antigens delivered by vaccines can be formulated in several ways, including live-attenuated or inactivated pathogens, proteins or protein subunits, and nucleic acids6,7. After vaccine injection, antigen-presenting cells (APCs) take up antigen or other components and drain to the local draining lymph nodes (LNs), where the antigen is presented to B cells and T cells to initiate the adaptive immune response. Despite cancer vaccines having many outstanding features, current clinical efficacy is still insufficient. Various factors, such as the immunogenicity of tumor antigen, the duration time of vaccine stimulation, the delivery efficacy of vaccine components, and the immune microenvironment at the local injection site or tumor tissue, can influence the final anti-tumor effects. To overcome these issues, many strategies have been developed, including sustained delivery of multiple vaccine components, targeting of specific immune cells or tissue, or activating APCs8,9,10,11.
Traditional bolus vaccines have often failed to supply sustained immune stimulation, requiring several booster injections to enhance specific immune responses. However, multi-dose injection leads to additional procedures for those people having limited access to healthcare service, and extra costs and pain to inoculated population. Hydrogels, as a unique type of drug delivery platform, have been widely studied for various applications12, including traditional chemotherapy, chemoimmunotherapy13,14, and cancer vaccination15,16,17,18. Hydrogels have considerable advantages as drug delivery systems, especially for the sustained, long-acting delivery of multiple drugs or immunomodulating components, which can mimic multi-dose injection. Moreover, in recent studies, it has been proved that hydrogels can also affect the phenotype of immune response19 through adjusting mechanical strength20, pore size16, degradation behavior21, and components22. Furthermore, hydrogel-based vaccines can provide an immune niche due to the intrinsic features of immune cell infiltration into the hydrogel16,17,23,24. Based on our previous studies on polypeptide hydrogels as drug vehicles15,25,26,27, we herein investigate the feasibility of utilizing polypeptide hydrogels as platforms for cancer vaccines.
As the basic unit of polypeptides, amino acids have the unique characteristic of being chiral enantiomers. Recently, chiral materials have been used in biomedical applications, including tissue repair and cancer therapy28,29,30,31. Indeed, the effect of amino acid chirality on immune response has been studied using the platforms of peptide-based nanovaccines30,31 and supramolecular oligopeptide hydrogels29. Several systems based on D-amino acids were shown to enhance anti-tumor immune responses through the promotion of endocytosis of antigen or nanoparticle and the secretion of inflammatory cytokines. However, the study on polypeptide hydrogel vaccines with varying residue chirality is lacking, and current studies have paid little attention to the impact of the local immune microenvironment caused by hydrogel itself32. Moreover, as a type of macroscopic implantable material, hydrogels can significantly influence the local immune microenvironment at injection sites by regulating the activation status of infiltrating immune cells, and subsequently affect the anti-tumor efficacy. In our previous work, we showed that varying chiral enantiomers led to significantly different hydrogel degradation behaviors in vivo33; however, the profound effect on host immune response has not been reported.
Herein, we design and synthesize methoxy-poly(ethylene glycol)-polypeptide (mPEG-polypeptide) hydrogels with different chiral residues and use them as cancer vaccines through loading antigens and adjuvant. The sol-gel transition and drug release behavior in vitro are studied to make sure that all polypeptide hydrogels exhibit similar properties. Degradation behaviors both in vitro and in vivo demonstrate the hydrogel with higher content of D-amino acid residues displays slower degradation. The effect of chirality of the amino acid residues on the recruitment of immune cells is investigated. Subsequently, the phenotypes of the recruited immune cells within hydrogels are analyzed to demonstrate the activation status of the immune cells and the local immune microenvironment. The tumor vaccines based on the chiral polypeptide hydrogels are prepared through loading specific antigens and adjuvants. The influence of hydrogel vaccines on the anti-tumor immune response and tumor inhibition efficiency is evaluated in prophylactic and postsurgical reoccurrence mice models. It is found that the polypeptide hydrogels based on L- or D-amino acid residue induce significantly different anti-tumor immune responses by regulating the local immune microenvironment (Fig. 1).
Results
Characterization of polypeptide copolymers and hydrogel properties
The mPEG-polypeptide diblock copolymer was synthesized according to our previously reported procedure (Supplementary Fig. 1)33. Three copolymers with varying proportions of L- or D-amino acid residues were synthesized, including mPEG-b-poly(γ-ethyl-L-glutamate) (mPEG-b-PELG), mPEG-b-poly(γ-ethyl-D-glutamate) (mPEG-b-PEDG), and mixed chiral residues at a molar ratio of 1:1 (mPEG-b-P(ELG0.5-co-EDG0.5)). All representative peaks in 1H NMR spectra of monomers and copolymers were assigned, indicating the successful synthesis of copolymers (Supplementary Fig. 2A–G). The degree of polymerization (DP) was calculated by comparing the integration of the proton peak at ~3.5 ppm (-CH2 of mPEG-NH2) with that at ~0.9 ppm (-CH3 of glutamate). The DPs of all the diblock copolymers were controlled at ~13 (13.3 for mPEG-b-PELG, 13.3 for mPEG-b-P(ELG0.5-co-EDG0.5), and 13.6 for mPEG-b-PEDG). Gel permeation chromatography (GPC) was performed to measure the polydispersity indexes (PDIs) of copolymers. The curves showed that all copolymers displayed unimodal distribution (Supplementary Fig. 2H–J).
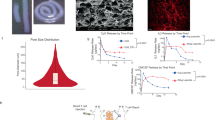
The formation of copolymer micelles in aqueous solution was detected by the pyrene fluorescence probe method, and the critical micelle concentrations (CMCs) were 0.0442, 0.0236, and 0.0159 g L−1 for mPEG-b-PELG, mPEG-b-P(ELG0.5-co-EDG0.5), and mPEG-b-PEDG, respectively (Fig. 2A). Scanning electron microscopy (SEM) showed that mPEG-b-PELG and mPEG-b-PEDG formed rodlike nanoparticles, and mPEG-b-P(ELG0.5-co-EDG0.5) tended to form spherical nanoparticles, which is consistent with our previous work (Supplementary Fig. 3). To assess whether there were differences in secondary structures of mPEG-polypeptides, circular dichroism (CD) spectra of the three copolymers were measured in 0.2 mg mL−1 aqueous solution at 25 °C (Fig. 2B). L-amino acid-based copolymers showed a negative peak at ~218 nm and a positive peak at ~195 nm, indicating that the predominant secondary structure of mPEG-b-PELG was a β-sheet; conversely, D-amino acid-based copolymers showed the opposite trend. mPEG-b-P(ELG0.5-co-EDG0.5) containing enantiomeric residues exhibited no obvious absorption within the whole wavelength. Thus, the chirality of glutamate residue of copolymers was preserved completely.
A Plot of the intensity ratios (I336/I333) to log10C in CMC test. B CD spectra of three copolymers in aqueous solution. The experiments were repeated two times and representative data from one of the experiments are shown. C Sol-gel phase diagrams of copolymer solutions (n = 4 experimental replicates in each group). D Rheological properties of copolymer solutions of 6.0 wt%. Release behavior of CpG-ODN (E) and OVA-Cy5 (F) in vitro (n = 3 experimental replicates in each group). The mass remaining of degradation behavior of hydrogels in vitro (G) and in vivo (H) (n = 3 experimental replicates in each group). I Representative image of hydrogels at 37 °C (top) and the porous structure of hydrogels photographed by cryo-SEM (bottom), scale bar: 5 μm. The experiments were repeated at least two times and representative data from one of the experiments are shown. J Hematoxylin-eosin (H&E) stain of hydrogels and surrounded tissues at 1 and 2 weeks in vivo, scale bar: 1 mm. Data are presented as the mean ± SEM. Source data are provided as a Source Data file.
Next, the gelation properties of the three copolymers were compared by assessing the temperature-dependent sol-gel transition of the copolymer in aqueous solutions at different concentrations (Fig. 2C and Supplementary Fig. 4). For convenience, the hydrogels based on mPEG-b-PELG, mPEG-b-P(ELG0.5-co-EDG0.5) and mPEG-b-PEDG were named L-Gel, LD-Gel and D-Gel, respectively. The gelation properties of the three copolymers were similar and the lowest gelation concentration for L-Gel and LD-Gel was 4 wt% whereas that of D-Gel was 5 wt%. Taking into consideration the stability of hydrogel, a polymer concentration of 6 wt% was chosen for further study. Furthermore, the sol-gel transition temperatures were 26 °C, 28 °C, and 24 °C for L-Gel, LD-Gel, and D-Gel, respectively, which are suitable for in vivo applications because the polymer solutions can be easily injected at a low temperature, while gelation occurs rapidly at the physiological temperature (37 °C) (Fig. 2I top). The porous structures of the hydrogels were observed by Cryo-SEM (Fig. 2I bottom) and exhibited similar pore sizes. Additionally, the pore sizes of hydrogels could be modulated by adjusting the polymer concentration (Supplementary Fig. 5). As the concentration of copolymer increases, the pore size gradually decreases.
Hydrogel stiffness has been shown to influence the immune response or cell phenotype20. Therefore, the consistency of mechanical properties of hydrogels was essential for comparing the immune response caused by hydrogels with different chiral residues in vivo. Rheology studies of 6 wt% polypeptide hydrogels showed that the three polypeptide hydrogels exhibited storage moduli (G′) at a comparable level at 37 °C (172, 92, and 269 Pa for L-Gel, LD-Gel, and D-Gel, respectively) (Fig. 2D).
The release behaviors of antigen and adjuvant were studied in vitro. Sustained release of the two immunomodulators (CpG-ODN and OVA) was observed over 7 days in vitro, with similar drug release curves from the three types of hydrogels (Fig. 2E, F). It would help to eliminate the influence of drug release behavior, when we study the effect of chirality on immune response. In mammals and human, almost all proteins and peptides are produced based on L-amino acids, and proteins or peptides constituted by D-amino acids are difficult to degrade in vivo33. Therefore, we studied the degradation properties of the three hydrogels both in vitro and in vivo (Fig. 2G, H and Supplementary Fig. 6). Additionally, all the three copolymers exhibited excellent cytocompatibility with various types of cells (Supplementary Fig. 7), indicating their suitability for in vivo applications. L-Gel degraded faster in the presence of proteinase K, demonstrating enzyme-mediated degradation. In contrast, the degradation behaviors of D-Gel were similar in media with or without proteinase K (Fig. 2G). Similarly, the hydrogel containing more D-amino acid residues degraded slower in vivo (Fig. 2H and Supplementary Fig. 6A). To evaluate the immune response around the hydrogel injection site, hematoxylin and eosin (H&E) staining of paraffin sections of the hydrogels was conducted (Fig. 2J and Supplementary Fig. 6B). A distinct aggregation of immune cells appeared around the hydrogels, with an increased number of immune cells as the content of D-amino acid residues increased. Therefore, hydrogels with different chiral residues would lead to significantly different host immune response in vivo.
Types of immune cells recruited by hydrogels in vivo
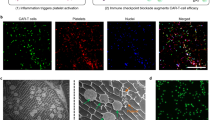
The composition of the recruited immune cells in the hydrogels was assessed by flow cytometry. After subcutaneous injection of L-Gel and D-Gel at the left and right flanks of mice, respectively, the hydrogels and the skin surrounding them were isolated after euthanasia of the mice on days 1, 3, and 7 post-injection. The infiltrated immune cells were detected by flow cytometry. The infiltration of CD45+ leukocytes in the hydrogels increased obviously at day 3. Additionally, D-Gel recruited more immune cells than L-Gel both in the hydrogel and skin, including macrophages (F4/80+), dendritic cells (DCs, CD11c+), T cells (CD3+), B cells (CD19+), and NK cells (NK1.1+) (Fig. 3A, B and Supplementary Figs. 8 and 9). D-Gel also caused a higher amount of protein absorption to BSA in the early stages compared to L-Gel (Supplementary Fig. 10). The proportions of the different immune cells in the hydrogels were counted (Supplementary Fig. 11). It was seen that macrophages were predominant in the recruited immune cells and the proportions of DCs and NK cells were moderate, while the proportions of T cells and B cells were obviously lower. This may be attributed to the fact that innate immunity mediated by macrophages, DCs, and NK cells occurred earlier than adaptive immunity related to T cells and B cells34. In addition, various types of cells around L-Gel and D-Gel on day 7 were also observed through immunofluorescence images (Fig. 3C and Supplementary Fig. 12), which indicated the infiltration of a larger number of macrophages and DCs in D-Gel than in L-Gel.
A The infiltration of different types of immune cells in hydrogels (CD45+ leukocyte, n = 6 mice in each group; F4/80+ macrophages, n = 6 mice in each group; CD11c+ DCs, n = 6 mice in each group; CD3+ T cells, n = 5 mice in each group; CD19+ B cells, n = 5 mice in each group; NK1.1+ NK cells, n = 5 mice in each group). The experiments were repeated at least two times and representative data from one of the experiments are shown. B The infiltration of different types of immune cells in skins surrounding hydrogels (n = 5 mice in each group). C Staining images of L-Gel (top) and D-Gel (bottom) after subcutaneous injection hydrogels into mice for 1 week. The inflammatory response to the hydrogels were evaluated by a hematoxylin-eosin (H&E) stain, scale bar: 1 mm. Leukocytes, Macrophages, DCs, and T cells were labeled by immunofluorescent biomarker (CD45, F4/80, CD11c, and CD3), scale bar: 100 µm. Nuclei were stained by DAPI to show blue fluorescence. The experiments were repeated at least two times and representative data from one of the experiments are shown. D Quantification of the fluorescence intensity of OVA-Cy5 in injection sites by IVIS Lumina LT (n = 3 mice in each group). The experiments were repeated two times and representative data from one of the experiments are shown. E The LNs reflux of OVA-Cy5 at different time points (n = 5 mice in each group). The experiments were repeated two times and representative data from one of the experiments are shown. Data are presented as the mean ± SEM. Statistical significance was analyzed by two-way ANOVA using Sidak’s posttest (A, B, E). Statistical significance was analyzed by one-way ANOVA using Tukey’s posttest for five groups of data at the same time point and the P value marks the significance difference between L-Gel OVA group and D-Gel OVA group (D). Source data are provided as a Source Data file.
Given the greater number of immune cells recruited by D-Gel, especially APCs (DCs and macrophages), we expected it could help the phagocytose and draining LNs reflux of antigens. Therefore, the release behavior of antigen was studied in vivo using Cy5-labeled OVA as a model antigen. Hydrogel vaccines based on L-Gel or D-Gel exhibited prolonged release behaviors of OVA-Cy5 compared to free vaccine or Imject Alum adjuvant (containing an aqueous solution of aluminum hydroxide (40 mg mL−1) and magnesium hydroxide (40 mg mL−1) plus inactive stabilizers) (Alum) (Fig. 3D). Antigen release was found to be sustained over 2 weeks in vivo, which may contribute to mimic multi-dose injection of the antigen. In addition, the fluorescence quantitative statistics of Cy5 at injection sites showed that the D-Gel vaccine had a fewer intensity than L-Gel at the same time point, likely due to the faster phagocytosis of antigen mediated by the greater number of recruited immune cells in D-Gel. Therefore, the reflux of antigen in draining LNs was further studied by flow cytometry analysis (Fig. 3E and Supplementary Fig. 13). The number of OVA-Cy5+ DCs increased with increasing time and the maximal levels were observed on day 7 in both L-Gel and D-Gel. The D-Gel vaccine caused higher proportions of OVA-Cy5+ DCs in LNs than L-Gel, with 17.0% and 20.4% induced by D-Gel vaccine on days 3 and 7, respectively. In contrast, only 12.0% and 12.7% of OVA-Cy5+ DCs in LNs elicited by L-Gel vaccine were observed at the same time points.
Thus, the above results demonstrate that both L-Gel and D-Gel can recruit several types of immune cells, especially APCs, and achieve sustained release of antigens over 2 weeks in vivo. The gels promoted antigen phagocytosis and generated an immune niche, which may contribute to the induction of humoral and cellular immunity16. Furthermore, compared to L-Gel, D-Gel induced greater immune cell infiltration, leading to faster antigen phagocytosis and LN reflux.
Systematic immune responses of hydrogel vaccines in vivo
The systemic immune responses induced by hydrogel vaccines from both humoral and cellular immunity were assessed. The immune stimulation of hydrogel vaccine was first assessed in vitro (Supplementary Fig. 14). All antigen-containing groups activated bone marrow-derived dendritic cells (BMDCs) in vitro. To determine whether hydrogel vaccines can effectively prime antigen-specific T cells, BMDCs that had previously been stimulated by OVA-peptide257-264 (SIINFEKL)-containing hydrogels were incubated with CD8+ T cells from OT-I transgenic mice. It was found that the hydrogel vaccines enhanced the proliferation of OT-I T cells and promoted the transformation of naïve T cells into memory phenotypes (Supplementary Fig. 15).
Furthermore, the general immune responses were investigated in detail in vivo. For humoral immunity, specific anti-OVA lgG antibodies were detected in the mouse serum at different time points after two injections of hydrogel vaccine on days 0 and 14 (Fig. 4A, D–F). In contrast with the free vaccine and alum adjuvant, both hydrogel vaccines arouse a robust anti-OVA response, attributed to the sustained release of antigen from the hydrogels. Interestingly, D-Gel vaccine did not induce a higher anti-OVA titer despite it causing stronger immune cell infiltration and follow-up antigen phagocytosis and reflux. Conversely, L-Gel vaccine induced the strongest anti-OVA titer. Consistent with the antibody titers, the immunofluorescence images of draining LNs also exhibited stronger humoral immunity in the L-Gel vaccine because the more germinal centers (GCs) represented by GL7+ cells occurred (Fig. 4B). In addition, to prove the prolonged release of antigen through hydrogel could mimic multi-dose injection, the influence of injection times on anti-OVA titers was assessed (Supplementary Fig. 16). Moreover, a widely-studied thermosensitive poly(D,L-lactide-co-glycolide)-PEG-poly(D,L-lactide-co-glycolide) (PLGA-PEG-PLGA) triblock copolymer hydrogel was used as the control group. However, the gelation concentration of PLGA-PEG-PLGA is relatively high (~20 wt%)35, which is much higher than mPEG-polypeptide (≥4 wt%). At a fixed total OVA dosage, triple injection of free vaccine exhibited a higher anti-OVA antibody titer than single injection of alum-based vaccine on day 21, but a single injection of OVA-loaded PLGA-PEG-PLGA hydrogel and L-Gel both displayed no significant differences to multiple injections of free vaccine. Moreover, after day 21, the titer of multi-dose of free vaccine decreased quickly, but a single dose of hydrogel vaccine retained relatively higher level of anti-OVA titers on day 42. Thus, the hydrogel vaccines can mimic multi-dose bolus injection through sustained antigen release.
A Schematic illustration of activated immune cells analysis and antibody titer detection. B Immunofluorescence images of inguinal lymph nodes to assess germinal centers 7 days after vaccination, scale bar: 1 mm. C Schematic illustration of in vivo T cell proliferation assay. Specific anti-OVA IgG antibody endpoint titers of different treatment groups in serum on days 10 (D), 14 (E), and 21 (F) (n = 6 mice in each group). The experiments were repeated two times and representative data from one of the experiments are shown. G, H The proportion of SIINFEKL+ DCs in lymph nodes (n = 4 mice in each group). I, J The proportion of IFN-γ+ T cells in spleen (n = 4 mice in each group). K, L The proportion of IFN-γ+ T cells in peripheral blood (n = 4 mice in each group). M The proliferation of CD8 T-cells isolated from transgenic OT-I mice, CFSE content of CD8+ T cells was determined as a measure of T-cell proliferation (n = 6 mice in each group). The experiments were repeated two times and representative data from one of the experiments are shown. N Representative flow cytometry images of the proliferation of CD8+ T cells of in vivo T cell proliferation assay. G1: PBS, G2: Free Vac, G3: Alum Vac, G4: D-Gel Vac and G5: L-Gel Vac. Data are presented as the mean ± SEM. Statistical significance was analyzed one-way ANOVA using Tukey’s posttest (D–M). Source data are provided as a Source Data file.
To assess systemic cellular immunity, the main peripheral immune organs, including spleen and draining LNs, were separated 7 days after vaccine injection, and the specific DCs and activated T cells were detected. Additionally, peripheral blood was also collected and analyzed after erythrocyte lysis. Both L-Gel and D-Gel vaccines induced more specific SIINFEKL+ DCs compared to control groups (Fig. 4G, H and Supplementary Fig. 17). In both the spleen and peripheral blood, L-Gel vaccine induced significantly increased proportion of IFN-γ+ in CD3+CD8+ T cells (Fig. 4I–L and Supplementary Figs. 18 and 19). To determine whether L-Gel vaccine can prime antigen-specific T cells at a higher efficiency, in vivo T cells proliferation assay was performed. CD8+ T cells isolated from OT-I transgenic mice were labeled with CFSE and reinfused into healthy mice. One day later, these mice were vaccinated with different hydrogel vaccines containing OVA-peptide257-264. One day after vaccination, the proliferation of CFSE-positive OT-I CD8+ T cells was assessed (Fig. 4C). L-Gel vaccine induced a higher level of cell proliferation compared to D-Gel vaccine (Fig. 4M, N and Supplementary Fig. 20). These results suggest that L-Gel vaccine improves the priming of antigen-specific T cells.
In vivo long-term prophylactic immunity of hydrogel vaccines
To assess the effect of immune response caused by L-Gel or D-Gel on tumor growth inhibition in vivo, the anti-tumor efficacy of hydrogel vaccines containing only OVA antigen was preliminarily studied using a prophylactic model (Supplementary Fig. 21). Healthy C57BL/6 mice were inoculated with 1 × 106 B16-OVA cells 10 days after subcutaneous injection with L-Gel or D-Gel vaccines, and two antigen doses (20 μg and 100 μg) were used to observe the influence on anti-tumor efficacy. The average tumor growth curve showed that both L-Gel and D-Gel vaccines had better tumor inhibition capacity compared to PBS and Alum adjuvant/OVA group at the same antigen dosage. Moreover, L-Gel vaccine exhibited superior tumor inhibition efficiency than D-Gel vaccine at both antigen dosages, and the anti-tumor efficacy increased with increasing antigen dose. Subsequently, hydrogel vaccines containing antigen (100 μg OVA) and adjuvant (10 μg CPG-ODN) were prepared and used. Similarly, 100 μL hydrogel vaccines were injected at the tail base 10 days before tumor inoculation, while mice injected with equivalent amounts of PBS, free vaccine (OVA + CpG-ODN), or Alum vaccine (Alum adjuvant + OVA + CpG-ODN) served as the control groups (Fig. 5A). L-Gel vaccine exhibited the strongest tumor inhibition ability (Fig. 5B, C). The tumor inhibition rate in the L-Gel vaccine group was 93.3% and half of mice appeared to have no tumor growth during the observation period (Fig. 5E and Supplementary Fig. 22B). However, D-Gel vaccine showed insufficient anti-tumor efficacy and the tumor inhibition rate was only 55.8%, similar to the other control groups (49.6% and 51.7% for free vaccine and alum vaccine, respectively). The body weight of mice showed no obvious changes during the whole experiment period (Supplementary Fig. 22A). Subsequently, the tumors were collected and weighed. Consistent with the tumor growth curves, the tumor weight of L-Gel vaccine-treated group was significantly lower than those of the control groups (Fig. 5D). The infiltration of immune cells in tumors was assessed through immunofluorescence staining to detect CD3+ T cells and CD11c+ DCs in tumor tissues. L-Gel vaccine induced the most robust immune responses, with a greater number of T cells and DCs infiltrating into tumor tissues (Supplementary Fig. 22C).
A Schematic illustration of the prophylactic treatment. B Individual tumor growth kinetics in each group (n = 8 mice in each group). CR was defined as complete disappearance of tumor on day 24. The experiments were repeated three times and representative data from one of the experiments are shown. C Average tumor growth curve (n = 8 mice in each group). The experiments were repeated three times and representative data from one of the experiments are shown. D The tumor weights of different groups on day 24 (n = 8 mice in each group). E Tumor images on day 24 in the prophylactic experiment (n = 8 mice in each group). F The SIINFEKL-H2Kb positive DCs in LNs (n = 6 mice in each group). The IFN-γ positive cells within CD8+ T cells (G) and CD4+ T cells (H) in peripheral blood (n = 6 mice in each group). The proportion of central memory T cells (CD44+CD62L+) within CD4+ T cells (I) and CD8+ T cells (J) in spleens (n = 6 mice in each group). K Representative flow cytometry images of memory T cells. Data are presented as the mean ± SEM. G1: PBS, G2: Free Vac, G3: Alum Vac, G4: D-Gel Vac and G5: L-Gel Vac. Statistical significance was analyzed by one-way ANOVA using LSD posttest (C, D) or one-way ANOVA using Tukey’s posttest (F–J). Source data are provided as a Source Data file.
The peripheral blood of mice and major peripheral immune organs, including spleen and draining LNs, were collected to investigate the systemic anti-tumor immunity on day 24. The expression of SIINFEKL on the surface of DCs was detected in LNs and was found to be higher for L-Gel vaccine (Fig. 5F). Subsequently, T cell activation and migration into the tumor site occurs through the circulatory system. On account of secreting IFN-γ was usually identified as the sign of activated T cells, we detected the proportion of IFN-γ+ cells in CD3+CD8+ and CD3+CD4+ T cells in peripheral blood. The proportion of CD8+IFN-γ+ T cells in L-Gel vaccine group increased by ~3.5-fold compared to the PBS-treated group and was ~1.8-fold higher than the D-Gel vaccine group (Fig. 5G). The similar results could also be observed in CD4+IFN-γ+ T cells (Fig. 5H). Thus, L-Gel vaccine aroused stronger anti-tumor immune responses in the circulatory system. Memory T cells are essential to long-term prophylactic immunity; therefore, the number of memory T cells in spleen was further assessed. The L-Gel vaccine-treated group had the highest number of the central memory T cells (Tcm) which expressed CD44+CD62L+ in CD3+CD8+ T cells, at ~4.9-fold, 2.7-fold, 2.0-fold, and 1.5-fold levels compared to PBS, free vaccine, alum vaccine, and D-Gel vaccine groups, respectively (Fig. 5I, K). Similar results were detected in CD3+CD4+ T cells (Fig. 5J, K). No significant difference in effector memory T cells (Tem) was observed in spleens likely because Tem preferentially resides in peripheral tissues rather than in secondary lymphoid organs like Tcm. Tcm residing in spleen would recall responses to antigens and rapidly proliferate and differentiate into effector T cells, while Tem usually produce effector cytokines immediately upon antigen challenge in peripheral tissues36. The survival experiment of mice in prophylactic model was performed with an additional parallel experiment (Supplementary Fig. 23). The tumor growth curves showed that L-Gel inhibited tumor growth more effectively, and L-Gel vaccine significantly prolonged the survival time of mice compared to the other groups.
The effect of hydrogel with different chiral residues on the local immune microenvironment
According to the above results, stronger anti-tumor immune responses were observed for the L-Gel vaccine than D-Gel vaccine. Thus, the underlying mechanism was investigated. Several studies have demonstrated that hydrogels could regulate the host immune response through the intrinsic properties of materials, including chirality29, pore sizes16, and electric charge37. Herein, the polypeptide hydrogels constructed using different chiral residues induced significant host immune responses. D-Gel caused a rapid influx of immune cells and a greater LN reflux of antigen but did not induce a strong anti-tumor immune response compared to L-Gel. Therefore, the local immune microenvironment induced by different types of hydrogels at injection sites was further assessed.
To analyze the local immune microenvironment within the hydrogels, 100 μL of L-Gel or D-Gel were injected on the flank of mice, and the hydrogels with infiltrated immune cells were collected at days 3 or 7 post-injection for flow cytometry analysis. The constituent of recruited immune cells in hydrogels have been analyzed in previous section (Supplementary Fig. 11); herein, the subtypes of immune cells, including DCs, macrophages, and T cells, were assessed. We firstly studied the immunosuppressive markers on the surface of APCs, including PD-L1 and PD-1 expression. A significant increase in PD-L1 expression on CD11c+ DCs and F4/80+ macrophages in D-Gel was observed compared to L-Gel (Fig. 6A, B). PD-L1 expression levels on DCs in D-Gel were ~2.5-fold and 2.3-fold higher compared to those in L-Gel on days 3 and 7, respectively, and PD-L1 expression in D-Gel increased with increasing time. Additionally, the expression of PD-L1 on F4/80+ macrophages showed a similar trend. The expressions in D-Gel were upregulated to ~ 9.9-fold and ~10-fold than in L-Gel on days 3 and 7, respectively. The tests by western blot showed similar results (Fig. 6D and Supplementary Fig. 24B). PD-L1/PD-1 pathway blockade has been shown to significantly enhance anti-tumor immune responses and tumor inhibition efficiency. In recent years, considerable efforts have been devoted to elucidating the mechanisms of how these therapies instigate anticancer immunity4. Although the PD-L1/PD-1 pathway is usually involved in inhibition of T cell function, recent studies have also demonstrated that PD-L1 expression on DCs is an important factor for immune suppression, and is thus regarded as a target for strengthening of anti-tumor efficacy38,39. PD-L1 has two receptors, PD-1 and B7.1 (CD80). The overexpression of PD-L1 on DCs would therefore sequestrate B7.1 in cis to inhibit the B7.1/CD28 interaction, which was adverse for priming T cells. The PD-L1+CD11c+ and PD-L1+F4/80+ cells in draining LNs were also analyzed on day 7. Consistent with the local injection sites of hydrogel, D-Gel led to the upregulation of PD-L1 expression in LNs (Supplementary Fig. 24A).
A PD-L1 expression on CD11c+ DCs and F4/80+ macrophages in hydrogels on days 3 and 7 (n = 5 mice in each group). The experiments were repeated two times and representative data from one of the experiments are shown. B Flow cytometry image of PD-L1 and PD−1 on infiltrating immune cells in hydrogels. C PD−1 expression on CD45+ leukocytes, F4/80+ macrophages, CD11c+ DCs, and CD3+ T cells in hydrogels on days 3 and 7 (n = 6 mice in each group). The experiments were repeated two times and representative data from one of the experiments are shown. D Western blot analysis of PD-L1 and PD-1 expression by immune cells infiltrated in the hydrogels (β-actin was used as the reference). The experiments were repeated two times and representative data from one of the experiments are shown. E Other T cell exhaustion markers (LAG-3, TIM-3, CTLA-4) expression of CD3+ T cells in subcutaneously injected hydrogels after 3 days and 7 days (n = 4 mice in each group). The experiments were repeated two times and representative data from one of the experiments are shown. F Volcano plot of differentially expressed genes (DEGs) between the D-Gel and L-Gel through RNA-seq (n = 3 mice in each group). G The Kyoto Encyclopedia of Genes and Genomes (KEGG) pathway enrichment analysis of DEGs (n = 3 mice in each group). H The expression of relative genes detected by q-PCR (n = 3 technical replicates, GAPDH was used as the reference). The experiments were repeated three times and representative data from one of the experiments are shown. I Immunohistochemistry and immunofluorescence staining of skin tissues surrounding with hydrogels. J Schematic illustration of the status of infiltrating immune cells. Data are presented as the mean ± SEM. Statistical significance was analyzed by two-way ANOVA using Sidak’s posttest. Source data are provided as a Source Data file.
Subsequently, the expression of PD-1 on the infiltrated immune cells in hydrogels on days 3 and 7 was assessed (Fig. 6B, C). PD-1 expression levels of CD45+ leukocytes in D-Gel were ~1.3-fold and ~1.4-fold levels compared to those in L-Gel on days 3 and 7, respectively. PD-1 expressions in D-Gel were also upregulated on other immune cells, including F4/80+ macrophages, CD11c+ DCs, and CD3+ T cells, with ~1.5-fold, ~1.9-fold, and ~2.3-fold levels, respectively, compared to those in L-Gel on day 7. Myeloid cells play an important role in T cell function through multiple mechanisms; indeed, the PD-L1/PD-1 pathway of myeloid cells has been shown to negatively regulate the activation and proliferation of T cells40,41. Wang et al. demonstrated that injection of TiO2 nanoparticles in mice upregulated the expression of PD-1 on T cells and induced T cell exhaustion through the recruitment of PD-1+ myeloid cells21. Therefore, other exhaustion markers on T cells, such as LAG-3, TIM-3, and CTLA-4, were further investigated herein (Fig. 6E and Supplementary Fig. 25). The expression of these exhaustion markers showed a negligible difference on day 3 but the expression of LAG-3 and TIM-3 on CD3+ T cells in D-Gel significantly increased compared to L-Gel on day 7. Thus, herein, the overexpression of PD-L1 and PD-1 on the surface of APCs and the exhaustion of T cells in D-Gel might be responsible for the inadequate anti-tumor immune response.
To further characterize the heterogeneity of the local immune microenvironment following injection with the different hydrogels, bulk RNA sequencing (RNA-seq) was performed with the Illumina sequencing platform. Principal component analysis revealed obviously different mRNA transcriptome patterns of L-Gel and D-Gel (Supplementary Fig. 26A). The Pearson correlation between different samples also indicated the heterogeneity of D-Gel and L-Gel, because the inter-group correlation coefficient is clearly lower than the intra-group correlation coefficient (Supplementary Fig. 26B). The volcano plot showed a total of 1035 differentially expressed genes (DEGs) were observed between the D-Gel and L-Gel containing infiltrated immune cells (Fig. 6F and Supplementary Fig. 26C). Compared to L-Gel, 494 genes were upregulated and 541 genes were downregulated in D-Gel.
The Kyoto Encyclopedia of Genes and Genomes (KEGG) pathway enrichment analysis identified the top 10 upregulated signaling pathways in D-Gel (Fig. 6G); the first four pathways were involved in graft-versus-host disease, phagosome, lysosome, and complement coagulation cascades (Padj < 0.001), all of which have been reported to participate in the progression of inflammation and immunogenicity21,22,42. Thus, the results indicate that D-Gel is more immunogenic and evokes a stronger inflammatory response than L-Gel.
Further, qPCR analyses of relative genes were performed to identify the local microenvironment. The expressions of IFN-γ, TNF-α, STAT1, and NF-κB were responsible for the induction of inflammation. Inflammation can cause locally increased levels of CXCL9, CXCL10, and CCR3 in D-Gel, leading to the recruitment of a large number of immune cells (Fig. 6H). The infiltrated immune cells established an inflammatory but immunosuppressive microenvironment through upregulating the expression of PD-L1 and PD-1 in D-Gel, and even induced T cell exhaustion (Fig. 6J). Immunohistochemistry and immunofluorescence staining of skin tissues also displayed higher expression of IFN-γ, PD-1, and PD-L1 in D-Gel (Fig. 6I). It has been demonstrated in previous studies that IFN-γ, a typical proinflammatory cytokine, would upregulate PD-L1 expression by JAK/STAT pathway43. In order to investigate whether PD-L1 expression can be decreased after reducing inflammation by inhibiting JAK/STAT pathway, hydrogels loaded with JAK inhibitors (Ruxolitinib) or STAT inhibitors (Fludarabine) were subcutaneously injected into the flank of mice. After 5 days, the PD-L1 expression of the infiltrated DCs or macrophages in hydrogels was evaluated through flow cytometry analysis. As shown in Supplementary Fig. 27, the PD-L1 expression of the infiltrated DCs or macrophages in D-Gel could be reduced extent through blocking the JAK/STAT pathway.
In vivo therapeutic efficacy of hydrogel vaccines combined with PD-L1 blockade
The therapeutic efficacy of hydrogel vaccines combined with PD-L1 blockade for established tumor was assessed. Vaccination was conducted on day 3 after injection of 8 × 105 B16-OVA cells into the flank of C57BL/6 mice. The free vaccine showed slight tumor inhibition compared with PBS, while alum vaccine exhibited a significant improvement. L-Gel vaccine was superior to other groups, but had no significant difference compared with D-Gel (Supplementary Fig. 28). This might be due to improvement in the local immunosuppressive microenvironment to a certain degree by aPD-L1. The body weight of mice showed no obvious changes during the treatment period.
Anti-tumor immunity was further analyzed by collecting tumor, spleen, and draining LN. Firstly, the infiltration of both CD3+CD4+ and CD3+CD8+ T cells in spleens increased in the L-Gel vaccine-treated group (Supplementary Fig. 29A, B). The maturation of DCs in LNs was identified through the proportion of CD86+CD80+ DCs. L-Gel vaccine treatment induced the most robust activation of DCs (Supplementary Fig. 29C, D); similar results were observed in tumors (Supplementary Fig. 29E–I). Thus, the L-Gel vaccine enhanced the activation of T cells in spleen and the maturation of DCs in LNs.
Hydrogel vaccines for the treatment of postsurgical tumor recurrence
Surgery remains the first choice for early-stage cancers in clinical treatment, but postsurgical recurrence always appears and causes the death of patients. To further assess the potency of hydrogel vaccination, postsurgical treatment was performed to observe tumor recurrence. B16F10 cells (2 × 106) were inoculated in the flank of C57BL/6 mice and the tumors were allowed to grow to an average of 150–200 mm3, after which the visible tumors were resected. Tumor membrane antigen (BMAg) was extracted from the excisional tumor tissues and hydrogel vaccines were prepared through mixing 100 μl of hydrogel with 100 μg of BMAg, 10 μg of CpG-ODN, and 60 μg of aPD-L1. Equivalent amounts of PBS, free vaccine, and Alum adjuvant-based vaccine were prepared for comparison. After 1 day of tumor resection, the different formulations of vaccination were injected into the surgical sites (Fig. 7A). PBS and free vaccine showed poor ability in resisting tumor recurrence and alum-based vaccine was insufficient. Both L-Gel and D-Gel vaccines led to suppression of tumor growth and prolonged the survival time of mice. L-Gel vaccine exhibited superior effects, and 53.3% mice were free from tumor recurrence. However, nine mice among fifteen in the D-Gel vaccine group showed recurrence and occurred earlier compared to L-Gel (Fig. 7B–D). At 40 days post tumor resection, 2 × 105 B16F10 cells were inoculated in the flank of cured mice for re-challenge. Mice treated with the L-Gel vaccine group could resist the growth of tumors and had the longest animal survival time (Fig. 7E–G). Thus, the L-Gel vaccine exhibited the strongest anti-tumor efficacy. After 100 days of the primary tumor inoculation, memory T cells in spleens were detected. The number of CD3+ T cells, CD4+ Tem cells, and CD8+ Tcm cells were significantly increased in mice treated with the L-Gel vaccine (Supplementary Fig. 30).
A Schematic illustration of the postsurgical recurrence model. B Average tumor growth curves (n = 15 mice in each group). C Survival curves of postsurgical recurrence model (n = 15 mice in each group). D Individual tumor growth kinetics in each group (n = 15 mice in each group). CR was defined as complete disappearance of tumor on day 40. E Average tumor growth curves of re-challenged tumors inoculated on day 40 post-surgery (n = 8 mice in each group). F Survival curves of re-challenged model (n = 6 mice in D-Gel Vac+aPD-1 group or n = 8 mice in PBS group and L-Gel Vac+aPD-1 group). G Individual tumor growth kinetics in each group (n = 2 mice in Free Vac+aPD-1 group, n = 3 mice in Alum Vac+aPD-1 group, n = 6 mice in D-Gel Vac+aPD-1 group or n = 8 mice in PBS group and L-Gel Vac+aPD-1 group). CR was defined as complete disappearance of tumor on day 100. Data are presented as the mean ± SEM. Statistical significance was analyzed by one-way ANOVA using LSD posttest for five groups of data at the same time point and the P value marks the significance difference on Day 18 (B). Statistical significance was analyzed by two-tailed Student’s t-test (E) or a log-rank test (C, F). Source data are provided as a Source Data file.
The impact of residue chirality in other mPEG-polypeptide-based hydrogels
To investigate the impact of residue chirality in other mPEG-polypeptide-based hydrogels, we additionally synthesized a series of mPEG-polypeptide block copolymers with various residue chirality and DPs. These peptide-based block copolymers included mPEG-poly(L-alanine) and mPEG-poly(D-alanine) derived from L-/D-alanine, and mPEG-poly(L-valine) and mPEG-poly(D-valine) derived from L-/D-valine, because the above peptide-based materials have been shown to form thermo-sensitive hydrogels under physiological conditions (Fig. 8A)32,44,45. All representative peaks in 1H NMR spectra of monomers and copolymers were assigned, indicating the successful synthesis of copolymers (Supplementary Figs. 31 and 32).
A The chemical structures of mPEG-poly(L/D-alanine) and mPEG-poly(L/D-valine) block copolymers (left) and the preparation of hydrogel vaccines (right). The peptide-based block copolymers included mPEG-poly(L-alanine), mPEG-poly(D-alanine), mPEG-poly(L-valine), and mPEG-poly(D-valine). B Representative images of different hydrogels at 0 °C or 37 °C. The experiments were repeated at least three times and representative data from one of the experiments are shown. C Sol-gel phase diagrams of different copolymer solutions, LA1: mPEG-poly(L-alanine) (DP = 23.6), LA2: mPEG-poly(L-alanine) (DP = 26.7), LA3: mPEG-poly(L-alanine) (DP = 28.0), DA1: mPEG-poly(D-alanine) (DP = 21.4), DA2: mPEG-poly(D-alanine) (DP = 25.5), DA3: mPEG-poly(D-alanine) (DP = 28.2), LV1: mPEG-poly(L-valine) (DP = 13.1), LV2: mPEG-poly(L-valine) (DP = 18.6), LV3: mPEG-poly(L-valine) (DP = 22.8), DV1: mPEG-poly(D-valine) (DP = 14.6), DV2: mPEG-poly(D-valine) (DP = 18.5), DV3: mPEG-poly(D-valine) (DP = 21.6); DP indicates the degree of polymerization of polypeptide block. CD spectra of mPEG-poly(L- or D-alanine) (D) and mPEG-poly(L- or D-valine) (E) in aqueous solution (0.2 mg mL−1, 25 °C). The experiments were repeated two times and representative data from one of the experiments are shown. F Average tumor growth curves in the C57BL/6 mice with treatments of different OVA antigen-containing vaccine formulations, followed by subcutaneous inoculation of B16OVA melanoma cells at 10 days post-vaccination (n = 7 mice in each group). G Survival curves of mice during the prophylactic treatment (n = 7 mice in each group). Data are presented as the mean ± SEM. Statistical significance was analyzed by one-way ANOVA using LSD posttest for five groups of data at the same time point and the P value marks the significance difference on Day 19 (F). Statistical significance was analyzed by a log-rank test (G). Source data are provided as a Source Data file.
According to the gelation properties of various copolymers with different DPs of polypeptide block and polymer concentrations (Fig. 8C and Supplementary Figs. 33 and 34), the optimal materials were selected for further experiments. For mPEG-poly(L-alanine) and mPEG-poly(D-alanine), the optimal DP was ~28, with a polymer concentration of 8.0 wt%. For mPEG-poly(L-valine) and mPEG-poly(D-valine), the optimal DPs were 13–15, with a polymer concentration of 4.0 wt%. The formation of copolymer micelles in aqueous solution was detected by the pyrene fluorescence probe method and SEM (Supplementary Fig. 35). CD spectra of various copolymers were recorded in 0.2 mg mL−1 aqueous solution at 25 °C, which confirmed that the chirality of residues in the copolymers was fully preserved (Fig. 8D, E). The chosen copolymers exhibited similar properties in terms of sol-gel transition, pore size, rheology, degradation behavior in PBS, and antigen release in vitro (Fig. 8B and Supplementary Figs. 36 and 37). Similarly, the hydrogel containing D-amino acid residues degraded slower in vivo (Supplementary Fig. 38). Additionally, all copolymers exhibited good cytocompatibility against various types of cells (Supplementary Fig. 39).
Further, anti-tumor efficacy of various hydrogel vaccines was evaluated using a prophylactic model (Fig. 8F, G and Supplementary Fig. 40). For mPEG-polypeptide hydrogel vaccines derived from alanine and valine, those based on L-chirality exhibited more effective tumor inhibition and prolonged survival time of mice compared to those based on D-chirality. These results were consistent with the hydrogel vaccines based on L/D-glutamates. Overall, a similar trend in the impact of chiral residues on tumor inhibition was observed in these two types of mPEG-polypeptide hydrogel vaccines.
In addition, the trends of local immune microenvironment of mPEG-polypeptide hydrogels derived from L/D-valines were consistent with the hydrogels based on L/D glutamates (Supplementary Fig. 41). It is worth mentioning that, although the anti-tumor efficacy of mPEG-poly(L-alanine) based hydrogel vaccines was better than that of mPEG-poly(D-alanine) based vaccines, the local immune microenvironment showed no obvious differences between L-Ala-based Gel and D-Ala-based Gel, except for the increased recruitment of macrophages and DCs in D-Ala-based Gel. The results indicated that there are still other factors that exhibit impact on the immune microenvironment and final tumor inhibition efficacy for the mPEG-polypeptide vaccines derived from alanine (Supplementary Fig. 42).
Discussion
Chirality has attracted wide attention in the development of bioinspired organic or inorganic materials. Bioinspired chiral inorganic materials have been designed for the applications in photonics, sensing, catalysis, and biomedicine, such as immune modulation46,47,48. For instance, it has been shown that the chirality of intrinsic structure of gold nanoparticles (NPs) is able to modulate the immune response through enantiomer-dependent receptor interaction. It was found that, even though both left-handed and right-handed gold nanoparticles could be endocytosed by BMDCs, the left-handed nanoparticles displayed stronger association with the CD97 and EGF-like module receptor 1, resulting in activation of potassium efflux channels and generation of inflammasomes49,50. Moreover, the enhanced interactions between the L-type gold NPs and DCs were able to promote the activation of NK cells and CD8+ T cells, resulting in a significantly stronger anti-tumor immunity, compared to D-type NPs50,51.
In addition, chiral polymers have also been developed for immune modulation. Ding et al. synthesized two kinds of chiral polypeptides, poly(L-phenylalanine)-b-poly(L-lysine) (PL-K) and poly(L-phenylalanine)-b-poly(D-lysine) (PD-K)30. After mixing with OVA, the PD-K-OVA nanovaccine induced more robust effect on the maturation of DCs and adaptive immune responses through the TLR4 and MyD88 signaling pathways compared with the PL-K-OVA nanovaccine. Liu et al. prepared supramolecular chiral polymer micelles through complexing antigens with L- or D-histidine-modified polyethyleneimine (PEI)31. Similarly, they demonstrated that D-histidine-modified PEI showed higher uptake level by DCs and enhanced maturation of DCs compared to L-histidine-modified PEI. Apart from the chiral nanovaccines, hydrogels based on chiral peptides have also been investigated as drug-delivery platforms for cancer vaccines. Yang et al. developed a supramolecular hydrogel by self-assembling a D-tetra-peptide as a vaccine adjuvant to promote immune responses29,52. The D-tetra-peptide hydrogel enhanced antigen uptake and promoted DC maturation and accumulation of antigen in LNs. However, the degradation behavior and local immune responses of the D-tetra-peptide hydrogel deserve to be further investigated. After hydrogel injection or implantation, varying immune responses and FBR may occur at the local site. The extent of these responses is influenced by several factors, including degradation33,45, chemical structure22,53,54,55,56, pore size16, electric charge37,57,58, and chirality59.
In this study, we investigated the difference in the in vivo host immune responses caused by the hydrogels based on L- or D-amino acid residues and the potential role of the local immune microenvironment in influencing the anti-tumor immune response and tumor inhibition efficacy. The polypeptide hydrogels based on chiral residues including L/D-glutamate, L/D-valine, and L/D-alanine showed comparable properties in chemical structure, pore size, gelation behavior, mechanical property, and drug-release behavior, except for the chirality and concomitant degradation behavior. The antigen and adjuvant in both hydrogel vaccines were released sustainedly over 2 weeks in vivo, mimicking the effects of multi-dose injection. In the subcutaneous layer of mice, D-Gel recruited a greater number of immune cells than L-Gel both in the hydrogel and surrounding skin, especially macrophages and DCs. Accordingly, D-Gel induced higher proportions of antigen reflux in LNs than L-Gel.
Although D-Gel recruited a larger number of immune cells, these cells tended to express more suppressive markers (PD-L1 and PD-1), especially on the surface of APCs, including DCs and macrophages. The high levels of these suppressive markers inhibited the interactions between APCs and T cells, leading to the weakening of the initiation of adaptive immune responses38,39. Moreover, D-Gel even induced T cell exhaustion through the expression of higher levels of exhaustion markers, such as PD-1, LAG-3, and TIM-3, on the surface of T cells. Based on the RNA-seq and q-PCR, it was confirmed that D-Gel upregulated multiple pathways related to inflammation and immunogenicity, including graft-versus-host disease, phagosome, lysosome, and complement coagulation cascades, altering the local immune microenvironment and finally establishing an inflammatory but immunosuppressive microenvironment.
When loaded with OVA and CpG, the L-Gel vaccine elicited higher levels of activation and proliferation of T cells as well as generation of specific anti-OVA lgG antibodies in C57BL/6 mice, compared to the D-Gel vaccine. Moreover, in the prophylactic model using C57BL/6 mice, the L-Gel vaccine caused significantly stronger anti-tumor immunity against B16OVA melanoma cells with enhanced levels of antigen-specific DCs, IFN-γ+ CD8+ T cells, central memory CD4+ and CD8+ T cells, compared to the D-Gel vaccine. Additionally, the enhanced tumor inhibition efficacy of the L-Gel vaccine containing tumor antigen, CpG, and aPD-L1 was also confirmed in a postsurgical reoccurrence model using B16F10-bearing C57BL/6 mice.
Biocompatibility is the fundamental element for the clinical applications of drug delivery systems. To confirm the biosafety of hydrogel vaccines for in vivo applications, further investigation is needed to comprehensively evaluate the long-term degradation and metabolism of the hydrogels in vivo. Furthermore, more comprehensive and systematical assessments on the hydrogel vaccines are needed to examine the anti-tumor immune response and tumor inhibition efficacy against different types of cancers.
In summary, this study describes the significant difference of the host immune responses in vivo caused by the polypeptide hydrogels with different chirality residues. D-Gel exhibited stronger immunogenicity and induced increased immune cell infiltration in vivo. However, due to the excessive inflammation, D-Gel established an immunosuppressive microenvironment and caused insufficient anti-tumor efficacy through the upregulation of the suppressive markers of APCs and exhausting T cells. Conversely, the milder host immune responses induced by L-Gel led to superior anti-tumor immunity. Overall, this study put forward a strategy for the design of polypeptide hydrogel vaccine platforms with different chiral residues for enhanced anti-tumor immunity.
Methods
Ethical regulations
All animal experiments were performed in accordance with guidelines and regulations for the administration of laboratory animals decreed by the National Science and Technology Commission of China. All animal studies were carried out according to the guidelines approved by the Animal Welfare and Ethics Committee of Changchun Institute of Applied Chemistry, Chinese Academy of Sciences (No. 2021-53). In this study, the maximal tumor burden is 2000 mm3 or 10% of the mouse body weight which was approved by the guideline of assessment for humane endpoints in animal experiment of China (No. RB/T 173-2018), and not exceeded during the whole experiment.
Chemicals and reagents
L-/D-glutamic acid, L-/D-alanine, and L-/D-valine were purchased from Aladdin (Shanghai, P. R. China). Methoxy PEG Amine (mPEG-NH2, Mn = 2000 Da) was purchased from Jenkem (Beijing, P. R. China). Triphosgene was purchased from Duodian Chemicals (Nanjing, P. R. China). Anhydrous tetrahydrofuran (THF) and dimethylformamide (DMF) were purchased from Energy Chemical (Shanghai, P. R. China). All other reagents and solvents were purchased from Sinopharm Chemical Reagent (Shanghai, P. R. China) and used directly without further purification.
Ovalbumin (OVA) was purchased from Sigma-Aldrich (Darmstadt, Germany). Murine class C CpG-ODN (sequence 5′-tcgtcgttttcggcgcgcgccg-3′) and OVA-peptide257-264 were purchased from Sangon Biotech (Shanghai, P. R. China). InVivoMab anti-mouse PD-L1 (B7-H1) (Cat. BE0101) was bought from BioXcell (New Hampshire, The United States of America). BMDC inducible cytokines including GM-CSF and IL-4 were obtained from PeproTech (New Jersey, The United States of America). Other antibodies used in FACS were purchased from BioLegend (California, The United States of America): APC/Cyanine 7 labeled anti-CD45 (Cat. 103116), PE/Cyanine 7 labeled anti-F4/80 (Cat. 123114), PE labeled anti-CD11c (Cat. 117308), FITC labeled anti-CD3 (Cat. 100204), APC labeled anti-CD19 (Cat. 115512), PE labeled anti-NK1.1 (Cat. 108707), FITC labeled anti-CD11c (Cat. 117306), PE labeled anti-mouse H-2Kb bound to SIINFEKL (Cat. 141604), APC labeled anti-CD8 (Cat. 100712), PE/Cyanine 7 labeled anti-CD4 (Cat. 100422), PE labeled anti-IFN-γ (Cat. 505808), APC/Cyanine 7 labeled anti-CD4 (Cat. 100414), PE labeled anti-CD44 (Cat. 103007), PE/Cyanine 7 labeled anti-CD62L (Cat. 104417), APC labeled anti-CD80 (Cat. 104714), APC/Cyanine 7 labeled anti-I-A/I-E (MHCII) (Cat. 107628), PE/Cyanine 7 labeled anti-CD86 (Cat. 105014), PE labeled anti-CD206 (Cat. 141706), PE labeled anti-PD-L1 (Cat. 124307), PE labeled anti-PD-1(Cat. 135205), PE/Cy7 labeled anti-LAG-3 Antibody (Cat. 125225), APC labeled anti-TIM-3 Antibody (Cat. 119705), APC labeled anti-CTLA-4 Antibody (Cat. 106309). Other antibodies used in western blot were purchased from Cell Signaling Technology: primary anti-PD-L1 Antibody (CST: 60475S), primary anti-PD-1 Antibody (CST: 84651S) and primary β-actin Antibody (CST: 8457T).
Cell lines
NIH 3T3 and DC2.4 were purchased from Shanghai GuanDao Biological Engineering Co., Ltd (catalog number: BNCC100843 and BNCC351939). B16F10 cell lines were purchased from the American Type Culture Collection (catalog number: CRL-6475). B16OVA cell lines were purchased from the Bohui Biotechnology (Guangzhou) Co., Ltd (catalog number: BH-C489). NIH 3T3, DC2.4, B16F10, and B16OVA cells were maintained in Roswell Park Memorial Institute medium 1640 (RPMI-1640) supplemented with 10% fetal bovine serum (FBS) and 1% penicillin/streptomycin. All cell lines were authenticated by Short Tandem Repeat profiling by the provider and routinely tested for mycoplasma contamination, with all tests confirming mycoplasma-free status.
Animals
C57BL/6 mice (female, 4–6 weeks or 6–8 weeks old) were obtained from Beijing Vital River Laboratory Animal Technology Co., Ltd. OT-I transgenic mice were kindly provided by the Tianmeng Sun’s group of State Key Laboratory of Supramolecular Structure and Materials, Jilin University, Changchun, Jilin, China.
Synthesis of polypeptides with different chiral residues
First, 20 g of L- or D-glutamic acid was mixed with 30 mL of ethanol and the mixture was stirred in the ice bath. Subsequently, 8 mL of 98% sulfuric acid was gradually added over a period of 30 min. The mixture was then allowed to return to room temperature and stirred overnight. The reaction solution was slowly poured into a 1:1 (v/v) mixture of triethylamine and ethanol, leading to the formation of a white precipitate. This crude product was collected by centrifugation and further purified by recrystallization from water and ethanol, yielding γ-ethyl-L-glutamate (ELG) with a yield of 46.7% and γ-ethyl-D-glutamate (EDG) with a yield of 47.5%. ELG 1H NMR (D2O, 500 MHz): δ 1.09 (t, 3H), 2.00 (m, 2H), 2.39 (t, 2H), 3.61 (t, 1H), 4.02 (q, 2H). EDG 1H NMR (D2O, 500 MHz): δ 1.10 (t, 3H), 2.01 (m, 2H), 2.40 (t, 2H), 3.61 (t, 1H), 4.02 (q, 2H).
ELG or EDG (10.0 g, 57.1 mmol) and triphosgene (16.7 g, 56.2 mmol) were added separately into a flame-dried three-neck flask with 150 mL of anhydrous THF. The white suspension was bubbled with a nitrogen flux and stirred at ~55 °C for 10 min, during which it transformed into a clear yellow solution. The solution was cooled to room temperature, with continued nitrogen bubbling for an additional 30 min. Subsequently, the mixture was poured into 1.5 L of cold petroleum ether and kept at −20 °C for 2 h. After decanting the supernatant, the yellow residue was dissolved in 150 mL of cold ethyl acetate and repeatedly washed with ice water in a separatory funnel. The organic phase was dried with anhydrous MgSO4 and stored overnight at −20 °C. The solution was then filtered, and the solvent was evaporated under vacuum to yield the crude product. Purified γ-ethyl-L-glutamate N-carboxyanhydride, (ELG-NCA, 84.9% in yield) or γ-ethyl-D-glutamate N-carboxyanhydride (EDG-NCA, 84.6% in yield) was obtained by recrystallizing the crude product using anhydrous THF and n-hexane (1:1, v/v). ELG-NCA 1H NMR (CDCl3, 500 MHz): δ 1.29 (t, 3H), 2.06–2.33 (m, 2H), 2.56 (t, 2H), 4.16 (q, 2H), 4.44 (t, 1H), 6.77 (s, NH). EDG-NCA 1H NMR (CDCl3, 500 MHz): δ 1.29 (t, 3H), 2.06–2.33 (m, 2H), 2.56 (t, 2H), 4.16 (q, 2H), 4.44 (t, 1H), 6.85 (s, NH).
The polypeptides with different chirality were prepared via the ring-opening polymerization of ELG-NCA or EDG-NCA under mPEG-NH2 initiation. Taking mPEG-block-poly(γ-ethyl-L-glutamate)n (mPEG-b-PELG) as an example, mPEG-NH2 (1 g, 0.5 mmol) was distilled in toluene (80 mL) to removal water for 4 h at ~130 °C. Toluene was then evaporated, followed by the addition of 50 mL of anhydrous DMF to the flask. After mPEG-NH2 was re-dissolved using anhydrous DMF, ELG-NCA (1.31 g, 6.5 mmol) was added to the flask. The system was reacted at room temperature for 72 h. The solution was then poured into cold diethyl ether and a white precipitate was obtained after filtration. The precipitate was dialyzed against deionized water (MWCO: 500 Da) and collected by lyophilization at a yield of 75.5%. mPEG-block-(poly(γ-ethyl-L-glutamate)0.5-co-poly(γ-ethyl-D-glutamate)0.5)n (mPEG-b-P(ELG0.5-co-EDG0.5)), and mPEG-block-poly(γ-ethyl-D-glutamate)n (mPEG-b-PEDG) were synthesized using a similar procedure by adjusting the feeding molar ratios of the two types of NCAs at a yield of 78.4% and 80.2%, respectively. The chemical compositions of polypeptides with different chirality in CF3COOD were determined by 1H NMR using a 500-MHz Bruker instrument. mPEG-b-PELG 1H NMR (CF3COOD, 500 MHz): δ 0.89 (t, 39.9H), 1.77–1.88 (m, 26H), 2.19 (m, 24.6H), 3.15 (m, 3H), 3.48 (m, 180H), 3.84 (m, 26.3H), 4.35 (m, 12.2H). mPEG-b-P(ELG0.5-co-EDG0.5) 1H NMR (CF3COOD, 500 MHz): δ 0.84 (t, 39.9H), 1.70–1.86 (m, 27.1H), 2.14 (m, 25.4H), 3.10 (m, 2.5H), 3.43 (m, 180H), 3.80 (m, 25.4H), 4.36 (m, 12.7H). mPEG-b-PEDG 1H NMR (CF3COOD, 500 MHz): δ 0.83 (t, 40.9H), 1.67–1.83 (m, 27H), 2.14 (m, 25.7H), 3.10 (m, 2.2H), 3.43 (m, 180H), 3.79 (m, 26.4H), 4.31 (m, 13.1H). The Mn and PDI of polypeptides of different chirality were measured by gel permeation chromatography using DMF as the eluent.
The synthesis methods of other mPEG-polypeptides derived from L-/D-alanine and L-/D-valine are consistent with those for L-/D-glutamic acid, but do not require an esterification reaction. L-Ala-NCA 1H NMR (CDCl3, 500 MHz): δ 1.58–1.60 (d, 3H), 4.41–4.48 (q, 1H), 6.61 (s, NH). D-Ala-NCA 1H NMR (CDCl3, 500 MHz): δ 1.58–1.60 (d, 3H), 4.41–4.48 (q, 1H), 6.76 (s, NH). L-Val-NCA 1H NMR (CDCl3, 500 MHz): δ 1.04–1.12 (dd, 6H), 2.23–2.33 (m, 1H), 4.24–4.25 (d, 1H), 6.83 (s, NH). D-Val-NCA 1H NMR (CDCl3, 500 MHz): δ 1.03–1.12 (dd, 6H), 2.22–2.33 (m, 1H), 4.24–4.26 (d, 1H), 7.09 (s, NH). mPEG-b-PLAla (DP = 28.0) 1H NMR (CF3COOD, 500 MHz): δ 1.53 (m, 84.7H), 3.60 (m, 3H), 3.92 (m, 182H), 4.66 (m, 23.7H). mPEG-b-PDAla (DP = 28.2) 1H NMR (CF3COOD, 500 MHz): δ 1.53 (m, 84.6H), 3.59 (m, 3H), 3.93 (m, 182H), 4.67 (m, 24.4H). mPEG-b-PLVal (DP = 13.1) 1H NMR (CF3COOD, 500 MHz): δ 0.98–1.02 (m, 78.8H), 2.12 (m, 12.5H), 3.58–3.60 (m, 2.9H), 3.92 (m, 182H), 4.45 (m, 12.4H). mPEG-b-PDVal (DP = 14.6) 1H NMR (CF3COOD, 500 MHz): δ 0.98–1.02 (m, 88.3H), 2.13 (m, 13.9H), 3.59–3.60 (m, 2.9H), 3.93 (m, 182H), 4.45 (m, 13.5H).
Critical micelle concentration (CMC) study
The CMC of copolymer was investigated through the pyrene fluorescence probe technique. In brief, 20 µL of a solution of pyrene in acetone (6.0 × 10–5 mol L−1) was separately added into several brown vials, and acetone was evaporated overnight. Afterwards, a series of different concentrations of copolymer aqueous solutions were obtained by half-diluting 14 times from 1.0 mg mL−1. Subsequently, 2 mL of copolymer solutions at different concentrations were added to the brown vials, separately, and the vials were shaken for 24 h in the dark. The fluorescence excitation spectra (280–360 nm) of copolymer solutions at different concentrations were recorded on a Fluoromax-4 spectrophotometer (Horiba Ltd., Kyoto, Japan) at a λem of 390 nm.
The circular dichroism (CD) detection
The ellipticity of copolymers was determined using a Chirascan CD spectrometer (Applied Photophysics, Leatherhead, UK) using aqueous solutions of the polymers (0.2 mg mL−1).
Thermosensitive sol-gel phase transition behavior
The hydrogel formation of the copolymer solutions at different concentrations was confirmed by the test tube inversion method. First, a series of copolymer solutions of different concentrations were prepared in a glass vial and stirred in the ice bath for 48 h. For the thermosensitive sol-gel phase transition tests, the vials were placed in a water bath with programmed temperature increase (2 °C 10 min−1). When the sample was tilted for 30 s and no fluidity was observed, the temperature was recorded as the gelation temperature.
The observation of self-assembly behaviors
For micelle of copolymers, the copolymers were first dissolved in water (0.5 mg mL−1) for 24 h. Subsequently, 10 μL of copolymer solution was dripped onto a silicon wafer and slowly evaporated at 37 °C. The self-assembly microstructure of the copolymers was observed using SEM (Gemini 2, Carl Zeiss, Germany).
For hydrogels, various concentrations of different types of copolymer solutions were prepared in a glass vial and stirred in the ice bath for 48 h. The microstructure of the hydrogel was investigated via cryogenic scanning electron microscopy (Cryo-SEM, ZEISS Sigma 300, ZEISS, Germany).
Rheological tests
Rheological tests were performed using an MCR 301 rheometer (Anton Paar GmbH., Graz, Austria). The copolymer solution (0.32 mL) was added between parallel plates with a diameter of 25 mm and a gap of 0.5 mm. The edge of the sample was covered with silicon oil to prevent the evaporation of water. The strain was set to 1%, and the frequency was 1 Hz. The temperature range was 0–70 °C, and the heating rate was 0.5 °C min−1.
In vitro gel degradation
First, 300 µL of the copolymer solutions in PBS were prepared in separate glass vials and stirred in an ice bath for 48 h. The hydrogels were then stabilized under 37 °C for 12 h. PBS (10 mM, pH 7.4) with or without Proteinase K (≥5 U mL−1) was chosen to degrade the hydrogel (degradation medium). Following the formation of the hydrogels, 1.8 mL of the degradation medium was placed into each glass vial. The vials were then shaken at 37 °C. The mass of the remaining hydrogels was recorded following removal of the degradation medium at set time intervals. Finally, 1.8 mL of fresh degradation medium was added into the vials.
In vivo gel degradation
In vivo hydrogel degradation was investigated using C57BL/6 female mice (6–8 weeks old). The dorsal area of mice was shaved, and 100 µL hypodermic injection of copolymer solution was conducted in the dorsal area of mice. The mice were then sacrificed at different time points. Subsequently, the hydrogels were separated for imaging and weighing. The skin tissues surrounding hydrogels were fixed with 4% PFA. The histopathology was observed on an inverted fluorescence microscope after the skin was sectioned and stained with H&E.
In vitro drug release properties
OVA was first labeled with Cy5 at a weight ratio of 10%. Briefly, 90 mg of OVA and 10 mg of Cy5-NHS were mixed and dissolved in water and stirred overnight at room temperature. The OVA-Cy5 stock solution was then obtained with further dialyzed with a 1000 Da dialysis bag in water.
OVA-Cy5 (1 mg mL−1) and CpG-ODN (5.32 μM) were mixed with 6.0 wt% copolymer solutions and the solution was stirred in the ice bath for 48 h. Afterwards, drug-containing solutions were transferred into glass vials (300 µL for each vial). The vials were rapidly placed at 37 °C to form stable drug-loaded hydrogels. PBS (10 mM; pH 7.4) was used as the release medium. After gel formation, 1 mL of the release media was added into the vials containing hydrogel. At given time intervals, the medium containing the drugs was collected. An equal volume of fresh-release medium was supplemented in each vial. Quantification of OVA-Cy5 and CpG-ODN was conducted by a UV-Vis spectrometer at a detection wavelength of 650 nm and 260 nm, respectively.
In vivo cell recruitment
C57BL/6 female mice (6–8 weeks old) received subcutaneous injection of 100 μL of L-Gel on the left flank and 100 μL of D-Gel on the right flank. On days 1, 3, and 7, the hydrogels were separated to evaluate the cell recruitment capability. For the detection of immune cells infiltrating hydrogels, the hydrogels were ground and filtrated into a single-cell suspension and stained with immune markers, including APC/Cy7-CD45, FITC-CD3, PE-CD11c, PE/Cy7-F4/80, APC-CD19, and PE-NK1.1 for 30 min on ice. The cells were then fixed by 4% PFA after washing twice by PBS containing 3% FBS for flow cytometry detection (BD FACSCalibur). For immunofluorescence staining, the skin surrounding the hydrogels was collected and fixed with 4% PFA. After embedding and slicing, the sections were labeled with immunofluorescent biomarkers (CD45, F4/80, CD11c, and CD3) for observation, a process performed by Wuhan Service Biotechnology Co., Ltd.
Protein adsorption
L-Gel or D-Gel precursor solutions (100 µL) were added into 48-cell plates and the hydrogels were allowed to form and stabilize under 37 °C for 12 h. Then, 400 µL of BSA-Cy5 (50 µg mL−1) was gently added in the surface of hydrogels and cultured at 37 °C. At different time points, the protein concentration of the supernate was detected by Microplate Reader (Tecan Spark, Switzerland). The adsorbed protein could be calculated with the number of proteins in initial solution and the residual supernate.
In vivo OVA release and characterization of lymph node reflux
To assess the in vivo OVA release properties, 100 μL of 6.0 wt% copolymer solution containing 20 μg of OVA-Cy5 was injected subcutaneously on the right flank of C57BL/6 female mice (6–8 weeks old). OVA-Cy5 fluorescence was imaged by IVIS Lumina LT, PerkinElmer, at different time points. For the LN reflux of OVA-Cy5, C57BL/6 female mice (6–8 weeks old) were injected with L-Gel or D-Gel (100 μg of OVA-Cy5 per mouse) subcutaneously at the tail base. On days 1, 3, 7, and 14, mice were sacrificed and inguinal LNs were excised. The LNs were ground and filtered to obtain single-cell suspensions, which were then stained with APC/Cy7-CD45 and PE-CD11c antibodies for 30 min on ice. Following staining, the cells were washed twice with PBS containing 3% FBS and fixed using 4% PFA before flow cytometry analysis.
In vitro DCs maturation
For BMDCs preparation, monocytes were isolated from the femur and tibia of 4- to 6-week-old C57BL/6 female mice. These monocytes were then cultured in a cell culture medium supplemented with GM-CSF (20 ng mL−1) and IL-4 (10 ng mL−1) for a period of 7 days to generate BMDCs. For DC stimulation experiments, 1 × 106 of myeloid-derived DCs (BMDCs) were co-cultured with PBS, L-Gel, D-Gel, soluble-free OVA (5 μg), OVA@L-Gel (5 μg), or OVA@D-Gel (5 μg) for 24 h. Following the different treatments, cells were collected and stained with PE-CD11c, APC-CD80, PE/Cy7-CD86, APC/Cy7-MHCII, and FITC-CD40 antibodies for 30 min on ice. Following staining, the cells were washed twice with PBS containing 3% FBS and fixed using 4% PFA. The stained cells were then analyzed using flow cytometry on a BD FACSCalibur instrument.
In vitro T-cell activation and proliferation
CD8+ T cells were extracted from the spleens of OT-I transgenic female mice (6–8 weeks old), following the manufacturer’s protocol (EasySep Mouse CD8+ T-cell Isolation Kit, STEMCELL Technologies). BMDCs were co-cultured for 1 day with either PBS, D-Gel containing 10 μg of OVA-peptide257-264, or L-Gel containing 10 μg of OVA-peptide257-264. After this initial co-culture, CFSE-stained OT-I cells were introduced. The co-cultures were then conducted for an additional 24 h to facilitate T-cell proliferation and activation. Following the incubation, the cells were collected and stained with APC/Cy7-CD62L and PE-CD44 antibodies for 30 min on ice. Following staining, the cells were washed twice with PBS containing 3% FBS and fixed using 4% PFA before flow cytometry analysis.
In vivo systemic immune activation of hydrogel vaccine
For specific antibody titer detection, C57BL/6 female mice (6–8 weeks old) were injected with PBS, free OVA, alum OVA, alum: Imject Alum adjuvant (containing an aqueous solution of aluminum hydroxide (40 mg mL−1) and magnesium hydroxide (40 mg mL−1) plus inactive stabilizers), OVA@L-Gel, and OVA@D-Gel on days 0 and 14 (100 µL, 20 μg of OVA per mouse in all groups). On days 10, 14, and 21, peripheral blood was collected from mice following vaccination, coagulated for 1 h at 37 °C, and centrifuged at 3000 rpm for 10 min to separate serum. High-binding 96-well plates (Corning Cat. 42592) were coated overnight at 4 °C with 10 µg mL−1 of ovalbumin in PBS (10 mM; pH 7.4). After washing by PBST (10 mM PBS containing 0.05% tween 20), the wells were blocked with blocking buffer (10 mM PBS containing 2% BSA) for 2 h and incubated with serum at a range of dilutions for 1 h at room temperature. Respective wells were incubated with HRP-conjugated goat anti-mouse IgG (H + L) secondary antibody (Thermo Fisher Cat. 31430) for 1 h, followed by a 10 min incubation with TMB substrate. Absorbance was measured at 450 nm, subtracting the background at 570 nm after terminating the reaction with 1 M H2SO4.
For immunofluorescence of GCs, after vaccination, C57BL/6 female mice (6–8 weeks old) were euthanized on day 7 and vaccine-draining inguinal LNs were collected. LNs were immediately stored at −80 °C, and cryo-sections were stored at −20 °C. Sections were blocked in blocking buffer (10 mM PBS containing 5% BSA) for 2 h at room temperature and then stained with Alexa Fluor® 488-conjugated anti-mouse/human GL7 (Biolegend Cat. 144612) and Alexa Fluor® 594-conjugated anti-mouse/human CD45R/B220 (Biolegend Cat. 103254) antibodies overnight at 4 °C. After washing with PBST, stained sections were mounted with antifade mounting medium with DAPI (Beyotime P0131) and imaged on a confocal laser scanning microscopy (CLSM, ZEISSLSM780, Germany).
For flow cytometry detection of systemic immune response, after vaccination, C57BL/6 female mice (6–8 weeks old) were euthanized on day 7. Inguinal LNs and spleen were collected and preserved in the 10 mM PBS containing 3% FBS. Peripheral blood samples were collected and mixed with 20 μL of a 10 mg mL−1 heparin sodium solution to prevent clotting. LNs were ground to a single-cell suspension and stained with FITC-anti-CD11c and PE-anti-SIINFEKL/H-2Kb antibodies for 30 min on ice. Following staining, the cells were washed twice with PBS containing 3% FBS and fixed using 4% PFA before flow cytometry analysis. Spleen was ground to a single-cell suspension and erythrocytes were lysed using ACK lysis buffer (Beyotime C3702). Single cells were firstly stained with FITC-CD3, APC-CD8, and PE/Cy7-CD4 antibodies for 30 min on ice. Subsequently, the cells were fixed by fixation buffer (Biolegend Cat. 420801) and washed twice by intracellular staining perm wash buffer (Biolegend Cat. 421002). Cells were then stained with PE-IFN-γ in intracellular staining perm wash buffer for 30 min at room temperature. Following staining, the cells were washed twice with PBS containing 3% FBS and resuspended for FACS analysis. For detection of IFN-γ+ T cells in blood, a single-cell suspension was obtained by lysing erythrocytes using ACK lysis buffer. Subsequently, the protocol followed was identical to that used for the spleen.
For in vivo OT-1 T-cell proliferation, CD8+ T cells were extracted from the spleens of OT-I transgenic female mice (6–8 weeks old), following the manufacturer’s protocol (EasySep Mouse CD8+ T-cell Isolation Kit, STEMCELL Technologies). On day 0, 1 × 106 CFSE-labeled OT-1 CD8+ T cells were intravenously injected into C57BL/6 female mice (6–8 weeks old). On day 1, these mice were administrated with different vaccine formulations including L-Gel containing 20 μg of OVA-peptide257-264 (100 µL), or D-Gel containing 20 μg of OVA-peptide257-264 (100 µL). On day 2, the mice were euthanized and the spleens of mice were harvested. Spleens were ground to single-cell suspension and erythrocytes were lysed using ACK lysis buffer. It was then initially stained for cell-surface makers with PE-CD8 antibodies for 30 min on ice. Following staining, the cells were washed twice with PBS containing 3% FBS and fixed using 4% PFA before flow cytometry analysis.
Prophylactic experiment
C57BL/6 female mice (6–8 weeks old) were randomly divided into five groups: PBS group, free vaccine group (Free vac), alum vaccine group (Alum vac), L-Gel vaccine group (L-Gel vac), and D-Gel vaccine group (D-Gel vac). On day −10, the prepared vaccine (100 µL) of the different formulations (10 μg of CpG ODN and 100 μg of OVA) was injected into the tail base of mice. B16-OVA melanoma cells (1 × 106) were injected subcutaneously on the right flank of mice. Tumor sizes and mice weights were measured every 2 days. Tumor volumes were calculated following the formula: short diameter2 × long diameter × 0.5. Animals were euthanized when the tumor exceeded 1.5 cm3 or when the tumor exhibited broken signals such as rupture and bleeding.
To assess the immune response induced by the various vaccines, on day 24, inguinal LNs and spleen were collected and preserved in 10 mM PBS containing 3% FBS. Peripheral blood samples were collected and mixed with 20 μL of a 10 mg mL−1 heparin sodium solution to prevent clotting. LNs were ground to a single-cell suspension and stained with FITC-anti-CD11c and PE-anti-SIINFEKL/H-2Kb antibodies for 30 min on ice. Following staining, the cells were washed twice with PBS containing 3% FBS and fixed using 4% PFA before flow cytometry analysis. Splenocytes were isolated by lysing erythrocytes with ACK lysis buffer, followed by staining with FITC-CD3, APC-CD8, PE/Cy7-CD4, APC/Cy7-CD62L, and PE-CD44 antibodies for 30 min on ice. After staining, the cells were washed twice with PBS containing 3% FBS and fixed using 4% PFA before flow cytometry analysis. For detection of IFN-γ+ T cells in blood, a single-cell suspension was obtained by lysing erythrocytes using ACK lysis buffer. Subsequently, single cells were firstly stained with FITC-CD3, APC-CD8, and PE/Cy7-CD4 antibodies for 30 min on ice. Subsequently, the cells were fixed by fixation buffer (Biolegend Cat. 420801) and washed twice by intracellular staining perm wash buffer (Biolegend Cat. 421002). Cells were then stained with PE-IFN-γ in intracellular staining perm wash buffer for 30 min at room temperature. Following staining, the cells were washed twice with PBS containing 3% FBS and resuspended for FACS analysis.
In vivo analysis of local immune microenvironment at hydrogel injection site
For flow cytometry detection, C57BL/6 female mice (6–8 weeks old) received subcutaneous injection of 100 μL of L-Gel or D-Gel on the flank. On days 3 or 7, the hydrogels were separated and ground to a single-cell suspension. The collected cells were divided into six groups for staining as follows: (1) FITC-CD45, PE-CD11c, APC-CD80, PE/Cy7-CD86, and APC/Cy7-MHCII; (2) APC/Cy7-CD45, PE/Cy7-F4/80, APC-CD80, and PE-CD206; (3) APC/Cy7-CD45, FITC-CD11c, PE/Cy7-F4/80, and PE-PD-L1; (4) APC/Cy7-CD45, FITC-CD11c, PE/Cy7-F4/80, and PE-PD-1; (5) APC/Cy7-CD45, FITC-CD3, PE-PD-1, PE/Cy7-LAG-3, and APC-TIM-3; and (6) APC/Cy7-CD45, FITC-CD3, and APC-CTLA-4. All cells were stained for 30 min on ice, then washed twice with PBS containing 3% FBS and fixed using 4% PFA before flow cytometry analysis. The skins containing hydrogel were fixed with 4% PFA, followed by embedding and sectioning. Immunohistochemical and immunofluorescence staining (IFN-γ, PD-L1, and PD-1) were performed by Wuhan Service Biotechnology Co., Ltd.
For western blot detection, C57BL/6 female mice (6–8 weeks old) received subcutaneous injection of 100 μL of L-Gel, L-Gel + Fludarabine, L-Gel + Ruxolitinib, D-Gel, D-Gel + Fludarabine, or D-Gel + Ruxolitinib on the flank. On day 5 or 7, the hydrogels were separated, ground, and lysed using RIPA buffer (Epizyme Biotech PC101) containing protease inhibitor and phosphatase inhibitor. The total amount of proteins was measured with a BCA protein kit (Thermo 23227). The proteins were separated using SDS-PAGE and transferred to a 0.45-μm PVDF membrane. The membrane was blocked using 10 mM PBS containing 5% BSA for 2 h at room temperature. It was then incubated overnight at 4 °C with primary antibodies against PD-L1 (CST 60475S), PD-1 (CST 84651S), and β-actin (CST 8457T), followed by incubation with anti-rabbit IgG, HRP-linked antibody (CST 7074S). Chemical luminescent reagents (Beyotime P0018S) were used for imaging (Amersham Imager 600).
For RNA-seq analysis of the immune microenvironment induced by hydrogels, 100 μL of L-Gel or D-Gel were injected into the back of C57BL/6 female mice (6–8 weeks old). After 7 days, formed hydrogel niches (without adjacent tissues) were collected in sterile tubes and stored in liquid nitrogen. RNA samples were qualified and quantified using a NanoDrop and Agilent 2100 bioanalyzer (Thermo Fisher Scientific, MA, USA). PCR amplification was performed using cDNA as a template for building a standard sequencing library. NEBNext® Ultra™ RNA Library Prep Kit for Illumina® was used as assay kit for building the sequencing library. High-volume sequencing of the library was performed using the two-end sequencing mode of an Illumina sequencing platform by Novogene Co., Ltd. The DEGs between the L-Gel and D-Gel were screened with Padj < 0.05 and |log2(fold change)| > 0.5 as the cut-off criteria. The top 10 pathways enriched in the DEGs were analyzed by KEGG (Padj < 0.05).
For the expression of mRNA, the hydrogel niches (without adjacent tissues) were collected on day 7. Total RNA was extracted from the hydrogel niches using RNAsimple Total RNA Kit (Tiangen DP419). A FastKing RT Kit (Tiangen KR116) was used to synthesize the first chain of cDNA by reverse transcription (Bio-Rad T100 Thermal Cycler) and amplify it by qPCR (LightCycler 480 II). The thermal cycler parameters were: 95 °C, 10 min; 95 °C, 10 s; and 65 °C, 30 s; 40 cycles. The primer sequences were as follows: mouse IFN-γ primers: forward primer 5′- CAGCAACAGCAAGGCGAAAAAGG-3′ and reverse primer 5′-TTTCCGCTTCCTGAGGCTGGAT-3′; mouse TNF-α primers: forward primer 5′- TTCTGCAAAGGGAGAGTGGTC-3′ and reverse primer 5′- TGAAGGTAGGAAGGCCTGAGAT-3′; mouse NF-κβ primers: forward primer 5′- GACCTGTATCAGACACCT-3′ and reverse primer 5′- CTGTCGTGTCCTTCTTTGG-3′; mouse CXCL9 primers: forward primer 5′- CGCTGTTCTTTTCCTCTTGGG-3′ and reverse primer 5′- CCTTATCACTAGGGTTCCTCGAA-3′; mouse CXCL10 primers: forward primer 5′- CGCTGCAACTGCATCCATA-3′ and reverse primer 5′- TAGGCTCGCAGGGATGATTTC-3′; mouse CXCR3 primers: forward primer 5′- TACGATCAGCGCCTCAATGCCA-3′ and reverse primer 5′- AGCAGGAAACCAGCCACTAGCT-3′; mouse STAT1 primers: forward primer 5′- GTCATCCCGCAGAGAGAACG-3′ and reverse primer 5′- GCAGAGCTGAAACGACCTAGA-3′; mouse PD-L1 primers: forward primer 5′- CCAGCCACTTCTGAGCATGA-3′ and reverse primer 5′- CTTCTCTTCCCACTCACGGG-3′. Gene expression levels were calculated using Log2 (relative fold change).
Therapeutic experiment
The therapeutic efficacy of the hydrogel vaccine was studied by using B16-OVA tumor-bearing C57BL/6 female mice (6–8 weeks old). On day 0, 8 × 105 B16-OVA melanoma cells were injected subcutaneously on the right flank of mice. On day 3, the prepared vaccines (100 µL) with the different formulations (PBS group, Free vac, Alum vac, L-Gel vac, and D-Gel vac; 10 μg of CpG ODN, 100 μg of OVA and 60 μg of anti-PD-L1) were injected into the tail base of mice. To assess the therapeutic efficacy of vaccination, the tumor growth and body weight of mice were monitored. The volume of the tumor was measured with a digital caliper every 2 days.
For flow cytometry detection, on day 0, 1 × 106 B16-OVA melanoma cells were injected subcutaneously on the right flank of mice. On day 10, the prepared vaccines (100 µL) with the different formulations (PBS group, Free vac, Alum vac, L-Gel vac, and D-Gel vac; 10 μg of CpG ODN, 100 μg of OVA) were administered. On day 24, the tumors, spleens, and LNs were collected. For evaluation of immune activation of tumors, single-cell suspensions were collected after grinding and filtration, then these cells were stained with antibody cocktail (APC/Cy7-CD45, FITC-CD3, APC-CD8, PE/Cy7-CD4 antibodies or APC/Cy7-CD45, FITC-CD11c, APC-CD80, PE/Cy7-CD86, and PE-SIINFEKL/H-2Kb antibodies) for 30 min on ice. After staining, these cells were washed twice with PBS containing 3% FBS and fixed using 4% PFA before flow cytometry analysis. For the detection of maturation of DCs in LNs, single-cell suspensions were collected after grinding and filtration, then these cells were stained with antibody cocktail (FITC-CD11c, APC-CD80, PE/Cy7-CD86, and PE-SIINFEKL/H-2Kb) for 30 min on ice. After staining, these cells were washed twice with PBS containing 3% FBS and fixed using 4% PFA before flow cytometry analysis. Spleen was ground to a single-cell suspension and erythrocytes were lysed using ACK lysis buffer (Beyotime C3702). Single cells were firstly stained with FITC-CD3, APC-CD8, and PE/Cy7-CD4 antibodies for 30 min on ice. Subsequently, the cells were fixed by fixation buffer (Biolegend Cat. 420801) and washed twice by intracellular staining perm wash buffer (Biolegend Cat. 421002). Cells were then stained with PE-IFN-γ in intracellular staining perm wash buffer for 30 min at room temperature. Following staining, the cells were washed twice with PBS containing 3% FBS and resuspended for FACS analysis.
Tumor recurrence model
To study the inhibition behavior of the hydrogel vaccines on tumor recurrence, 1 × 106 of B16F10 cells were transplanted into the right flank of C57BL/6 female mice (6–8 weeks old) 10 days prior. Tumors in all groups were completely resected and the harvested tumor was grounded into single-cell suspensions and incubated in 60 μM NaClO at 37 °C with gentle shaking. The B16F10 tumor cell membrane proteins (BMAg) were then extracted by using a cell membrane and cytoplasmic protein extraction kit (Beyotime, Cat. P0033) and quantified by BCA protein assay (Beyotime, Cat. P0010). 100 µL of different formulations, including the PBS group, Free vac, Alum vac, L-Gel vac, and D-Gel vac (10 μg of CpG ODN, 100 μg of BMAg, and 60 μg of anti-PD-L1 per mouse), were directly injected into the resection site. Tumor volumes and body weights of mice were recorded every 2 days. After 40 days, 2 × 105 of B16F10 cells were re-challenged and tumor volumes of mice were recorded every 2 days. On day 100, spleens were removed. Single splenocytes suspension was collected after grinding, filtration, and lysis of red blood cells, which were then labeled with antibody cocktail (FITC-CD3, APC-CD8, PE/Cy7-CD4, APC/Cy7-CD62L, and PE-CD44 antibodies) for 30 min on ice. After staining, these cells were washed twice with PBS containing 3% FBS and fixed using 4% PFA before flow cytometry analysis.
Statistics and reproducibility
The data were expressed as mean ± SEM. Statistical analysis was performed by a two-tailed Student’s t-test or one-way ANOVA using the LSD or Tukey’s posttest or two-way ANOVA using Sidak’s posttest. Animal survival time was plotted using a Kaplan–Meier curve, and significant differences were determined with the log-rank test. The exact P values have been reported within the main figures itself. The times of those experiments which were repeated at least two times were included in the legends of figures. Other experiments were conducted once.
Reporting summary
Further information on research design is available in the Nature Portfolio Reporting Summary linked to this article.
Data availability
NMR data of the monomers and the copolymers derived from L/D-glutamate is included in Supplementary Fig. 2A–G. NMR data of the monomers and the copolymers derived from L/D-alanine is included in Supplementary Fig. 31B–E. NMR data of the monomers and the copolymers derived from L-/D-valine is included in Supplementary Fig. 32B–E. The data that support the findings of this study are available within the paper, Supplementary Information, and Source Data File. The RNASeq data is deposited on the Gene Expression Omnibus (GEO) repository with the accession code of GSE261299. Source data are provided with this paper.
References
Siegel, R. L., Miller, K. D., Wagle, N. S. & Jemal, A. Cancer statistics, 2023. CA Cancer J. Clin. 73, 17–48 (2023).
Sahin, U. et al. An RNA vaccine drives immunity in checkpoint-inhibitor-treated melanoma. Nature 585, 107–112 (2020).
Mandal, R. et al. Genetic diversity of tumors with mismatch repair deficiency influences anti-PD-1 immunotherapy response. Science 364, 485–491 (2019).
Xu, W., Atkins, M. B. & McDermott, D. F. Checkpoint inhibitor immunotherapy in kidney cancer. Nat. Rev. Urol. 17, 137–150 (2020).
Waldman, A. D., Fritz, J. M. & Lenardo, M. J. A guide to cancer immunotherapy: from T cell basic science to clinical practice. Nat. Rev. Immunol. 20, 651–668 (2020).
Lin, M. J. et al. Cancer vaccines: the next immunotherapy frontier. Nat. Cancer. 3, 911–926 (2022).
Blass, E. & Ott, P. A. Advances in the development of personalized neoantigen-based therapeutic cancer vaccines. Nat. Rev. Clin. Oncol. 18, 215–229 (2021).
Irvine, D. J., Aung, A. & Silva, M. Controlling timing and location in vaccines. Adv. Drug Deliv. Rev. 158, 91–115 (2020).
Tan, J., Ding, B., Teng, B., Ma, P. A. & Lin, J. Understanding structure-function relationships of nanoadjuvants for enhanced cancer vaccine efficacy. Adv. Funct. Mater. 32, 2111670 (2022).
Liu, J., Liew, S. S., Wang, J. & Pu, K. Bioinspired and biomimetic delivery platforms for cancer vaccines. Adv. Mater. 31, 2103790 (2021).
Zhang, Y. et al. Supramolecular assembled programmable nanomedicine as in situ cancer vaccine for cancer immunotherapy. Adv. Mater. 33, 2007293 (2021).
Ren, H. et al. Injectable, self-healing hydrogel adhesives with firm tissue adhesion and on-demand biodegradation for sutureless wound closure. Sci. Adv. 9, eadh4327 (2023).
Yu, S. et al. Injectable bioresponsive gel depot for enhanced immune checkpoint blockade. Adv. Mater. 30, e1801527 (2018).
Wang, T. et al. An adhesive immune-stimulating multifunctional hydrogel for potent tumor chemoimmunotherapy and postoperative wound healing promotion. Adv. Funct. Mater. 34, 2312360 (2023).
Shi, Y., Li, D., He, C. & Chen, X. Design of an injectable polypeptide hydrogel depot containing the immune checkpoint blocker anti-PD-L1 and doxorubicin to enhance antitumor combination therapy. Macromol. Biosci. 21, 2100049 (2021).
Zhang, Y. et al. 3D printing scaffold vaccine for antitumor immunity. Adv. Mater. 33, 2106768 (2021).
Najibi, A. J. et al. Scaffold vaccines for generating robust and tunable antibody responses. Adv. Func. Mater. 32, 2110905 (2022).
Super, M. et al. Biomaterial vaccines capturing pathogen-associated molecular patterns protect against bacterial infections and septic shock. Nat. Biomed. Eng. 6, 8–18 (2022).
Zhang, Z., He, C. & Chen, X. Designing hydrogels for immunomodulation in cancer therapy and regenerative medicine. Adv. Mater. 36, 2308894 (2023).
Guimarães, C. F., Gasperini, L., Marques, A. P. & Reis, R. L. The stiffness of living tissues and its implications for tissue engineering. Nat. Rev. Mater. 5, 351–370 (2020).
Fan, Q. et al. Degradation-resistant implanted biomaterials establish an immunosuppressive microenvironment that induces T cell exhaustion by recruiting myeloid cells. Fundam. Res. 2, 648–658 (2022).
Zhang, D. et al. Bio-inspired poly-DL-serine materials resist the foreign-body response. Nat. Commun. 12, 5327 (2021).
Fan, Q. et al. An implantable blood clot-based immune niche for enhanced cancer vaccination. Sci. Adv. 6, eabb4639 (2020).
Majedi, F. S. et al. Systemic enhancement of antitumour immunity by peritumourally implanted immunomodulatory macroporous scaffolds. Nat. Biomed. Eng. 7, 56–71 (2023).
Ding, J. et al. Enhanced antitumor chemo‐immunotherapy by local co‐delivery of chemotherapeutics, immune checkpoint blocking antibody and IDO inhibitor using an injectable polypeptide hydrogel. J. Polym Sci. 60, 1595–1609 (2022).
Chen, Z. et al. Injectable polypeptide hydrogel depots containing dual immune checkpoint inhibitors and doxorubicin for improved tumor immunotherapy and post-surgical tumor treatment. Pharmaceutics 15, 428 (2023).
Zhao, D., Rong, Y., Li, D., He, C. & Chen, X. Thermo-induced physically crosslinked polypeptide-based block copolymer hydrogels for biomedical applications. Regen. Biomater. 10, rbad039 (2023).
Griffin, D. R. et al. Activating an adaptive immune response from a hydrogel scaffold imparts regenerative wound healing. Nat. Mater. 20, 560–569 (2021).
Luo, Z. et al. A powerful CD8+ T-cell stimulating D-tetra-peptide hydrogel as a very promising vaccine adjuvant. Adv. Mater. 29, 1601776 (2016).
Su, Y. et al. Chiral polypeptide nanoparticles as nanoadjuvants of nanovaccines for efficient cancer prevention and therapy. Sci. Bull. 68, 284–294 (2023).
Jiang, H. et al. Chiral-selective antigen-presentation by supramolecular chiral polymer micelles. Adv. Mater. 35, e2208157 (2023).
Song, H. et al. Injectable polypeptide hydrogel for dual-delivery of antigen and TLR3 agonist to modulate dendritic cells in vivo and enhance potent cytotoxic T-lymphocyte response against melanoma. Biomaterials 159, 119–129 (2018).
Li, D., Zhao, D., He, C. & Chen, X. Crucial impact of residue chirality on the gelation process and biodegradability of thermoresponsive polypeptide hydrogels. Biomacromolecules 22, 3992–4003 (2021).
Singh, A. & Peppas, N. A. Hydrogels and scaffolds for immunomodulation. Adv. Mater. 26, 6530–6541 (2014).
Cao, D. et al. Unified therapeutic‐prophylactic vaccine demonstrated with a postoperative filler gel to prevent tumor recurrence and metastasis. Adv. Funct. Mater. 32, 2206084 (2022).
Sallusto, F., Lenig, D., Förster, R., Lipp, M. & Lanzavecchia, A. Two subsets of memory T lymphocytes with distinct homing potentials and effector functions. Nature 401, 708–712 (1999).
Lopez-Silva, T. L. et al. Chemical functionality of multidomain peptide hydrogels governs early host immune response. Biomaterials 231, 119667 (2020).
Mayoux, M. et al. Dendritic cells dictate responses to PD-L1 blockade cancer immunotherapy. Sci. Transl. Med. 12, eaav7431 (2020).
Oh, S. A. et al. PD-L1 expression by dendritic cells is a key regulator of T-cell immunity in cancer. Nat. Cancer. 1, 681–691 (2020).
Wherry, E. J. & Kurachi, M. Molecular and cellular insights into T cell exhaustion. Nat. Rev. Immunol. 15, 486–499 (2015).
Fujiwara, M., Garo, L. P. & Murugaiyan, G. PD1 blockade in cancer: impact on myeloid cells. Trends Cancer 6, 443–444 (2020).
Yang, M. et al. An immunomodulatory polypeptide hydrogel for osteochondral defect repair. Bioact. Mater. 19, 678–689 (2023).
Lu, C., Talukder, A., Savage, N. M., Singh, N. & Liu, K. JAK-STAT-mediated chronic inflammation impairs cytotoxic T lymphocyte activation to decrease anti-PD-1 immunotherapy efficacy in pancreatic cancer. Oncoimmunology 6, 1291106 (2017).
Wang, Y. et al. Chiral polypeptide thermogels induce controlled inflammatory response as potential immunoadjuvants. ACS Appl. Mater. Inter. 11, 8725–8730 (2019).
Kang, E. Y., Yeon, B., Moon, H. J. & Jeong, B. PEG-l-PAF and PEG-d-PAF: comparative study on thermogellation and biodegradation. Macromolecules 45, 2007–2013 (2012).
Ayuso, D. et al. Synthetic chiral light for efficient control of chiral light–matter interaction. Nat. Photonics. 13, 866–871 (2019).
Sachs, J., Günther, J.-P., Mark, A. G. & Fischer, P. Chiroptical spectroscopy of a freely diffusing single nanoparticle. Nat. Commun. 11, 4513 (2020).
Li, S. et al. Single- and multi-component chiral supraparticles as modular enantioselective catalysts. Nat. Commun. 10, 4826 (2019).
Cho, N. H. et al. Bioinspired chiral inorganic nanomaterials. Nat. Rev. Bioeng. 1, 88–106 (2023).
Xu, L. et al. Enantiomer-dependent immunological response to chiral nanoparticles. Nature 601, 366–373 (2022).
Wang, W. et al. The development of chiral nanoparticles to target NK cells and CD8(+) T cells for cancer immunotherapy. Adv. Mater. 34, 2109354 (2022).
Li, X. et al. A supramolecular “trident” for cancer immunotherapy. Adv. Funct. Mater. 31, 2100729 (2021).
Zhang, D. et al. Silk-inspired beta-peptide materials resist fouling and the foreign-body response. Angew. Chem. Int. Ed. 59, 9586–9593 (2020).
Rostam, H. M. et al. Immune-instructive polymers control macrophage phenotype and modulate the foreign body response in vivo. Matter 2, 1564–1581 (2020).
Lopez-Silva, T. L., Leach, D. G., Li, I. C., Wang, X. & Hartgerink, J. D. Self-assembling multidomain peptides: design and characterization of neutral peptide-based materials with pH and ionic strength independent self-assembly. ACS Biomater. Sci. Eng. 5, 977–985 (2019).
Vegas, A. J. et al. Combinatorial hydrogel library enables identification of materials that mitigate the foreign body response in primates. Nat. Biotechnol. 34, 345–352 (2016).
Pearce, H. A. et al. Thermogelling hydrogel charge and lower critical solution temperature influence cellular infiltration and tissue integration in an ex vivo cartilage explant model. J. Biomed. Mater. Res. Part A 111, 15–34 (2023).
Pogostin, B. H. et al. Multidomain peptide hydrogel adjuvants elicit strong bias towards humoral immunity. Biomater. Sci. 10, 6217–6229 (2022).
Wei, Y. et al. Chirality controls mesenchymal stem cell lineage diversification through mechanoresponses. Adv. Mater. 31, 1900582 (2019).
Acknowledgements
This work was financially supported by the National Natural Science Foundation of China (Projects 51973218, 52425312, C.H., 52203202, Y.R.), and the Scientific and Technological Development Projects of Jilin Province (20210204136YY, C.H.).
Author information
Authors and Affiliations
Contributions
Conceptualization: J.D., Y.R., X.C., and C.H. Methodology: J.D., Y.R., X.C., and C.H. Investigation: J.D., T.W., Z. Lin, Z. Li, J.Y, and F.L. Visualization: J.D. Supervision: Y.R., X.C., and C.H. Writing—original draft: J.D. Writing—review and editing: Y.R., X.C., and C.H.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Communications thanks Saman Maleki Vareki who co-reviewed with Megan Hong, Liming Bian and the other, anonymous, reviewer(s) for their contribution to the peer review of this work. A peer review file is available.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Source data
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Ding, J., Wang, T., Lin, Z. et al. Chiral polypeptide hydrogels regulating local immune microenvironment and anti-tumor immune response. Nat Commun 16, 1222 (2025). https://doi.org/10.1038/s41467-025-56137-w
Received:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s41467-025-56137-w
This article is cited by
-
Turning cold tumors into hot tumors to ignite immunotherapy
Molecular Cancer (2025)
-
Therapeutic vaccine strategies in cancer: advances, challenges, and future directions
Clinical Cancer Bulletin (2025)